Abstract
Substantial evidence indicates that antibodies to Plasmodium falciparum merozoite antigens play a role in protection from malaria, although the precise targets and mechanisms mediating immunity remain unclear. Different malaria antigens induce distinct immunoglobulin G (IgG) subclass responses, but the importance of different responses in protective immunity from malaria is not known and the factors determining subclass responses in vivo are poorly understood. We examined IgG and IgG subclass responses to the merozoite antigens MSP1-19 (the 19-kDa C-terminal region of merozoite surface protein 1), MSP2 (merozoite surface protein 2), and AMA-1 (apical membrane antigen 1), including different polymorphic variants of these antigens, in a longitudinal cohort of children in Papua New Guinea. IgG1 and IgG3 were the predominant subclasses of antibodies to each antigen, and all antibody responses increased in association with age and exposure without evidence of increasing polarization toward one subclass. The profiles of IgG subclasses differed somewhat for different alleles of MSP2 but not for different variants of AMA-1. Individuals did not appear to have a propensity to make a specific subclass response irrespective of the antigen. Instead, data suggest that subclass responses to each antigen are generated independently among individuals and that antigen properties, rather than host factors, are the major determinants of IgG subclass responses. High levels of AMA-1-specific IgG3 and MSP1-19-specific IgG1 were strongly predictive of a reduced risk of symptomatic malaria and high-density P. falciparum infections. However, no antibody response was significantly associated with protection from parasitization per se. Our findings have major implications for understanding human immunity and for malaria vaccine development and evaluation.
Effective immunity against Plasmodium falciparum malaria in humans develops slowly over time after repeated exposure and protects against the development of symptomatic and severe illness. Although the targets of protective immunity in humans remain ill-defined, substantial evidence suggests that antibodies against merozoite antigens play an important role, and several merozoite antigens are leading vaccine candidates (5, 15, 29, 35, 37, 45). Antibodies to merozoite antigens are thought to function in vivo by inhibition of merozoite invasion of erythrocytes, opsonization of merozoites for phagocytosis, and antibody-dependent cellular inhibition (3, 9, 13, 21, 24).
The subclass of antibodies produced against antigens is likely to be important for protective activity, as immunoglobulin G (IgG) subclasses differ in their structures and mediate different immune effector functions (32). Knowledge of subclass responses associated with protection against malaria is important for understanding immunity and guiding vaccine development. IgG1 and IgG3 are the predominant subclasses produced in response to merozoite antigens (31, 37, 40, 43, 46, 48). IgG1 and IgG3 are cytophilic and T cell dependent, have high affinity for Fc receptors, and mediate phagocyte activation and complement fixation (7). It has been suggested that IgG3 is more efficient at mediating these processes (7). For reasons that are not well understood, different merozoite antigens induce different relative levels of IgG1 and IgG3 (14, 29, 31, 37, 40, 46, 48). It is unclear whether individuals have a bias toward producing a specific subclass regardless of the antigen or if instead the IgG subclass response is generated independently for each antigen and how this relates to protective immunity. While factors determining subclass responses to antigens are not clearly defined, antigen properties, host age, cumulative exposure, and genetic determinants have been linked with the nature of subclass responses (2, 4, 17, 33, 34, 41, 42, 47, 48). Some studies have suggested that increasing age (and therefore malaria exposure) leads to an increasing polarization of IgG subclass responses to merozoite antigens (41, 48).
Antibodies to merozoite antigens have been linked with protection from malaria in humans in some longitudinal studies (6, 11, 15, 23, 25, 29, 31, 35, 37-39, 45). Results from these studies have been conflicting, which results partly from the use of different endpoints for evaluating the protective role of antibodies (i.e., different parasitemia thresholds versus symptomatic illness). It is thought that acquired immunity largely targets blood-stage antigens and acts by limiting parasite replication, thereby preventing the development of high-density parasitemia, but is less effective at protecting from parasitization per se (26). However, there are limited data that directly address this question and few studies have evaluated antibody associations with protection from symptomatic malaria, high-density parasitemia, and parasitization per se in the same cohort because of challenges in performing these studies in community-based settings. Additionally, the detection of parasitization has generally been performed using light microscopy, which is not sufficiently sensitive to detect parasitemias of very low density. The development of high-throughput molecular methods to detect parasitemia in cohort studies has provided new opportunities to better define these associations between immune responses and parasitization and symptomatic malaria. Furthermore, most studies of immunity have been conducted in sub-Saharan Africa, and there are little data from populations in Asia, where a large portion of the global malaria burden occurs (44).
We addressed these important issues in a treatment-reinfection study of 206 children resident in an area of malaria endemicity in Papua New Guinea. We prospectively examined associations between subclass-specific responses to P. falciparum merozoite antigens (the 19-kDa C-terminal region of merozoite surface protein 1 [MSP1-19], apical membrane antigen 1 [AMA-1], and merozoite surface protein 2 [MSP2]) and the risks of high-density parasitemia, symptomatic malaria, and reinfection, as detected by sensitive molecular-based methods. Furthermore, we evaluated the influences of host age, exposure, and concurrent P. falciparum infection on the nature of responses and assessed whether individuals demonstrated a bias toward specific subclass responses and whether polymorphisms in antigens influenced the nature of subclass responses.
MATERIALS AND METHODS
Study population.
A prospective treatment-reinfection study was undertaken in the Mugil and Megiar area 50 km north of Madang, Papua New Guinea. Details of the study are described elsewhere (30). Briefly, 206 children aged 5 to 14 years (median, 9 years; interquartile range, 8.1 to 10.3 years) were enrolled in the study, and venous blood samples were collected. All children were treated with 7 days of artesunate taken orally and were monitored for 6 months by active (twice-weekly) and passive case detection for reinfection and symptomatic illness. New infections were distinguished from treatment failures by MSP2-based genotyping. A symptomatic episode of P. falciparum malaria was defined as the presence of fever and parasitemia of >5,000 parasites/μl. Parasitemia was determined by a semiquantitative post-PCR ligase detection reaction-fluorescent microsphere assay (LDR-FMA) (28), and light microscopy. All analyses were performed using parasitemia determined by LDR-FMA, unless otherwise indicated. P. falciparum was detected in 67.5% of subjects at enrollment by LDR-FMA and in 40.3% by light microscopy. Samples collected at baseline and from the first symptomatic infection were genotyped to identify MSP2 alleles (FC27 or 3D7), according to methods published previously (16, 22).
Samples were also collected from children and adults (0 to 3 years old [n = 50; median age of 2 years], 4 to 6 years old [n = 48; median age of 5 years], 7 to 9 years old [n = 50; median age of 7.9 years], and ≥10 years old [n = 59; range from 10 to 56 years; median age of 17.7 years]) in the Madang area of Papua New Guinea to further evaluate the associations between age and IgG subclass response to merozoite antigens. Plasma samples were obtained from anonymous Melbourne, Australia, residents with no known previous exposure to malaria to act as negative controls in all assays.
Informed consent was obtained from all participants in the studies, and ethics approval was obtained from the Medical Research Advisory Council, PNG, and the Human Research Ethics Committee, The Walter and Eliza Hall Institute.
ELISA.
Samples collected from the enrollment bleed were used in an enzyme-linked immunosorbent assay (ELISA). All available samples were tested for total levels of IgG, IgG1, and IgG3 to each antigen. A subset of samples was tested for IgG2 and IgG4 (n = 129 for MSP1-19 and MSP2 IgG2 and IgG4; n = 121 for AMA-1 IgG2; n = 120 for AMA-1 IgG4). AMA-1 was expressed as a His-tagged recombinant protein in Escherichia coli, using the full ectodomains of 3D7 and W2mef, and was purified and refolded as described previously (21). Recombinant MSP1-19 (3D7 sequence) was expressed as a His-tagged protein in E. coli, purified over nickel-nitrilotriacetic acid resin (Qiagen, Victoria, Australia), and refolded, as described previously (12). Full-length MSP2 (corresponding to the 3D7 or FC27 gene sequence) was expressed in E. coli as a C-terminally His-tagged protein which was purified by nickel chelate, anion-exchange, and reversed-phase chromatography. Schizont parasite protein extract was prepared from P. falciparum (3D7). Schizonts were lysed with saponin (0.09% in RPMI-HEPES) on ice for 10 min and then centrifuged at 4°C, and the pellet was washed in cold phosphate-buffered saline (PBS). Schizonts were resuspended in cold PBS, vortexed and freeze-thawed on dry ice twice, and sonicated for 30 s to solubilize proteins. This mixture was then centrifuged, and the supernatant was collected and used in the assays.
ELISAs were performed using established methods (36). Ninety-six-well plates (Immulon 4 plates [ThermoLabsystems, MA] or Maxisorp plates [Nunc, Roskilde, Denmark]) were coated with 0.5 μg/ml of recombinant antigen or 4.6 μg/ml schizont lysate in PBS and incubated overnight at 4°C. Skim milk-PBS-0.05% Tween was used for blocking and diluting plasma and antibodies. Plasma was added in duplicate at previously determined dilutions. For measurement of total IgG, horseradish peroxidase-conjugated sheep anti-human IgG (Chemicon, Melbourne, Australia) was used at a 1:2,500 concentration. For measurement of IgG subclasses, secondary antibodies were added at a dilution of 1:1,000 using mouse anti-human IgG subclass antibodies (IgG1 clone HP6069 [Invitrogen Corporation, CA], IgG3 clone HP6047 [Invitrogen Corporation, CA], IgG4 clone HP6023 [Calbiochem-Novabiochem Corp., CA], and IgG2 clone HP6002 [Caltag Laboratories, CA]). The tertiary antibody for the subclass assays was a sheep anti-mouse antibody (Chemicon International, CA), added at 1:2,500. Finally, ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] substrate (Sigma, Castle Hill, Australia) was added to the plates and the reaction stopped with 1% sodium dodecyl sulfate. The optical density (OD) was determined at 405 nm. All samples were tested in duplicate, and samples were retested if there was a discrepancy of greater than 25% between duplicates. Standardization of the plates was achieved using positive-control plasma pools on each plate. Background (determined from the wells with no plasma) was deducted from the mean of each sample and a cutoff threshold for positivity determined as the mean plus 3 standard deviations from the nine negative-control plasma samples (Melbourne residents) included in each assay.
Analysis.
As antibody levels were not normally distributed, nonparametric tests were used for analyses. Correlations between ODs of different subclasses and/or to different antigens were determined using Spearman's rank correlation, and differences in the median ODs with age and infection status were compared using a two-sample Wilcoxon rank sum test. Differences in the proportions of children positive for different subclasses and associations between age and infection status and antibody prevalence were assessed using the chi-square or Fisher's exact test. To determine how exposure influences the subclass profile, children were grouped into four equal groups (quartiles) according to their IgG responses to P. falciparum schizont extract and the subclass responses within each group examined.
For determining the association between antibody levels and P. falciparum infection and symptomatic malaria, children were stratified into three equal groups (tertiles), reflecting low, medium, and high responders according to OD values for each antigen (tertile cutoffs are given in Table S1 in the supplemental material). A Poisson regression was used to test for associations between antibody levels and incidence of disease, while associations with time to first infection were assessed by Cox regression, adjusting for known confounders, as previously described (30). In all multivariate analyses, backward selection and likelihood ratio tests were used to identify the best-fitting models. All statistical analyses were performed using STATA 8 statistical analysis software (Stata Corporation, College Station, TX). P values of ≤0.05 were considered statistically significant, and P values of >0.1 were classified as not significant (NS).
RESULTS
IgG subclass responses to merozoite antigens and effect of polymorphisms on these responses.
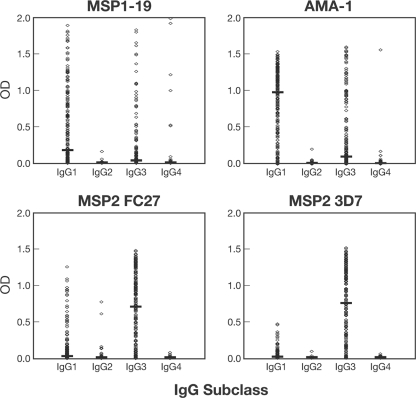
In agreement with previous studies, antibody responses were predominantly IgG1 and IgG3 and there was little IgG2 and IgG4 reactivity (Fig. 1). Therefore, analyses were restricted to IgG1 and IgG3. The levels of predominance of IgG1 versus IgG3 varied among antigens (Fig. 1; also see Table S1 in the supplemental material). For MSP1-19 and AMA-1, IgG1 levels were significantly higher than IgG3 levels (median IgG1 OD of 0.17 versus median IgG3 OD of 0.03 [P < 0.001] for MSP1-19; median IgG1 OD of 0.97 versus median IgG3 OD of 0.09 [P < 0.001] for AMA-1). In contrast, IgG3 was the dominant subclass for both allelic forms of MSP2 (median IgG1 OD of 0.01 versus median IgG3 OD of 0.74 for the 3D7 allele; median IgG1 OD of 0.02 versus median IgG3 OD of 0.70 for the FC27 allele [P < 0.001]).
FIG. 1.
IgG subclass profiles for the levels of reactivity to merozoite antigens of P. falciparum among the study cohort, as measured by ELISA. Horizontal bars indicate the median antibody levels. The 3D7 allele of MSP1-19 and AMA-1 was used.
We next evaluated the effect of antigen polymorphisms on subclass responses. Polymorphisms in AMA-1 appeared to have little effect on the profile of IgG subclass responses. In a subset of children (n = 73), IgG1 and IgG3 were measured against both the 3D7 and the W2mef AMA-1 variants, which have been shown to be antigenically different (20). For both AMA-1 variants, IgG1 was predominant, and similar numbers of individuals were positive for each allele (93.2% IgG1 versus 82.2% IgG3 for 3D7; 93.2% IgG1 versus 76.7% IgG3 for W2mef). For MSP2, responses were less strongly biased toward IgG3 for the FC27 allele than for the 3D7 allele. The prevalence of IgG1 was significantly higher for the FC27 than for the 3D7 allele (41.3% and 51.5% IgG1 [P = 0.04] for MSP2 3D7 and FC27, respectively), whereas the prevalences of IgG3 were similar (84.2% and 80.7% IgG3 for MSP2 3D7 and FC27, respectively [P = 0.35]).
IgG subclass responses are associated with age, exposure, and concurrent parasitemia.
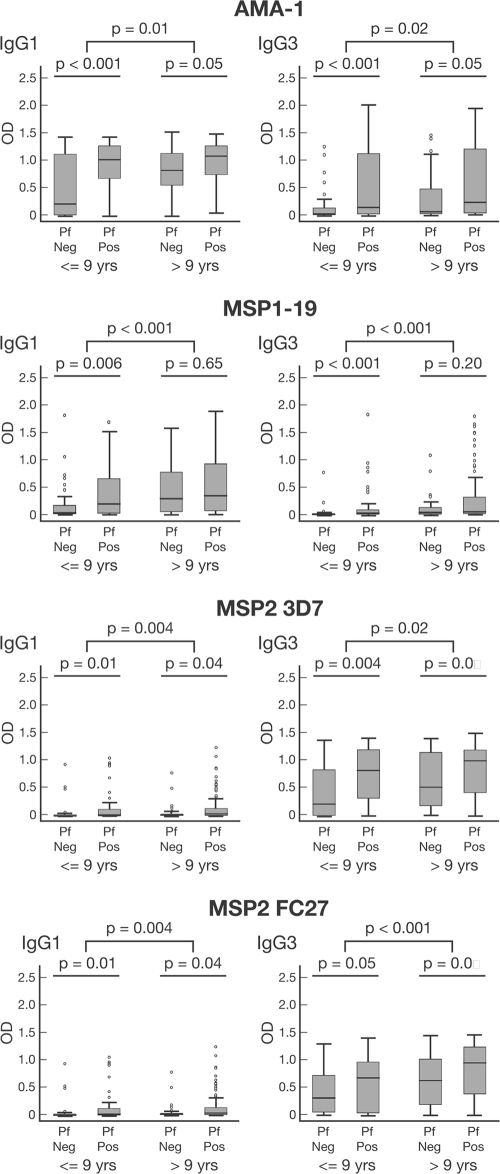
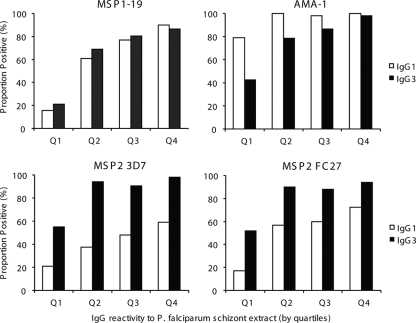
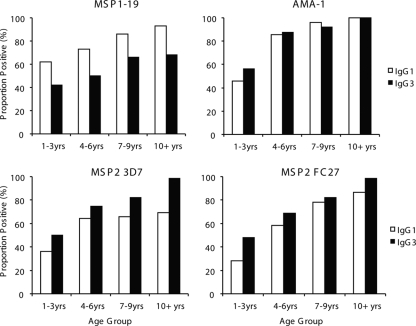
Antibody responses were significantly associated with age in the cohort; both IgG1 and IgG3 levels were significantly higher among older children than among younger children for all four antigens (Fig. 2). Antibody prevalence was also significantly higher for older children for all responses except that of IgG1 to MSP2 (see Table S2 in the supplemental material). We did not find any evidence that increasing age or malaria exposure leads to an increasing polarization of responses from a mixed IgG1/IgG3 profile to a particular IgG subclass response, as reported from studies of African populations (45, 48). Higher levels of IgG to schizont protein extract (reflecting greater exposure to malaria) were significantly correlated with higher levels and prevalence of IgG1 and IgG3 for all antigens (Fig. 3 and Table 1; also see Fig. S1 in the supplemental material). Because the age range in the cohort was narrow, we tested an additional set of samples from 207 randomly selected individuals aged 0 to 56 years resident in the same geographical area to further examine this relationship. For all four antigens, both IgG1 and IgG3 increased significantly in association with age (Fig. 4; also see Fig. S2 in the supplemental material).
FIG. 2.
Associations between age and presence of parasitemia and IgG subclass responses to merozoite antigens of P. falciparum. Children were divided into two age groups, ≤9 years (n = 91) and >9 years (n = 115), to examine associations with age. The presence (Pf Pos [n = 139]) or absence (Pf Neg [n = 67]) of P. falciparum infection was determined by PCR. Data are plotted as box-and-whisker plots (boxes show medians and interquartile ranges; error bars show 95% confidence intervals).
FIG. 3.
Effect of exposure on prevalence of IgG subclass responses to different merozoite antigens. Exposure was determined by IgG reactivity to P. falciparum schizont extract. Children were grouped into quartiles (Q1 to Q4, where Q1 represents the group with the lowest IgG response to P. falciparum schizont extract), and the proportions of individuals who were positive for IgG1 and IgG3 for each quartile are shown. P < 0.0001 for differences in prevalence of IgG1 or IgG3 between groups for all antigens, except P = 0.001 for MSP2 3D7 IgG1. The 3D7 allele was used for MSP1-19 and AMA-1.
TABLE 1.
Correlations between antibody responses to merozoite antigens of P. falciparum
| Antigen and antibody | Correlation coefficienta
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMA-1
|
MSP1-19
|
MSP2 3D7
|
MSP2 FC27
|
|||||||||
| IgG | IgG1 | IgG3 | IgG | IgG1 | IgG3 | IgG | IgG1 | IgG3 | IgG | IgG1 | IgG3 | |
| AMA-1 | ||||||||||||
| IgG | 0.87 | 0.46 | 0.48 | 0.52 | 0.43 | 0.49 | 0.20** | 0.48 | 0.48 | 0.37 | 0.41 | |
| IgG1 | 0.47 | 0.46 | 0.50 | 0.40 | 0.48 | 0.24 | 0.49 | 0.48 | 0.39 | 0.41 | ||
| IgG3 | 0.40 | 0.44 | 0.55 | 0.42 | 0.22** | 0.42 | 0.42 | 0.16* | 0.47 | |||
| MSP1-19 | ||||||||||||
| IgG | 0.86 | 0.32 | 0.41 | 0.14* | 0.42 | 0.47 | 0.31 | 0.38 | ||||
| IgG1 | 0.34 | 0.48 | 0.21** | 0.48 | 0.43 | 0.37 | 0.37 | |||||
| IgG3 | 0.43 | 0.26 | 0.44 | 0.54 | 0.31 | 0.56 | ||||||
| MSP2 3D7 | ||||||||||||
| IgG | 0.48 | 0.89 | 0.46 | 0.36 | 0.39 | |||||||
| IgG1 | 0.39 | 0.32 | 0.36 | 0.25 | ||||||||
| IgG3 | 0.40 | 0.29 | 0.37 | |||||||||
| MSP2 FC27 | ||||||||||||
| IgG | 0.72 | 0.91 | ||||||||||
| IgG1 | 0.6 | |||||||||||
| IgG3 | ||||||||||||
| Schizont protein extract IgG | 0.52 | 0.58 | 0.68 | 0.52 | 0.36 | 0.58 | 0.44 | 0.58 | ||||
Correlation coefficients were determined by Spearman's method, using samples that had complete data only (n = 198). All correlations are significant at a P value of <0.001 unless otherwise indicated (*, P < 0.05; **, P < 0.01).
FIG. 4.
Association between age and IgG subclass reactivity to different merozoite antigens. The proportions of individuals who were positive for IgG1 and IgG3 for each age group are shown (0 to 3 years [n = 50], 4 to 6 years [n = 48], 7 to 9 years [n = 50], and >10 years [n = 59]). Associations between IgG subclass responses and age were significant for all antigens (for MSP1-19, P < 0.001 and P = 0.018 for IgG1 and IgG3, respectively; for AMA-1, P < 0.0001 for IgG1 and IgG3; for MSP2 3D7, P = 0.002 and P < 0.0001 for IgG1 and IgG3, respectively; and for MSP2 FC27, P < 0.0001 for IgG1 and IgG3). The 3D7 allele of MSP1-19 and AMA-1 was used.
In the main cohort, levels of IgG1 and IgG3 to AMA-1, MSP1-19, and MSP2 were higher among children with concurrent P. falciparum infection at enrollment than among uninfected children; for MSP1-19, this association was observed only in children of <9 years (Fig. 2). For AMA-1 and MSP1-19, the prevalences of IgG1 and IgG3 were also higher in infected children than in uninfected children (see Table S2 in the supplemental material). For MSP2, only the prevalence of the less dominant IgG1 was higher among infected children (see Table S2 in the supplemental material). When the MSP2 allelic type was considered, a higher prevalence of allele-specific IgG3 was associated with infection only by the corresponding genotype (i.e., the prevalence of MSP2 3D7 IgG3 was higher among children with 3D7-type infections than among children with FC27-type infections) (Fig. 5). A similar, although less pronounced, strain-specific boosting was observed for IgG1 (data not shown).
FIG. 5.
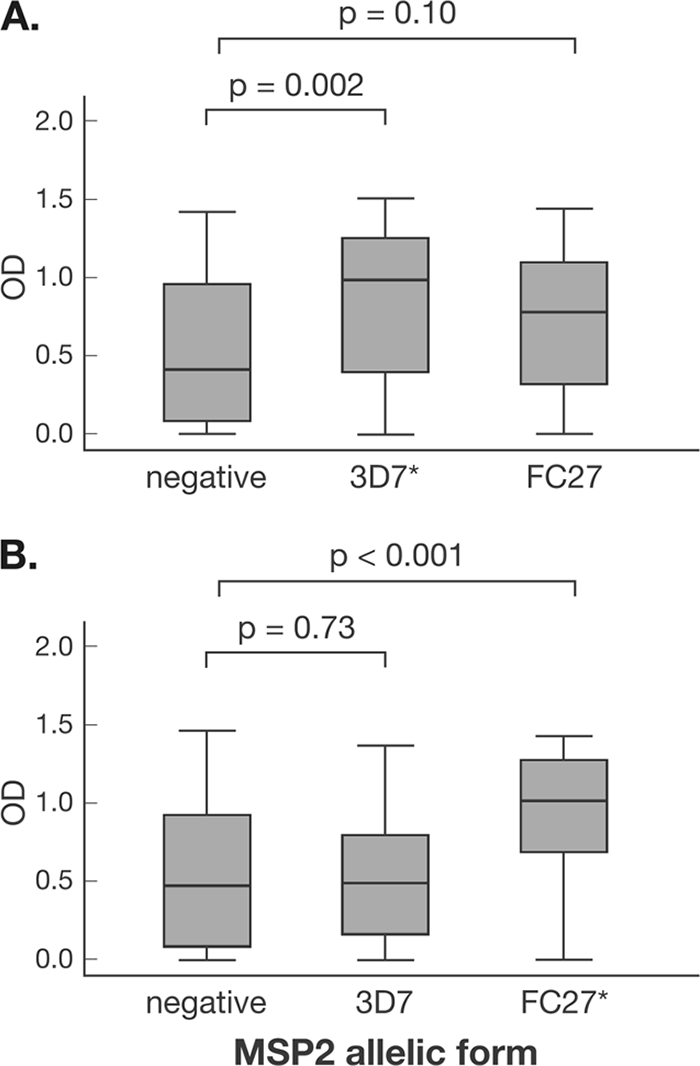
Association between MSP2 3D7 (A) and MSP2 FC27 (B) IgG3 responses and MSP2 genotype of concurrent P. falciparum infection. P. falciparum infection status was determined by PCR. *, results include mixed 3D7/FC27 infections. Data are plotted as box-and-whisker plots (boxes show medians and interquartile ranges; error bars show 95% confidence intervals).
Relatedness of subclass responses to different antigens within individuals.
For all antigens, the total IgG levels were strongly correlated with the IgG1 and IgG3 responses (P < 0.001) (Table 1). Correlations with the total IgG levels were highest for the predominant subclass for each antigen (higher for IgG1 than IgG3 for AMA-1 and MSP1-19, but higher for IgG3 than IgG1 for MSP2). Antibody responses were also significantly correlated between different antigens, although the strengths of the correlations varied substantially (median rho = 0.42; interquartile range, 0.36 to 0.48) (Table 1).
As subclass responses varied between different individuals, we examined whether individuals might have a propensity to make a particular subclass response irrespective of the antigen. When comparing individual responses to different merozoite antigens, we found no evidence that this was the case. Individuals who were high responders (defined as >50th percentile; n = 103) for IgG3 to AMA-1 (3D7 variant) were no more likely to be high responders for IgG3 to MSP1-19 than for IgG1 to MSP1-19 (67% were high responders for IgG3, versus 64% for IgG1; P value was NS). Similarly, high responders for IgG1 to AMA-1 (3D7) were no more likely to be high responders for IgG1 to MSP1-19 than for IgG3 to MSP1-19 (70% were high responders for IgG1, versus 62% for IgG3; P value was NS). Similar results were obtained when comparing AMA-1 3D7 subclass responses of high responders for IgG1 and IgG3 to MSP2 (data not shown).
We then examined the relatedness of subclass responses between different forms of the same antigen. High responders for IgG3 to AMA-1 3D7 were significantly more likely to be high responders for IgG3 to AMA-1 W2mef than high responders for IgG1 to AMA-1 W2mef (92% were high responders for IgG3, versus 64% for IgG1; P = 0.005). A similar association was observed when comparing high responders for IgG1 to AMA-1 3D7 with high responders for IgG1 to AMA-1 W2mef (86% were high responders for IgG1, versus 67% for IgG3; P = 0.052). The IgG subclass responses to the two AMA-1 variants were also strongly correlated (n = 73; IgG1 rho = 0.92; IgG3 rho = 0.92; P < 0.001). Conversely, there was little evidence of relatedness between IgG subclass responses to the two MSP2 alleles. High responders for IgG1 to MSP2 3D7 were no more likely to be high responders for IgG1 to MSP2 FC27 than for IgG3 to MSP2 FC27 (67% were high responders for IgG1, versus 61% for IgG3; P value was NS). Similar observations were made with MSP2 IgG3 responses (data not shown). IgG subclass reactivities against the 3D7 and FC27 alleles were significantly correlated (n = 199; IgG1 rho = 0.36; IgG3 rho = 0.37; P < 0.001) but not as strongly as those observed for AMA-1.
Association between subclass response and risk of reinfection, high-density parasitemia, and symptomatic malaria.
We examined associations between antibodies and symptomatic malaria, reinfection, or parasitemias of different densities (Fig. 6). Univariate analyses established that the risk of P. falciparum malaria was associated with older age and location of residence but not with other demographic parameters (30); therefore, analyses of associations between antibodies and malaria risk were adjusted for location and age. Erythrocyte genetic polymorphisms (SAO, Gerbich, α+-thalassemia, and CR1 polymorphisms) were not associated with risk of P. falciparum malaria (E. Lin, P. Michon, and I. Mueller, unpublished data).
FIG. 6.
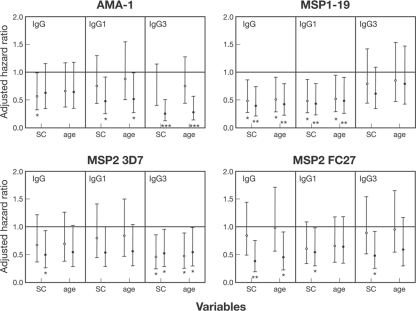
Association between IgG antibody responses to merozoite antigens and protection against symptomatic P. falciparum malaria. Children were stratified into groups of low, moderate, and high responders (based on tertiles) in order to test for associations between antibody responses and risk of symptomatic malaria (defined as parasitemia of >5,000 parasites/μl and fever). Values represent AHRs ± 95% confidence intervals, adjusted for spatial confounders (SC) (attendance at Mugil school and living 1 km from seaboard) or age and SC. Open circles, AHRs for medium versus low responders; filled circles, AHRs for high versus low responders. *, P < 0.05; **, P < 0.01; ***, P < 0.001. The 3D7 allele of MSP1-19 and AMA-1 was used.
Antibody levels were grouped as high, medium, or low, based on tertiles (which divide the data into three equally sized groups), and related to the risk of symptomatic malaria. The strongest association between IgG subclass reactivity to merozoite antigens and protection from symptomatic malaria was observed for IgG3 to AMA-1. After adjustment for location, a strong reduction in risk (i.e., evidence of protection) was found for high (compared to low) levels of AMA-1 IgG3 (adjusted hazard ratio [AHR] of 0.23, P < 0.001). There was also a smaller reduction in risk among children with high IgG1 responses (AHR of 0.51, P = 0.028). However, in a model combining IgG1 and IgG3 responses, only high IgG3 responses were predictive of protection. Total IgG to AMA-1 was associated only weakly with protection against symptomatic malaria (AHR of 0.56 for low versus moderate levels, P = 0.045; AHR of 0.63 for high versus low levels, P = 0.14). Individuals with high levels of IgG1 and IgG to MSP1-19 had a significantly reduced risk of malaria compared to those with low levels, after adjusting for location (AHR of 0.43 for IgG1, P = 0.007; AHR of 0.39 for total IgG, P = 0.004). Individuals with medium IgG1 and IgG levels also had a reduced risk of malaria compared to those with low levels (AHR of 0.48 for IgG1, P = 0.015; AHR of 0.48 for total IgG, P = 0.014). In contrast, IgG3 responses to MSP1-19 were not associated with a reduced risk of malaria.
Medium (AHR of 0.47, P = 0.015) and high (AHR of 0.53, P = 0.037) IgG3 responses to MSP2 3D7 were associated with protection, after adjusting for location (AHR of 0.53, P = 0.037), whereas IgG1 was not. In contrast, high IgG1 (AHR of 0.55, P = 0.046) and IgG3 (AHR of 0.49, P = 0.027) responses to MSP2 FC27 were associated with a reduced risk of symptomatic malaria. Performing the analysis using the combined IgG3 responses to both MSP2 alleles did not substantially change the strength of the association with malaria risk. High IgG responses to MSP2 FC27 were significantly associated with protection against symptomatic malaria, but the association with IgG to MSP2 3D7 was of borderline significance (3D7 AHR of 0.50, P = 0.031; FC27 AHR of 0.39, P = 0.006).
We further investigated the association between antibodies to MSP2 and protection from malaria by examining the MSP2 genotype of the first symptomatic episode in relation to MSP2 allele-specific antibodies at baseline. Of 96 clinical episodes with available DNA, 56 (58.3%) were caused by a 3D7-type infection, 24 (25%) were caused by an FC27-type infection, and 16 (16.7%) by mixed 3D7/FC27 infections. If only episodes containing 3D7-type infections were considered, high levels of IgG3 to MSP2 3D7 were associated with a reduced risk of symptomatic malaria (AHR of 0.43, P = 0.026). However, high levels of IgG1 (AHR of 0.42, P = 0.03) and IgG3 (AHR of 0.35, P = 0.009) to MSP2 FC27 were also associated with a reduced risk of symptomatic malaria containing 3D7-type infections. Due to the low numbers of FC27-type infections, it was not possible to examine the associations between MSP2 FC27 antibodies and FC27-type malaria episodes.
After further adjusting analyses for age in addition to location, the associations between the reduced risk of symptomatic malaria (comparing high and low responders) and IgG3 to AMA-1 (AHR of 0.28, P < 0.021), IgG1 to AMA-1 (AHR of 0.49, P = 0.025), and IgG1 to MSP1-19 (AHR of 0.48, P = 0.024) remained significant (Fig. 6). Age adjustment had a more substantial effect on associations between MSP2 antibodies and protection; associations with high levels of IgG3 to MSP2 3D7 remained significant (AHR of 0.54, P = 0.041), whereas associations with high levels of IgG1 or IgG3 to MSP2 FC27 (AHR of 0.58 [P = 0.070] or AHR of 0.54 [P = 0.065], respectively) were of borderline significance.
To further understand the contribution of specific antibody responses to protection from symptomatic malaria, we performed a multivariate analysis (adjusted for location) including subclass-specific responses to all four antigens. Only high anti-AMA-1 IgG3 responses (AHR of 0.30 for high versus medium and low levels, P = 0.001) and moderate and high anti-MSP1-19 IgG1 responses (AHR of 0.58 for high and medium versus low levels, P = 0.036) were predictive of a reduced risk of symptomatic malaria. Adjustment for age did not change the significance or size of these associations (data not shown).
We also examined associations between antibodies and reinfection with a parasitemia of any density (as detected by PCR or light microscopy) or episodes of moderate-density (>500 parasites/μl) or high-density (>5,000 parasites/μl) parasitemia (Fig. 7) (analysis was based on the time to first episode). There was a weak association of borderline statistical significance between high AMA-1 IgG3 levels and time to reinfection, as detected by PCR (HR of 0.72, P = 0.071) (Fig. 7). There were no other total IgG or subclass responses significantly associated with time to reinfection, as determined by PCR or light microscopy (data not shown for MSP2). In contrast, high AMA-1 IgG3 levels were more strongly associated with protection from moderate-density parasitemia (HR of 0.60, P = 0.017) and most strongly associated with protection from high-density parasitemia (HR of 0.24, P < 0.001). High levels of IgG1 to MSP1-19 were significantly associated with protection from only high-density parasitemia (HR of 0.40, P = 0.003) and not moderate-density parasitemia.
FIG. 7.
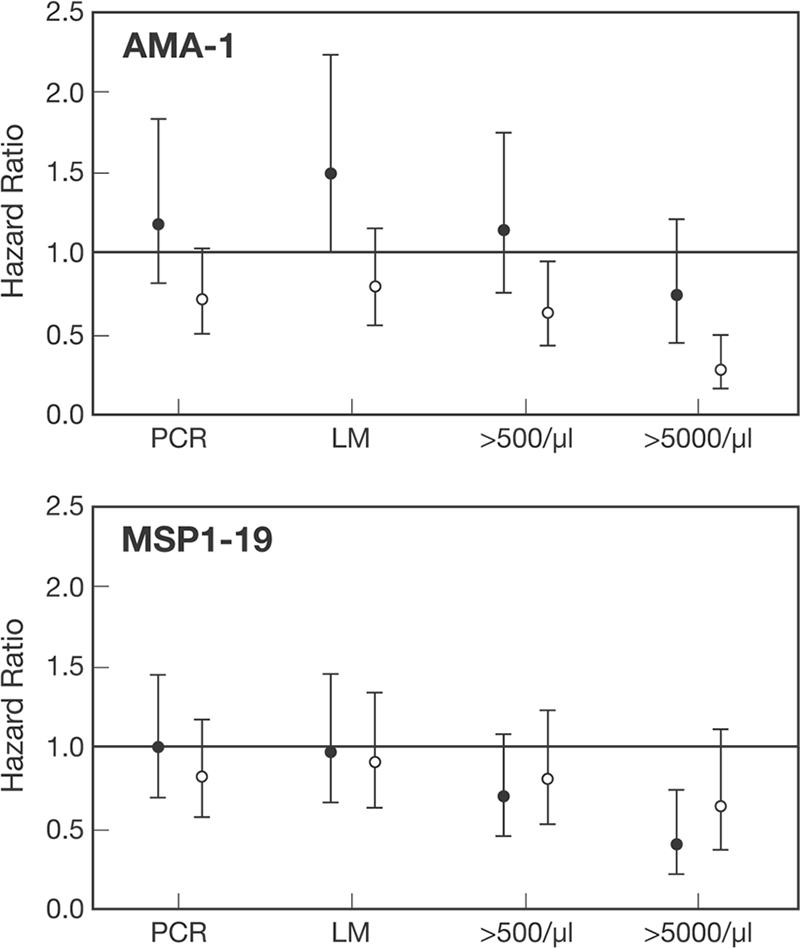
Association between subclass-specific antibodies against merozoite antigens and P. falciparum blood-stage infections of different densities. Values represent HRs ± 95% confidence intervals. Filled circles, IgG1; open circles, IgG3. PCR, P. falciparum infection determined by PCR; LM, P. falciparum infection determined by light microscopy; >500/μl, P. falciparum infection with a density of >500 parasites/μl; >5000/μl, P. falciparum infection with a density of >5,000 parasites/μl. The 3D7 allele was used for each antigen.
DISCUSSION
An important question in understanding human immunity to malaria is whether antibodies to merozoite antigens may contribute to protection against parasitization per se or only act to prevent symptomatic malaria. Our results demonstrate that IgG subclass-specific responses to merozoite antigens are significantly associated with protection from high-density parasitemia and symptomatic malaria but not against parasitization per se. Several previous studies have examined associations between merozoite antibodies and risk of malaria illness, but very few have examined associations with reinfection. To our knowledge, none have examined associations between IgG subclass-specific responses and reinfection, high-density parasitemia, and symptomatic malaria in the same study. To address these important questions in our study, we cleared parasitemia among participants at enrollment with a highly effective treatment (>90% cure rate), actively screened for reparasitization using sensitive molecular methods, and genotyped infecting parasites to distinguish reinfection from treatment failure. Furthermore, we examined IgG subclass responses in addition to total IgG responses. Although there was a weak association between IgG3 to AMA-1 and reinfection, this was of borderline statistical significance. The association with risk of high-density parasitemia was much stronger and highly significant for IgG3 to AMA-1 and IgG1 to MSP1-19. No other IgG or subclass responses were associated with risk of reinfection, as detected by PCR or light microcopy. Instead, our results suggest that antibodies to merozoite antigens mediate their protective effect by control of blood-stage parasitemia, thereby preventing high-density infections, which has important implications for understanding immunity. High-density parasitemia and overall parasite biomass are linked with the pathogenesis of severe malaria (19), suggesting that an ability to control parasitemia, but not necessarily prevent blood-stage parasitemia, may afford substantial protection from severe and complicated malaria.
The strongest association with protection from symptomatic malaria in multivariate analysis was for IgG3 against AMA-1. Although IgG3 was predictive of protection, IgG1 was only weakly associated with protection, even though it was the dominant subclass and correlated with age and exposure. Furthermore, total IgG to AMA-1 was only weakly associated with protection, emphasizing the value of examining subclasses and not just total IgG in studies of human immunity. The association between protection and IgG3 is unlikely to be explained simply by these antibodies being a marker of an alternate protective immune response. AMA-1 IgG3 was not predictive of the IgG1 or IgG3 response to MSP1-19 or MSP2 and did not indicate an overall propensity of protected individuals to have a greater IgG3 response to malaria antigens; the IgG3 response to AMA-1 and its association with protection appeared specific to AMA-1. These results have important implications for AMA-1 vaccine development, as a recent phase 1 clinical trial demonstrated that the vaccine construct induced predominantly IgG1 (27). Our finding differs from a recent study (31) in which IgG1, but not IgG3, to AMA-1 was associated with protection from symptomatic malaria in an African population. Differences in population genetics, transmission levels, and age groups studied may account for the differences between studies.
IgG1 responses to MSP1-19 were also associated with protection against symptomatic malaria, which remained significant in multivariate analysis. This finding is in agreement with some studies conducted previously with African populations, but not others (5, 11, 15, 25, 31, 39). IgG3 reactivity to MSP1-19 was low overall, which may also explain why it was not associated with a reduced risk of malaria. Consistent with the association with protection, AMA-1 and MSP1-19 are thought to be essential for erythrocyte invasion (10) and have been identified as targets of human invasion-inhibitory antibodies (for example, see references 13 and 21).
Antibodies to MSP2 had a weaker association with protection, and this association did not remain significant in a multivariate analysis of antibody responses. Although there was evidence that levels of allele-specific antibodies were higher if infected with that particular allele, there was not clear evidence of allele-specific protection by antibodies to MSP2 when the genotype of episodes was prospectively related to allele-specific antibodies. MSP2 3D7 IgG3 and MSP2 FC27 IgG1 and IgG3 were all associated with a reduced risk of symptomatic 3D7-type malaria. This may indicate that a component of the protective antibody response is directed against the conserved region of MSP2. It has been shown in previous studies that total IgG and IgG3 to MSP2 are associated with a reduced risk of symptomatic malaria (1, 18, 29, 37, 45). Antibodies to the variable region of MSP2 3D7 are thought to have contributed to the efficacy of the combination B malaria vaccine (18).
In this study, IgG1 and IgG3 levels of reactivity to each antigen increased with age and exposure and were higher among those with active infection. In previous studies, it has been suggested that increasing age (and malaria exposure) is accompanied by an increasing polarization of the subclass response to malaria antigens (45, 48); that is, an individual's ability to produce both IgG1 and IgG3 diminishes and only IgG1 or IgG3 is produced. For example, it has been suggested that a mixed IgG1/IgG3 response to MSP2 in young children evolves into an IgG3-only response as age increases (45, 48). In our cohort, we did not observe polarization of responses; for all antigens, the prevalence and levels of IgG1 and IgG3 increased significantly with age and exposure (measured as antibody reactivity to P. falciparum schizont extract). As the age range of our primary cohort was narrow, we studied a second cohort of residents of the same geographical area with a wider age range. Both the dominant and subdominant antibody responses increased with age for all antigens, supporting our previous observation. Reasons for differences between the studies may relate to differences in host population genetics, malaria transmission intensity, and sensitivity of reagents used.
We report here that subclass-specific responses to different antigens are not related among individuals; antigen-specific subclass responses appear to be generated independently. Only in the instance of responses to different AMA-1 variants was there evidence of relatedness. This may be because antibodies are largely directed against conserved epitopes of AMA-1 rather than due to an inherent bias among individuals to produce a particular subclass. The lack of a predisposition to produce a particular subclass response regardless of the antigen further supports the hypothesis that the factors influencing the subclass response are determined mainly by the antigen rather than the host. Prior studies of mice identified a T-cell epitope in MSP2 that appears to promote cytophilic IgG subclass responses (47). Although polymorphisms in AMA-1 were not associated with differences in subclass responses, it is conceivable that the component of the antibody response directed against the conserved epitopes may have affected our ability to detect differences in subclass responses to allele-specific epitopes. Some difference in the subclass responses to MSP2 alleles was observed, and IgG1 and IgG3 responses to the two alleles were not as strongly correlated as other responses and were not significantly related. These observations suggest that responses to each allele may be determined independently and that, in principle, polymorphisms in antigens can influence the profile of IgG subclasses induced.
Our observation that the subclass response to MSP1-19 is a mixed IgG1/IgG3 response, with IgG1 being predominant, and that the response to MSP2 is predominantly IgG3 is generally in agreement with previous studies (8, 11, 15, 25, 29, 31, 37, 42, 43, 46, 48). Studies of African populations report that IgG1 is preferentially induced, with some IgG3 (31, 37); however, one study reported very little or no IgG3 to AMA-1 (48). In our study, although IgG1 was the predominant response to AMA-1, it was also a mixed IgG1/IgG3 response. To confirm our results, we validated our subclass typing reagents against two other commercially available reagents; furthermore, testing plasma from Kenyan children and adults, we detected both IgG1 and IgG3 to AMA-1, with IgG1 being predominant (D. Stanisic and F. McCallum, unpublished data).
In conclusion, these findings have significant implications for understanding and measuring immunity and for the development and evaluation of blood-stage vaccines. They provide strong support for the role of IgG subclass-specific antibodies to merozoite antigens in protection from symptomatic malaria and high-density infections and important insights into how IgG subclass responses are acquired.
Supplementary Material
Acknowledgments
Thanks to all participants in the study and to Joanne Chesson for technical assistance. Thanks to Danny Wilson and Jack Taraika for assistance with sample processing.
Funding was provided by the National Health and Medical Research Council of Australia (project grant and career development award to J. Beeson; postgraduate research fellowships to F. McCallum and J. Richards).
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 12 January 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.al-Yaman, F., B. Genton, R. F. Anders, M. Falk, T. Triglia, D. Lewis, J. Hii, H. P. Beck, and M. P. Alpers. 1994. Relationship between humoral response to Plasmodium falciparum merozoite surface antigen-2 and malaria morbidity in a highly endemic area of Papua New Guinea. Am. J. Trop. Med. Hyg. 51593-602. [DOI] [PubMed] [Google Scholar]
- 2.Aucan, C., Y. Traore, F. Fumoux, and P. Rihet. 2001. Familial correlation of immunoglobulin G subclass responses to Plasmodium falciparum antigens in Burkina Faso. Infect. Immun. 69996-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouharoun-Tayoun, H., P. Attanath, A. Sabchareon, T. Chongsuphajaisiddhi, and P. Druilhe. 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 1721633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouharoun-Tayoun, H., and P. Druilhe. 1992. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect. Immun. 601473-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braga, E., R. Barros, T. Reis, C. Fontes, C. Morais, M. Martins, and A. Krettli. 2002. Association of the IgG response to Plasmodium falciparum merozoite protein (C-terminal 19kD) with clinical immunity to malaria in the Brazilian Amazon region. Am. J. Trop. Med. Hyg. 66461-466. [DOI] [PubMed] [Google Scholar]
- 6.Branch, O., V. Udhayakumar, A. Hightower, A. Oloo, W. Hawley, B. Nahlen, P. Bloland, D. Kaslow, and A. Lal. 1998. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kilodalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia and anemia. Am. J. Trop. Med. Hyg. 58211-219. [DOI] [PubMed] [Google Scholar]
- 7.Bredius, R. G., C. A. Fijen, M. De Haas, E. J. Kuijper, R. S. Weening, J. G. Van de Winkel, and T. A. Out. 1994. Role of neutrophil Fc gamma RIIa (CD32) and Fc gamma RIIIb (CD16) polymorphic forms in phagocytosis of human IgG1-and IgG3-opsonised bacteria and erythrocytes. Immunology 83624-630. [PMC free article] [PubMed] [Google Scholar]
- 8.Cavanagh, D. R., C. Dobaño, I. M. Elhassan, K. Marsh, A. Elhassan, L. Hviid, E. At Tahir, G. Khalil, T. G. Theander, D. E. Arnot, and J. S. McBride. 2001. Differential patterns of human immunoglobulin G subclass responses to distinct regions of a single protein, the merozoite surface protein 1 of Plasmodium falciparum. Infect. Immun. 691207-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, S., G. Butcher, and R. Crandall. 1969. Action of malarial antibody in vitro. Nature 223368-371. [DOI] [PubMed] [Google Scholar]
- 10.Cowman, A., and B. Crabb. 2006. Invasion of red blood cells by malaria parasites. Cell 124755-766. [DOI] [PubMed] [Google Scholar]
- 11.Dodoo, D., T. G. Theander, J. A. Kurtzhals, K. Koram, E. Riley, B. D. Akanmori, F. K. Nkrumah, and L. Hviid. 1999. Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein 1 in Ghanaian children are not associated with protection from clinical malaria. Infect. Immun. 672131-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutta, S., D. Kaushal, L. Ware, S. Puri, N. Kaushal, A. Narula, D. Upadhyaya, and D. Lanar. 2005. Merozoite surface protein 1 of Plasmodium vivax induces a protective response against Plasmodium cynomolgi challenge in rhesus monkeys. Infect. Immun. 735936-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan, A., P. Burghaus, P. Druilhe, A. Holder, and E. Riley. 1999. Human antibodies to the 19kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 21133-139. [DOI] [PubMed] [Google Scholar]
- 14.Egan, A. F., J. A. Chappel, P. A. Burghaus, J. S. Morris, J. S. McBride, A. A. Holder, D. C. Kaslow, and E. M. Riley. 1995. Serum antibodies from malaria-exposed people recognize conserved epitopes formed by the two epidermal growth factor motifs of MSP1-19, the carboxy-terminal fragment of the major merozoite surface protein of Plasmodium falciparum. Infect. Immun. 63456-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egan, A. F., J. Morris, G. Barnish, S. Allen, B. M. Greenwood, D. C. Kaslow, A. A. Holder, and E. M. Riley. 1996. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J. Infect. Dis. 173765-769. [DOI] [PubMed] [Google Scholar]
- 16.Felger, I., and H. Beck. 2002. Genotyping of Plasmodium falciparum: RFLP analysis. Methods Mol. Med. 72117-129. [DOI] [PubMed] [Google Scholar]
- 17.Garraud, O., R. Perraut, A. Diouf, W. S. Nambei, A. Tall, A. Spiegel, S. Longacre, D. C. Kaslow, H. Jouin, D. Mattei, G. M. Engler, T. B. Nutman, E. M. Riley, and O. Mercereau-Puijalon. 2002. Regulation of antigen-specific immunoglobulin G subclasses in response to conserved and polymorphic Plasmodium falciparum antigens in an in vitro model. Infect. Immun. 702820-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genton, B., I. Betuela, I. Felger, F. Al-Yaman, R. Anders, A. Saul, L. Rare, M. Baisor, K. Lorry, G. Brown, D. Pye, D. Irving, T. Smith, H. Beck, and M. Alpers. 2002. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J. Infect. Dis. 185820-827. [DOI] [PubMed] [Google Scholar]
- 19.Gravenor, M., M. van Hensbroek, and D. Kwiatkowski. 1998. Estimating sequestered parasite population dynamics in cerebral malaria. Proc. Natl. Acad. Sci. USA 957620-7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Healer, J., V. Murphy, A. Hodder, R. Masciantonio, A. Gemmill, R. Anders, A. Cowman, and A. Batchelor. 2004. Allelic polymorphisms in apical membrane antigen-1 are responsible for evasion of antibody-mediated inhibition in Plasmodium falciparum. Mol. Microbiol. 52159-168. [DOI] [PubMed] [Google Scholar]
- 21.Hodder, A., P. Crewther, and R. Anders. 2001. Specificity of the protective antibody response to apical membrane antigen 1. Infect. Immun. 693286-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irion, A., I. Felger, S. Abdulla, T. Smith, R. Mull, M. Tanner, C. Hatz, and H. Beck. 1998. Distinction of recrudescences from new infections from PCR-RFLP analysis in a comparative trial of CGP56697 and chloroquine in Tanzanian children. Trop. Med. Int. Health 3490-497. [DOI] [PubMed] [Google Scholar]
- 23.John, C. C., A. M. Moormann, D. C. Pregibon, P. O. Sumba, M. M. McHugh, D. L. Narum, D. E. Lanar, M. D. Schluchter, and J. W. Kazura. 2005. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am. J. Trop. Med. Hyg. 73222-228. [PubMed] [Google Scholar]
- 24.Khusmith, S., P. Druilhe, and M. Gentilini. 1982. Enhanced Plasmodium falciparum merozoite phagocytosis by monocytes from immune individuals. Infect. Immun. 35874-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitua, A. Y., H. Urassa, M. Wechsler, T. Smith, P. Vounatsou, N. A. Weiss, P. L. Alonso, and M. Tanner. 1999. Antibodies against Plasmodium falciparum vaccine candidates in infants in an area of intense and perennial transmission: relationships with clinical malaria and with entomological inoculation rates. Parasite Immunol. 21307-317. [DOI] [PubMed] [Google Scholar]
- 26.Langhorne, J., F. Ndungu, A. Sponaas, and K. Marsh. 2008. Immunity to malaria: more questions than answers. Nat. Immunol. 9725-732. [DOI] [PubMed] [Google Scholar]
- 27.Malkin, E., D. Diemert, J. McArthur, J. Perreault, A. Miles, B. Giersing, G. Mullen, A. Orcutt, O. Muratova, M. Awkal, H. Zhou, J. Wang, A. Stowers, C. Long, S. Mahanty, L. Miller, A. Saul, and A. Durbin. 2005. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect. Immun. 733677-3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNamara, D., J. Thomson, L. Kasehagen, and P. Zimmerman. 2004. Development of multiplex PCR-ligase detection reaction assay for diagnosis of infection by the four parasite species causing malaria in humans. J. Clin. Microbiol. 422403-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metzger, W., D. Okenu, D. Cavanagh, J. Robinson, K. Bojang, H. Weiss, J. McBride, B. Greenwood, and D. Conway. 2003. Serum IgG3 to the Plasmodium falciparum merozoite surface protein 2 is strongly associated with a reduced prospective risk of malaria. Parasite Immunol. 25307-312. [DOI] [PubMed] [Google Scholar]
- 30.Michon, P., J. Cole-Tobian, E. Dabod, S. Schoepflin, J. Igu, M. Susapu, N. Tarongka, P. Zimmerman, J. Reeder, J. G. Beeson, L. Schofield, C. King, and I. Mueller. 2007. The risk of malarial infections and disease in Papua New Guinean children. Am. J. Trop. Med. Hyg. 76997-1008. [PMC free article] [PubMed] [Google Scholar]
- 31.Nebie, I., A. Diarra, A. Ouedraogo, I. Soulama, E. Bougouma, A. Tiono, A. Konate, R. Chilengi, M. Theisen, D. Dodoo, E. Remarque, S. Bosomprah, P. Milligan, and S. Sirima. 2008. Humoral responses to Plasmodium falciparum blood-stage antigens and association with incidence of clinical malaria in children living in an area of seasonal malaria transmission in Burkina Faso, West Africa. Infect. Immun. 76759-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nimmerjahn, F., and J. Ravetch. 2008. Fc gamma receptors as regulators of the immune response. Nat. Rev. Immunol. 834-47. [DOI] [PubMed] [Google Scholar]
- 33.Ntoumi, F., M. Ekala, M. Makuwa, F. Lekoulou, O. Mercereau-Puijalon, and P. Deloron. 2002. Sickle cell trait carriage: imbalanced distribution of IgG subclass antibodies reactive to Plasmodium falciparum family-specific MSP2 peptides in serum samples from Gabonese children. Immunol. Lett. 849-16. [DOI] [PubMed] [Google Scholar]
- 34.Ntoumi, F., L. Flori, P. Mayengue, D. Maya, S. Issifou, P. Deleron, B. Lell, P. Kremsner, and P. Rihet. 2005. Influence of carriage of hemoglobin AS and the FcG receptor IIa-R131 allele on levels of immunoglobulin G2 antibodies to Plasmodium falciparum merozoite antigens in Gabonese children. J. Infect. Dis. 1921975-1980. [DOI] [PubMed] [Google Scholar]
- 35.Perraut, R., L. Marrama, B. Diouf, C. Sokhna, A. Tall, P. Nabeth, J. Trappe, S. Longacre, and O. Mercereau-Puijalon. 2005. Antibodies to the conserved C-terminal domain of the Plasmodium falciparum merozoite surface protein 1 and to the merozoite extract and their relationship with in vitro inhibitory antibodies and protection against clinical malaria in a Senegalese village. J. Infect. Dis. 191264-271. [DOI] [PubMed] [Google Scholar]
- 36.Persson, K., F. McCallum, L. Reiling, N. Lister, J. Stubbs, A. Cowman, K. Marsh, and J. G. Beeson. 2008. Variation in use of erythrocyte pathways by Plasmodium falciparum mediates evasion of human inhibitory antibodies. J. Clin. Investig. 118342-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polley, S., D. Conway, D. Cavanagh, J. McBride, B. Lowe, T. Williams, T. Mwangi, and K. Marsh. 2006. High levels of serum antibodies to merozoite surface protein 2 of Plasmodium falciparum are associated with reduced risk of clinical malaria in coastal Kenya. Vaccine 244233-4246. [DOI] [PubMed] [Google Scholar]
- 38.Polley, S., T. Mwangi, C. Kocken, A. Thomas, S. Dutta, D. Lanar, E. Remarque, A. Ross, T. Williams, G. Mwambingu, B. Lowe, D. Conway, and K. Marsh. 2004. Human antibodies to recombinant protein constructs of Plasmodium falciparum apical membrane antigen 1 (AMA1) and their associations with protection from malaria. Vaccine 23718-728. [DOI] [PubMed] [Google Scholar]
- 39.Roussilhon, C., C. Oeuvray, C. Muller-Graf, A. Tall, C. Rogier, J. Trape, M. Theisen, A. Balde, J. Perignon, and P. Druilhe. 2007. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. 41791-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rzepczyk, C. M., K. Hale, N. Woodroffe, A. Bobogare, P. Csurhes, A. Ishii, and A. Ferrante. 1997. Humoral immune responses of Solomon Islanders to the merozoite surface antigen 2 of Plasmodium falciparum show pronounced skewing towards antibodies of the immunoglobulin G3 subclass. Infect. Immun. 651098-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scopel, K., C. Fontes, M. Ferreira, and E. Braga. 2006. Factors associated with immunoglobulin G subclass polarization in naturally acquired antibodies in Plasmodium falciparum merozoite surface proteins: a cross-sectional survey in Brazilian Amazonia. Clin. Vaccine Immunol. 13810-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scopel, K., C. Fontes, M. Ferreira, and E. Braga. 2005. Plasmodium falciparum: IgG subclass antibody response to merozoite surface protein-1 among Amazonian gold miners, in relation to infection status and disease expression. Exp. Parasitol. 109124-134. [DOI] [PubMed] [Google Scholar]
- 43.Shi, Y., U. Sayed, S. Quary, J. Roberts, V. Udhayakumar, A. Oloo, W. Hawley, D. Kaslow, B. Nahlem, and A. Lal. 1996. Natural immune response to the C-terminal 19-kilodalton domain of Plasmodium falciparum merozoite surface protein 1. Infect. Immun. 642716-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor, R. R., S. J. Allen, B. M. Greenwood, and E. M. Riley. 1998. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am. J. Trop. Med. Hyg. 58406-413. [DOI] [PubMed] [Google Scholar]
- 46.Taylor, R. R., D. B. Smith, V. J. Robinson, J. S. McBride, and E. M. Riley. 1995. Human antibody response to Plasmodium falciparum merozoite surface protein 2 is serogroup specific and predominantly of the immunoglobulin G3 subclass. Infect. Immun. 634382-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tongren, J., P. Corran, W. Jarra, J. Langhorne, and E. Riley. 2005. Epitope-specific regulation of immunoglobulin class switching in mice immunized with malarial merozoite surface proteins. Infect. Immun. 738119-8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tongren, J., C. Drakeley, S. McDonald, H. Reyburn, A. Manjurano, W. Nkya, M. Lemnge, C. Gowda, J. Todd, P. Corran, and E. Riley. 2006. Target antigen, age, and duration of antigen exposure independently regulate immunoglobulin G subclass switching in malaria. Infect. Immun. 74257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.