Abstract
Eighteen clustered cases of meningococcal disease associated with B:2a:P1.5 strains doubled the annual incidence up to 4.3 × 105 in Navarra, Spain, in 2007. Eleven percent of cases were fatalities, and 74% of cases were individuals 10 to 24 years old. This is the third cluster associated with this strain in northern Spain since 2001.
Neisseria meningitidis is an exclusively human pathogen causing sepsis and meningitis (13). Its capsular polysaccharide is one of the most important virulence factors, defining the serogroup and being targeted for the currently available A, C, Y, and W-135 vaccines. N. meningitidis meningococci from serogroups B, C, Y, and W-135 express capsules composed of polysialic acid or sialic acid linked to glucose or galactose, while the capsules of meningococci from serogroup A are composed of N-acetyl mannosamine-1-phosphate. Meningococcal capsule switching appears to occur among sialic acid-expressing strains by allelic replacement of the sialic acid capsule polymerase. Since the genetic structure responsible for capsule expression in group A meningococci is different, homologous recombination with conversion of serogroup A strains to sialic acid capsule-expressing strains (by introduction of a sialic acid capsule biosynthetic operon) may be less likely (14). This type of genetic event has been described to occur in both outbreak and nonoutbreak situations, and it might happen extraordinarily fast in vivo (16).
In Spain, an increasing number of serogroup C meningococcal strains was observed in the second half of the 1990s (5), first associated with C:2b:P1.5,2 ST8 strains until 1999 (3) and since then with C:2a:P1.5 ST11 isolates. In response, a mass immunization campaign focusing on members of the population that were between 18 months and 19 years old was implemented, first with the polysaccharide A+C vaccine in most of the country during 1997 and then with the new C conjugate polysaccharide vaccines introduced into the Spanish routine vaccination schedule in 2000 (4). The efficacy of both interventions was quite high (6, 15), but immune pressure might have the potential to select those organisms that have their capsules replaced (4, 8, 10). Although the frequency of the capsular switching event is not known in nature, in a population immunized against meningococci from serogroup C, replacement of serogroup C 2a:P1.5 ST11 by serogroup B 2a:P1.5 ST11 meningococci might easily occur. In fact, since 2000 there have been two clusters associated with B:2a:P1.5 ST11 strains in northern Spain (7, 10, 12).
The aim of this study was to analyze an increase in the incidence rate of meningococcal disease (MD) associated with B:2a:P1.5 strains from 2007 to January 2008 in Navarra, a region located in northern Spain that has 600,000 inhabitants.
The cases were assigned to Navarra when the patients were in this region during the incubation period, i.e., 2 to 10 days before the onset of illness (13).
We analyzed all probable or confirmed cases of MD. A probable case was defined as a clinically compatible case in which gram-negative diplococci from a normally sterile site (e.g., cerebrospinal fluid, blood, or skin scrapings of purpuric lesions) were seen. A confirmed case was defined as a clinically compatible case with either isolation of N. meningitidis or detection of meningococcal DNA in a specimen from a normally sterile site.
For all MD cases, chemoprophylaxis was applied immediately for those who had come in close contact with the patient, both in the household and in school (11).
In Navarra, the MD incidence rate ranged between 1.8 and 3.4 per 105 inhabitants (10 to 19 annual cases) over the 1998 to 2006 period. Following the introduction of group C conjugate vaccine in 2000, the number of cases of serogroup C strains declined from 9 in 1998 to 0 in 2007. In contrast, the number of serogroup B cases was quite stable (6 to 11 annual cases) until 2005, increasing to 16 cases in 2006 and 24 in 2007 (Table 1).
TABLE 1.
MD cases by serogroup and year of diagnosis in Navarra, Spain, from 1998 through January 2008
| Yr | No. of MD cases (incidence rate per 100,000 inhabitants) caused by:
|
Total no. of MD cases (incidence rate per 100,000 inhabitants) | |||
|---|---|---|---|---|---|
| Strain from serogroup C | Strain 2a:P1.5 from serogroup B | Other serogroup B strain | Strain from other/ unknown serogroup | ||
| 1998 | 9 (1.7) | 0 (0) | 6 (1.2) | 0 (0) | 15 (2.9) |
| 1999 | 8 (1.5) | 0 (0) | 7 (1.3) | 3 (0.6) | 18 (3.4) |
| 2000 | 2 (0.4) | 0 (0) | 6 (1.1) | 4 (0.7) | 12 (2.2) |
| 2001 | 3 (0.5) | 0 (0) | 6 (1.1) | 1 (0.2) | 10 (1.8) |
| 2002 | 4 (0.7) | 1 (0.2) | 11 (1.9) | 0 (0) | 16 (2.8) |
| 2003 | 5 (0.9) | 2 (0.3) | 8 (1.4) | 0 (0) | 15 (2.6) |
| 2004 | 2 (0.3) | 1 (0.2) | 7 (1.2) | 1 (0.2) | 11 (1.9) |
| 2005 | 2 (0.3) | 2 (0.3) | 8 (1.3) | 1 (0.2) | 13 (2.2) |
| 2006 | 1 (0.2) | 1 (0.2) | 15 (2.5) | 2 (0.3) | 19 (3.2) |
| 2007 | 0 (0) | 14 (2.3) | 10 (1.7) | 2 (0.3) | 26 (4.3) |
| January 2008 | 0 (0) | 4 (0.7) | 2 (0.3) | 1 (0.2) | 7 (1.2) |
All isolates were sent to the Spanish Reference Laboratory (SRL) for serotyping and, when an isolate was not available, clinical samples were sent for real-time PCR diagnosis using the gene ctrA as a target. Cases were characterized by serotyping/serosubtyping with monoclonal antibodies (3) or genotyping/genosubtyping by porB and porA gene sequencing (1, 2). All B:2a:P1.5 strains isolated were analyzed by multilocus sequence typing (9) and pulsed-field gel electrophoresis after DNA digestion with BglII and compared with those strains isolated in sporadic cases and previous clusters in Spain.
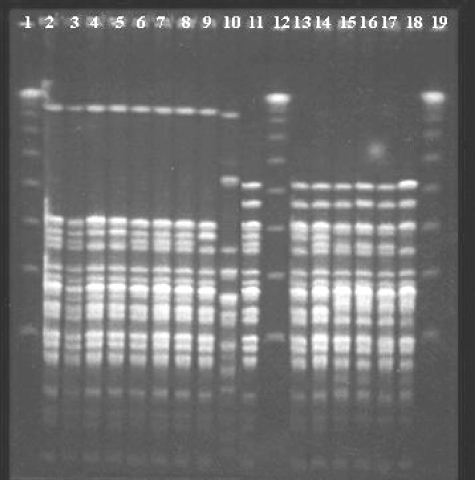
B:2a:P1.5 strains have been isolated from sporadic cases all over Spain since 2001, most of them showing closely related pulse types (PTs) (Fig. 1), representing around 6% of all serogroup B cases analyzed in the SRL during 2006 and 2007. In the 13-month period, from 2007 through January 2008, 18/30 strains were identified as B:2a:P1.5 strains in Navarra, and all 18 of them belonged to clonal complex ST11 (ET15 variant). In Spain, there have been two previous clusters of MD cases associated with B:2a:P1.5 strains, one in the Basque Country (2001 to 2002) and the other one in Castilla-Leon (2006), two regions near Navarra. All isolates from Navarra showed closely related PTs representing closely related patterns of those PTs previously found in either sporadic or outbreak cases (Fig. 1). However, those B:2a:P1.5 strains associated with previous clusters and also those isolated from sporadic cases look like homogeneous clones, showing some bands of difference with the strains associated with the cluster in Navarra. These isolates from Navarra might represent an evolutionary line evolving from the strains associated with previous clusters or they might have originated in an independent event representing a different line (Fig. 1).
FIG. 1.
Pattern profiles obtained by pulsed-field gel electrophoresis after digestion with BglII in B:2a:P1.5 strains. Lanes 1, 12, and 19, molecular weight markers; lanes 2 to 9, B:2a:P1.5 strains isolated in Navarra during the cluster; lane 10, B:NT:P1.2 strain isolated in Navarra during the cluster; lane 11, B:2a:P1.5 strain isolated in the Castilla-Leon cluster in 2006; lanes 13 and 14, B:2a:P1.5 strains isolated in the Basque Country cluster in 2002; lanes 15 to 18, B:2a:P1.5 strains associated with sporadic cases in other Spanish regions.
The comparison of cases caused by B:2a:P1.5 (n = 19) and cases caused by other B strains (n = 25) over the period of 2006 to January 2008 in Navarra showed that infection with B:2a:P1.5 was most frequent among young people between 10 and 24 years old (74% versus 28%; P = 0.005), and cases appeared exclusively in the capital city and in the northern part of the region (100% versus 72%; P = 0.014). All cases caused by B:2a:P1.5 presented with sepsis or meningitis, while 24% of cases caused by other B strains presented with bacteremia (P = 0.0289). Cases associated with B:2a:P1.5 strains showed a quite high but not statistically significant fatality rate (11% versus 0%; P = 0.181). The frequency of associated secondary cases was also higher among B:2a:P1.5-associated cases than among other serogroup B-associated cases but without statistical significance (11% versus 0%; P = 0.181).
Only three cases occurred among close contacts of primary cases. One was a case whose symptoms debuted before chemoprophylaxis was started. The other two had received chemoprophylaxis more than 10 days after the onset of symptoms, and the isolated strains had developed decreased susceptibility to rifampin (MIC, 0.125 mg/liter), which might explain the failure to eradicate nasopharyngeal carriage of the bacteria. However, new contacts with the bacteria also may have occurred in both cases.
Fourteen of the 18 cases of MD caused by B:2a:P1.5 appeared in three well-delimited temporal-spatial clusters; the first one occurred in January 2007 with four cases in the northern part of the region, the second one between June and August 2007 with six cases in the capital city and central area of the region, and the third one in January 2008 with four cases in the northeast part of the region. From February to June 2008, seven additional MD cases caused by serogroup B appeared, but only one was characterized as being caused by B:2a:P1.5.
The results show the spread of these types of isolates in successive clusters of cases that affect mainly adolescents and young adults with severe clinical forms of MD and a high case fatality rate.
The emergence of isolation of these B:2a:P1.5 strains in Spain, coinciding with a progressive extension of meningococcal C vaccination, suggests that strong immunological pressure might be selecting these types of strains that evade the immune response produced by the vaccine. However, other explanations, such as the emergence of new, more virulent variants, could not be totally ruled out. No similar situation has been observed in other European countries using serogroup C conjugate vaccines (8). Use of meningococcal C conjugate vaccines has been successful in Spain, with a dramatic reduction in the number of cases associated with this serogroup. However, we have to be alert to the appearance and spread of these types of meningococcal variants evading immune response.
Acknowledgments
This study was supported in part by the Spanish Fund for Health Research (FIS PI061346).
We do not have any conflicts of interest to disclose with respect to this publication.
Footnotes
Published ahead of print on 17 December 2008.
REFERENCES
- 1.Abad, R., B. Alcalá, C. Salcedo, R. Enríquez, M. J. Uría, P. Diez, and J. A. Vázquez. 2006. Sequencing of the porB gene: a step toward a true characterization of Neisseria meningitidis. Clin. Vaccine Immunol. 131087-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcalá, B., C. Salcedo, L. Arreaza, R. Abad, R. Enríquez, L. De La Fuente, M. J. Uría, and J. A. Vázquez. 2004. Antigenic and/or phase variation of PorA protein in non-subtypable Neisseria meningitidis strains isolated in Spain. J. Med. Microbiol. 53515-518. [DOI] [PubMed] [Google Scholar]
- 3.Alcalá, B., C. Salcedo, L. Arreaza, S. Berrón, L. De La Fuente, and J. A. Vázquez. 2002. The epidemic wave of meningococcal disease in Spain in 1996-1997: probably a consequence of strain displacement. J. Med. Microbiol. 511102-1106. [DOI] [PubMed] [Google Scholar]
- 4.Alcalá, B., L. Arreaza, C. Salcedo, M. J. Uría, L. De La Fuente, and J. A. Vázquez. 2002. Capsule switching among C:2b:P1.2,5 meningococcal epidemic strains after mass immunization campaign, Spain. Emerg. Infect. Dis. 81512-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berrón, S., L. De La Fuente, E. Martín, and J. A. Vázquez. 1998. Increasing incidence of meningococcal disease in Spain associated with a new variant of serogroup C. Eur. J. Clin. Microbiol. Infect. Dis. 1785-89. [DOI] [PubMed] [Google Scholar]
- 6.Cano, R., A. Larrauri, S. Mateo, B. Alcalá, C. Salcedo, and J. A. Vázquez. 2004. Impact of the meningococcal C conjugate vaccine in Spain: an epidemiological and microbiological decision. Euro. Surveill. 911-15. [PubMed] [Google Scholar]
- 7.Cano Portero, R. 2007. Enfermedad meningocócica en España. Análisis de la temporada 2005-2006. Bol. Epidemiol. Semanal. 15169-172. [Google Scholar]
- 8.European Union Invasive Bacterial Surveillance (EU-IBIS) Network. 2006. Invasive Neisseria meningitidis in Europe 2006. Health Protection Agency, London, United Kingdom. http://www.euibis.org/documents/2006_meningo.pdf.
- 9.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 953140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pérez-Trallero, E., D. Vicente, M. Montes, and R. Cisterna. 2002. Positive effect of meningococcal C vaccination on serogroup replacement in Neisseria meningitidis. Lancet 360953. [DOI] [PubMed] [Google Scholar]
- 11.Purcell, B., S. Samuelsson, S. J. Hahné, I. Ehrhard, S. Heuberger, I. Camaroni, A. Charlett, and J. M. Stuart. 2004. Effectiveness of antibiotics in preventing meningococcal disease after a case: systematic review. BMJ 3281339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz Sopeña, C., J. A. Gómez de Caso Canto, L. Mateos Baruque, J. L. Yáñez Ortega, M. J. Rodríguez Recio, A. Dorado Díaz, C. Ruiz Cosín, R. Cano Portero, and J. A. Vázquez. 2007. Estudio de portadores de meningococo ante una agregación temporo-espacial de enfermedad meningocócica en Castilla y León. Gac. Sanit. 2111. [Google Scholar]
- 13.Stephens, D. S., B. Greenwood, and P. Brandtzaeg. 2007. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 3692196-2210. [DOI] [PubMed] [Google Scholar]
- 14.Swartley, J. S., A. A. Marfin, S. Edupuganti, L. J. Liu, P. Cieslak, B. Perkins, J. D. Wenger, and D. S. Stephens. 1997. Capsule switching of Neisseria meningitidis. Proc. Natl. Acad. Sci. USA 94271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vázquez, J. A. 2002. Development of vaccinations against meningococcus: a long, winding, and still unfinished road. Enferm. Infecc. Microbiol. Clin. 20313-315. (In Spanish.) [PubMed] [Google Scholar]
- 16.Vogel, U., H. Claus, and M. Frosch. 2000. Rapid serogroup switching in Neisseria meningitidis. N. Engl. J. Med. 342219-220. [DOI] [PubMed] [Google Scholar]