Abstract
Plague is a zoonosis transmitted by fleas and caused by the gram-negative bacterium Yersinia pestis. During infection, the plasmidic caf1M1A1 operon that encodes the Y. pestis F1 protein capsule is highly expressed, and anti-F1 antibodies are protective. Surprisingly, the capsule is not required for virulence after injection of cultured bacteria, even though it is an antiphagocytic factor and capsule-deficient Y. pestis strains are rarely isolated. We found that a caf-negative Y. pestis mutant was not impaired in either flea colonization or virulence in mice after intradermal inoculation of cultured bacteria. In contrast, absence of the caf operon decreased bubonic plague incidence after a flea bite. Successful development of plague in mice infected by flea bite with the caf-negative mutant required a higher number of infective bites per challenge. In addition, the mutant displayed a highly autoaggregative phenotype in infected liver and spleen. The results suggest that acquisition of the caf locus via horizontal transfer by an ancestral Y. pestis strain increased transmissibility and the potential for epidemic spread. In addition, our data support a model in which atypical caf-negative strains could emerge during climatic conditions that favor a high flea burden. Human infection with such strains would not be diagnosed by the standard clinical tests that detect F1 antibody or antigen, suggesting that more comprehensive surveillance for atypical Y. pestis strains in plague foci may be necessary. The results also highlight the importance of studying Y. pestis pathogenesis in the natural context of arthropod-borne transmission.
Plague, caused by the gram-negative bacterium Yersinia pestis, is a disease that primarily affects rodents and occasionally humans (7). Bubonic plague, the most common form of the disease, is usually transmitted by fleas. It is characterized by a painful, hypertrophied, and swollen lymph node called a bubo. Without early treatment, the disease evolves rapidly to a life-threatening septicemia. Flea-borne transmission may also lead to primary septicemic plague, which is characterized by a deadly septicemia without bubo production (55). Occasionally, the hematogenous dissemination of bacteria leads to a pulmonary infection, which results in a fatal airborne disease, pneumonic plague.
Y. pestis strains usually contain three plasmids: the pesticin plasmid (pPst), the Yersinia virulence plasmid (pYV), and the fraction 1 (pFra) plasmid (49). All of these replicons harbor at least one gene required for the propagation of plague. pPst carries the plasminogen activator gene (pla), which encodes a surface protease/adhesin that enables bacterial dissemination from the flea bite site and therefore bubonic plague production (59). pYV encodes a type III secretion system (T3SS) that injects the Yersinia outer proteins (Yops) into host cells to inhibit phagocytosis and cytokine production and to induce apoptosis (13). pFra harbors the ymt gene that enhances bacterial survival in the flea gut (31) and also encodes a fimbrial protein (the Caf or fraction 1 [F1] antigen) that accumulates on the bacterial surface to form an amorphous capsule (12, 45, 53).
F1 capsule synthesis requires the caf1M1A1 operon and the caf1R transcriptional activator gene (24, 25, 38, 39). The caf operon products constitute a fimbrial chaperone-usher system that acts to assemble and export F1 subunits on the bacterial surface. The caf1-encoded F1 fimbrial subunits are translocated from the cytoplasm into the periplasm, where they interact with the Caf1M chaperone and dimerize prior to exportation to the surface of the bacteria by the outer-membrane usher protein Caf1A (46, 72). Further addition of Caf1 dimers results in capsule formation. F1 capsule production is strongly influenced by temperature—little or no capsule is detected at <35°C in vitro or in the flea vector, an ambient-temperature environment (6, 8, 12, 19, 34). In contrast, the F1 subunit gene, caf1, is one of the most highly expressed genes during infection of the mammal; Y. pestis is surrounded by F1 capsule in vivo, and free F1 antigen can also be detected in tissues, indicating that it is shed in large amounts from the surface of the bacteria (10, 11, 14, 53, 56). In addition, anti-F1 antibody provides protection against plague, and F1 is a major component of second-generation plague vaccines (64). The fact that F1 capsule is produced in large amounts during infection suggests that it is a virulence factor, which is corroborated by several studies showing that the capsule acts in concert with the T3SS to make Y. pestis highly resistant to uptake by phagocytes (6, 14, 19, 70). However, although some genetically undefined F1-negative strains show decreased virulence (5, 16, 23, 66, 68, 71), studies using genetically defined caf-negative mutant strains have uniformly shown that lack of F1 capsule has no effect on virulence in mouse and guinea pig models of bubonic plague or in the African green monkey model of pneumonic plague (14, 18, 23). The lack of correlation between F1 and virulence is all the more striking because natural selection of encapsulated strains seems to occur. Indeed, only one F1 antigen-deficient Y. pestis strain has been isolated from humans, and very few natural isolates of Y. pestis lack the caf operon (47, 71). These inconsistencies prompted us to assess the role of the caf operon in Y. pestis infectivity in the natural context of the flea-borne transmission route.
MATERIALS AND METHODS
Mutant production.
A caf-negative mutant of the fully virulent Y. pestis 195/P strain in which a 3,848-bp DNA fragment encompassing 18 bp of the caf1M1A1 operon promoter and 3,830 bp of the 3,896-bp caf1M1A1 operon was replaced by a kanamycin resistance cassette (aph). The mutant was produced by allelic exchange using the plasmid pCVD442 (4) harboring the aph gene flanked by ∼500 bp of Y. pestis sequence upstream and downstream of the intended deletion. The mutation was verified by PCR analysis, and loss of capsule production was verified by electron microscopy and immunofluorescence assay using anti-F1 antibody (data not shown). The effect of the F1 capsule on the ability to produce a transmissible infection in fleas was assessed using a Caf-negative Y. pestis 195/P variant lacking pFra (29) transformed with pCH16, a recombinant plasmid carrying the Y. pestis ymt gene (32).
Flea infections.
Xenopsylla cheopis fleas were infected with Y. pestis using a previously described artificial feeding system (30). The infection rates were monitored at 0 and 28 days after the infectious blood meal by enumerating CFU from dilutions of 20 individually triturated female fleas plated on brain heart infusion containing 1 μg/ml Irgasan. Flea blockage was also monitored during the 4-week period using a separate sample of 50 female and 50 male fleas. Blockage was determined by microscopic examination of individual fleas immediately after they fed. Blocked fleas were diagnosed by the presence of fresh blood in the esophagus but not in the midgut (30).
Mouse infections.
All experiments were performed using Rocky Mountain Laboratories Swiss-Webster mice at biosafety level 3 and were approved by the Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, National Institutes of Health Biosafety and Animal Care and Use Committees in accordance with National Institutes of Health guidelines. Mouse infections were done in conjunction with a previously reported study with a Y. pestis pla mutant (55), and the data reported here for the wild-type 195/P strain are from that study.
For 50% lethal dose (LD50) determination, Y. pestis strains cultured in LB overnight at 21°C without shaking were quantified using a Petroff-Hausser bacterial-counting chamber, and serial dilutions of bacterial suspensions in sterile phosphate-buffered saline were inoculated intradermally (i.d.) (0.03 ml) into groups of five 8-week-old female mice. The number of bacteria injected was confirmed by the CFU count. Infected animals were observed three times daily and euthanized upon signs of terminal plague (evidence of lethargy, hunched posture, and reluctance to respond to external stimuli) for 3 weeks. Blood was collected by cardiac puncture immediately after euthanasia and plated on Yersinia selective agar (YSAB; Difco) to confirm infection. LD50s were calculated according to the Reed-Muench equation (52) and the Probit method (StatPlus software; AnalystSoft, Vancouver, Canada).
A previously described (37, 55) flea-borne transmission model was used to determine Y. pestis infectivity after challenge by flea bite. Briefly, infected fleas were applied to a restrained mouse and allowed to feed for 60 min. The fleas were then recovered and examined under a dissecting microscope to determine how many had taken a normal blood meal (unblocked, or noninfective, fleas) and how many were blocked (infective fleas). After challenge, the mice were euthanized upon the appearance of signs of terminal illness. Mice that did not develop any symptoms after 1 week following a challenge were rechallenged.
Bacteriology and histology.
All tissue samples were collected immediately after euthanasia. One-half of each spleen was completely triturated through sterile wire mesh into phosphate-buffered saline, and dilutions of the triturate were plated on Yersinia selective agar (YSAB) for CFU counts. Blood samples were similarly diluted and plated for CFU counts. The remaining portion of the spleens, the inguinal and axillary lymph nodes, and the livers were fixed in neutral buffered 10% formalin and then embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Immunohistochemical (IHC) staining was performed on additional paraffin-embedded sections using a Dako autostainer. Anti-Y. pestis antiserum and the secondary antibody horseradish peroxidase-conjugated goat-anti-rabbit immunoglobulin G (H+L) (Pierce Biotechnology, Rockford, IL) were used to specifically detect bacteria (36).
Statistical analysis.
Comparisons of death rates after flea bite challenge were calculated by using a chi-square test. A P value of <0.05 was considered statistically significant.
RESULTS
The caf1M1A1 operon is not required to produce a transmissible infection in fleas.
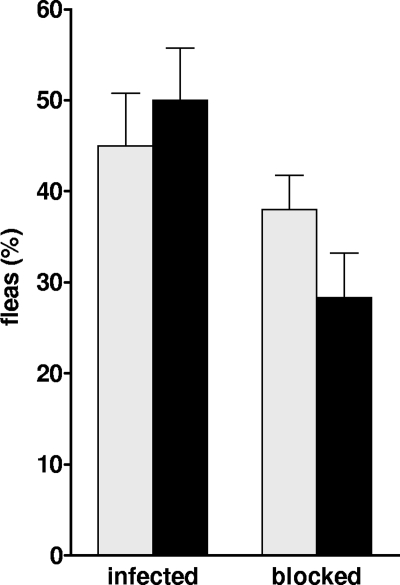
Y. pestis ingested by fleas after a blood meal replicate actively in the flea gut to produce a bacterial mass that blocks the proventricular valve in the foregut. Complete or partial blockage is critical for efficient biological transmission of the plague bacillus to the mammalian host (3, 50), as opposed to early-phase transmission, which does not require colonization of the flea (20-22, 67). Therefore, it was necessary to first evaluate the effect of the caf operon on the ability of Y. pestis to block fleas prior to assessing its role in Y. pestis infectivity by flea bite. For this, groups of X. cheopis rat fleas were infected with caf-positive or caf-negative Y. pestis 195/P. During the 4-week period after the infectious blood meal, the fleas developed infection and blockage of the proventriculus at similar rates regardless of the Y. pestis strain used (Fig. 1). Hence, the caf operon is not required to produce a transmissible infection in the flea.
FIG. 1.
Infection of X. cheopis fleas with encapsulated and unencapsulated Y. pestis strain 195/P. The percentages of fleas blocked and still infected 4 weeks after a single blood meal containing the Y. pestis 195/P wild-type (black bars) or pFra-negative (gray bars) strains are shown. The averages and standard deviations were determined from three independent experiments.
The caf1M1A1 operon is required for full virulence after flea bite.
Prior to determining the role of the caf operon in the virulence of Y. pestis in a flea-borne-transmission model, we confirmed previous reports (18, 23) that the caf operon is not required for virulence in mice needle inoculated i.d. with cultured bacteria (Table 1). Next, we fed fleas with mouse blood containing either the wild-type or the Δcaf1M1A1-negative strain. Beginning 10 days after their infectious blood meal (the time required by Y. pestis to block X. cheopis), the fleas were applied to mice and allowed to feed (37). Mice challenged with fleas infected with the wild-type and the caf-negative mutant received the same number of challenges on median and approximately the same number of infective and noninfective bites (Tables 2 and 3). Plague developed in 8 of 10 mice challenged by wild-type-infected fleas but in only 4 of 10 mice challenged by the mutant-infected fleas. The time to terminal disease was 2 to 4 days after flea challenge regardless of the presence of the caf operon (Tables 2 and 3).
TABLE 1.
Effects of transmission route on virulence of caf-negative Y. pestis
| Y. pestis strain | LD50 by transmission routea:
|
||
|---|---|---|---|
| i.v. | i.d. | Flea bite | |
| Wild type | <10 (<10) | 46 (66 ± 21) | 8/10 |
| Δcaf1M1A1 | <12 (<12) | 55 (86 ± 48) | 4/10 |
LD50 values measured using the Reed-Muench equation and (in parentheses) the Probit method (95% confidence interval) are given for intravenous (i.v.) and i.d. injection, and disease incidence is given for fleabite transmission (number of mice that developed terminal disease/number of mice challenged).
TABLE 2.
Incidence of plague in mice infected with the wild-type Y. pestis 195/P strain after flea bite challenge
| Mouse no. | No. of challenges (median, 3) | Total no. of flea bites (cumulative) (median, 57.5) | No. of blocked flea bites (cumulative) (median, 6) | Time to onset of disease (days) (median, 3) | Outcomea |
|---|---|---|---|---|---|
| 1 | 1 | 4 | 1 | 2 | B |
| 2 | 1 | 13 | 2 | 3 | B |
| 3 | 1 | 20 | 2 | 3 | B |
| 4 | 3 | 87 | 10 | 3 | B |
| 5 | 3 | 70 | 6 | 4 | B |
| 6 | 4 | 68 | 9 | 4 | B |
| 7 | 1 | 17 | 3 | 3 | S |
| 8 | 3 | 51 | 10 | 3 | S |
| 9 | 3 | 64 | 6 | − | |
| 10 | 4 | 69 | 7 | − |
B, bubonic plague; S, septicemic plague; −, no disease.
TABLE 3.
Incidence of plague in mice infected with the Y. pestis 195/P Δcaf1M1A1::aph strain after flea bite challenge
| Mouse no. | No. of challenges (median, 3) | Total no. of flea bites (cumulative) (median, 49) | No. of blocked flea bites (cumulative) (median, 7.5) | Time to onset of disease (days) (median, 3) | Outcomea |
|---|---|---|---|---|---|
| 1 | 1 | 17 | 7 | 3 | B |
| 2 | 2 | 30 | 8 | 3 | B |
| 3 | 2 | 37 | 8 | 4 | B |
| 4 | 2 | 42 | 11 | 3 | S |
| 5 | 3 | 56 | 7 | − | |
| 6 | 3 | 63 | 8 | − | |
| 7 | 4 | 71 | 4 | − | |
| 8 | 4 | 59 | 5 | − | |
| 9 | 4 | 41 | 6 | − | |
| 10 | 4 | 79 | 8 | − |
B, bubonic plague; S, septicemic plague; −, no disease.
Consistent with a previous study (37), development of the disease in mice infected with the wild-type strain was correlated with the total number of infective (from blocked fleas) flea bites per challenge (r2 = 0.95) and not with the total number of noninfective bites (r2 = 0.03). The same observation was noted with mice infected with the caf-negative strain, but a statistically significantly higher number of infective bites per challenge was required to establish an infection (P = 0.01891) (Fig. 2). With the wild-type strain, one infective bite in a single challenge was sufficient to cause plague, whereas with the caf-negative mutant, a minimum of four infective bites in a single challenge was required for disease production. Altogether, these observations suggest that the caf operon is required for full virulence after transmission by flea bite.
FIG. 2.
Frequency distribution of plague incidence in mice that experienced zero to seven bites in a single challenge by blocked fleas infected with the Y. pestis wild-type (gray bars) or the Δcaf1M1A1 (black bars) strain. The number of mice that were bitten by x blocked fleas per challenge is indicated above each bar (x = 0 to 5 for wild-type-infected flea bites and 0 to 5 or 7 for the caf mutant. None of the mice received six infective bites, indicated by NA.).
Both bubonic and primary septicemic plague can result from flea-borne transmission of caf-negative Y. pestis.
A previous study indicated that blocked fleas attempting to obtain a blood meal deposit bacteria either in the dermis or in a dermal blood vessel, which leads to bubonic or primary septicemic plague, respectively (55). Development of bubonic plague depends on the plasminogen activator Pla, whereas primary septicemic plague does not (55, 59). Like pla, the caf operon could encode factors that are specifically required for the production of bubonic plague. Therefore, we diagnosed the form of disease that developed in mice after challenge by flea bite. Three of four sick mice infected with the caf mutant had bubonic plague, evidenced by myriad bacteria in their draining lymph nodes; the other mouse had primary septicemic plague, with no bacteria in its lymph nodes and no sign of lymphadenitis (Table 3). Six of eight sick mice infected with wild-type Y. pestis had bubonic plague and two had primary septicemic plague (Table 2). Regardless of the lymph node colonization, the blood, spleens, and livers of all sick mice were heavily colonized (Fig. 3, 4, 5, and 6). The equivalent incidence rates of the two forms of flea-borne plague indicate that, unlike pla, the caf operon is not essential for bubonic plague.
FIG. 3.
Bacterial loads in the spleens and the blood of mice that developed terminal plague after being bitten by fleas infected with the wild-type (WT) or the Δcaf1M1A1 (Δcaf) Y. pestis strain.
FIG. 4.
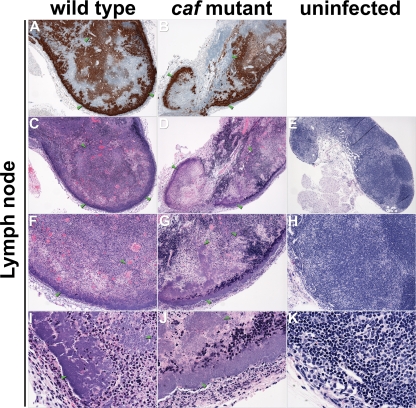
Differential histopathologies in the proximal lymph nodes of mice. Sections of infected (A to D, F to G, and I to J) and uninfected (E, H, and K) lymph nodes were stained by IHC using Y. pestis-specific antibody (A and B) or by H&E (C and K). Masses of bacteria, indicated by green arrowheads, strained brown by IHC and blue by H&E. Magnification, ×40 (A to E), ×100 (F to H), and ×400 (I to K).
FIG. 5.
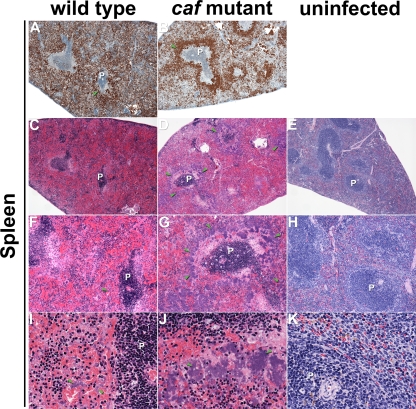
Differential histopathologies in the spleens of mice. Sections of infected (A to D, F to G, and I to J) and uninfected (E, H, and K) spleens were stained by IHC using Y. pestis-specific antibody (A and B) or by H&E (C to K). Masses of brown (A and B)- and blue (C, F, and I)-stained wild-type and Δcaf1M1A1 (D, G, and J) bacteria were widely dispersed in the infected tissue, but the Δcaf1M1A1 mutant tended to form larger colonies and was more localized to the marginal zone of the periateriolar lymphoid sheath (P). The green arrowheads indicate examples of bacteria. Magnification, ×40 (A to E), ×100 (F to H), and ×400 (I to K).
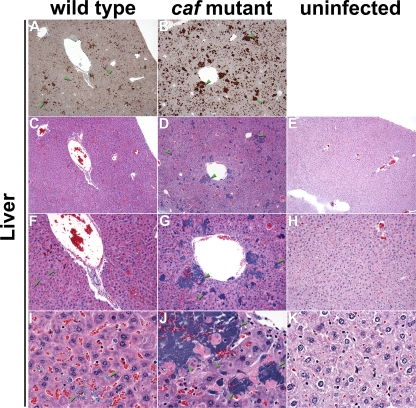
FIG. 6.
Differential histopathologies in the livers of mice. Sections of infected (A to D, F to G, and I to J) and uninfected (E, H, and K) livers were stained by IHC using Y. pestis-specific antibody (A and B) or by H&E (C to K). Wild-type (C, F, and I) and Δcaf1M1A1 (D, G, and J) bacteria stained brown by IHC (A and B) and blue by H&E (C, D, F, G, I, and J) were widely dispersed in the infected tissue, but the Δcaf1M1A1 mutant tended to form larger and more numerous colonies. The green arrowheads indicate examples of bacteria. Magnification, ×40 (A to E), ×100 (F to H), and ×400 (I to K).
The caf1M1A1 mutant autoaggregates in infected tissues.
The spleen, the liver, and the lymph node proximal to the flea bite site showed overall similar histopathologies in mice infected with the wild type or the caf-negative mutant (Fig. 4, 5, and 6). In the lymph node, the normal nodal architecture was severely obscured or completely effaced by larges masses of bacteria admixed with abundant necrotic cellular debris, which is typical of bubonic plague. In the spleen, the red pulp was diffusely obscured by moderate hemorrhage, numerous bacteria, and moderate amounts of cellular debris. In the white pulp, moderate to severe lymphocytolysis with loss of periarteriolar lymphoid sheaths was noted multifocally, with the affected architecture replaced by cellular debris, fibrin, and numerous bacteria. Diffuse hepatocellular degeneration was seen in the liver, with mildly swollen and vacuolated hepatocytes. Variably sized bacterial colonies expanding sinusoids or replacing lost hepatocytes were also noted multifocally. Frequently, hepatocytes with increased eosinophilia and pyknotic or fragmented nuclei were present next to the bacterial colonies, which was interpreted as hepatocellular necrosis. Many intravascular bacteria were noted within medium-size veins, and blood vessels occasionally contained fibrin thrombi.
Similar pathologies were noted in the lymph nodes, spleens, and livers of mice infected with the wild type and the caf-negative mutant, but the localizations and the morphologies of the extracellular masses of bacteria differed. In the spleen, the mutant formed large discrete colonies confined to the marginal zone of the white pulp, whereas the myriad wild-type Y. pestis organisms were more loosely and evenly distributed (Fig. 5). In the liver, the mutant colonies appeared larger and more numerous (Fig. 6). In the lymph node, the autoaggregative phenotype was less obvious (Fig. 4).
DISCUSSION
A variety of functions that are related to virulence have been proposed for the F1 capsule of Y. pestis. Early studies led to a model in which the bacteria are subject to phagocytosis immediately after transmission because the antiphagocytic systems (T3SS or F1) are not produced in the flea. Bacteria ingested by macrophages are not destroyed, however. Instead, they replicate and produce the T3SS and F1 capsule, which allow Y. pestis to multiply extracellularly once released from the phagocytic cell (5, 6, 8, 51). This model was supported by the demonstration of antiphagocytic effects of F1 capsule (19, 23, 70). More recently, evidence of possible immunomodulatory effects of caf gene products has been reported. Interactions between Caf1 dimers bound to the Caf1M chaperone and interleukin 1 (IL-1) receptors on epithelial cells and macrophages, and between the Caf1A outer-membrane usher protein and IL-1β, have been demonstrated (1, 2, 73). Proinflammatory cytokine induction due to macrophage activation by Caf1 has also been reported (57, 58, 60). However, these in vitro studies are in conflict with others (40, 41). Shielding of other Y. pestis surface antigens from the immune system by the F1 capsule and the removal of circulating F1 antibody by the shed capsule may also contribute to virulence (45).
Despite these proposed virulence functions, we replicated previous studies showing that deletion of the caf operon does not reduce the LD50 for mice following i.d. injection of cultured bacteria. Because it seems unlikely that the caf operon, and the associated energy costs of capsule synthesis, would be maintained unless it conferred a selective advantage on Y. pestis during its life cycle, we hypothesized that the F1 capsule might be important in the natural context of flea-borne transmission. In contrast to needle inoculation, deletion of the caf operon resulted in reduced incidence of plague following flea bite (Tables 2 and 3). Productive disease with the caf mutant required a minimum of four infective flea bites in a single challenge, whereas disease with the wild-type strain often resulted after one to three infective flea bites per challenge (Fig. 2). These results could be due either to a defective ability of the mutant to produce a transmissible infection in the flea vector or to lower virulence in the host. We think the latter is more likely, because F1 capsule is not detected in the flea (8, 34) and the caf mutant was able to infect and block fleas as well as the wild-type strain (Fig. 1).
The requirement for the caf operon for maximum disease transmission may reflect specific aspects of the Y. pestis phenotype in the flea and the microenvironment at the flea bite site. F1 capsule is not detected either in the flea or in low-temperature cultures, but the caf genes are transcribed in the flea (R. Rebeil and B. J. Hinnebusch, unpublished results). Small amounts of F1 subunits and the Caf1A outer membrane usher protein showed affinity to the IL-1 receptor on macrophages and epithelial cells and to IL-1β, respectively. This adhesin or anti-immunity potential has been suggested to be important early in infection (1, 2, 73). In addition, Y. pestis has evolved to be transmitted along with flea saliva into the dermis, which is not well mimicked by subcutaneous or i.d. injection by needle. For example, the bite and components of the saliva of blood-feeding arthropods may attract a heightened innate immune response (15, 48, 54). Finally, transmission by flea bite may simply represent a more sensitive way to detect small differences in the LD50. Blocked X. cheopis fleas often transmit fewer than 10 bacteria, which corresponds to the LD50 of Y. pestis (44). Less than a 10-fold increase in this very low LD50 is technically difficult to measure with confidence, but even a 2-fold increase in the LD50 might explain the requirement for the increased number of flea bites required to produce productive infection by the caf mutant. Given the low efficiency of transmission by fleas, such a small increase in the LD50 could significantly affect the transmission cycle.
The role of the F1 capsule in virulence appears to be confined to overcoming some bottleneck early after transmission. Although the incidence of disease was lower following flea bite transmission of the caf mutant, the time to disease onset and the bacterial loads achieved in tissue and blood were not affected (Tables 1 and 2 and Fig. 3). However, lack of F1 capsule was correlated with an autoaggregative in vivo phenotype (Fig. 5 and 6). This has also been observed in the lungs of monkeys infected with a caf mutant and has been suggested to be a consequence of loss of the negatively charged capsule (14).
Accretion of genetic material via horizontal transfer accelerated the abilities of many bacteria to colonize diverse ecological niches and to become pathogenic (26). For Y. pestis, sequential plasmid transfers from unrelated bacteria were crucial for its emergence (9). Acquisition of a plasmid encoding a phospholipase D (Ymt) would have allowed the ancestral Y. pestis strain to colonize fleas and to become an arthropod-borne pathogen causing a disease with low incidence (31). Later acquisition of pPst encoding a plasminogen activator increased the invasiveness of the bacteria from the flea bite site, enabling bubonic plague and increasing the potential for epidemic spread (55, 59). Our results suggest that a Y. pestis progenitor harboring a pYV, a pPst, and an ancestral pFra plasmid containing ymt but not the caf operon could persist in nature. The mosaic structure of pFra supports the idea that ymt and caf could have been successively acquired by Y. pestis (42). Addition of the caf locus by horizontal transfer may have brought supplementary anti-immune properties (antiphagocytosis and anti-IL-1) that would have increased plague incidence in host populations.
The persistence of arthropod-borne disease depends on host abundance and the vector density per host, which depend on each other and are driven by environmental conditions. For plague, an estimated minimum of five X. cheopis fleas per host is required to maintain a wild-type Y. pestis strain in a rat population (20, 44). The average number of X. cheopis fleas per host (the flea index) typically ranges from 0 to 14 but varies with climatic conditions, and flea indices of 20 to 80 have been recorded (44, 61). A minimum of four fleas infected with Y. pestis lacking the caf locus was required to produce plague, whereas only one flea infected with a wild-type strain can lead to disease. Hence, 20 (5 × 4) fleas per rat would be required to maintain a caf-negative strain in a population. Our results imply that Y. pestis lacking the caf locus could emerge and persist in a rodent population only when unusual long-term environmental conditions favor high flea populations. These atypical strains should disappear when environmental conditions return to normal. Such a model could explain why unencapsulated strains are rarely isolated.
Plague remains an international public health concern and is classified as a reemerging disease (63). Two new concerns add to the threat of Y. pestis plague epidemics: (i) the emergence of Y. pestis strains resistant to antibiotics used as the first-line treatment against plague and (ii) the potential use of Y. pestis as a bioweapon (27, 35). In this context, many efforts have been made to develop rapid diagnostic procedures. The caf1 and pla genes, as well as their products, are the most frequent targets used to detect Y. pestis in many different kinds of samples (11, 17, 28, 33, 43, 62, 65). Thus, even though diagnostic procedures based on caf1 and pla have been shown to have great value in the field (11), our current work, and our previous results showing that pla is not essential to establish an infection after flea bite (54), suggests that supplementary diagnostic procedures should be developed to detect atypical Y. pestis and that better surveillance of atypical Y. pestis strains in plague foci is needed, as was stressed more than 20 years ago (69).
Acknowledgments
We thank Nadine Lemaître, David Erickson, and Cyril Guyard for critical review of the manuscript and Laurent Heliot for the use of the Interdisciplinary Research Institute's microscope platform.
This work was supported by the Division of Intramural Research, NIAID, NIH; the Ellison Medical Foundation (New Scholars Award in Global Infectious Diseases to B.J.H.); and INSERM.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 22 December 2008.
REFERENCES
- 1.Abramov, V. M., A. M. Vasiliev, V. S. Khlebnikov, R. N. Vasilenko, N. L. Kulikova, I. V. Kosarev, A. T. Ishchenko, J. R. Gillespie, I. S. Millett, A. L. Fink, and V. N. Uversky. 2002. Structural and functional properties of Yersinia pestis Caf1 capsular antigen and their possible role in fulminant development of primary pneumonic plague. J. Proteome Res. 1307-315. [DOI] [PubMed] [Google Scholar]
- 2.Abramov, V. M., A. M. Vasiliev, R. N. Vasilenko, N. L. Kulikova, I. V. Kosarev, V. S. Khlebnikov, A. T. Ishchenko, S. MacIntyre, J. R. Gillespie, R. Khurana, T. Korpela, A. L. Fink, and V. N. Uversky. 2001. Structural and functional similarity between Yersinia pestis capsular protein Caf1 and human interleukin-1 beta. Biochemistry 406076-6084. [DOI] [PubMed] [Google Scholar]
- 3.Bacot, A. W., and C. J. Martin. 1914. Observations on the mechanism of the transmission of plague by fleas. J. Hyg. Plague 13(Plague Suppl. 3)423-439. [PMC free article] [PubMed] [Google Scholar]
- 4.Blomfield, I. C., V. Vaughn, R. F. Rest, and B. I. Eisenstein. 1991. Allelic exchange in Escherichia coli using the Bacillus subtilis sacB gene and a temperature-sensitive pSC101 replicon. Mol. Microbiol. 51447-1457. [DOI] [PubMed] [Google Scholar]
- 5.Burrows, T. W. 1957. Virulence of Pasteurella pestis. Nature 1791246-1247. [DOI] [PubMed] [Google Scholar]
- 6.Burrows, T. W., and G. A. Bacon. 1956. The basis of virulence in Pasteurella pestis: the development of resistance to phagocytosis in vitro. Br. J. Exp. Pathol. 37286-299. [PMC free article] [PubMed] [Google Scholar]
- 7.Butler, T. 1983. Plague and other Yersinia infections. Plenum Medical Book Company, New York, NY.
- 8.Cavanaugh, D. C., and R. Randall. 1959. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J. Immunol. 83348-363. [PubMed] [Google Scholar]
- 9.Chain, P. S., E. Carniel, F. W. Larimer, J. Lamerdin, P. O. Stoutland, W. M. Regala, A. M. Georgescu, L. M. Vergez, M. L. Land, V. L. Motin, R. R. Brubaker, J. Fowler, J. Hinnebusch, M. Marceau, C. Medigue, M. Simonet, V. Chenal-Francisque, B. Souza, D. Dacheux, J. M. Elliott, A. Derbise, L. J. Hauser, and E. Garcia. 2004. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 10113826-13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chanteau, S., L. Rabarijaona, T. O'Brien, L. Rahalison, J. Hager, P. Boisier, J. Burans, and M. Rasolomaharo. 1998. F1 antigenaemia in bubonic plague patients, a marker of gravity and efficacy of therapy. Trans. R. Soc. Trop. Med. Hyg. 92572-573. [DOI] [PubMed] [Google Scholar]
- 11.Chanteau, S., L. Rahalison, L. Ralafiarisoa, J. Foulon, M. Ratsitorahina, L. Ratsifasoamanana, E. Carniel, and F. Nato. 2003. Development and testing of a rapid diagnostic test for bubonic and pneumonic plague. Lancet 361211-216. [DOI] [PubMed] [Google Scholar]
- 12.Chen, T. H., and S. S. Elberg. 1977. Scanning electron microscopic study of virulent Yersinia pestis and Yersinia pseudotuberculosis type 1. Infect. Immun. 15972-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M. P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 621315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis, K. J., D. L. Fritz, M. L. Pitt, S. L. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Pathology of experimental pneumonic plague produced by fraction 1-positive and fraction 1-negative Yersinia pestis in African green monkeys (Cercopithecus aethiops). Arch. Pathol. Lab. Med. 120156-163. [PubMed] [Google Scholar]
- 15.Demeure, C. E., K. Brahimi, F. Hacini, F. Marchand, R. Peronet, M. Huerre, P. St-Mezard, J. F. Nicolas, P. Brey, G. Delespesse, and S. Mecheri. 2005. Anopheles mosquito bites activate cutaneous mast cells leading to a local inflammatory response and lymph node hyperplasia. J. Immunol. 1743932-3940. [DOI] [PubMed] [Google Scholar]
- 16.Donavan, J. E., D. Ham, G. M. Fukui, and M. J. Surgalla. 1961. Role of the capsule of Pasteurella pestis in bubonic plague in the guinea pig. J. Infect. Dis. 109154-157. [DOI] [PubMed] [Google Scholar]
- 17.Drancourt, M., G. Aboudharam, M. Signoli, O. Dutour, and D. Raoult. 1998. Detection of 400-year-old Yersinia pestis DNA in human dental pulp: an approach to the diagnosis of ancient septicemia. Proc. Natl. Acad. Sci. USA 9512637-12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drozdov, I. G., A. P. Anisimov, S. V. Samoilova, I. N. Yezhov, S. A. Yeremin, A. V. Karlyshev, V. M. Krasilnikova, and V. I. Kravchenko. 1995. Virulent non-capsulate Yersinia pestis variants constructed by insertion mutagenesis. J. Med. Microbiol. 42264-268. [DOI] [PubMed] [Google Scholar]
- 19.Du, Y., R. Rosqvist, and A. Forsberg. 2002. Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infect. Immun. 701453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisen, R. J., S. W. Bearden, A. P. Wilder, J. A. Montenieri, M. F. Antolin, and K. L. Gage. 2006. Early-phase transmission of Yersinia pestis by unblocked fleas as a mechanism explaining rapidly spreading plague epizootics. Proc. Natl. Acad. Sci. USA 10315380-15385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisen, R. J., J. N. Borchert, J. L. Holmes, G. Amatre, K. Van Wyk, R. E. Enscore, N. Babi, L. A. Atiku, A. P. Wilder, S. M. Vetter, S. W. Bearden, J. A. Montenieri, and K. L. Gage. 2008. Early-phase transmission of Yersinia pestis by cat fleas (Ctenocephalides felis) and their potential role as vectors in a plague-endemic region of Uganda. Am. J. Trop. Med. Hyg. 78949-956. [PubMed] [Google Scholar]
- 22.Eisen, R. J., A. P. Wilder, S. W. Bearden, J. A. Montenieri, and K. L. Gage. 2007. Early-phase transmission of Yersinia pestis by unblocked Xenopsylla cheopis (Siphonaptera: Pulicidae) is as efficient as transmission by blocked fleas. J. Med. Entomol. 44678-682. [DOI] [PubMed] [Google Scholar]
- 23.Friedlander, A. M., S. L. Welkos, P. L. Worsham, G. P. Andrews, D. G. Heath, G. W. Anderson, Jr., M. L. Pitt, J. Estep, and K. Davis. 1995. Relationship between virulence and immunity as revealed in recent studies of the F1 capsule of Yersinia pestis. Clin. Infect. Dis. 21(Suppl.)S178-S181. [DOI] [PubMed] [Google Scholar]
- 24.Galyov, E. E., A. V. Karlishev, T. V. Chernovskaya, D. A. Dolgikh, O. Smirnov, K. I. Volkovoy, V. M. Abramov, and V. P. Zav'yalov. 1991. Expression of the envelope antigen F1 of Yersinia pestis is mediated by the product of caf1M gene having homology with the chaperone protein PapD of Escherichia coli. FEBS Lett. 28679-82. [DOI] [PubMed] [Google Scholar]
- 25.Galyov, E. E., O. Smirnov, A. V. Karlishev, K. I. Volkovoy, A. I. Denesyuk, I. V. Nazimov, K. S. Rubtsov, V. M. Abramov, S. M. Dalvadyanz, and V. P. Zav'yalov. 1990. Nucleotide sequence of the Yersinia pestis gene encoding F1 antigen and the primary structure of the protein. Putative T and B cell epitopes. FEBS Lett. 277230-232. [DOI] [PubMed] [Google Scholar]
- 26.Groisman, E. A., and J. Casadesus. 2005. The origin and evolution of human pathogens. Mol. Microbiol. 561-7. [DOI] [PubMed] [Google Scholar]
- 27.Guiyoule, A., G. Gerbaud, C. Buchrieser, M. Galimand, L. Rahalison, S. Chanteau, P. Courvalin, and E. Carniel. 2001. Transferable plasmid-mediated resistance to streptomycin in a clinical isolate of Yersinia pestis. Emerg. Infect. Dis. 743-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins, J. A., J. Ezzell, B. J. Hinnebusch, M. Shipley, E. A. Henchal, and M. S. Ibrahim. 1998. 5′ Nuclease PCR assay to detect Yersinia pestis. J. Clin. Microbiol. 362284-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinnebusch, B. J., E. R. Fischer, and T. G. Schwan. 1998. Evaluation of the role of the Yersinia pestis plasminogen activator and other plasmid-encoded factors in temperature-dependent blockage of the flea. J. Infect. Dis. 17814006-14015. [DOI] [PubMed] [Google Scholar]
- 30.Hinnebusch, B. J., R. D. Perry, and T. G. Schwan. 1996. Role of the Yersinia pestis hemin storage (hms) locus in the transmission of plague by fleas. Science 273367-370. [DOI] [PubMed] [Google Scholar]
- 31.Hinnebusch, B. J., A. E. Rudolph, P. Cherepanov, J. E. Dixon, T. G. Schwan, and A. Forsberg. 2002. Role of Yersinia murine toxin in survival of Yersinia pestis in the midgut of the flea vector. Science 296733-735. [DOI] [PubMed] [Google Scholar]
- 32.Hinnebusch, J., P. Cherepanov, Y. Du, A. Rudolph, J. D. Dixon, T. Schwan, and A. Forsberg. 2000. Murine toxin of Yersinia pestis shows phospholipase D activity but is not required for virulence in mice. Int. J. Med. Microbiol. 290483-487. [DOI] [PubMed] [Google Scholar]
- 33.Hinnebusch, J., and T. G. Schwan. 1993. New method for plague surveillance using polymerase chain reaction to detect Yersinia pestis in fleas. J. Clin. Microbiol. 311511-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hudson, B. W., L. Kartman, and F. M. Prince. 1966. Pasteurella pestis detection in fleas by fluorescent antibody staining. Bull. W. H. O. 34709-714. [PMC free article] [PubMed] [Google Scholar]
- 35.Inglesby, T. V., D. T. Dennis, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, J. F. Koerner, M. Layton, J. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, M. Schoch-Spana, K. Tonat, et al. 2000. Plague as a biological weapon: medical and public health management. JAMA 2832281-2290. [DOI] [PubMed] [Google Scholar]
- 36.Jarrett, C. O., E. Deak, K. E. Isherwood, P. C. Oyston, E. R. Fischer, A. R. Whitney, S. D. Kobayashi, F. R. DeLeo, and B. J. Hinnebusch. 2004. Transmission of Yersinia pestis from an infectious biofilm in the flea vector. J. Infect. Dis. 190783-792. [DOI] [PubMed] [Google Scholar]
- 37.Jarrett, C. O., F. Sebbane, J. J. Adamovicz, G. P. Andrews, and B. J. Hinnebusch. 2004. Flea-borne transmission model to evaluate vaccine efficacy against naturally acquired bubonic plague. Infect. Immun. 722052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karlyshev, A. V., E. E. Galyov, V. M. Abramov, and V. P. Zav'yalov. 1992. caf1R gene and its role in the regulation of capsule formation of Y. pestis. FEBS Lett. 30537-40. [DOI] [PubMed] [Google Scholar]
- 39.Karlyshev, A. V., E. E. Galyov, O. Smirnov, A. P. Guzayev, V. M. Abramov, and V. P. Zav'yalov. 1992. A new gene of the f1 operon of Y. pestis involved in the capsule biogenesis. FEBS Lett. 29777-80. [DOI] [PubMed] [Google Scholar]
- 40.Kingston, R., F. Burke, J. H. Robinson, P. A. Bedford, S. M. Jones, S. C. Knight, and E. D. Williamson. 2007. The fraction 1 and V protein antigens of Yersinia pestis activate dendritic cells to induce primary T cell responses. Clin. Exp. Immunol. 149561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krakauer, T., and D. Heath. 1998. Lack of IL-1 receptor antagonistic activity of the capsular F1 antigen of Yersinia pestis. Immunol. Lett. 60137-142. [DOI] [PubMed] [Google Scholar]
- 42.Lindler, L. E., G. V. Plano, V. Burland, G. F. Mayhew, and F. R. Blattner. 1998. Complete DNA sequence and detailed analysis of the Yersinia pestis KIM5 plasmid encoding murine toxin and capsular antigen. Infect. Immun. 665731-5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loiez, C., S. Herwegh, F. Wallet, S. Armand, F. Guinet, and R. J. Courcol. 2003. Detection of Yersinia pestis in sputum by real-time PCR. J. Clin. Microbiol. 414873-4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lorange, E. A., B. L. Race, F. Sebbane, and B. J. Hinnebusch. 2005. Poor vector competence of fleas and the evolution of hypervirulence in Yersinia pestis. J. Infect. Dis. 1911907-1912. [DOI] [PubMed] [Google Scholar]
- 45.MacIntyre, S., S. D. Knight, and L. J. Fooks. 2004. Structure, assembly and applications of the polymeric F1 antigen of Yersinia pestis, p. 363-408. In E. Carniel and B. J. Hinnebusch (ed.), Yersinia molecular and cellular biology. Horizon Sciences, Norfolk, United Kingdom.
- 46.MacIntyre, S., I. M. Zyrianova, T. V. Chernovskaya, M. Leonard, E. G. Rudenko, V. P. Zav'Yalov, and D. A. Chapman. 2001. An extended hydrophobic interactive surface of Yersinia pestis Caf1M chaperone is essential for subunit binding and F1 capsule assembly. Mol. Microbiol. 3912-25. [DOI] [PubMed] [Google Scholar]
- 47.Meka-Mechenko, T. V. 2003. F1-negative natural Y. pestis strains. Adv. Exp. Med. Biol. 529379-381. [DOI] [PubMed] [Google Scholar]
- 48.Owhashi, M., M. Harada, S. Suguri, H. Ohmae, and A. Ishii. 2001. The role of saliva of Anopheles stephensi in inflammatory response: identification of a high molecular weight neutrophil chemotactic factor. Parasitol. Res. 87376-382. [DOI] [PubMed] [Google Scholar]
- 49.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis—etiologic agent of plague. Clin. Microbiol. Rev. 1035-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pollitzer, R. 1954. Plague. World Health Organization, Geneva, Switzerland.
- 51.Pujol, C., and J. B. Bliska. 2005. Turning Yersinia pathogenesis outside in: subversion of macrophage function by intracellular yersiniae. Clin. Immunol. 114216-226. [DOI] [PubMed] [Google Scholar]
- 52.Reed, L. J., and H. A. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27493-497. [Google Scholar]
- 53.Runco, L. M., S. Myrczek, J. B. Bliska, and D. G. Thanassi. 2008. Biogenesis of the fraction 1 capsule and analysis of the ultrastructure of Yersinia pestis. J. Bacteriol. 1903381-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schoeler, G. B., and S. K. Wikel. 2001. Modulation of host immunity by haematophagous arthropods. Ann. Trop. Med. Parasitol. 95755-771. [DOI] [PubMed] [Google Scholar]
- 55.Sebbane, F., C. O. Jarrett, D. Gardner, D. Long, and B. J. Hinnebusch. 2006. Role of the Yersinia pestis plasminogen activator in the incidence of distinct septicemic and bubonic forms of flea-borne plague. Proc. Natl. Acad. Sci. USA 1035526-5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sebbane, F., N. Lemaitre, D. E. Sturdevant, R. Rebeil, K. Virtaneva, S. F. Porcella, and B. J. Hinnebusch. 2006. Adaptive response of Yersinia pestis to extracellular effectors of innate immunity during bubonic plague. Proc. Natl. Acad. Sci. USA 10311766-11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma, R. K., A. Sodhi, and H. V. Batra. 2005. Involvement of c-Jun N-terminal kinase in rF1 mediated activation of murine peritoneal macrophages in vitro. J. Clin. Immunol. 25215-223. [DOI] [PubMed] [Google Scholar]
- 58.Sharma, R. K., A. Sodhi, H. V. Batra, and U. Tuteja. 2005. Phosphorylation of p42/44 MAP kinase is required for rF1-induced activation of murine peritoneal macrophages. Mol. Immunol. 421385-1392. [DOI] [PubMed] [Google Scholar]
- 59.Sodeinde, O. A., Y. V. Subrahmanyam, K. Stark, T. Quan, Y. Bao, and J. D. Goguen. 1992. A surface protease and the invasive character of plague. Science 2581004-1007. [DOI] [PubMed] [Google Scholar]
- 60.Sodhi, A., R. K. Sharma, H. V. Batra, and U. Tuteja. 2004. Recombinant fraction 1 protein of Yersinia pestis activates murine peritoneal macrophages in vitro. Cell Immunol. 22952-61. [DOI] [PubMed] [Google Scholar]
- 61.Soliman, S., A. J. Main, A. S. Marzouk, and A. A. Montasser. 2001. Seasonal studies on commensal rats and their ectoparasites in a rural area of Egypt: the relationship of ectoparasites to the species, locality, and relative abundance of the host. J. Parasitol. 87545-553. [DOI] [PubMed] [Google Scholar]
- 62.Stevenson, H. L., Y. Bai, M. Y. Kosoy, J. A. Montenieri, J. L. Lowell, M. C. Chu, and K. L. Gage. 2003. Detection of novel Bartonella strains and Yersinia pestis in prairie dogs and their fleas (Siphonaptera: Ceratophyllidae and Pulicidae) using multiplex polymerase chain reaction. J. Med. Entomol. 40329-337. [DOI] [PubMed] [Google Scholar]
- 63.Tikhomirow, E. 1999. Epidemiology and distribution of plague, p. 11-41. In D. T. Dennis, K. L. Gage, N. Gratz, J. D. Poland, and E. Tikhomirov (ed.), Plague manual: epidemiology, distribution, surveillance and control. World Health Organization, Geneva, Switzerland.
- 64.Titball, R. W., and E. D. Williamson. 2001. Vaccination against bubonic and pneumonic plague. Vaccine 194175-4184. [DOI] [PubMed] [Google Scholar]
- 65.Tomaso, H., E. C. Reisinger, S. Al Dahouk, D. Frangoulidis, A. Rakin, O. Landt, and H. Neubauer. 2003. Rapid detection of Yersinia pestis with multiplex real-time PCR assays using fluorescent hybridisation probes. FEMS Immunol. Med. Microbiol. 38117-126. [DOI] [PubMed] [Google Scholar]
- 66.Welkos, S. L., G. P. Andrews, L. E. Lindler, N. J. Snellings, and S. D. Strachan. 2004. Mu dI1(Ap lac) mutagenesis of Yersinia pestis plasmid pFra and identification of temperature-regulated loci associated with virulence. Plasmid 511-11. [DOI] [PubMed] [Google Scholar]
- 67.Wilder, A. P., R. J. Eisen, S. W. Bearden, J. A. Montenieri, K. L. Gage, and M. F. Antolin. 2008. Oropsylla hirsuta (Siphonaptera: Ceratophyllidae) can support plague epizootics in black-tailed prairie dogs (Cynomys ludovicianus) by early-phase transmission of Yersinia pestis. Vector Borne Zoonotic Dis. 8359-367. [DOI] [PubMed] [Google Scholar]
- 68.Williams, J. E., and D. C. Cavanaugh. 1984. Potential for rat plague from nonencapsulated variants of the plague bacillus (Yersinia pestis). Experientia 40739-740. [DOI] [PubMed] [Google Scholar]
- 69.Williams, J. E., D. N. Harrison, T. J. Quan, J. L. Mullins, A. M. Barnes, and D. C. Cavanaugh. 1978. Atypical plague bacilli isolated from rodents, fleas, and man. Am. J. Public Health 68262-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Williams, R. C., Jr., H. Gewurz, and P. G. Quie. 1972. Effects of fraction I from Yersinia pestis on phagocytosis in vitro. J. Infect. Dis. 126235-241. [DOI] [PubMed] [Google Scholar]
- 71.Winter, C., W. Cherry, and M. Moody. 1960. An unusual strain of Pasteurella pestis isolated from a fatal human case of plague. Bull. W. H. O. 23408-409. [PMC free article] [PubMed] [Google Scholar]
- 72.Zavialov, A. V., J. Kersley, T. Korpela, V. P. Zav'yalov, S. MacIntyre, and S. D. Knight. 2002. Donor strand complementation mechanism in the biogenesis of non-pilus systems. Mol. Microbiol. 45983-995. [DOI] [PubMed] [Google Scholar]
- 73.Zav'yalov, V. P., T. V. Chernovskaya, E. V. Navolotskaya, A. V. Karlyshev, S. MacIntyre, A. M. Vasiliev, and V. M. Abramov. 1995. Specific high affinity binding of human interleukin 1 beta by Caf1A usher protein of Yersinia pestis. FEBS Lett. 37165-68. [DOI] [PubMed] [Google Scholar]