Abstract
The capacity of Neisseria gonorrhoeae to cause disseminated gonococcal infection requires that such strains resist the bactericidal action of normal human serum. The bactericidal action of normal human serum against N. gonorrhoeae is mediated by the classical complement pathway through an antibody-dependent mechanism. The mechanism(s) by which certain strains of gonococci resist normal human serum is not fully understood, but alterations in lipooligosaccharide structure can affect such resistance. During an investigation of the biological significance of phosphoethanolamine extensions from lipooligosaccharide, we found that phosphoethanolamine substitutions from the heptose II group of the lipooligosaccharide β-chain did not impact levels of gonococcal (strain FA19) resistance to normal human serum or polymyxin B. However, loss of phosphoethanolamine substitution from the lipid A component of lipooligosaccharide, due to insertional inactivation of lptA, resulted in increased gonococcal susceptibility to polymyxin B, as reported previously for Neisseria meningitidis. In contrast to previous reports with N. meningitidis, loss of phosphoethanolamine attached to lipid A rendered strain FA19 susceptible to complement killing. Serum killing of the lptA mutant occurred through the classical complement pathway. Both serum and polymyxin B resistance as well as phosphoethanolamine decoration of lipid A were restored in the lptA-null mutant by complementation with wild-type lptA. Our results support a role for lipid A phosphoethanolamine substitutions in resistance of this strict human pathogen to innate host defenses.
Neisseria gonorrhoeae causes over 60 million cases of the sexually transmitted disease gonorrhea each year worldwide (6). Although most infections are uncomplicated and usually restricted to the lower urogenital tract, more invasive forms of disease that result in significant medical complications can occur. For example, entrance of N. gonorrhoeae into the bloodstream followed by dissemination, termed disseminated gonococcal infection (DGI), can occur in 1 to 3% of cases when particular strains are endemic in the community (26, 33, 38).
In contrast to gonococcal strains that cause pelvic inflammatory disease or salpingitis in women and to a lesser extent uncomplicated, urogenital tract infections in men or women, DGI strains can stably resist the bactericidal action of normal human serum (NHS) (33, 38). In some cases the bactericidal activity of NHS is mediated by natural immunoglobulin M (IgM) antibodies (14) that activate the classical complement pathway (CCP) (16, 40, 41). Bactericidal activity in NHS is an important host defense mechanism for prevention of invasive bloodstream disease due to the pathogenic Neisseria species (gonococci and meningococci) (9, 32, 34). For example, patients with defects in their terminal complement components often have recurrent bacteremias with these strict human pathogens (26). Although the multiplicity of mechanisms by which gonococci can resist killing by NHS remains to be fully defined, there is evidence that certain stably serum-resistant gonococci fail to bind bactericidal IgM (36). Further, a strong correlation exists between the ability of gonococci to bind the CCP regulatory protein C4b binding protein (C4BP), which dampens activation of the CCP, and stably serum-resistant phenotypes of N. gonorrhoeae (29).
Variations in surface structures of the pathogenic neisseriae have been invoked as being important in the capacity of these strict human pathogens to resist innate host defenses that function during infection both at mucosal surfaces and in the bloodstream. For instance, the structure of the lipooligosaccharide (LOS) possessed by gonococci (53, 54) and meningococci (17, 27) can vary at high frequencies (1-3, 13, 37, 48). LOS can also be modified by sialylation (43) or by the addition of phosphoethanolamine (PEA) to the heptose (HepII) group in the β-chain of the core oligosaccharide (2, 24, 25, 52) as well as to the 1 and 4′ positions of lipid A (8). Changes in LOS structure can have a profound impact on bacterial interactions with host cells and/or defensive systems (18, 20, 35, 45, 46). As an example, the NHS resistance expressed by certain gonococci can be lost by high-frequency, spontaneous mutations within the lgtABCDE operon (39), which encodes the glycosyl transferases responsible for extending the LOS α-chain (13).
We hypothesized that the PEA substitutions of gonococcal LOS could influence the susceptibility of this pathogen to mediators of innate host defense. In support of this hypothesis, previous work (28) has shown that PEA attached to position 6 of HepII of meningococcal LOS can form an amide linkage with complement component C4b and enhances susceptibility of N. meningitidis to the bactericidal action of NHS. Additionally, PEA attached to the lipid A of meningococcal LOS enhances bacterial resistance to cationic antimicrobial peptides (CAMPs), including the human cathelicidin LL-37 (49), but does not influence resistance to killing by NHS (8). Based on these examples with meningococci, we tested whether loss of PEA from the HepII group of the LOS β-chain or lipid A would alter gonococcal susceptibility to NHS or CAMPs. We report that loss of PEA substitution of lipid A significantly increases gonococcal susceptibility to both polymyxin B (PB) and NHS and propose that the presence of PEA on lipid A contributes to the ability of gonococci to resist mediators of innate host defense.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
NHS-resistant Neisseria gonorrhoeae strain FA19 (4) was the main gonococcal strain used in all experiments. It was grown in GCB broth containing defined supplements I and II or on GCB agar with supplements under 3.8% CO2 (vol/vol) at 37°C as described previously (4, 40). Escherichia coli strain DH5α MCR or TOP10 (Invitrogen, Carlsbad, CA) was cultured in Luria-Bertani (LB) broth or on LB agar (12, 19).
Construction of mutant strains and complementation analysis.
Piliated colony variants of strain FA19 were transformed with plasmid or chromosomal DNA preparations bearing insertionally inactivated lpt3, lpt6, or lptA genes that had been prepared previously from N. meningitidis strain NMB (17, 50). Transformation was conducted by the method of Gunn and Stein (15). Kanamycin- or spectinomycin (Spc)-resistant transformants with inactivated lpt3 or lptA genes were selected on GCB agar containing 50 μg of kanamycin or Spc per ml, respectively, while erythromycin (Ery)-resistant transformants bearing an inactivated lpt6 gene were selected using 1 μg of Ery per ml. Antibiotic-resistant transformants were screened on GCB plates containing the appropriate antibiotic. Inactivation of specific genes was verified by PCR using the following oligonucleotide primer sets: LPT3F (5′-CCGACAAATGACAAACCACTT-3′) and LPT3R (5′-CCGCGTACTTGGTTTTTCATA-3′) for lpt3, LPT6A (5′-CTTCGGTCTGGTTTGTGGTGC-3′) and LPT6B (5′-GCAGATAACGGTCGAAACTTTCC-3′) for lpt6, and LPTA1 (5′-GGCGGTGTCTTACCAAGAAAT-3′) and LPTA2 (5′-TCGGGTGTTTCGGACACATAT-3′) for lptA. PCR products were subjected to agarose gel electrophoresis and visualized by staining with ethidium bromide.
Complementation analysis employed the neisserial insertional complementation system (NICS) described by Skaar et al. (42) and used pGCC4 (kindly provided by H. Seifert, Northwestern University, Chicago, IL), which contains an isopropyl-β-d-thiogalactoside (IPTG)-regulated lac promoter. pGCC4 was digested with PacI and PmeI and purified by agarose gel electrophoresis for subsequent cloning of lptA. The lptA coding sequence and 93 bp of upstream DNA (containing all of the predicted lptA promoter except three bases in the −35 region) were PCR amplified from a chromosomal DNA preparation obtained from N. meningitidis strain M7 (19) using oligonucleotide primers LPTAFPAC (5′-TTAATTAACCCTGCTTTGCTCCGTT-3′) and LPTARPME (5′-GTTTAAACACATATGCCGTGAAGG-3′). The resulting PCR product was purified by agarose gel electrophoresis and cloned into pBAD (Invitrogen, Carlsbad, CA) using the TOPO TA reporter kit. Plasmid DNA was prepared from a representative transformant, and the insert DNA was removed by digestion with PacI and PmeI. The resulting fragment was cloned into pGCC4 that had been digested previously with PacI and PmeI. Plasmid DNA was then prepared from a transformant and digested with ClaI to remove the DNA region containing the origin of replication (19). The digestion reaction mixture was subjected to agarose gel electrophoresis, and the 8.2-kb fragment containing lptA and the NICS region was recovered. This fragment was then used to transform FA19 lptA::spc for resistance to Ery (1 μg per ml) as described previously (12). The presence of the complementing lptA between lctP and aspC was confirmed by PCR using oligonucleotide primers LCTP and ASPC1 as previously described (12, 19). The nucleotide sequence of the complementing lptA gene was confirmed.
In order to examine expression of lptA in the complemented strain, gonococci were grown in GCB broth in the presence or absence of 1 mM IPTG as described previously (19) and total RNA was extracted after 2 hours of growth. Reverse transcriptase PCR (RT-PCR) was used to detect the full-length transcript. As described previously (19), the rnpB transcript was used as a control to demonstrate equivalent amounts of RNA in the reaction and specificity of IPTG induction of lptA gene expression. The rnpB transcript was reverse transcribed using oligonucleotide primer 5′-GGACAGGCGGTAAGCCGGTTC-3′, and PCR was performed with this primer and 5′-CGGGACGGGCAGACAGTCGC-3′. RT-PCRs were performed in the absence of RT to confirm the lack of contaminating DNA.
Bacterial killing by NHS and sensitivity to PB.
The bactericidal assay employed in this investigation used pooled NHS from healthy donors as described previously (23, 30). To distinguish complement pathway-specific killing, C1q-depleted or factor B-depleted serum (Complement Technologies, Inc., Tyler, TX) was used to selectively inactivate the CCP and mannan-binding lectin (MBL) pathways or the alternative complement pathway (ACP), respectively. In control experiments, depleted sera were reconstituted with purified C1q (final concentration of 100 μg/ml) or factor B (final concentration of 200 μg/ml) as required.
The MIC of PB was determined as described by Tzeng et al. (49) using GCB agar. In some PB assays, GCB agar was supplemented with 1 mM IPTG.
LOS and lipid A chemical analyses.
Gonococci were grown in 12-liter batch cultures using GCB broth with defined supplements as described above. Bacteria were harvested by centrifugation, washed with sterile distilled water, and treated with formalin. LOS was extracted from the formalin-treated dry cell pellet by hot-phenol water extraction (51). The phenol-saturated water layer (top layer) was dialyzed extensively to remove phenol and was sequentially treated with DNase, RNase, and proteinase K. Enzyme-treated LOS was dialyzed extensively against water using 2,000-molecular-weight-cutoff dialysis membranes; dialysate was centrifuged at 100,000 × g for 4 h at room temperature, and the precipitate was dissolved in water and lyophilized. The yield was 20 mg of LOS from 1 g of dried cell preparation. Constituent sugar and fatty acid composition analyses of all the LOS samples were done by gas chromatography-mass spectrometry as analyses of their trimethyl silyl methyl glycosides and fatty acid methyl esters, respectively (55). Glycosyl linkages were determined by the preparation and gas chromatography-mass spectrometry analysis of partially methylated alditol acetates. Partially methylated alditol acetates were prepared as described by Ciucanu and Kerek (7).
To isolate oligosaccharides and lipid A separately from LOS preparations, samples were extracted with a 9:1 ethanol-water mixture to remove contaminating phospholipids and then lyophilized. Oligosaccharides were released from LOS by mild acid hydrolysis (1% [vol/vol] acetic acid at 100°C for 2 h). The lipid A portions were precipitated by low-speed centrifugation, and supernatants containing oligosaccharides were lyophilized and used for further analysis. Because mild acid hydrolysis partially removes the aglycone from the reducing end of the lipid A, LOS was hydrolyzed using milder conditions, which allows retention of the aglycone. This milder hydrolysis procedure was performed with 20 mM sodium acetate buffer, pH 4.5, containing 1% sodium dodecyl sulfate (SDS) at 100°C for 1 h as described by Caroff et al. (5). Oligosaccharide mass and lipid A mass were determined by matrix-assisted laser desorption ionization—time of flight mass spectrometry (MALDI-TOF MS). Oligosaccharides were dissolved in water and mixed in a 1:1 (vol/vol) ratio with 0.5 M 2,5-dihydroxybenzoic acid and spotted on a 100-well stainless steel MALDI plate. Lipid A was dissolved in a 3:1 chloroform-methanol mixture, mixed in 1:1 (vol/vol) ratio with 0.5 M trihydroxyacetophenone matrix, and spotted on a 100-well MALDI plate. The spectra were collected in positive mode for oligosaccharides and in the negative mode for lipid A. Analysis was done in delayed reflectron mode by using a 337-nm N2 laser.
In certain experiments, proteinase K digests of whole gonococci or purified LOS were subjected to SDS-polyacrylamide gel electrophoresis and the resolved LOS species were visualized by silver staining (22).
RESULTS AND DISCUSSION
NHS and PB resistance in gonococcal strain FA19 requires expression of lptA.
PEA substitution of lipid A in Salmonella enterica serovar Typhimurium and N. meningitidis is known to be important in enabling these pathogens to resist killing by CAMPs (21, 47, 49); in these studies PB was used as a model CAMP. In order to determine if PEA residues substituted on the oligosaccharide or the lipid A of gonococcal LOS are important in causing gonococci to be susceptible to CAMPs, we created mutants of N. gonorrhoeae strain FA19 that lacked PEA substitution at the 3 or 6 position (or both) of the HepII group in the LOS β-chain or on lipid A. We found that insertional inactivation of lpt3 (lpt3::kan) or lpt6 (lpt6::ery), which is responsible for transferring PEA to HepII at the 3 and 6 positions, respectively, or inactivation of both genes did not increase gonococcal susceptibility to PB (Table 1). We found that loss of 6-PEA resulted in a modest (twofold) increase in PB resistance. In contrast, insertional inactivation of lptA (lptA::spc), which abrogated the addition of PEA to the 4′ position of lipid A (see below), either in a wild-type (wt) background (strain FA19) or in strains bearing coresident lpt3 and/or lpt6 mutations significantly increased (64-fold) gonococcal susceptibility to PB (Table 1).
TABLE 1.
Loss of lipid A PEA enhances gonococcal susceptibility to PB
| Strain | PB MIC (μg/ml)a |
|---|---|
| FA19 (wt) | 100 |
| FA19 lptA::spc | 1.56 |
| FA19 lpt3::kan | 100 |
| FA19 lpt6::ery | 200 |
| FA19 lptA::spc lpt3::kan | 1.56 |
| FA19 lptA::spc lpt6::ery | 1.56 |
| FA19 lpt3::kan lpt6::ery | 200 |
| FA19 lptA::spc lpt3::kan lpt6::ery | 1.56 |
The results are representative of three independent experiments.
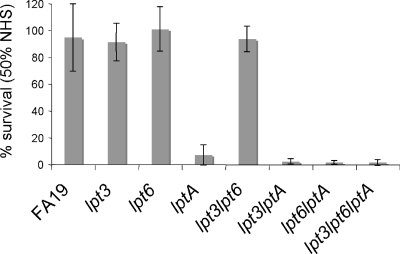
NHS killing of gonococci depends on natural bactericidal antibody, primarily IgM, which is directed against an epitope(s) located within the LOS inner core region (14). Pathogenic neisserial LOS is also a target for complement components C3 and C4b (11, 23). C4b binds to meningococci through an amide linkage with PEA attached to HepII (6-PEA) (28). To determine if loss of PEA from the HepII of the gonococcal LOS β-chain and/or lipid A would change the NHS resistance property of strain FA19 (4, 40), transformants bearing inactivated lpt3, lpt6, or lptA genes described above were examined in serum bactericidal assays. The results (Fig. 1) showed that the lptA-null mutant, but not parental strain FA19 or its individual lpt3 and lpt6 mutants or the double lpt3 lpt6 mutant, was susceptible to NHS. Additionally, lptA::spc strains bearing coresident lpt3 and/or lpt6 mutations were also highly sensitive to NHS (Fig. 1).
FIG. 1.
NHS susceptibility of 4′-PEA-deficient gonococcal strains. Serum bactericidal assays were performed using the wt strain FA19 and FA19 mutant strains that lack PEA substitutions (the lpt3 strain lacks 3-PEA from HepII, the lpt6 strain lacks 6-PEA from HepII, and the lptA strain lacks PEA attached to lipid A) using 50% (vol/vol) NHS. Values represent the average percent survival calculated from three or more independent experiments.
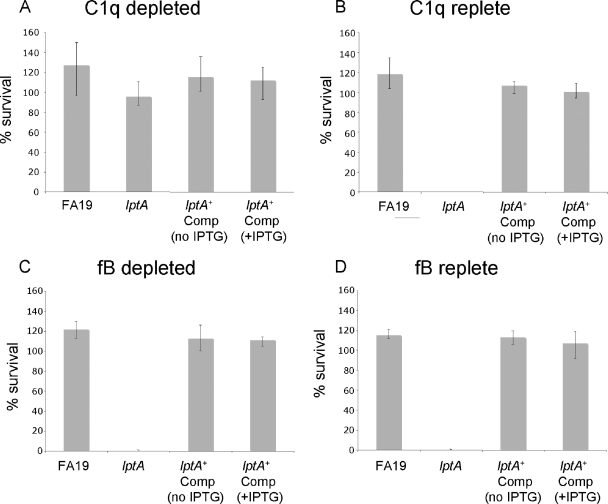
Gonococci can activate both the CCP and ACP (10), but killing involves the CCP predominantly (16, 40). To determine the specific complement pathway involved in killing the lptA mutant, serum bactericidal assays were performed using either C1q (CCP and MBL inactivated) or factor B-depleted (ACP-selectively inactivated) sera. C1q-depleted serum (Fig. 2A), but not the factor B-depleted serum (Fig. 2C), lacked bactericidal activity against the lptA mutant, indicating that the CCP is required for NHS killing of the lptA mutant (Fig. 2). Adding purified C1q back to C1q-depleted serum restored bactericidal activity (Fig. 2B).
FIG. 2.
The CCP (but not the ACP) is required for serum killing of the lptA::spc mutant. Serum bactericidal assays were performed using FA19, its lptA mutant (lptA::spc; labeled lptA), and the complemented lptA mutant (lptA::spc lptA+; labeled lptA+ Comp) using 20% (vol/vol) C1q-depleted (C1q−) NHS without CCP and MBL-deficient (A) or factor B-depleted (fB−) NHS that was ACP deficient (C). In control assays CCP activity was restored to C1q− serum by the addition of purified C1q (final concentration of 100 μg/ml) (B), and ACP activity was restored to fB− sera by the addition of fB (final concentration of 200 μg/ml) (D). Values represent the average percent survival calculated from three or more independently performed experiments. Where indicated, the assay was performed in the presence or absence of 1 mM IPTG.
Complementation of lptA::spc restores resistance to NHS and CAMP.
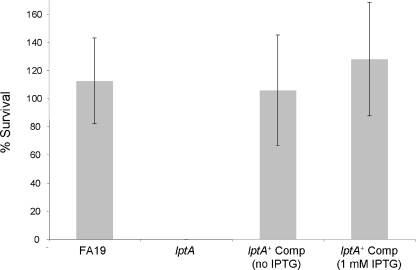
LptA-mediated resistance to NHS and PB was verified in gonococcal strain FA19 by complementing the lptA mutation in FA19 lptA::spc with a wt lptA gene at a second site in the gonococcal chromosome (see Materials and Methods). Using the complemented strain of FA19 lptA::spc (FA19 lptA::spc lptA+), we found that ectopic expression of lptA restored resistance to NHS to a level similar to resistance in wt strain FA19 (Fig. 2 and 3). We noted, however, that NHS resistance expressed by the complemented strain was independent of the presence of IPTG (Fig. 2 and 3), suggesting that the 93-bp DNA sequence upstream of the lptA sequence inserted between lctP and aspC contains a promoter element that can drive transcription of the inserted lptA sequence, independently of the lac promoter that can also be used to transcribe lptA. In this respect, RT-PCR analysis confirmed expression of lptA in FA19 lptA::spc lptA+ in the absence of IPTG, but expression was lower than that in the presence of IPTG (data not shown). As a control, we also created a transformant of FA19 lptA::spc bearing the lctP-aspC sequence in pGCC4 DNA and found that this transformant remained sensitive to NHS (data not presented).
FIG. 3.
Complementation of the lptA::spc mutant restores NHS resistance. Serum bactericidal assays were performed using FA19, its lptA mutant (lptA::spc; labeled lptA), and the complemented lptA mutant (lptA::spc lptA+; labeled lptA+ Comp) using 50% (vol/vol) NHS. Values represent the average percent survival calculated from three or more independent experiments. Where indicated, the assay was performed in the presence or absence of 1 mM IPTG.
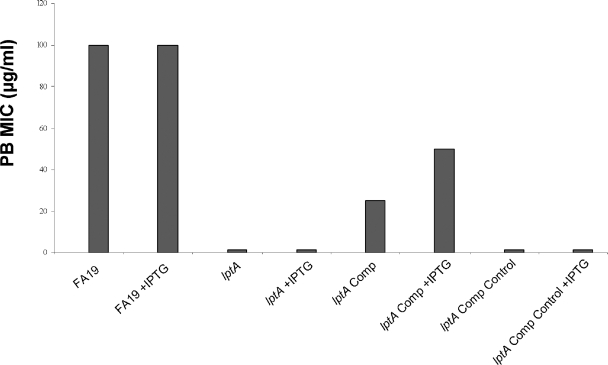
The lptA::spc lptA+ complemented strain also expressed increased (16- to 32-fold) resistance to PB (Fig. 4), but the level of resistance was two- to fourfold lower than that in the wt strain FA19 and depended on the presence of IPTG in the GCB agar. The control strain bearing the pGCC4 sequence but lacking lptA between aspC and lctP (identified as “Comp Control” in Fig. 4) remained highly sensitive to PB, indicating that expression of the inserted lptA sequence in the lptA::spc lptA+ strain was responsible for increased resistance to PB in the complemented strain.
FIG. 4.
PB susceptibility (PB MIC) of gonococcal strains expressing lptA. PB susceptibility was determined by the method of Tzeng et al. (49) using wt strain FA19, its lptA::spc mutant (labeled lptA), the lptA+ complemented derivative (labeled lptA Comp), and the complemented control (labeled Comp Control), which lacks the ectopically expressed lptA gene but contains the NICS sequence from pGCC4 (see Materials and Methods). The assay was performed in the presence or absence of 1 mM IPTG, and the results are representative of two independent experiments.
PEA modification of lipid A due to expression of lptA.
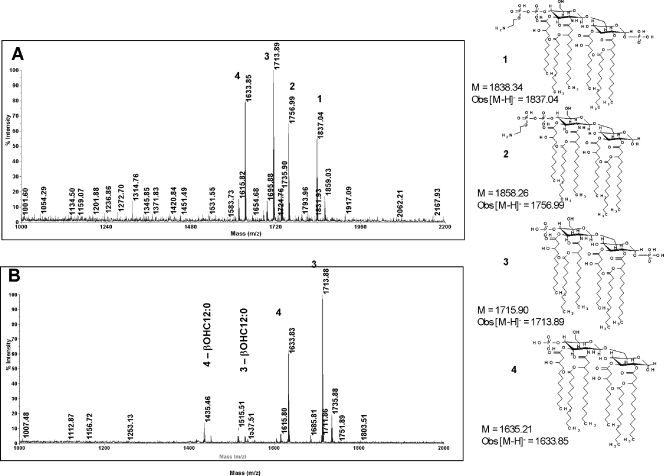
In meningococci, inactivation of lptA results in a loss of PEA residues from both the 1 and 4′ positions of lipid A (8, 49). Meningococci lacking lipid A PEA exhibit decreased resistance to CAMPs but are not altered in their ability to resist NHS (8). Although the meningococcal LptA is homologous (98 to 99% identity at the LptA amino acid level) to gonococcal LptA, the role of LptA in adding PEA to gonococcal LOS has not been established unambiguously. To confirm that the lptA::spc mutation results in loss of PEA substitution on gonococcal lipid A without changing PEA substitutions at other locations within LOS or otherwise altering LOS structures, chemical analysis of LOS-derived carbohydrates (oligosaccharides) and lipid A from strains FA19 (wt), FA19 lptA::spc, and FA19 lptA::spc lptA+ was performed; the lptA::spc lptA+ complemented strain was analyzed following growth in both the presence and the absence of IPTG. All strains produced a predominant 3.6-kDa LOS species as judged by silver staining of proteinase K digests that were separated by SDS-polyacrylamide gel electrophoresis (data not presented). Structural and compositional analyses of the LOS carbohydrate from the three strains also did not reveal any differences (data not presented). However, analyses of the lipid A showed that the lptA::spc mutant lacked PEA attached to the 4′ position (4′-PEA) that was present in parent strain FA19 (Fig. 5). Lipid A from FA19 (wt) LOS shows major [M-H]− ions of m/z 1,837.04, 1,756.99, 1,713.89, and 1,633.85. These ions correspond to structures 1, 2, 3, and 4, respectively, shown in Fig. 5. Structures 1 and 2 contain the 4′-PEA substituent, while structures 3 and 4 lack this substituent. The lipid A from FA19 lptA::spc mutant LOS contains major ions of m/z 1,633.83 and 1,713.88, which are consistent with structures 3 and 4, which are devoid of the 4′-PEA substituent. Minor ions of m/z 1,436.46 and 1,515.51 are also present, which are due to the loss of a β-OH C12:0 fatty acyl component from structures 3 and 4, respectively. Unlike meningococci (49, 50), none of the major lipid A species produced by gonococcal strain FA19 contained PEA substituents at the 1 position (Fig. 5). A minor species containing both 1-PEA and 4′-PEA was detected in the FA19 lipid A preparation. This minor species was absent in the lipid A prepared from the FA19 lptA::spc mutant (data not presented), suggesting that the gonococcal LptA may be bifunctional, able to add PEA to both the 1 and 4′ positions of lipid A, but that, in gonococci, PEA addition to the 4′ position of lipid A is the preferred substitution. Alternatively, a second lipid A PEA transferase may exist in Neisseria, but its function may require the presence of 4′-PEA a priori on the lipid A substrate. More detailed genetic and chemical studies will be required to fully understand the complexity (and potential differences) of lipid A biosynthesis in gonococci and meningococci, particularly as the differences relate to phosphoforms that contain PEA.
FIG. 5.
MALDI-TOF MS spectra for gonococcal lipid A. Shown are the data for the lipid A from parent strain FA19 (A) and transformant strain FA19 lptA::spc (B). The lipid A structures that are consistent with the [M-H]− ions observed in the MALDI-TOF MS spectra are also shown in this figure. The calculated molecular weights for the structures (M) are given along with the observed [M-H]− ion for the lipid A from FA19 (wt); the single-digit numbers above ion peaks refer to structures shown to the right of panels A and B. Obs, observed.
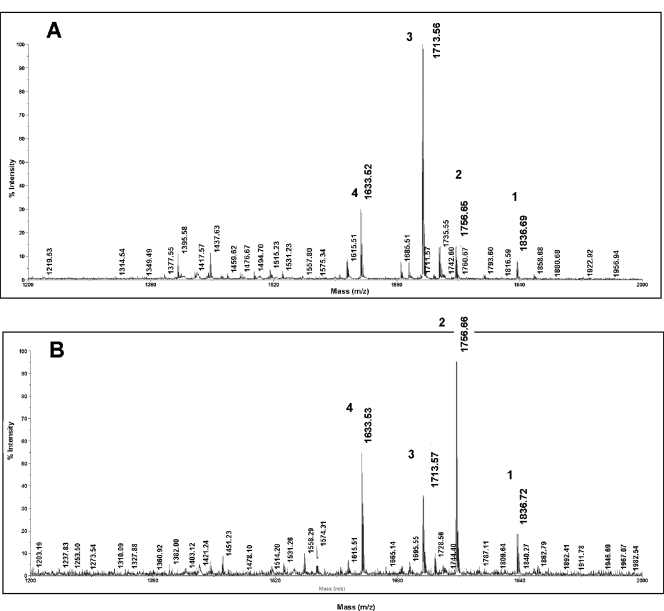
The lipid A from the complemented strain, FA19 lptA::spc lptA+, showed the same ions (Fig. 6B) and, therefore, contained the same lipid A structures 1, 2, 3, and 4 as those observed for FA19 lipid A (Fig. 5). Of note, significantly less 4′-PEA (compare [M-H]− ions of m/z 1,756.66 and 1,836.72 in Fig. 6A and B) was detected on the lipid A from the lptA complemented strain when it was grown in the absence of IPTG (Fig. 6A) than when it was grown in the presence of IPTG (Fig. 6B). This finding is consistent with RT-PCR expression studies that detected less lptA mRNA in the absence of IPTG (data not shown). Despite this difference in 4′-PEA levels, the amount produced in the absence of IPTG appears to be sufficient to confer full resistance to NHS but insufficient for wt levels of resistance to the CAMP PB. Nevertheless, the addition of IPTG to the complemented strain, but not the control strain lacking the ectopically expressed lptA, increased the amount of lipid A 4′-PEA and resulted in increased resistance to PB (Fig. 4).
FIG. 6.
Complementation of lptA::spc restores 4-PEA substitution of lipid A. Lipid A was purified from complemented strain FA19 lptA::spc lptA+ grown in the absence (A) or presence (B) of 1 mM IPTG. Shown are the MALDI-TOF MS spectra for the lipid A species. Note that for the lipid A prepared from gonococci grown in the presence of IPTG, the levels of the two major PEA-containing species, with [M-H]− ions of m/z 1,836.72 and 1,756.66, are elevated compared to the species in the same strain grown in the absence of IPTG. The single-digit numbers above ion peaks refer to structures shown in Fig. 5B.
Tzeng et al. (49) reported a correlation between susceptibility to CAMP and PEA-deficient lipid A meningococci. PEA modification of gonococcal lipid A was also found to be important in the relative resistance of strain FA19 to a model CAMP, PB (Table 1 and Fig. 4), presumably because it reduces simultaneously the local electronegativity and hydrophobicity of lipid A. These characteristics will influence both the ionic binding of CAMPs to the bacterial cell surface (44) and insertion of membrane-damaging agents such as CAMPs and the membrane attack complex (C5b-9) of complement. Substitution of PEA of the lipopolysaccharides produced by enteric pathogens (21, 47) also enhances CAMP resistance. The ability of bacteria to modify their surface structure by reducing negatively charged surface groups can diminish binding of CAMPs, resulting in prolonged survival of bacteria during infection (44).
The ability of certain strains of gonococci (e.g., DGI isolates) to stably resist the bactericidal action of NHS has been linked, in part, to structural changes in the carbohydrate component of the core oligosaccharide of LOS (28, 31, 39, 41, 46). Phase-variable changes in the nucleotide repeat sequences within the lgtA and lgtC genes of the lgtABCDE operon (13) are important in determining resistance to killing by NHS expressed by strain FA19 (39). In particular, strain FA19 with a phase-off lgtA produces a 3.6-kDa LOS species that contains an α-chain of Gal-Glc-HepI-Kdo (3-deoxy-d-manno-octulosonic acid) and is resistant to NHS, while FA19 with a phase-on lgtA produces an extended α-chain of Gal-GlcNac-Gal-Glc-HepI-Kdo and is susceptible to NHS. An antibody-dependent process involving the CCP mediates NHS killing of the phase-on derivative. Natural IgM antibodies that mediate killing of NHS-sensitive gonococci are directed against LOS species that contain extended α-chain species (e.g., Gal-GlcNac-Gal-Glc-HepI-Kdo). An assumption has been that the NHS resistance property of gonococci producing the truncated, 3.6-kDa LOS is due to the loss (or shielding) of a LOS epitope recognized by IgM bactericidal antibody (36). Our data demonstrating that loss of PEA from lipid A in a strain that produces the 3.6-kDa LOS results in NHS susceptibility require a reevaluation of this hypothesis. In particular, the complement regulatory protein C4BP, which interacts with most gonococcal porin 1A (Por1A) and select Por1B molecules on gonococci, contributes significantly to the NHS resistance property of gonococci, including strain FA19. The structure of LOS, particularly the length of the HepI chain, modulates the ability of C4BP to bind to Por (28), and we hypothesize that loss of PEA from lipid A may impact similarly on the efficacy of C4BP binding. In support of this, we have recently found that loss of lipid A PEA significantly reduces C4BP binding to intact gonococci (L. A. Lewis et al., unpublished data). Based on the data presented herein, we propose that gonococcal lipid A substitution with PEA is an important determinant in the capacity of this strict human pathogen to resist innate host defenses such as the CCP and CAMPs. In other experiments, we observed (data not presented) that insertional inactivation of lptA in other naturally occurring NHS-resistant gonococci resulted in loss of NHS and PB resistance, indicating that PEA modification of lipid A is broadly important in the ability of gonococci to resist killing by NHS and CAMPs. In contrast to our results with gonococci, Cox et al. (8) have reported no difference in survival of the wt and lptA mutants of encapsulated N. meningitidis when isogenic strains were exposed to serum. Thus, we propose that the lack of encapsulation of gonococci, in addition to other differences in the chemistry and perhaps the organization of the outer membranes of gonococci and meningococci, influences the interaction of complement proteins on these bacterial surfaces, thereby impacting resistance to complement-mediated killing.
Acknowledgments
We thank L. Pucko for help in manuscript preparation and P. Johnson for help with the figures.
This work was supported by funds from a VA Merit Award grant to W.M.S. from the Department of Veterans Affairs; NIH grants AI322725 (L.A.L., S.R., and P.A.R.), AI054544 (L.A.L. and S.R.), AI 33517 (D.S.S.), and AI062755 (W.M.S.); and DOE grant DE-FG02-98ER20307 to the CCRC. W.M.S. was supported by a Senior Research Career Scientist Award from the VA Medical Research Service.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 29 December 2008.
REFERENCES
- 1.Apicella, M. A., M. Shero, G. A. Jarvis, J. M. Griffiss, R. E. Mandrell, and H. Schneider. 1987. Phenotypic variation in epitope expression of the Neisseria gonorrhoeae lipooligosaccharide. Infect. Immun. 551755-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee, A., R. Wang, S. N. Uljon, P. A. Rice, E. C. Gotschlich, and D. C. Stein. 1998. Identification of the gene (lgtG) encoding the lipooligosaccharide beta chain synthesizing glucosyl transferase from Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. USA 9510872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burch, C. L., R. J. Danaher, and D. C. Stein. 1997. Antigenic variation in Neisseria gonorrhoeae: production of multiple lipooligosaccharides. J. Bacteriol. 179982-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannon, J. G., T. J. Lee, L. F. Guymon, and P. F. Sparling. 1981. Genetics of serum resistance in Neisseria gonorrhoeae: the sac-1 genetic locus. Infect. Immun. 32547-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caroff, M., A. Tacken, and L. Szabo. 1988. Detergent-accelerated hydrolysis of bacterial endotoxins and determination of the anomeric configuration of the glycosyl phosphate present in the “isolated lipid A” fragment of the Bordetella pertussis endotoxin. Carbohydr. Res. 175273-282. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2007. Sexually transmitted disease surveillance 2005 supplement. Gonococcal Isolate Surveillance Project (GISP) annual report 2005. Centers for Disease Control and Prevention, Atlanta, GA.
- 7.Ciucanu, I., and F. Kerek. 1984. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 131209-217. [Google Scholar]
- 8.Cox, A. D., J. C. Wright, J. Li, D. W. Hood, E. R. Moxon, and J. C. Richards. 2003. Phosphorylation of the lipid A region of meningococcal lipopolysaccharide: identification of a family of transferases that add phosphoethanolamine to lipopolysaccharide. J. Bacteriol. 1853270-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Densen, P. 1989. Interaction of complement with Neisseria meningitidis and Neisseria gonorrhoeae. Clin. Microbiol. Rev. 2(Suppl.)S11-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Densen, P., C. M. McRill, and S. C. Ross. 1988. Assembly of the membrane attack complex promotes decay of the alternative pathway C3 convertase on Neisseria gonorrhoeae. J. Immunol. 1413902-3909. [PubMed] [Google Scholar]
- 11.Edwards, J. L., and M. A. Apicella. 2002. The role of lipooligosaccharide in Neisseria gonorrhoeae pathogenesis of cervical epithelia: lipid A serves as a C3 acceptor molecule. Cell. Microbiol. 4585-598. [DOI] [PubMed] [Google Scholar]
- 12.Folster, J. P., V. Dhulipala, R. A. Nicholas, and W. M. Shafer. 2007. Differential regulation of ponA and pilMNOPQ expression by the MtrR transcriptional regulatory protein in Neisseria gonorrhoeae. J. Bacteriol. 1894569-4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gotschlich, E. C. 1994. Genetic locus for the biosynthesis of the variable portion of Neisseria gonorrhoeae lipooligosaccharide. J. Exp. Med. 1802181-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffiss, J. M., G. A. Jarvis, J. P. O'Brien, M. M. Eads, and H. Schneider. 1991. Lysis of Neisseria gonorrhoeae initiated by binding of normal human IgM to a hexosamine-containing lipooligosaccharide epitope(s) is augmented by strain-specific, properdin-binding-dependent alternative complement pathway activation. J. Immunol. 147298-305. [PubMed] [Google Scholar]
- 15.Gunn, J. S., and D. C. Stein. 1996. Use of a non-selective transformation technique to construct a multiply restriction/modification-deficient mutant of Neisseria gonorrhoeae. Mol. Gen. Genet. 251509-517. [DOI] [PubMed] [Google Scholar]
- 16.Joiner, K. A., K. A. Warren, E. J. Brown, J. Swanson, and M. M. Frank. 1983. Studies on the mechanism of bacterial resistance to complement-mediated killing. IV. C5b-9 forms high molecular weight complexes with bacterial outer membrane constituents on serum-resistant but not on serum-sensitive Neisseria gonorrhoeae. J. Immunol. 1311443-1451. [PubMed] [Google Scholar]
- 17.Kahler, C. M., A. Datta, Y. L. Tzeng, R. W. Carlson, and D. S. Stephens. 2005. Inner core assembly and structure of the lipooligosaccharide of Neisseria meningitidis: capacity of strain NMB to express all known immunotype epitopes. Glycobiology 15409-419. [DOI] [PubMed] [Google Scholar]
- 18.Kahler, C. M., and D. S. Stephens. 1998. Genetic basis for biosynthesis, structure, and function of meningococcal lipooligosaccharide (endotoxin). Crit. Rev. Microbiol. 24281-334. [DOI] [PubMed] [Google Scholar]
- 19.Kamal, N., C. Rouquette-Loughlin, and W. M. Shafer. 2007. The TolC-like protein of Neisseria meningitidis is required for extracellular production of the repeats-in-toxin toxin FrpC but not for resistance to antimicrobials recognized by the Mtr efflux pump system. Infect. Immun. 756008-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerwood, D. E., H. Schneider, and R. Yamasaki. 1992. Structural analysis of lipooligosaccharide produced by Neisseria gonorrhoeae, strain MS11mk (variant A): a precursor for a gonococcal lipooligosaccharide associated with virulence. Biochemistry 3112760-12768. [DOI] [PubMed] [Google Scholar]
- 21.Lee, H., F. F. Hsu, J. Turk, and E. A. Groisman. 2004. The PmrA-regulated pmrC gene mediates phosphoethanolamine modification of lipid A and polymyxin resistance in Salmonella enterica. J. Bacteriol. 1864124-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesse, A. J., A. A. Campagnari, W. E. Bittner, and M. A. Apicella. 1990. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J. Immunol. Methods 126109-117. [DOI] [PubMed] [Google Scholar]
- 23.Lewis, L. A., S. Ram, A. Prasad, S. Gulati, S. Getzlaff, A. M. Blom, U. Vogel, and P. A. Rice. 2008. Defining targets for complement components C4b and C3b on the pathogenic neisseriae. Infect. Immun. 76339-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackinnon, F. G., A. D. Cox, J. S. Plested, C. M. Tang, K. Makepeace, P. A. Coull, J. C. Wright, R. Chalmers, D. W. Hood, J. C. Richards, and E. R. Moxon. 2002. Identification of a gene (lpt-3) required for the addition of phosphoethanolamine to the lipooligosaccharide inner core of Neisseria meningitidis and its role in mediating susceptibility to bactericidal killing and opsonophagocytosis. Mol. Microbiol. 43931-943. [DOI] [PubMed] [Google Scholar]
- 25.O'Connor, E. T., A. Piekarowicz, K. V. Swanson, J. M. Griffiss, and D. C. Stein. 2006. Biochemical analysis of Lpt3, a protein responsible for phosphoethanolamine addition to the lipooligosaccharide of pathogenic Neisseria. J. Bacteriol. 1881039-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen, B. H., T. J. Lee, R. Snyderman, G. F. Brooks, and P. F. Sparling. 1979. N. meningitidis and N. gonorrhoeae bacteremia associated with C6, C7 or C8 deficiency. Ann. Intern. Med. 90917-920. [DOI] [PubMed] [Google Scholar]
- 27.Rahman, M. M., D. S. Stephens, C. M. Kahler, J. Glushka, and R. W. Carlson. 1998. The lipooligosaccharide (LOS) of Neisseria meningitidis serogroup B strain NMB contains L2, L3, and novel oligosaccharides, and lacks the lipid-A 4′-phosphate substituent. Carbohydr. Res. 307311-324. [DOI] [PubMed] [Google Scholar]
- 28.Ram, S., A. D. Cox, J. C. Wright, U. Vogel, S. Getzlaff, R. Boden, J. Li, J. S. Plested, S. Meri, S. Gulati, D. C. Stein, J. C. Richards, E. R. Moxon, and P. A. Rice. 2003. Neisserial lipooligosaccharide is a target for complement component C4b. Inner core phosphoethanolamine residues define C4b linkage specificity. J. Biol. Chem. 27850853-50862. [DOI] [PubMed] [Google Scholar]
- 29.Ram, S., M. Cullinane, A. M. Blom, S. Gulati, D. P. McQuillen, B. G. Monks, C. O'Connell, R. Boden, C. Elkins, M. K. Pangburn, B. Dahlback, and P. A. Rice. 2001. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J. Exp. Med. 193281-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ram, S., D. P. McQuillen, S. Gulati, C. Elkins, M. K. Pangburn, and P. A. Rice. 1998. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J. Exp. Med. 188671-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ram, S., J. Ngampasutadol, A. D. Cox, A. M. Blom, L. A. Lewis, F. St. Michael, J. Stupak, S. Gulati, and P. A. Rice. 2007. Heptose I glycan substitutions on Neisseria gonorrhoeae lipooligosaccharide influence C4b-binding protein binding and serum resistance. Infect. Immun. 754071-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rice, P. A. 1989. Molecular basis for serum resistance in Neisseria gonorrhoeae. Clin. Microbiol. Rev. 2(Suppl.)S112-S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice, P. A., W. M. McCormack, and D. L. Kasper. 1980. Natural serum bactericidal activity against Neisseria gonorrhoeae isolates from disseminated, locally invasive, and uncomplicated disease. J. Immunol. 1242105-2109. [PubMed] [Google Scholar]
- 34.Rice, P. A., H. Vayo, M. Tam, and M. S. Blake. 1986. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J. Exp. Med. 1641735-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider, H., J. M. Griffiss, J. W. Boslego, P. J. Hitchcock, K. M. Zahos, and M. A. Apicella. 1991. Expression of paragloboside-like lipooligosaccharides may be a necessary component of gonococcal pathogenesis in men. J. Exp. Med. 1741601-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider, H., J. M. Griffiss, R. E. Mandrell, and G. A. Jarvis. 1985. Elaboration of a 3.6-kilodalton lipooligosaccharide, antibody against which is absent from human sera, is associated with serum resistance of Neisseria gonorrhoeae. Infect. Immun. 50672-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider, H., T. L. Hale, W. D. Zollinger, R. C. Seid, Jr., C. A. Hammack, and J. M. Griffiss. 1984. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect. Immun. 45544-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoolnik, G. K., T. M. Buchanan, and K. K. Holmes. 1976. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J. Clin. Investig. 581163-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shafer, W. M., A. Datta, V. S. Kolli, M. M. Rahman, J. T. Balthazar, L. E. Martin, W. L. Veal, D. S. Stephens, and R. Carlson. 2002. Phase variable changes in genes lgtA and lgtC within the lgtABCDE operon of Neisseria gonorrhoeae can modulate gonococcal susceptibility to normal human serum. J. Endotoxin Res. 847-58. [PubMed] [Google Scholar]
- 40.Shafer, W. M., L. F. Guymon, and P. F. Sparling. 1982. Identification of a new genetic site (sac-3+) in Neisseria gonorrhoeae that affects sensitivity to normal human serum. Infect. Immun. 35764-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shafer, W. M., K. Joiner, L. F. Guymon, M. S. Cohen, and P. F. Sparling. 1984. Serum sensitivity of Neisseria gonorrhoeae: the role of lipopolysaccharide. J. Infect. Dis. 149175-183. [DOI] [PubMed] [Google Scholar]
- 42.Skaar, E. P., M. P. Lazio, and H. S. Seifert. 2002. Roles of the recJ and recN genes in homologous recombination and DNA repair pathways of Neisseria gonorrhoeae. J. Bacteriol. 184919-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith, H., N. J. Parsons, and J. A. Cole. 1995. Sialylation of neisserial lipopolysaccharide: a major influence on pathogenicity. Microb. Pathog. 19365-377. [DOI] [PubMed] [Google Scholar]
- 44.Spitznagel, J. K. 1990. Antibiotic proteins of human neutrophils. J. Clin. Investig. 861381-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stein, D. C., M. Griffiss, and H. Schneider. 1987. Alteration of serum sensitivity in Neisseria gonorrhoeae strain DOV by transformation, p. 599-604. In J. Poolman (ed.), Gonococci and meningococci. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 46.Stephens, D. S., and W. M. Shafer. 1987. Evidence that the serum resistance genetic locus sac-3 of Neisseria gonorrhoeae is involved in lipopolysaccharide structure. J. Gen. Microbiol. 1332671-2678. [DOI] [PubMed] [Google Scholar]
- 47.Tamayo, R., B. Choudhury, A. Septer, M. Merighi, R. Carlson, and J. S. Gunn. 2005. Identification of cptA, a PmrA-regulated locus required for phosphoethanolamine modification of the Salmonella enterica serovar Typhimurium lipopolysaccharide core. J. Bacteriol. 1873391-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tong, Y., D. Arking, S. Ye, B. Reinhold, V. Reinhold, and D. C. Stein. 2002. Neisseria gonorrhoeae strain PID2 simultaneously expresses six chemically related lipooligosaccharide structures. Glycobiology 12523-533. [DOI] [PubMed] [Google Scholar]
- 49.Tzeng, Y. L., K. D. Ambrose, S. Zughaier, X. Zhou, Y. K. Miller, W. M. Shafer, and D. S. Stephens. 2005. Cationic antimicrobial peptide resistance in Neisseria meningitidis. J. Bacteriol. 1875387-5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tzeng, Y. L., A. Datta, K. Ambrose, M. Lo, J. K. Davies, R. W. Carlson, D. S. Stephens, and C. M. Kahler. 2004. The MisR/MisS two-component regulatory system influences inner core structure and immunotype of lipooligosaccharide in Neisseria meningitidis. J. Biol. Chem. 27935053-35062. [DOI] [PubMed] [Google Scholar]
- 51.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharides. Methods Carbohydr. Chem. 583-91. [Google Scholar]
- 52.Wright, J. C., D. W. Hood, G. A. Randle, K. Makepeace, A. D. Cox, J. Li, R. Chalmers, J. C. Richards, and E. R. Moxon. 2004. lpt6, a gene required for addition of phosphoethanolamine to inner-core lipooligosaccharide of Neisseria meningitidis and Haemophilus influenzae. J. Bacteriol. 1866970-6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamasaki, R., B. E. Bacon, W. Nasholds, H. Schneider, and J. M. Griffiss. 1991. Structural determination of oligosaccharides derived from lipooligosaccharide of Neisseria gonorrhoeae F62 by chemical, enzymatic, and two-dimensional NMR methods. Biochemistry 3010566-10575. [DOI] [PubMed] [Google Scholar]
- 54.Yamasaki, R., D. E. Kerwood, H. Schneider, K. P. Quinn, J. M. Griffiss, and R. E. Mandrell. 1994. The structure of lipooligosaccharide produced by Neisseria gonorrhoeae, strain 15253, isolated from a patient with disseminated infection: evidence for a new glycosylation pathway of gonococcal lipooligosaccharide. J. Biol. Chem. 26930345-30351. [PubMed] [Google Scholar]
- 55.York, W. S., A. G. Darvill, M. McNeil, T. T. Stevenson, and P. Albershein. 1985. Isolation and characterization of plant cell walls and cell wall components. Methods Enzymol. 1183-40. [Google Scholar]