Abstract
Multiple microbial components trigger the formation of an inflammasome complex that contains pathogen-specific nucleotide oligomerization and binding domain (NOD)-like receptors (NLRs), caspase-1, and in some cases the scaffolding protein ASC. The NLR protein Nalp1b has been linked to anthrax lethal toxin (LT)-mediated cytolysis of murine macrophages. Here we demonstrate that in unstimulated J774A.1 macrophages, caspase-1 and Nalp1b are membrane associated and part of ∼200- and ∼800-kDa complexes, respectively. LT treatment of these cells resulted in caspase-1 recruitment to the Nalp1b-containing complex, concurrent with processing of cytosolic caspase-1 substrates. We further demonstrated that Nalp1b and caspase-1 are able to interact with each other. Intriguingly, both caspase-1 and Nalp1b were membrane associated, while the caspase-1 substrate interleukin-18 was cytosolic. Caspase-1-associated inflammasome components included, besides Nalp1b, proinflammatory caspase-11 and the caspase-1 substrate α-enolase. Asc was not part of the Nalp1b inflammasome in LT-treated macrophages. Taken together, our findings suggest that LT triggers the formation of a membrane-associated inflammasome complex in murine macrophages, resulting in cleavage of cytosolic caspase-1 substrates and cell death.
Multiple microbial pathogens, including Salmonella, Francisella, Listeria, and Staphylococcus species, activate specific nucleotide oligomerization and binding domain (NOD)-like receptors (NLRs) and elicit an inflammatory response characterized by caspase-1 activation (40, 42). The NLR protein Nalp1b has also been linked to caspase-1 activation and macrophage cytolysis mediated by anthrax lethal toxin (LT) (6, 36). However, it is unclear how LT activates the proinflammatory protein Nalp1b and how this results in caspase-1 activation in murine macrophages.
LT is considered the primary virulence factor produced by the gram-positive organism Bacillus anthracis. In fact, challenge with LT alone mimics disease progression seen in mammalian hosts infected with B. anthracis spores. LT is a protein toxin consisting of two subunits, protective antigen (PA) and lethal factor (LF) (10). PA binds to specific cell surface receptors and mediates endocytosis of LF, a zinc protease. The proteolytic activity of LF is essential for the cytopathic and lethal effects observed in LT-treated mice (15, 20).
The response of murine macrophages to LT exposure is mouse strain dependent. Murine macrophages are either susceptible or resistant to LT-mediated caspase-1 activation and cytolysis (29, 30). Genetic mapping experiments have identified a single gene, Nalp1b, which is linked to the strain-specific LT responsiveness of murine macrophages (6, 36). The expression of dominant Nalp1b alleles from susceptible murine strains in the resistant C57BL/6 background renders the resulting macrophages susceptible to rapid LT killing (6).
Nalp1b belongs to the NLR family of intracellular surveillance proteins, which are able to recognize pathogen-associated molecular patterns, including lipopolysaccharide (LPS) (25, 34, 40). In contrast to murine Nalp1b, the human NLR proteins NALP1 and NALP3 have been well characterized (25, 40). Stimulation of NALP1 or NALP3 results in the recruitment of downstream components and the formation of the inflammasome complex, which appears to be a critical event associated with caspase-1 activation (1, 24, 26). The NOD of NALP proteins is required for dimerization, and the LRR domain has a microbe-sensing function (40). The pyrin domain (PYD) and the caspase recruitment domain (CARD) of NALP1 and NALP3 are essential for the recruitment of ASC and caspase-1, respectively (24, 26). In contrast to human NALP1, the PYD is absent in murine Nalp1b, and the involvement of murine Asc in Nalp1b inflammasome activation is therefore questionable (6).
NLR stimulation by specific ligands results in activation of proinflammatory caspase-1 and cell death (11, 12, 16). Activated caspase-1 then processes pro-interleukin-1β (pro-IL-1β), IL-18, and IL-33 into their mature forms (22, 37). Consistent with a role for Nalp1b in LT susceptibility, caspase-1 is activated in susceptible LT-treated macrophages but not in resistant cells (31). Studies with caspase-1-deficient murine macrophages and caspase-1 inhibitors suggest that caspase-1 is essential for LT killing of susceptible murine macrophages (6, 31, 41).
The mechanism by which microbial components, including LT, activate the inflammasome and the way in which this results in caspase-1 activation are poorly understood. In contrast to bacteria, which contain multiple virulence factors that simultaneously activate several NLRs, LT is a single virulence factor and appears to represent an ideal model system to study microbial inflammasome induction and caspase-1 activation. Our findings indicate that LT triggers the formation of an inflammasome complex containing Nalp1b and caspase-1 in murine macrophages. In untreated macrophages, caspase-1 was part of low-molecular-weight fractions and shifted toward high-molecular-weight fractions following LT treatment. Formation of the high-molecular-weight complex, presumably the inflammasome, coincides with caspase-1 activation and macrophage lysis. Caspase-1-associated proteins also included caspase-11 and the caspase-1 target α-enolase in LT-treated macrophages.
MATERIALS AND METHODS
Cell lines and plasmids.
The BALB/c-derived murine macrophage cell line J774A.1 and the human kidney fibroblast line 293T were obtained from the American Type Tissue Culture Collection (ATCC, Manassas, VA). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Mediatech, Herndon, VA) containing 10% fetal bovine serum (HyClone, Logan, UT) supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco, Grand Island, NY). The procaspase-1 expression vector was purchased from ATCC. The Nalp1b-V5 expression construct was kindly provided by Bill Dietrich (Novartis, Boston, MA). PA and LT were purchased from List Biological Laboratories (Campbell, CA) and used at 500 and 250 ng/ml, respectively.
DNA transfection.
For transfection assays, 293T cells were grown in 100-mm tissue culture dishes at 80% confluence. 293T cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's guidelines. Transfected cells were cultured for 48 h before processing.
Antibodies.
Rabbit anti-procaspase-1, goat anti-α-enolase, goat anti-caspase-11, and mouse antiactin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); mouse anti-IL-18 antibodies were from Biovision (Mountain View, CA); mouse antiflotillin antibodies were from BD Pharmingen (Franklin Lanes, NJ); mouse anti-V5 antibodies were from Invitrogen; and polyclonal rabbit anti-Toll-like receptor-4 (TLR-4) antibodies were from eBioscience (San Diego, CA). Antibodies were used for Western blotting and immunoprecipitation according to manufacturers' recommendations. Murine anti-Nalp1b and anti-Asc peptide antibodies were generated in rabbits against the following peptides: for Nalp1b, SPPKQKSNTKVAQHEGQ, and for Asc, QALKEIHPYLVMDLEQS (EzBiolab, Westfield, IN). The resulting antibodies against Nalp1b and Asc were affinity purified against the immunogenic peptide immobilized to epoxy-activated agarose beads (Sigma, St. Louis, MO).
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis of caspase-1 and IL-18.
Caspase-1 activation was examined in J774A.1 macrophages treated with LT. Cells were washed with phosphate-buffered saline (PBS) at designated time points and lysed in radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 7.4, 0.1% sodium dodecyl sulfate [SDS], 0.5% sodium deoxycholate, 1% NP-40, 150 mM NaCl, 1 mM EDTA) containing a protease inhibitor cocktail (Roche, Basel, Switzerland). Cell lysates were then centrifuged at 10,000 rpm for 15 min, and 15 μg of protein was run on 4 to 20% SDS-polyacrylamide gradient gels (Bio-Rad, Hercules, CA). Immunoblotting was performed with antibody dilutions recommended by the manufacturer.
PI exclusion assay.
Murine J774A.1 macrophages were washed three times with PBS and seeded at 8 × 105 cells/ml on 96-well fluorescent plates in DMEM with 10% fetal calf serum and antibiotics (Mediatech, Herndon, VA). Cytopathic effects were assessed by a propidium iodide (PI) exclusion assay at different time points after LT exposure. PA treatment alone and LF treatment alone were used as controls. PI (25 μM) was added to each well, and fluorescence was determined with a Wallac Victor II multititer plate reader at 590 nm (PerkinElmer Life Sciences).
Fluorescence microscopy.
Staining using phalloidin, PI, and Hoechst 33342 (Invitrogen) was performed according to the manufacturer's recommendations. Immunostaining was performed as described previously (2). Briefly, cells were grown overnight on Lab-Tek II coverslip chambers (Nalgene, Rochester, NY) to a density of ∼105 cells/ml. Cells were stained with Hoechst 33342 (10 μg/ml) for 15 min and washed twice with PBS prior to LT treatment. LT and PI were then added to cells for the PI exclusion assay. Cells were fixed with 4% paraformaldehyde and permeabilized with 1% Triton X-100 and 0.05% SDS in PBS at different time points after LT exposure. For major histocompatibility complex class I (MHC-I) immunostaining, cells were incubated with anti-MHC-I antibody in DMEM containing preimmunized mouse antibody (10 μg/ml; Sigma) for 20 min, followed by 10 washes with PBS. Subsequently, the secondary antibody was added for an additional 20 min and the cells were washed 10 times with PBS and fixed. For intracellular immunostaining, cells were fixed, permeabilized, and blocked with 10% fetal bovine serum in PBS and preimmune mouse immunoglobulin G (10 μg/ml; Sigma) and then incubated with primary antibody for 1 h at room temperature. Cells were then washed 10 times with PBS and incubated with secondary antibodies (30 min). Finally, cells were washed and mounted using Prolong (Invitrogen). As controls, we used nonmatching secondary antibodies and secondary antibodies alone.
Subcellular fractionation.
Subcellular fractions were prepared according to the method of Yeung et al. (48). Briefly, J774A.1 macrophages were grown in DMEM on 100-mm tissue culture dishes. Upon reaching 90% confluence, cells were exposed to LT for 90 min. After LT exposure, cells were swollen in hypotonic buffer (10 mM sodium pyrophosphate, 10 mM sodium fluoride, 5 mM Tris-HCl, pH 7.4) in the presence of a protease inhibitor cocktail (Roche) for 5 min on ice. Cells were homogenized to more than 95% breakage with a Dounce homogenizer (Kimble/Kontes, Vineland, NJ), as monitored by phase-contrast microscopy. Finally, 0.25 volume of compensation buffer (0.95 M sucrose, 60 mM sodium pyrophosphate, 150 mM sodium fluoride, 20 mM Tris-HCl, pH 7.4) was added, the homogenate was centrifuged for 5 min at 1,000 × g to pellet the nuclei, and the supernatant was saved as postnuclear supernatant (PNS). The resultant pellet was washed with the compensated homogenization buffer to remove contamination by the other subcellular fractions and solubilized in SDS loading buffer. The PNS was subfractionated by centrifugation at 100,000 × g at 4°C for 1 h, and the supernatant was saved as the cytosolic fraction. Pellets (the postnuclear particulate [PNP] fraction) were homogenized in hypotonic fractionation buffer with compensation buffer and 1% Triton X-100 with a Dounce homogenizer. The homogenate was centrifuged at 100,000 × g for 30 min at 4°C, and the supernatant was saved as the Triton X-100-soluble membrane (TXS) fraction. The Triton X-100-insoluble particulate fraction was further separated into Triton X-100-insoluble lipid raft (LR) and cytoskeletal (CSK) fractions (19). The LR fraction was diluted 10 times in buffer A (130 mM NaCl, 10 mM Tris-HCl, pH 7.2), pelleted by centrifugation at 40,000 rpm at 4°C for 1 h, and resuspended in a small volume of buffer A prior to solubilization in SDS-PAGE loading buffer. Protein concentrations of the fractions were determined using a bicinchoninic acid protein assay kit (Pierce Biotech, Rockfort, IL). The purity of each subcellular fraction was determined using lactate dehydrogenase (LDH) (34 kDa) as a cytosolic marker, flotillin-1 (48 kDa) as a marker for LRs, and TLR-4 (100 kDa) as a marker for the nonraft TXS fraction.
SEC.
Sephacryl S-300 (100- by 2.5-cm) and S-200 (100- by 2.5-cm) columns (Amersham, Piscataway, NJ) were used for size exclusion chromatography (SEC) of macrophage lysates. Briefly, J774A.1 macrophages were grown on 100-mm tissue culture dishes in DMEM in the presence or absence of LT. The PNSs from these cells were collected after treatment with SEC buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% octylglucoside) containing a protease inhibitor cocktail and centrifuged (10,000 rpm, 4°C, 15 min) to remove insoluble cellular debris. The protein concentration was determined using a bicinchoninic acid protein assay kit (Pierce). Ten milligrams of solubilized PNS from either treated or untreated cells was loaded onto the SEC column.
Cross-linking antibody to protein A and immunoaffinity purification.
Cross-linking of antibodies to protein A-Sepharose was performed as previously described (38). Briefly, 1 mg of the antibody was incubated with 1 ml protein A-Sepharose beads (Invitrogen), with continuous mixing at 4°C for 2 h. The beads were then centrifuged and washed five times with PBS and once with 200 mM sodium borate, pH 9. Equal volumes of 40 mM dimethylsuberimidate (Pierce) in 200 mM sodium borate were mixed with the beads, and the suspension was incubated, with continuous mixing, at room temperature for 30 min. The reaction was terminated by addition of equal volumes of 400 mM ethanolamine, and the sample was incubated for an additional 30 min. The beads were packed into a 1-cm-diameter column (Bio-Rad) and washed three times with 2 bed volumes of PBS and once with 4 bed volumes of 100 mM glycine-HCl at pH 2.4 to remove non-cross-linked antibodies. Finally, the cross-linked antibody beads were washed with PBS until the pH reached 7 and stored at 4°C in PBS containing 0.02% sodium azide. Rabbit anti-caspase-1 antibodies cross-linked to protein A-Sepharose beads and packed into columns (Bio-Rad) were used to immunoaffinity purify the caspase-1-associated complex. To reduce nonspecific protein-protein interactions, samples were precleared with protein A beads cross-linked to rabbit immunoglobulin G. Eluates from the affinity column were concentrated using a 10-kDa-cutoff Amicon Ultra-4 concentrator (Millipore, Billerica, MA).
Mass spectrometry.
Concentrated samples from casapse-1 affinity chromatography eluates were digested with trypsin, and the tryptic peptides were analyzed by electrospray ionization-tandem mass spectrometry (Keck Foundation, Proteomics Facility, Yale University).
Immunoprecipitation and pull-down assays.
293T cells were cotransfected with V5-His-tagged Nalp1b and caspase-1 expression plasmids, and 36 h posttransfection, transfected cells were lysed with immunoprecipitation buffer (1% NP-40, 150 mM NaCl, 50 mM Tris, pH 8.4, in the presence of 2× protease inhibitors [Roche]). For immunoprecipitation from J774A.1 cells, cells were treated either with LPS (2 μg/ml) for 8 h or with LT (250 ng/ml LF and 500 ng/ml PA) for 90 min. Cells were lysed with immunoprecipitation buffer. Samples were mixed with anti-caspase-1 antibody-conjugated beads for 4 h at 4°C. The beads were collected and washed five times with the same lysis buffer and one time with doubly distilled H2O before elution with protein loading buffer. Samples were resolved on 4 to 15% SDS-polyacrylamide gels and electroblotted to polyvinylidene difluoride membranes (Bio-Rad) according to the manufacturer's protocol. After that, the blotted membranes were probed with anti-V5 and anti-procaspase-1 antibodies. Nickel columns were used to pull down His-tagged Nalp1b proteins according to the manufacturer's recommendations (Pierce). After elution, samples were concentrated over a 10-kDa-cutoff Amicon Ultra-4 concentrator (Millipore, Billerica, MA) before electrophoresis and probing with anti-V5 and anti-caspase-1 antibodies.
RESULTS
LT triggers the formation of a high-molecular-weight complex containing caspase-1 in murine macrophages.
Several microbial toxins and components, including LT, stimulate the innate immune response via activation of specific NLRs. In the case of LT, the dominant isoform of the NLR protein Nalp1b controls the susceptibility of murine macrophages to rapid cell death (6). We are using LT and murine macrophages as a tool to study inflammasome activation by microbial toxins. It remains unknown how LT triggers Nalp1b activation and how this leads to macrophage killing. We postulate that LT triggers the formation of a specific inflammasome complex in susceptible murine macrophages, resulting in caspase-1 activation and cell death.
To date, the best-characterized inflammasome complex is associated with the human NLR NALP3. Stimulation of this receptor leads to the recruitment of the downstream components ASC and caspase-1 (1, 24). Here we tested whether LT triggers the formation of a specific inflammasome complex containing Nalp1b, Asc (the murine homolog of human ASC), and caspase-1.
To analyze the formation of an inflammasome complex in LT-treated macrophages, we performed SEC. Untreated or LT-treated J774A.1 macrophages were homogenized, and the PNSs from these cells were subjected to SEC under nondenaturing conditions. To observe the formation of a Nalp1b inflammasome complex, we generated antibodies against murine Nalp1b and Asc proteins. Polyclonal antibodies raised against Nalp1b and Asc peptides detected the corresponding proteins in Western blot analyses of subcellular-fractionation assays (see Fig. 1 and 3). The anti-Nalp1b antibody also immunostained endogenous Nalp1b in murine J774A.1 macrophages and in 293T cells transiently transfected with Nalp1b expression constructs, while the anti-Asc antibody detected endogenous Asc in murine J774A.1 macrophages (data not shown). While subcellular-fractionation assays localized Nalp1b to the membrane fraction, we detected punctate as well as cytosolic staining of Nalp1b in J774A.1 cells (data not shown), possibly due to the presence of a truncated version of Nalp1b (see Fig. 5).
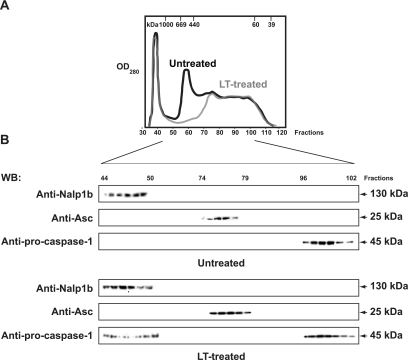
FIG. 1.
LT triggers the formation of a high-molecular-weight complex containing caspase-1 and Nalp1b in J774A.1 macrophages. The PNP fractions were isolated from untreated and LT-treated macrophages 120 min after LT exposure and fractionated by SEC on an S-300 column under nondenaturing conditions at 4°C. (A) Elution profiles of proteins (optical density at 280 nm [OD280]) from untreated and LT-treated macrophages. The molecular mass scale was determined using a wide range of molecular mass markers. (B) SDS-PAGE of fractions of untreated and LT-treated macrophages immunoblotted with anti-procaspase-1, anti-Asc, and anti-Nalp1b antibodies. All fractions were immunoblotted, but only those with detected bands are shown. WB, Western blotting.
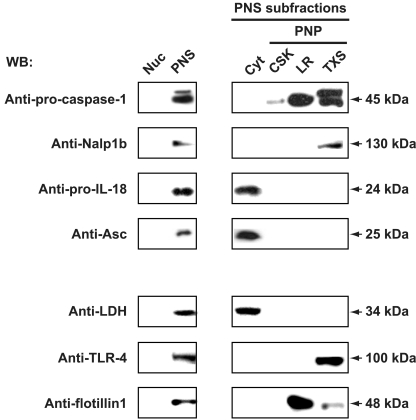
FIG. 3.
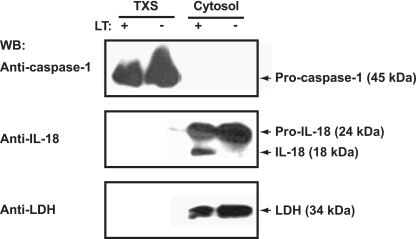
Inflammasome proteins Nalp1b and caspase-1 localize to the membrane fraction of J774A.1 murine macrophages. Cells fractionated into cytosolic (Cyt), nuclear (Nuc), and PNP fractions, and the PNP was further fractionated into CSK, LR, and TXS subfractions. Fifteen micrograms of protein from each fraction was subjected to 4 to 20% SDS-PAGE and immunoblotting using antibodies against Nalp1b, Asc, and caspase-1. The purity of subcellular fractions was demonstrated with markers for cytosolic (LDH), LR (flotillin), and TXS (TLR-4) fractions. WB, Western blotting.
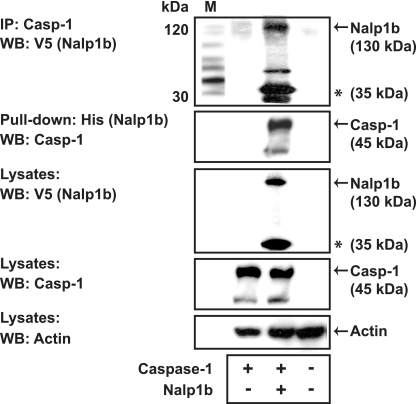
FIG. 5.
Nalp1b interacts directly with caspase-1. Coimmunoprecipitation and pull-down assays were performed using lysates from 293T cells that had been transiently transfected with plasmids encoding murine caspase-1 (Casp-1) and V5-His-tagged Nalp1b, as indicated. Lysates were immunoprecipitated using an anti-caspase-1 antibody, and following elution, samples were subjected to SDS-PAGE and immunoblotting using anti-V5 antibodies. Lysates were pulled down with a nickel column, and the eluates were subjected to immunoblotting using anti-caspase-1 antibodies. A truncated version of Nalp1b is indicated with an asterisk. IP, immunoprecipitation; WB, Western blotting.
The SEC fractions of untreated and LT-treated macrophages showed distinct protein profiles (Fig. 1A) and were further subjected to SDS-PAGE and Western blotting using antibodies against the putative inflammasome components. Procaspase-1 was found in an ∼200-kDa complex, Asc in an ∼350-kDa complex, and Nalp1b in a complex of ∼800 kDa in unstimulated J774A.1 cells (Fig. 1B, top). While the distributions of Nalp1b and Asc did not change following LT treatment of murine macrophages, procaspase-1 shifted from the low-molecular-mass fractions (∼200 kDa) to the high-molecular-mass fractions (∼800 kDa) following LT exposure. Accordingly, the high-molecular-mass fractions in LT-treated macrophages contained caspase-1 and Nalp1b but not Asc (Fig. 1B, bottom). The shift of caspase-1 toward the high-molecular-mass fraction is consistent with the hypothesis that caspase-1 is recruited to a putative inflammasome complex in LT-treated macrophages. Consistent with the inflammasome-dependent caspase-1 activation observed with LPS-stimulated monocytes (24), we found activated caspase-1 (p10) exclusively in the high-molecular-mass fraction of LT-treated macrophages (data not shown). It should be noted that in unstimulated macrophages, the 45-kDa procaspase-1 was eluted from the ∼200-kDa SEC fractions, suggesting that procaspase-1 is not monomeric and forms a complex, presumably in association with other proteins, in unstimulated cells.
Inflammasome components caspase-1 and Nalp1b localized to the membrane fraction of murine macrophages.
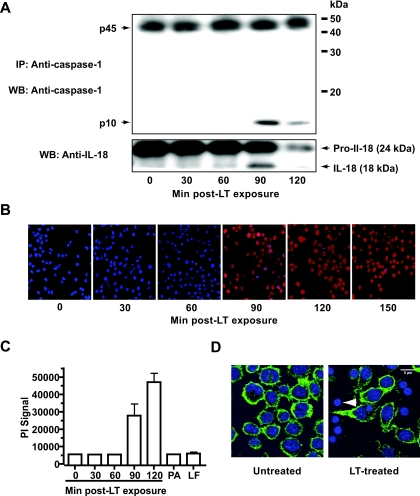
Our findings suggested that the formation of the inflammasome complex in LT-treated cells requires recruitment of caspase-1 to a putative inflammasome complex. We therefore tested whether the cellular localization of caspase-1 and other putative inflammasome components changed following LT treatment of murine macrophages. In order to address this question, we first determined the kinetics of caspase-1 activation in LT-treated macrophages. Murine J774A.1 macrophages were challenged with a cytotoxic dose of LT (250 and 500 ng/ml of LF and PA, respectively), and caspase-1 activation and cell lysis were examined. As only a minor fraction of caspase-1 is activated in LT-treated cells, we enriched for active caspase-1 by immunoprecipitation. We detected a peak in caspase-1 activation 90 min after LT exposure (Fig. 2A). Strikingly, the peak in caspase-1 activation was concurrent with a drastic increase in IL-18 processing and in the number of PI-positive cells and a breakdown of the cytoskeleton 90 min after LT exposure (Fig. 2B to D). No increase in PI signal (Fig. 2C) or caspase-1 activation (data not shown) was detected when J774A.1 macrophages were treated with PA or LF only, indicating that the activity was LT specific and not due to LPS contamination. These findings indicate the close correlation between caspase-1 activation and cytolytic events in LT-treated macrophages. Subsequently, we analyzed the subcellular localization of inflammatory components in untreated cells and in stimulated cells at the peak of caspase-1 activation (90 min after LT exposure).
FIG. 2.
The peak of caspase-1 activation is concurrent with membrane impairment in LT-treated murine macrophages. (A) Caspase-1 was immunoprecipitated from LT-treated murine J774A.1 macrophages. Triton X-100-soluble PNP fractions of J774A.1 macrophages were immunoprecipitated using anti-caspase-1 antibodies, and proteins were analyzed by SDS-PAGE and immunoblotting. (Bottom) Immunoblotting of whole-cell lysates from untreated and LT-treated macrophages was done using anti-IL-18 antibodies. IP, immunoprecipitation; WB, Western blotting. (B) Membrane integrity of murine macrophages in response to LT treatment, as determined by PI (red) exclusion. Nuclei were stained with the membrane-permeable Hoechst stain (blue). (C) PI uptake in J774A.1 macrophages following treatment with LT (250 and 500 ng/ml of LF and PA, respectively) for the indicated times. PI uptake was determined using a plate reader (Victor 3V; PerkinElmer). As a control, macrophages were treated with PA and LF only (250 and 500 ng/ml of LF and PA, respectively) for 120 min. Error bars indicate standard deviations. (D) Changes in the J774A.1 cytoskeleton 90 min after LT treatment. The changes in the cytoskeleton were determined by F-actin staining with phalloidin (green), and the nuclei were stained with Hoechst stain (blue). An LT-treated cell lacking its cytoskeleton is marked with a white arrowhead.
To investigate the cellular localization of putative inflammasome components, we subjected untreated and LT-treated macrophages to subcellular-fractionation assays. In untreated macrophages, caspase-1 and Nalp1b were found exclusively in membrane-associated fractions (Fig. 3). More specifically, we detected caspase-1 in the TXS and LR fractions, with only trace amounts of caspase-1 in the CSK fraction (Fig. 3). Caspase-1 and Nalp1b were both in the TXS fraction, suggesting that the inflammasome forms in this subcellular fraction. However, unlike caspase-1, Nalp1b was not present in the CSK and LR fractions. In contrast to Nalp1b and caspase-1, the inflammatory proteins Asc and IL-18 were found exclusively in the cytosolic fraction (Fig. 3). Notably, we did not detect any caspase-1 or Nalp1b in the cytosolic fraction. The subcellular compartmentalization of caspase-1 or IL-18 did not change upon LT treatment of murine macrophages (Fig. 4). As expected, the cellular localization of Nalp1b and Asc did not change following LT exposure of J774A.1 cells (data not shown). While LT treatment resulted in caspase-1 recruitment to the high-molecular-weight fraction, it did not alter the subcellular localization of caspase-1 (Fig. 4).
FIG. 4.
The subcellular localization of caspase-1 and IL-18 does not change upon LT exposure of J774A.1 murine macrophages. Untreated (−) and LT-treated (+) macrophages were subjected to subcellular fractionation at 90 min after LT exposure, as described in Materials and Methods. SDS-PAGE and immunoblotting were performed with anti-IL-18, anti-caspase-1, and anti-LDH antibodies. WB, Western blotting.
Nalp1b and caspase-1 interactions.
To determine whether Nalp1b and caspase-1 are able to interact with each other, we performed pull-down experiments with 293T cells expressing plasmids for V5-His-tagged Nalp1b and murine caspase-1. Nalp1b was coimmunoprecipitated with anti-caspase-1 antibody from lysates of cells expressing Nalp1b and caspase-1 (Fig. 5). Similar results were obtained in a reciprocal experiment in which we pulled down caspase-1 using a nickel column to capture His-tagged Nalp1b complexes (Fig. 5). Together, these data suggest that caspase-1 and Nalp1b interact to form a protein complex.
Identification of protein components of the inflammasome.
Our SEC fractionation assays have demonstrated that Nalp1b is part of a high-molecular-weight complex in unstimulated J774A.1 cells and that caspase-1 shifts toward this high-molecular-weight fraction upon LT exposure (Fig. 1). In LPS-stimulated human monocytes, the human ortholog of murine Nalp1, NALP1, recruits caspase-1 via the caspase-1 recruitment protein ASC (24). Murine Nalp1b, however, lacks a functional Asc recruitment domain, and Asc does not shift toward the high-molecular-weight fractions in LT-treated macrophages (Fig. 1), suggesting that inflammasome formation is Asc independent in LT-treated macrophages. Moreover, our pull-down experiments suggested that caspase-1 is able to interact directly with Nalp1b. However, the high molecular weight of the complex suggested that in addition to Nalp1b and caspase-1, there are other, unidentified components. To analyze the composition of the inflammasome complex in LT-treated murine macrophages, we subjected low- and high-molecular-weight SEC fractions from LT-treated J774A.1 macrophages to immunoaffinity purification by using anti-caspase-1 antibodies cross-linked to protein A beads. The eluted fractions were digested with trypsin, and the resulting peptides were subjected to electrospray ionization-tandem mass spectrometry for identification.
The ∼800-kDa fractions from LT-stimulated cells contained the inflammatory proteins Nalp1b, α-enolase, caspase-1, and caspase-11, as determined by mass spectrometry (Table 1). As expected, the ∼800-kDa complex from LT-treated macrophages did not contain the scaffolding protein Asc. As a control, we also identified caspase-1-associated components of the low-molecular-mass (∼200-kDa) complex in LT-treated macrophages (Fig. 1). Strikingly, the only protein from the ∼800-kDa complex that forms in LT-treated cells that was also found in the ∼200-kDa complex in untreated cells was caspase-1.
TABLE 1.
Identification of caspase-1-associated proteins from high-molecular-weight complexes of LT-treated macrophages, as determined by mass spectrometrya
| Category | Protein | Accession no.b |
|---|---|---|
| Inflammatory | Pro-caspase-11 | NP_031635 |
| proteins | Pro-caspase-1 | NP_033937 |
| Nalp1b | ABI18117 | |
| Glycolytic enzymes | α-Enolase | P17182 |
| Membrane-associated | AP-2 subunit α-1 | NP_001070732 |
| proteins | Annexin A1 | NP_034860 |
| Potassium channel subfamily K member 4 | O88454 | |
| ABC1 transport protein | NP_038482 |
Octylglucoside-solubilized PNP fractions of LT-treated macrophages were fractionated by SEC, and high-molecular-weight fractions were immunoaffinity purified using anti-caspase-1 antibodies. Proteins were identified by electrospray ionization-tandem mass spectrometry, as described in Materials and Methods.
Accession numbers are from the PubMed and ExPASY databases.
Caspase-11 and α-enolase are caspase-1-associated proteins in LT-treated macrophages.
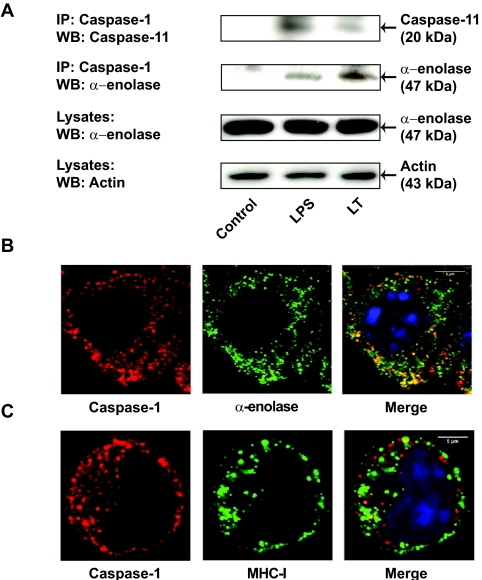
The caspase-1-associated proteins isolated from the high-molecular-weight fractions of LT-treated macrophages contained caspase-11 and the caspase-1 substrate α-enolase. Strikingly, caspase-11-deficient mice are highly resistant to LPS-induced septic shock and show reduced levels of IL-1β following LPS exposure (17, 47). Moreover, caspase-11 has been shown to be required for caspase-1 activation and IL-1β processing in LPS-treated mice (17, 47). To confirm that caspase-11 is part of the inflammasome complex formed in response to LT, we immunoprecipitated caspase-1 from LT-treated J774A.1 macrophages by using a protein A-cross-linked anti-caspase-1 antibody. As a positive control, we used LPS-treated J774A.1 cells. Caspase-1 coimmunoprecipitated with the active p20 subunit of caspase-11 from both LT- and LPS-treated J774A.1 macrophages (Fig. 6A). These results suggested that caspase-11 is required for caspase-1 activation by LPS and LT in murine J774A.1 macrophages.
FIG. 6.
Caspase-1 is able to pull down caspase-11 and α-enolase in LT-treated J774A.1 macrophages. (A) Pull-down assays were performed using lysates from untreated, LPS (2 μg/ml)-treated, and LT-treated J774A.1 macrophages. Lysates were immunoprecipitated using an anti-caspase-1 antibody, and following elution, samples were subjected to SDS-PAGE and immunoblotting using anti-caspase-11 and anti-α-enolase antibodies. Levels of expression of α-enolase and actin in J774A.1 lysates are shown as controls. IP, immunoprecipitation; WB, Western blotting. (B) LT-treated J774A.1 murine macrophages were subjected to immunostaining with anti-α-enolase (fluorescein isothiocyanate [FITC] conjugated, green) and anti-caspase-1 (Alexa Fluor 555 labeled, red) antibodies 90 min after LT exposure. Nuclei were stained with Hoechst (blue). (C) LT-treated J774A.1 cells were incubated with anti-MHC-I antibodies (FITC conjugated, green) for 20 min at 37°C to allow endocytosis of MHC-I. Subsequently, cells were immunostained using anti-caspase-1 antibodies (red). Colocalization of caspase-1 and α-enolase or MHC-1 is shown in yellow. As a negative control for the MHC-I immunostaining, cells were incubated with the secondary antibody alone (data not shown).
Our proteomics results indicated that the glycolytic enzyme and caspase-1 substrate α-enolase is a caspase-1-associated protein in LT-treated J774A.1 macrophages. To confirm that α-enolase was part of the high-molecular-weight complex assembled in LT-treated macrophages, we performed immunoprecipitation experiments using anti-procaspase-1 antibodies. Consistent with our proteomics results, we found that caspase-1 was able to coimmunoprecipitate α-enolase from both LT-treated and LPS-treated (control) cells but not from untreated cells (Fig. 6A).
Consistent with the recruitment of α-enolase to an inflammasome complex, we found that α-enolase partially localized with caspase-1 in LT-treated J774A.1 macrophages (Fig. 6B), while no colocalization of α-enolase and caspase-1 was observed in untreated cells (data not shown). These findings are consistent with previous studies showing changes in the cellular localization of α-enolase in cells stimulated with phorbol myristate acetate or LPS (3, 13, 32).
Taken together, our findings indicate that LT triggers the formation of a high-molecular-weight complex, presumably the inflammasome. Our data suggest that this complex is membrane bound and contains Nalp1b, caspase-1, caspase-11, and α-enolase. Our findings indicate that the activation of this complex results in processing of caspase-1 substrates and macrophage killing.
DISCUSSION
Genetic mapping experiments have linked the LT susceptibility of murine macrophages to the NLR protein Nalp1b. Human NLR proteins closely related to Nalp1b, such as NALP1 and NALP3, have been shown to stimulate the innate immune response via caspase-1 activation. Several findings have indicated that caspase-1 controls the LT-mediated cytolysis of murine macrophages: caspase-1 inhibitors block LT killing of susceptible murine macrophages (41), proteasome inhibitors prevent cytolysis of LT-treated macrophages by blocking caspase-1 (31, 41), and caspase-1-deficient macrophages are highly resistant to LT-mediated cytolysis (6). However, no studies to date have shown the direct involvement of proinflammatory proteins, such as Nalp1b and caspase-1, in LT-mediated cell killing of murine macrophages. Here we present data suggesting that LT triggers the formation of a high-molecular-weight complex and that this complex contains the proinflammatory proteins Nalp1b and caspase-1. We found that caspase-1 was part of a low-molecular-weight complex in unstimulated cells and that caspase-1 shifted to a high-molecular-weight complex in LT-treated macrophages. Intriguingly, this complex contained Nalp1b both before and after LT challenge. We assume that caspase-1 is recruited to the Nalp1b-containing complex upon LT treatment. Formation of high-molecular-weight complexes containing NLR proteins and caspase activation have been described to occur in macrophages following stimulation with microbial components (18, 23).
The assembly of high-molecular-weight protein complexes has been shown to result in caspase activation and cell death (5, 8). For example, following activation of the intrinsic apoptotic pathway, the NLR protein Apaf-1 forms the apoptosome complex with procaspase-9, an essential step for caspase-3 activation and apoptosis induction (7). It is reasonable to assume that caspase-1 recruitment to the Nalp1b inflammasome complex is also essential for caspase-1 activation. Here we show that caspase-1 activation is concurrent with a loss of membrane integrity, IL-18 release, and a breakdown of the cytoskeleton, all markers of necrotic cell death. These findings suggest a close correlation between caspase-1 activation and induction of necrosis.
Different NLR proteins have been shown to recruit caspase-1 via the scaffolding protein ASC (40). For example, the PYD of human NALP1 through Nalp14 is required for recruitment of ASC, which then recruits caspase-1 via its CARD (40, 44). We found that, unlike caspase-1, murine Asc did not shift toward the high-molecular-weight fraction following LT treatment of murine macrophages. This result suggests that Asc is not part of the Nalp1b inflammasome complex, consistent with the fact that murine Nalp1b lacks a PYD (6) and that LT kills Asc-deficient macrophages (33, 35). Therefore, we propose that Nalp1b recruits caspase-1 in an Asc-independent fashion. Both Nalp1b and caspase-1 contain a CARD, and these proteins are therefore able to interact directly via their CARDs. Consistent with a direct interaction between these proteins, we were able to pull down Nalp1b with anti-caspase-1 antibodies from 293T cells transiently transfected with caspase-1 and Nalp1b expression plasmids. Moreover, Nalp1b was among the caspase-1-associated proteins in LT-treated J774A.1 macrophages. While unlikely, we cannot rule out that Nalp1b and caspase-1 interact indirectly with each other and require a bridge protein for signaling. However, CARD-CARD interactions are highly specific (9, 43). For example, the CARD of human NALP1 does not interact with the caspase-1 CARD, but it interacts efficiently with the CARD of caspase-5 (24). While it remains to be shown definitively whether Nalp1b recruits caspase-1 directly via its CARD or via an alternative Nalp1b-specific protein in murine macrophages, our pull-down experiments support a direct-recruitment model.
Finally, subcellular-fractionation assays suggested that LT triggers the formation of a membrane-associated inflammasome complex. Fractionation assays further indicated that Nalp1b and caspase-1 were both present in the TXS fractions, indicating the colocalization of these proteins. Intriguingly, LT treatment did not alter the subcellular localization of Nalp1b and caspase-1. Caspase-1 has previously been shown to localize to the plasma membrane in human THP1 monocytes (39). The membrane localization of Nalp1b is consistent with the reported membrane association of the NLR protein NOD-2 (4). Furthermore, NOD-1 has also been shown to localize to the plasma membrane of human epithelial cells (21).
In contrast to the membrane association of caspase-1 and Nalp1b, we detected the caspase-1 substrate IL-18 in the cytosol of murine macrophages. This finding is consistent with studies showing the cytosolic localization of IL-18 and IL-1β (14). Our data indicate that membrane-bound caspase-1 cleaves cytosolic IL-18 in LT-treated macrophages. It is therefore very conceivable that that the inflammasome complex is on the cytosol-facing side of intracellular membrane structures. The secretion mechanism of the caspase-1 substrates IL-1β and IL-18 is controversial, as these cytokines lack leader sequences and do not follow traditional secretion pathways. While an active-secretion model has been proposed for IL-18 and IL-1β, several findings support a passive-release model for activated IL-18. First, we did not detect any unprocessed and cleaved IL-18 in the membrane fraction, as would be expected in an active secretion system. Moreover, LT treatment resulted in the breakdown of the cytoskeleton, which is required for vesicle trafficking to the plasma membrane, an essential step in the active-release model. Also, the presence of actin and LDH in the supernatant of LT-treated macrophages further supports a passive-release model. Finally, LT-induced caspase-1 activation was concurrent with a loss of membrane integrity and a release of cytosolic proteins. Taken together, LT appears to trigger the necrotic release of cytosolic proteins, including processed cytokines.
Caspase-11 was also among the caspase-1-associated components of the high-molecular-weight complex in LT-treated macrophages. We were also able to pull down caspase-11 from LT-treated murine macrophages by using anti-caspase-1 antibodies. Caspase-11 is highly homologous to human caspase-5 and caspase-4 (45). It has been reported that caspase-11 is required for the activation of caspase-1 (47). While caspase-11 cannot process pro-IL-1β directly, its overexpression stimulates the processing of IL-1β by caspase-1 (17, 47). Interestingly, caspase-5, the human homolog to caspase-11, has been identified as a part of the NALP1 inflammasome complex that forms following LPS stimulation of human monocytes (24). It is thus reasonable to deduce that caspase-1 and caspase-11 are part of the Nalp1b inflammasome.
We also found that the glycolytic enzyme and caspase-1 substrate α-enolase was part of the high-molecular-weight complex in LT-treated macrophages. Moreover, we pulled down α-enolase from LT-treated macrophages by using anti-caspase-1 antibodies. These findings are consistent with the partial colocalization of α-enolase with caspase-1 in LT-treated macrophages but not in untreated cells. Strikingly, caspase-1 has been reported to alter its cellular localization upon stimulation with phorbol myristate acetate and LPS, other NLR agonists (3, 13, 27, 28, 32, 46, 49). This suggests that α-enolase recruitment is a general process of inflammasome activation.
Taken together, we discovered that LT treatment triggers the formation of a high-molecular-weight complex containing proinflammatory proteins in murine macrophages. We further demonstrated that the activation of this membrane-associated complex results in processing of cytosolic IL-18 and macrophage death. Formation of the inflammasome is a critical step in initiating both pyroptosis and the innate immune response. However, it remains to be shown how the formation of this complex triggers downstream events leading to cell death and possibly disease progression.
Acknowledgments
We thank Kathryn Stone and Erol E. Gulcicek from the Keck facility at Yale for their help with the mass spectrometric analysis. Also, we thank Lee Jacobson, Raynal Squires, and Stefan Muehlbauer for their critical reading of the manuscript and Jack Lenz and Vinayaka Prasad for helpful discussions. We thank Yan Deng from the Analytical Imaging Facility at AECOM for technical assistance.
A.M.N. designed the immunogenic peptides for generation of the murine anti-Nalp1b and anti-Asc antibodies and performed the cell killing, Western blotting, immunoprecipitation, pull-down, SEC, immunopurification, immunostaining, and subcellular-fractionation assays.
E.R.S. and Y.-G.Y. were supported by NIH grant CA26504, and J.B. and A.M.N. were supported by NIH grant NIAID-AI075222-01A1.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 5 January 2009.
REFERENCES
- 1.Agostini, L., F. Martinon, K. Burns, M. F. McDermott, P. N. Hawkins, and J. Tschopp. 2004. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20319-325. [DOI] [PubMed] [Google Scholar]
- 2.Allan, V. J. 2002. Protein localization by fluorescence microscopy. Oxford University Press, Oxford, United Kingdom.
- 3.Arza, B., J. Felez, R. Lopez-Alemany, L. A. Miles, and P. Munoz-Canoves. 1997. Identification of an epitope of alpha-enolase (a candidate plasminogen receptor) by phage display. Thromb. Haemost. 781097-1103. [PubMed] [Google Scholar]
- 4.Barnich, N., J. E. Aguirre, H. C. Reinecker, R. Xavier, and D. K. Podolsky. 2005. Membrane recruitment of NOD2 in intestinal epithelial cells is essential for nuclear factor-{kappa}B activation in muramyl dipeptide recognition. J. Cell Biol. 17021-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beere, H. M., B. B. Wolf, K. Cain, D. D. Mosser, A. Mahboubi, T. Kuwana, P. Tailor, R. I. Morimoto, G. M. Cohen, and D. R. Green. 2000. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2469-475. [DOI] [PubMed] [Google Scholar]
- 6.Boyden, E. D., and W. F. Dietrich. 2006. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 38240-244. [DOI] [PubMed] [Google Scholar]
- 7.Cain, K., S. B. Bratton, C. Langlais, G. Walker, D. G. Brown, X. M. Sun, and G. M. Cohen. 2000. Apaf-1 oligomerizes into biologically active approximately 700-kDa and inactive approximately 1.4-MDa apoptosome complexes. J. Biol. Chem. 2756067-6070. [DOI] [PubMed] [Google Scholar]
- 8.Cain, K., C. Langlais, X. M. Sun, D. G. Brown, and G. M. Cohen. 2001. Physiological concentrations of K+ inhibit cytochrome c-dependent formation of the apoptosome. J. Biol. Chem. 27641985-41990. [DOI] [PubMed] [Google Scholar]
- 9.Chou, J. J., H. Matsuo, H. Duan, and G. Wagner. 1998. Solution structure of the RAIDD CARD and model for CARD/CARD interaction in caspase-2 and caspase-9 recruitment. Cell 94171-180. [DOI] [PubMed] [Google Scholar]
- 10.Collier, R. J., and J. A. Young. 2003. Anthrax toxin. Annu. Rev. Cell Dev. Biol. 1945-70. [DOI] [PubMed] [Google Scholar]
- 11.Fink, S. L., and B. T. Cookson. 2006. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 81812-1825. [DOI] [PubMed] [Google Scholar]
- 12.Fink, S. L., and B. T. Cookson. 2007. Pyroptosis and host cell death responses during Salmonella infection. Cell. Microbiol. 92562-2570. [DOI] [PubMed] [Google Scholar]
- 13.Fontan, P. A., V. Pancholi, M. M. Nociari, and V. A. Fischetti. 2000. Antibodies to streptococcal surface enolase react with human alpha-enolase: implications in poststreptococcal sequelae. J. Infect. Dis. 1821712-1721. [DOI] [PubMed] [Google Scholar]
- 14.Gardella, S., C. Andrei, A. Poggi, M. R. Zocchi, and A. Rubartelli. 2000. Control of interleukin-18 secretion by dendritic cells: role of calcium influxes. FEBS Lett. 481245-248. [DOI] [PubMed] [Google Scholar]
- 15.Hanna, P. C., S. Kochi, and R. J. Collier. 1992. Biochemical and physiological changes induced by anthrax lethal toxin in J774 macrophage-like cells. Mol. Biol. Cell 31269-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inohara, N., M. Chamaillard, C. McDonald, and G. Nunez. 2005. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu. Rev. Biochem. 74355-383. [DOI] [PubMed] [Google Scholar]
- 17.Kang, S. J., S. Wang, K. Kuida, and J. Yuan. 2002. Distinct downstream pathways of caspase-11 in regulating apoptosis and cytokine maturation during septic shock response. Cell Death Differ. 91115-1125. [DOI] [PubMed] [Google Scholar]
- 18.Kanneganti, T. D., M. Lamkanfi, Y. G. Kim, G. Chen, J. H. Park, L. Franchi, P. Vandenabeele, and G. Nunez. 2007. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26433-443. [DOI] [PubMed] [Google Scholar]
- 19.Kim, S., J. Jin, and S. P. Kunapuli. 2004. Akt activation in platelets depends on Gi signaling pathways. J. Biol. Chem. 2794186-4195. [DOI] [PubMed] [Google Scholar]
- 20.Klimpel, K. R., N. Arora, and S. H. Leppla. 1994. Anthrax toxin lethal factor contains a zinc metalloprotease consensus sequence which is required for lethal toxin activity. Mol. Microbiol. 131093-1100. [DOI] [PubMed] [Google Scholar]
- 21.Kufer, T. A., E. Kremmer, A. C. Adam, D. J. Philpott, and P. J. Sansonetti. 2008. The pattern-recognition molecule Nod1 is localized at the plasma membrane at sites of bacterial interaction. Cell. Microbiol. 10477-486. [DOI] [PubMed] [Google Scholar]
- 22.Li, H., S. B. Willingham, J. P. Ting, and F. Re. 2008. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J. Immunol. 18117-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mariathasan, S., D. S. Weiss, K. Newton, J. McBride, K. O'Rourke, M. Roose-Girma, W. P. Lee, Y. Weinrauch, D. M. Monack, and V. M. Dixit. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440228-232. [DOI] [PubMed] [Google Scholar]
- 24.Martinon, F., K. Burns, and J. Tschopp. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10417-426. [DOI] [PubMed] [Google Scholar]
- 25.Martinon, F., and J. Tschopp. 2007. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 1410-22. [DOI] [PubMed] [Google Scholar]
- 26.Martinon, F., and J. Tschopp. 2004. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 117561-574. [DOI] [PubMed] [Google Scholar]
- 27.Mears, R., R. A. Craven, S. Hanrahan, N. Totty, C. Upton, S. L. Young, P. Patel, P. J. Selby, and R. E. Banks. 2004. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics 44019-4031. [DOI] [PubMed] [Google Scholar]
- 28.Miles, L. A., C. M. Dahlberg, J. Plescia, J. Felez, K. Kato, and E. F. Plow. 1991. Role of cell-surface lysines in plasminogen binding to cells: identification of alpha-enolase as a candidate plasminogen receptor. Biochemistry 301682-1691. [DOI] [PubMed] [Google Scholar]
- 29.Moayeri, M., D. Haines, H. A. Young, and S. H. Leppla. 2003. Bacillus anthracis lethal toxin induces TNF-alpha-independent hypoxia-mediated toxicity in mice. J. Clin. Investig. 112670-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moayeri, M., N. W. Martinez, J. Wiggins, H. A. Young, and S. H. Leppla. 2004. Mouse susceptibility to anthrax lethal toxin is influenced by genetic factors in addition to those controlling macrophage sensitivity. Infect. Immun. 724439-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muehlbauer, S. M., T. H. Evering, G. Bonuccelli, R. C. Squires, A. W. Ashton, S. A. Porcelli, M. P. Lisanti, and J. Brojatsch. 2007. Anthrax lethal toxin kills macrophages in a strain-specific manner by apoptosis or caspase-1-mediated necrosis. Cell Cycle 6758-766. [DOI] [PubMed] [Google Scholar]
- 32.Pancholi, V., and V. A. Fischetti. 1998. Alpha-enolase, a novel strong plasmin(ogen) binding protein on the surface of pathogenic streptococci. J. Biol. Chem. 27314503-14515. [DOI] [PubMed] [Google Scholar]
- 33.Pelegrin, P., C. Barroso-Gutierrez, and A. Surprenant. 2008. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J. Immunol. 1807147-7157. [DOI] [PubMed] [Google Scholar]
- 34.Petrilli, V., C. Dostert, D. A. Muruve, and J. Tschopp. 2007. The inflammasome: a danger sensing complex triggering innate immunity. Curr. Opin. Immunol. 19615-622. [DOI] [PubMed] [Google Scholar]
- 35.Reig, N., A. Jiang, R. Couture, F. S. Sutterwala, Y. Ogura, R. A. Flavell, I. Mellman, and F. G. van der Goot. 2008. Maturation modulates caspase-1-independent responses of dendritic cells to anthrax lethal toxin. Cell. Microbiol. 101190-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts, J. E., J. W. Watters, J. D. Ballard, and W. F. Dietrich. 1998. Ltx1, a mouse locus that influences the susceptibility of macrophages to cytolysis caused by intoxication with Bacillus anthracis lethal factor, maps to chromosome 11. Mol. Microbiol. 29581-591. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz, J., A. Owyang, E. Oldham, Y. Song, E. Murphy, T. K. McClanahan, G. Zurawski, M. Moshrefi, J. Qin, X. Li, D. M. Gorman, J. F. Bazan, and R. A. Kastelein. 2005. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23479-490. [DOI] [PubMed] [Google Scholar]
- 38.Simanis, V., and D. P. Lane. 1985. An immunoaffinity purification procedure for SV40 large T antigen. Virology 14488-100. [DOI] [PubMed] [Google Scholar]
- 39.Singer, I. I., S. Scott, J. Chin, E. K. Bayne, G. Limjuco, J. Weidner, D. K. Miller, K. Chapman, and M. J. Kostura. 1995. The interleukin-1 beta-converting enzyme (ICE) is localized on the external cell surface membranes and in the cytoplasmic ground substance of human monocytes by immuno-electron microscopy. J. Exp. Med. 1821447-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sirard, J. C., C. Vignal, R. Dessein, and M. Chamaillard. 2007. Nod-like receptors: cytosolic watchdogs for immunity against pathogens. PLoS Pathog. 3e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Squires, R. C., S. M. Muehlbauer, and J. Brojatsch. 2007. Proteasomes control caspase-1 activation in anthrax lethal toxin-mediated cell killing. J. Biol. Chem. 28234260-34267. [DOI] [PubMed] [Google Scholar]
- 42.Sutterwala, F. S., and R. A. Flavell. 2009. NLRC4/IPAF: a CARD carrying member of the NLR family. Clin. Immunol. 1302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tibbetts, M. D., L. Zheng, and M. J. Lenardo. 2003. The death effector domain protein family: regulators of cellular homeostasis. Nat. Immunol. 4404-409. [DOI] [PubMed] [Google Scholar]
- 44.Tschopp, J., F. Martinon, and K. Burns. 2003. NALPs: a novel protein family involved in inflammation. Nat. Rev. Mol. Cell Biol. 495-104. [DOI] [PubMed] [Google Scholar]
- 45.Van de Craen, M., P. Vandenabeele, W. Declercq, I. Van den Brande, G. Van Loo, F. Molemans, P. Schotte, W. Van Criekinge, R. Beyaert, and W. Fiers. 1997. Characterization of seven murine caspase family members. FEBS Lett. 40361-69. [DOI] [PubMed] [Google Scholar]
- 46.van der Goot, F. G., and J. Gruenberg. 2006. Intra-endosomal membrane traffic. Trends Cell Biol. 16514-521. [DOI] [PubMed] [Google Scholar]
- 47.Wang, S., M. Miura, Y. K. Jung, H. Zhu, E. Li, and J. Yuan. 1998. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92501-509. [DOI] [PubMed] [Google Scholar]
- 48.Yeung, Y. G., P. T. Jubinsky, and E. R. Stanley. 1986. Solubilization and assay of a colony-stimulating factor receptor from murine macrophages. J. Cell. Biochem. 31259-269. [DOI] [PubMed] [Google Scholar]
- 49.Yu, J., D. A. Fischman, and T. L. Steck. 1973. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J. Supramol. Struct. 1233-248. [DOI] [PubMed] [Google Scholar]