Abstract
porB DNA sequence analysis and Neisseria gonorrhoeae multiantigen sequence typing (NG-MAST) methods were compared for their abilities to discriminate strains and to identify epidemiologically congruent pairs of N. gonorrhoeae. Both methods provided high-level discrimination of strains. NG-MAST further differentiated large porB-based clusters. However, considerations of cost suggest that porB DNA sequence analysis is a useful tool for preliminary molecular analysis of the epidemiology of N. gonorrhoeae.
Molecular typing methods that differentiate Neisseria gonorrhoeae isolates, coupled with traditional epidemiological methods, have been used to identify circulating clusters of strains and transmission networks (3, 24). DNA sequence analysis of various genes is currently the method of choice for distinguishing N. gonorrhoeae strains, as it provides unambiguous and reproducible information and high discriminatory power, and data can be stored or shared electronically, permitting reliable comparisons to be made between laboratories (10, 11, 23). porB DNA sequence analysis is now commonly used for studying the molecular epidemiology of N. gonorrhoeae (1, 9, 13, 15, 16, 19, 20, 23) and involves the sequencing of either the entire gene or various regions of porB (8, 17-19). The N. gonorrhoeae multiantigen sequence typing (NG-MAST) methodology, which has been applied since 2004 (2, 9, 13, 20, 21), is based on limited DNA sequence analyses of two highly polymorphic loci, porB and tbpB (11). The NG-MAST database, available online (www.ng-mast.net), allows public access for sequence submission and the assignment of sequence types either for porB or tbpB individually or for the assignment of strain types (STs) using a combination of the two loci (11). The objective of the present study was to compare NG-MAST with porB DNA sequence analysis (∼82% of the full-length porB gene) to identify circulating clusters of N. gonorrhoeae isolates in Shanghai, China, and to evaluate the correlation between self-reported sexual contacts and genotypes of N. gonorrhoeae isolates.
The N. gonorrhoeae isolates (n = 199) were collected from males with gonorrhea (n = 157) and their positive female partners (n = 42), as previously described (8, 25). These isolates included 39 pairs and one triplet (one male with two female partners) from patients with self-reported sexual contacts. The identification and growth of N. gonorrhoeae isolates were described previously (8, 25). DNA was extracted from the isolates and used for PCR amplification of porB and tbpB. Amplicons were analyzed as previously described (8, 11, 25). porB DNA sequence analysis covered ∼82% of the nucleotides encoding surface-exposed loops I to VII and interspace regions II to VII as described and analyzed previously (8, 15, 22), and porB DNA sequences were deposited in the GenBank database (8). Each isolate was arbitrarily assigned a porB DNA sequence type (PST), and isolates were sorted into groups based on PSTs; groups also defined common clusters of isolates. For NG-MAST analysis, DNA sequences of porB and tbpB fragments as described previously were submitted to the NG-MAST database (www.ng-mast.net; last accessed 2 May 2008) (11). NG-MAST STs were assigned for each N. gonorrhoeae isolate, and STs new to the NG-MAST database were identified. Simpson's index of diversity (ID) was used to determine the discriminatory abilities of each method (4, 7). To minimize clonal effects, only porB DNA sequences and STs from isolates from male patients (n = 157) were used in ID analyses.
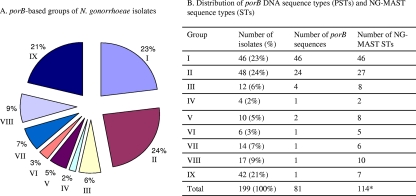
The IDs of porB-based analyses and NG-MAST for N. gonorrhoeae isolates from male patients were 0.942 and 0.982, respectively. Among the 199 clinical isolates, including female partners, 81 PSTs were identified (see Table S1 in the supplemental material). Isolates were separated into nine groups based on their PSTs (Fig. 1A). Group I included 46 (23%) isolates each having a distinct PST. Group II comprised 24 porB DNA sequences with two isolates per PST, i.e., 48 isolates (24%) having 24 PSTs. Group III contained 12 isolates (6%) with four PSTs, with each PST comprising three isolates. Group V included 10 isolates (5%) divided into two porB groups (i.e., each PST with five isolates). Isolates in groups IV (n = 4; 2%), VI (n = 6; 3%), VII (n = 14; 7%), VIII (n = 17; 9%), and IX (n = 42; 21%) had an identical PST in each group, and PSTs were distinct between the groups (Fig. 1A and B).
FIG. 1.
porB-based groups and distributions of PSTs and NG-MAST STs of 199 N. gonorrhoeae isolates. (A) porB-based groups. (B) Distribution of porB PSTs and NG-MAST STs. Isolates were grouped based on the number of isolates with identical porB PSTs. For group I, each isolate has a distinct PST (n = 46). For group II, each two isolates have an identical PST (n = 48). For group III, each three isolates have an identical PST (n = 12). For group IV, four isolates have an identical PST. For group V, each five isolates have an identical PST (n = 10). For group VI, six isolates have an identical PST. For group VII, 14 isolates have an identical PST. For group VIII, 17 isolates have an identical PST. For group IX, 42 isolates have an identical PST. Percentages represent the proportions of total isolates. *, some NG-MAST STs were further differentiated by porB typing.
Among the 199 N. gonorrhoeae isolates, 114 NG-MAST STs were identified, and 71 (62%) of these STs had not been previously reported (Fig. 1B; see also Table S1 in the supplemental material). Groups of N. gonorrhoeae isolates identified by porB-based analysis and NG-MAST were compared; a discordance of clusters between PSTs and NG-MAST STs was noted (see vertical double arrows in Table S1 in the supplemental material). Each isolate in group I (n = 46) exhibited a distinct ST. There were 24 PSTs in group II and 27 NG-MAST STs with 2 PSTs distinguished by NG-MAST. Isolates in group III (n = 12) with four PSTs were further differentiated into eight STs, with three of the PSTs being further differentiated by NG-MAST. Each of the two group V PSTs (n = 10) were differentiated into four different NG-MAST STs, for a total of eight STs from group V. Isolates with a single PST in groups IV (n = 4), VI (n = 6), VII (n = 14), VIII (n = 17), and IX (n = 42) were further differentiated into 2, 5, 6, 10, and 7 NG-MAST STs, respectively (Fig. 1B; see also Table S1 in the supplemental material). On the other hand, a few isolates with identical NG-MAST STs were further differentiated by porB DNA sequence analysis. ST567 (two isolates), ST641 (four isolates), ST1691 (six isolates), and ST2066 (three isolates) were differentiated into two PSTs, respectively.
The congruence of genetic types and sexual connections identified by contact tracing was analyzed. Thirty porB PSTs and 36 NG-MAST STs were identified among 81 isolates comprising 40 sexual contacts (39 pairs and one trio) (see Table S2 in the supplemental material). porB PSTs in the majority of isolates having sexual connections (37 pairs and the trio) were identical, while two pairs (pairs 141/141F and 41/41F) had different PSTs (see isolates in boldface type in Table S2 in the supplemental material). NG-MAST typing revealed that the majority of pairs (37/39 pairs) and the trio had identical STs (see Table S2 in the supplemental material). As expected, the two pairs with different PSTs also had different NG-MAST STs. Noticeably, each of three PSTs (i.e., PST15 [nine pairs], PST67 [three pairs], and PST71 [two pairs and the trio]) were differentiated into three NG-MAST STs (see open boxes in Table S2 in the supplemental material), although seven of nine pairs of PST15 also had an identical NG-MAST ST (i.e., ST421).
This study shows that both porB DNA sequence analysis and NG-MAST analysis have high discriminatory powers sufficient to distinguish N. gonorrhoeae isolates and to identify circulating clusters of strains. Among isolates with identified epidemiological links, both methods were congruent with epidemiological findings. These results validate the use of porB DNA sequence analysis for epidemiological studies, in agreement with data from previous studies (1, 5, 8, 11, 13, 15, 18-21, 23). NG-MAST further differentiated porB types due to the sequence variations present in tbpB alleles (6), which contributed to a different NG-MAST ST. However, it is uncertain whether the differences detected in tbpB using NG-MAST are indicative of epidemiologically distinct groups of isolates, and further study is required. The majority of isolates in the largest porB-based cluster (PST15; n = 42) exhibited a single NG-MAST ST (ST421; n = 27), demonstrating that the two methods are congruent in defining predominant clusters of transmission. Other isolates of this large porB cluster (n = 15) displayed six distinct NG-MAST STs, which warrants further epidemiological investigation to determine whether these isolates are truly related or not.
porB DNA sequence analysis is less costly and quicker to perform, as this method can be determined by one PCR and two DNA sequencing reactions (one reaction for each strand). NG-MAST typing involves two PCR and four DNA sequencing reactions. porB DNA sequences are associated with two gonococcal porB isoforms (i.e., porB1a and porB1b) (8) and would additionally provide isolate information with clinical relevance, as porB serovar analysis was formerly used (18). However, the association between porB sequence types and serovar types remains to be elucidated (17). It should be noted that the analysis of various lengths of porB DNA sequence can produce different discriminatory abilities. Olsen et al. previously reported that an analysis of the entire porB DNA sequence had an ID of 97.8% (13); however, four sequencing reactions (two for each DNA strand) were required to ascertain the entire porB gene sequence. By analyzing DNA sequences of defined regions covering ∼82% of the N. gonorrhoeae porB gene, we obtained an ID of 94.2%, which we consider to provide sufficiently high discrimination for identifying transmission clusters of N. gonorrhoeae (4, 7). This method also confirmed epidemiologically identified sexual contacts (8).
Standardized guidelines should be established if porB DNA sequence analysis is to be used for widely studying N. gonorrhoeae molecular epidemiology, to facilitate interlaboratory comparisons, and to differentiate N. gonorrhoeae clusters from different geographical regions. An independent N. gonorrhoeae porB DNA sequence database has not yet been established; a publicly accessible N. gonorrhoeae porB database is needed for depositing of sequence data and for sequence type assignment, as well as for spatial and temporal characterizations of N. gonorrhoeae isolate distribution. Furthermore, some common porB type assignments should be established between NG-MAST-assigned types and porB types based on larger porB segments. Overall, porB DNA sequence analysis is a useful tool for determining N. gonorrhoeae molecular epidemiology, particularly in resource-limited settings. NG-MAST analysis can be used if higher discrimination is required for the further differentiation of large porB-based clusters of N. gonorrhoeae. Opa typing is another highly discriminatory method that has been used for the molecular typing of N. gonorrhoeae (12, 14). However, DNA sequencing methods are replacing the Opa typing approach, which differentiates N. gonorrhoeae isolates based on banding patterns of amplified/restriction endonuclease-digested opa genes (14).
Nucleotide sequence accession numbers.
The porB DNA sequences reported here were deposited in the GenBank database under accession numbers EF540591 to EF540669 and EU719202 to EU719208. tbpB and porB sequences for NG-MAST analysis have been submitted to the NG-MAST database.
Supplementary Material
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (grant no. 77748).
Ethical approvals were obtained from the Ottawa Hospital Research Ethics Board, the Ethics Committee of the Shanghai Municipal Bureau of Public Health (Shanghai, China), and the Biomedical Ethics Board of the University of Saskatchewan (Saskatoon, Saskatchewan, Canada).
Footnotes
Published ahead of print on 3 December 2008.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Bash, M., P. Zhu, S. Gulati, D. McKnew, P. Rice, and F. Lynn. 2005. por variable-region typing by DNA probe hybridization is broadly applicable to epidemiologic studies of Neisseria gonorrhoeae. J. Clin. Microbiol. 431522-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilek, N., I. Martin, G. Bell, G. Kinghorn, C. Ison, and B. Spratt. 2007. Concordance between Neisseria gonorrhoeae genotypes recovered from known sexual contacts. J. Clin. Microbiol. 453564-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choudhury, B., C. Risley, A. Ghani, C. Bishop, H. Ward, K. Fenton, C. Ison, and B. Spratt. 2006. Identification of individuals with gonorrhoea within sexual networks: a population-based study. Lancet 368139-146. [DOI] [PubMed] [Google Scholar]
- 4.Dillon, J., M. Rahman, and K. Yeung. 1993. Discriminatory power of typing schemes based on Simpson's index of diversity for Neisseria gonorrhoeae. J. Clin. Microbiol. 312831-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fredlund, H., L. Falk, M. Jurstrand, and M. Unemo. 2004. Molecular genetic methods for diagnosis and characterisation of Chlamydia trachomatis and Neisseria gonorrhoeae: impact on epidemiological surveillance and interventions. APMIS 112771-784. [DOI] [PubMed] [Google Scholar]
- 6.Harrison, O., M. Maiden, and B. Rokbi. 2008. Distribution of transferrin binding protein B gene (tbpB) variants among Neisseria species. BMC Microbiol. 866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter, P., and M. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 262465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao, M. M., K. Bell, W. M. Gu, Y. Yang, N. F. Eng, W. K. Fu, L. Wu, C. G. Zhang, Y. Chen, A. M. Jolly, and J. A. R. Dillon. 2008. Clusters of circulating Neisseria gonorrhoeae strains and association with antimicrobial resistance in Shanghai. J. Antimicrob. Chemother. 61478-487. [DOI] [PubMed] [Google Scholar]
- 9.Lundbäck, D., H. Fredlund, T. Berglund, B. Wretlind, and M. Unemo. 2006. Molecular epidemiology of Neisseria gonorrhoeae—identification of the first presumed Swedish transmission chain of an azithromycin-resistant strain. APMIS 11467-71. [DOI] [PubMed] [Google Scholar]
- 10.Maiden, M. 2006. Multilocus sequence typing of bacteria. Annu. Rev. Microbiol. 60561-588. [DOI] [PubMed] [Google Scholar]
- 11.Martin, I., C. Ison, D. Aanensen, K. Fenton, and B. Spratt. 2004. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J. Infect. Dis. 1891497-1505. [DOI] [PubMed] [Google Scholar]
- 12.Morris, A., H. Palmer, and H. Young. 2008. Opa-typing can identify epidemiologically distinct subgroups within Neisseria gonorrhoeae multi-antigen sequence type (NG-MAST) clusters. Epidemiol. Infect. 136417-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen, B., R. Hadad, H. Fredlund, and M. Unemo. 2008. The Neisseria gonorrhoeae population in Sweden during 2005—phenotypes, genotypes and antibiotic resistance. APMIS 116181-189. [DOI] [PubMed] [Google Scholar]
- 14.O'Rourke, M., C. Ison, A. Renton, and B. Spratt. 1995. Opa-typing: a high-resolution tool for studying the epidemiology of gonorrhoea. Mol. Microbiol. 17865-875. [DOI] [PubMed] [Google Scholar]
- 15.Pérez-Losada, M., R. Viscidi, J. Demma, J. Zenilman, and K. Crandall. 2005. Population genetics of Neisseria gonorrhoeae in a high-prevalence community using a hypervariable outer membrane porB and 13 slowly evolving housekeeping genes. Mol. Biol. Evol. 221887-1902. [DOI] [PubMed] [Google Scholar]
- 16.Posada, D., K. Crandall, M. Nguyen, J. Demma, and R. Viscidi. 2000. Population genetics of the porB gene of Neisseria gonorrhoeae: different dynamics in different homology groups. Mol. Biol. Evol. 17423-436. [DOI] [PubMed] [Google Scholar]
- 17.Unemo, M., P. Olcén, J. Albert, and H. Fredlund. 2003. Comparison of serologic and genetic porB-based typing of Neisseria gonorrhoeae: consequences for future characterization. J. Clin. Microbiol. 414141-4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Unemo, M., P. Olcén, T. Berglund, J. Albert, and H. Fredlund. 2002. Molecular epidemiology of Neisseria gonorrhoeae: sequence analysis of the porB gene confirms presence of two circulating strains. J. Clin. Microbiol. 403741-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unemo, M., P. Olcén, J. Jonasson, and H. Fredlund. 2004. Molecular typing of Neisseria gonorrhoeae isolates by pyrosequencing of highly polymorphic segments of the porB gene. J. Clin. Microbiol. 422926-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unemo, M., A. Sjöstrand, M. Akhras, B. Gharizadeh, E. Lindbäck, N. Pourmand, B. Wretlind, and H. Fredlund. 2007. Molecular characterization of Neisseria gonorrhoeae identifies transmission and resistance of one ciprofloxacin-resistant strain. APMIS 115231-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unemo, M., V. Vorobieva, N. Firsova, T. Ababkova, I. Leniv, B. Haldorsen, H. Fredlund, and V. Skogen. 2007. Neisseria gonorrhoeae population in Arkhangelsk, Russia: phenotypic and genotypic heterogeneity. Clin. Microbiol. Infect. 13873-878. [DOI] [PubMed] [Google Scholar]
- 22.van der Ley, P., J. Heckels, M. Virji, P. Hoogerhout, and J. Poolman. 1991. Topology of outer membrane porins in pathogenic Neisseria spp. Infect. Immun. 592963-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viscidi, R., J. Demma, J. Gu, and J. Zenilman. 2000. Comparison of sequencing of the por gene and typing of the opa gene for discrimination of Neisseria gonorrhoeae strains from sexual contacts. J. Clin. Microbiol. 384430-4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward, H., C. Ison, S. Day, I. Martin, A. Ghani, G. Garnett, G. Bell, G. Kinghorn, and J. Weber. 2000. A prospective social and molecular investigation of gonococcal transmission. Lancet 3561812-1817. [DOI] [PubMed] [Google Scholar]
- 25.Yang, Y., M. Liao, W. Gu, K. Bell, L. Wu, N. Eng, C. Zhang, Y. Chen, A. Jolly, and J. Dillon. 2006. Antimicrobial susceptibility and molecular determinants of quinolone resistance in Neisseria gonorrhoeae isolates from Shanghai. J. Antimicrob. Chemother. 58868-872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.