Abstract
Foot and ankle infections are the most common cause of hospitalization among diabetic patients, and Staphylococcus aureus is a major pathogen implicated in these infections. Patients with insulin-resistant (type 2) diabetes are more susceptible to bacterial infections than nondiabetic subjects, but the pathogenesis of these infections is poorly understood. C57BL/6J-Leprdb/Leprdb (hereafter, db/db) mice develop type 2 diabetes due to a recessive, autosomal mutation in the leptin receptor. We established a S. aureus hind paw infection in diabetic db/db and nondiabetic Lepr+/+ (+/+) mice to investigate host factors that predispose diabetic mice to infection. Nondiabetic +/+ mice resolved the S. aureus hind paw infection within 10 days, whereas db/db mice with persistent hyperglycemia developed a chronic infection associated with a high bacterial burden. Diabetic db/db mice showed a more robust neutrophil infiltration to the infection site and higher levels of chemokines in the infected tissue than +/+ mice. Blood from +/+ mice killed S. aureus in vitro, whereas db/db blood was defective in bacterial killing. Compared with peripheral blood neutrophils from +/+ mice, db/db neutrophils demonstrated a diminished respiratory burst when stimulated with S. aureus. However, bone marrow-derived neutrophils from +/+ and db/db mice showed comparable phagocytosis and bactericidal activity. Our results indicate that diabetic db/db mice are more susceptible to staphylococcal infection than their nondiabetic littermates and that persistent hyperglycemia modulates innate immunity in the diabetic host.
Approximately 2 million of the estimated 16 million individuals with diabetes in the United States will develop chronic foot ulcers or infections during the course of their disease (38). An infection is initiated when the skin barrier is breached and bacteria, mostly skin commensals, gain access to the underlying tissues. Although limb-threatening infections are usually polymicrobial, Staphylococcus aureus is a major determinant of these infections (21). S. aureus is the predominant pathogen in non-limb-threatening infections, particularly in patients who have not received antimicrobial therapy (5, 26, 27). The emergence of S. aureus strains resistant to multiple antibiotics has made treatment of staphylococcal infections especially problematic. Methicillin-resistant S. aureus strains have become increasingly prevalent among both nosocomial and community-acquired infections within the United States (22, 36, 43). The prevalence of methicillin-resistant S. aureus is higher among diabetic patients than in the general population (11, 44, 45). Seven S. aureus strains resistant to vancomycin have been isolated in the United States, and four of these strains were isolated from patients with diabetes (42, 50). Complications of type 2 diabetes such as peripheral neuropathy and vasculopathy contribute to delayed wound healing. Although the increased susceptibility of the diabetic host to bacterial infections is well established, the chronicity of these infections is poorly understood. A consistent defect in the humoral or cell-mediated host immune system of diabetic patients has not been demonstrated. However, deficiencies in the host innate immune response are apparent since clinical investigations have indicated that phagocytes from type 2 diabetic patients are in a heightened state of oxidative stress and have impaired bactericidal activity and chemotaxis (6, 15, 41, 48). Clearly, numerous pathophysiologic perturbations contribute to the recurrence of soft tissue and bone infections in the lower extremity of patients with diabetes.
C57BL/6J-Leprdb/Leprdb (hereafter, db/db) mice are a valuable model of type 2 diabetes since they are hyperglycemic and resistant to insulin, and they experience peripheral neuropathy, delayed wound healing, and myocardial disease. In this study we inoculated the hind paws of wild-type Lepr+/+ (+/+) and diabetic db/db mice with S. aureus and evaluated the course of the ensuing infection in each host type, as well as the resultant host innate immune response to infection. Diabetic mice that were ≥4 months of age were more susceptible to staphylococcal infection than age-matched nondiabetic control animals. The db/db mice showed a heightened inflammatory response that was characterized by defects in phagocyte function.
MATERIALS AND METHODS
Mice.
db/db mice develop type 2 diabetes due to a lack of a functional hypothalamic leptin receptor (7, 8). The animals become obese around 3 to 4 weeks of age, elevations of plasma insulin begin at 10 to 14 days of age, and blood sugar levels start to rise at 4 to 8 weeks. Control nondiabetic mice included wild-type (+/+) and Leprdb/+ heterozygous (db/+) littermates. Male mice (8 weeks of age) were purchased from The Jackson Laboratory (Bar Harbor, ME) and were housed in a modified barrier facility under viral antibody-free conditions. The animals were given food and water ad libitum, and blood glucose levels were measured by the glucose oxidase test with glucostrips (Bayer, Elkhart, IN). Animal care and experimental procedures complied with guidelines established by Harvard Medical School.
Mouse model of S. aureus hind paw infection.
S. aureus strain PS80 (streptomycin resistant) is a capsular serotype 8 isolate that has been shown to be virulent in a surgical wound infection model (32), a rat model of abscess formation (46), and a hind paw infection model described previously (39). S. aureus PS80 was cultivated for 24 h at 37°C on Columbia agar supplemented with 2% NaCl. A bacterial suspension was prepared in phosphate-buffered saline (PBS), and the number of CFU/ml was confirmed by quantitative plate counts. Mice were anesthetized subcutaneously with 100 mg of ketamine/kg of body weight and 10 mg/kg xylazine. The left hind paw was cleansed with 70% ethanol, and a syringe with a 29.5-gauge hypodermic needle was used to inject 10 μl of the bacterial suspension into the plantar-proximal aspect of the hind paw. At various time points after inoculation, the mice were euthanized by CO2 asphyxiation, and the hind paws were amputated and defleshed. The excised tendon and muscle tissues were weighed and homogenized in tryptic soy broth with a mechanical homogenizer. The homogenates were serially diluted and plated quantitatively on tryptic soy agar. The lower limit of detection was ∼1.6 log10 CFU/g of tissue.
Histological examination of hind paw tissues.
Infected hind paws were excised and fixed in formaldehyde. The tissues were decalcified, embedded in paraffin, and stained with hematoxylin and eosin. Immunohistochemical staining was performed on tissue sections that were deparaffinized and pretreated with 10 mM sodium citrate buffer (pH 6.0). After tissue samples were rinsed in distilled water, endogenous peroxidase activity was quenched by treatment with Peroxidase Block (Dako, Carpinteria, CA). Mouse neutrophils were detected with rat monoclonal anti-mouse Ly-6G (Gr-1; BD Pharmingen, San Diego, CA) applied at a 1:100 dilution for 1 h. After samples were washed in 50 mM Tris-Cl (pH 7.4), rabbit anti-rat antibody was applied at a 1:750 dilution for 1 h. Slides were washed again and treated with goat anti-rabbit horseradish peroxidase-conjugated antibody (Envision Plus detection kit; Dako). After another washing step, immunoperoxidase staining was developed with a diaminobenzedene chromogen (Dako), and nuclei were counterstained with hematoxylin. A myeloperoxidase (MPO) assay (30) was used to quantify neutrophil accumulation in the hind paw tissues.
Tissue chemokine measurements.
Excised hind paw tissues were homogenized in lysis buffer (1% Nonidet P-40, 500 mM NaCl, 50 mM HEPES, 1% Igepal detergent, pH 7.2 to 7.4) containing a mammalian protease inhibitor cocktail (Sigma, St. Louis, MO). Soluble tissue extracts were stored at −70°C before testing. Concentrations of CXCL1/KC (keratinocyte-derived chemokine) and CXCL2/macrophage inflammatory protein 2 were measured by a sandwich enzyme-linked immunosorbent assay (ELISA) from R&D Systems (Minneapolis, MN). The detection limit of the ELISA was 16 ng/ml, and the results were normalized to the weight of the hind paw tissue samples.
Functional analyses of blood leukocytes.
Heparinized blood was collected by nicking the tail vein of mice. Blood killing assays were performed in polypropylene tubes containing 200 μl of mouse blood and 100 μl of S. aureus to yield a final concentration of 105 CFU/ml. The samples were incubated at 37°C on a rotator, and aliquots were removed for quantitative culture after 0, 60, and 120 min. The data were expressed as percent survival of the initial inoculum. The respiratory burst of the neutrophils was assessed by the oxidation of dihydrorhodamine-123 as described previously (39). Neutrophils were identified and gated on the basis of their forward and side scatter and by staining with phycoerythrin-conjugated anti-mouse Ly-6G (eBioscience, San Diego, CA). The samples were analyzed on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) with CellQuest Pro software.
Functional analyses of bone marrow-derived neutrophils.
Bone marrow-derived neutrophils were isolated as described by Looney et al. (29). Mice were euthanized by CO2 asphyxiation, and their femurs and tibias were flushed with PBS. After hypotonic lysis of erythrocytes, the cell suspension was layered over a 62% Percoll (Sigma) gradient and centrifuged for 30 min at 1,300 × g. The resulting neutrophil pellet was washed in PBS and suspended in minimum essential medium (MEM; Invitrogen, Grand Island, NY) with 1% endotoxin-free bovine serum albumin (BSA). Neutrophils were counted on a hemacytometer, and cell purity was confirmed by Diff Quick staining of cytospin preparations. S. aureus was opsonized with fresh frozen 10% mouse serum for 20 min at 37°C. For phagocytosis assays, 5 × 106 opsonized bacteria were incubated with 5 × 105 neutrophils in 300 μl of MEM-1% BSA for 30 min at 37°C on a rotator. Cytospin preparations of each sample were stained with Diff Quick solution and evaluated under a light microscope. The percentage of 200 neutrophils with cell-associated or internalized S. aureus was estimated, and the average number of bacteria per neutrophil was calculated. For the S. aureus killing assays, 2 × 106 opsonized bacteria were incubated with 2 × 106 neutrophils in 500 μl of MEM-1% BSA at 37°C on a rotator. Aliquots were removed at 0, 30, and 60 min; diluted in ice-cold sterile water with 0.05% saponin; vortexed vigorously; and plated on tryptic soy agar plates.
Statistical analyses.
Data collected from three groups of animals were analyzed by analysis of variance with Bonferroni posthoc analysis. Comparisons between two groups were analyzed by the unpaired Student's t test with Welch's correction.
RESULTS
Susceptibility of diabetic db/db mice to S. aureus infection.
The blood glucose levels in 8- to 11-week-old +/+ wild-type and db/+ heterozygous nondiabetic control mice were 3.9 to 7.3 and 4.7 to 6.3 mmol/liter (71 to 131 and 84 to 113 mg/dl, respectively), respectively. In contrast, db/db mice of the same age had blood glucose levels that ranged from 25.5 mmol/liter to >33.3 mmol/liter (459 to >599 mg/dl). Our initial studies were aimed at evaluating whether mice with type 2 diabetes showed greater susceptibility to S. aureus infection of the hind paw than nondiabetic mice. The animals were challenged with S. aureus strain PS80 at doses ranging from 8 × 104 CFU to 8 × 106 CFU. Most of the nondiabetic +/+, db/+, and diabetic db/db mice inoculated with 8 × 104 CFU of S. aureus resolved the infection within 5 days, as estimated by quantitative cultures of the homogenized hind paw tissue (data not shown). In contrast, animals (three mice/group) challenged with 8 × 106 CFU of S. aureus developed an acute, purulent infection, and the bacterial burden in the infected tissues was uniformly high. The mean log CFU/g of tissue ± standard error of the mean (SEM) was 7.48 ± 0.98, 7.73 ± 0.66, and 7.56 ± 0.60 for the db/db, db/+, and +/+ mice, respectively. At an inoculum of 8 × 105 CFU, the mean log CFU/g of tissue was highest for the diabetic db/db animals (5.57 ± 0.59). The nondiabetic mice had fewer viable S. aureus cells recovered from the tissues (Table 1), although the differences among the groups did not reach statistical significance by analysis of variance (P = 0.0655). An inoculum dose of ∼106 CFU per mouse was considered optimal and chosen for further experiments.
TABLE 1.
Mean bacterial loads in the hind paw tissues of db/db, db/+, and +/+ mice inoculated with ∼106 CFU of S. aureus PS80 and euthanized for quantitative cultures on day 5
| Mouse age (wks) | Bacterial load by genotype (log CFU/g of tissue ± SEM)
|
P valuea | ||
|---|---|---|---|---|
| db/db (n)b | db/+ (n)b | +/+ (n)b | ||
| 8-11 | 5.57 ± 0.59 (5) | 3.19 ± 0.68 (7) | 4.56 ± 0.65 (6) | 0.0655 |
| 20 | 6.71 ± 0.98 (4) | 2.59 ± 0.47 (4) | 3.50 ± 0.23 (4) | 0.0035 |
| 34-35 | 6.68 ± 0.41 (7) | 3.79 ± 0.57 (4) | 4.91 ± 0.56 (9) | 0.0103 |
P values were calculated with one-way analysis of variance. At 20 weeks of age, the bacterial burden of db/db mice was significantly increased compared to that of db/+ (P < 0.05) or +/+ (P < 0.01) mice (Bonferroni multiple comparisons test). The numbers of CFU/g of tissue were not significantly different between db/+ and +/+ mice.
n, number of mice.
Because 8- to 11-week-old db/db mice did not exhibit significantly greater susceptibility to staphylococcal infection than nondiabetic littermates, we considered that animals sustained in a prolonged diabetic state might be more vulnerable to S. aureus infection. Therefore, we housed the mice for periods up to 35 weeks before hind paw challenge with S. aureus. As shown in Table 1, db/db mice 20 weeks of age were more susceptible to S. aureus infection than db/db animals 8 to 11 weeks of age. The mean bacterial burden in the infected hind paws of db/db mice 20 weeks of age was 3 to 4 logs higher than that of age-matched db/+ or +/+ control mice (P = 0.0035). Housing the mice up to 35 weeks did not further enhance the susceptibility of the db/db animals to staphylococcal infection (Table 1), and so mice 16 to 20 weeks of age were utilized for subsequent experiments.
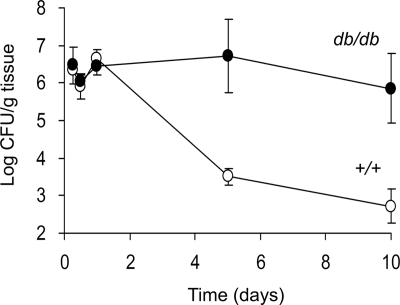
Figure 1 shows the time course of S. aureus infection in the hind paw of groups of 4 to 10 diabetic db/db or +/+ mice 19 to 20 weeks of age. Whereas the tissue bacterial burden peaked 24 h after bacterial challenge in both groups of mice, the wild-type animals showed a greater ability to clear the infection thereafter. The db/db animals had significantly more S. aureus recovered from the infected tissues on both days 5 (P = 0.05) and 10 (P = 0.0168). Furthermore, 6 of 10 +/+ mice had sterile hind paw cultures on day 10 compared with only 1 of 7 db/db mice.
FIG. 1.
Time course of hind paw infection in +/+ and db/db mice challenged with ∼106 CFU of S. aureus PS80. The animals were euthanized at different time points, and the excised tissue was cultured quantitatively. Each point represents the mean ± SEM of cultures performed on groups of 4 to 10 animals. The bacterial burden in the hind paws of db/db mice (black circles) was higher than that of the +/+ mice (white circles) on days 5 (P = 0.05) and 10 (P = 0.0168), as determined by the unpaired Student's t test with Welch's correction for samples with unequal variances.
Inflammatory response to S. aureus in infected hind paws.
Neutrophils play an important role in the initial host responses to bacterial infection. We evaluated whether the impaired clearance of bacteria from the hind paw tissues of db/db animals was associated with an aberrant inflammatory response. The hind paws of +/+ and db/db mice were inoculated with ∼106 CFU of S. aureus, and the animals were euthanized for tissue harvest after 6, 12, 24, or 48 h. An examination of the stained tissue sections revealed that both groups of animals responded to the S. aureus challenge with a robust neutrophil infiltration to the infected tissue. However, the temporal course of the host response indicated that the inflammatory response was more pronounced in the db/db mice 6 and 12 h after bacterial challenge. Twelve hours after bacterial inoculation, the inflammatory response in the infected tissues of diabetic mice was markedly greater than in nondiabetic +/+ mice (Fig. 2A and B), exhibiting an influx of neutrophils (Ly-6G positive) to the infection site (Fig. 2C and D). Neutrophil infiltration was quantified by MPO assays performed on the tissues of mice euthanized at 6, 12, or 24 h. Although neutrophils could be seen in the hind paw tissue as early as 6 h postinfection (data not shown), MPO activity was barely detectable in the tissue homogenates then (Fig. 2E). Tissue extracts from both mouse groups had detectable MPO activity by 12 h, but the hind paw tissues from infected db/db mice showed higher (P = 0.0154) MPO activity than those of +/+ mice, despite similarities in the tissue bacterial burden (Fig. 1). By 24 h, the tissue MPO activities were comparable in both mouse groups (Fig. 2E), confirming the similarities in the influx of neutrophils by 24 h that we observed histologically (data not shown). These results suggest that db/db mice exhibit a greater early inflammatory response to S. aureus infection than the nondiabetic +/+ mice. However, the results of quantitative cultures (Table 1 and Fig. 1) show that this early inflammatory response in the db/db animals was not effective in bacterial clearance.
FIG. 2.
Inflammation seen at 12 h in the hind paw tissues from +/+ (A) and db/db (B, C, and D) mice challenged with ∼106 CFU of S. aureus. Hematoxylin and eosin staining was used in panels A, B, and C. Immunohistochemical staining of neutrophils with anti-mouse Ly-6G antibody was used in panel D. Panel C shows a higher magnification (×100) of the boxed region in panel B showing neutrophils as the predominant cell type. Images in panels A, B, and D are at a magnification of ×10. (E) MPO activity in homogenates of hind paw tissues from mice (n = 2 to 4) challenged with 106 CFU of S. aureus. MPO activity was higher (P = 0.0154) at 12 h in the db/db mice (black circles) than in the nondiabetic +/+ mice (white circles).
Chemokine levels in infected hind paws.
To determine whether enhanced chemokine production might explain the excessive neutrophil influx in the hind paw tissues of infected db/db mice, we measured CXCL1 and CXCL2 levels in hind paw tissue extracts from infected mice by an ELISA. Consistent with a greater influx of neutrophils to the infected tissues, CXCL1 levels were significantly higher (P = 0.0089) in db/db mice than in +/+ mice 12 h after S. aureus inoculation (Fig. 3A). Similarly, tissue CXCL2 levels at 12 h were approximately twofold higher in db/db mice than in nondiabetic +/+ mice (Fig. 3B), but the difference was not quite significant (P = 0.0689). Chemokine levels at 6 h and 24 h were comparable in both diabetic and nondiabetic mice (Fig. 3), consistent with the MPO activities measured at these same time points (Fig. 2E).
FIG. 3.
Chemokine levels were higher in the hind paw extracts from diabetic db/db mice (n = 10) 12 h after inoculation with S. aureus than in those from nondiabetic (+/+) mice (n = 10). P = 0.0089 for CXCL1 (A) and P = 0.0669 for CXCL2 (B) at 12 h. Values are means ± SEM.
Functional analyses of blood leukocytes.
The paradoxical finding that the greater inflammatory response of db/db mice was accompanied by an inability to clear the bacterial infection led us to examine neutrophil function in the db/db mouse. Neutrophils recovered from patients with type 2 diabetes have been shown to exhibit impaired bactericidal activity (6, 15, 41, 48). To determine whether leukocytes from db/db mice would show a similar defect, we performed S. aureus killing assays with mouse blood. Blood samples were tested on one to three occasions for 22 +/+ mice and 37 db/db mice. As shown in Fig. 4, blood from +/+ mice killed 52% of the S. aureus inoculum after a 2-h incubation at 37°C. Similar levels of staphylococcal killing were observed previously in the blood from ICR (outbred mice derived at the Institute for Cancer Research) and nonobese diabetic (NOD) mice (39, 47). The blood from db/db animals killed only 33% of the S. aureus cells (P = 0.0029). We along with others have shown that preincubation with up to 33 mmol/liter α-d-glucose did not impair the in vitro respiratory burst or bactericidal activity of neutrophils from nondiabetic mice (39, 41).
FIG. 4.
In vitro bactericidal activity of blood from +/+ and db/db mice against 105 CFU/ml S. aureus. The data (means ± SEM) represent the mean values from 22 +/+ mice and 37 db/db mice.
To determine whether the impaired bactericidal activity of blood from db/db mice could be attributed to defects in phagocyte function, we performed respiratory burst assays. Blood from +/+ or db/db mice was incubated for 30 min with MEM-1% BSA or S. aureus, and the respiratory burst was assessed by the oxidation of dihydrorhodamine and analyzed by flow cytometry. Figure 5A shows the results of a typical experiment wherein unstained neutrophils and neutrophils stained with reduced dye are shown in the R2 region, and the cells that oxidized the dye appear in the R3 region. Neutrophils from the +/+ animal showed a marked shift in fluorescence in response to S. aureus, indicative of a robust respiratory burst (Fig. 5A). In contrast, neutrophils from the diabetic db/db mouse responded poorly to the S. aureus stimulus.
FIG. 5.
Differential respiratory burst activity of neutrophils from +/+ and db/db mice. (A) Representative results from an assay performed with mouse blood stimulated for 30 min with MEM-BSA (gray shading) or S. aureus (black shading). Unshaded peaks represent unstained cells. Cells within the R2 region did not undergo an oxidative burst. Cells within the R3 region show a shift in fluorescence intensity characteristic of an oxidative burst. Neutrophils from the db/db animal responded poorly to S. aureus compared with neutrophils from the +/+ animal. (B) The percentage of blood neutrophils from +/+ (n = 6) or diabetic db/db (n = 13) mice that responded to stimulation with medium alone, S. aureus, or PMA is shown. (C) The magnitude of the respiratory burst, shown as the mean fluorescence index (MFI), of neutrophils activated by S. aureus was significantly lower in db/db mice than in +/+ controls.
The data in Fig. 5B and C summarize the neutrophil response of 13 db/db mice that were compared with six nondiabetic +/+ control mice. The percentage of resting neutrophils (stimulated with MEM-1% BSA only) that oxidized the dye was higher for db/db mice than +/+ mice, but this difference was not significant (P = 0.1148). All of the animals showed a similar percentage of neutrophils that responded to the S. aureus stimulus (Fig. 5B); however, the magnitude of the response (measured by the mean fluorescence index) was significantly lower (P = 0.0494) for the diabetic mice. The addition of 0.1 μM phorbol myristate acetate (PMA), a protein kinase C (PKC) agonist, induced a similar phagocyte respiratory burst in both db/db and +/+ mice (Fig. 5B and 5C).
Functional analyses of bone marrow-derived neutrophils.
Since neutrophils comprise only ∼28% of mouse peripheral leukocytes (10), we purified neutrophils from the bone marrow of 16- to 20-week-old +/+ and db/db mice. At a multiplicity of infection (MOI) of 10 (10 CFU per neutrophil), >90% of the neutrophils from diabetic and nondiabetic animals had ingested S. aureus opsonized with 10% serum after 30 min. The number of bacteria associated with or internalized by each neutrophil ranged from 9 to 18 for cells recovered from both +/+ and db/db mice. If the MOI was decreased to 1 or if the staphylococci were opsonized with 4% serum, the phagocytic uptake of staphylococci by neutrophils from +/+ or db/db mice remained comparable (data not shown).
The bactericidal activity of bone marrow-derived neutrophils from +/+ and db/db mice was assessed with an in vitro opsonophagocytic killing assay. The bacteria were opsonized with 10% normal mouse serum and incubated with or without neutrophils. As shown in Fig. 6, bone marrow-derived neutrophils from +/+ and db/db mice killed ∼75% of the S. aureus inoculum within 60 min. Without opsonizing mouse serum, no killing was observed (data not shown). Additional experiments wherein the bacteria were opsonized with 5% serum or with S. aureus capsule-specific antibodies failed to show differences in the bactericidal activity of bone marrow-derived neutrophils from diabetic and nondiabetic mice (data not shown).
FIG. 6.
Serum-opsonized S. aureus cells were incubated in medium alone (white squares) or at an MOI of 1 in the presence of 2 × 106 bone marrow-derived neutrophils from db/db or +/+ mice. Bacterial survival is expressed as a percentage of the inoculum, and the data shown are means ± SEM. PMN, polymorphonuclear neutrophils.
DISCUSSION
Individuals with type 2 diabetes may develop lower-extremity infections and ulcerations that can be severely debilitating, often leading to functional disability, depression, increased risk of amputation, and associated mortality. Many factors influence the susceptibility of diabetic patients to infection, including vascular pathology, peripheral neuropathy, and nephropathy. Foot and ankle infections are the most common cause of hospitalization in diabetic patients (37), and S. aureus is a frequent cause of these infections. Recurrent infection and delayed wound healing associated with diabetic foot ulcers may ultimately result in lower-extremity amputation.
Previously, we established a chronic S. aureus infection in the hind paws of NOD mice (39). These animals develop type 1 diabetes between 14 and 23 weeks of age. In contrast to age-matched nondiabetic littermates, the diabetic NOD mice were unable to resolve the staphylococcal hind paw infection over a 10-day period. The diabetic animals exhibited a delayed inflammatory response to S. aureus, and this finding was consistent with diminished CXC chemokine levels in the infected tissues. The impaired killing of S. aureus in the tissues and blood of diabetic mice correlated with a diminished in vitro leukocytic respiratory burst.
Like diabetic NOD mice, db/db mice with type 2 diabetes were also more susceptible to staphylococcal infection than age-matched +/+ littermates. This susceptibility was more apparent in db/db mice that were ≥20 weeks of age than in younger animals, perhaps as a result of their sustained hyperglycemic state. The host response to the S. aureus hind paw infection in the db/db animals was quite distinct from that observed in diabetic NOD mice. Levels of the proinflammatory chemokines CXCL1 and CXCL2, which recruit neutrophils (23, 49), were elevated in the hind paw tissues of db/db mice 12 h after bacterial inoculation. Accordingly, db/db mice showed a greater inflammatory response to S. aureus infection than +/+ mice. The early neutrophil influx into the infected hind paw tissues of diabetic mice exceeded that of wild-type mice, and this observation was confirmed by MPO assays. Since others have reported diminished intracellular MPO activity in neutrophils from patients with type 2 diabetes (40), this quantitative assay may have actually underestimated the numbers of neutrophils in the tissues of the db/db animals.
Neutrophils are the major inflammatory cells in acute bacterial infections. However, the heightened inflammatory response observed in db/db mice did not result in resolution of the staphylococcal infection. Similar observations using other staphylococcal infection models have been reported, wherein a neutrophil-rich environment actually exacerbated the infection as a result of S. aureus survival within neutrophils (16, 31, 32). Consistent with the inefficient clearance of the bacteria from the infected hind paw tissues, phagocytic killing of S. aureus in the blood from db/db mice was impaired (Fig. 5). Several groups have reported that patients with diabetes or hyperglycemia exhibit aberrant neutrophil responses (1, 3, 12, 14, 33, 34). Other investigators, however, failed to detect a defect in neutrophil function (18, 28). These discrepancies could be attributed to the metabolic status of the diabetic patients, differences in methodology, or underlying diseases or complications among the population groups.
The phagocyte respiratory burst is key to neutrophil function since reactive oxygen species generated by the NADPH oxidase membrane complex effectively kill the engulfed bacteria (17). Individuals with chronic granulomatous disease lack NADPH oxidase activity and suffer recurrent, often life-threatening bacterial infections (25). Likewise, mice lacking one of the subunits of the NADPH oxidase complex exhibit enhanced susceptibility to S. aureus infection (13, 35). The respiratory burst of neutrophils from the blood of db/db mice was aberrant compared with that of nondiabetic +/+ mice (Fig. 5). Whereas only ∼20% of resting neutrophils from control mice were activated in the presence of medium alone, ∼40% of resting neutrophils from the blood of db/db mice were activated. Several studies have demonstrated that resting neutrophils from diabetic patients exhibit an augmented oxidative burst activity compared with resting neutrophils from nondiabetic controls (2, 18, 24). Chronic exposure to low levels of reactive oxygen species may contribute to vascular complications and the development of chronic low-grade inflammation commonly found in diabetic hosts (41). In our study, these “primed” neutrophils exhibited a blunted response to stimulation with S. aureus. Similar numbers of neutrophils responded to S. aureus in both mouse groups, but the magnitude of the oxidative burst for neutrophils from the db/db mice was only about 60% of the wild-type response, consistent with results from Shurtz-Swirski and colleagues (41), who stimulated human neutrophils in vitro with zymosan. The measured rate of superoxide release from the neutrophils of type 2 diabetic patients was significantly lower than that of age- and sex-matched normal control subjects. Consistent with the findings of Zykova et al. (51), we observed no difference in the PMA-induced respiratory burst in neutrophils from db/db and +/+ mice. PMA enters neutrophils and activates PKC by directly binding to its diacylglycerol (natural activator) site (19). Because the S. aureus-induced respiratory burst also requires activated PKC (20), the decreased respiratory burst of db/db mice in response to S. aureus stimulus may derive from a defect upstream of PKC in the process of NADPH oxidase activation. Alternatively, activation of NADPH oxidase by PMA versus S. aureus may involve different signaling pathways or different isotypes of PKC.
To further investigate the phagocytic and bactericidal activities of neutrophils from nondiabetic and diabetic mice, we collected bone marrow-derived neutrophils from each mouse group. Notably, neutrophils from the bone marrow of diabetic db/db mice ingested and killed opsonized S. aureus as well as neutrophils from +/+ mice. Likewise, the results of respiratory burst assays comparing +/+ and db/db bone marrow-derived neutrophil responses to S. aureus were similar (data not shown). Since neutrophils from the bone marrow are considered fully functional (4), the diminished bactericidal activity and respiratory burst characteristic of neutrophils in the blood may be due to modulation of neutrophil function by cytokines secreted by blood mononuclear cells. Zykova et al. (51) reported that stimulated macrophages from db/db mice produced lower levels of tumor necrosis factor alpha and interleukin-1β than cells from db/+ mice. Blood glucose concentrations do not directly affect the respiratory burst or the bactericidal activity of neutrophils (39, 41). However, Shurtz-Swirski (41) did show a correlation between the rate of superoxide release and blood levels of hemoglobin A1c. Prolonged exposure of neutrophils to elevated blood glucose concentrations may cause the formation of advanced glycation end products that may compromise neutrophil function (9). Treatment of neutrophils with advanced glycation end product albumin resulted in increased phagocytic activity; however, the treatment abrogated the production of S. aureus-induced reactive oxygen species, thereby protecting ingested bacteria from intracellular killing (9). Whether this mechanism or others contribute to the defective neutrophil function of db/db mice is currently under investigation in our laboratory.
In summary, our results suggest that defects in innate immunity predispose diabetic db/db mice to staphylococcal infection. db/db mice showed a heightened inflammatory response to S. aureus, but the recruited neutrophils failed to resolve the bacterial infection, a result that correlated with an impaired neutrophil respiratory burst. The factors that contribute to the impaired function of neutrophils derived from the blood and tissues of diabetic mice merit further investigation.
Acknowledgments
This research was supported by NIH grant AI29040. J.R. was the recipient of a Juvenile Diabetes Research Foundation award (3-2004-546), and J.R. and S.P. were supported by NIH Infectious Disease Training Grant T32 AI07061. F.H. was supported by a grant from German Academy of Sciences Leopoldina.
We gratefully acknowledge Rachel McLoughlin for discussions and helpful suggestions.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 22 December 2008.
REFERENCES
- 1.Alba-Loureiro, T. C., C. D. Munhoz, J. O. Martins, G. A. Cerchiaro, C. Scavone, R. Curi, and P. Sannomiya. 2007. Neutrophil function and metabolism in individuals with diabetes mellitus. Braz. J. Med. Biol. Res. 401037-1044. [DOI] [PubMed] [Google Scholar]
- 2.Bagdade, J. D., R. K. Root, and R. J. Bulger. 1974. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes 239-15. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharya, S. K., S. Shastri, P. Mahajan, S. V. Madhu, A. K. Tripathi, G. P. Rauniar, B. P. Das, and K. R. Paudel. 2007. Polymorphonuclear leukocyte function in type-2 diabetes mellitus patients and its correlation with glycaemic control. Nepal Med. Coll. J. 9111-116. [PubMed] [Google Scholar]
- 4.Boxio, R., C. Bossenmeyer-Pourie, N. Steinckwich, C. Dournon, and O. Nusse. 2004. Mouse bone marrow contains large numbers of functionally competent neutrophils. J. Leukoc. Biol. 75604-611. [DOI] [PubMed] [Google Scholar]
- 5.Caputo, G. M., J. Ulbrecht, P. Cavanagh, and P. J. Juliano. 2000. The role of cultures in mild diabetic foot cellulitis. Infect. Dis. Clin. N. Am. 9241-243. [Google Scholar]
- 6.Chang, F. Y., and M. F. Shaio. 1995. Respiratory burst activity of monocytes from patients with non-insulin-dependent diabetes mellitus. Diabetes Res. Clin. Pract. 29121-127. [DOI] [PubMed] [Google Scholar]
- 7.Chen, H., O. Charlat, L. A. Tartaglia, E. A. Woolf, X. Weng, S. J. Ellis, N. D. Lakey, J. Culpepper, K. J. Moore, R. E. Breitbart, G. M. Duyk, R. I. Tepper, and J. P. Morgenstern. 1996. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84491-495. [DOI] [PubMed] [Google Scholar]
- 8.Chua, S. C., Jr., W. K. Chung, X. S. Wu-Peng, Y. Zhang, S. M. Liu, L. Tartaglia, and R. L. Leibel. 1996. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 271994-996. [DOI] [PubMed] [Google Scholar]
- 9.Collison, K. S., R. S. Parhar, S. S. Saleh, B. F. Meyer, A. A. Kwaasi, M. M. Hammami, A. M. Schmidt, D. M. Stern, and F. A. Al-Mohanna. 2002. RAGE-mediated neutrophil dysfunction is evoked by advanced glycation end products (AGEs). J. Leukoc. Biol. 71433-444. [PubMed] [Google Scholar]
- 10.Cotter, M. J., K. E. Norman, P. G. Hellewell, and V. C. Ridger. 2001. A novel method for isolation of neutrophils from murine blood using negative immunomagnetic separation. Am. J. Pathol. 159473-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dang, C. N., Y. D. Prasad, A. J. Boulton, and E. B. Jude. 2003. Methicillin-resistant Staphylococcus aureus in the diabetic foot clinic: a worsening problem. Diabet. Med. 20159-161. [DOI] [PubMed] [Google Scholar]
- 12.Delamaire, M., D. Maugendre, M. Moreno, M. C. Le Goff, H. Allannic, and B. Genetet. 1997. Impaired leucocyte functions in diabetic patients. Diabet. Med. 1429-34. [DOI] [PubMed] [Google Scholar]
- 13.Ellson, C. D., K. Davidson, G. J. Ferguson, R. O'Connor, L. R. Stephens, and P. T. Hawkins. 2006. Neutrophils from p40phox−/− mice exhibit severe defects in NADPH oxidase regulation and oxidant-dependent bacterial killing. J. Exp. Med. 2031927-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallacher, S. J., G. Thomson, W. D. Fraser, B. M. Fisher, C. G. Gemmell, and A. C. MacCuish. 1995. Neutrophil bactericidal function in diabetes mellitus: evidence for association with blood glucose control. Diabet. Med. 12916-920. [DOI] [PubMed] [Google Scholar]
- 15.Geerlings, S. E., and A. I. Hoepelman. 1999. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 26259-265. [DOI] [PubMed] [Google Scholar]
- 16.Gresham, H. D., J. H. Lowrance, T. E. Caver, B. S. Wilson, A. L. Cheung, and F. P. Lindberg. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 1643713-3722. [DOI] [PubMed] [Google Scholar]
- 17.Hampton, M. B., A. J. Kettle, and C. C. Winterbourn. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 923007-3017. [PubMed] [Google Scholar]
- 18.Hand, W. L., D. L. Hand, and Y. Vasquez. 2007. Increased polymorphonuclear leukocyte respiratory burst function in type 2 diabetes. Diabetes Res. Clin. Pract. 7644-50. [DOI] [PubMed] [Google Scholar]
- 19.Heyworth, P. G., and J. A. Badwey. 1990. Protein phosphorylation associated with the stimulation of neutrophils. Modulation of superoxide production by protein kinase C and calcium. J. Bioenerg. Biomembr. 221-26. [DOI] [PubMed] [Google Scholar]
- 20.Kampen, A. H., T. Tollersrud, and A. Lund. 2004. Flow cytometric measurement of neutrophil respiratory burst in whole bovine blood using live Staphylococcus aureus. J. Immunol. Methods 28947-55. [DOI] [PubMed] [Google Scholar]
- 21.Karchmer, A. 2002. Microbiology and treatment of diabetic foot infections, p. 207-219. In A. Veves, J. M. Giurini, and F. W. LoGerfro (ed.), The diabetic foot: medical and surgical treatment. Humana Press, Totowa, NJ.
- 22.Klevens, R. M., M. A. Morrison, J. Nadle, S. Petit, K. Gershman, S. Ray, L. H. Harrison, R. Lynfield, G. Dumyati, J. M. Townes, A. S. Craig, E. R. Zell, G. E. Fosheim, L. K. McDougal, R. B. Carey, and S. K. Fridkin. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2981763-1771. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi, Y. 2008. The role of chemokines in neutrophil biology. Front. Biosci. 132400-2407. [DOI] [PubMed] [Google Scholar]
- 24.Larijani, B., P. Shooshtarizadeh, N. Mosaffa, and R. Heshmat. 2007. Polymorphonuclear leucocyte respiratory burst activity correlates with serum zinc level in type 2 diabetic patients with foot ulcers. Br. J. Biomed. Sci. 6413-17. [DOI] [PubMed] [Google Scholar]
- 25.Liese, J., S. Kloos, V. Jendrossek, T. Petropoulou, U. Wintergerst, G. Notheis, M. Gahr, and B. H. Belohradsky. 2000. Long-term follow-up and outcome of 39 patients with chronic granulomatous disease. J. Pediatr. 137687-693. [DOI] [PubMed] [Google Scholar]
- 26.Lipsky, B. A., R. E. Pecoraro, S. A. Larson, M. E. Hanley, and J. H. Ahroni. 1990. Outpatient management of uncomplicated lower-extremity infections in diabetic patients. Arch. Intern. Med. 150790-797. [PubMed] [Google Scholar]
- 27.Lipsky, B. A., R. E. Pecoraro, and L. J. Wheat. 1990. The diabetic foot. Soft tissue and bone infection. Infect. Dis. Clin. N. Am. 4409-432. [PubMed] [Google Scholar]
- 28.Llorente, L., H. De La Fuente, Y. Richaud-Patin, C. Alvarado-De La Barrera, A. Diaz-Borjon, A. Lopez-Ponce, I. Lerman-Garber, and J. Jakez-Ocampo. 2000. Innate immune response mechanisms in non-insulin dependent diabetes mellitus patients assessed by flow cytoenzymology. Immunol. Lett. 74239-244. [DOI] [PubMed] [Google Scholar]
- 29.Looney, M. R., X. Su, J. A. Van Ziffle, C. A. Lowell, and M. A. Matthay. 2006. Neutrophils and their Fc gamma receptors are essential in a mouse model of transfusion-related acute lung injury. J. Clin. Investig. 1161615-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnotti, L. J., J. S. Upperman, D. Z. Xu, Q. Lu, and E. A. Deitch. 1998. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann. Surg. 228518-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLoughlin, R. M., J. C. Lee, D. L. Kasper, and A. O. Tzianabos. 2008. IFN-gamma regulated chemokine production determines the outcome of Staphylococcus aureus infection. J. Immunol. 1811323-1332. [DOI] [PubMed] [Google Scholar]
- 32.McLoughlin, R. M., R. M. Solinga, J. Rich, K. J. Zaleski, J. L. Cocchiaro, A. Risley, A. O. Tzianabos, and J. C. Lee. 2006. CD4+ T cells and CXC chemokines modulate the pathogenesis of Staphylococcus aureus wound infections. Proc. Natl. Acad. Sci. USA 10310408-10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mowat, A., and J. Baum. 1971. Chemotaxis of polymorphonuclear leukocytes from patients with diabetes mellitus. N. Engl. J. Med. 284621-627. [DOI] [PubMed] [Google Scholar]
- 34.Nielson, C. P., and D. A. Hindson. 1989. Inhibition of polymorphonuclear leukocyte respiratory burst by elevated glucose concentrations in vitro. Diabetes 381031-1035. [DOI] [PubMed] [Google Scholar]
- 35.Pollock, J. D., D. A. Williams, M. A. Gifford, L. L. Li, X. Du, J. Fisherman, S. H. Orkin, C. M. Doerschuk, and M. C. Dinauer. 1995. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 9202-209. [DOI] [PubMed] [Google Scholar]
- 36.Popovich, K. J., R. A. Weinstein, and B. Hota. 2008. Are community-associated methicillin-resistant Staphylococcus aureus (MRSA) strains replacing traditional nosocomial MRSA strains? Clin. Infect. Dis. 46787-794. [DOI] [PubMed] [Google Scholar]
- 37.Ramsey, S. D., K. Newton, D. Blough, D. K. McCulloch, N. Sandhu, and E. H. Wagner. 1999. Patient-level estimates of the cost of complications in diabetes in a managed-care population. Pharmacoeconomics 16285-295. [DOI] [PubMed] [Google Scholar]
- 38.Reiber, G. E., L. Vileikyte, E. J. Boyko, M. del Aguila, D. G. Smith, L. A. Lavery, and A. J. Boulton. 1999. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care 22157-162. [DOI] [PubMed] [Google Scholar]
- 39.Rich, J., and J. C. Lee. 2005. The pathogenesis of Staphylococcus aureus infection in the diabetic NOD mouse. Diabetes 542904-2910. [DOI] [PubMed] [Google Scholar]
- 40.Sato, N., K. Kashima, Y. Tanaka, H. Shimizu, and M. Mori. 1997. Effect of granulocyte-colony stimulating factor on generation of oxygen-derived free radicals and myeloperoxidase activity in neutrophils from poorly controlled NIDDM patients. Diabetes 46133-137. [DOI] [PubMed] [Google Scholar]
- 41.Shurtz-Swirski, R., S. Sela, A. T. Herskovits, S. M. Shasha, G. Shapiro, L. Nasser, and B. Kristal. 2001. Involvement of peripheral polymorphonuclear leukocytes in oxidative stress and inflammation in type 2 diabetic patients. Diabetes Care 24104-110. [DOI] [PubMed] [Google Scholar]
- 42.Sievert, D. M., J. T. Rudrik, J. B. Patel, L. C. McDonald, M. J. Wilkins, and J. C. Hageman. 2008. Vancomycin-resistant Staphylococcus aureus in the United States, 2002-2006. Clin. Infect. Dis. 46668-674. [DOI] [PubMed] [Google Scholar]
- 43.Skiest, D. J., K. Brown, T. W. Cooper, H. Hoffman-Roberts, H. R. Mussa, and A. C. Elliott. 2007. Prospective comparison of methicillin-susceptible and methicillin-resistant community-associated Staphylococcus aureus infections in hospitalized patients. J. Infect. 54427-434. [DOI] [PubMed] [Google Scholar]
- 44.Stanaway, S., D. Johnson, P. Moulik, and G. Gill. 2007. Methicillin-resistant Staphylococcus aureus (MRSA) isolation from diabetic foot ulcers correlates with nasal MRSA carriage. Diabetes Res. Clin. Pract. 7547-50. [DOI] [PubMed] [Google Scholar]
- 45.Tentolouris, N., G. Petrikkos, N. Vallianou, C. Zachos, G. L. Daikos, P. Tsapogas, G. Markou, and N. Katsilambros. 2006. Prevalence of methicillin-resistant Staphylococcus aureus in infected and uninfected diabetic foot ulcers. Clin. Microbiol. Infect. 12186-189. [DOI] [PubMed] [Google Scholar]
- 46.Tzianabos, A. O., J. Y. Wang, and J. C. Lee. 2001. Structural rationale for the modulation of abscess formation by Staphylococcus aureus capsular polysaccharides. Proc. Natl. Acad. Sci. USA 989365-9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watts, A., D. Ke, Q. Wang, A. Pillay, A. Nicholson-Weller, and J. C. Lee. 2005. Staphylococcus aureus strains that express serotype 5 or serotype 8 capsular polysaccharides differ in virulence. Infect. Immun. 733502-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wykretowicz, A., B. Wierusz-Wysocka, J. Wysocki, A. Szczepanik, and H. Wysocki. 1993. Impairment of the oxygen-dependent microbicidal mechanisms of polymorphonuclear neutrophils in patients with type 2 diabetes is not associated with increased susceptibility to infection. Diabetes Res. Clin. Pract. 19195-201. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, X. W., Q. Liu, Y. Wang, and H. Thorlacius. 2001. CXC chemokines, MIP-2 and KC, induce P-selectin-dependent neutrophil rolling and extravascular migration in vivo. Br. J. Pharmacol. 133413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu, W., N. C. Clark, L. K. McDougal, J. Hageman, L. C. McDonald, and J. B. Patel. 2008. Vancomycin-resistant Staphylococcus aureus isolates associated with Inc18-like vanA plasmids in Michigan. Antimicrob. Agents Chemother. 52452-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zykova, S. N., T. G. Jenssen, M. Berdal, R. Olsen, R. Myklebust, and R. Seljelid. 2000. Altered cytokine and nitric oxide secretion in vitro by macrophages from diabetic type II-like db/db mice. Diabetes 491451-1458. [DOI] [PubMed] [Google Scholar]