Abstract
We investigated the mechanisms that lead to the production of proinflammatory mediators by human monocytes when these cells are exposed in vitro to live Borrelia burgdorferi spirochetes. We first focused on myeloid differentiation primary response protein 88 (MyD88), an adapter molecule that is essential in the Toll-like receptor (TLR) pathway. Real-time PCR, flow cytometry, and confocal microscopy experiments revealed that MyD88 was maximally expressed in THP-1 cells after 24-h stimulation of these cells with live B. burgdorferi. Silencing of the MYD88 gene by using small interfering RNA resulted in 24%, 35%, and 84% down-modulation of the production of tumor necrosis factor alpha (TNF-α), interleukin-8 (IL-8), and IL-6, respectively, in THP-1 cells stimulated with live B. burgdorferi. Specific silencing of the TLR1, TLR2, or TLR5 gene by RNA interference further revealed that silencing of the TLR1 and TLR2 genes alone or combined, but not the TLR5 gene, caused a downregulation of IL-6, IL-8, and TNF-α in live B. burgdorferi-stimulated THP-1 cells. Overall, similar results were obtained for THP-1 cells stimulated with purified lipoproteins. Our results indicate that the TLR pathway mediates, at least in part, the release of inflammatory mediators in human monocytes stimulated with live B. burgdorferi spirochetes and furthermore suggest that the TLR-dependent interaction between these cells and live spirochetes is mediated by spirochetal lipoproteins but not by flagellin.
Borrelia burgdorferi, the spirochete that causes Lyme disease, is spread to humans and other mammals through the bite of infected Ixodes ticks (8). Invasion of the mammalian host by B. burgdorferi results in the release of inflammatory mediators and the influx of inflammatory cells (23, 25, 26) in multiple organs such as the skin, heart, joints, and central and peripheral nervous systems (15, 20, 46, 55). It is thought that inflammation, induced either by the spirochete or by the spirochetal antigens left in tissues after bacterial demise, plays a major role in disease pathogenesis.
Recognition of microbial pathogens by cells of the innate immune system occurs in part via pattern recognition receptors such as those belonging to the germ line-encoded Toll-like receptor (TLR) family (31). To date, 13 mammalian TLRs (TLR1 to TLR13) have been identified (2, 33, 61). TLR2 recognizes bacterial lipopeptides and lipoproteins in a heterodimeric complex with TLR1 or TLR6 (4, 30, 42, 52, 56, 57); TLR4 recognizes lipopolysaccharide (48); and TLR5 detects bacterial flagellin (27). TLR3, TLR7, and TLR8 are specialized primarily for viral RNA detection (3, 18, 28, 41); TLR9 recognizes unmethylated CpG DNA (29, 59). TLR11 recognizes uropathogenic bacteria and apicomplexan profilins in mice (37, 47, 61), but it is nonfunctional in humans because of the presence of a stop codon in the gene (37, 47). The ligands for TLR10, TLR12, and TLR13 are as yet unknown (58).
The signaling events downstream of TLRs proceed through at least four adapter proteins: myeloid differentiation factor 88 (MyD88), MyD88 adapter-like/Toll interleukin-1 receptor-associated protein (MAL/TIRAP), TLR-associated activator of interferon (TRIF), and TLR-associated molecule (TRAM) (54). Notable among the TLR-mediated activation processes of innate immune cells is the induction of the transcription factor NF-κB, which in turn triggers the expression of many proinflammatory mediators such as cytokines, chemokines, and costimulatory molecules (1, 2, 19, 22, 34, 36, 49). Perhaps the most important TLR-expressing cells are dendritic cells and macrophages. These are major effectors and mediators of innate and acquired immunity and, as such, are crucial players at the host-pathogen interface (31, 45).
Studies addressing the mechanisms of TLR signaling by B. burgdorferi in innate immune cells have focused predominantly on spirochetal surface lipoproteins. These molecules have been shown to play an important role in the inflammatory response to and host defense against spirochetal infection. Lipoproteins of B. burgdorferi interact with TLR2/TLR1 heterodimers (4, 56), resulting in the activation of NF-κB and release of inflammatory mediators (30). Although purified microbial motifs selectively activate TLRs, recent data indicate that the interaction of live organisms with TLR-bearing cells is more complex than initially anticipated, as different components of the same organism can activate several different TLRs, as well as other receptors, and can lead to the activation of multiple signaling cascades and different patterns of gene expression (1, 31). In this regard, it has been shown that B. burgdorferi binds to integrin α3β1 and that binding of the spirochete to this integrin results in the induction of inflammatory cytokines and chemokines in primary human chondrocytes (6).
Elucidating the molecular basis of signaling events caused by live B. burgdorferi spirochetes in innate immune cells is crucial to understanding inflammation in Lyme disease. Thus, in the present study, we used human monocytic THP-1 cells and live B. burgdorferi spirochetes as a model for monocyte-pathogen interactions to mimic the initial phase of the immune response during the course of a spirochetal infection. We focused on the candidates most likely to recognize live B. burgdorferi spirochetes, namely, TLR1, TLR2, and TLR5, and determined whether their involvement was dependent on the well-characterized downstream adapter protein MyD88 for inflammatory mediator production. First, we verified the ability of live B. burgdorferi to activate the expression of the MYD88 gene in THP-1 cells and then correlated this expression level with the production of concomitantly elicited inflammatory mediators. We then used RNA interference (RNAi) to silence specifically the MYD88 gene and evaluated the resulting effect on the production of inflammatory mediators induced by live B. burgdorferi. Next, kinetics studies were conducted to determine the ability of live B. burgdorferi to induce the expression of TLR1 and TLR2 in THP-1 cells. The TLR1, TLR2, and TLR5 genes were silenced to evaluate the contribution of these genes to the production of inflammatory mediators induced by live B. burgdorferi. Finally, for the purpose of comparison, the effect of silencing MYD88, TLR1, and TLR2 expression on induction of inflammatory mediators by purified recombinant lipidated outer surface protein A (L-OspA) was quantified. The results of this study are presented herein.
MATERIALS AND METHODS
Bacteria and lipoprotein.
B. burgdorferi spirochetes (strain B31, clone 5A19, with the complete plasmid content) were grown in vitro in Barbour-Stoenner-Kelly (BSK)-H medium, as previously described (16). Purified recombinant L-OspA protein was kindly provided by GlaxoSmithKline Biologicals (Rixensart, Belgium). The L-OspA preparation contained less than 0.25 endotoxin unit per mg of protein, as assessed by Limulus amoebocyte assay (Associates of Cape Cod Inc., Woods Hole, MA).
Cell stimulation and culture conditions.
The THP-1 monocytic cell line was the same as that described previously (43) and was obtained from the American Type Culture Collection (Manassas, VA). THP-1 cells were pretreated with 0.05 μM 1α,25-dihydroxyvitamin D3 (Calbiochem-Nova Biochem International, La Jolla, CA) for 48 h prior to being used in this study. Cell culture medium consisted of RPMI 1640 medium (Gibco Invitrogen, Carlsbad, CA), 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT), 1 mM HEPES (Gibco Invitrogen), 2 mM l-glutamine (Gibco Invitrogen), and 1 μg/ml antibiotic/antimycotic (complete medium). To determine the kinetics of cytokine production and gene expression, THP-1 cells (3 × 106 cells/ml/well) were cultured in 24-well plates (Costar, Cambridge, MA) with either L-OspA at 1 μg/ml or with live B. burgdorferi spirochetes at a multiplicity of infection (MOI) of 10:1 for 2, 4, 6, and 24 h. Viable spirochetes were incubated with cells in antibiotic-free medium. The organisms remained viable for up to 24 h in culture, as assessed by dark-field microscopy and by their ability to grow successfully upon transfer to BSK-H medium. All stimulated and unstimulated cell cultures were subsequently centrifuged at 400 × g for 10 min at 4°C, and cell-free culture supernatants were collected, aliquoted, and stored at −70°C until they were used. RNA was extracted from the cell pellet using a Qiagen RNeasy kit (Qiagen Inc., Valencia, CA), which included a DNase I digestion step.
RNAi.
A HiPerfect transfection reagent kit (Qiagen) containing positive and nontargeting control small interfering RNAs (siRNAs) was used in all studies. ON-TARGETplus SMARTpool siRNAs targeting human MyD88, TLR1, TLR2, or TLR5 transcripts were obtained from Dharmacon (Chicago, IL). A volume of 100 μl of THP-1 cell suspension (containing 2 × 104 cells) in antibiotic-free medium was seeded in each well of a 24-well plate. Transfection complexes (100 μl) were generated by vortexing and incubating 100 nM siRNA with 3 μl of HiPerfect transfection reagent at room temperature for 10 min in serum and antibiotic-free medium. A volume of 100 μl of the complex was used to transfect each well containing 100 μl of cells. The plates were incubated at 37°C for 6 h, after which 400 μl of fresh antibiotic-free medium was added to each well, and were incubated for an additional 18 h prior to stimulation. Transfected cells were stimulated for 24 h with live B. burgdorferi (MOI of 10) or 1 μg/ml of L-OspA to collect cell-free culture supernatants or RNA. RNA was isolated from cell pellets using an Allprep RNA/protein kit (Qiagen). Supernatants and RNA samples were stored at −70°C until used. All siRNA studies included negative and positive control siRNAs. Transfection complexes were not cytotoxic to THP-1 cells, as assessed by the trypan blue exclusion assay.
Cytokine ELISA.
Cytokine enzyme-linked immunosorbent assay (ELISA) paired antibodies (BD-Pharmingen, San Jose, CA) were employed to detect interleukin-6 (IL-6), IL-8, and tumor necrosis factor alpha (TNF-α) secretion in cell-free culture supernatants of THP-1 cells. The ELISA protocol was described previously (43).
qRT-PCR.
Purified RNA (50 ng) was used as the template in the quantitative real-time PCR (qRT-PCR) mixture according to the manufacturer's standard protocol for QuantiFast SYBR one-step RT-PCR (Qiagen). MAPK1, GAPDH, MyD88, TLR1, TLR2, and TLR5 QuantiTect primers were used (Qiagen), and quantifications using SYBR Green were performed using an ABI model 7700 apparatus (Applied Biosystems, Foster City, CA). The specificity of the PCR was controlled by no-template controls. Specific cDNA was quantified by using standard curves based on known amounts of product. Threshold values were normalized to the expression of GAPDH. qRT-PCR results are expressed as the fold increase in induction (normalized copy number of stimulated cells/normalized copy number of unstimulated cells). The percentage of relative gene expression was calculated as the relative amount of MyD88, TLR1, TLR2, or TLR5 mRNA in cultures transfected with MyD88, TLR1, TLR2, or TLR5 siRNA (and stimulated with live B. burgdorferi) compared to that of cells transfected with the nontargeting control siRNA (and stimulated with live B. burgdorferi), which was set to 100%.
Confocal microscopy.
THP-1 cells (1.0 × 107), either unstimulated or stimulated with live B. burgdorferi, were washed and fixed in a microcentrifuge tube with isotonic 2% paraformaldehyde. Cells were washed, resuspended in 100 mM glycine solution, washed again, and subsequently subjected to immunostaining with rabbit anti-MyD88 primary antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) diluted to 1:50 in a 10% normal goat serum-phosphate-buffered-fish skin gelatin and Triton X-100 solution and a goat anti-rabbit secondary antibody (1:1,000) coupled to either Alexa-488 or Alexa-568 (Invitrogen-Molecular probes). Nuclei were stained with ToPro-3 or BoPro-1 nuclear stain. For some studies, THP-1 cells were stained with fluorescein isothiocyanate-labeled affinity-purified anti-B. burgdorferi antibody (Kirkegaard & Perry, Gaithersburg, MD) or with human anti-CD14 antibody (produced from an ATCC hybridoma) coupled to Alexa-633. Protein expression was visualized by using a Leica True Confocal Laser Scanning Microscope SP2 equipped with three lasers (Leica Microsystems, Exton, PA). MyD88 protein expression levels were semiquantified by measuring the fluorescence intensity for the stained cells, using Image-Pro analysis software (Media Cybernetics, Silver Spring, MD).
Flow cytometry.
The expression of MyD88 and the effect of silencing MYD88 in THP-1 cells (0.5 × 106) after 24 h of stimulation with live B. burgdorferi were examined by flow cytometry using a rabbit anti-MyD88 primary antibody (Santa Cruz Biotechnology), followed by a goat anti-rabbit secondary antibody (1:1,000) coupled to Alexa-488 (Invitrogen-Molecular probes). As MyD88 is known to be recruited to the cell surface for colocalization with TLRs (35), both surface and intracellular staining assays were performed using a Cytofix/Cytoperm kit (BD-Pharmingen). Stimulated cells were also stained with secondary antibody alone. Unstimulated control cell cultures (medium) were kept as background controls. All samples were fixed with 1% paraformaldehyde prior to data acquisition. Data acquisition was performed using a FACScalibur flow cytometer and Cell Quest software (Becton Dickinson Immunocytometry Systems, Mountain View, CA). For each sample, a minimum of 1 × 105 cells were analyzed with FlowJo software version 8.6.3 (Tree Star Inc., Ashland, OR) by gating the cluster of cells based on forward and side scatters. All gated cells were further analyzed for their expression of MyD88, and the mean fluorescent intensities were determined.
Statistical analysis.
All data were analyzed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA), applying a one-way analysis of variance nonparametric test with Tukey's multiple comparison test. Differences were considered significant if the probability that they occurred by chance was less than 5%. (P < 0.05).
RESULTS
Live B. burgdorferi bacteria activate MyD88 expression in THP-1 cells.
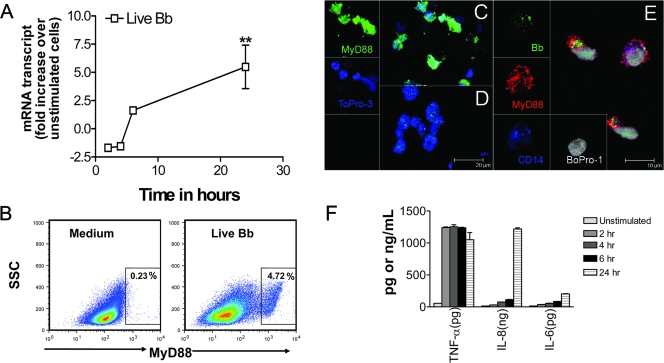
To determine whether or not live B. burgdorferi bacteria activate components of the TLR pathway, we first focused on MyD88, an adapter protein that serves as a bridge from most TLRs to the downstream signaling elements, resulting in the activation of NF-κB and the production of inflammatory mediators. We used qRT-PCR to determine the level of MyD88 mRNA expression in THP-1 cells as a function of time at 2, 4, 6, and 24 h after stimulation of these cells with live B. burgdorferi (MOI of 10). The highest level of MyD88 expression (P < 0.01) was observed after 24 h of stimulation (Fig. 1A). MyD88 expression was also evaluated at the protein level by flow cytometry, showing that the percentage of MyD88-dependent positive cells increased upon live B. burgdorferi stimulation for 24 h at an MOI of 10 (Fig. 1B). This was also evident qualitatively, as assessed by confocal microscopy (Fig. 1C to E). Thus, the MyD88 expression level was upregulated in cells stimulated with live B. burgdorferi (Fig. 1C) compared to that in unstimulated cells (Fig. 1D). The use of CD14, a surface receptor that has been shown to mediate phagocytosis of both gram-negative bacteria and apoptotic cells (17, 24, 50), revealed a partial overlap between the live B. burgdorferi signal and that of CD14 (Fig. 1E). This suggests that spirochetes are internalized or, alternatively, are colocalized with CD14 and MyD88 at the cell surface. MyD88 is known to be recruited to the cell surface for colocalization with TLRs (35).
FIG. 1.
Live B. burgdorferi spirochetes (MOI of 10) activate MyD88 expression in THP-1 cells. Vitamin D3-treated THP-1 cells (3 × 106 cells/ml) were incubated in supplemented antibiotic-free RPMI medium alone or with live B. burgdorferi (Bb). (A) RNA samples were collected after 2, 4, 6, and 24 h of incubation, and the MYD88 transcripts were quantified by qRT-PCR. All values were normalized with respect to the GAPDH “housekeeping” gene mRNA levels. Results are presented as the fold increase over control (the level in unstimulated cells). Each line symbol represents the means ± standard deviations (SDs) of three independent experiments. Asterisks indicate significant upregulation (P < 0.01). (B) THP-1 cells (0.5 × 106) incubated with live B. burgdorferi for 24 h and then fixed and stained with anti-MyD88 antibody and Alexa-488-labeled secondary antibody and subjected to flow cytometric analysis revealed upregulation of MyD88 expression in cells compared with that in the medium control cells. Numbers in the insets are percentages of MyD88-positive cells. (C and D) After 24 h of stimulation with live B. burgdorferi, cells were fixed and stained with anti-MyD88 antibody and Alexa-488-labeled secondary antibody (green), and nuclei were counterstained with ToPro3 (blue). Both stimulated (C) and unstimulated (D) cells were visualized by confocal fluorescence microscopy. (E) Cells were fixed and triple stained with anti-MyD88 antibody and Alexa-568-labeled secondary antibody (red), fluorescein isothiocyanate-labeled anti-B. burgdorferi antibody (green), and anti-CD14 antibody and Alexa-633-labeled secondary antibody (blue); nuclei were counterstained with BoPro-1 (gray) and were visualized by confocal fluorescence microscopy. (F) Cell-free supernatants were harvested from the culture medium at 2, 4, 6, and 24 h and analyzed by antibody capture ELISA for TNF-α, IL-8, and IL-6 production. The lower limit of detection of the ELISA was 15 pg/ml. Each bar represents the means ± SDs of three independent experiments.
We next correlated the level of expression of MyD88 in monocytes with inflammatory mediator production by measuring the concentrations of TNF-α, IL-6, and IL-8 in the cell-free culture supernatants as a function of time after stimulation with live B. burgdorferi (Fig. 1F). At all time points, all of the tested cytokines were below detection limits in the absence of live B. burgdorferi. In cultures in which live B. burgdorferi was added, TNF-α production was observed at 2 h poststimulation and remained significantly elevated thereafter (P < 0.001). In contrast, IL-6 and IL-8 levels gradually increased (P values of <0.05 to <0.001) from 2 to 24 h poststimulation. The expression level of MyD88 was also elevated at 24 h in THP-1 cells stimulated with L-OspA (12-fold increase) and, as with the live B. burgdorferi, a similar pattern of cytokine production by L-OspA-stimulated THP-1 cells was observed (data not shown). Our results indicate that live B. burgdorferi can activate MyD88 expression in human monocytes. MyD88 expression as induced by live B. burgdorferi or by L-OspA in THP-1 cells correlated with the enhanced production of TNF-α, IL-6, and IL-8 proteins.
MyD88 is involved in the induction of inflammatory mediators by THP-1 cells stimulated with live B. burgdorferi.
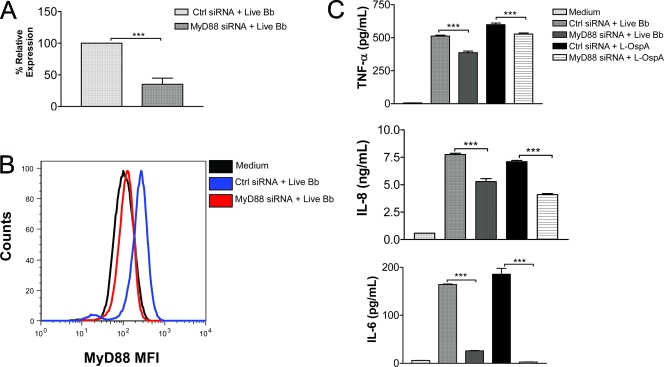
To assess the direct involvement of MyD88 in mediating the production of inflammatory mediators by live B. burgdorferi, we used RNAi to inhibit the expression of MyD88 in THP-1 cells. Transfection of THP-1 cells with MyD88-specific siRNA significantly reduced the expression of the MYD88 gene transcript that was induced in these cells by live B. burgdorferi (P < 0.001) (Fig. 2A). Expression of the MyD88 protein was also diminished (Fig. 2B). The mean fluorescence intensity due to MyD88 expression in control siRNA-transfected cells stimulated with live B. burgdorferi increased by 2.23-fold with respect to that of unstimulated cells but increased only by 1.10-fold in cells transfected with MyD88 siRNA, further validating the efficiency of the MYD88 knockdown in cells (Fig. 2B).
FIG. 2.
MyD88 siRNA transfection of THP-1 cells inhibits the production of inflammatory mediators elicited in response to stimulation with live B. burgdorferi. Vitamin D3-treated THP-1 cells (0.5 × 106) were transfected with 100 nM nontargeting control siRNA or with 100 nM of MyD88 siRNA and cultured in medium alone for 24 h. They were then stimulated with live B. burgdorferi (MOI of 10) for an additional 24 h. (A) RNA was extracted from transfected cells and analyzed by qRT-PCR to assess MYD88 expression in relation to that of GAPDH as the housekeeping gene. The percentage of relative expression was calculated as the amount of MyD88 mRNA in cultures transfected with MyD88 siRNA and stimulated with live B. burgdorferi compared to that of cells transfected with nontargeting control siRNA and stimulated with live B. burgdorferi. Results are the means ± standard deviations (SDs) of three independent experiments. (B) Cells transfected as described above were analyzed by flow cytometry to assess the silencing of MyD88 expression at the protein level. The mean fluorescence intensity of MyD88 siRNA-transfected cells was markedly decreased compared with that of medium or control siRNA-transfected cells. (C) Cell-free culture supernatants were harvested from transfected cells after 24 h of stimulation with live B. burgdorferi (MOI of 10) or with L-OspA of B. burgdorferi at 1 μg/ml. Supernatants were analyzed by antibody capture ELISA for TNF-α, IL-8, and IL-6 production. The lower limit of detection of the ELISA was 15 pg/ml. Each bar represents the means ± SDs of three independent experiments. Asterisks indicate significant differences (P < 0.001) in the production of inflammatory mediators in cells transfected with MyD88 siRNA compared to that of the control siRNA-transfected cells.
After transfection for 24 h, as described above, with either control siRNA or MyD88 siRNA and stimulation of cells with live B. burgdorferi, cell culture supernatants were collected at 24 h poststimulation to determine the levels of production of TNF-α, IL-6, and IL-8. Unstimulated cells did not produce significant levels of any of these mediators. Silencing of MYD88 led to significant inhibition of TNF-α, IL-8, and IL-6 secretion (P < 0.001) induced by live B. burgdorferi in THP-1 cells, namely, by 24%, 35%, and 84%, respectively, compared to that in control siRNA-transfected cells stimulated with live B. burgdorferi (Fig. 2C). MYD88-silenced cells stimulated with L-OspA showed a similar pattern of inhibition of these inflammatory mediators (Fig. 2C). Our results indicate that recognition of live B. burgdorferi by human monocytes and the downstream secretion of TNF-α, IL-8, and IL-6 inflammatory mediators require, in part, the MyD88 adapter molecule. The data further suggest that the TLR pathway is involved in the induction of inflammatory mediators by live B. burgdorferi in THP-1 cells in a MyD88-dependent fashion.
Transfection of THP-1 cells with TLR1 and TLR2 siRNAs reduces the production of inflammatory mediators in response to live B. burgdorferi.
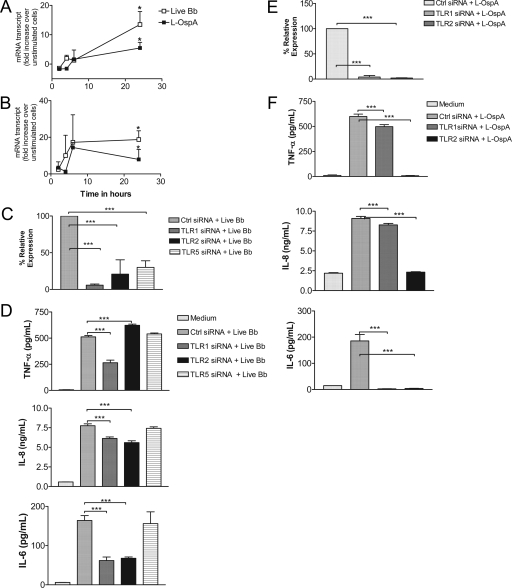
Because MyD88 is essential to the TLR signaling pathway, we investigated which TLR is recognized by live B. burgdorferi for the induction of inflammatory mediators by THP-1 cells. We focused initially on TLR1 and TLR2, the prototype TLRs used by lipoproteins of B. burgdorferi. We employed qRT-PCR to determine the expression levels of the TLR1 and TLR2 gene transcripts in THP-1 cells stimulated with live B. burgdorferi. In addition, we used L-OspA, a known ligand of TLR1 and TLR2, as a positive control for a direct comparison. We observed that expression of both TLR1 and TLR2 was significantly upregulated after 24 h of stimulation with live B. burgdorferi (P < 0.01); transcript activation kinetics were comparable to those induced by L-OspA (Fig. 3A and B).
FIG. 3.
TLR1 or TLR2 siRNA transfection of THP-1 cells affects the production of inflammatory mediators in response to stimulation with live B. burgdorferi. Vitamin D3-treated THP-1 cells (3 × 106 cells/ml) were incubated with supplemented antibiotic-free RPMI medium alone or with live B. burgdorferi at an MOI of 10 or with L-OspA at 1 μg/ml in complete medium. RNA samples were collected after 2, 4, 6, and 24 h of incubation and qRT-PCR was performed for TLR1 (A) and TLR2 (B) mRNA transcript levels. Results are presented as the increase over control (the level in unstimulated cells). Each data point represents the mean ± standard deviation (SD) of two independent experiments. (C) Cells were transfected and analyzed for TLR1, TLR2, and TLR5 knockdown as described in the legend to Fig. 2A. (D) Supernatants were analyzed by antibody capture ELISA for TNF-α, IL-8, and IL-6 production as described in the legend to Fig. 2C. (E) Cells were transfected with nontargeting control siRNA or with TLR1 or TLR2 siRNA and cultured in complete medium alone for 24 h. They were then stimulated with L-OspA (1 μg/ml) for an additional 24 h, and RNA was extracted from transfected cells and used in qRT-PCR to assess TLR1 and TLR2 knockdown as described in the legend to Fig. 2A. Results are the means ± SDs of three independent experiments. (F) Supernatants were analyzed by antibody capture ELISA for TNF-α, IL-8, and IL-6 production as described in the legend to Fig. 2C.
To determine if TLR1 and TLR2 are mediators of live-B. burgdorferi signaling in THP-1 cells, we inhibited the expression of the TLR1 and TLR2 gene transcripts with their specific siRNAs (Fig. 3C). In addition, and because whole bacteria contain agonists for multiple TLRs, we also silenced the expression of TLR5 (Fig. 3C). The relative expression of the three gene transcripts was significantly reduced by this procedure (P < 0.001). Silencing of TLR1 caused a significant downregulation of TNF-α in cells stimulated with live B. burgdorferi compared to that of the control siRNA. Surprisingly, production of TNF-α was increased significantly (P < 0.0001) when TLR2 was silenced (Fig. 3D). On the other hand, silencing of either TLR1 or TLR2 resulted in a significant downregulation of both IL-8 and IL-6 (P < 0.001) compared with their production levels induced in control cells (Fig. 3D). The TLR1 TLR2 double knockdown yielded results similar to those obtained when these genes were knocked down individually (data not shown).
The silencing of TLR5 had no effect on the production of TNF-α, IL-8, or IL-6 by these cells upon stimulation with live B. burgdorferi (Fig. 3D). Because lipoproteins of B. burgdorferi are known to signal through TLR1 TLR2 heterodimers, we also individually silenced both receptors in experiments in which L-OspA was used as the stimulant (Fig. 3E). Silencing of either TLR1 or TLR2 in L-OspA-stimulated THP-1 cells significantly affected the protein expression levels of TNF-α, IL-8, and IL-6 (P < 0.001) (Fig. 3F). The downregulatory effect was more pronounced in TLR2-transfected cell cultures.
DISCUSSION
Substantial efforts have been made to understand the molecular mechanism by which the lipoproteins of B. burgdorferi elicit the production of inflammatory mediators in cells of the human innate immune system. In contrast, much has yet to be learned about how live B. burgdorferi participates in this process. Here, we investigated the molecular mechanisms that lead to the production of proinflammatory mediators by human monocytes when these cells are exposed in vitro to live B. burgdorferi. We showed in kinetics studies that live B. burgdorferi upregulates the expression of components of the TLR pathway, such as MyD88, TLR1, and TLR2. We further demonstrated that MYD88, TLR1, and TLR2, but not TLR5, transcript knockdown significantly reduced the production of proinflammatory mediators by human monocytes in response to live B. burgdorferi stimulation. THP-1 cells stimulated with lipoproteins yielded similar results but with some interesting differences that we discuss below.
Our results indicate that live B. burgdorferi activates MYD88 transcription and translation in human monocytes. Using RNAi, we showed that specific silencing of the MYD88 gene significantly downmodulates the production of TNF-α, IL-6, and IL-8, suggesting that live B. burgdorferi stimulation of monocytes for the production of these inflammatory mediators is MyD88 dependent. MyD88 is an adapter molecule that plays a critical role in the downstream signaling of several TLRs in innate immune cells. In vitro, studies using cells from MyD88-deficient mice have shown that MyD88 is an important mediator of inflammatory signaling in several bacterial diseases (38, 40, 53). Studies conducted by Shin and coworkers (53) elegantly demonstrated that MyD88 was necessary for the internalization and phagocytosis of live B. burgdorferi and for the production of several inflammatory mediators in mouse bone marrow-derived macrophages. To our knowledge, our study is the first to demonstrate a MyD88-mediated innate immune signaling pathway activated by live B. burgdorferi in human monocytes. Our findings and those of Shin et al. (53) together ascribe a critical role to MyD88 during the initial phase of B. burgdorferi infection.
Silencing of the TLR5 gene revealed that flagellin, the TLR5 ligand, is not involved in the monocyte response to live B. burgdorferi, as measured by the production of TNF-α, IL-6, and IL-8. Our finding, however, contrasts with that of Shin et al., (53), who observed that the knockdown of the TLR5 gene resulted in diminished expression of cytokine (TNF-α, IL-6, and IL-β) but not chemokine (MCP-1 and CXCL-2) transcripts in murine bone marrow macrophages. Differences recently shown between TLR signaling of mouse and human macrophages (10, 44) may explain this discrepancy.
Evaluation of the roles of TLR1 and TLR2 as mediators of inflammation in Lyme disease has been limited in most instances to examining the stimulation of innate immune cells with B. burgdorferi lipoproteins or lysates (4, 9, 30, 32, 51, 53, 60). Our study is one of the few to address the roles of both TLR1 and TLR2 in inflammatory mediator production by human monocytes stimulated with live organisms. Another study (21) focused only on TLR2 and showed that TLR2 was essential in mediating the production of matrix metalloproteinases from human monocytes in response to live spirochete stimulation.
While the inhibition of TLR1 and TLR2 transcription by the corresponding siRNAs under conditions of live B. burgdorferi stimulation was 94 and 79%, respectively (Fig. 3C), cytokine/chemokine production was less inhibited. Thus, IL-6 production was decreased by 63% upon TLR1 silencing and by 60% upon silencing of TLR2, whereas IL-8 production was reduced by only 21% (TLR1) and 28% (TLR2), respectively. TNF-α behaved differently, in that its production was 48% inhibited by the silencing of TLR1 but was enhanced by 21% upon TLR2 knockdown. These results indicate that alternative receptors and pathways are involved in mediating inflammation as induced by live B. burgdorferi in human monocytes. In mice, Lyme disease inflammation can proceed via a TLR-independent pathway(s), since ablation of TLR or MyD88 signaling failed to decrease the influx of inflammatory cells or inflammation in response to B. burgdorferi infection (6, 7, 39). To this end, B. burgdorferi has been shown to bind to various integrin receptors such as αIIbβ3, αvβ3, α3β1 (11, 12, 13, 14), and α3β1 (6) on host cells, suggesting the importance of these molecules in the non-TLR-dependent pathogenesis of Lyme disease. In fact, a recent study uncovered the α3β1 integrin as a receptor utilized by live spirochetes, specifically the BBB07 protein of B. burgdorferi, for the induction of matrix metalloproteinases and several inflammatory mediators in human chondrocytes (5, 6). In experiments in vivo, those same investigators also showed significant production of several chemokines and cytokines in the joints of MYD88 knockout mice, thus validating the occurrence of inflammation in a MyD88-independent manner (6).
The probable utilization of receptors other than TLR1 and TLR2 by live B. burgdorferi in its interaction with human monocytes is further substantiated by the results obtained with L-OspA. For example, while the silencing of TLR2 led to the complete inhibition of the production of TNF-α by THP-1 cells that were stimulated with L-OspA (Fig. 3F), it resulted, as mentioned above, in an increase in the production of this cytokine upon stimulation with live B. burgdorferi. The increase in TNF-α production in TLR2-silenced cells stimulated with live B. burgdorferi and not in those stimulated with L-OspA would indicate the utilization of a TLR-independent pathway by nonlipoproteins of B. burgdorferi for the induction of TNF-α. Studies by Behera et al. (6) may help to corroborate our findings, as those investigators demonstrated via siRNA technology that the integrin α3 mediated the B. burgdorferi-induced expression of several inflammatory mediators, including TNF-α in human chondrocytes. Our present study provides additional evidence that the induction of inflammation in Lyme disease occurs via both TLR-dependent and -independent pathways (5, 6, 7, 39).
The other two mediators whose production we evaluated in this study, IL-8 and IL-6, responded to L-OspA stimulation upon TLR1 and TLR2 silencing with a trend that was comparable to that shown in response to live B. burgdorferi. In addition, silencing of MYD88 resulted in a similar response pattern of THP-1 cells when these cells were stimulated with either live B. burgdorferi or L-OspA. However, the inhibition of mediator production upon TLR2 silencing was complete after stimulation with L-OspA, whereas it was partial when THP-1 cells were stimulated with live B. burgdorferi. Thus, it is possible that B. burgdorferi lipoproteins, as well as other surface proteins such as the recently identified BBB07 protein of B. burgdorferi (5), are involved in the interaction of human monocytes with live spirochetes. In conclusion, our results indicate that the TLR pathway mediates, at least in part, the release of inflammatory mediators in human monocytes stimulated with live B. burgdorferi spirochetes and further suggest that the TLR-dependent interaction between these cells and spirochetes is mediated by spirochetal lipoproteins but not by flagellin.
Acknowledgments
This work was supported by grant UO1-CI000153 from the Centers for Disease Control and Prevention and by grants NS048952, RR00164, G20 R018397-01, and G20 R019607-01 from the National Institutes of Health.
We thank Avery MacLean for excellent secretarial help. We also thank Robin Rodriguez for help with confocal microscopy images.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 12 January 2009.
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4499-511. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2675-680. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413732-738. [DOI] [PubMed] [Google Scholar]
- 4.Alexopoulou, L., V. Thomas, M. Schnare, Y. Lobet, J. Anguita, R. T. Schoen, R. Medzhitov, E. Fikrig, and R. A. Flavell. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8878-884. [DOI] [PubMed] [Google Scholar]
- 5.Behera, A. K., E. Durand, C. Cugini, S. Antonara, L. Bourassa, E. Hildebrand, L. T. Hu, and J. Coburn. 2008. Borrelia burgdorferi BBB07 interaction with integrin α3β1 stimulates production of pro-inflammatory mediators in primary human chondrocytes. Cell Microbiol. 10320-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behera, A. K., E. Hildebrand, S. Uematsu, S. Akira, J. Coburn, and L. T. Hu. 2006. Identification of a TLR-independent pathway for Borrelia burgdorferi-induced expression of matrix metalloproteinases and inflammatory mediators through binding to integrin α3β1. J. Immunol. 177657-664. [DOI] [PubMed] [Google Scholar]
- 7.Bolz, D. D., R. S. Sundsbak, Y. Ma, S. Akira, C. J. Kirschning, J. F. Zachary, J. H. Weis, and J. J. Weis. 2004. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J. Immunol. 1732003-2010. [DOI] [PubMed] [Google Scholar]
- 8.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease—a tick-borne spirochetosis? Science 2161317-1319. [DOI] [PubMed] [Google Scholar]
- 9.Cassiani-Ingoni, R., E. S. Cabral, J. D. Lunemann, Z. Garza, T. Magnus, H. Gelderblom, P. J. Munson, A. Marques, and R. Martin. 2006. Borrelia burgdorferi induces TLR1 and TLR2 in human microglia and peripheral blood monocytes but differentially regulates HLA-class II expression. J. Neuropathol. Exp. Neurol. 65540-548. [DOI] [PubMed] [Google Scholar]
- 10.Chang, S., A. Dolganiuc, and G. Szabo. 2007. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J. Leukoc. Biol. 82479-487. [DOI] [PubMed] [Google Scholar]
- 11.Coburn, J., S. W. Barthold, and J. M. Leong. 1994. Diverse Lyme disease spirochetes bind integrin αIIbβ3 on human platelets. Infect. Immun. 625559-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coburn, J., and C. Cugini. 2003. Targeted mutation of the outer membrane protein P66 disrupts attachment of the Lyme disease agent, Borrelia burgdorferi, to integrin αvβ3. Proc. Natl. Acad. Sci. USA 1007301-7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coburn, J., J. M. Leong, and J. K. Erban. 1993. Integrin αIIbβ3 mediates binding of the Lyme disease agent Borrelia burgdorferi to human platelets. Proc. Natl. Acad. Sci. USA 907059-7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coburn, J., L. Magoun, S. C. Bodary, and J. M. Leong. 1998. Integrins αvβ3 and α5β1 mediate attachment of Lyme disease spirochetes to human cells. Infect. Immun. 661946-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coyle, P. K. 1993. Lyme disease. Mosby-Year Book, St. Louis, MO.
- 16.Dennis, V. A., A. Jefferson, S. R. Singh, F. Ganapamo, and M. T. Philipp. 2006. Interleukin-10 anti-inflammatory response to Borrelia burgdorferi, the agent of Lyme disease: a possible role for suppressors of cytokine signaling 1 and 3. Infect. Immun. 745780-5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devitt, A., O. D. Moffatt, C. Raykundalia, J. D. Capra, D. L. Simmons, and C. D. Gregory. 1998. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature 392505-509. [DOI] [PubMed] [Google Scholar]
- 18.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 3031529-1531. [DOI] [PubMed] [Google Scholar]
- 19.Dunne, A., and L. A. O'Neill. 2003. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci. STKE 2003re3. [DOI] [PubMed] [Google Scholar]
- 20.England, J. D., R. P. Bohm, Jr., E. D. Roberts, and M. T. Philipp. 1997. Mononeuropathy multiplex in rhesus monkeys with chronic Lyme disease. Ann. Neurol. 41375-384. [DOI] [PubMed] [Google Scholar]
- 21.Gebbia, J. A., J. L. Coleman, and J. L. Benach. 2004. Selective induction of matrix metalloproteinases by Borrelia burgdorferi via toll-like receptor 2 in monocytes. J. Infect. Dis. 189113-119. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16225-260. [DOI] [PubMed] [Google Scholar]
- 23.Glickstein, L., B. Moore, T. Bledsoe, N. Damle, V. Sikand, and A. C. Steere. 2003. Inflammatory cytokine production predominates in early Lyme disease in patients with erythema migrans. Infect. Immun. 716051-6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grunwald, U., X. Fan, R. S. Jack, G. Workalemahu, A. Kallies, F. Stelter, and C. Schutt. 1996. Monocytes can phagocytose Gram-negative bacteria by a CD14-dependent mechanism. J. Immunol. 1574119-4125. [PubMed] [Google Scholar]
- 25.Grygorczuk, S., S. Pancewicz, J. Zajkowska, M. Kondrusik, R. Rwierzbinska, and T. Hermanowska-Szpakowicz. 2004. Concentrations of macrophage inflammatory proteins MIP-1α and MIP-1β and interleukin 8 (IL-8) in lyme borreliosis. Infection 32350-355. [DOI] [PubMed] [Google Scholar]
- 26.Guerau-de-Arellano, M., J. Alroy, and B. T. Huber. 2005. β2 Integrins control the severity of murine Lyme carditis. Infect. Immun. 733242-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 4101099-1103. [DOI] [PubMed] [Google Scholar]
- 28.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 3031526-1529. [DOI] [PubMed] [Google Scholar]
- 29.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408740-745. [DOI] [PubMed] [Google Scholar]
- 30.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol. 1632382-2386. [PubMed] [Google Scholar]
- 31.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5987-995. [DOI] [PubMed] [Google Scholar]
- 32.Izadi, H., A. T. Motameni, T. C. Bates, E. R. Olivera, V. Villar-Suarez, I. Joshi, R. Garg, B. A. Osborne, R. J. Davis, M. Rincon, and J. Anguita. 2007. c-Jun N-terminal kinase 1 is required for Toll-like receptor 1 gene expression in macrophages. Infect. Immun. 755027-5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jared, C. R., G. Gustavo, R. Lee, K. Amardeep, K. P. Maureen, D. S. Kelly, E. H. Leroy, and A. Alan. 2005. The evolution of vertebrate toll-like receptors. Proc. Natl. Acad. Sci. USA 1029577-9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang, Z., M. Zamanian-Daryoush, H. Nie, A. M. Silva, B. R. Williams, and X. Li. 2003. Poly(I-C)-induced Toll-like receptor 3 (TLR3)-mediated activation of NF-κB and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6-TAK1-TAB2-PKR. J. Biol. Chem. 27816713-16719. [DOI] [PubMed] [Google Scholar]
- 35.Kagan, J. C., and R. Medzhitov. 2006. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell 125943-955. [DOI] [PubMed] [Google Scholar]
- 36.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18621-663. [DOI] [PubMed] [Google Scholar]
- 37.Lauw, F. N., D. R. Caffrey, and D. T. Golenbock. 2005. Of mice and man: TLR11 (finally) finds profilin. Trends Immunol. 26509-511. [DOI] [PubMed] [Google Scholar]
- 38.Lehnardt, S., P. Henneke, E. Lien, D. L. Kasper, J. J. Volpe, I. Bechmann, R. Nitsch, J. R. Weber, D. T. Golenbock, and T. Vartanian. 2006. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J. Immunol. 177583-592. [DOI] [PubMed] [Google Scholar]
- 39.Liu, N., R. R. Montgomery, S. W. Barthold, and L. K. Bockenstedt. 2004. Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi-infected mice. Infect. Immun. 723195-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macedo, G. C., D. M. Magnani, N. B. Carvalho, O. Bruna-Romero, R. T. Gazzinelli, and S. C. Oliveira. 2008. Central role of MyD88-dependent dendritic cell maturation and proinflammatory cytokine production to control Brucella abortus infection. J. Immunol. 1801080-1087. [DOI] [PubMed] [Google Scholar]
- 41.Mogensen, T. H., and S. R. Paludan. 2005. Reading the viral signature by Toll-like receptors and other pattern recognition receptors. J. Mol. Med. 83180-192. [DOI] [PubMed] [Google Scholar]
- 42.Moore, L. J., A. M. Gilbey, C. G. Dowson, A. C. Pridmore, S. K. Dower, and R. C. Read. 2007. Proinflammatory activation of Toll-like receptor-2 during exposure of penicillin-resistant Streptococcus pneumoniae to beta-lactam antibiotics. J. Antimicrob. Chemother. 5935-42. [DOI] [PubMed] [Google Scholar]
- 43.Murthy, P. K., V. A. Dennis, B. L. Lasater, and M. T. Philipp. 2000. Interleukin-10 modulates proinflammatory cytokines in the human monocytic cell line THP-1 stimulated with Borrelia burgdorferi lipoproteins. Infect. Immun. 686663-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nahori, M. A., E. Fournie-Amazouz, N. S. Que-Gewirth, V. Balloy, M. Chignard, C. R. Raetz, I. Saint Girons, and C. Werts. 2005. Differential TLR recognition of leptospiral lipid A and lipopolysaccharide in murine and human cells. J. Immunol. 1756022-6031. [DOI] [PubMed] [Google Scholar]
- 45.Pasare, C., and R. Medzhitov. 2003. Toll-like receptors: balancing host resistance with immune tolerance. Curr. Opin. Immunol. 15677-682. [DOI] [PubMed] [Google Scholar]
- 46.Philipp, M. T., and B. J. Johnson. 1994. Animal models of Lyme disease: pathogenesis and immunoprophylaxis. Trends Microbiol. 2431-437. [DOI] [PubMed] [Google Scholar]
- 47.Plattner, F., F. Yarovinsky, S. Romero, D. Didry, M. F Carlier, A. Sher, and D. Soldati-Favre. 2008. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe 377-87. [DOI] [PubMed] [Google Scholar]
- 48.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 2822085-2088. [DOI] [PubMed] [Google Scholar]
- 49.Sato, S., M. Sugiyama, M. Yamamoto, Y. Watanabe, T. Kawai, K. Takeda, and S. Akira. 2003. Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-κB and IFN-regulatory factor-3, in the Toll-like receptor signaling. J. Immunol. 1714304-4310. [DOI] [PubMed] [Google Scholar]
- 50.Schiff, D. E., L. Kline, K. Soldau, J. D. Lee, J. Pugin, P. S. Tobias, and R. J. Ulevitch. 1997. Phagocytosis of gram-negative bacteria by a unique CD14-dependent mechanism. J. Leukoc. Biol. 62786-794. [DOI] [PubMed] [Google Scholar]
- 51.Schroder, N. W., I. Diterich, A. Zinke, J. Eckert, C. Draing, V. von Baehr, D. Hassler, S. Priem, K. Hahn, K. S. Michelsen, T. Hartung, G. R. Burmester, U. B. Gobel, C. Hermann, and R. R. Schumann. 2005. Heterozygous Arg753Gln polymorphism of human TLR-2 impairs immune activation by Borrelia burgdorferi and protects from late stage Lyme disease. J. Immunol. 1752534-2540. [DOI] [PubMed] [Google Scholar]
- 52.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 27417406-17409. [DOI] [PubMed] [Google Scholar]
- 53.Shin, O. S., R. R. Isberg, S. Akira, S. Uematsu, A. K. Behara, and L. T. Hu. 2008. Distinct roles for MyD88 and Toll-like receptors 2, 5, and 9 in phagocytosis of Borrelia burgdorferi and cytokine induction. Infect. Immun. 762341-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh, S. K., and H. J. Girschick. 2006. Toll-like receptors in Borrelia burgdorferi-induced inflammation. Clin. Microbiol. Infect. 12705-717. [DOI] [PubMed] [Google Scholar]
- 55.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345115-125. [DOI] [PubMed] [Google Scholar]
- 56.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21335-376. [DOI] [PubMed] [Google Scholar]
- 57.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401811-815. [DOI] [PubMed] [Google Scholar]
- 58.van Maren, W. W., J. F. Jacobs, I. J. de Vries, S. Nierkens, and G. J. Adema. 2008. Toll-like receptor signalling on Tregs: to suppress or not to suppress? Immunology 124445-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wagner, H. 2004. The immunobiology of the TLR9 subfamily. Trends Immunol. 25381-386. [DOI] [PubMed] [Google Scholar]
- 60.Wang, X., Y. Ma, A. Yoder, H. Crandall, J. F. Zachary, R. S. Fujinami, J. H. Weis, and J. J. Weis. 2008. T cell infiltration is associated with increased Lyme arthritis in TLR2−/− mice. FEMS Immunol. Med. Microbiol. 52124-133. [DOI] [PubMed] [Google Scholar]
- 61.Zhang, D., G. Zhang, M. S. Hayden, M. B. Greenblatt, C. Bussey, R. A. Flavell, and S. Ghosh. 2004. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 3031522-1526. [DOI] [PubMed] [Google Scholar]