Abstract
The ability of Brucella spp. to infect human osteoblasts and the cytokine response of these cells to infection were investigated in vitro. Brucella abortus, B. suis, B. melitensis, and B. canis were able to infect the SaOS-2 and MG-63 osteoblastic cell lines, and the first three species exhibited intracellular replication. B. abortus internalization was not significantly affected by pretreatment of cells with cytochalasin D but was inhibited up to 92% by colchicine. A virB10 mutant of B. abortus could infect but not replicate within osteoblasts, suggesting a role for the type IV secretion system in intracellular survival. Infected osteoblasts produced low levels of chemokines (interleukin-8 [IL-8] and macrophage chemoattractant protein 1 [MCP-1]) and did not produce proinflammatory cytokines (IL-1β, IL-6, and tumor necrosis factor alpha [TNF-α]). However, osteoblasts stimulated with culture supernatants from Brucella-infected human monocytes (THP-1 cell line) produced chemokines at levels 12-fold (MCP-1) to 17-fold (IL-8) higher than those of infected osteoblasts and also produced IL-6. In the inverse experiment, culture supernatants from Brucella-infected osteoblasts induced the production of IL-8, IL-1β, IL-6, and TNF-α by THP-1 cells. The induction of TNF-α and IL-1β was largely due to granulocyte-macrophage colony-stimulating factor produced by infected osteoblasts, as demonstrated by inhibition with a specific neutralizing antibody. This study shows that Brucella can invade and replicate within human osteoblastic cell lines, which can directly and indirectly mount a proinflammatory response. Both phenomena may have a role in the chronic inflammation and bone and joint destruction observed in osteoarticular brucellosis.
Brucella spp. are gram-negative facultative intracellular bacteria that infect domestic and wild animals and can be transmitted to humans, in whom they produce a debilitating and eventually chronic disease. The most common clinical features of human brucellosis are undulant fever, sweats, arthralgias, myalgias, lymphadenopathy, and hepatosplenomegaly (35). Osteoarticular brucellosis is the most common localization of active brucellosis, although its reported prevalence varies widely. The three most common forms of osteoarticular involvement are sacroiliitis, spondylitis, and peripheral arthritis (1, 15, 23, 28, 38).
Brucellar arthritis is frequently polyarticular and usually affects knees, sacroiliac joints, shoulders, and hips (28). In some cases, brucellar arthritis may be destructive, with associated osteopenia and cartilage damage. Brucellar spondylitis, which is more destructive than arthritis and causes more serious complications than arthritis does (7), typically begins at the disco-vertebral junction but may spread to the whole vertebrae and to adjacent vertebral bodies (29, 50).
While the clinical and imaging aspects of osteoarticular brucellosis have been described widely, the pathogenic mechanisms of joint and bone disease caused by Brucella have not been investigated at the molecular and cellular levels. Regarding brucellar arthritis, a septic form and a reactive form have been proposed (15). The septic form is supported by the isolation of Brucella spp. from synovial fluid or tissue.
In osteoarticular infections by pathogens such as Staphylococcus aureus and Mycobacterium tuberculosis, bone and joint damage results mainly from the inflammatory reaction elicited by the infection. In the mouse model of S. aureus arthritis, polymorphonuclear leukocytes and macrophages are seen in the synovial tissue early in the infection (5, 44). Similarly, an infiltrate of highly activated polymorphonuclear leukocytes has been observed in posttraumatic infectious osteomyelitis in humans (45). These cells produce not only proinflammatory cytokines and chemokines but also a series of tissue-degrading enzymes, including metalloproteinases, which can contribute to joint and bone destruction (13, 48). High levels of tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) are detected in the synovial fluid of patients with bacterial arthritis (34, 40). Increased local levels of TNF-α mRNA have also been detected in a rat model of osteomyelitis (27). These cytokines stimulate the release of proteases by inflammatory cells (41). In addition, TNF-α and IL-1β, together with IL-6, stimulate osteoclast differentiation and bone resorption in a synergistic fashion (19, 26). In human brucellar arthritis, synovial fluid usually presents an increased leukocyte count, and the synovial membrane frequently exhibits a nonspecific inflammatory change (28).
Given the central role of inflammatory cells in bone and joint destruction in osteomyelitis and arthritis, the recruitment and activation of these cells are of utmost importance for the development of these pathological conditions. Besides their role in bone formation, osteoblasts have also been shown to respond to bacterial infection or bacterial products by secreting proinflammatory cytokines, such as IL-6 and IL-12 (2, 20), and chemokines, such as macrophage chemoattractant protein 1 (MCP-1), IL-8, IP-10, and RANTES (4, 31, 47, 49), which recruit macrophages, neutrophils, and T lymphocytes. Overall, these data point to an active role of osteoblasts in the immune responses elicited during osteoarticular infections.
Staphylococcus aureus and Mycobacterium tuberculosis, which are common etiological agents of osteoarticular infections, can infect human osteoblasts in vitro (11, 12, 22, 46, 47). The intracellular persistence of these bacteria in bone cells may facilitate disease progression by protecting these organisms from extracellular host defenses and antibiotic therapy and may help to explain the recurrent nature of osteomyelitis (12). Brucella spp. are known to survive and replicate within mononuclear phagocytes (32) and also in nonphagocytic cells, including epithelial cells and fibroblasts (37). In contrast, there are no data on invasion and/or intracellular replication of Brucella spp. within osteoblasts.
In the present study, we investigated whether Brucella spp. can infect and survive within human osteoblastic cell lines and whether this infection elicits the secretion of proinflammatory cytokines and chemokines that might be involved in the osteoarticular manifestations of brucellosis. Since many of these aspects have been described widely for S. aureus, which is a frequent etiological agent of septic arthritis and osteomyelitis, this bacterium was included in parallel in most experiments for comparison.
MATERIALS AND METHODS
Bacterial culture.
Brucella abortus 2308, its isogenic virB10 polar mutant (kindly provided by Diego Comerci), Brucella suis 1330, Brucella melitensis H38, and a local clinical isolate of Brucella canis were grown overnight in 10 ml of tryptic soy broth with constant agitation at 37°C. Bacteria were harvested by centrifugation for 15 min at 6,000 × g at 4°C and washed twice in 10 ml of phosphate-buffered saline (PBS). Bacterial numbers in cultures were estimated by comparing the optical densities at 600 nm with a standard curve. To prepare inocula, cultures were diluted in sterile PBS to the desired bacterial concentration on the basis of the optical density readings, but the precise concentrations of inocula were determined by plating cells on tryptic soy agar. All live Brucella manipulations were performed in biosafety level 3 facilities. A clinical isolate of Staphylococcus aureus was used. Before experiments, S. aureus was cultured overnight (16 h) in 10 ml of Luria-Bertani broth (LB) at 37°C with agitation, and inocula were prepared as described for Brucella.
Cell culture.
The human osteoblastic cell lines SaOS-2 (ATCC, Rockville, MD) and MG-63 (European Collection of Animal Cell Cultures) were cultured as monolayers in a 5% CO2 atmosphere at 37°C in Dulbecco's modified Eagle's medium (Gibco, Grand Island, NY) supplemented with 2 mM l-glutamine, 10% heat-inactivated fetal bovine serum (FBS) (Gibco), 100 U/ml penicillin, and 100 μg/ml streptomycin. Monocytic human THP-1 cells were cultured in a 5% CO2 atmosphere at 37°C in RPMI 1640 (Gibco) supplemented with 2 mM l-glutamine, 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. All cell lines were seeded at 5 × 105 cells/well in 24-well plates.
For cocultures, SaOS-2 osteoblasts were harvested by gentle trypsinization with 0.05% trypsin-0.02% EDTA (Gibco) and were mixed with THP-1 monocytes to obtain monocyte/osteoblast ratios of 1:10 and 1:100. Cocultures were maintained in 24-well plates (1 × 106 cells per well in 1 ml) at 37°C in a 5% CO2 atmosphere in Dulbecco's modified Eagle's medium supplemented with 2 mM l-glutamine and 10% heat-inactivated FBS. To control for the individual contributions of osteoblasts and monocytes to chemokine secretion during cocultures (see below), the same number of each cell type used for the coculture was plated in separated wells.
Cellular infections.
B. abortus infections of SaOS-2 and MG-63 osteoblasts and infections of osteoblast-monocyte cocultures were set up at different multiplicities of infection (MOIs) (10:1, 100:1, and 1,000:1 [bacteria:cell]), while an MOI of 100:1 was used for S. aureus infections. Both species were used at an MOI of 100:1 to infect THP-1 monocytes. After the bacterial suspension was dispensed, the plates were centrifuged for 10 min at 2,000 rpm and then incubated for 2 h at 37°C under a 5% CO2 atmosphere. Cells were extensively washed with RPMI to remove extracellular bacteria and incubated in medium supplemented with 100 μg/ml gentamicin and 50 μg/ml streptomycin to kill extracellular bacteria. At different times postinfection (p.i.) (2, 24, 48, or 72 h), the supernatants from infections of individual cell types or cocultures were harvested for measurement of cytokines and chemokines.
At the end of the infection period, each well was washed three times with sterile PBS. To monitor Brucella intracellular survival, cells were lysed with a sterile solution of 0.1% (vol/vol) Triton X-100 in H2O and serial dilutions of lysates were rapidly plated on tryptic soy agar plates to enumerate CFU.
Inhibition of internalization.
Infection experiments in the presence of specific inhibitors were carried out to examine whether B. abortus internalization by the osteoblastic cell lines depends on actin polymerization (cytochalasin D) or microtubules (colchicine). Cytochalasin D was solubilized in dimethyl sulfoxide (DMSO) and was used at 20, 8, and 2 μM. Colchicine was solubilized in water and was used at 10, 5, and 1 μM. Inhibitors were obtained from Sigma (St. Louis, MO), and the concentrations used were based on previous reports on internalization by cultured osteoblasts or epithelial cells (22, 33). To examine the effects of such inhibitors, the osteoblastic cell lines were exposed to each compound during the whole infection period (2 h). Cell viability after incubation with these inhibitors was higher than 90%, as assessed by staining with trypan blue. After infection, the culture medium was removed and replaced with medium containing gentamicin and streptomycin for 2 h, and the cells were processed as described above for quantification of intracellular bacteria. To account for any possible effect of DMSO (cytochalasin vehicle) on osteoblast viability, cell cultures not treated with the inhibitors were treated with the highest final concentration of DMSO used in these studies (0.5%), and the results were compared to those for osteoblast cultures not exposed to DMSO.
Confocal microscopy.
SaOS-2 and MG-63 cells seeded onto glass coverslips were infected with B. abortus 2308 as described above (MOI, 100) and were fixed with 4% paraformaldehyde. The THP-1 monocytic cell line was infected in parallel for comparison. To label internalized bacteria, cells were incubated with a monoclonal antibody against Brucella lipopolysaccharide obtained in our laboratory, followed by incubation with a fluorescein isothiocyanate-conjugated antibody against mouse immunoglobulin G (IgG; Jackson Immunoresearch, West Grove, PA). Coverslips were mounted in PBS-glycerine (9:1 [vol/vol]) and were analyzed by confocal microscopy (C1 confocal microscope; Nikon, Melville, NY), using a ×60 Plan oil immersion lens. Pictures were acquired and processed using Photoshop software (Adobe System Inc., Mountain View, CA).
Flow cytometry analysis.
SaOS-2 cells were infected at an MOI of 100 as indicated above. At 24 and 48 h p.i., the cells were detached from the wells by treatment with 0.05% trypsin-0.02% EDTA solution for 10 min, followed by treatment with culture medium containing 10% fetal calf serum to inactivate trypsin. After the cells were washed with sterile PBS, cell permeabilization was performed with a solution of 0.05% saponin in PBS containing 0.1% bovine serum albumin (BSA). Cells were then incubated for 1 h with a monoclonal antibody, raised in our laboratory, against B. abortus lipopolysaccharide (0.5 mg/ml in PBS containing 2% BSA). After three washes with PBS containing 0.05% Tween 20 (PBS-T), the cells were incubated with a fluorescein isothiocyanate-conjugated antibody to mouse immunoglobulins (Jackson Immunoresearch, West Grove, PA) diluted 1/200 in PBS-2% BSA. After three washes with PBS-T and a further wash with PBS, cells were resuspended in 4% paraformaldehyde for 1 h at room temperature. Samples were analyzed in a flow cytometer (PAS III; Partec, Münster, Germany). Results were analyzed using WinMDI 2.8 software.
Stimulation with conditioned media.
Culture supernatants (CS) from Brucella-infected THP-1 monocytes (CSBIM) and Brucella-infected osteoblastic SaOS-2 cells (CSBIO) were harvested at 24 h p.i., sterilized by filtration through a 0.22-μm nitrocellulose filter, and used to stimulate noninfected SaOS-2 and THP-1 cells, respectively. Supernatants were used diluted 1/2, 1/5, 1/10, or 1/100 in complete medium. Parallel experiments were performed with CS from S. aureus-infected cells. After 24 h, the supernatants from these stimulated cultures were harvested to measure cytokines and chemokines. In other experiments, SaOS-2 cells stimulated for 24 h with supernatants from infected THP-1 cells were washed and infected with B. abortus or S. aureus, and supernatants were harvested at 24 h p.i.
Measurement of cytokine concentrations.
Human IL-1β, IL-6, IL-8, MCP-1, TNF-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF) were measured in culture supernatants by sandwich enzyme-linked immunosorbent assay, using paired cytokine-specific monoclonal antibodies, according to the manufacturer's instructions (BD Pharmingen, San Diego, CA).
Statistical analysis.
Data were analyzed using analysis of variance (ANOVA). Multiple comparisons between all pairs of groups were made with Tukey's posttest, and those against a control group were made with Dunnett's posttest. All statistical analyses were performed with GraphPad software (San Diego, CA).
RESULTS
Smooth Brucella species invade and multiply in human osteoblastic cell lines.
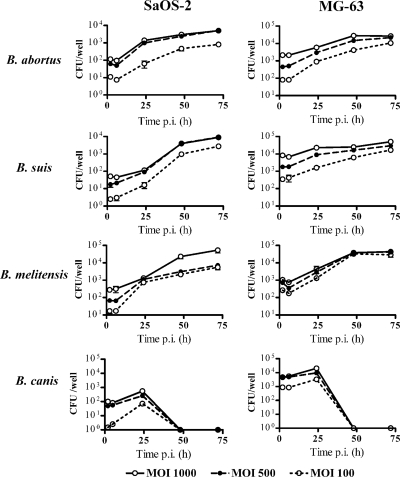
Infection experiments showed that Brucella abortus 2308, B. suis 1330, B. melitensis H38, and B. canis virulent strains are internalized by human osteoblastic cell lines in vitro. The first three Brucella species (naturally smooth species) were also able to multiply efficiently within MG-63 and SaOS-2 cells, while B. canis was not (Fig. 1). The magnitude of the infection (intracellular CFU) was directly related to the MOI used, but both infection and intracellular replication were observed even for MOIs as low as 100. For B. abortus, internalization was confirmed by confocal microscopy of infected cells, using immunodetection with an antibody to Brucella smooth lipopolysaccharide (Fig. 2). S. aureus also invaded both osteoblastic cell lines but did not multiply inside these cells (not shown), in agreement with previous reports (11).
FIG. 1.
Infection and replication of different Brucella species within human osteoblasts. After infection at different MOIs, cells were incubated with antibiotics to kill extracellular bacteria. Cells were lysed at different times p.i. and plated on agar to determine intracellular CFU. Values are means ± standard errors of the means (SEM) for triplicate determinations from one experiment, which was repeated twice with similar results.
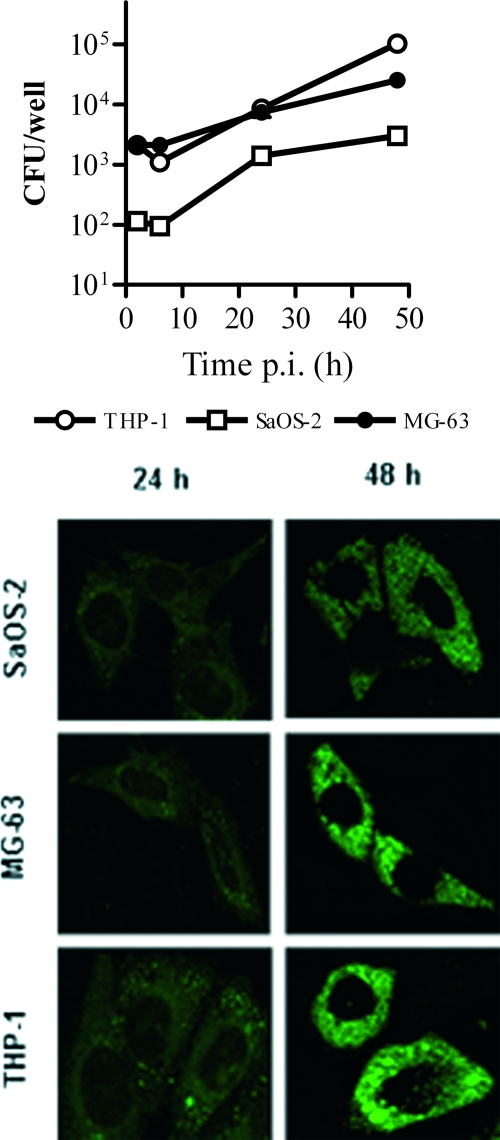
FIG. 2.
Comparison of intracellular replication of B. abortus within osteoblastic cell lines and the monocytic cell line THP-1 as assessed by CFU (top) and confocal microscopy (bottom). Data in the upper panel represent means ± SEM of CFU measured in triplicate in one experiment, which was repeated twice with similar results.
The number of bacteria internalized into MG-63 cells was higher than that observed for the SaOS-2 line after 2 h of infection (MOI, 1,000) with B. abortus (2,130 ± 240.41 versus 128 ± 8.48 CFU per well), B. suis (8,260 ± 2,064.75 versus 43 ± 9.90 CFU per well), B. melitensis (1,060 ± 90 versus 66 ± 8.48), or B. canis (4,695 ± 1,053.59 versus 1.5 ± 0.71 CFU per well) (not shown). For both cell lines, the number of intracellular bacteria had increased significantly at 48 h p.i. for infections with B. abortus (28,250 ± 3,750 CFU/well for MG-63 cells and 2,993 ± 10 CFU/well for SaOS-2 cells), B. suis (25,500 ± 500 and 4,050 ± 50 CFU/well, respectively), and B. melitensis (38,500 ± 500 and 23,150 ± 6,050 CFU/well, respectively), but no viable bacteria were recovered from cells infected with B. canis. The increase of intracellular bacteria with time was confirmed by confocal microscopy of SaOS-2 and MG-63 cells infected with B. abortus (Fig. 2).
The percentage of osteoblasts infected with B. abortus was determined from confocal microscopy images and also by flow cytometry (not shown). Determinations were performed at 24 h and 48 h p.i. because the small numbers of bacteria limited the sensitivity of detection at earlier time points. By flow cytometry, the percentage of infected SaOS-2 cells was 0.76% at 24 h and 4.22% at 48 h p.i. By confocal microscopy, the corresponding values were 2% and 8%, respectively. For MG-63 cells, the percentage of infected cells according to flow cytometry was 1.32% and 9.28%, respectively, and by confocal microscopy the results were 8% and 22%, respectively.
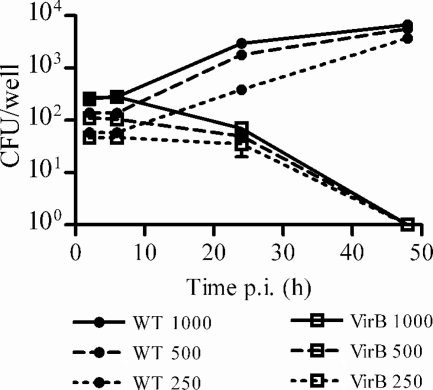
Since the type IV secretion system (T4SS) encoded by the virB genes has been shown to be involved in the capacity of different Brucella species to establish an intracellular replication niche (14), we decided to test whether the T4SS is involved in the ability of B. abortus to replicate within human osteoblastic cell lines. SaOS-2 cells were infected at different MOIs (up to 1,000 bacteria/cell) with B. abortus 2308 (wild type) and an isogenic virB10 polar mutant that has been shown to be incapable of intracellular survival and replication in HeLa cells (8). At 24 h p.i., CFU counts were significantly lower in osteoblasts infected with the mutant than in those infected with the parental strain (Fig. 3). While CFU from the latter had increased at 48 h p.i., no CFU were recovered at this time point from cells infected with the mutant. Collectively, these results showed that smooth Brucella species can infect and replicate in human osteoblastic cell lines and that such intracellular replication depends on the T4SS.
FIG. 3.
Involvement of the T4SS in B. abortus infection of SaOS-2 cells. Osteoblasts were infected at different MOIs with either wild-type B. abortus or an isogenic virB10 polar mutant and were processed as indicated in the legend to Fig. 1. Values are means ± SEM of CFU measured in triplicate at different times p.i. Data correspond to a representative experiment of two with similar results.
Internalization depends on microtubules.
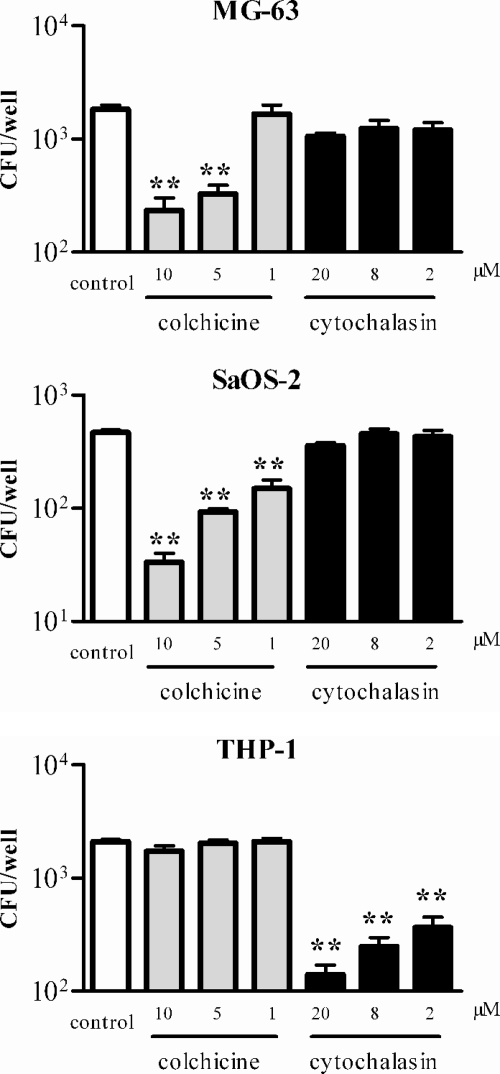
To assess the role of the cytoskeleton in internalization of B. abortus by the SaOS-2 and MG-63 cell lines, infections were performed in the presence of cytochalasin D and colchicine (Fig. 4). B. abortus internalization was not significantly affected by cytochalasin D, a drug that disrupts actin microfilaments. The lack of effect of cytochalasin D on internalization was specific to osteoblast-like cells, since this drug inhibited Brucella internalization by THP-1 cells, in agreement with previous reports (25). In contrast, colchicine, which causes depolymerization of microtubules and therefore inhibits transport of endocytic vesicles, interfered in a dose-dependent manner with the uptake of B. abortus by osteoblastic cell lines. Internalization in SaOS-2 was inhibited 92%, 80%, and 60% by colchicine added at 10 μM, 5 μM, and 1 μM, respectively. Internalization in MG63 was inhibited 85%, 80%, and 60%, respectively.
FIG. 4.
Effects of cytoskeleton inhibitors on B. abortus internalization in osteoblasts. Osteoblasts were exposed to each compound during the whole infection period (2 h) with B. abortus at an MOI of 1,000. After being washed, the cells were incubated for 2 h in the presence of gentamicin and processed as indicated in the legend to Fig. 1. Data represent means ± SEM of CFU measured in triplicate in one experiment, which was repeated twice with similar results. Significant differences relative to the control infection (no inhibitor added) are indicated with asterisks (*, P < 0.05; **, P < 0.01 [ANOVA followed by Dunnett's multiple comparison test]).
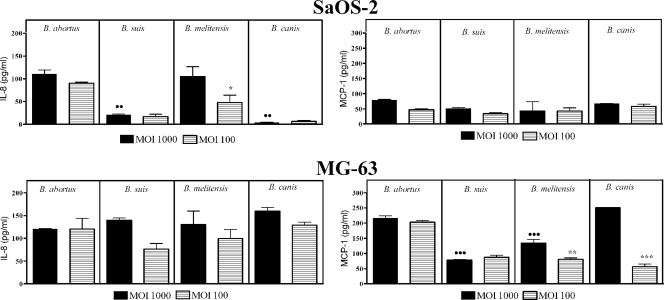
Brucella infection induces a low level of chemokine production by osteoblasts.
Infection of the MG-63 and SaOS-2 cell lines with the different Brucella strains at MOIs of 1,000 and 100 elicited low levels of secretion of IL-8 and MCP-1. For both cell lines, maximum levels (stimulus-specific levels) of these chemokines in CS were detected 48 h after infection with all Brucella strains assayed (Fig. 5). No further increase of the IL-8 or MCP-1 level was detected for any infection at 72 h p.i. (not shown). In general, chemokine levels elicited by infections at an MOI of 100 were comparable to those obtained at an MOI of 1,000, and this was particularly true for B. abortus infections. Stimulation of these cell lines with heat-killed B. abortus did not result in IL-8 or MCP-1 secretion compared with unstimulated cells (not shown).
FIG. 5.
Chemokine production by osteoblasts infected with different Brucella species at MOIs of 100 and 1,000. Levels measured at 48 h p.i. are depicted. Values represent specific chemokine production (spontaneous chemokine release by noninfected cells has been subtracted) and are expressed as means ± SEM of duplicate determinations in a representative experiment of two with similar results. Differences were analyzed by ANOVA followed by Tukey's multiple comparison test. Significant differences between B. abortus and other species at an MOI of 1,000 are shown with closed circles (••, P < 0.01; •••, P < 0.001). Significant differences between MOIs of 100 and 1,000 for the same species are shown with asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Infection with S. aureus also induced IL-8 secretion by both the MG-63 and SaOS-2 cell lines, with maximum levels detected at 72 h p.i. (297 ± 88 and 462 ± 55 pg/ml, respectively) (not shown). MCP-1 levels were relatively low in supernatants of SaOS-2 cells infected with S. aureus, but high levels were detected at 24 h p.i. in supernatants of MG-63 cells, in agreement with previous reports (47). At 72 h p.i., the MCP-1 level was 220 ± 9 pg/ml for SaOS-2 cells and 55,010 ± 2,938 pg/ml for MG-63 cells.
TNF-α production was not detected after infection with Brucella spp. in any osteoblastic cell line. Similarly, neither IL-1 nor IL-6 was detected in supernatants of SaOS-2 cells after B. abortus infection (not shown).
Osteoblast-monocyte interactions enhance cytokine responses to bacterial challenge.
While isolated osteoblastic cell lines seem to produce low levels of chemokines and no proinflammatory cytokines upon Brucella infection, the situation may be different in vivo, where other cell types are present at the site of infection. From an immunological point of view, an important cell type likely to be involved in interactions with osteoblasts are monocytes/macrophages. Therefore, we decided to test whether the presence of monocytes might modify the cytokine response of osteoblasts to Brucella infection. First, we tested whether factors secreted by Brucella-infected monocytes might induce the secretion of cytokines by osteoblasts and vice versa. Second, we tested whether the stimulation of osteoblasts with conditioned medium from Brucella-infected monocytes modifies the response of osteoblasts to Brucella infection. Third, we tested whether the cytokine response of monocyte-osteoblast cocultures to Brucella infection differs from that of each cell type alone. These experiments were performed using the THP-1 monocytic cell line and the SaOS-2 osteoblastic cell line.
(i) Culture supernatants from infected monocytes induce cytokine production by osteoblasts.
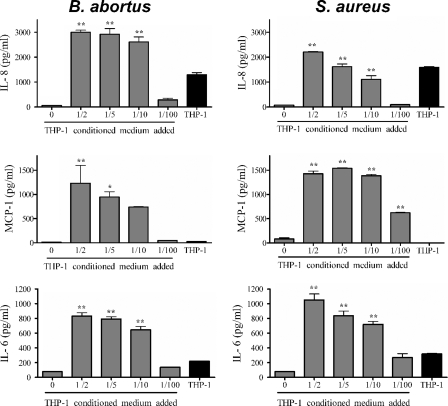
The addition of CS from B. abortus- or S. aureus-infected monocytes to uninfected SaOS-2 cells induced a significant secretion of MCP-1 by the latter cells compared to that in unstimulated cultures (Fig. 6). For stimulations with CSBIM added at a 1/2, 1/5, 1/10, or 1/100 dilution, the levels of MCP-1 produced by stimulated osteoblasts were significantly higher than those produced by osteoblasts infected with B. abortus at an MOI of either 1,000 or 100 (P < 0.001 for stimulations at 1/2 and 1/5; P < 0.01 for stimulations at 1/10). MCP-1 levels in CS of SaOS-2 cells stimulated at a 1/2 dilution with CSBIM were about 12-fold higher than those detected in Brucella-infected osteoblasts at 48 h postinfection. A similar difference in MCP-1 levels was found in the case of S. aureus between stimulated and infected SaOS-2 osteoblasts (1,543 ± 7 pg/ml for 1/2 stimulation versus 220 ± 9 pg/ml for infection). No MCP-1 was detected in supernatants from B. abortus- or S. aureus-infected macrophages, indicating that the MCP-1 measured in stimulated osteoblasts was produced exclusively by the latter cells.
FIG. 6.
Chemokine production by noninfected SaOS-2 osteoblasts stimulated with CS from B. abortus- or S. aureus-infected THP-1 monocytes. The proportion of CS added is indicated. Results are expressed as means ± SEM of duplicate measures in a representative experiment of two with similar results. Significant differences in chemokine production between cultures stimulated with CS and wells with no CS added were determined by ANOVA followed by Dunnett's multiple comparison test (*, P < 0.05; **, P < 0.01). The concentration of each chemokine in the undiluted CS is indicated by the black bars (labeled THP-1).
CS from infected monocytes also induced significant IL-8 production by SaOS-2 cells (Fig. 6). In this case, the transferred supernatants already contained IL-8 (1,300 ± 108 pg/ml for B. abortus infection and 1,594 ± 45 pg/ml for S. aureus infection). However, IL-8 levels found in supernatants from stimulated osteoblasts were even higher, indicating specific IL-8 production by these cells. The osteoblast-specific production of IL-8 upon stimulation with CSBIM at 1/2, 1/5, and 1/10 was around 1,700 pg/ml, 1,620 pg/ml and 1,310 pg/ml, respectively. These values were between 13-fold and 17-fold higher than those found at 48 h p.i. in supernatants from osteoblasts infected with B. abortus at an MOI of either 1,000 or 100 (P < 0.001 for 1/2 and 1/5 dilutions; P < 0.01 for 1/10 dilution). For SaOS-2 cells stimulated with CS from S. aureus-infected monocytes, IL-8 levels in the stimulated culture were somewhat lower than those for the equivalent experiment with CSBIM (Fig. 6).
While SaOS-2 cells did not seem to secrete IL-6 upon B. abortus infection, the stimulation of these cells with CSBIM resulted in the specific production of IL-6 (Fig. 6). After correcting for the IL-6 already present in the CSBIM (219 ± 7 pg/ml), the osteoblast-specific production of IL-6 upon stimulation with CSBIM at 1/2, 1/5, and 1/10 was calculated to be around 720 pg/ml, 750 pg/ml, and 625 pg/ml, respectively.
TNF-α and IL-1β were detected in CSBIM (236 ± 2 and 359 ± 13 pg/ml, respectively) and in CS from S. aureus-infected monocytes (431 ± 84 and 338 ± 25 pg/ml, respectively). After correcting for such levels, no specific production of these cytokines was detected in SaOS-2 cell supernatants after stimulation with THP-1 cell conditioned medium.
In all of the experiments described above, cytokine secretion was not stimulated by CS from noninfected monocytes.
(ii) The chemokine response induced in osteoblastic cells by culture supernatants from infected monocytes is not modified by subsequent infection.
Once it was established that CSBIM induces the production of chemokines by uninfected SaOS-2 cells, we wanted to explore whether such pretreatment also modifies the chemokine response of osteoblasts to subsequent Brucella infection. SaOS-2 cells preincubated for 24 h with different proportions of CSBIM were washed and subsequently infected or not (control) for 2 h with B. abortus. Supernatants were harvested 24 h later to measure chemokines. The levels of IL-8 in supernatants from infected osteoblasts did not differ significantly from those found in noninfected osteoblasts for pretreatments with CSBIM at 1/2 (452 ± 5 versus 466 ± 16 pg/ml), 1/5 (456 ± 6 versus 458 ± 10 pg/ml), and 1/10 (454 ± 11 versus 458 ± 12 pg/ml). A similar behavior was observed for MCP-1 secretion (533 ± 37 versus 575 ± 72 pg/ml, 510 ± 31 versus 582 ± 18 pg/ml, and 425 ± 39 versus 549 ± 60 pg/ml, respectively). Therefore, B. abortus infection did not produce a further increase of chemokine production by SaOS-2 cells over that induced by stimulation with CSBIM.
(iii) Culture supernatants from infected osteoblasts induce cytokine production by monocytes.
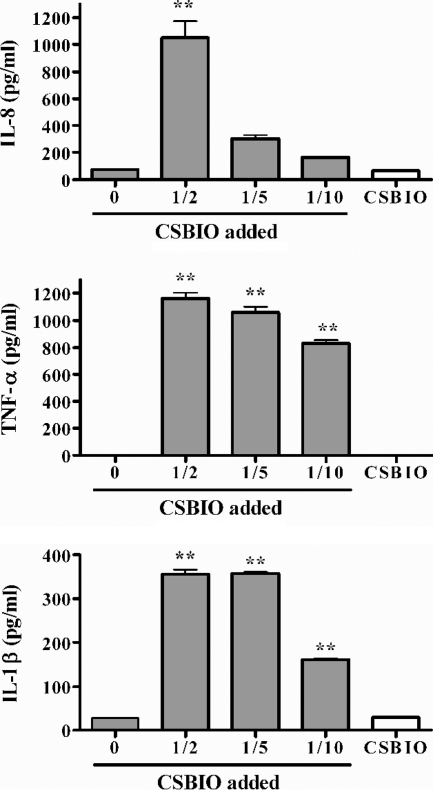
The inverse experiment, i.e., the stimulation of noninfected monocytes with supernatants from infected osteoblasts, was also performed. CSBIO added in different proportions induced the production of IL-8 by THP-1 cells compared to unstimulated cultures (Fig. 7), and the same happened with CS from S. aureus-infected osteoblasts (not shown). After subtracting the small amount of IL-8 already present in CSBIO, the monocyte-specific production of IL-8 upon stimulation with CSBIO at dilutions of 1/2, 1/5, and 1/10 was calculated to be around 980 pg/ml, 275 pg/ml, and 150 pg/ml, respectively. For stimulations with CS from S. aureus-infected osteoblasts, the monocyte-specific production of IL-8 was 481 pg/ml for the 1/2 dilution and 167 pg/ml for the 1/5 dilution.
FIG. 7.
IL-8 and TNF-α production by noninfected THP-1 monocytes stimulated with CSBIO. The proportion of supernatant added is indicated. Results are expressed as means ± SEM of duplicate measures in a representative experiment of two with similar results. Statistical significance was determined by ANOVA followed by Dunnett's multiple comparison test. Means were compared to data for wells with no CSBIO added (**, P < 0.01). The concentration of each chemokine in the undiluted CSBIO is also indicated.
THP-1 cells did not produce MCP-1 in response to stimulation with CS from either B. abortus- or S. aureus-infected SaOS-2 cells.
CSBIO added at 1/2, 1/5, 1/10, and 1/100 dilutions also induced a significant dose-dependent production of TNF-α by THP-1 cells compared to unstimulated monocytes (Fig. 7). The same effect was elicited by CS from S. aureus-infected SaOS-2 cells (1,162 ± 59, 1,058 ± 59, 831 ± 30, and 84 ± 6 pg/ml, respectively). Since TNF-α was not detected in CS from B. abortus- or S. aureus-infected osteoblasts, the measured levels are attributable only to production by THP-1 cells.
CSBIO added at a 1/2, 1/5, or 1/10 dilution induced the production of IL-1β by THP-1 cells (366 ± 28 and 361 ± 9 pg/ml for 1/2 and 1/5 dilutions, respectively) (Fig. 7). The same effect was elicited by CS from S. aureus-infected SaOS-2 cells (368 ± 7 and 351 ± 7 pg/ml, respectively) (not shown). Since IL-1β was not detected in CS from B. abortus- or S. aureus-infected osteoblasts, the measured levels are attributable only to production by the stimulated monocytes.
CSBIO also stimulated a low specific production of IL-6 by THP-1 monocytes (137 ± 5 pg/ml for stimulation at the 1/2 dilution) (not shown).
In all of the experiments described above, cytokine secretion was not stimulated by CS from noninfected SaOS-2 cells.
Since CSBIO induced the production of proinflammatory cytokines by THP-1 cells, experiments were performed to assess the effect of CSBIO on the intracellular proliferation of Brucella inside these cells. Two types of experiments were performed, in which supernatants from infected SaOS-2 cells were (i) transferred to Brucella-infected THP-1 cells at 2 h or 24 h p.i. (with CFU counts determined 24 and 48 h after transfer) or (ii) transferred to noninfected THP-1 cells for 24 h, followed by Brucella infection and a further 24-h incubation in the presence or absence of osteoblast supernatants. In every case, the CFU did not differ significantly from those found in Brucella-infected THP-1 cells that were not treated with CSBIO at any time of the infection (not shown).
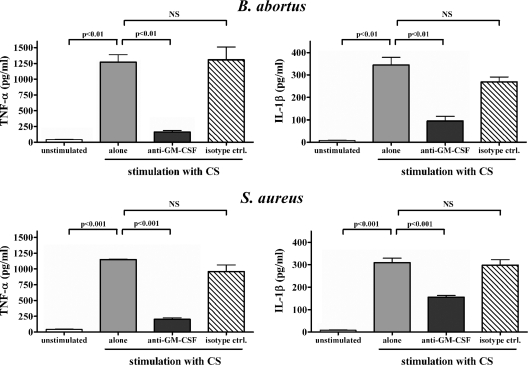
(iv) Induction of TNF-α and IL-1β by CSBIO is due largely to GM-CSF.
GM-CSF has been shown to stimulate TNF-α secretion by monocytes and has also been reported to be secreted by human osteoblasts in response to infection (3, 9). To determine whether GM-CSF is involved in the ability of CSBIO to induce TNF-α secretion by monocytes, GM-CSF levels in CSBIO were measured by enzyme-linked immunosorbent assay. GM-CSF was detected, albeit at low levels, in CS from SaOS-2 cells infected with B. abortus (16 ± 1 pg/ml) or S. aureus (23 ± 1 pg/ml) but was absent in CS from uninfected osteoblasts. To confirm that such GM-CSF levels can stimulate TNF-α secretion by monocytes, these cells were incubated in the presence of CSBIO alone or CSBIO preincubated for 1 h with either an anti-GM-CSF monoclonal neutralizing antibody or an isotype control. As shown in Fig. 8, neutralization of GM-CSF significantly reduced the ability of CSBIO to stimulate TNF-α secretion by monocytes (87% reduction compared to stimulation with untreated CSBIO), while the isotype control had no effect. Similar results were obtained with CS from S. aureus-infected osteoblasts (74% reduction of TNF-α secretion upon neutralization of GM-CSF) (Fig. 8). In addition, neutralization of GM-CSF significantly reduced the ability of CSBIO to stimulate IL-1β secretion by monocytes (72% reduction compared to stimulation with untreated CSBIO), while the isotype control had only a small effect (nonsignificant). The effect of GM-CSF neutralization on IL-1β secretion was somewhat lower (50% reduction) in the case of stimulation with CS from S. aureus-infected osteoblasts. These results indicate that GM-CSF is a major mediator of the stimulating effect of CSBIO on TNF-α and IL-1β secretion by monocytes.
FIG. 8.
Inhibition of the stimulating effect of CS from B. abortus- and S. aureus-infected osteoblasts on monocytic TNF-α and IL-1β production by pretreatment of CS with a neutralizing antibody to GM-CSF. CS were incubated with either the neutralizing antibody or an isotype control for 1 h before addition to THP-1 cultures. Results are expressed as means ± SEM of duplicate measures in a representative experiment of two with similar results. Statistical significance was determined by ANOVA followed by Tukey's multiple comparison test. NS, no significance.
(v) Cytokine response of osteoblast-monocyte cocultures to Brucella infection.
Since the in vivo ratio of monocytes to osteoblasts during osteoarticular brucellosis is unknown, two ratios were tested in coculture experiments (monocytes to osteoblasts, 1:100 and 1:10). Basal (uninfected) levels of cytokines secreted by cells grown in coculture were very low and did not differ significantly from those of SaOS-2 or THP-1 cells cultured separately. During the infection of the coculture model, monocytes and osteoblasts retained their normal morphology. The viability of the cells making up the coculture model was evaluated by trypan blue exclusion. No obvious cytotoxic effect following infections was detected, and cell viability was ≥98% in all experiments (data not shown).
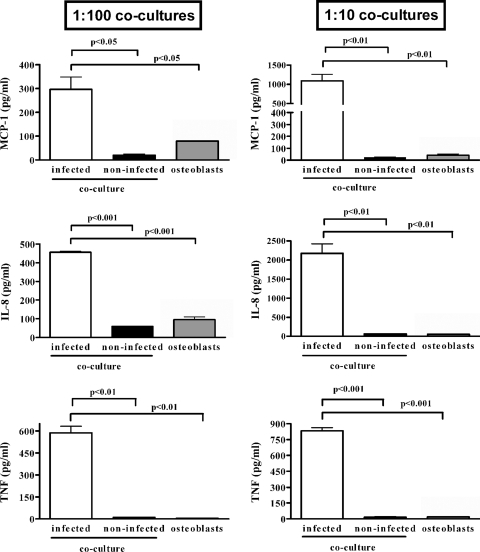
For MCP-1, which is produced by SaOS-2 cells but not by monocytes, levels in supernatants from infected 1:100 monocyte-osteoblast cocultures at 48 h p.i. were significantly higher (P < 0.05) than those for the same number of infected SaOS-2 cells cultured alone for both B. abortus infection (282 ± 94 versus 79 ± 33 pg/ml; MOI, 1,000) (Fig. 9) and S. aureus infection (282 ± 34 versus 84 ± 5 pg/ml; MOI, 100) (not shown). The difference was even higher (P < 0.01) for 1:10 monocyte-osteoblast cocultures (1,339 ± 591 versus 40 ± 14 pg/ml for B. abortus).
FIG. 9.
Cytokine production by monocyte-osteoblast cocultures in response to B. abortus infection. These cell types were mixed in the proportion indicated (1:10 or 1:100) to total 1 × 106 cells/well and were infected or not with B. abortus (MOI, 1,000) for 2 h. In parallel, the same number of osteoblasts cultured alone were also infected (osteoblasts). Cytokines were measured in culture supernatants harvested at 48 h p.i. Results are expressed as means ± SEM of duplicate measures in a representative experiment of two with similar results. Statistical significance was determined by ANOVA followed by Tukey's multiple comparison test.
A similar tendency toward increased production in coculture relative to that in osteoblasts cultured alone was observed for IL-8 (Fig. 9). However, since this chemokine is produced by both cell types, no definitive conclusions can be drawn from these data regarding the relative contribution of each cell type.
Levels of TNF-α in supernatants from infected 1:100 monocyte-osteoblast cocultures at 48 h p.i. were significantly higher (P < 0.05) than those for the same number of infected SaOS-2 cells cultured alone for both B. abortus infection (588 ± 44 versus 4 ± 2 pg/ml; MOI, 1,000) (Fig. 9) and S. aureus infection (370 ± 30 versus 17 ± 5 pg/ml; MOI, 100) (not shown). The difference was even higher (P < 0.01) for 1:10 monocyte-osteoblast cocultures (832 ± 30 versus 19 ± 1 pg/ml for B. abortus).
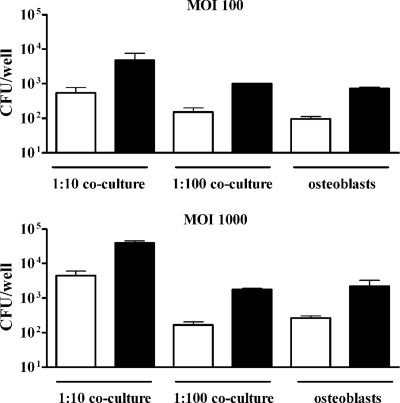
To assess whether the direct interaction between THP-1 cells and SaOS-2 cells may hamper the intracellular replication of Brucella within these cell lines, monocyte-osteoblast cocultures (1:100 and 1:10) were infected with B. abortus and CFU were measured at 24 and 48 h p.i. As shown in Fig. 10, Brucella organisms replicated within the cells present in the coculture. Furthermore, replication was similar between the culture of osteoblasts alone and the 1:100 coculture, which differed only by the presence of 1% THP-1 cells in the latter. Therefore, Brucella replication does not seem to be affected by the interaction between these cell types.
FIG. 10.
Proliferation of Brucella abortus in monocyte-osteoblast cocultures. These cell types were mixed in the proportion indicated (1:10 or 1:100) to total 1 × 106 cells/well and were infected or not with B. abortus (MOI, 100 or 1,000) for 2 h. In parallel, the same number of osteoblasts cultured alone were also infected (osteoblasts). After infection, cells were incubated with antibiotics to kill extracellular bacteria. Cells were lysed at 24 h (white bars) or 48 h (black bars) and plated on agar to determine the intracellular CFU. Experiments were performed three times in triplicate. Data are means ± SEM from a representative experiment.
DISCUSSION
While osteoarticular disease is the most common complication of human brucellosis, no studies have been performed on the cellular and molecular mechanisms involved in the pathogenesis of this condition. Since osteoblasts have been shown to play a pivotal role in the pathogenesis of osteoarticular diseases caused by other bacteria, the main goal of the present study was to assess whether Brucella can infect and survive within human osteoblastic cell lines and whether these infected cells secrete proinflammatory cytokines and chemokines.
To our best knowledge, the present study is the first to show that Brucella species can infect and eventually multiply within human osteoblastic cell lines. Brucella species naturally present a smooth or a rough phenotype, depending on the composition of their lipopolysaccharide molecules. While B. abortus and B. suis are naturally smooth, B. canis is naturally rough. In the present study, smooth strains were able to invade and replicate within human osteoblasts, while B. canis invaded these cells but did not exhibit intracellular replication. These differences between smooth and rough strains agree with those observed by others in Brucella infections of macrophages and nonphagocytic cell types (10, 39). It has been proposed that the intracellular persistence of S. aureus and M. tuberculosis within osteoblasts may protect these organisms from extracellular host defenses and antibiotic therapy and may contribute to the recurrent nature of the osteomyelitis that they cause (12). Similarly, the capacity of Brucella to survive and replicate within osteoblasts may be relevant to the chronic nature of brucellar osteomyelitis. As shown in this study, such a capacity depended on the expression of the T4SS encoded by virB genes, in agreement with results obtained by other authors for epithelial cells and macrophages (8, 14, 32).
The MG-63 cell line seemed to be infected more efficiently by Brucella than the SaOS-2 cell line was. The reasons for such a difference are unclear at present. To our best knowledge, there are no reports of parallel infections of the SaOS-2 and MG-63 cell lines with any bacterial species, which could allow a comparison with our results. A study of the phenotypic characteristics of these cell lines determined that both exhibit osteoblastic features such as the expression of osteocalcin, bone sialoprotein, decorin, and procollagen I (36). Collagen III was expressed by >95% of MG-63 cells but only 15% of SaOS-2 cells, and collagen VI was detected only in MG-63 cells. It was concluded that SaOS-2 cells exhibit the most mature osteoblastic phenotype, while MG-63 cells have both mature and immature osteoblastic features. Therefore, these cell lines have some different features that may be involved in their different susceptibilities to Brucella infection.
In the few studies that reported quantitative data on the intensity of bacterial infection of osteoblasts, two types of measures were used, namely, the number of intracellular bacteria per cell and the percentage of infected cells. In the present study, the percentage of osteoblasts infected by B. abortus was 8% for MG-63 cells (by confocal microscopy at 24 h p.i.), which is in line with values reported for MG-63 cells infected by Mycobacterium tuberculosis (4% and 15% at 4 and 24 h p.i., respectively) (47). For MG-63 cells, the CFU/cell ratio immediately after infection was 0.004 for B. abortus and 0.016 for B. suis, which are considerably lower than the value reported for Salmonella enterica serovar Dublin infections of the same cell line (0.7 bacteria/cell) (3). Therefore, Brucella species seem to invade a similar proportion of osteoblasts to those for other pathogens involved in bone infections, but the number of internalized bacteria is comparatively lower. Interestingly, a previous study showed that nonopsonized B. suis cells are poorly internalized in human monocytes compared to Escherichia coli cells (25).
The invasion of both SaOS-2 and MG-63 cell lines by B. abortus was inhibited by colchicine but not by cytochalasin D, suggesting that invasion depends on microtubules but not on microfilament formation. These results contrast with those obtained with HeLa cells, in which both colchicine and cytochalasin D reduced B. abortus invasion (18). To our best knowledge, the only previous studies on the effects of cytoskeleton inhibitors on the bacterial invasion of osteoblasts are those performed with S. aureus, which has a different lifestyle from that of Brucella but constitutes a common cause of osteomyelitis. Our results differed from those found for S. aureus infection of MG-63 osteoblasts, in which internalization was inhibited by both colchicine and cytochalasin D (22). The concentrations of inhibitors used in the present study were equal to those used in the study on S. aureus internalization in osteoblasts and were also similar to those used in studies on B. abortus internalization by epithelial cells (18). In the present study, the lack of effect of cytochalasin D on internalization was specific for osteoblast-like cells, since this agent inhibited Brucella internalization in THP-1 cells, in agreement with previous findings for macrophages (25). These results suggest that the mechanism of internalization of B. abortus in human osteoblasts differs from that reported for other cell types.
Recent studies have shown that osteoblasts play an important role in the pathogenesis of osteoarticular infectious diseases by several mechanisms, including the production of chemokines that attract inflammatory cells to the site of infection (30). In the present study, infection with each of the three Brucella strains used elicited relatively low levels of secretion of chemokines from both SaOS-2 and MG-63 cells. The production of chemokines in response to Brucella seemed to depend on bacterial viability, since stimulation of osteoblasts with heat-killed B. abortus did not elicit any chemokine response (not shown). The low chemokine production level in response to Brucella spp. may be related to the small number of bacteria per infected cell compared to that for osteoblast infections by other pathogens. Chemokine levels were about twofold to threefold higher for S. aureus infection, except for MCP-1 production by MG-63 cells, which was much higher, in agreement with previous reports (47). Interestingly, the high production level of MCP-1 by MG-63 cells was restricted to S. aureus and did not happen with any of the three Brucella strains. Except for this difference, chemokine production by the osteoblastic cell lines in response to Brucella infection was on the same order of magnitude (three- to fivefold lower) as that in response to S. aureus, although it was lower than that reported for M. tuberculosis infections (47).
Inflammatory cells play a significant role in the damage produced to bone and synovial tissues during infectious osteomyelitis and arthritis (19, 41). In experimental arthritis by S. aureus, mononuclear phagocytes migrate to the site of infection slightly later than neutrophils do. Macrophages are involved in the arthritic damage in this model, since mice depleted of monocytes/macrophages exhibit a significantly less severe arthritis (43). Similar studies have not been performed with Brucella, but a nonspecific inflammatory infiltrate has been found in synovial membrane and bone in patients with brucellar arthritis and osteomyelitis, respectively (28). Therefore, it can be speculated that macrophages can interact with osteoblasts at the site of osteoarticular Brucella infection and that both cell types can mutually modify their response to the pathogen. Our results show that CSBIM stimulates osteoblasts to secrete MCP-1 and IL-8. Chemokine levels in supernatants from CSBIM-stimulated osteoblasts were between 12-fold and 20-fold higher than those found in Brucella-infected osteoblasts. Therefore, while direct infection by B. abortus induces only low chemokine production in osteoblasts, the interaction of these cells with infected monocytes can induce a significantly greater chemokine response. This enhanced chemokine response as a result of the interaction with monocytes was also observed when both cell types were cultured and infected together in a coculture model. Previous studies (6, 16, 17) have shown that IL-8 and MCP-1 production by normal osteoblasts and osteoblastic cell lines can be stimulated by TNF-α and IL-1β, both of which were produced by Brucella-infected monocytes in the present study.
Notably, the inverse interaction between osteoblasts and macrophages was also verified. CSBIO and also CS from S. aureus-infected osteoblasts stimulated THP-1 monocytes to secrete IL-8. This effect was not elicited by CS from noninfected osteoblasts, showing that it occurs only in an infectious environment. Overall, these results agree with those obtained in a previous study with monocytes stimulated with conditioned medium from M. tuberculosis-infected osteoblasts (48). In that study, the stimulating effect was partially mediated by IL-1 and TNF-α. Since neither of these cytokines was detected in CSBIO, other secreted factors seem to mediate the stimulation of IL-8 secretion in this case. In the present study, IL-8 secretion by monocytes in response to CSBIO was higher than that induced by direct infection of SaOS-2 cells but was lower than that produced by osteoblasts stimulated with CSBIM (either subsequently infected or not with B. abortus). Overall, these results suggest that in this model of osteoblast-monocyte interaction in response to B. abortus challenge, IL-8 is produced mainly by osteoblasts. MCP-1 was not produced by THP-1 cells in response to Brucella infection or to stimulation with CSBIO. Therefore, osteoblasts also seem to constitute the source of MCP-1 in this model.
We also wanted to determine whether pretreatment with CSBIM may increase the chemokine response of osteoblasts to B. abortus infection relative to the response produced by nonpretreated cells. However, similar chemokine responses were observed in both situations. The lack of additional stimulation of chemokine production by Brucella infection may be due to the low potential of the bacteria to induce chemokines in osteoblasts, as shown in this study.
A novel finding of this study was that CSBIO also stimulated monocytes to produce TNF-α and IL-1β, which were not produced by the osteoblasts themselves. Therefore, osteoblasts may contribute to the inflammatory process of Brucella osteoarticular infections not only by recruiting phagocytes to the site of infection but also by stimulating these cells to secrete proinflammatory chemokines and cytokines. In our experiments, the stimulation produced by Brucella on TNF-α secretion by macrophages was indirect and likely to be mediated by soluble factors produced by Brucella-infected osteoblasts. Several soluble cellular factors, including GM-CSF, M-CSF, IL-2, gamma interferon, and IL-12, have been reported to stimulate, alone or in combination, the secretion of TNF-α and/or IL-1β by monocytes (9, 21, 24). Among these factors, GM-CSF, M-CSF, and IL-12 have been reported to be secreted by human osteoblasts in response to infection (2, 3). In agreement with those studies, GM-CSF was detected in CS from osteoblasts infected with B. abortus or S. aureus. Neutralization assays with specific antibodies to GM-CSF demonstrated that this factor is a major mediator of the stimulating effect of CSBIO on TNF-α and IL-1β production by monocytes. Since these cytokines can in turn stimulate the production of chemokines by osteoblasts, this cytokine networking may help to amplify the inflammatory reaction to Brucella infection in the bone. In addition, the TNF-α produced by stimulated monocytes might induce an autocrine stimulation of IL-1β production (9, 42).
As shown in this study, different Brucella species can invade, survive, and replicate within osteoblasts. We also show that Brucella-infected osteoblasts secrete proinflammatory chemokines, which in the in vivo situation might recruit phagocytic cells to the site of infection, initiating the inflammatory response. Our results suggest that such a response may be amplified by subsequent interactions between osteoblasts and monocytes in the face of Brucella infection. While the physiological role of the inflammatory response is the eradication of the infecting agent, the intracellular persistence of Brucella within osteoblasts may stimulate a localized chronic inflammation. Further studies will be needed to determine whether the innate immune responses described here, alone or associated with adaptive immune processes, have a role in the chronic inflammation and bone and joint destruction observed in osteoarticular brucellosis.
Acknowledgments
We thank Horacio Salomón and the staff of the Centro Nacional de Referencia del Sida, University of Buenos Aires, for their assistance with biosafety level 3 laboratory use. We thank Cesar Bogado from Instituto de Investigaciones Metabólicas (IDIM), Buenos Aires, Argentina, for kindly providing the SaOS-2 and MG-63 cells.
This work was supported by grants PICT 05-14305 and PICT 05-38237 from Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT), by grant PIP 5212 from the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), and by a grant from Fundación Roemmers. M.V.D., C.A.F., and P.C.B. are members of the Research Career of CONICET. C.A.F. is also a member of the Facultad de Ciencias Exactas, Universidad Nacional de La Plata.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 22 December 2008.
REFERENCES
- 1.Aydin, M., A. Fuat Yapar, L. Savas, M. Reyhan, A. Pourbagher, T. Y. Turunc, Y. Ziya Demiroglu, N. A. Yologlu, and A. Aktas. 2005. Scintigraphic findings in osteoarticular brucellosis. Nucl. Med. Commun. 26639-647. [DOI] [PubMed] [Google Scholar]
- 2.Bost, K. L., W. K. Ramp, N. C. Nicholson, J. L. Bento, I. Marriott, and M. C. Hudson. 1999. Staphylococcus aureus infection of mouse or human osteoblasts induces high levels of interleukin-6 and interleukin-12 production. J. Infect. Dis. 1801912-1920. [DOI] [PubMed] [Google Scholar]
- 3.Bost, K. L., J. L. Bento, J. K. Ellington, I. Marriott, and M. C. Hudson. 2000. Induction of colony-stimulating factor expression following Staphylococcus or Salmonella interaction with mouse or human osteoblasts. Infect. Immun. 685075-5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bost, K. L., J. L. Bento, C. C. Petty, L. W. Schrum, M. C. Hudson, and I. Marriott. 2001. Monocyte chemoattractant protein-1 expression by osteoblasts following infection with Staphylococcus aureus or Salmonella. J. Interferon Cytokine Res. 21297-304. [DOI] [PubMed] [Google Scholar]
- 5.Bremell, T., A. Abdelnour, and A. Tarkowski. 1992. Histopathological and serological progression of Staphylococcus aureus arthritis. Infect. Immun. 602976-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhary, L. R., T. C. Spelsberg, and B. L. Riggs. 1992. Production of various cytokines by normal human osteoblast-like cells in response to interleukin-1β and tumor necrosis factor-α: lack of regulation by 17β-estradiol. Endocrinology 1302528-2534. [DOI] [PubMed] [Google Scholar]
- 7.Colmenero, J. D., J. D. Ruiz-Mesa, A. Plata, P. Bermúdez, P. Martín-Rico, M. I. Queipo-Ortuño, and J. M. Reguera. 2008. Clinical findings, therapeutic approach, and outcome of brucellar vertebral osteomyelitis. Clin. Infect. Dis. 46426-433. [DOI] [PubMed] [Google Scholar]
- 8.Comerci, D. J., M. J. Martínez-Lorenzo, R. Sieira, J. P. Gorvel, and R. A. Ugalde. 2001. Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell. Microbiol. 3159-168. [DOI] [PubMed] [Google Scholar]
- 9.Danis, V. A., G. M. Franic, D. A. Rathjen, and P. M. Brooks. 1991. Effects of granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-2, interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α) and IL-6 on the production of immunoreactive IL-1 and TNF-α by human monocytes. Clin. Exp. Immunol. 85143-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Detilleux, P. G., B. L. Deyoe, and N. F. Cheville. 1990. Entry and intracellular localization of Brucella spp. in Vero cells: fluorescence and electron microscopy. Vet. Pathol. 27317-328. [DOI] [PubMed] [Google Scholar]
- 11.Ellington, J. K., M. Harris, L. Webb, B. Smith, T. Smith, K. Tan, and M. Hudson. 2003. Intracellular Staphylococcus aureus. A mechanism for the indolence of osteomyelitis. J. Bone Joint Surg. Br. 85918-921. [PubMed] [Google Scholar]
- 12.Ellington, J. K., M. Harris, M. C. Hudson, S. Vishin, L. X. Webb, and R. Sherertz. 2006. Intracellular Staphylococcus aureus and antibiotic resistance: implications for treatment of staphylococcal osteomyelitis. J. Orthop. Res. 2487-93. [DOI] [PubMed] [Google Scholar]
- 13.Gjertsson, I., M. Innocenti, L. M. Matrisian, and A. Tarkowski. 2005. Metalloproteinase-7 contributes to joint destruction in Staphylococcus aureus induced arthritis. Microb. Pathog. 3897-105. [DOI] [PubMed] [Google Scholar]
- 14.Gorvel, J. P., and E. Moreno. 2002. Brucella intracellular life: from invasion to intracellular replication. Vet. Microbiol. 90281-297. [DOI] [PubMed] [Google Scholar]
- 15.Gotuzzo, E., G. S. Alarcón, T. S. Bocanegra, C. Carrillo, J. C. Guerra, I. Rolando, and L. R. Espinoza. 1982. Articular involvement in human brucellosis: a retrospective analysis of 304 cases. Semin. Arthritis Rheum. 12245-255. [DOI] [PubMed] [Google Scholar]
- 16.Graves, D. T., Y. Jiang, and A. J. Valente. 1999. Regulated expression of MCP-1 by osteoblastic cells in vitro and in vivo. Histol. Histopathol. 141347-1354. [DOI] [PubMed] [Google Scholar]
- 17.Graves, D. T., Y. Jiang, and A. J. Valente. 1999. The expression of monocyte chemoattractant protein-1 and other chemokines by osteoblasts. Front. Biosci. 4571-580. [DOI] [PubMed] [Google Scholar]
- 18.Guzmán-Verri, C., E. Chaves-Olarte, C. von Eichel-Streiber, I. López-Goñi, M. Thelestam, S. Arvidson, J. P. Gorvel, and E. Moreno. 2001. GTPases of the Rho subfamily are required for Brucella abortus internalization in nonprofessional phagocytes. J. Biol. Chem. 27644435-44443. [DOI] [PubMed] [Google Scholar]
- 19.Haynes, D. R. 2004. Bone lysis and inflammation. Inflamm. Res. 53596-600. [DOI] [PubMed] [Google Scholar]
- 20.Ishimi, Y., C. Miyaura, C. H. Jin, T. Akatsu, E. Abe, Y. Nakamura, A. Yamaguchi, S. Yoshiki, T. Matsuda, and T. Hirano. 1990. IL-6 is produced by osteoblasts and induces bone resorption. J. Immunol. 1453297-3303. [PubMed] [Google Scholar]
- 21.Jana, M., S. Dasgupta, R. N. Saha, X. Liu, and K. Pahan. 2003. Induction of tumor necrosis factor-α (TNF-α) by interleukin-12 p40 monomer and homodimer in microglia and macrophages. J. Neurochem. 86519-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jevon, M., C. Guo, B. Ma, N. Mordan, S. P. Nair, M. Harris, B. Henderson, G. Bentley, and S. Meghji. 1999. Mechanisms of internalization of Staphylococcus aureus by cultured human osteoblasts. Infect. Immun. 672677-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khateeb, M. I., G. F. Araj, S. A. Majeed, and A. R. Lulu. 1990. Brucella arthritis: a study of 96 cases in Kuwait. Ann. Rheum. Dis. 49994-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimball, E. S., E. Kovacs, M. C. Clark, and C. R. Schneider. 1995. Activation of cytokine production and adhesion molecule expression on THP-1 myelomonocytic cells by macrophage colony-stimulating factor in combination with interferon-gamma. J. Leukoc. Biol. 58585-594. [DOI] [PubMed] [Google Scholar]
- 25.Kusumawati, A., C. Cazevieille, F. Porte, S. Bettache, J. P. Liautard, and J. Sri Widada. 2000. Early events and implication of F-actin and annexin I associated structures in the phagocytic uptake of Brucella suis by the J-774A.1 murine cell line and human monocytes. Microb. Pathog. 28343-352. [DOI] [PubMed] [Google Scholar]
- 26.Kwan Tat, S., M. Padrines, S. Théoleyre, D. Heymann, and Y. Fortun. 2004. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 1549-60. [DOI] [PubMed] [Google Scholar]
- 27.Littlewood-Evans, A. J., M. R. Hattenberger, C. Luscher, A. Pataki, O. Zak, and T. O'Reilly. 1997. Local expression of tumor necrosis factor alpha in an experimental model of acute osteomyelitis in rats. Infect. Immun. 653438-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madkour, M. M. 2001. Osteoarticular brucellosis, p. 74-84. In M. M. Madkour (ed.), Madkour's brucellosis, 2nd ed. Springer-Verlag, Berlin, Germany.
- 29.Madkour, M. M., and H. Sharif. 2001. Bone and joint imaging, p. 90-132. In M. M. Madkour (ed.), Madkour's brucellosis, 2nd ed. Springer-Verlag, Berlin, Germany.
- 30.Marriott, I. 2004. Osteoblast responses to bacterial pathogens. A previously unappreciated role for bone-forming cells in host defense and disease progression. Immunol. Res. 30291-308. [DOI] [PubMed] [Google Scholar]
- 31.Marriott, I., D. L. Gray, D. M. Rati, V. G. Fowler, Jr., M. E. Stryjewski, L. S. Levin, M. C. Hudson, and K. L. Bost. 2005. Osteoblasts produce monocyte chemoattractant protein-1 in a murine model of Staphylococcus aureus osteomyelitis and infected human bone tissue. Bone 37504-512. [DOI] [PubMed] [Google Scholar]
- 32.O'Callaghan, D., C. Cazevieille, A. Allardet-Servent, M. L. Boschiroli, G. Bourg, V. Foulongne, P. Frutos, Y. Kulakov, and M. Ramuz. 1999. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 331210-1220. [DOI] [PubMed] [Google Scholar]
- 33.Oelschlaeger, T. A., P. Guerry, and D. J. Kopecko. 1993. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc. Natl. Acad. Sci. USA 906884-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osiri, M., K. Ruxrungtham, S. Nookhai, Y. Ohmoto, and U. Deesomchok. 1998. IL-1beta, IL-6 and TNF-alpha in synovial fluid of patients with non-gonococcal septic arthritis. Asian Pac. J. Allergy Immunol. 16155-160. [PubMed] [Google Scholar]
- 35.Pappas, G., N. Akritidis, M. Bosilkovski, and E. Tsianos. 2005. Brucellosis. N. Engl. J. Med. 3522325-2336. [DOI] [PubMed] [Google Scholar]
- 36.Pautke, C., M. Schieker, T. Tischer, A. Kolk, P. Neth, W. Mutschler, and S. Milz. 2004. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res. 243743-3748. [PubMed] [Google Scholar]
- 37.Pizarro-Cerdá, J., E. Moreno, and J. P. Gorvel. 2000. Invasion and intracellular trafficking of Brucella abortus in nonphagocytic cells. Microbes Infect. 2829-835. [DOI] [PubMed] [Google Scholar]
- 38.Pourbagher, A., M. A. Pourbagher, L. Savas, T. Turunc, Y. Z. Demiroglu, I. Erol, and D. Yalcintas. 2006. Epidemiologic, clinical, and imaging findings in brucellosis patients with osteoarticular involvement. Am. J. Roentgenol. 187873-880. [DOI] [PubMed] [Google Scholar]
- 39.Rittig, M. G., A. Kaufmann, A. Robins, B. Shaw, H. Sprenger, D. Gemsa, V. Foulongne, B. Rouot, and J. Dornand. 2003. Smooth and rough lipopolysaccharide phenotypes of Brucella induce different intracellular trafficking and cytokine/chemokine release in human monocytes. J. Leukoc. Biol. 741045-1055. [DOI] [PubMed] [Google Scholar]
- 40.Saez-Llorens, X., M. M. Mustafa, O. Ramilo, C. Fink, B. Beutler, and J. T. Nelston. 1990. Tumor necrosis factor alpha and interleukin 1 beta in synovial fluid of infants and children with suppurative arthritis. Am. J. Dis. Child. 144353-356. [DOI] [PubMed] [Google Scholar]
- 41.Shirtliff, M. E., and J. T. Mader. 2002. Acute septic arthritis. Clin. Microbiol. Rev. 15527-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith, D. M., G. A. Lackides, and L. B. Epstein. 1990. Coordinated induction of autocrine tumor necrosis factor and interleukin 1 in normal human monocytes and the implications for monocyte-mediated cytotoxicity. Cancer Res. 503146-3153. [PubMed] [Google Scholar]
- 43.Tarkowski, A., M. Bokarewa, L. V. Collins, I. Gjertsson, O. H. Hultgren, T. Jin, I. M. Jonsson, E. Josefsson, E. Sakiniene, and M. Verdrengh. 2002. Current status of pathogenetic mechanisms in staphylococcal arthritis. FEMS Microbiol. Lett. 217125-132. [DOI] [PubMed] [Google Scholar]
- 44.Verdrengh, M., H. Carlsten, C. Ohlsson, and A. Tarkowski. 2006. Rapid systemic bone resorption during the course of Staphylococcus aureus-induced arthritis. J. Infect. Dis. 1941597-1600. [DOI] [PubMed] [Google Scholar]
- 45.Wagner, C., K. Kondella, T. Bernschneider, V. Heppert, A. Wentzensen, and G. M. Hänsch. 2003. Post-traumatic osteomyelitis: analysis of inflammatory cells recruited into the site of infection. Shock 20503-510. [DOI] [PubMed] [Google Scholar]
- 46.Webb, L. X., W. Wagner, D. Carroll, H. Tyler, F. Coldren, and E. Martin. 2007. Osteomyelitis and intraosteoblastic Staphylococcus aureus. J. Surg. Orthop. Adv. 1673-78. [PubMed] [Google Scholar]
- 47.Wright, K. M., and J. S. Friedland. 2002. Differential regulation of chemokine secretion in tuberculous and staphylococcal osteomyelitis. J. Bone Min. Res. 171680-1690. [DOI] [PubMed] [Google Scholar]
- 48.Wright, K. M., and J. S. Friedland. 2004. Regulation of monocyte chemokine and MMP-9 secretion by proinflammatory cytokines in tuberculous osteomyelitis. J. Leukoc. Biol. 751086-1092. [DOI] [PubMed] [Google Scholar]
- 49.Yang, L. C., F. M. Huang, C. S. Lin, C. M. Liu, C. C. Lai, and Y. C. Chang. 2003. Induction of interleukin-8 gene expression by black-pigmented Bacteroides in human pulp fibroblasts and osteoblasts. Int. Endod. J. 36774-779. [DOI] [PubMed] [Google Scholar]
- 50.Young, E. J. 1989. Clinical manifestations of human brucellosis, p. 97-126. In E. J. Young and M. J. Corbel (ed.), Brucellosis: clinical and laboratory aspects. CRC Press, Boca Raton, FL.