Abstract
Strategies to limit complement deposition on Streptococcus pneumoniae are established as virulence features for invasive disease, but their role in respiratory tract infection requires further analysis. We evaluated complement C3 protein deposition on discordant S. pneumoniae isolates of the same serotype (6A) and their capacity to cause nasopharyngeal (NP) colonization and experimental otitis media (EOM) in an animal model. We compared C3 binding to five 6A isolates from asymptomatic NP carriers with five 6A strains that caused invasive disease, and we observed less C3 (∼10-fold less fluorescence) binding to invasive isolates. We selected two high-level C3-binding carriage and two low-level C3-binding invasive 6A isolates for further study. In the EOM model, 11/12 (92%) ears challenged with a low-level C3-binding 6A strain became infected. Only 2/8 (25%) ears challenged with the discordant high-level C3-binding 6A isolate developed disease (P = 0.005). Results with the second discordant 6A isolate pair were comparable. Cobra venom factor (CoVF) treatment, which depletes C3 and consumes complement, restored virulence of the high-level C3-binding strain; 8/8 (100%) ears in CoVF-treated animals developed EOM compared to only 25% of ears in naïve animals (P = 0.007). These studies demonstrate the critical role for complement evasion in pneumococcal EOM. Colonization with carriage isolates that bound high levels of C3 caused EOM in fewer animals compared to low-level C3-binding invasive strains. Thus, limiting C3 deposition on the surface of S. pneumoniae correlates with increased incidence of EOM following NP colonization and barotrauma in the animal model.
The pathogenesis of Streptococcus pneumoniae infection involves initial colonization of the nasopharynx, followed by its spreading to the middle ear, sinus, or lower respiratory tract and, in some cases, invasion of the bloodstream. To successfully cause disease, the pneumococcus has evolved a number of mechanisms to avert complement-mediated opsonization and phagocytosis. Pneumococci possess a broad variety of specialized surface proteins, some of which are adapted to interact with host defenses during colonization or dissemination in humans. Being a gram-positive bacterium, it is resistant to the bactericidal activity of complement (24) because its rigid cell wall prevents lysis by the membrane attack complex. The capsular polysaccharide is critical for resistance to complement deposition (32) and may also mask cell wall-associated complement from being recognized by the complement receptors on phagocytes (6). Additionally, select surface proteins can degrade native C3 proteins, thereby preventing or diminishing binding of C3b and iC3b to the bacterial surface, which are necessary components for opsonization (3). Furthermore, an important role for complement is suggested by the association of increased risk for invasive infections in individuals (or animal models) with deficiencies of complement proteins such as C2 and C3 and of complement receptors such as CR3 (2, 16). Type-specific antibody formation is an important host defense mechanism against infections caused by S. pneumoniae. However, the efficacy of opsonization of pneumococci by either immunoglobulin M (IgM) or IgG is related to their ability to enhance complement deposition on the bacterial surface, thus making complement essential for recovery from pneumococcal disease (6, 9).
Colonization of mucosal surfaces is often the first step in the development of disease. Studies of S. pneumoniae support recent acquisition as the critical event preceding the development of pneumococcal otitis media. S. pneumoniae has evolved specific characteristics that are critical in dictating initial success for establishing colonization within a competitive niche of the mucosal surface of the nasopharynx. Often the success of an organism in establishing carriage depends on its ability to resist innate clearance mechanisms generated in the setting of polymicrobial stimulation. Lysenko and colleagues have demonstrated that complement and polymorphonuclear leukocytes are necessary host defenses for the elimination of S. pneumoniae from the nasopharynx in the presence of nontypeable Haemophilus influenzae (NTHi) (29).
Prior work from our laboratory demonstrated that complement was an important host defense mechanism against protection of the middle ear from infection with NTHi (15). We hypothesized that complement would also be relevant for protection against S. pneumoniae respiratory tract infection (RTI). We evaluated the role of complement by comparing the capacities of four isolates of S. pneumoniae, all belonging to serotype 6A but differing in their abilities to bind complement to their surface to cause otitis media following nasopharyngeal (NP) colonization. Our goal was to determine whether evasion of complement deposition was an important virulence feature in the pathogenesis of RTI, using a model for experimental otitis media (EOM). The model requires initial NP colonization followed by ascension through the Eustachian tube after barotrauma for establishing middle ear infection.
MATERIALS AND METHODS
Bacterial isolates and growth conditions.
S. pneumoniae serotype 6A strains that were isolated from patients with either invasive pneumococcal disease (n = 5) or asymptomatic NP carriage (n = 5) were used for C3 binding studies. Four of these 6A strains (nasopharynx and invasive) were selected for further animal studies. Colony morphology and optochin sensitivity were used to initially identify bacteria as S. pneumoniae. Serotyping was performed using quellung reaction with Danish antisera (Statens Seruminstitute, Copehagen, Denmark). Additional confirmation of the serotype was confirmed by Moon Nahm's laboratory by inhibition enzyme-linked immunosorbent assay (ELISA) using monoclonal antibodies (MAbs) Hyp6AG1 and Hyp6AM3 that specifically reacted with the 6A capsule (34). S. pneumoniae isolates were grown for 15 h overnight on blood agar in 5% CO2 at 37°C, followed by subculture in brain heart infusion (BHI) broth supplemented with 10 μg/ml hemin and 2 μg/ml NAD, and grown at 37°C to mid-log phase in a shaking water bath.
Sera and complement reagents.
Immune serum was obtained from two healthy volunteers 4 weeks after vaccination with the 23-valent pneumococcal polysaccharide vaccine (includes serotype 6A capsular polysaccharide). Human complement was purchased from Sigma Chemical Co. (St. Louis, MO). In some experiments, the alternative pathway was selectively activated by adding EGTA and Mg2+ to serum, both to a final concentration of 10 mM (35). Heat inactivation of human complement (56°C for 30 min) was used as a control in some experiments.
Antibodies.
MAbs directed against serotype 6A pneumococcal polysaccharides were obtained as a courtesy from Wyeth Research Division (Wyeth Pharmaceuticals Inc., Pearl River, NY). Fluorescein isothiocyanate (FITC)-conjugated sheep anti-human C3c and anti-human C4 (Biodesign) were used in flow cytometry assays as described previously (39, 40). Anti-goat IgG, anti-human IgG, and anti-mouse IgG conjugated to FITC (Sigma) were used as secondary antibodies.
Multilocus sequence typing.
The genotypes of the pneumococcal strains used in this study were analyzed by multilocus sequence typing (MLST) (13). MLST of the NP strains was performed at the Imperial College London, whereas the invasive strains were first sequenced and MSLT was performed using web-based software (http://www.mlst.net/).
Colony morphology.
Bacteria grown overnight in BHI broth supplemented with 10 μg/ml hemin and 2 μg/ml NAD at 31°C were reinoculated into fresh media the next day and grown to the mid-log phase. Bacteria were inoculated onto tryptic soy agar plates (Remel), each containing 100 μl (3,000 U) of catalase (Worthington Biochemical Co., Freehold, NJ) (48). The strains were grown in a candle extinction jar. Colony morphology was assessed with a stereo-zoom microscope (magnification of ×67.5) with a halogen illuminator and adjustable angle mirror, and an attached Canon camera was used to record colony morphology.
Evaluation of complement and antibody deposition on surface of S. pneumoniae.
Flow cytometry was utilized to quantitate C3 and IgG binding to the surface of pneumococci, as described previously (39, 40). Briefly, 2 × 108 bacteria were washed twice in Hanks' balanced salt solution (HBSS; Cambrex, Inc.) containing 2% bovine serum albumin (Sigma). Bacteria were then incubated with antibody, followed by the addition of complement. Controls included bacteria incubated with complement alone (no antibody added). In some experiments, the role of alternative pathway-mediated C3 deposition was evaluated using serum that was treated with Mg2+ and EGTA (35). MAb directed against serotype 6A pneumococcal polysaccharide (stock solution of a concentration of 24.3 mg/ml) was added to a final concentration of 0.5%. Human complement was added to a final concentration of 5%. The final volume of the reaction mixture in every instance was 500 μl. C3 fragments bound to the bacterial surface were detected using anti-human C3c-FITC (Biodesign, Saco, ME) as described previously (15). The amount of MAb or immune human IgG bound to bacteria was detected using anti-mouse IgG-FITC or anti-human IgG-FITC (both from Sigma).
EOM model.
An experimental chinchilla model of acute otitis media was used (4). All procedures and manipulations were performed using sedation analgesia with a mixture of ketamine and xylazine given intramuscularly in accordance with approved IACUC protocols at Boston University Medical Center (5). Baseline plasma samples were obtained through the cephalic sinus 24 h prior to bacterial inoculation. Isolates of S. pneumoniae grown to the mid-log phase were diluted in HBSS, and approximately 107 CFU in 100 μl was introduced into each nare. Forty-eight hours after intranasal bacterial inoculation, barotrauma was created by withdrawing 250 μl of air from the middle ear through the superior bullae of both ears, which promotes ascension of bacteria into the middle ear. Daily tympanometry and otomicroscopy were performed to determine the presence of fluid behind the tympanic membrane and signs of infection including bulging tympanic membrane and erythema. Once abnormality was identified, the middle ear cavity was accessed 48 to 72 h later as described previously (4). A direct culture of middle ear was obtained with a calcium alginate swab and immediately streaked onto a blood agar plate. Middle ear fluid (MEF) was obtained with a 22-gauge angiocatheter connected to an empty tuberculin syringe, 10 μl of MEF was diluted 1:10 in HBSS, and three serial 10-fold dilutions were prepared. One hundred microliters of each dilution was plated onto blood agar. The lower limit of detection of viable organisms in MEF using this dilution series was 100 CFU/ml (5). If MEF was absent, the middle ear was flushed with 0.25 ml HBSS, and the contents were sampled, as described above. Direct and indirect ear examination was performed every 3 to 4 days until the middle-ear cultures were sterile on two consecutive samples. In subsequent experiments, animals were randomly assigned to receive either one dose of cobra venom factor (CoVF) (CompTech, Tyler, TX) at a dose of 300 μg/kg of body weight administered intraperitoneally 24 h prior to barotrauma to deplete complement or sterile normal saline (control animals). Serial plasma samples were obtained to confirm the extent and duration of C3 depletion. CoVF-treated and control animals were challenged as described above, using NP colonization followed by barotraumas to induce EOM.
Western blotting to monitor C3 depletion in CoVF-treated chinchillas.
Plasma samples from chinchillas were immediately diluted 1:100 in phosphate-buffered saline containing 10 mM EDTA to prevent C3 activation and degradation ex vivo. Following the addition of 4× NuPAGE LDS sample buffer (Invitrogen) containing 10% 2-mercaptoethanol, proteins were separated on a NuPAGE 4 to 12% bis-Tris gel (Invitrogen) with MOPS (morpholinepropanesulfonic acid) running buffer and transferred to an Immobilon polyvinylidene difluoride membrane (Millipore, Bedford, MA). Chinchilla C3 was detected using goat anti-rat C3 antibody (MP Biomedicals), followed by anti-goat IgG-alkaline phosphatase, both at a dilution of 1:1,000 in phosphate-buffered saline-0.05% Tween 20, as described previously (15).
Capsule quantification.
Capsule quantification, both that on intact bacteria and that released into culture supernatants during growth in liquid media, was performed by an inhibition ELISA using MAb (Hyp6AG1) against 6A polysaccharide and purified serotype 6A capsular polysaccharide (51). In brief, bacteria are grown in 50 ml Todd-Hewitt broth supplemented with 1% (wt/vol) yeast extract and centrifuged, and bacterial pellets are lysed using lysing buffer (0.2% sodium deoxycholate, 0.02% sodium dodecyl sulfate, 0.1% sodium azide, and 0.3 M sodium citrate [pH 7.8]) as previously described (51). Bacterial culture supernatants were also collected for capsular polysaccharide measurements. Purified 6A polysaccharide (100 ng/well) is used to coat ELISA plates, and Hyp6AG1 antibody culture supernatant is used at a 1:200 dilution. Both bacterial cell lysate and culture supernatant are used as inhibitors. Substrate (nitrophenyl phosphate) reaction is stopped after incubation for 80 min. The optical density value is read at a 405-nm wavelength, and then the final concentration of capsular polysaccharide was compared to the 6A polysaccharide standard titration curve (web-based software from http://www.imtech.res.in/raghava/abag/).
Statistical analysis.
Fisher's exact test was used to calculate statistical significance for the differences in the proportions of culture-positive middle ears in animals challenged with high- and low-level C3-binding 6A strains and animals treated with CoVF compared with untreated animals.
RESULTS
Discordant complement binding to carriage versus invasive 6A isolates.
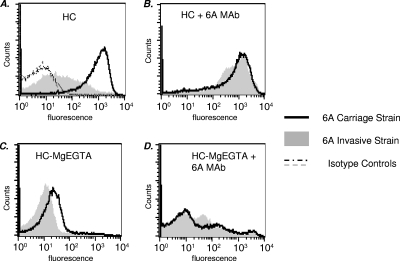
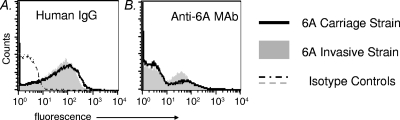
We evaluated C3 binding to five carriage and five invasive 6A isolates. Interestingly, there was good correlation between the amount of C3 binding and the site of isolation of the 6A isolates. The mean geometric C3-binding fluorescence of the five NP strains was 301 (range, 250 to 475) via flow cytometry. For invasive strains, the mean geometric fluorescence was 24 (range, 14 to 40). Control experiments performed with heat-inactivated complement showed minimal (fluorescence, <10) C3 binding (data not shown). Thus, the carriage strains bound large amounts of C3, while invasive isolates bound small amounts of C3. Characteristics of the four strains used for further studies in the animal model are shown in Table 1. All the isolates used in this study were genotypically heterogeneous. Histograms of C3 binding to a representative carriage isolate and an invasive isolate that were incubated with human complement are shown in Fig. 1A. The addition of a specific MAb against serotype 6A capsular polysaccharide to the human complement resulted in markedly increased C3 binding to the invasive strain to levels that approached the levels of binding seen with the carriage strain (Fig. 1B). In contrast, there was no augmentation in the amount of C3 that bound to the carriage strain that bound high levels of C3 even without the addition of a specific MAb. To determine the contribution of the alternative pathway to the high level of C3 deposition on the carriage strain, we incubated bacteria with Mg-EGTA-treated human serum (blocks classical and lectin pathways). We noted an ∼2 log10 decrease in C3 binding to the carriage strain, and C3-binding levels were now comparable to those seen with the invasive strain (Fig. 1C), suggesting that the alternative pathway alone was not responsible for high-level C3 binding to the carriage strains. Finally, we sought to determine whether the enhancement of C3 binding to the invasive isolate mediated by the anticapsular MAb required the classical pathway. Bacteria were incubated with MAb and Mg-EGTA-treated human complement. The majority of bacteria did not show any increase in C3 binding over baseline levels; only a small population of bacteria showed increased C3 binding (Fig. 1D). These results contrasted with the marked increase in C3 binding to the entire bacterial population seen when all complement pathways were intact (Fig. 1B). We measured binding of IgG in the human complement source to exclude the possibility that differences in C3 binding between the invasive and carriage isolates were because of differences in antibody binding. As seen in Fig. 2, both strains showed similar levels of human IgG (left graph) and anti-6A MAb (right graph) binding. We tested two additional carriage isolates and an invasive isolate by using these assay conditions and noted similar results (data not shown). Lower levels of C3 binding to the invasive isolate despite Ig-binding levels similar to those of carriage isolates suggest that invasive isolates possess a mechanism(s) to subvert complement activation. The two strains used for experiments in Fig. 1 and Fig. 2 and another carriage isolate and invasive isolate (all listed in Table 1) were selected for subsequent in vivo studies.
TABLE 1.
Characteristics of pneumococcal strainsa
| Serotype 6A strain | MLST | Type (source) | Colony morphology | Virulence in chinchilla model | C3 binding |
|---|---|---|---|---|---|
| 2445 | 5007 | Invasive (blood) | Transparent | ++++ | + |
| MD5023 | 376 | Carriage (NP) | Transparent | + | ++++ |
| AR213 | 690 | Invasive (blood) | Transparent | ++++ | + |
| LE4007 | 1538 | Carriage (NP) | Transparent | + | ++++ |
+, low level; ++++, high level.
FIG. 1.
C3 binding to representative serotype 6A carriage and invasive isolates. (A) Bacteria were incubated with 5% human complement (HC). C3 binding to the carriage strain is represented by the solid line, binding to the invasive strain by the gray-shaded histogram, and their isotype controls (serum samples excluded) by dashed lines. (B) C3 binding to bacteria incubated with 5% HC and anticapsular 6A MAb (0.5%). (C) Bacteria incubated with HC containing 10 mM Mg-EGTA (only alternative pathway active). (D) Bacteria incubated with HC containing Mg-EGTA with anticapsular 6A MAb. The results for one experiment representative of two separate and comparable experiments are shown. The x axis represents fluorescence on a log10 scale, and the y axis represents the number of events (counts). Isotype controls (no human complement in the reaction mixture) yielded fluorescence values that lay within the first decade (<10) and, for simplicity, are shown only in panel A.
FIG. 2.
IgG and anti-serotype 6A MAb binding to serotype 6A carriage and invasive strains. (A) Bacteria were incubated with human complement (4%), and IgG binding to carriage (solid line) and invasive (gray-shaded histogram) isolates was measured. Isotype controls (serum samples excluded) are shown by the dashed lines. (B) Anti-6A MAb binding to carriage (solid line) and invasive (gray-shaded histogram) strains. Binding to controls (no MAb added) is similar as that shown in panel A and is omitted in panel B for simplicity. Axes are described in the legend for Fig. 1.
NP colonization by S. pneumoniae appears independent of surface binding of C3.
We evaluated the role of C3 deposition on NP colonization by measuring the density of colonization following intranasal challenge with either the high- or low-level C3-binding 6A isolate. No significant difference in NP colonization between discordant C3-binding S. pneumoniae serotype 6A isolates was observed following bilateral inoculation with ∼107 CFU/100 μl per nare. NP lavage at 24 h following challenge identified a mean concentration of 104 CFU/ml in all four groups of animals. By day 4, the density of NP colonization increased to 106 to 107 CFU/ml in both groups; no differences between animals challenged with low- or high-level C3-binding S. pneumoniae serotype 6A isolates were observed (data not shown).
High-level C3-binding S. pneumoniae isolates demonstrate reduced capacity to invade middle ear following barotrauma compared to that demonstrated by low-level C3-binding S. pneumoniae isolates.
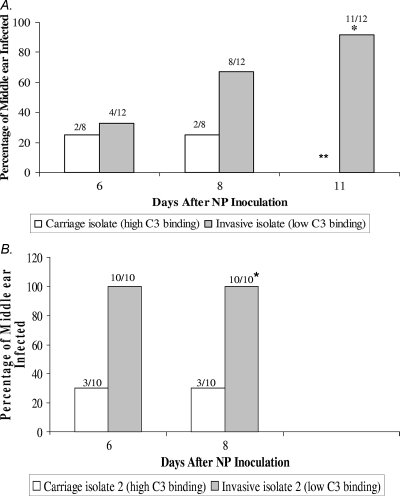
Eleven of 12 (92%) ears challenged with the low-level C3-binding 6A strain developed culture-positive EOM by day 11, and four of six (66%) of these animals developed bacteremia. One animal was euthanized prior to obtaining MEF from one side due to the presence of bacteremia. Only two of eight (25%) ears challenged with the high-level C3-binding 6A strain developed culture-positive EOM by day 8 (P = 0.005) (Fig. 3), and none developed bacteremia (P = 0.076). In animals challenged with the high-level C3-binding S. pneumoniae serotype 6A isolate, six ears from three animals were culture negative on days 4 and 8 following barotrauma and were not opened again. In addition to the difference in incidence of culture-positive EOM, middle ear disease due to the high-level C3-binding 6A strain was characterized by low-density infection (102 to 103 CFU/ml) (data not shown) compared to 105 to 106 CFU/ml in animals challenged with the low-level C3-binding isolate.
FIG. 3.
(A) Development of middle ear infection following NP challenge with an invasive or carriage serotype 6A isolate. The percentage of animals that developed middle ear infection following NP challenge with an S. pneumoniae high-level (carriage) or low-level (invasive) C3-binding isolate is shown. Fisher's exact test was used to compare the two groups. * signifies a P value of 0.005. ** indicates that the MEF from the animals challenged with the carriage isolates yielded negative culture results on two consecutive occasions and were not subsequently sampled according to the IACUC protocol. (B) Development of middle ear infection following NP challenge with the second invasive or carriage serotype 6A isolate. The percentage of animals that developed middle ear infection following NP challenge with an S. pneumoniae high-level (carriage) or low-level (invasive) C3-binding isolate is shown. Fisher's exact test was used to compare the two groups. * signifies a P value of 0.003.
In the second group, 10/10 (100%) ears challenged with the second low-level C3-binding 6A strain developed culture-positive EOM by day 8, and 1/4 (25%) of these animals developed bacteremia. Only 3/10 (30%) ears challenged with the second high-level C3-binding strain developed disease by day 8 (P = 0.003) (Fig. 3B), and none of the animals developed bacteremia. Thus, limited C3 deposition on the surface of S. pneumoniae correlates with increased capacity to cause EOM.
Complement depletion renders high-level C3-binding S. pneumoniae serotype 6A virulent.
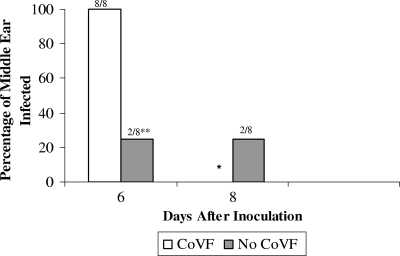
We next examined whether complement depletion permitted a high-level C3-binding isolate to cause EOM. Complement depletion was confirmed by Western blotting (see Fig. S1 in the supplemental material). We challenged CoVF-treated animals and normal animals with a 6A carriage isolate and monitored animals for the development of EOM. One-hundred percent (8/8 ears) of animals pretreated with CoVF developed culture-positive EOM compared to only 25% of untreated control animals (P = 0.007) (Fig. 4). The density of middle ear infection caused by high-level C3-binding S. pneumoniae in CoVF-treated animals was comparable to that seen with the low-level C3-binding strain in untreated animals (106 to 107 CFU/ml). These data suggest that complement is an essential arm of innate immune defenses against S. pneumoniae in the middle ear.
FIG. 4.
Depletion of complement results in virulence of the high-level C3-binding S. pneumoniae carriage isolate. The percentage of animals treated with CoVF that developed middle ear infection following intranasal challenge with a carriage isolate is shown. The control group included animals with an intact complement system (no CoVF treatment). * indicates that all four animals in the CoVF-treated group developed bacteremia and had to be euthanized, which precluded further middle ear sampling. ** signifies a P value of 0.007.
Colony morphology.
No variation in the appearance of colonies derived from four isolates was noted (see Fig. S2 in the supplemental material). All the strains appeared transparent when visualized under a stereo-zoom microscope with oblique, indirect illumination. The typical colony was small, more transparent in the center, umbilicated, and as previously described, had a bulls-eye appearance (48).
Capsule quantification.
Both the invasive and carriage strains expressed similar amounts of bacterium-bound capsular polysaccharide (see Fig. S3A in the supplemental material). Furthermore, the amounts of capsule found in culture supernatants of the two strains were similar (see Fig. S3B in the supplemental material). Together, these data suggest that the amount of capsule associated with the surfaces of the carriage and invasive strains that we have tested and the total amount of capsule synthesized by these two strains are similar.
DISCUSSION
Specific attributes that enable pneumococci to breach host epithelial and tissue barriers during the progression from colonization to invasive infection are not fully characterized. Pneumococcal isolates display phase variation manifested by two distinct morphologies that can be distinguished by their opaque or transparent colony appearance that is related to the quantity of polysaccharide capsule produced (48). During the initial stages of colonization, transparent pneumococci that express less capsule and possess other characteristics which promote binding to mucosal surfaces prevail over opaque variants (48, 49). When present at the mucosal surface, the expression of a thick capsule appears to be disadvantageous for the pneumococcus, likely due to its inhibitory effect on adherence (23, 32). In both mouse models and in humans, opaque variants that express increased amounts of capsular polysaccharide and are more resistant to opsonophagocytic killing are selected for during the transition from colonization of the mucosal surface to invasion of the bloodstream (25, 49). A correlation between increased amounts of capsular polysaccharide and greater virulence in mice has been described (30). Conceptually, larger amounts of capsule protect the organism from the deposition of complement and phagocytosis by polymorphonuclear white blood cells. More recent data suggest that the capsule can restrict the total amount of complement that is deposited on the bacterial surface (1, 32).
Interestingly, all strains used in this study had a transparent morphology and expressed similar levels of capsular polysaccharide in vitro, and yet the invasive isolates showed markedly lower levels of C3 binding than did carriage strains, suggesting an important role for bacterial factors other than capsule in inhibiting C3 deposition. Differences in C3 binding were primarily mediated by the ability of the invasive isolates to inhibit the classical pathway. The reason for differences in classical pathway activation is not clear and merits further study, but it is not because of differences in antibody binding (Fig. 2). The alternative pathway can amplify the amount of C3 initially deposited on the organism. The alternative pathway is inhibited by factor H, and previous studies have shown that pneumococci can bind to factor H (28, 36-38). Both carriage and invasive strains bound similar amounts of the alternative pathway inhibitor factor H (data not shown), thus making it unlikely that differences in regulation of the alternative pathway positive feedback loop accounted for differences in C3 binding.
Our studies describe differences in complement deposition among four isolates of S. pneumoniae serotype 6A and show an inverse correlation between C3 surface binding and virulence in a model of EOM that requires bacteria to ascend from the nasopharynx to the middle ear. Our findings confirm a critical role for complement as part of the host defense against pneumococcal middle ear infection. We have previously demonstrated the critical role of complement for defense of the middle ear when challenged with NTHi (15). Animals with an intact complement system that were inoculated with S. pneumoniae serotype 6A strains permissive for a large amount of C3 surface deposition develop culture-positive middle ear disease significantly less often than those challenged with S. pneumoniae serotype 6A strains that have the capacity to restrict complement C3 deposition on their surfaces. In addition, the two strains that bind high quantities of C3 do not produce bloodstream infection, in contrast with the low-level C3-binding invasive 6A strains. Complement depletion with CoVF enables the otherwise less-virulent high-level C3-binding isolate to establish disease with rates and densities similar to those seen with the low-level C3-binding strain. Thus, evasion of complement deposition is necessary for development of both EOM and bacteremia in the chinchilla model.
The colonization of mucosal surfaces is most often the first event in the development of either mucosal or invasive pneumococcal disease (14, 18). The rate of S. pneumoniae clearance from the nasopharynx was not affected in C3 knockout mice (46). These findings suggest that complement may not play a major role in the clearance of NP colonization and are consistent with our observations, which show that all animals became colonized with similar densities after NP challenge.
Even though type-specific antibody formation is an important host defense mechanism against infections caused by S. pneumoniae, effective opsonization of pneumococci by either IgM or IgG requires host complement deposition on the bacterial surface, making complement essential for recovery from pneumococcal disease (9, 27). Hostetter demonstrated that strains with different capsular types differ in the amounts and sites of bound C3b, as well as of the C3b degradation products, potentially affecting opsonophagocytosis (20). The polysaccharide capsule is the most important virulence factor of pneumococci because it protects the bacteria from phagocytosis (47). Reduced expression of capsule presumably results in greater access of antibodies and complement to the pneumococcal surface (31, 32) and, hence, increased clearance by the immune system. Differences in virulence may be related to the ability of the specific capsular polysaccharide structure to block the accessibility of antibodies and complement to surface components or to mask cell wall-bound C3b from recognition by phagocytic receptors (7, 8, 17, 50). We quantified the amount of capsular polysaccharide in the high- and low-level C3-binding 6A strains using an inhibition type ELISA and found no differences. Thus, differences in C3 binding among the 6A isolates in this study were not attributable to differences in the amounts of capsular polysaccharide expression. Our data suggest that subcapsular components also play a critical role in modulating complement activation.
Complement deposition is the result of a complex series of events that is regulated by surface proteins in addition to the polysaccharide capsule. Binding of the alternative pathway inhibitor factor H to PspC (10, 11, 21, 22, 36), cleavage of the C3 α chain by PhpA (19, 53), and decreased C3 binding as a result of PspA expression (26, 41-43, 45, 52) all contribute to decreased C3 binding. Isogenic cppA mutants have substantially increased amounts of iC3b on their surfaces and are readily killed in vitro and in outbred Swiss Webster mice (19). Further studies will be necessary to determine what, if any, role variations in the above-mentioned surface proteins play in complement regulation. The central role for complement in defense against pneumococcal disease in humans is illustrated by clinical observations that deficiencies of either antibodies or components of the complement pathway that lead to decreased C3 binding to bacteria and/or affect opsonophagocytic killing of the bacteria lead to an increased risk of disease, often with a higher mortality rate (2, 12, 16, 19, 33, 44).
The observed increase in invasive pneumococcal disease in individuals with a spectrum of complement deficiencies is consistent with our finding of the ability of strains that limit C3 deposition on their surfaces to more readily cause EOM (and, in some instances, bacteremia). Further identification of the mechanisms of complement evasion is needed to better understand disease pathogenesis. Variations in surface proteins known to modulate complement activation could explain differences in the amounts of C3 binding among the invasive and carriage isolates. Further investigation of bacterial factors that decrease C3 binding may shed light on differences in the pathogenic potentials of carriage and invasive strains.
Supplementary Material
Acknowledgments
These studies were supported in part by a research grant from the Shereta R. Seelig Charitable Foundation Trust. S.R. was supported by NIH grant AI054544. I.H.P. was supported by NIH grant AI-31473.
We thank Loc Truong for expert technical assistance. We thank Phil Fernsten from Wyeth Inc. for providing MAbs. We thank Moon Nahm and his team for their confirmation of our isolates as serotype 6A.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 12 January 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Abeyta, M., G. G. Hardy, and Y. Yother. 2003. Genetic alteration of capsule type but not PspA type affects accessibility of surface-bound complement and surface antigens of Streptococcus pneumoniae. Infect. Immun. 71218-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alper, C. A., J. Xu, K. Cosmopoulos, B. Dolinski, R. Stein, G. Uko, C. E. Larsen, D. P. Dubey, P. Densen, L. Truedsson, G. Sturfelt, and A. G. Sjoholm. 2003. Immunoglobulin deficiencies and susceptibility to infection among homozygotes and heterozygotes for C2 deficiency. J. Clin. Immunol. 23297-305. [DOI] [PubMed] [Google Scholar]
- 3.Angel, C. S., M. Ruzek, and M. K. Hostetter. 1994. Degradation of C3 by Streptococcus pneumoniae. J. Infect. Dis. 170600-608. [DOI] [PubMed] [Google Scholar]
- 4.Babl, F. E., S. I. Pelton, and Z. Li. 2002. Experimental acute otitis media due to nontypeable Haemophilus influenzae: comparison of high and low azithromycin doses with placebo. Antimicrob. Agents Chemother. 462194-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouchet, V., D. W. Hood, J. Li, J. R. Brisson, G. A. Randle, A. Martin, Z. Li, R. Goldstein, E. K. Schweda, S. I. Pelton, J. C. Richards, and E. R. Moxon. 2003. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc. Natl. Acad. Sci. USA 1008898-8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, E. J., S. W. Hosea, and M. M. Frank. 1983. The role of antibody and complement in the reticuloendothelial clearance of pneumococci from the bloodstream. Rev. Infect. Dis. 5(Suppl. 4)S797-S805. [DOI] [PubMed] [Google Scholar]
- 7.Brown, E. J., S. W. Hosea, C. H. Hammer, C. G. Burch, and M. M. Frank. 1982. A quantitative analysis of the interactions of antipneumococcal antibody and complement in experimental pneumococcal bacteremia. J. Clin. Investig. 6985-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, E. J., K. A. Joiner, R. M. Cole, and M. Berger. 1983. Localization of complement component 3 on Streptococcus pneumoniae: anti-capsular antibody causes complement deposition on the pneumococcal capsule. Infect. Immun. 39403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruyn, G. A., B. J. Zegers, and R. van Furth. 1992. Mechanisms of host defense against infection with Streptococcus pneumoniae. Clin. Infect. Dis. 14251-262. [DOI] [PubMed] [Google Scholar]
- 10.Dave, S., A. Brooks-Walter, M. K. Pangburn, and L. S. McDaniel. 2001. PspC, a pneumococcal surface protein, binds human factor H. Infect. Immun. 693435-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dave, S., S. Carmicle, S. Hammerschmidt, M. K. Pangburn, and L. S. McDaniel. 2004. Dual roles of PspC, a surface protein of Streptococcus pneumoniae, in binding human secretory IgA and factor H. J. Immunol. 173471-477. [DOI] [PubMed] [Google Scholar]
- 12.Dee, T. H., G. Schiffman, M. I. Sottile, and M. W. Rytel. 1977. Immunologic studies in pneumococcal disease. J. Lab. Clin. Med. 891198-1207. [PubMed] [Google Scholar]
- 13.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144(Pt. 11)3049-3060. [DOI] [PubMed] [Google Scholar]
- 14.Faden, H., L. Duffy, R. Wasielewski, J. Wolf, D. Krystofik, Y. Tung, et al. 1997. Relationship between nasopharyngeal colonization and the development of otitis media in children. J. Infect. Dis. 1751440-1445. [DOI] [PubMed] [Google Scholar]
- 15.Figueira, M. A., S. Ram, R. Goldstein, D. W. Hood, E. R. Moxon, and S. I. Pelton. 2007. Role of complement in defense of the middle ear revealed by restoring the virulence of nontypeable Haemophilus influenzae siaB mutants. Infect. Immun. 75325-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figueroa, J. E., and P. Densen. 1991. Infectious diseases associated with complement deficiencies. Clin. Microbiol. Rev. 4359-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine, D. P. 1975. Pneumococcal type-associated variability in alternate complement pathway activation. Infect. Immun. 12772-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray, B. M., G. M. Converse III, and H. C. Dillon, Jr. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142923-933. [DOI] [PubMed] [Google Scholar]
- 19.Hostetter, M. K. 2008. Interactions of Streptococcus pneumoniae with complement proteins, p. 83-92. In G. R. Siber et al. (ed.), Pneumococcal vaccines: the impact of conjugate vaccine. ASM Press, Washington, DC.
- 20.Hostetter, M. K. 1986. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J. Infect. Dis. 153682-693. [DOI] [PubMed] [Google Scholar]
- 21.Janulczyk, R., F. Iannelli, A. G. Sjoholm, G. Pozzi, and L. Bjorck. 2000. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J. Biol. Chem. 27537257-37263. [DOI] [PubMed] [Google Scholar]
- 22.Jarva, H., R. Janulczyk, J. Hellwage, P. F. Zipfel, L. Bjorck, and S. Meri. 2002. Streptococcus pneumoniae evades complement attack and opsonophagocytosis by expressing the pspC locus-encoded Hic protein that binds to short consensus repeats 8-11 of factor H. J. Immunol. 1681886-1894. [DOI] [PubMed] [Google Scholar]
- 23.Kadioglu, A., J. N. Weiser, J. C. Paton, and P. W. Andrew. 2008. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 6288-301. [DOI] [PubMed] [Google Scholar]
- 24.Kemper, C. A. 1994. The immunology of pneumococcal pneumonia, p. 29-49. Pulmonary infections and immunity. Plenum Press, New York, NY.
- 25.Kim, J. O., S. Romero-Steiner, U. B. Sorensen, J. Blom, M. Carvalho, S. Barnard, G. Carlone, and J. N. Weiser. 1999. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect. Immun. 672327-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, J., D. T. Glover, A. J. Szalai, S. K. Hollingshead, and D. E. Briles. 2007. PspA and PspC minimize immune adherence and transfer of pneumococci from erythrocytes to macrophages through their effects on complement activation. Infect. Immun. 755877-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, J., A. J. Szalai, S. K. Hollingshead, M. H. Nahm, and D. E. Briles. 2009. Antibody to the type 3 capsule facilitates immune adherence of pneumococci to erythrocytes and augments their transfer to macrophages. Infect. Immun. 77464-471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu, L., Z. Ma, T. S. Jokiranta, A. R. Whitney, F. R. DeLeo, and J. R. Zhang. 2008. Species-specific interaction of Streptococcus pneumoniae with human complement factor H. J. Immunol. 1817138-7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lysenko, E. S., A. J. Ratner, A. L. Nelson, and J. N. Weiser. 2005. The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces. PLoS Pathog. 1e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacLeod, C. M., and M. R. Kraus. 1950. Relation of virulence of pneumococcal strains for mice to the quantity of capsular polysaccharide formed in vitro. J. Exp. Med. 921-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magee, A. D., and J. Yother. 2001. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect. Immun. 693755-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melin, M., H. Jarva, L. Siira, S. Meri, H. Kayhty, and M. Vakevainen. Streptococcus pneumoniae capsular serotype 19F is more resistant to C3 deposition and less sensitive to opsonophagocytosis than serotype 6B. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]
- 33.Naked, G. M., M. P. Florido, P. Ferreira de Paula, A. M. Vinet, J. S. Inostroza, and L. Isaac. 2000. Deficiency of human complement factor I associated with lowered factor H. Clin. Immunol. 96162-167. [DOI] [PubMed] [Google Scholar]
- 34.Park, I. H., D. G. Pritchard, R. Cartee, A. Brandao, M. C. Brandileone, and M. H. Nahm. 2007. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 451225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Platts-Mills, T. A., and K. Ishizaka. 1974. Activation of the alternate pathway of human complements by rabbit cells. J. Immunol. 113348-358. [PubMed] [Google Scholar]
- 36.Quin, L. R., S. Carmicle, S. Dave, M. K. Pangburn, J. P. Evenhuis, and L. S. McDaniel. 2005. In vivo binding of complement regulator factor H by Streptococcus pneumoniae. J. Infect. Dis. 1921996-2003. [DOI] [PubMed] [Google Scholar]
- 37.Quin, L. R., C. Onwubiko, S. Carmicle, and L. S. McDaniel. 2006. Interaction of clinical isolates of Streptococcus pneumoniae with human complement factor H. FEMS Microbiol. Lett. 26498-103. [DOI] [PubMed] [Google Scholar]
- 38.Quin, L. R., C. Onwubiko, Q. C. Moore, M. F. Mills, L. S. McDaniel, and S. Carmicle. 2007. Factor H binding to PspC of Streptococcus pneumoniae increases adherence to human cell lines in vitro and enhances invasion of mouse lungs in vivo. Infect. Immun. 754082-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ram, S., A. D. Cox, J. C. Wright, U. Vogel, S. Getzlaff, R. Boden, J. Li, J. S. Plested, S. Meri, S. Gulati, D. C. Stein, J. C. Richards, E. R. Moxon, and P. A. Rice. 2003. Neisserial lipooligosaccharide is a target for complement component C4b. Inner core phosphoethanolamine residues define C4b linkage specificity. J. Biol. Chem. 27850853-50862. [DOI] [PubMed] [Google Scholar]
- 40.Ram, S., M. Cullinane, A. M. Blom, S. Gulati, D. P. McQuillen, B. G. Monks, C. O'Connell, R. Boden, C. Elkins, M. K. Pangburn, B. Dahlback, and P. A. Rice. 2001. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J. Exp. Med. 193281-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren, B., M. A. McCrory, C. Pass, D. C. Bullard, C. M. Ballantyne, Y. Xu, D. E. Briles, and A. J. Szalai. 2004. The virulence function of Streptococcus pneumoniae surface protein A involves inhibition of complement activation and impairment of complement receptor-mediated protection. J. Immunol. 1737506-7512. [DOI] [PubMed] [Google Scholar]
- 42.Ren, B., A. J. Szalai, S. K. Hollingshead, and D. E. Briles. 2004. Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect. Immun. 72114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren, B., A. J. Szalai, O. Thomas, S. K. Hollingshead, and D. E. Briles. 2003. Both family 1 and family 2 PspA proteins can inhibit complement deposition and confer virulence to a capsular serotype 3 strain of Streptococcus pneumoniae. Infect. Immun. 7175-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roy, S., K. Knox, S. Segal, D. Griffiths, C. E. Moore, K. I. Welsh, A. Smarason, N. P. Day, W. L. McPheat, D. W. Crook, and A. V. Hill. 2002. MBL genotype and risk of invasive pneumococcal disease: a case-control study. Lancet 3591569-1573. [DOI] [PubMed] [Google Scholar]
- 45.Tu, A. H., R. L. Fulgham, M. A. McCrory, D. E. Briles, and A. J. Szalai. 1999. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect. Immun. 674720-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Rossum, A. M., E. S. Lysenko, and J. N. Weiser. 2005. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect. Immun. 737718-7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watson, D. A., and D. M. Musher. 1999. A brief history of the pneumococcus in biomedical research. Semin. Respir. Infect. 14198-208. [PubMed] [Google Scholar]
- 48.Weiser, J. N., R. Austrian, P. K. Sreenivasan, and H. R. Masure. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 622582-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiser, J. N., D. Bae, H. Epino, S. B. Gordon, M. Kapoor, L. A. Zenewicz, and M. Shchepetov. 2001. Changes in availability of oxygen accentuate differences in capsular polysaccharide expression by phenotypic variants and clinical isolates of Streptococcus pneumoniae. Infect. Immun. 695430-5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winkelstein, J. A., A. S. Abramovitz, and A. Tomasz. 1980. Activation of C3 via the alternative complement pathway results in fixation of C3b to the pneumococcal cell wall. J. Immunol. 1242502-2506. [PubMed] [Google Scholar]
- 51.Yu, J., G. Carvalho Mda, B. Beall, and M. H. Nahm. 2008. A rapid pneumococcal serotyping system based on monoclonal antibodies and PCR. J. Med. Microbiol. 57171-178. [DOI] [PubMed] [Google Scholar]
- 52.Yuste, J., M. Botto, J. C. Paton, D. W. Holden, and J. S. Brown. 2005. Additive inhibition of complement deposition by pneumolysin and PspA facilitates Streptococcus pneumoniae septicemia. J. Immunol. 1751813-1819. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, Y., A. W. Masi, V. Barniak, K. Mountzouros, M. K. Hostetter, and B. A. Green. 2001. Recombinant PhpA protein, a unique histidine motif-containing protein from Streptococcus pneumoniae, protects mice against intranasal pneumococcal challenge. Infect. Immun. 693827-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.