Abstract
The obligate intracellular parasite Toxoplasma gondii can actively infect any nucleated cell type, including cells from the immune system. In the present study, we observed that a large number of natural killer (NK) cells were infected by T. gondii early after intraperitoneal inoculation of parasites into C57BL/6 mice. Interestingly, one mechanism of NK cell infection involved NK cell-mediated targeting of infected dendritic cells (DC). Perforin-dependent killing of infected DC led to active egress of infectious parasites that rapidly infected adjacent effector NK cells. Infected NK cells were not efficiently targeted by other NK cells. These results suggest that rapid transfer of T. gondii from infected DC to effector NK cells may contribute to the parasite's sequestration and shielding from immune recognition shortly after infection.
Toxoplasma gondii causes chronic infections in up to one-third of the human population and in animals (22, 31). In healthy individuals, primary T. gondii infection causes relatively mild symptoms, whereas in the immunocompromised patient or in the developing fetus, life-threatening manifestations lead to severe neurological and ocular damage (11, 28, 37). Following oral infection, T. gondii parasites typically pass across restrictive biological barriers and rapidly disseminate (13). In this process, T. gondii actively infects a great variety of cell types, including epithelial cells and blood leukocytes (12, 21). In infected cells, the parasites establish nonfusigenic parasitophorous vacuoles, where they can replicate (27, 32, 38).
Natural killer (NK) cells and dendritic cells (DC) are two important cell types of the innate immune system. DC-NK cell interactions are important not only in host defense but also for the development of adaptive immune responses (5, 9). The activation of DC by pathogens leads to cytokine secretion, which activates NK cells, which in turn, via cytokines or by direct cell-cell contact, may determine the adaptive immune responses that follow (9, 29). DC are sensitive to NK cell-mediated lysis in vitro and can be eliminated by NK cells in vivo (4, 6, 17, 19, 33, 43). Viral or bacterial infection of DC can reduce their sensitivity to NK cell-mediated lysis by increasing the expression of classical and nonclassical major histocompatibility complex class I molecules on the cell surface (14, 35, 43).
DC and NK cells play critical roles in innate immunity during acute Toxoplasma infection, being early sources of interleukin-12 (IL-12) and gamma interferon (IFN-γ), respectively (16, 20, 24, 34, 40). It has recently been suggested that infected DC, and possibly other leukocytes, can act as Trojan horses, potentiating the dissemination of the parasite from the point of infection to distal parts (8, 26). In the early phase of infection with T. gondii, NK cell recruitment to the site of infection is mediated by CCR5-binding chemokines (24). IFN-γ production by NK cells, induced by IL-12 from infected DC or macrophages, has been suggested to be the primary contribution of NK cells to the host defense against T. gondii (18, 25, 39). It can also drive cytotoxic CD8+ T-cell immunity to T. gondii even in the absence of CD4+ T cells (7). NK cells can also kill T. gondii-infected target cells (42), and perforin has been demonstrated to be important in protecting mice in the chronic stage of infection (10). In the present study, we investigated NK cell interactions with T. gondii-infected DC and, surprisingly, demonstrated how this interaction leads to T. gondii infection of NK cells.
MATERIALS AND METHODS
Animals.
C57BL/6 (B6), B6.recombination activating gene-1 (B6.RAG1)−/−, and B6.perforin (B6.pfp)−/− mice (6 to 10 weeks old) were housed under standard conditions at the Department of Microbiology, Tumor and Cell Biology at the Karolinska Institutet and at the Karolinska University Hospital Huddinge, Stockholm, Sweden. All procedures were performed in conformance with both institutional and national guidelines.
Antibodies.
Anti-FAS-L and anti-TRAIL monoclonal antibodies (MAbs) (41) were purified from cell culture media. Anti-FAS-L and anti-TRAIL MAbs were injected intraperitoneally (i.p.) at 500 μg/mouse 24 h prior to inoculation of parasites or adoptive transfer of parasite-infected DC. All labeled antibodies used for flow cytometry were obtained from Becton Dickinson (San Diego, CA).
Parasites and infection.
Green fluorescent protein (GFP)-expressing type I RH-LDM (1) and type II PTG-GFPS65T (31) T. gondii tachyzoites were maintained by serial 2-day passage in human foreskin fibroblast monolayers. Human foreskin fibroblasts were propagated in Dulbecco's modified Eagle's medium (Invitrogen, Paisley, United Kingdom) with 10% fetal calf serum (BioWhittaker, Verviers, Belgium), 20 μg/ml gentamicin, 2 mM glutamine, and 0.01 M HEPES (Invitrogen).
For infection of DC or NK cells in vitro, cells were harvested and incubated with freshly egressed GFP-expressing T. gondii tachyzoites at the indicated multiplicities of infection (MOI) for 16 to 24 h unless stated otherwise. Infection rates were assessed by flow cytometry by counting GFP+ cells. To inhibit parasite replication, 50 μM pyrimethamine (Sigma-Aldrich, Steinheim, Germany) was added to the cultures of GFP-expressing T. gondii tachyzoites and DC for the 16 to 24 h (30). Replication of parasites was assessed by flow cytometry and epifluorescence microscopy. Heat-killed parasites was generated as previously described (26).
Preparation of bone marrow-derived DC.
Bone marrow-derived DC were generated as described previously (19). Briefly, bone marrow-derived cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) containing 10 ng/ml recombinant granulocyte-macrophage colony-stimulating factor (Biosource, Brussels, Belgium). The cells were harvested after 6 days and replated overnight. DC were further purified with anti-CD11c MAb-coated beads (Militeny Biotec, Bergisch Gladbach, Germany).
NK cell preparation.
DX5+ cells from spleens of B6, B6.RAG1−/−, and B6.pfp−/− mice were purified by using the MACS separation system (Miltenyi Biotech) according to the manufacturer's guidelines. Purified cells were resuspended in complete αMEM medium (10 mM HEPES, 2 × 10−5 M 2-mercaptoethanol, 10% fetal calf serum, 100 U/ml penicillin, 100 U/ml streptomycin) and cultured in 1,000 U recombinant IL-2 (Biosource)/ml for 6 days.
Cytotoxicity assays.
Target cells (DC or NK cells) were incubated for 1 h in the presence of 51Cr (Amersham, Oxford, United Kingdom) and then washed thoroughly in phosphate-buffered saline (PBS). After 4 h of effector and target cell coincubation, cell culture supernatants were taken from these wells and analyzed by using a gamma radiation counter (Wallac Oy, Turku, Finland). Specific lysis was calculated according to the following formula: % specific lysis = [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100.
Egress of T. gondii from DC in vitro.
DC were infected with GFP+ T. gondii tachyzoites and extensively washed to remove free parasites before mixing with IL-2-stimulated NK cells or splenocytes at a 3:1 ratio. After 2 h, the cells were collected and examined by flow cytometry (FACScalibur; BD, San Diego, CA). In some experiments, dithiothreitol (Sigma-Aldrich) was added at 10 mM to DC prior to incubation with NK cells. For flow cytometry, cells were labeled with anti-NK1.1 and anti-CD11c MAbs. Dead cells were gated away by using propidium iodide.
Ex vivo microscopy of infected lymphocytes.
For visualization of in vivo Toxoplasma-infected NK and T cells, DX5+ NK cells and CD3+ T cells were sorted from the peritoneal cavity with the MACS separation system and then seeded on glass coverslips coated with poly-l-lysine (Sigma-Aldrich). After 30 min at 37°C, the cells were washed once with BRB80 buffer [80 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), pH 6.9; 1 mM MgCl2; 1 mM EGTA] and then fixed with 0.3% glutaraldehyde (TAAB Laboratories, Berkshire, United Kingdom) in BRB80 for 10 min at room temperature. Next, the cells were permeabilized with 0.1% Triton X-100 in PBS (PBST; Sigma, Steinheim, Germany) for 5 min at room temperature. Following a brief wash with PBS, pH 7.4, the coverslips were treated with 1 mg/ml sodium borohydride (Merck, Hohenbrunn, Germany) in PBS three times for 5 min each. The coverslips were then washed twice with PBST and incubated with phalloidin-Alexa 594 (Invitrogen, Carlsbad, CA) in PBST. Twenty minutes later, the coverslips were mounted with Vector Shield with 4′,6′-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA). Images were taken with a Leica DMRB microscope equipped with a Qimaging Q20780 camera and processed with OpenLab software.
Real-time confocal microscopy.
Consequences of NK cell interaction with infected DC were visualized with a spinning-disk confocal setup (Ultraview LCI-3 Tandem Scanning Unit; Perkin-Elmer, United Kingdom) on an Axiovert 200 M (Carl Zeiss, Germany) connected to a charge-coupled device camera (OrcaER; Hamamatsu, Japan). Cells were placed in a minichamber system (POCmini; LaCon, Germany) with a heating stage. Image acquisition and analysis of motility were performed with Openlab software (version 4.0.2) and Volocity software (Improvision Inc., United States).
Statistical analysis.
Statistical analyses were performed with Prism Graph Version 4 (GraphPad Software Inc., La Jolla, CA).
RESULTS
Infection of NK cells in mice inoculated i.p. with T. gondii.
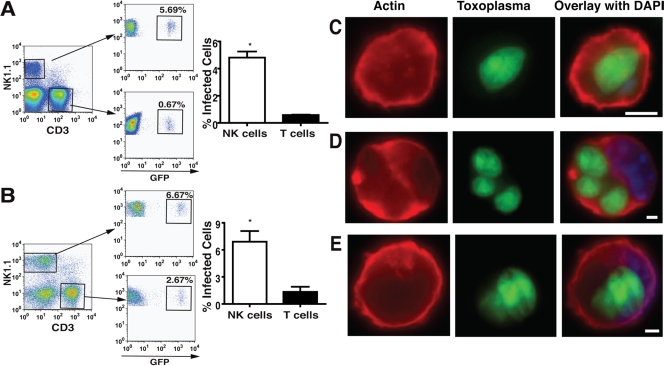
Freshly egressed type I GFP+ RH-LDM T. gondii tachyzoites were injected into the peritoneal cavities of B6 mice. After 48 h, approximately 30% of the CD11b+ myeloid cells were infected (data not shown and reference 26). Surprisingly, when gating on the lymphocyte population, a significant number of NK cells were also infected with GFP+ T. gondii tachyzoites. Among the total lymphocytes, the relative number of NK cells infected was significantly greater than that of infected T cells (Fig. 1A and Table 1). Interestingly, similar results were obtained when GFP+ RH-LDM T. gondii tachyzoite-infected DC were injected into the peritoneal cavities of B6 mice (Fig. 1B and Table 1). Results were similar with type II GFP+ PTG/ME49 T. gondii tachyzoites, both when inoculated as free parasites and in DC (Table 1). Ex vivo examination of the infected NK cells showed proliferating intracellular tachyzoites (Fig. 1C). Some NK cells had multiple vacuoles with replicating parasites (Fig. 1D). These data demonstrate that not only can myeloid cells, including macrophages and DC, become infected but also lymphocytes, including NK cells, following i.p. injection of T. gondii tachyzoites in B6 mice. During the 72-h period that we examined, the number of T cells in the peritoneal cavity remained relatively constant, whereas the number of NK cells increased, as previously observed by Khan et al. for NK cell recruitment into the spleen and liver (24).
FIG. 1.
NK cells are infected with GFP+ T. gondii tachyzoites. Flow cytometry analysis of the lymphocyte population of peritoneal exudates at 48 h postinfection. (A) Infection of NK cells and T cells following i.p. injection of 5 × 105 GFP+ tachyzoites. (B) Infection of NK cells and T cells following i.p. injection of 5 × 105 DC infected with GFP+ tachyzoites (MOI of 1). Bar graphs show the differences between infected NK cells and T cells (n = 6 mice; *, P < 0.05 [Student's t test]). Ex vivo examination of infected NK and T cells. (C and D) Infected NK cells. (E) Infected T cell. Scale bars = 3 μm.
TABLE 1.
Numbers of T. gondii-infected NK cells and T cells from the peritonea of infected mice
| Exptl conditions | No. of cellsa
|
Infected/uninfected cell ratio | |
|---|---|---|---|
| Infected | Uninfected | ||
| Free T. gondii | |||
| LDM | |||
| NK cellsb | 1,425 | 29,055 | 0.045 |
| T cells | 1,197 | 181,224 | 0.006 |
| PTG | |||
| NK cellsb | 669 | 29,324 | 0.022 |
| T cells | 729 | 80,349 | 0.008 |
| T. gondii-infected DC | |||
| LDM | |||
| NK cellsb | 2,069 | 33,323 | 0.062 |
| T cells | 1,534 | 151,221 | 0.01 |
| PTG | |||
| NK cellsb | 2,419 | 21,197 | 0.114 |
| T cells | 1,558 | 60,099 | 0.03 |
Total number of cells from six mice.
Chi-square analysis comparing the total number of infected and uninfected NK cells with the total number of infected and uninfected T cells: P < 0.001.
Lysis of infected DC by NK cells in vitro leads to active infection of NK cells.
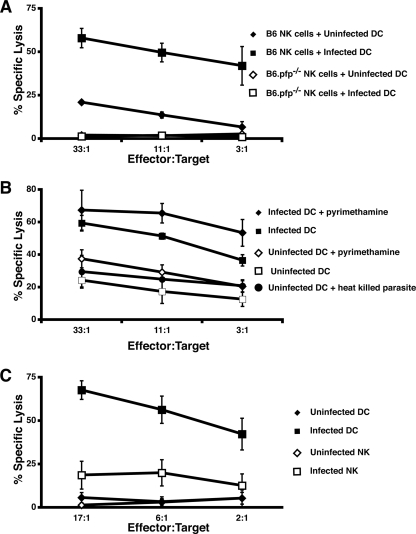
Since NK cells became infected in mice inoculated with T. gondii-infected DC, we investigated whether NK cell-mediated lysis of DC could facilitate the transmission of parasites from DC to NK cells. Although T. gondii tachyzoites could stimulate DC maturation (data not shown and reference 26), T. gondii-infected DC were more sensitive to NK cell-mediated lysis than were uninfected DC in vitro (Fig. 2A to C). NK cell-mediated lysis of DC in vitro is dependent on perforin (6). Accordingly, lysis of uninfected and infected DC was completely abolished when NK cells derived from B6.pfp−/− mice were used (Fig. 2A). We next determined if lysis of infected DC was related to parasite replication. Inhibition of tachyzoite replication was performed by pretreatment of DC and freshly egressed tachyzoites with pyrimethamine that was maintained throughout the experiment (30). Infected DC were still hypersensitive to NK cell-mediated lysis (Fig. 2B). DC incubated overnight with heat-killed tachyzoites did not exhibit enhanced killing by NK cells (Fig. 2B). Although T. gondii-infected DC were sensitive to NK cell killing, T. gondii-infected NK cells were resistant to NK cell-mediated killing under similar conditions (Fig. 2C).
FIG. 2.
NK cell-mediated lysis of DC is enhanced upon infection with T. gondii and is dependent on perforin and live-parasite infection. (A) Lysis of DC infected with tachyzoites (MOI of 3) by NK cells from B6 and B6.pfp−/− mice versus the effector-to-target cell ratio. The results of three separate experiments are shown ± the standard error of the mean. (B) Lysis of DC infected with tachyzoites, tachyzoites pretreated with pyrimethamine, or heat-killed tachyzoites versus the effector-to-target cell ratio. The results of three separate experiments are shown ± the standard error of the mean. (C) Lysis of tachyzoite-infected B6 NK cells and DC by uninfected B6 NK cells versus the effector-to-target cell ratio. The results of three separate experiments are shown ± the standard error of the mean.
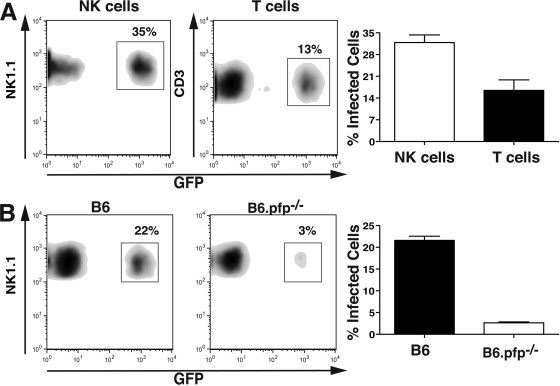
Since NK cells were preferentially infected in vitro and in vivo compared to T cells (Fig. 1A and 3A), and since NK cells readily lysed infected DC (Fig. 2A), we tested whether NK cells would become infected upon lysis of DC. NK cells sorted from B6 or B6.pfp−/− mice were mixed with DC infected with GFP+ RH-LDM tachyzoites in vitro. After 2 h, NK cells were analyzed by flow cytometry. At this time point, approximately 20% of the wild-type NK cells had become infected, as determined by GFP expression (Fig. 3B, left side). In contrast, only a small proportion of NK cells from B6.pfp−/− mice were infected under similar conditions (Fig. 3B, right side). Upon treatment with the parasite egress-promoting agent dithiothreitol, a significant increase in the transfer of parasites from DC to NK cells from B6.pfp−/− mice was observed. Under these conditions, NK cell infection levels were almost in line with those of NK cells from B6 mice (data not shown). Altogether, these data suggest that perforin-dependent lysis of infected DC by NK cells leads to the egress of tachyzoites that then infect surrounding NK cells.
FIG. 3.
NK cells become infected following lysis of infected DC in vitro. (A) Lymphokine-activated killer cell cultures containing one NK cell (left side) to three T cells (right side) following 2 h of culture with GFP+ tachyzoite-infected DC (MOI of 1). Bar graphs demonstrate the difference between infected NK cells and T cells. Accumulated data from three experiments are shown (P < 0.01 [Student's t test]). (B) Infection of B6 (left side) and B6.pfp−/− (right side) NK cells following 2 h of culture with DC infected with GFP+ tachyzoites (MOI of 1). One representative of three experiments is shown. The NK cell/DC ratio was 3:1. Bar graphs demonstrate the difference between infected B6 and B6.pfp−/− NK cells. Accumulated data from three separate experiments are shown (P < 0.01 [Student's t test]).
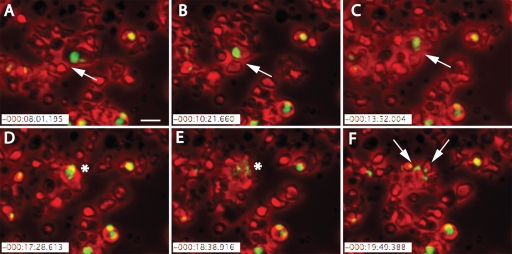
The transfer of parasites from infected DC to NK cells was further analyzed by live imaging. In the resulting films, motile NK cells physically interacted with infected DC (Fig. 4A to C). After several minutes of interaction, the NK cells lysed the infected DC (Fig. 4D and E), after which the release of GFP+ T. gondii tachyzoites was visible (Fig. 4E), soon followed by the spread of infection to surrounding NK cells (Fig. 4F; see videos S1 to S3 in the supplemental material). These data suggest that T. gondii can exit DC upon lysis by NK cells and that egressing parasites can infect the NK cells.
FIG. 4.
Real-time confocal analysis shows parasites' escape from DC and subsequent infection of NK cells. GFP+ T. gondii tachyzoites were allowed to infect DC (MOI of 1) for 12 h before the addition of NK cells. Mixed cell populations were visualized for approximately 2 h by time-lapse microscopy with artificial red colored phase contrast. Shortly after the addition of NK cells, egress of parasites from motile infected DC led to the infection of effector NK cells. (A to C) White arrows indicate a motile DC harboring GFP+ T. gondii tachyzoites seen as a green vacuole inside the DC. Interaction between the smaller NK cells and larger DC for approximately 5 min was followed by (D and E) lysis of the infected DC and rapid egress of parasites (indicated by an asterisk). (F) Shortly after the parasites' egress, surrounding NK cells became infected by the GFP-expressing parasites (white arrows). Scale bar = 25 μm.
NK cell-mediated lysis of infected DC leads to NK cell infection in vivo.
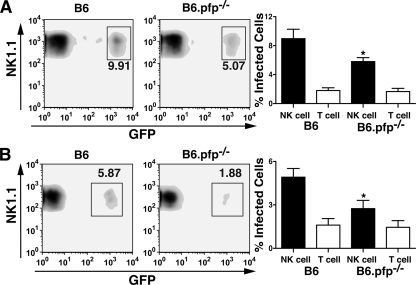
Lysis of DC by NK cells in vitro is mediated primarily through perforin (6). In contrast, lysis of DC by NK cells in vivo can be mediated by perforin or TRAIL (19). To test whether the spread of parasites from cell to cell in vivo could be mediated through killing of infected DC by NK cells, GFP+ RH-LDM tachyzoite-infected DC were injected into B6.pfp−/− mice treated with anti-TRAIL and anti-FAS-L MAbs. Inoculation with T. gondii-infected DC yielded significantly fewer infected NK cells in the B6.pfp−/− mice treated with anti-TRAIL and anti-FAS-L MAbs than in untreated B6 mice (Fig. 5A). Similarly, after inoculation of free parasites i.p., infection of NK cells was also reduced in B6.pfp−/− mice treated with anti-TRAIL and anti-FAS-L MAb compared to that in untreated B6 mice (Fig. 5B). In contrast, similar levels of T-cell infection were observed in B6 and B6.pfp−/− mice receiving either infected DC or free parasites (Fig. 5A and B). In summary, these observations suggest that NK cell-mediated killing of infected cells, such as DC, contributes to parasite infection of NK cells in vivo.
FIG. 5.
NK cell-mediated lysis of infected DC leads to infection of NK cells in vivo. Flow cytometry analysis of the NK1.1+ CD3− lymphocyte population of peritoneal exudates at 72 h postinfection. (A) Infection of B6 and B6.pfp−/− NK cells after i.p. injection of 5 × 105 DC infected with GFP+ tachyzoites (MOI of 1). (B) Infection of B6 and B6.pfp−/− NK cells 72 h after i.p. injection with 5 × 105 GFP+ tachyzoites. Bar graphs represent the different extents of infection of NK and T cells between B6 and B6.pfp−/− mice (n = 6 or 7 mice). *, P < 0.05 (analysis of variance).
DISCUSSION
NK cells are one important component of the immune system involved in the control of T. gondii infection (16, 20, 24, 40). In the present study, we demonstrate that T. gondii tachyzoites are rapidly transferred to NK cells during infection. We show here that NK cell-mediated killing of infected DC leads to rapid egress of viable parasites, which can then infect effector NK cells, possibly enabling them to resist immune elimination.
CD4+ and CD8+ T cells are also important in the protection of the host from T. gondii infection (15, 16). However, we recently reported that T-cell-mediated cytotoxicity triggers rapid egress of parasites from their host cells in vitro and in vivo, an active process mediated by intracellular fluxes of Ca2+ induced by death signals from FAS-L, TRAIL, and perforin (36). Thus, primed but not naive CD8+ T cells could be infected by T. gondii upon interaction with infected cells (36). In the present study, we have focused on examining the first 24 to 72 h of infection, a time when the cytotoxic response is dominated by NK cells. This and the previous study raise new questions about the role of cell-mediated immunity in the establishment of acute and chronic Toxoplasma infections. From the pathogen's perspective, its rapid transfer from infected DC into NK cells is intriguing. It may be argued that transmission of parasites from DC to NK cells contributes to the Toxoplasma parasite's efficacy in establishing a primary infection while avoiding clearance as immune control mounts. Also, NK cells are not as well equipped to handle intracellular infections as antigen-presenting cells are, since NK cells do not possess intracellular killing pathways such as, e.g., nitric oxide. Since NK cells did not appear to target infected NK cells, NK cell infection may provide a reservoir in which the parasites proliferate. Thus, even though the rapid transfer of T. gondii from DC to NK cells may not mediate systemic dissemination per se, it may promote persistence of the parasite in a less hostile intracellular environment.
Additionally, NK cells are likely poorer at stimulating naïve T cells than are DC, since they lack high levels of the necessary costimulatory molecules (3). Therefore, T. gondii parasites may selectively recruit NK cells (24) and be strong activators of NK cells. This activation could lead to NK cell-mediated lysis of infected cells and production of IFN-γ that could eliminate the majority of the parasites. In the process, though, NK cells could become infected, thus creating a niche for the parasites. Therefore, parasites that have secluded themselves within NK cells could reach distant organs directly upon the migration of NK cells or indirectly upon the lysis of infected NK cells after parasite replication.
In terms of host defense, antibody responses to T. gondii may be more critical than generally appreciated in protecting the host by preventing cell-cell transmission of the parasite. In line with this hypothesis, B-cell-deficient mice survive past the early stage of infection by T. gondii but die 3 to 4 weeks postinfection (23). Therefore, the development of effective immunizations against the parasite may require the ability to evoke antibody-mediated responses to prevent chronic infection, since NK cell and T-cell cytotoxic responses may in fact aid the parasites' survival, dissemination, and persistence.
This study demonstrates that T. gondii can use NK cells and potentially other lymphocytes to survive and multiply in the host. This may not be an isolated mechanism of immune evasion used by T. gondii. It has recently been shown that Neospora, a related apicomplexan parasite, also enhances the susceptibility of infected fibroblasts to NK cells (2) and can infect NK cells. However, we still need to determine if other pathogens similarly increase the NK cell sensitivity of targeted cells and, if so, provide an advantage for the persistence of infection. Two distinct possible mechanisms can be hypothesized by which pathogens can evade NK cell-mediated responses. Infections that promote the maturation of DC and NK cell resistance may use this strategy to bypass early elimination and thereby disseminate in the host. Additionally, pathogens that induce NK cell-activating ligands may take advantage of NK cell-mediated killing to continuously infect other cells and further the pathogen's survival. In conclusion, the present data suggest a mechanism by which NK cells paradoxically may promote the dissemination of the parasite T. gondii.
Supplementary Material
Acknowledgments
We thank everyone who provided fruitful advice throughout this project.
This work was funded by the Swedish Cancer Society (B.J.C.), the Swedish Research Council (A.B.), the Karolinska Institutet Foundations, the Swedish Foundation for Strategic Research, and the Karolinska Institutet Infection Biology Network.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 12 January 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Barragan, A., and L. D. Sibley. 2002. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J. Exp. Med. 1951625-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boysen, P., S. Klevar, I. Olsen, and A. K. Storset. 2006. The protozoan Neospora caninum directly triggers bovine NK cells to produce gamma interferon and to kill infected fibroblasts. Infect. Immun. 74953-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caminschi, I., F. Ahmet, K. Heger, J. Brady, S. L. Nutt, D. Vremec, S. Pietersz, M. H. Lahoud, L. Schofield, D. S. Hansen, M. O'Keeffe, M. J. Smyth, S. Bedoui, G. M. Davey, J. A. Villadangos, W. R. Heath, and K. Shortman. 2007. Putative IKDCs are functionally and developmentally similar to natural killer cells, but not to dendritic cells. J. Exp. Med. 2042579-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbone, E., G. Terrazzano, G. Ruggiero, D. Zanzi, A. Ottaiano, C. Manzo, K. Karre, and S. Zappacosta. 1999. Recognition of autologous dendritic cells by human NK cells. Eur. J. Immunol. 294022-4029. [DOI] [PubMed] [Google Scholar]
- 5.Chambers, B. J., and H. G. Ljunggren. 1999. NK cells, p. 257-268. In M. T. Lotze and A. W. Thomson (ed.), Dendritic cells. Academic Press, San Diego, CA.
- 6.Chambers, B. J., M. Salcedo, and H. G. Ljunggren. 1996. Triggering of natural killer cells by the costimulatory molecule CD80 (B7-1). Immunity 5311-317. [DOI] [PubMed] [Google Scholar]
- 7.Combe, C. L., T. J. Curiel, M. M. Moretto, and I. A. Khan. 2005. NK cells help to induce CD8+-T-cell immunity against Toxoplasma gondii in the absence of CD4+ T cells. Infect. Immun. 734913-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courret, N., S. Darche, P. Sonigo, G. Milon, D. Buzoni-Gatel, and I. Tardieux. 2006. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 107309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degli-Esposti, M. A., and M. J. Smyth. 2005. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat. Rev. Immunol. 5112-124. [DOI] [PubMed] [Google Scholar]
- 10.Denkers, E. Y., G. Yap, T. Scharton-Kersten, H. Charest, B. A. Butcher, P. Caspar, S. Heiny, and A. Sher. 1997. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J. Immunol. 1591903-1908. [PubMed] [Google Scholar]
- 11.Desmonts, G., and J. Couvreur. 1974. Congenital toxoplasmosis. A prospective study of 378 pregnancies. N. Engl. J. Med. 2901110-1116. [DOI] [PubMed] [Google Scholar]
- 12.Dobrowolski, J. M., and L. D. Sibley. 1996. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 84933-939. [DOI] [PubMed] [Google Scholar]
- 13.Dubey, J. P. 1998. Advances in the life cycle of Toxoplasma gondii. Int. J. Parasitol. 281019-1024. [DOI] [PubMed] [Google Scholar]
- 14.Ferlazzo, G., B. Morandi, A. D'Agostino, R. Meazza, G. Melioli, A. Moretta, and L. Moretta. 2003. The interaction between NK cells and dendritic cells in bacterial infections results in rapid induction of NK cell activation and in the lysis of uninfected dendritic cells. Eur. J. Immunol. 33306-313. [DOI] [PubMed] [Google Scholar]
- 15.Gazzinelli, R., Y. Xu, S. Hieny, A. Cheever, and A. Sher. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol. 149175-180. [PubMed] [Google Scholar]
- 16.Gazzinelli, R. T., S. Hieny, T. A. Wynn, S. Wolf, and A. Sher. 1993. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc. Natl. Acad. Sci. USA 906115-6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geldhof, A. B., M. Moser, and P. De Baetselier. 1998. IL-12-activated NK cells recognize B7 costimulatory molecules on tumor cells and autologous dendritic cells. Adv. Exp. Med. Biol. 451203-210. [DOI] [PubMed] [Google Scholar]
- 18.Guan, H., M. Moretto, D. J. Bzik, J. Gigley, and I. A. Khan. 2007. NK cells enhance dendritic cell response against parasite antigens via NKG2D pathway. J. Immunol. 179590-596. [DOI] [PubMed] [Google Scholar]
- 19.Hayakawa, Y., V. Screpanti, H. Yagita, A. Grandien, H. G. Ljunggren, M. J. Smyth, and B. J. Chambers. 2004. NK cell TRAIL eliminates immature dendritic cells in vivo and limits dendritic cell vaccination efficacy. J. Immunol. 172123-129. [DOI] [PubMed] [Google Scholar]
- 20.Hunter, C. A., C. S. Subauste, V. H. Van Cleave, and J. S. Remington. 1994. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect. Immun. 622818-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joiner, K. A. 1993. Cell entry by Toxoplasma gondii: all paths do not lead to success. Res. Immunol. 14434-38. [DOI] [PubMed] [Google Scholar]
- 22.Joynson, D. H., and T. J. Wreghitt. 2001. Toxoplasmosis: a comprehensive clinical guide. Cambridge University Press, Cambridge, United Kingdom.
- 23.Kang, H., J. S. Remington, and Y. Suzuki. 2000. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-γ, TNF-α, and inducible nitric oxide synthase. J. Immunol. 1642629-2634. [DOI] [PubMed] [Google Scholar]
- 24.Khan, I. A., S. Y. Thomas, M. M. Moretto, F. S. Lee, S. A. Islam, C. Combe, J. D. Schwartzman, and A. D. Luster. 2006. CCR5 is essential for NK cell trafficking and host survival following Toxoplasma gondii infection. PLoS Pathog. 2e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korbel, D. S., O. C. Finney, and E. M. Riley. 2004. Natural killer cells and innate immunity to protozoan pathogens. Int. J. Parasitol. 341517-1528. [DOI] [PubMed] [Google Scholar]
- 26.Lambert, H., N. Hitziger, I. Dellacasa, M. Svensson, and A. Barragan. 2006. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell. Microbiol. 81611-1623. [DOI] [PubMed] [Google Scholar]
- 27.Lingelbach, K., and K. A. Joiner. 1998. The parasitophorous vacuole membrane surrounding Plasmodium and Toxoplasma: an unusual compartment in infected cells. J. Cell Sci. 111(Pt. 11)1467-1475. [DOI] [PubMed] [Google Scholar]
- 28.Luft, B. J., and J. S. Remington. 1992. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 15211-222. [DOI] [PubMed] [Google Scholar]
- 29.Martín-Fontecha, A., L. L. Thomsen, S. Brett, C. Gerard, M. Lipp, A. Lanzavecchia, and F. Sallusto. 2004. Induced recruitment of NK cells to lymph nodes provides IFN-γ for TH1 priming. Nat. Immunol. 51260-1265. [DOI] [PubMed] [Google Scholar]
- 30.Meneceur, P., M. A. Bouldouyre, D. Aubert, I. Villena, J. Menotti, V. Sauvage, J. F. Garin, and F. Derouin. 2008. In vitro susceptibility of various genotypic strains of Toxoplasma gondii to pyrimethamine, sulfadiazine, and atovaquone. Antimicrob. Agents Chemother. 521269-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montoya, J. G., and O. Liesenfeld. 2004. Toxoplasmosis. Lancet 3631965-1976. [DOI] [PubMed] [Google Scholar]
- 32.Mordue, D. G., S. Hakansson, I. Niesman, and L. D. Sibley. 1999. Toxoplasma gondii resides in a vacuole that avoids fusion with host cell endocytic and exocytic vesicular trafficking pathways. Exp. Parasitol. 9287-99. [DOI] [PubMed] [Google Scholar]
- 33.Parajuli, P., Y. Nishioka, N. Nishimura, S. M. Singh, M. Hanibuchi, H. Nokihara, H. Yanagawa, and S. Sone. 1999. Cytolysis of human dendritic cells by autologous lymphokine-activated killer cells: participation of both T cells and NK cells in the killing. J. Leukoc. Biol. 65764-770. [DOI] [PubMed] [Google Scholar]
- 34.Pepper, M., F. Dzierszinski, E. Wilson, E. Tait, Q. Fang, F. Yarovinsky, T. M. Laufer, D. Roos, and C. A. Hunter. 2008. Plasmacytoid dendritic cells are activated by Toxoplasma gondii to present antigen and produce cytokines. J. Immunol. 1806229-6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Persson, C. M., E. Assarsson, G. Vahlne, P. Brodin, and B. J. Chambers. 2008. Critical role of Qa1b in the protection of mature dendritic cells from NK cell-mediated killing. Scand. J. Immunol. 6730-36. [DOI] [PubMed] [Google Scholar]
- 36.Persson, E. K., A. M. Agnarson, H. Lambert, N. Hitziger, H. Yagita, B. J. Chambers, A. Barragan, and A. Grandien. 2007. Death receptor ligation or exposure to perforin trigger rapid egress of the intracellular parasite Toxoplasma gondii. J. Immunol. 1798357-8365. [DOI] [PubMed] [Google Scholar]
- 37.Roberts, F., and R. McLeod. 1999. Pathogenesis of toxoplasmic retinochoroiditis. Parasitol. Today 1551-57. [DOI] [PubMed] [Google Scholar]
- 38.Sacks, D., and A. Sher. 2002. Evasion of innate immunity by parasitic protozoa. Nat. Immunol. 31041-1047. [DOI] [PubMed] [Google Scholar]
- 39.Sher, A., C. Collazzo, C. Scanga, D. Jankovic, G. Yap, and J. Aliberti. 2003. Induction and regulation of IL-12-dependent host resistance to Toxoplasma gondii. Immunol. Res. 27521-528. [DOI] [PubMed] [Google Scholar]
- 40.Sher, A., I. P. Oswald, S. Hieny, and R. T. Gazzinelli. 1993. Toxoplasma gondii induces a T-independent IFN-γ response in natural killer cells that requires both adherent accessory cells and tumor necrosis factor-alpha. J. Immunol. 1503982-3989. [PubMed] [Google Scholar]
- 41.Smyth, M. J., E. Cretney, K. Takeda, R. H. Wiltrout, L. M. Sedger, N. Kayagaki, H. Yagita, and K. Okumura. 2001. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon gamma-dependent natural killer cell protection from tumor metastasis. J. Exp. Med. 193661-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subauste, C. S., L. Dawson, and J. S. Remington. 1992. Human lymphokine-activated killer cells are cytotoxic against cells infected with Toxoplasma gondii. J. Exp. Med. 1761511-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson, J. L., L. C. Heffler, J. Charo, A. Scheynius, M. T. Bejarano, and H. G. Ljunggren. 1999. Targeting of human dendritic cells by autologous NK cells. J. Immunol. 1636365-6370. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.