Cellular infrastructure is often compared to a highly organized city. Organelles act as molecular factories to produce proteins, lipids, nucleic acids and energy (ATP). The distribution of these products throughout the cell is critical to cell viability and function. Due to the density of the host cytoskeleton and cellular organelles, there is a formidable barrier to the free diffusion of macromolecular complexes (protein density in the cytoplasm approaches 300 mg/ml [51], analogous to the viscosity of wet sand). The cytoskeleton provides a highly dynamic, adaptable system that provides mechanical strength to the cell as well as the molecular framework for localized and long-distance trafficking of vesicle cargo and cellular organelles (10). The complex process of traffic control is maintained by three “basic” filament systems: actin filaments, microtubules, and intermediate filaments (IFs) (which provide a scaffold for proteins involved in polarized trafficking) (77). Molecular motor proteins facilitate the movement of various cargoes by hydrolyzing ATP and virtually walking along actin streets and microtubule highways. These active transport mechanisms are crucial to the efficient transport of molecules larger than 500 kDa (54, 55).
The Herpesviridae family, comprising the alpha-, beta-, and gammaherpesvirus subfamilies, is a large and diverse group of double-stranded DNA viruses. Commonly studied members of the alphaherpesvirus subfamily include the human pathogens herpes simplex virus (HSV) and varicella-zoster virus (VZV) and the animal pathogens pseudorabies virus (PRV), bovine herpesvirus, and Marek's disease virus. The betaherpesvirus subfamily includes, among others, human and mouse cytomegalovirus (HCMV and MCMV, respectively), while the gammaherpesvirus subfamily contains the lymphotropic Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV).
Despite the different tropisms of the three viral subfamilies, all share a key survival strategy: herpesviruses replicate and package their viral DNA inside the nuclei of infected cells (84). Therefore, transport to and away from the nucleus is the crux of successful propagation and transmission to an uninfected cell/host. Virus particles must attach at the cell surface and be actively trafficked through a crowded cytoplasm to the nuclear pore complex, where viral DNA is released into the nucleus (scale drawing, Fig. 1). Efficient diffusion of capsids is virtually impossible; for example, it has been calculated that an HSV capsid would take >200 years to diffuse 1 cm in cytoplasm (97). Upon replication of the viral genome and subsequent packaging of genomes into viral nucleocapsids, immature particles egress the nucleus and again must be efficiently transported to the trans-Golgi network (TGN) or an endosomal compartment, the site of secondary envelopment (62). Virions then are exocytosed and released from the cell surface (or, for alphaherpesviruses, can be sorted into axons for long-distance transport and release). This review addresses how herpesvirus proteins engage the host cytoskeleton and associated motor and remodeling proteins to move complex viral structures in and out of cells with remarkable efficiency.
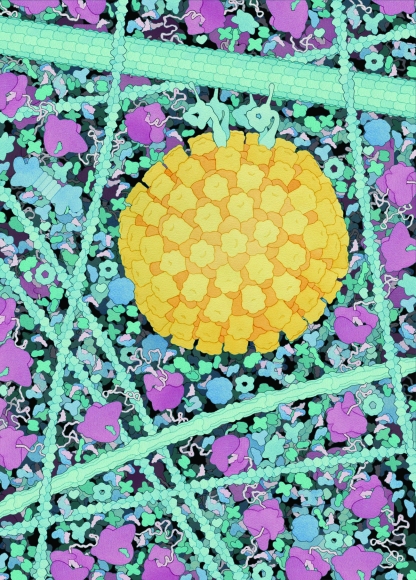
FIG. 1.
Scale drawing of an alphaherpesvirus capsid (yellow) transported along a microtubule by dynein motors (the width of the image represents 250 nm of cytoplasmic space). Note the crowded nature of the host cytosol, including actin filaments (narrow vertical cables to the right and left of the herpesvirus capsid), an IF (medium-sized cable below the capsid), ribosomes (light red), de novo proteins (string-like structures emerging from ribosomes), chaperones (round blue proteins), a proteosome (dumbbell-like structure near the left edge of the image), tRNAs (pink, crescent-shaped proteins), aminoacyl-tRNA synthetases (small blue protein in complex with tRNAs), and assorted enzymes of glycolysis and biosynthesis. The illustration is by David S. Goodsell, Scripps Research Institute.
TRANSPORT ON MICROTUBULES: RIDING CELLULAR HIGHWAYS
Microtubules provide tracks for the long-distance transport of endocytic/exocytic vesicles, organelles, and chromosomes. Microtubules are stiff, polar cylinders comprising α-tubulin and β-tubulin heterodimers. Single protofilaments are assembled through the head-to-tail association of tubulin dimers; 13 protofilaments then join to form a hollow microtubule filament with an outer diameter of 25 nm (2). Microtubules have a distinct structural polarity, with a highly dynamic “plus” end that can grow and shrink rapidly (a process known as dynamic instability), as well as a relatively stable “minus” end that is tethered to the microtubule-organizing center. The dynamics and distribution of microtubules within a cell are controlled by a variety of factors, including GTP hydrolysis, posttranslational modification of tubulin subunits, and microtubule-associated proteins (41, 74). Furthermore, the organization of microtubule networks varies dramatically from cell type to cell type (e.g., that of an epithelial cell versus a neuron) (26).
Directional transport along microtubules is governed by two classes of molecular motors. Plus-end-directed motors, i.e., those involved in transport to the cell periphery, are known as kinesins. The kinesin superfamily includes up to 45 members in mammalian cells (65). Conventional kinesin is a tetrameric molecule composed of two heavy chains and two light chains. These motors are highly processive and relatively powerful (each motor generates ∼6 pN of force) (103). Thus, few motors are required to transport cargo efficiently. Minus-end transport, i.e., traffic directed from the periphery to the cell center, is mediated by cytoplasmic dynein. This complex motor protein consists of two dynein heavy chains, two intermediate chains, and multiple light intermediate chains and light chains (80). The processivity of dynein is also enhanced by a large protein complex known as dynactin, which also interacts with certain cargo proteins (103). Cytoplasmic dynein was originally reported to generate up to 1 pN of force per motor (58). However, more recent studies suggest that the force generated is ∼6 to 8 pN, roughly equal to that of kinesin (32, 100).
LOCAL TRANSPORT ON ACTIN FILAMENTS
Whereas microtubules are essential for long-distance transport, actin filaments play a crucial role in short-range movements of cargo inside the cell, especially in areas of high actin/low microtubule density (4, 45, 50). Actin filaments (F-actin) are composed of monomer subunits (G-actin), each bound to a molecule of ATP, that assemble into two protofilaments (the two filaments wind around each other and are stabilized by lateral contacts). These flexible structures have diameters of 5 to 9 nm and can be further arranged into two- and three-dimensional networks inside the cell (2). Like microtubules, actin filaments have an intrinsic polarity consisting of plus and minus ends (also designated “barbed” and “pointed,” ends, respectively). Though actin filaments can be found throughout the cell body, they are highly concentrated at the cell cortex, which is the main site of polar actin networks (e.g., filopodia, lamellipodia, growth cones) (19). In general, cortical actin is arranged with the barbed/plus end of the actin filament directed toward the cell surface and with the minus end directed toward the cell center.
Directed movement of vesicles and protein complexes along actin filaments is mediated by the myosin superfamily of motor proteins (49), notably myosin V (MyoV) and MyoVI. MyoV is a highly processive, plus-end-directed motor that moves organelles and secretory vesicles outward toward the cell surface (15, 24). Like kinesin-1, MyoV exists as a dimer with two head regions (each head is an ATPase that binds directly to the actin filament), a stalk region, and a tail region (important for cargo binding) (59). MyoVI is involved in transporting endocytic vesicles toward the pointed ends of actin filaments (12).
INTERMEDIATE FILAMENT PROTEINS: A CELLULAR SCAFFOLD
IFs are ropelike fibers with an average diameter of 10 nm, an “intermediate” size in relation to actin filaments and microtubules (38, 46). IFs are largely responsible for providing mechanical integrity to a cell and do not serve as “rails” for transport of secretory and endocytic vesicles. Several well-known examples include keratins, neurofilaments, vimentin, desmins, and lamin A/C (33). IFs are formed through the winding of two monomeric polypeptides into a coiled-coil dimer. Two dimers then align in antiparallel fashion to form a staggered tetrameric structure. This tetramer is the basic unit for IF formation and is analogous to the tubulin dimer/actin monomer subunit (2). Staggered tetramers form protofilaments, and eight protofilaments form a mature IF (33). This unique, nonpolar structure makes IFs extremely resistant to bending and stretching forces within the cell.
CYTOSKELETON REMODELING DURING HERPESVIRUS ENTRY
The impact of herpesvirus infection on the host cytoskeleton is immediate, and it commences with certain viral glycoproteins binding their cognate receptor on the surface of an uninfected cell (Fig. 2). Fusion of the herpes virus envelope with the host plasma membrane requires the coordinated activity of at least three viral glycoproteins, namely, gB and gH/gL (the core fusion machinery) (99). All alphaherpesviruses, with the exception of VZV and Marek's disease virus, also require the viral gD envelope protein for entry (18). This viral protein is known to bind several host receptors, including nectins (cell adhesion molecules that induce the formation of adherens junctions in fibroblasts and epithelial cells), as well as synaptic junctions in neurons (71). Nectins regulate the reorganization of the actin cytoskeleton by activation of actin-remodeling proteins, such as Ras and Rho GTPases (e.g., Rap1, Cdc42, and Rac1) (86).
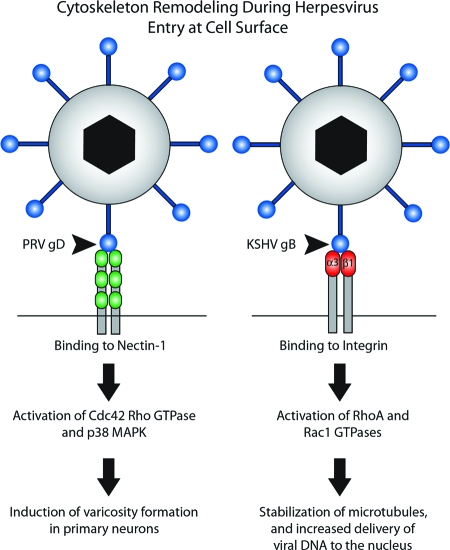
FIG. 2.
Binding of herpesvirus glycoproteins to host receptors during viral entry induces cytoskeletal rearrangements inside the cell. Binding of PRV gD to nectin-1 on the surfaces of swine trigeminal ganglion neurons activates certain Rho GTPase and mitogen-activated protein kinase (MAPK) signaling pathways, leading to the formation of varicosities/synaptic boutons. This event may prime neurons for egress of progeny virus particles. The attachment of KSHV gB to α3β1 integrin during viral entry activates RhoA and Rac1 GTPases, leading to the acetylation (stabilization) of microtubules and increased delivery of viral capsids to the nucleus.
Binding of PRV gD to nectin-1 during entry into porcine trigeminal ganglion neurons induces the formation of varicosities (synaptic boutons) (22) via an actin-based remodeling process (102). This effect can be blocked by the treatment of neurons with secramine A, an inhibitor of Cdc42 Rho GTPase. Early during infection, this type of actin remodeling in neurons may prepare neurons for virus egress at synaptic boutons (known sites for virus egress from axons) (16, 22, 87).
The gB glycoprotein of KSHV (human herpesvirus 8 [HHV-8]) utilizes the α3β1 integrin as one of its entry receptors. Binding of gB to this receptor led to the activation of cell signaling cascades and a marked rearrangement of the host cytoskeleton (72, 92). Binding and internalization of HHV-8 induced the acetylation of microtubules and the activation of the Rho GTPases RhoA and Rac1 (73). The inactivation of Rho GTPases decreased microtubular stability, thereby limiting the delivery of viral DNA to the nucleus. Conversely, the activation of Rho GTPases increased the efficiency of viral trafficking along microtubules to the nucleus (73). These studies emphasize a role for the host cytoskeleton during HHV-8 entry in addition to the cross talk that can occur between actin-remodeling proteins and microtubule formation (34).
A role for actin was also reported for a novel “phagocytosis-like” entry pathway for HSV type 1 (HSV-1) (17). Attachment of virus particles to human corneal fibroblasts and nectin-1-expressing CHO cells activated both Cdc42 and RhoA, leading to filopodium-like protrusions from the cell membrane. Virus particles preferentially associated with these protrusions, and treating cells with actin-depolymerizing drugs blocked HSV-1 internalization (17). Entry of HSV-1 into Madin-Darby canine kidney II cells also triggered Rac1/Cdc42 signaling, and expression of Rac1/Cdc42 mutants (prior to infection) decreased HSV-1 infectivity (40).
It is clear that early events in the herpesvirus viral life cycle (e.g., attachment at the cell surface) trigger cytoskeletal rearrangements that facilitate efficient virus entry and, in the case of alphaherpesviruses, potential downstream egress events from synaptic boutons. After traversing the cell cortex, herpesvirus particles transition from actin-mediated transport (local streets) to microtubule-based “highways” that lead to the host nucleus.
MICROTUBULES, A DYNEIN MOTOR, AND VIRAL ENTRY
Nuclear targeting of many viruses is mediated by microtubules and cytoplasmic dynein (reviewed in reference 26). Viral cargo traffics in the retrograde direction (microtubule minus ends) toward the microtubule-organizing center, which is often juxtaposed to the nucleus (20, 26). A crucial study by Sodeik et al. revealed that HSV-1 capsids associated with microtubules while in transit from the plasma membrane to the nucleus: capsids colocalized with cytoplasmic dynein, and microtubule-disrupting drugs strongly reduced the transport of HSV-1 capsids to the nucleus (98). An ultrastructural study of PRV entry demonstrated that capsids were tightly associated with microtubules in the cytoplasm (35). Nuclear targeting of CMV (a betaherpesvirus) is also dependent on an intact microtubule network, but is not dependent on actin filaments (75). Unfortunately, little is known about the transport of gammaherpesviruses to the nucleus. The speculation is that it is similar to that of other herpesviruses (42).
The direct interaction of various herpesvirus structural proteins with the dynein motor has been reported (26) (Fig. 3A). The outer HSV capsid protein, VP26, was reported to interact with the dynein light chains RP3 and Tctex1 by yeast two-hybrid analyses and in vitro pull-down assays (27). Recombinant herpesvirus capsids lacking VP26 also failed to move toward the nucleus when microinjected into HEp-2 cells (27). However, in another study, an HSV-1 mutant lacking VP26 was capable of efficient retrograde transport to sensory ganglia after inoculation into the mouse cornea, suggesting that VP26 does not play a major role in nuclear targeting of capsids (23). A VP26-null mutant in PRV also moved to the nucleus with wild-type kinetics in sensory neurons, leading to the conclusion that VP26 has no role in retrograde transport to the nucleus (3). Thus, if VP26 does interact directly with dynein light chains RP3 and Tctex1, this interaction may reflect another process distinct from capsid transport. The HSV-1 UL34 protein (a primary envelope component) was reported to interact with the dynein intermediate chain. The relevance of this interaction to virion transport is unknown, as the UL34 protein is not present in cytoplasmic and extracellular virions (83). HSV-1 VP5 and UL9 also interact with the dynein light-chain LC8 by Pepscan analysis using a technique in which recombinant LC8 protein was incubated with cellulose membrane bearing 75 overlapping spots of synthetic viral dodecapeptides. However, these putative interactions were not confirmed to occur inside infected cells (60).
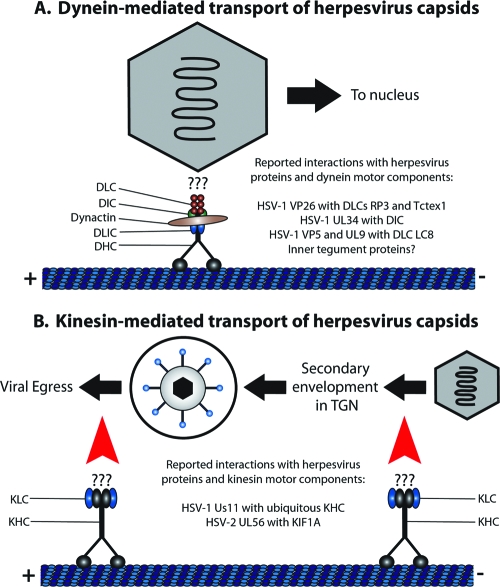
FIG. 3.
Dynein and kinesin-mediated transport of herpesvirus capsids along microtubules. (A) Transport of herpesvirus capsids along microtubules to the nucleus is mediated by the cytoplasmic dynein-dynactin complex. Dynein consists of two heavy chains (DHC; black), two intermediate chains (DIC; green), two light intermediate chains (DLIC; blue), and six light chains (DLC; red). Dynactin consists of eleven distinct subunits, mainly Arp1, dynamitin, and p150GLUED (brown). Several herpesvirus proteins have been reported to interact with dynein motor components, though their relevance during infection remains ill-defined. Inner tegument proteins have also surfaced as ideal candidates for interacting with the dynein-dynactin complex. (B) Kinesin-mediated transport of herpesvirus capsids is likely involved in the transport of capsid/tegument structures to sites of secondary envelopment in the TGN and in the trafficking of enveloped virus particles, contained within a vesicle, to the cell periphery (highlighted with red arrows). Kinesin-1 is represented in the diagram with two heavy chains (KHC; black) and two light chains (KLC; blue), though other kinesin motors may be involved in different stages of herpesvirus egress.
More-recent evidence supports the idea that inner tegument proteins promote capsid transport along microtubules and therefore are strong candidates for interaction with the dynein-dynactin complex (57, 109). Efficient movement of capsids along microtubules in vitro required the HSV inner tegument proteins UL36 (VP1/2) and UL37, but capsids devoid of tegument proteins showed virtually no movement. Furthermore, capsids with inner tegument proteins bound strongly to dynein intermediate-chain and dynactin components from cytosolic extracts of Xenopus (109). Live-cell imaging experiments examining the retrograde transport of monomeric red fluorescent protein-tagged PRV capsids and green fluorescent protein (GFP)-tagged tegument proteins (in sensory neurons) revealed that outer tegument proteins, such as VP13/14, VP16, and VP22, were lost during viral entry and did not move with capsids to the nucleus. In contrast, the inner tegument proteins VP1/2 and UL37 remained associated with capsids as they entered neurons, trafficked to the nucleus, and subsequently docked at the nuclear rim (57). Thus, these two alphaherpesvirus proteins are ideal candidates for interactions with microtubule transport machinery.
NUCLEAR EGRESS OF VIRAL CAPSIDS
Upon delivery into the nucleus, herpesvirus DNA is transcribed, replicated, and packaged into newly assembled capsids. These capsids leave the nucleus by moving to the nuclear periphery and budding through the nuclear envelope (herpesvirus capsids are likely too large to exit via the nuclear pore complex) (61, 63). Several components of the host cytoskeleton are involved in this process.
Infection of neurons with PRV or HSV-1 results in the formation of actin filaments within the nucleus, a process that depends on viral transcription but not viral DNA synthesis (29). PRV capsids are associated with these filaments as shown by serial block-face scanning electron microscopy, i.e., acquiring a z-stack of electron-microscopic images. PRV GFP-tagged capsids in the nucleus also colocalized with the MyoV motor protein, suggesting that transport of capsids along actin filaments was dependent on actin-based motor proteins (29). HSV-1 capsids were shown to be actively transported within the nuclei of infected HEp-2 cells, and this movement was sensitive to actin depolymerization and treatment with 2,3-butanedione monoxime, a putative inhibitor of myosin motors (30). Nuclear actin also plays a role in the formation of higher-order capsid assemblies of alphaherpesviruses (29, 30), though paradoxically, treatment of cells with actin-depolymerizing drugs does not alter/reduce infectious virus yields (94). Taken together, these findings indicate that capsid movement to the periphery of the nucleus may be driven (at least in part) by myosin motors trafficking along actin filaments.
Egress from the nucleus requires herpesvirus capsids to traverse the inner nuclear membrane (INM), an IF meshwork composed of lamins and lamin-associated polypeptides, e.g., LAP1 and LAP2β (56). This formidable barrier is roughly 20 to 80 nm in width and is normally disrupted by phosphorylation by cellular kinases during mitosis (69). Muranyi et al. showed that MCMV overcame this obstacle via its M50/p35 and M53/p38 proteins (69). M50 was inserted into the INM and concentrated into distinct sites by M53 (69). M50 then recruited cellular protein kinase C (PKC), which phosphorylated lamins, thereby destabilizing the nuclear lamina layer (69). Efficient nuclear egress is a crucial step in the life cycle of all herpesviruses, as reflected by the fact that M50 and M53 have homologs in both alpha- and gammaherpesvirus subfamilies, namely, UL34 and -31 for HSV and PRV and ORF67 and -69 for KSHV (88, 89).
It is well documented that HSV-1 infection also results in the dramatic relocalization of INM proteins. Scott and O'Hare reported that a “major morphological distortion” occurs by 8 hours postinfection in COS-1 cells of such proteins as lamin B receptor and lamins A/C, B1, and B2 (91). The HSV-1 UL31, UL34, and Us3 gene products were later implicated in this process, as production of these proteins in uninfected cells was sufficient to disrupt lamin A/C localization, UL31 and UL34 could bind lamin A/C in vitro, and UL31 and UL34 caused conformational changes in the nuclear lamina during infection (9, 82). As with MCMV, PKC was recruited to the nuclear rim after HSV-1 infection (a process that depends on the presence of UL31 and UL34) (78). Emerin, an INM protein that binds directly to lamin A/C, was also phosphorylated and delocalized during HSV infection, contributing to the egress of viral capsids from the nucleus (68).
MICROTUBULES, KINESIN MOTORS, AND VIRAL EGRESS
Upon penetration of the nuclear lamina, mature nucleocapsids undergo envelopment and deenvelopment steps at the nuclear membrane before being released into the cytoplasm (11, 61, 95). Viral capsids acquire a secondary/mature envelope in the cytoplasm and move to the cell periphery for release from the infected cell. The movement of cellular and viral cargo to the cell periphery occurs toward the plus end of microtubules (anterograde movement) and is mediated by a variety of kinesin motor proteins (14, 26) (Fig. 3B). Herpesvirus capsids acquire a mature envelope by budding into vesicles derived from the TGN or endosomes, a process that is dependent on intact microtubules (62, 101). Lee et al. reconstituted the anterograde trafficking of HSV-containing vesicles in vitro by isolating cytoplasmic organelles from infected epithelial cells (52). These vesicles were heavily enriched in the Golgi marker TGN46 (but not endosomal markers) and shown to be the main destination for enveloping HSV capsids after they enter the cytosol (37). As shown by time-lapse video microscopy, many of the vesicles containing GFP-tagged HSV trafficked along rhodamine-labeled microtubules in vitro in a manner consistent with anterograde movement of HSV in living cells (52).
The anterograde transport of alphaherpesvirus proteins in axons is a microtubule-dependent process and can be disrupted with nocodazole treatment (66, 79). Individual PRV capsids move in axons of sensory neurons with fast axonal kinetics (∼2 μm/s), a process that is dependent on microtubule motor proteins (39, 96). It is largely unknown which viral proteins bind kinesin motors to mediate this process, though several candidates have been reported. The major problem is that neurons are difficult to propagate and often do not provide sufficient material for biochemical analyses. Pulldown assays from HSV-infected HEp-2 cells, using the cargo-binding domain of kinesin heavy chain (KHC) as bait, demonstrated that four HSV proteins bound to the tail of KHC in vitro: the major capsid protein VP5 and the tegument proteins VP16, VP22, and Us11 (25). VP5 was an unlikely candidate, as capsid proteins are predicted to have restricted access to bind kinesin (the capsid is heavily coated with tegument proteins) (25, 26). VP16 and VP22 bound to Us11 and did not bind directly to KHC. Thus, Us11 was considered to interact directly with conventional KHC and play a major role in anterograde transport of HSV nucleocapsids (25). However, the significance of this finding is obscured by the fact that Us11 can bind to multiple proteins in vitro. In addition to binding KHC, Us11 also interacts with PAT1 (a microtubule binding protein), mRNAs, double-stranded RNA, rRNA, protein kinase R (PKR), PACT kinase activator, and certain proteins that arrest cell growth (8, 25, 26, 67). Therefore, the role of Us11 in anterograde transport of viral structures is not clear, despite its affinity for KHC in vitro. The UL56 gene product of HSV-2 was reported to bind to KIF1A, a kinesin motor protein responsible for the axonal transport of synaptic vesicle precursors (48). KIF1A colocalized with UL56 in transiently transfected cells and interacted with UL56 in yeast two-hybrid and glutathione S-transferase pulldown assays. However, the impact of UL56 in anterograde transport of viral components inside infected cells was not examined. Thus, the functional relevance of this interaction remains unknown.
INTERACTIONS WITH HERPESVIRUS PROTEINS AND MOTOR COMPLEXES DURING INFECTION
Transport of cargo (organelles, viruses, vesicles, etc.) by molecular motors involves the coordination of multiple trafficking events to ensure proper targeting within cells. For example, movement of cargo in and out of the cell involves switching between microtubules and actin filaments, specifically at the cell cortex and actin-microtubule intersections (85). Movement of certain cargoes can also be bidirectional and saltatory (proceeding by leaps rather than smooth gradual transitions), a consequence of binding both kinesin and dynein motors at the same time (108). In this case, the net movement of cargoes is achieved through winning a molecular “tug-of-war,” or coordinated release of one motor type so the other dominates (85). The mechanism of cargo attachment to motor complexes also varies significantly, with motors binding cargo through a coat protein, molecular scaffolds, transmembrane proteins, or GTPases (47).
Several putative in vitro interactions with alphaherpesvirus proteins and dynein and kinesin motor components have been described, and they have been studied using a variety of cell types: rat neonatal neurons and HEp-2, MDCK, COS-1, and Vero cells (8, 25, 27, 48, 104). Considering the dynamic nature of cargo transport inside cells, it is unclear if these interactions represent transient events within certain compartments of the cell (e.g., transport of cytoplasmic nucleocapsids to the site of secondary envelopment) or are cell type specific (occur in epithelial cells but not neurons or vice versa). Furthermore, it is not certain if these interactions tether viral cargo to the motor or regulate the association of the motor with the microfilament (i.e., motor processivity). It is also possible that viral proteins bind molecular motors indirectly through accessory proteins. If this scenario is correct, then screenings using motor subunits as “bait” may not identify these viral proteins. These questions highlight the difficult task for herpesvirus researchers: connecting the molecular interactions between viral structural proteins and the motor protein (the snapshot) and applying these findings to the cell biology of the infected cell (the movie).
ROLE FOR ACTIN DURING THE LATE STAGES OF INFECTION
Infection of most susceptible cells by herpesviruses causes a dramatic rearrangement of actin filaments. This cytopathic effect is particularly visible late in infection. HSV-infected Vero cells showed a reorganization of actin filaments as early as 4 hours postinfection and a continuous decrease of F-actin over the next 12 to 16 h (and a subsequent increase in G-actin pools) (7). The dissociation of actin stress fibers during alphaherpesvirus infection is promoted by the viral Us3 serine/threonine kinase (70, 90, 105), a multifunctional protein that also plays a role in egress of primary virions from the nucleus (90, 107) and protection from virus-induced apoptosis (1, 31, 53, 76). In addition to actin stress fiber disassembly, PRV Us3 promoted the formation of long projections from the surfaces of sparsely plated epithelial cells (13, 28). These cell projections contained both actin and microtubule filaments, and GFP-tagged capsids were transported toward their tips (which were in contact with neighboring, uninfected cells). Inhibiting the formation of these processes reduced the cell-to-cell spread capability of the virus (28).
Similar protrusions were observed for HSV-1- and VZV-infected cells, though it is unclear if these cytoskeletal rearrangements are driven by a Us3 ortholog (104, 111). van Leeuwen et al. reported that the major tegument protein of HSV-1, VP22, interacted with the actin-associated motor protein nonmuscle MyoIIA (NMIIA). These proteins colocalized in a perinuclear vesicular pattern and within virus-induced projections emanating from the cell surface of infected Vero cells (104). Inhibition of NMIIA also impaired the release of virus into the extracellular medium. Thus, the rearrangement of the actin cytoskeleton and motor proteins (late in infection) may assist in the egress of alphaherpesviruses from epithelial cells.
It is noteworthy that the UL37x1 protein of HCMV also reduces actin polymerization indirectly by inducing the release of Ca2+ stores from the endoplasmic reticulum, an event that causes dramatic morphological changes within the cell, namely, cell rounding and reorganization of F-actin (81, 93). The effects of infection on Ca2+ stores by other Herpesviridae members remain to be determined.
CYTOSKELETAL COMPONENTS IN VIRIONS
Proteins from the host cytoskeleton are incorporated into purified virions of all three subfamilies of Herpesviridae, though it is unclear what role these proteins play in particle formation and viral egress or in subsequent infection. F-actin was efficiently incorporated into wild-type PRV virions (110) and was more enriched in virions of mutants lacking the tegument proteins Us3, UL47, and UL49 (21, 64). These findings suggested that actin may compensate structurally for the loss of certain tegument proteins during virus assembly/budding (21). Thin filaments (up to 40 nm in length) were also observed within the tegument region of isolated HSV virions by cryoelectron tomography. Filaments had a width of ∼7 nm, leading the authors to suggest that they were F-actin (36). Electron tomography showed that nascent HSV nucleocapsids, undergoing envelopment at the INM, were connected to the membrane by fibers (bridging rods) that were potentially composed of actin or lamins (5).
Host cytoskeletal proteins were also associated with purified particles of the betaherpesvirus family. Mass spectrometry of HCMV virions identified α-actin, β-actin, α-tubulin, β-tubulin, cofilin, keratin, and vimentin as cellular components of the HCMV proteome (6, 106). A similar, but more limited, repertoire of cytoskeletal proteins was also identified for the virions of MCMV (44).
Studies of purified virions from several gammaherpesviruses, e.g., EBV and KSHV, revealed a similar trend. EBV virions purified from infected lymphocytes showed a large amount of β-actin, making it one of the most abundant tegument proteins within virus particles; tubulin and cofilin were also associated within the EBV tegument (43). Nonmuscle β-actin and class II myosin heavy chain were both detected in KSHV virions, suggesting that “the myosin-actin cytoskeleton is involved in intracellular capsid transport and assembly and egress of virions” (112).
In view of the wide array of cellular proteins that are incorporated into herpes virions, it is unclear whether packaging of certain cytoskeletal proteins has a functional role in viral egress or is “residual” material that is incorporated nonspecifically into virus particles during primary and secondary envelopment. There is intimate contact with viral particles and the host cytoskeleton during each stage of viral egress. Whether the incorporation of these cellular proteins into virions is important for efficient egress, particle viability, or subsequent entry events remains to be determined.
CONCLUSION
Herpesviruses are dependent on the host cytoskeleton for efficient entry, replication, and egress. To facilitate these processes, viral structures must associate with host motor protein complexes in the cytoplasm and nucleus to direct viral cargo to the appropriate site within the infected cell. Multiple viral cargoes must be moved in regulated processes. Therefore, the challenge is to design biochemical assays that identify the composition and function of the transported complexes at each stage in the virus life cycle.
QUESTIONS FOR FUTURE RESEARCH
Future research should address the following questions. What herpesvirus proteins interact directly or indirectly with host motor proteins (or associated motor proteins)? How do these interactions facilitate trafficking within the cell? How are nucleocapsids directed to sites of primary envelopment in the nucleus and sites of secondary envelopment in the cytoplasm? Is egress from an infected cell “random,” or are virions moved to and released from sites in close contact with other cells? What is the function, if any, of the cytoskeletal components found within purified virions? How is transport of viral cargo regulated along microtubules and actin filaments? Are viral mRNA transcripts moved to locations in cells by cytoskeletal systems?
Acknowledgments
We thank David S. Goodsell (The Scripps Research Institute) for the molecular artwork. We also thank Jane Flint for critical reading of the manuscript.
M.G.L. was supported by an American Cancer Society Eastern Division—Mercer Board postdoctoral fellowship (PF-08-264-01-MBC). L.W.E. acknowledges support from the National Institutes of Health (grant R01 33506).
Footnotes
Published ahead of print on 8 October 2008.
REFERENCES
- 1.Ahmed, M., M. Lock, C. G. Miller, and N. W. Fraser. 2002. Regions of the herpes simplex virus type 1 latency-associated transcript that protect cells from apoptosis in vitro and protect neuronal cells in vivo. J. Virol. 76717-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberts, B., A. Johnson, J. Lewis, M. Raff, K. Roberts, and P. Walter. 2002. Molecular biology of the cell, 4th ed. Garland Science, New York, NY.
- 3.Antinone, S. E., G. T. Shubeita, K. E. Coller, J. I. Lee, S. Haverlock-Moyns, S. P. Gross, and G. A. Smith. 2006. The herpesvirus capsid surface protein, VP26, and the majority of the tegument proteins are dispensable for capsid transport toward the nucleus. J. Virol. 805494-5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkinson, S. J., S. K. Doberstein, and T. D. Pollard. 1992. Moving off the beaten track. Curr. Biol. 2326-328. [DOI] [PubMed] [Google Scholar]
- 5.Baines, J. D., C. E. Hsieh, E. Wills, C. Mannella, and M. Marko. 2007. Electron tomography of nascent herpes simplex virus virions. J. Virol. 812726-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldick, C. J., Jr., and T. Shenk. 1996. Proteins associated with purified human cytomegalovirus particles. J. Virol. 706097-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedows, E., K. M. Rao, and M. J. Welsh. 1983. Fate of microfilaments in Vero cells infected with measles virus and herpes simplex virus type 1. Mol. Cell. Biol. 3712-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benboudjema, L., M. Mulvey, Y. Gao, S. W. Pimplikar, and I. Mohr. 2003. Association of the herpes simplex virus type 1 Us11 gene product with the cellular kinesin light-chain-related protein PAT1 results in the redistribution of both polypeptides. J. Virol. 779192-9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjerke, S. L., and R. J. Roller. 2006. Roles for herpes simplex virus type 1 UL34 and US3 proteins in disrupting the nuclear lamina during herpes simplex virus type 1 egress. Virology 347261-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown, S. S. 1999. Cooperation between microtubule- and actin-based motor proteins. Annu. Rev. Cell Dev. Biol. 1563-80. [DOI] [PubMed] [Google Scholar]
- 11.Browne, H., S. Bell, T. Minson, and D. W. Wilson. 1996. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J. Virol. 704311-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buss, F., G. Spudich, and J. Kendrick-Jones. 2004. Myosin VI: cellular functions and motor properties. Annu. Rev. Cell Dev. Biol. 20649-676. [DOI] [PubMed] [Google Scholar]
- 13.Calton, C. M., J. A. Randall, M. W. Adkins, and B. W. Banfield. 2004. The pseudorabies virus serine/threonine kinase Us3 contains mitochondrial, nuclear and membrane localization signals. Virus Genes 29131-145. [DOI] [PubMed] [Google Scholar]
- 14.Caviston, J. P., and E. L. Holzbaur. 2006. Microtubule motors at the intersection of trafficking and transport. Trends Cell Biol. 16530-537. [DOI] [PubMed] [Google Scholar]
- 15.Cheney, R. E., M. K. O'Shea, J. E. Heuser, M. V. Coelho, J. S. Wolenski, E. M. Espreafico, P. Forscher, R. E. Larson, and M. S. Mooseker. 1993. Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell 7513-23. [DOI] [PubMed] [Google Scholar]
- 16.Ch'ng, T. H., and L. W. Enquist. 2005. Neuron-to-cell spread of pseudorabies virus in a compartmented neuronal culture system. J. Virol. 7910875-10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clement, C., V. Tiwari, P. M. Scanlan, T. Valyi-Nagy, B. Y. Yue, and D. Shukla. 2006. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J. Cell Biol. 1741009-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole, N. L., and C. Grose. 2003. Membrane fusion mediated by herpesvirus glycoproteins: the paradigm of varicella-zoster virus. Rev. Med. Virol. 13207-222. [DOI] [PubMed] [Google Scholar]
- 19.Cramer, L. P. 1999. Organization and polarity of actin filament networks in cells: implications for the mechanism of myosin-based cell motility. Biochem. Soc. Symp. 65173-205. [PubMed] [Google Scholar]
- 20.Dammermann, A., A. Desai, and K. Oegema. 2003. The minus end in sight. Curr. Biol. 13R614-R624. [DOI] [PubMed] [Google Scholar]
- 21.del Rio, T., C. J. DeCoste, and L. W. Enquist. 2005. Actin is a component of the compensation mechanism in pseudorabies virus virions lacking the major tegument protein VP22. J. Virol. 798614-8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Regge, N., H. J. Nauwynck, K. Geenen, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, T. C. Mettenleiter, and H. W. Favoreel. 2006. Alpha-herpesvirus glycoprotein D interaction with sensory neurons triggers formation of varicosities that serve as virus exit sites. J. Cell Biol. 174267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desai, P., N. A. DeLuca, and S. Person. 1998. Herpes simplex virus type 1 VP26 is not essential for replication in cell culture but influences production of infectious virus in the nervous system of infected mice. Virology 247115-124. [DOI] [PubMed] [Google Scholar]
- 24.Desnos, C., S. Huet, and F. Darchen. 2007. ‘Should I stay or should I go?’: myosin V function in organelle trafficking. Biol. Cell 99411-423. [DOI] [PubMed] [Google Scholar]
- 25.Diefenbach, R. J., M. Miranda-Saksena, E. Diefenbach, D. J. Holland, R. A. Boadle, P. J. Armati, and A. L. Cunningham. 2002. Herpes simplex virus tegument protein US11 interacts with conventional kinesin heavy chain. J. Virol. 763282-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dohner, K., C. H. Nagel, and B. Sodeik. 2005. Viral stop-and-go along microtubules: taking a ride with dynein and kinesins. Trends Microbiol. 13320-327. [DOI] [PubMed] [Google Scholar]
- 27.Douglas, M. W., R. J. Diefenbach, F. L. Homa, M. Miranda-Saksena, F. J. Rixon, V. Vittone, K. Byth, and A. L. Cunningham. 2004. Herpes simplex virus type 1 capsid protein VP26 interacts with dynein light chains RP3 and Tctex1 and plays a role in retrograde cellular transport. J. Biol. Chem. 27928522-28530. [DOI] [PubMed] [Google Scholar]
- 28.Favoreel, H. W., G. Van Minnebruggen, D. Adriaensen, and H. J. Nauwynck. 2005. Cytoskeletal rearrangements and cell extensions induced by the US3 kinase of an alphaherpesvirus are associated with enhanced spread. Proc. Natl. Acad. Sci. USA 1028990-8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feierbach, B., S. Piccinotti, M. Bisher, W. Denk, and L. W. Enquist. 2006. Alpha-herpesvirus infection induces the formation of nuclear actin filaments. PLoS Pathog. 2e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forest, T., S. Barnard, and J. D. Baines. 2005. Active intranuclear movement of herpesvirus capsids. Nat. Cell Biol. 7429-431. [DOI] [PubMed] [Google Scholar]
- 31.Geenen, K., H. W. Favoreel, L. Olsen, L. W. Enquist, and H. J. Nauwynck. 2005. The pseudorabies virus US3 protein kinase possesses anti-apoptotic activity that protects cells from apoptosis during infection and after treatment with sorbitol or staurosporine. Virology 331144-150. [DOI] [PubMed] [Google Scholar]
- 32.Gennerich, A., A. P. Carter, S. L. Reck-Peterson, and R. D. Vale. 2007. Force-induced bidirectional stepping of cytoplasmic dynein. Cell 131952-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godsel, L. M., R. P. Hobbs, and K. J. Green. 2008. Intermediate filament assembly: dynamics to disease. Trends Cell Biol. 1828-37. [DOI] [PubMed] [Google Scholar]
- 34.Goode, B. L., D. G. Drubin, and G. Barnes. 2000. Functional cooperation between the microtubule and actin cytoskeletons. Curr. Opin. Cell Biol. 1263-71. [DOI] [PubMed] [Google Scholar]
- 35.Granzow, H., B. G. Klupp, and T. C. Mettenleiter. 2005. Entry of pseudorabies virus: an immunogold-labeling study. J. Virol. 793200-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grunewald, K., P. Desai, D. C. Winkler, J. B. Heymann, D. M. Belnap, W. Baumeister, and A. C. Steven. 2003. Three-dimensional structure of herpes simplex virus from cryo-electron tomography. Science 3021396-1398. [DOI] [PubMed] [Google Scholar]
- 37.Harley, C. A., A. Dasgupta, and D. W. Wilson. 2001. Characterization of herpes simplex virus-containing organelles by subcellular fractionation: role for organelle acidification in assembly of infectious particles. J. Virol. 751236-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herrmann, H., H. Bar, L. Kreplak, S. V. Strelkov, and U. Aebi. 2007. Intermediate filaments: from cell architecture to nanomechanics. Nat. Rev. Mol. Cell Biol. 8562-573. [DOI] [PubMed] [Google Scholar]
- 39.Hirokawa, N. 1998. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279519-526. [DOI] [PubMed] [Google Scholar]
- 40.Hoppe, S., M. Schelhaas, V. Jaeger, T. Liebig, P. Petermann, and D. Knebel-Morsdorf. 2006. Early herpes simplex virus type 1 infection is dependent on regulated Rac1/Cdc42 signalling in epithelial MDCKII cells. J. Gen. Virol. 873483-3494. [DOI] [PubMed] [Google Scholar]
- 41.Howard, J., and A. A. Hyman. 2003. Dynamics and mechanics of the microtubule plus end. Nature 422753-758. [DOI] [PubMed] [Google Scholar]
- 42.Hutt-Fletcher, L. M. 2007. Epstein-Barr virus entry. J. Virol. 817825-7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johannsen, E., M. Luftig, M. R. Chase, S. Weicksel, E. Cahir-McFarland, D. Illanes, D. Sarracino, and E. Kieff. 2004. Proteins of purified Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 10116286-16291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kattenhorn, L. M., R. Mills, M. Wagner, A. Lomsadze, V. Makeev, M. Borodovsky, H. L. Ploegh, and B. M. Kessler. 2004. Identification of proteins associated with murine cytomegalovirus virions. J. Virol. 7811187-11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelleher, J. F., and M. A. Titus. 1998. Intracellular motility: how can we all work together? Curr. Biol. 8R394-R397. [DOI] [PubMed] [Google Scholar]
- 46.Kim, S., and P. A. Coulombe. 2007. Intermediate filament scaffolds fulfill mechanical, organizational, and signaling functions in the cytoplasm. Genes Dev. 211581-1597. [DOI] [PubMed] [Google Scholar]
- 47.Klopfenstein, D. R., R. D. Vale, and S. L. Rogers. 2000. Motor protein receptors: moonlighting on other jobs. Cell 103537-540. [DOI] [PubMed] [Google Scholar]
- 48.Koshizuka, T., Y. Kawaguchi, and Y. Nishiyama. 2005. Herpes simplex virus type 2 membrane protein UL56 associates with the kinesin motor protein KIF1A. J. Gen. Virol. 86527-533. [DOI] [PubMed] [Google Scholar]
- 49.Krendel, M., and M. S. Mooseker. 2005. Myosins: tails (and heads) of functional diversity. Physiology (Bethesda) 20239-251. [DOI] [PubMed] [Google Scholar]
- 50.Langford, G. M. 1995. Actin- and microtubule-dependent organelle motors: interrelationships between the two motility systems. Curr. Opin. Cell Biol. 782-88. [DOI] [PubMed] [Google Scholar]
- 51.Lanni, F., A. S. Waggoner, and D. L. Taylor. 1985. Structural organization of interphase 3T3 fibroblasts studied by total internal reflection fluorescence microscopy. J. Cell Biol. 1001091-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee, G. E., J. W. Murray, A. W. Wolkoff, and D. W. Wilson. 2006. Reconstitution of herpes simplex virus microtubule-dependent trafficking in vitro. J. Virol. 804264-4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leopardi, R., C. Van Sant, and B. Roizman. 1997. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc. Natl. Acad. Sci. USA 947891-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luby-Phelps, K. 2000. Cytoarchitecture and physical properties of cytoplasm: volume, viscosity, diffusion, intracellular surface area. Int. Rev. Cytol. 192189-221. [DOI] [PubMed] [Google Scholar]
- 55.Lukacs, G. L., P. Haggie, O. Seksek, D. Lechardeur, N. Freedman, and A. S. Verkman. 2000. Size-dependent DNA mobility in cytoplasm and nucleus. J. Biol. Chem. 2751625-1629. [DOI] [PubMed] [Google Scholar]
- 56.Lusk, C. P., G. Blobel, and M. C. King. 2007. Highway to the inner nuclear membrane: rules for the road. Nat. Rev. Mol. Cell Biol. 8414-420. [DOI] [PubMed] [Google Scholar]
- 57.Luxton, G. W., S. Haverlock, K. E. Coller, S. E. Antinone, A. Pincetic, and G. A. Smith. 2005. Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. Proc. Natl. Acad. Sci. USA 1025832-5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mallik, R., B. C. Carter, S. A. Lex, S. J. King, and S. P. Gross. 2004. Cytoplasmic dynein functions as a gear in response to load. Nature 427649-652. [DOI] [PubMed] [Google Scholar]
- 59.Mallik, R., and S. P. Gross. 2004. Molecular motors: strategies to get along. Curr. Biol. 14R971-R982. [DOI] [PubMed] [Google Scholar]
- 60.Martinez-Moreno, M., I. Navarro-Lerida, F. Roncal, J. P. Albar, C. Alonso, F. Gavilanes, and I. Rodriguez-Crespo. 2003. Recognition of novel viral sequences that associate with the dynein light chain LC8 identified through a pepscan technique. FEBS Lett. 544262-267. [DOI] [PubMed] [Google Scholar]
- 60a.Maurer, U. E., B. Sodeik, and K. Grünewald. 2008. Native 3D intermediates of membrane fusion in herpes simplex virus 1 entry. Proc. Natl. Acad. Sci. USA. 10510559-10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 761537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mettenleiter, T. C., B. G. Klupp, and H. Granzow. 2006. Herpesvirus assembly: a tale of two membranes. Curr. Opin. Microbiol. 9423-429. [DOI] [PubMed] [Google Scholar]
- 63.Mettenleiter, T. C., and T. Minson. 2006. Egress of alphaherpesviruses. J. Virol. 801610-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Michael, K., B. G. Klupp, T. C. Mettenleiter, and A. Karger. 2006. Composition of pseudorabies virus particles lacking tegument protein US3, UL47, or UL49 or envelope glycoprotein E. J. Virol. 801332-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miki, H., Y. Okada, and N. Hirokawa. 2005. Analysis of the kinesin superfamily: insights into structure and function. Trends Cell Biol. 15467-476. [DOI] [PubMed] [Google Scholar]
- 66.Miranda-Saksena, M., P. Armati, R. A. Boadle, D. J. Holland, and A. L. Cunningham. 2000. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J. Virol. 741827-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mohr, I. 2004. Neutralizing innate host defenses to control viral translation in HSV-1 infected cells. Int. Rev. Immunol. 23199-220. [DOI] [PubMed] [Google Scholar]
- 68.Morris, J. B., H. Hofemeister, and P. O'Hare. 2007. Herpes simplex virus infection induces phosphorylation and delocalization of emerin, a key inner nuclear membrane protein. J. Virol. 814429-4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muranyi, W., J. Haas, M. Wagner, G. Krohne, and U. H. Koszinowski. 2002. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science 297854-857. [DOI] [PubMed] [Google Scholar]
- 70.Murata, T., F. Goshima, T. Daikoku, H. Takakuwa, and Y. Nishiyama. 2000. Expression of herpes simplex virus type 2 US3 affects the Cdc42/Rac pathway and attenuates c-Jun N-terminal kinase activation. Genes Cells 51017-1027. [DOI] [PubMed] [Google Scholar]
- 71.Nakanishi, H., and Y. Takai. 2004. Roles of nectins in cell adhesion, migration and polarization. Biol. Chem. 385885-892. [DOI] [PubMed] [Google Scholar]
- 72.Naranatt, P. P., S. M. Akula, C. A. Zien, H. H. Krishnan, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-ζ-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J. Virol. 771524-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naranatt, P. P., H. H. Krishnan, M. S. Smith, and B. Chandran. 2005. Kaposi's sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus. J. Virol. 791191-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nogales, E. 2000. Structural insights into microtubule function. Annu. Rev. Biochem. 69277-302. [DOI] [PubMed] [Google Scholar]
- 75.Ogawa-Goto, K., K. Tanaka, W. Gibson, E. Moriishi, Y. Miura, T. Kurata, S. Irie, and T. Sata. 2003. Microtubule network facilitates nuclear targeting of human cytomegalovirus capsid. J. Virol. 778541-8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ogg, P. D., P. J. McDonell, B. J. Ryckman, C. M. Knudson, and R. J. Roller. 2004. The HSV-1 Us3 protein kinase is sufficient to block apoptosis induced by overexpression of a variety of Bcl-2 family members. Virology 319212-224. [DOI] [PubMed] [Google Scholar]
- 77.Oriolo, A. S., F. A. Wald, V. P. Ramsauer, and P. J. Salas. 2007. Intermediate filaments: a role in epithelial polarity. Exp. Cell Res. 3132255-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park, R., and J. D. Baines. 2006. Herpes simplex virus type 1 infection induces activation and recruitment of protein kinase C to the nuclear membrane and increased phosphorylation of lamin B. J. Virol. 80494-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Penfold, M. E., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. USA 916529-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pfister, K. K., P. R. Shah, H. Hummerich, A. Russ, J. Cotton, A. A. Annuar, S. M. King, and E. M. Fisher. 2006. Genetic analysis of the cytoplasmic dynein subunit families. PLoS Genet. 2e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Poncet, D., A. L. Pauleau, G. Szabadkai, A. Vozza, S. R. Scholz, M. Le Bras, J. J. Briere, A. Jalil, R. Le Moigne, C. Brenner, G. Hahn, I. Wittig, H. Schagger, C. Lemaire, K. Bianchi, S. Souquere, G. Pierron, P. Rustin, V. S. Goldmacher, R. Rizzuto, F. Palmieri, and G. Kroemer. 2006. Cytopathic effects of the cytomegalovirus-encoded apoptosis inhibitory protein vMIA. J. Cell Biol. 174985-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reynolds, A. E., L. Liang, and J. D. Baines. 2004. Conformational changes in the nuclear lamina induced by herpes simplex virus type 1 require genes UL31 and UL34. J. Virol. 785564-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reynolds, A. E., E. G. Wills, R. J. Roller, B. J. Ryckman, and J. D. Baines. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 768939-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roizman, B., and P. E. Pellet. 2001. The family Herpesviridae: a brief introduction. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 85.Ross, J. L., M. Y. Ali, and D. M. Warshaw. 2008. Cargo transport: molecular motors navigate a complex cytoskeleton. Curr. Opin. Cell Biol. 2041-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sakisaka, T., W. Ikeda, H. Ogita, N. Fujita, and Y. Takai. 2007. The roles of nectins in cell adhesions: cooperation with other cell adhesion molecules and growth factor receptors. Curr. Opin. Cell Biol. 19593-602. [DOI] [PubMed] [Google Scholar]
- 87.Saksena, M. M., H. Wakisaka, B. Tijono, R. A. Boadle, F. Rixon, H. Takahashi, and A. L. Cunningham. 2006. Herpes simplex virus type 1 accumulation, envelopment, and exit in growth cones and varicosities in mid-distal regions of axons. J. Virol. 803592-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Santarelli, R., A. Farina, M. Granato, R. Gonnella, S. Raffa, L. Leone, R. Bei, A. Modesti, L. Frati, M. R. Torrisi, and A. Faggioni. 2008. Identification and characterization of the product encoded by ORF69 of Kaposi's sarcoma-associated herpesvirus. J. Virol. 824562-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schnee, M., Z. Ruzsics, A. Bubeck, and U. H. Koszinowski. 2006. Common and specific properties of herpesvirus UL34/UL31 protein family members revealed by protein complementation assay. J. Virol. 8011658-11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schumacher, D., B. K. Tischer, S. Trapp, and N. Osterrieder. 2005. The protein encoded by the US3 orthologue of Marek's disease virus is required for efficient de-envelopment of perinuclear virions and involved in actin stress fiber breakdown. J. Virol. 793987-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scott, E. S., and P. O'Hare. 2001. Fate of the inner nuclear membrane protein lamin B receptor and nuclear lamins in herpes simplex virus type 1 infection. J. Virol. 758818-8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharma-Walia, N., P. P. Naranatt, H. H. Krishnan, L. Zeng, and B. Chandran. 2004. Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J. Virol. 784207-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharon-Friling, R., J. Goodhouse, A. M. Colberg-Poley, and T. Shenk. 2006. Human cytomegalovirus pUL37x1 induces the release of endoplasmic reticulum calcium stores. Proc. Natl. Acad. Sci. USA 10319117-19122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Simpson-Holley, M., R. C. Colgrove, G. Nalepa, J. W. Harper, and D. M. Knipe. 2005. Identification and functional evaluation of cellular and viral factors involved in the alteration of nuclear architecture during herpes simplex virus 1 infection. J. Virol. 7912840-12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Skepper, J. N., A. Whiteley, H. Browne, and A. Minson. 2001. Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment→deenvelopment→reenvelopment pathway. J. Virol. 755697-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith, G. A., S. P. Gross, and L. W. Enquist. 2001. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc. Natl. Acad. Sci. USA 983466-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sodeik, B. 2000. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. 8465-472. [DOI] [PubMed] [Google Scholar]
- 98.Sodeik, B., M. W. Ebersold, and A. Helenius. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 1361007-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 7710179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Toba, S., T. M. Watanabe, L. Yamaguchi-Okimoto, Y. Y. Toyoshima, and H. Higuchi. 2006. Overlapping hand-over-hand mechanism of single molecular motility of cytoplasmic dynein. Proc. Natl. Acad. Sci. USA 1035741-5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Turcotte, S., J. Letellier, and R. Lippé. 2005. Herpes simplex virus type 1 capsids transit by the trans-Golgi network, where viral glycoproteins accumulate independently of capsid egress. J. Virol. 798847-8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Udo, H., I. Jin, J. H. Kim, H. L. Li, T. Youn, R. D. Hawkins, E. R. Kandel, and C. H. Bailey. 2005. Serotonin-induced regulation of the actin network for learning-related synaptic growth requires Cdc42, N-WASP, and PAK in Aplysia sensory neurons. Neuron 45887-901. [DOI] [PubMed] [Google Scholar]
- 103.Vale, R. D. 2003. The molecular motor toolbox for intracellular transport. Cell 112467-480. [DOI] [PubMed] [Google Scholar]
- 104.van Leeuwen, H., G. Elliott, and P. O'Hare. 2002. Evidence of a role for nonmuscle myosin II in herpes simplex virus type 1 egress. J. Virol. 763471-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Van Minnebruggen, G., H. W. Favoreel, L. Jacobs, and H. J. Nauwynck. 2003. Pseudorabies virus US3 protein kinase mediates actin stress fiber breakdown. J. Virol. 779074-9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Varnum, S. M., D. N. Streblow, M. E. Monroe, P. Smith, K. J. Auberry, L. Pasa-Tolic, D. Wang, D. G. Camp II, K. Rodland, S. Wiley, W. Britt, T. Shenk, R. D. Smith, and J. A. Nelson. 2004. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 7810960-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wagenaar, F., J. M. Pol, B. Peeters, A. L. Gielkens, N. de Wind, and T. G. Kimman. 1995. The US3-encoded protein kinase from pseudorabies virus affects egress of virions from the nucleus. J. Gen. Virol. 761851-1859. [DOI] [PubMed] [Google Scholar]
- 108.Welte, M. A. 2004. Bidirectional transport along microtubules. Curr. Biol. 14R525-R537. [DOI] [PubMed] [Google Scholar]
- 109.Wolfstein, A., C. H. Nagel, K. Radtke, K. Dohner, V. J. Allan, and B. Sodeik. 2006. The inner tegument promotes herpes simplex virus capsid motility along microtubules in vitro. Traffic 7227-237. [DOI] [PubMed] [Google Scholar]
- 110.Wong, M. L., and C. H. Chen. 1998. Evidence for the internal location of actin in the pseudorabies virion. Virus Res. 56191-197. [DOI] [PubMed] [Google Scholar]
- 111.Zapata, H. J., M. Nakatsugawa, and J. F. Moffat. 2007. Varicella-zoster virus infection of human fibroblast cells activates the c-Jun N-terminal kinase pathway. J. Virol. 81977-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhu, F. X., J. M. Chong, L. Wu, and Y. Yuan. 2005. Virion proteins of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79800-811. [DOI] [PMC free article] [PubMed] [Google Scholar]