Abstract
In C57BL/6 (B6) mice, most herpes simplex virus (HSV)-specific CD8 T cells recognize a strongly immunodominant epitope on glycoprotein B (gB498) and can inhibit HSV type 1 (HSV-1) reactivation from latency in trigeminal ganglia (TG). However, half of the CD8 T cells retained in latently infected TG of B6 mice are not gB498 specific and have been largely ignored. The following observations from our current study indicate that these gB498-nonspecific CD8 T cells are HSV specific and may contribute to the control of HSV-1 latency. First, following corneal infection, OVA257-specific OT-1 CD8 T cells do not infiltrate the infected TG unless mice are simultaneously immunized with OVA257 peptide, and then they are not retained. Second, 30% of CD8 T cells in acutely infected TG that produce gamma interferon in response to HSV-1 stimulation directly ex vivo are gB498 nonspecific, and these cells maintain an activation phenotype during viral latency. Finally, gB498-nonspecific CD8 T cells are expanded in ex vivo cultures of latently infected TG and inhibit HSV-1 reactivation from latency in the absence of gB498-specific CD8 T cells. We conclude that many of the CD8 T cells that infiltrate and are retained in infected TG are HSV specific and potentially contribute to maintenance of HSV-1 latency. Identification of the viral proteins recognized by these cells will contribute to a better understanding of the dynamics of HSV-1 latency.
The generation and maintenance of a CD8 T-cell response represent an important line of defense against many viral pathogens. Such responses are typically initiated when host antigen-presenting cells at the site of infection capture and process viral proteins and transport them to local draining lymph nodes (DLN). There the antigen-presenting cells either directly present viral antigens to naïve CD8 T cells or pass them to a distinct LN-resident dendritic cell (DC) subset for antigen presentation in the context of major histocompatibility complex class I (1). Antigen-specific CD8 T cells then undergo robust division and differentiation into effector populations armed to infiltrate infected tissue and eliminate the invading pathogen. The magnitude of the CD8 T-cell response against different viral epitopes is typically aligned within a defined hierarchy. Those epitopes recognized by the largest portion of the pathogen-specific CD8 T-cell population are referred to as immunodominant, while those inciting lesser responses are referred to as subdominant (17). Manipulation of this hierarchal system by the elimination of an immunodominant epitope often results in the expansion of a normally silent or “cryptic” determinant (2, 17, 21).
Although the HSV-1 genome contains at least 84 open reading frames (13), it is estimated that 70 to 95% of the acute CD8 T-cell response in lymphoid organs of B6 mice is directed against the single immunodominant gB498 epitope (11, 21, 24, 26, 27). The remaining HSV-specific CD8 T cells are thought to be directed against a subdominant epitope on the viral ribonucleotide reductase (RR1822) (16). These conclusions are derived from studies characterizing the specificity of CD8 T cells at the peak of the effector response in lymphoid tissue. Interestingly, a recombinant HSV-1 lacking the immunodominant gB498 epitope induced an HSV-specific CD8 T-cell response of normal magnitude, while the RR1822 epitope remained subdominant (21), suggesting the emergence of previously unrecognized or cryptic epitopes.
Following HSV-1 corneal infection of B6 mice, virus is transmitted to the trigeminal ganglia (TG), where it replicates briefly (up to 6 days postinfection [dpi]) and then establishes a latent infection. CD8 effector T cells accumulate to peak levels in the TG by 8 dpi and then undergo contraction, and then a memory population of constant size is maintained for the life of the animal. While 50% of both the effector and memory CD8 T-cell populations are specific for the immunodominant gB498 epitope (11, 18), the remaining TG-resident CD8 T cells are specific for neither the dominant gB498 nor the subdominant RR1822 epitope. Although the phenotype and function of the gB498-specific CD8 T cells in sensory ganglia and their role in maintaining HSV-1 latency have been well characterized (3, 5, 9, 11, 12, 14, 18, 19, 22, 24, 25, 27), the properties of the gB498-nonspecific TG-resident CD8 T-cell population and their role in maintaining viral latency remain unexplored. Here we demonstrate that many of the gB498-nonspecific CD8 T cells in latently infected TG proliferate and some produce gamma interferon (IFN-γ) when stimulated with HSV-1 antigens directly ex vivo. These cells also persistently exhibit an activation phenotype within latently infected TG, are expanded in ex vivo cultures of latently infected TG, and can block HSV-1 reactivation in TG neurons in the absence of gB498-specific CD8 T cells.
MATERIALS AND METHODS
Mice and virus.
Wild-type HSV-1 strain RE and a previously described recombinant virus on the RE background, pICP0-EGFP HSV-1 (6), were grown in Vero cells, and intact virions were isolated on Optiprep gradients according to manufacturer's instructions (Accurate Chemical & Scientific). Six- to 8-week-old female C57BL/6 (CD45.2) mice, B6.129S2-Cd8atm1Mak/J (CD8−/−) mice, and B6.SJL-PtprcaPepcb/BoyJ (B6.SJL; CD45.1) mice (The Jackson Laboratory) were anesthetized by intraperitoneal (i.p.) injection of 2.0 mg of ketamine hydrochloride and 0.04 mg of xylazine (Phoenix Scientific) in 0.2 ml of Hanks balanced salt solution (BioWhittaker). The abraded central corneas of anesthetized mice were infected by topical application of 3 μl of RPMI 1640 (BioWhittaker) containing 1 × 105 PFU of wild-type HSV-1 or pICP0-EGFP HSV-1. gB-T1.1 (kindly provided by Francis Carbone) and C57BL/6-Tg(TcraTcrb)1100Mjb/J (OT-1; The Jackson Laboratory) transgenic mice were used as donors for adoptive transfer assays. All animal experiments were conducted in accordance with guidelines established by the University of Pittsburgh Institutional Animal Care and Use Committee.
Reagents.
The gB498 (SSIEFARL) and RR1822 (QTFDFGRL) peptides were purchased from Research Genetics (Invitrogen Life Technologies). Phycoerythrin (PE)-conjugated H-2Kb tetramers complexed with the gB498 peptide were provided by the National Institute of Allergy and Infectious Diseases Tetramer Core Facility (Emory University Vaccine Center, Atlanta, GA). Rat anti-mouse allophycocyanin (APC)-Alexa Fluor 750-conjugated or Pacific Blue-conjugated anti-CD8α (clone 53-6.7), APC-conjugated anti-CD3 (145-2C11), APC-conjugated anti-IFN-γ (XMG1.2), peridinin chlorophyll protein-conjugated anti-CD45 (30-F11), fluorescein isothiocyanate (FITC)-conjugated anti-CD69 (H1.2F3), FITC-conjugated anti-CD62L (MEL-14), anti-human APC-conjugated anti-granzyme B (anti-grz B) (GB11), the bromodeoxyuridine (BrdU) flow cytometry kit (clone 3D4), and the Vβ T-cell receptor (TCR) flow cytometry kit were purchased from BD Pharmingen. APC-conjugated anti-CD127 (A7R34), APC-conjugated anti-CD27 (LG.7F9), APC-conjugated anti-KLRG1 (2F1), and FITC-conjugated anti-CD86 (GL1) were purchased from eBioscience. The appropriate isotype control antibodies were purchased from BD Pharmingen, eBioscience, or Biolegend. All flow cytometry samples were collected on a FACSAria cytometer and analyzed by FACSDiva software (BD Biosciences).
Tissue preparation.
Anesthetized mice were injected with 0.3 ml of 1,000-U/ml heparin and euthanized by exsanguination. TG were harvested and digested in 100 μl per ganglia of Dulbecco's modified Eagle medium (BioWhittaker) containing 10% fetal calf serum (FCS) and 400 U/ml collagenase type I (Sigma-Aldrich) for 1 h at 37°C. TG were dispersed into single-cell suspensions and treated with red blood cell (RBC) lysis buffer prior to use. Spleens were dispersed mechanically and treated with RBC lysis buffer prior to use. Tissue harvest and preparation were performed under sterile conditions for all adoptive transfers and those ex vivo culture assays lasting longer than 6 h.
Phenotypic analysis of T cells.
For all phenotypic analyses, TG were stained for CD45 to permit gating exclusively on bone marrow-derived cells. Surface staining was performed on single-cell suspensions as previously described (18). All samples were treated with unconjugated anti-CD16/CD32 for 10 min on ice prior to surface staining to prevent nonspecific antibody binding.
Cell sorting.
TG or spleens were excised and pooled, and single-cell suspensions were prepared as described above. Tissues were stained with anti-CD8α and gB498 tetramer for 1 h at 4°C. Cell sorting was performed on a FACSAria to obtain pure samples of CD8 T cells that either bound gB498 tetramer (gB498 specific) or did not (gB498 nonspecific). In some assays, gB498-nonspecific CD8 T cells were subdivided based on expression of CD86. Samples were sorted to 88 to 95% purity in this manner and then used in stimulation/BrdU assays or ex vivo reactivation assays.
DC immunization.
Bone marrow was isolated from the femurs and tibiae of B6 mice. RBCs were lysed with ACK lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA, pH 7.2). Cells were washed and resuspended in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 mM l-glutamine (all purchased from Invitrogen, Carlsbad, CA) supplemented with 1,000 U/ml granulocyte-macrophage colony-stimulating factor (Schering Plough, Kenilworth, NJ), as previously described (20). On day 6 to 7, CD11c+ DCs were isolated using anti-mouse CD11c-coated magnetic beads and MACS separation columns (Miltenyi Biotech), according to the manufacturer's protocol. The DCs were then matured for 24 h in the presence of 250 ng/ml lipopolysaccharide (Sigma-Aldrich), 1,000 U/ml interleukin-4 (IL-4) (Schering Plough), and 1,000 U/ml IFN-γ (Peprotech). All maturation conditions included 1,000 U/ml granulocyte-macrophage colony-stimulating factor to sustain cell viability. For the induction of OVA-specific immune responses, the dominant H-2Kb-restricted OVA epitope, OVA257 (SIINFEKL), was synthesized by the University of Pittsburgh Peptide Synthesis Facility. Matured DCs were pulsed with OVA257 epitope, washed twice with phosphate-buffered saline, and injected subcutaneously (3 × 103 DCs in 0.2 ml phosphate-buffered saline).
Adoptive transfers.
Spleens and LN were harvested from naïve gB-T1.1 and OT-1 mice. CD8 T cells from cell suspensions were isolated by magnetic bead separation on a MACS column. In different assays either 1 × 106 gB-T1.1 and 3 × 106 OT-1 CD8 T cells were transferred into CD8-deficient mice or 1 × 105 OT-1 CD8 T cells were transferred into B6 mice via intravenous (i.v.) injection and allowed to acclimate for 1 day prior to infection and/or immunization.
Administration of BrdU.
Mice were administered 1 mg of BrdU i.p. daily for indicated length of time prior to tissue excision. For in situ analysis of proliferation, mice received 1 mg of BrdU i.v. 4 h prior to tissue harvest. Single-cell suspensions were surface stained as indicated and then stained for intracellular BrdU according to the manufacturer's protocol (BD Pharmingen).
Intracellular cytokine staining/CFSE dilution.
TG or DLN were obtained at 8 dpi, dispersed into single-cell suspensions, and stained with gB498 tetramers. The cells were either directly stimulated or sorted into tetramer-positive and tetramer-negative cells and then stimulated. Stimulation was with 5 × 105 HSV-infected B6/T-350 fibroblasts, B6/T-350 fibroblasts pulsed with 10−6 M gB498 peptide or RR1822 peptide, B6/T-350 fibroblasts transfected with a plasmid expressing full-length gB, or unmanipulated B6/T-350 cells (unstimulated control) in the presence of RPMI 1640 containing 10% FCS and GolgiPlug (BD Biosciences) for 6 h at 37°C with 5% CO2. Following stimulation, cells were stained for CD8 surface expression, permeabilized and fixed with Cytofix/Cytoperm (BD Biosciences), and stained for intracellular IFN-γ. In proliferation assays, sorted populations were labeled with 1.0 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen) according to the manufacturer's instructions and stimulated with indicated targets for 3 days at 37°C with 5% CO2 in RPMI 1640 containing 10% FCS. To ensure specificity, nonoverlapping fluorochromes were used for pre- and postsort staining.
Ex vivo reactivation assays.
TG were excised from mice harboring latent pICP0-EGFP HSV-1 and depleted of >95% of CD45-expressing cells by complement fixation as previously described (4, 15). The CD45-depleted TG cells were plated in Iscove's modified Dulbecco's medium containing 10% FCS, 500 U/ml recombinant IL-2 (R & D Systems), and 50 μM 2-mercaptoethanol (Fischer Scientific) at 0.2 TG equivalent per well in a 48-well plate. Sorted populations of CD8 T cells were added to these cultures and incubated for 11 days at 37°C with 5% CO2. Cultures were monitored for HSV-1 reactivation from latency based both on spread of enhanced green fluorescent protein (EGFP) from neurons to surrounding fibroblasts as assessed by epifluorescence microscopy and on the presence of infectious virus in culture supernatants as assessed by a viral plaque assay as previously described (6). Spread of EGFP was always accompanied, though often with up to a 24-h delay, by detection of infectious virus in culture supernatants. At 11 days postexplant, cells were recovered and pooled from cultures exhibiting HSV-1 reactivation (EGFP spread) and from nonreactivated cultures. The pooled cells were stained with anti-CD8α monoclonal antibody and gB498 tetramers and analyzed by flow cytometry.
RESULTS
A population of gB498-nonspecific CD8 T cells in the HSV-1-infected TG expresses an activation phenotype consistent with antigenic experience.
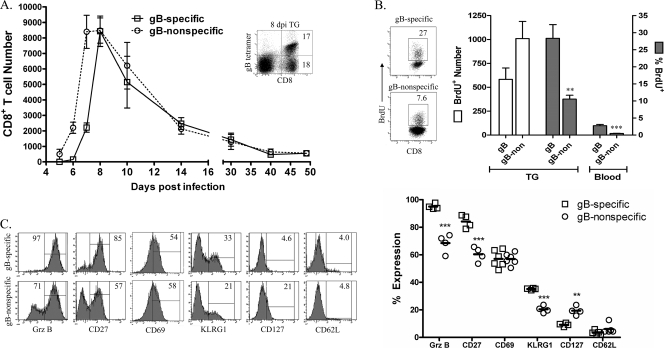
Tetramer staining and flow cytometric analysis were used to differentially quantify CD8 T cells specific for the immunodominant gB498 epitope and gB498-nonspecific CD8 T cells in the TG at various times after HSV-1 corneal infection. The gB498-nonspecific CD8 T cells infiltrated the TG 1 day earlier than their gB498-specific counterparts, but the two populations were equivalent in numbers by 8 dpi and remained equivalent through contraction and homeostasis (Fig. 1A). A 4-h i.v. BrdU pulse at 7 dpi resulted in a significantly increased frequency of BrdU+ gB498-specific and gB498-nonspecific CD8 T cells in the TG relative to the blood, suggesting expansion of both populations within the infected TG (Fig. 1B). Moreover, a comparable absolute number of gB498-specific and gB498-nonspecific CD8 T cells proliferated in the TG during the 4-h BrdU pulse.
FIG. 1.
The gB498-nonspecific CD8 T-cell population within infected TG is activated and undergoes proliferation. (A) TG were harvested at indicated dpi and stained with gB498 tetramers for quantification of gB498-specific and gB498-nonspecific CD8 T cells. The inset shows staining of CD8 T cells in TG at 8 dpi and illustrates the comparable sizes of the gB498-specific and gB498-nonspecific CD8 T-cell populations observed at all times beyond 8 dpi. Data are presented as the mean absolute number of each CD8 T-cell population in individual TG ± standard error of the mean. (B) HSV-1-infected mice received 1 mg BrdU i.v. 4 h prior to TG excision, and in situ proliferation of CD8 T-cell populations was assessed on 7 dpi by intracellular staining for incorporated BrdU and flow cytometry. Representative dot plots are gated on either gB498-specific or gB498-nonspecific CD8 T cells as demonstrated in the inset to panel A, gates are based on appropriate isotype controls, and numbers within dot plots represent the percentage of cells within the positive gate. Data from individual mice are represented as indicated (mean ± standard error of the mean). (C) Phenotypic characterization of CD8 T-cell populations within the TG at 7 dpi. Representative histograms are gated on either gB498-specific or gB498-nonspecific CD8 T cells as illustrated in the inset to panel A, gates are based on appropriate isotype controls, and numbers within histograms correspond to the percentage of cells within the positive gate. Data from individual mice are presented in the scatter plot as percent expression of indicated marker within the gB498-specific and gB498-nonspecific CD8 T-cell populations (solid line = mean). ***, P < 0.001; **, P < 0.01 (Student's t test).
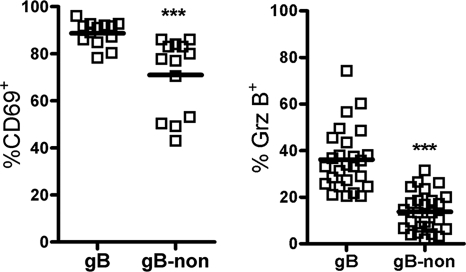
A majority of the cells in both the gB498-specific and gB498-nonspecific populations exhibited an activation phenotype characteristic of recent TCR stimulation within the acutely infected TG (Fig. 1C), including elevated expression of grz B, CD27, and CD69 and low expression of CD127 and CD62L. KLRG1 was also expressed on a small portion of cells in each population, possibly identifying the presence of short-lived effector cells in the TG (8, 10). Notably, the gB498-nonspecific CD8 T-cell population exhibited only a slightly lower proportion of activated cells than did the gB498-specific population. In addition, the activation status of the gB498-nonspecific CD8 T-cell population was maintained during latency, with elevated expression of CD69 and grz B (Fig. 2). These findings are consistent with the notion that gB498-nonspecific CD8 T-cell populations in the TG are HSV specific and that both the gB498-specific and gB498-nonspecific CD8 T cells receive antigenic stimulation within the infected TG.
FIG. 2.
Maintained activation of gB498-nonspecific CD8 T cells within the latent ganglia. B6 mice were allowed to establish latency (>34 days) prior to excision of the TG. Single-cell suspensions were stained for expression of CD8, for expression of CD69, with gB498 tetramers, and intracellularly for grz B. Data from individual mice are presented in the scatter plot as percent expression of the indicated marker within the gB498-specific and gB498-nonspecific CD8 T-cell populations (solid line = mean). ***, P < 0.001 (Student's t test).
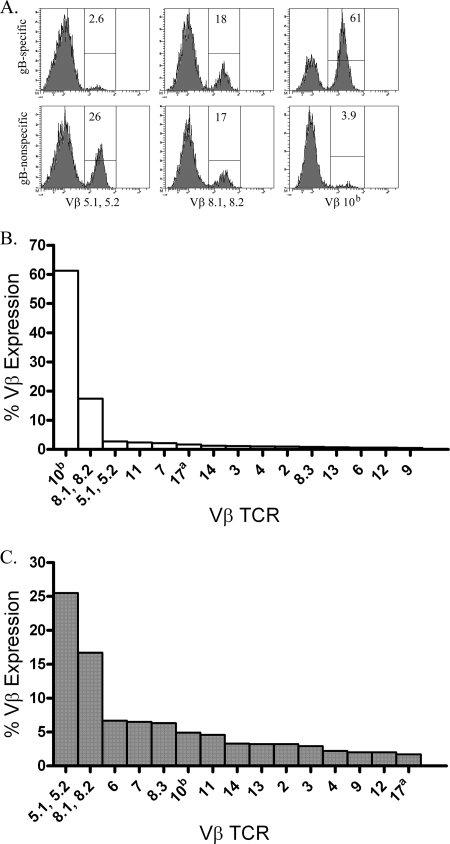
The gB498-nonspecific CD8 T-cell population in the infected TG utilizes a less restricted Vβ TCR repertoire.
The gB498-specific CD8 T-cell population in HSV-infected TG employs a highly restricted TCR Vβ repertoire, in which the Vβ 10b or Vβ 8.2 TCR elements are employed by nearly 80% of the cells (Fig. 3A and B) (23, 26, 27). In contrast, the gB498-nonspecific CD8 T-cell population in the TG exhibited a somewhat more diverse TCR Vβ repertoire (Fig. 3A and C), with 40% employing Vβ 5.1 and/or 5.2 or 8.1 and/or 8.2 TCR elements. The remaining 60% demonstrate diverse TCR Vβ utilization.
FIG. 3.
The gB498-nonspecific CD8 T-cell population displays a more diverse Vβ TCR usage. TG were harvested at 8 dpi, pooled, and stained with gB498 tetramers, for CD8, and for TCR Vβ utilization. (A) Representative histograms illustrate utilization of three prominent Vβ (Vβ 5.1, 5.2, Vβ 8.1, 8.2, and Vβ 10b) TCR segments by gB498 tetramer-positive (gB498-specific) and gB498 tetramer-negative (gB498-nonspecific) CD8 T cells. Numbers within histograms correspond to the percentage of cells within the positive gate. (B and C) Data are presented graphically as the percent expression of indicated Vβ TCR among gB498-specific CD8 T cells (B) or gB498-nonspecific CD8 T cells (C).
Bystander activation does not promote the migration of known HSV-1-nonspecific CD8 T cells into the HSV-1 infected TG.
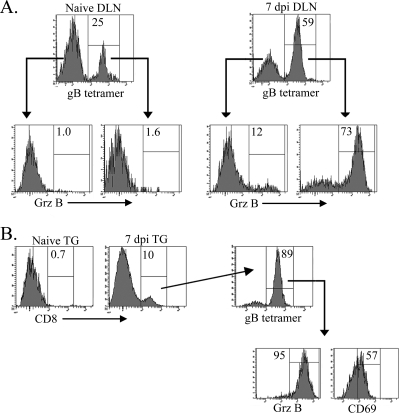
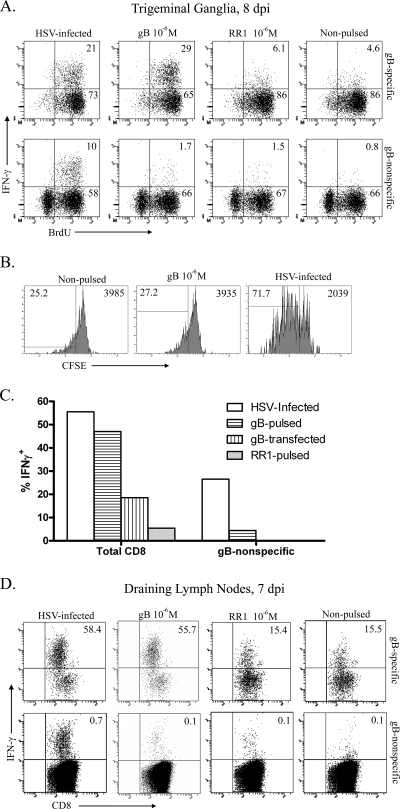
Selective accumulation of HSV-specific CD8 T cells in infected TG could result from preferential infiltration and/or retention of HSV-specific cells. To determine if known HSV-nonspecific CD8 T cells can be induced through bystander activation to infiltrate the infected TG, a mixture of naïve CD8 T cells from the spleens of gBT1.1 mice (CD8 T cells are HSV gB498 specific) and OT-1 mice (CD8 T cells are OVA257 specific) were adoptively transferred into CD8−/− recipient mice and allowed to acclimate for 1 day. The corneas of recipient mice were then infected with HSV-1 or mock infected, and the DLN and TG were excised at 7 dpi, the peak of the gB498-nonspecific CD8 T-cell accumulation in the TG. Single-cell suspensions of both tissues were surface stained for CD8, for CD69, and with gB498 tetramers; permeabilized; and then stained for intracellular grz B. In the LN of mock-infected mice, OT-1 and gBT1.1 represented 75% and 25% of the CD8 T cells, respectively, and neither population expressed grz B (Fig. 4A). A selective expansion of the HSV-specific gBT1.1 CD8 T cells was observed in the DLN of HSV-1-infected mice, where they represented 59% of CD8 T cells at 7 dpi. Moreover, grz B was expressed by 73% of the gB498-specific CD8 T cells in DLN, but only 12% of the OT-1 (HSV-nonspecific) CD8 T cells expressed grz B. Thus, following HSV-1 corneal infection, expansion and activation of CD8 T cells in the DLN appear to be primarily HSV specific, with minimal bystander activation of OVA257-specific CD8 T cells. In addition, 89% of the CD8 T cells in the infected TG were gB498 specific, with minimal if any infiltration of OVA257-specific cells (Fig. 4B). Consistent with observations in the dorsal root ganglia (24), these data demonstrate that bystander activation does not significantly promote the migration of HSV-nonspecific CD8 T cells into the TG following HSV-1 corneal infection.
FIG. 4.
Bystander activation does not appear to account for CD8 T-cell infiltration into the infected TG. CD8 T cells were isolated from spleens of noninfected gB-T1.1 and OT-1 mice and transferred into CD8−/− mice 1 day prior to mock infection or infection with HSV-1. Both the DLN (A) and TG (B) were excised at 7 dpi, and gB498-specific CD8 T cells were quantified using gB498 tetramers. Mock-infected recipient mice are labeled naïve. Parent histograms from representative mice are gated on CD8 T cells (A) or CD45 cells (B) with the gating strategy depicted. Numbers within histograms correspond to the percentage of cells within the positive gate.
Antigen stimulation promotes infiltration but not retention of HSV-nonspecific CD8 T cells in HSV-1-infected TG.
Since bystander activation did not appear to promote migration of CD8 T cells that are not HSV-1 specific into the infected TG, we next asked if any CD8 T cell stimulated by its cognate antigen at the time of HSV-1 corneal infection could enter the infected TG. To address this point, CD45.1+ B6.SJL mice received an adoptive transfer of CD45.2+ OVA257-specific OT-1 CD8 T cells 1 day before simultaneous HSV-1 corneal infection and immunization with OVA257-pulsed DCs. At 8 dpi the TG were excised and T-cell populations were determined by flow cytometry. The recipient CD45.1+ CD8 T cells in the TG showed the expected 1:1 ratio of gB498-specific to gB498-nonspecific cells (Fig. 5A), but there was also an equivalent population of donor OVA257-specific CD45.2+ cells. Thus, infiltration of the TG is not HSV specific but is selective for cells that recently received antigenic stimulation.
FIG. 5.
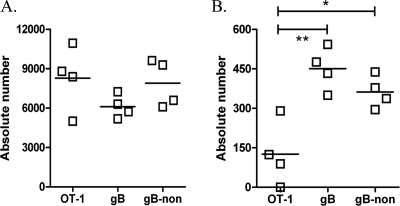
Known HSV-1-nonspecific CD8 T cells are not retained in the latent ganglia. CD45.2+ OT-1 CD8 T cells were isolated from naïve spleens, and 105 were transferred into B6.SJL (CD45.1) mice 1 day prior to simultaneous infection with HSV-1 and DC-OVA257 immunization. TG of these mice were harvested 8 (A) and 40 (B) days later for characterization of the infiltrating populations. Host and donor populations were distinguished based on congenic CD45 markers, and host populations are further delineated by recognition with gB498 mulitmers. The absolute number of each CD8 T-cell population in individual TG is represented graphically, with a solid line indicating the mean. **, P < 0.01; *, P < 0.05 (one-way analysis of variance with Bonferroni post hoc t test).
This provided a model in which to determine if CD8 T cells that specifically do not recognize HSV-1 antigens can be retained in the latently infected TG. As depicted in Fig. 1A, the gB498-specific and gB498-nonspecific CD8 T-cell populations in the TG undergo contraction between 8 to 30 dpi and then maintain populations of constant and similar size thereafter. This pattern is recapitulated by host gB498-specific and gB498-nonspecific CD8 T cells within the TG in the current model (Fig. 5B). However, the donor OVA257-specific CD8 T cells were selectively diminished in TG by 40 dpi. Thus, CD8 T cells known to lack HSV-1 specificity are selectively lost during latency. The fact that gB498-nonspecific CD8 T cells are retained in similar proportion to gB498-specific cells lends further support to the notion that these cells are indeed HSV specific.
A population of gB498-nonspecific CD8 T cells in the TG recognizes HSV-1 antigens.
To directly test whether gB498-nonspecific CD8 T cells can respond to targets presenting HSV-1 antigens, infected mice received twice-daily i.p. injections of BrdU starting at 6 dpi, and TG were excised at 8 dpi. Single-cell suspensions of TG were stained with gB498 tetramers and sorted into gB498-specific and gB498-nonspecific CD8 T cells by fluorescence-activated cell sorting. Both CD8 T-cell populations were then stimulated for 6 h with HSV-1-infected or noninfected targets or targets pulsed with gB498 or RR1822 peptides. Cells were then stained for intracellular IFN-γ and BrdU and analyzed by flow cytometry (Fig. 6A). Among gB498-nonspecific CD8 T cells in the TG, 68% proliferated during the 2 days prior to excision, and 10% produced IFN-γ in response to HSV-infected targets but not in response to gB498-pulsed, RR1822-pulsed, or nonpulsed targets. Among gB498-specific CD8 T cells, 94% divided during the 2 days prior to TG excision, and 21 and 29% produced IFN-γ when stimulated with HSV-infected or gB498-pulsed targets, respectively. A low level of IFN-γ production was observed in gB498-specific cells exposed to RR1822-pulsed or nonpulsed targets, which probably reflects mild stimulation by gB498 tetramers during the sorting and incubation periods (Fig. 6A). These data demonstrate that gB498-nonspecific CD8 T cells represent up to one-third of the CD8 T cells present in the TG at 8 dpi that are capable of producing IFN-γ in response to HSV-1 antigens. Moreover, the relatively low level of IFN-γ production by gB498-specific cells suggests that IFN-γ production greatly underestimates the frequency of HSV-specific CD8 T cells in the TG at this time.
FIG. 6.
A proportion of gB498-nonspecific CD8 T cells recognize other HSV-1 epitopes. (A) B6 mice were infected with HSV-1 and given 1 mg BrdU i.p. daily beginning at 5 dpi. Pooled TG were harvested at indicated dpi, sorted into either gB498-specific or gB498-nonspecific CD8 T cells, and then stimulated with the indicated targets for 6 h in the presence of brefeldin A. Cell suspensions were then stained for surface expression of CD45 and CD8, permeabilized, and stained intracellularly for IFN-γ and BrdU. Representative dot plots show the percentage of cells within the respective quadrants. (B) B6 mice were infected with HSV-1, and pooled TG were harvested at 8 dpi. A pure population of gB498-nonspecific CD8 T cells was obtained by sorting, labeled with 1.0 μM CFSE, and stimulated with the indicated targets for 3 days at 37°C with 5% CO2. Cell suspensions were collected and stained for surface expression of CD45 and CD8 and analyzed for CFSE dilution. Histograms show the percentage of cells within the respective quadrant on the left and the CFSE MFI of the entire CD8 population on the right. (C) Pooled TG obtained at 8 dpi were dispersed into single cell suspensions, stained for 1 h with PE- conjugated gB498 tetramers, and stimulated for 6 h with syngeneic targets that were HSV-1 infected, gB498 pulsed, RR1822 pulsed, or transfected to express full-length gB in the presence of brefeldin A. The cells were then permeabilized, fixed, and stained for intracellular IFN-γ. (D) DLN cells obtained at 7 dpi were stained with gB498 tetramers; sorted into tetramer-positive (gB498-specific) and tetramer-negative (gB498-nonspecific) populations; stimulated for 6 h with HSV-1 infected, gB498-pulsed, RR1822-pulsed, or unmanipulated targets in the presence of brefeldin A; stained for intracellular IFN-γ; and analyzed by flow cytometry. Representative dot plots show the percentage of sorted CD8 T cells positive for intracellular IFN-γ.
As a further measure of HSV-1 specificity, the gB498-nonspecific CD8 T cells sorted from the infected TG at 8 dpi were stained with CFSE; stimulated for 72 h with HSV-infected, gB498-pulsed, or nonpulsed targets; and analyzed by flow cytometry for proliferation as measured by CFSE dilution. Approximately 72% of gB498-nonspecific CD8 T cells from infected TG underwent one or more rounds of proliferation in response to HSV-infected targets, compared to 25% and 27% background proliferation in response to gB498-pulsed and nonpulsed targets, respectively (Fig. 6B). Thus, at least 50% of gB498-nonspecific CD8 T cells are responsive to HSV-1 antigens. Furthermore, the gB498-nonspecific CD8 T-cell population underwent more rounds of proliferation in response to HSV-infected targets, as indicated by an approximately 50% reduction in CFSE mean fluorescence intensity (MFI) among cells stimulated with HSV-1-infected targets compared to gB498-pulsed or nonpulsed targets (Fig. 6B).
These HSV-specific but gB498-nonspecific CD8 T cells did not respond to syngeneic targets that were transfected with plasmids expressing full-length gB (Fig. 6C). Total CD8 T cells isolated from the TG were stimulated by the transfected targets, although the response was less robust than the response to HSV-1-infected or gB498-pulsed targets (Fig. 6C). Thus, we were unable to demonstrate responsiveness of gB498-nonspecific CD8 T cells to other gB epitopes.
gB498-nonspecific CD86+ T cells can prevent HSV-1 reactivation in ex vivo cultures of latently infected TG.
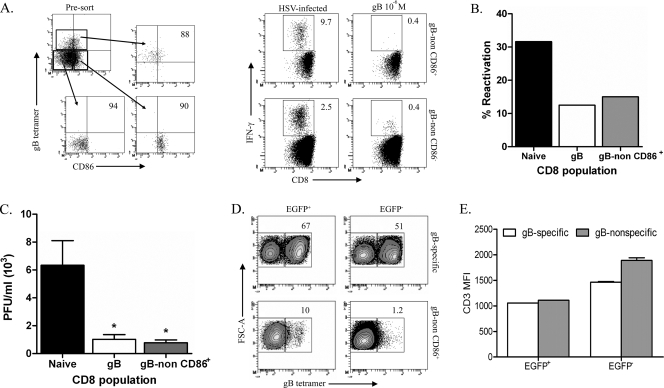
HSV-specific CD8 T cells represent a small proportion of the gB498-nonspecific CD8 T cells in the lymphoid organs (Fig. 6D), rendering functional studies difficult. Because the TG harbor too few HSV-specific CD8 T cells to permit functional studies, we attempted to enrich for HSV-specific CD8 T cells in the splenic gB498-nonspecific cells based on reported CD86 expression on antigen-experienced CD8 T cells (C. Rooney, personal communication). Spleens obtained at 8 dpi were sorted into three distinct CD8 T-cell populations: (i) gB498 tetramer-positive cells, (ii) gB498 tetramer-negative cells that express CD86, and (iii) gB498 tetramer-negative cells lacking CD86 expression. All three populations were approximately 90% pure based on postsort gating (Fig. 7A). The sorted populations were then stimulated for 6 h with either HSV-1-infected or gB498-pulsed targets and assayed for IFN-γ production. The sorted CD86+ gB498-nonspecific population was enriched approximately fourfold for HSV-specific CD8 T cells, accounting for approximately 10% of the population based on IFN-γ production (Fig. 7A).
FIG. 7.
gB498-nonspecific CD8 T cells are capable of preventing reactivation and impairing viral spread following TG explant. (A) Pooled spleens from mice at 8 dpi were stained with anti-CD8, anti-CD86, and gB498 tetramer. Cells were then sorted into three populations: (i) gB498-specific CD8 T cells, (ii) gB498-nonspecific CD86+ CD8 T cells, and (ii) gB498-nonspecific CD86− CD8 T cells. Pre- and postsort dot plots and purities are shown. Sorted populations were then stimulated for 6 h in the presence of brefeldin A with the indicated targets. Following stimulation, cell suspensions were stained for surface expression of CD45 and CD8 and then intracellularly for IFN-γ. Representative dot plots are shown with the percentage of IFN-γ-producing cells. (B) HSV-1 pICP0-EGFP latently infected TG cultures depleted of CD45-expressing cells via complement fixation were plated in 0.2 TG equivalent in 48-well plates. Cells sorted as described above were added to the cultures, and the cultures were monitored for reactivation by spread of fluorescence from neurons to surrounding fibroblasts and by detection of infectious virus in culture supernatants in a viral plaque assay with equivalent results. The graph depicts cumulative reactivation frequencies during 11 days in culture. (C and D) After 11 days in culture, infectious virus in supernatants of individual cultures that exhibited HSV-1 reactivation (based on EGFP spread) was quantified (C) and cells were collected from pooled wells in which the virus reactivated (EGFP+) and from those in which the virus did not reactivate (EGFP−) (D). Cells were stained with anti-CD8, anti-CD45, anti-CD3, and gB498 tetramers. Representative contour plots show the CD8 T cells isolated from pooled wells which received either gB498-specific or gB498-nonspecific CD86+ CD8 T cells. Numbers within contour plots represent the percentage of CD8 T cells that bound gB498 tetramer. (E) Quantification of CD3 MFI for pooled wells as described above is represented graphically as the mean CD3 MFI (± standard error of the mean). *, P < 0.05 (one-way analysis of variance with a Bonferroni post hoc t test).
We next tested the ability of this enriched HSV-specific, gB498-nonspecific CD8 T-cell population to block HSV-1 reactivation from latency in ex vivo cultures of CD45-depleted latently infected TG. These studies employed TG that were latently infected with a recombinant HSV-1 RE that expresses EGFP from the ICP0 promoter, permitting monitoring of HSV-1 reactivation from latency based on spread of EGFP from a neuron to surrounding fibroblasts as previously described (6). The CD45-depleted TG cultures were reconstituted with splenic CD8 T cells from noninfected mice (naïve) or with gB498-specific CD8 T cells or gB498-nonspecific CD86+ CD8 T cells obtained from spleens of infected mice at 8 dpi. Cultures received 10-fold more naïve CD8 T cells and gB498-nonspecific CD86+ CD8 T cells based on the estimate that approximately 10% of the latter are HSV specific. As illustrated in Fig. 7B, the reactivation frequency was reduced by approximately 67% in cultures that received gB498-specific CD8 T cells and those receiving gB498-nonspecific CD86+ CD8 T cells relative to cultures receiving naïve CD8 T cells. We conclude that gB498-nonspecific CD8 T cells can block HSV-1 reactivation in sensory neurons and might be as effective as gB498-specific CD8 T cells on a per-cell basis. The latter conclusion awaits more reliable quantification of HSV-specific cells among the gB498-nonspecific CD86+ population.
At the end of the 11-day observation period, supernatant fluids and cells were obtained from cultures in which HSV-1 reactivated (EGFP spread from neurons to surrounding fibroblasts) or failed to reactivate (no EGFP or EGFP confined to neurons). Quantification of infectious HSV-1 in supernatant fluids from reactivated cultures demonstrated that gB498-nonspecific CD8 T cells are as effective as their gB498-specific counterparts at inhibiting HSV-1 spread following a reactivation event (Fig. 7C). Both gB498-specific and gB498-nonspecific CD8 T cells were expanded two- to fivefold in cultures in which HSV-1 reactivated and in those not exhibiting HSV-1 reactivation (Fig. 7D). Interestingly, a small contaminating population of gB498-nonspecific cells was greatly expanded in both reactivated and nonreactivated cultures that received enriched gB498-specific CD8 T cells. Within these cultures CD3 was expressed to comparable extents on gB498-specific and gB498-nonspecific CD8 T cells, ruling out the possibility that gB498-nonspecific CD8 T cells represented gB498-specific cells that downregulated TCR expression (Fig. 7E). In contrast, very few gB498-specific CD8 T cells were expanded in TG cultures receiving gB498-nonspecific CD8 T cells. Thus, gB498-nonspecific cells appear to be preferentially expanded when responding to latently infected neurons in ex vivo TG cultures.
DISCUSSION
The data presented here provide strong evidence that many of the CD8 T cells in latently HSV-1-infected TG are specific for HSV, despite the fact that only about 50% recognize known HSV-1 epitopes. Our findings further support the concept that preferential accumulation of HSV-specific CD8 T cells in infected TG can result from both selective infiltration and selective retention of HSV-specific cells. Selective infiltration does not reflect antigen-specific extravasation but rather reflects a requirement for recent antigenic exposure. This conclusion is based on the observation that OVA257-specific OT-1 CD8 T cells can infiltrate HSV-1-infected TG, but only when HSV-1 infection is accompanied by OVA257 peptide immunization. The fact that OVA257-specific CD8 T cells can infiltrate the infected TG suggests that the antigen-specific extravasation of CD8 T cells observed in the central nervous system is not manifest in the peripheral nervous system (7). A previous study also demonstrated infiltration of OT-1 cells into HSV-1-infected dorsal root ganglia (24). In that study a large bolus (10 × 106) of in vitro-activated OT-1 T cells was administered i.v. to HSV-1-infected mice. Our findings confirm this observation under more physiologic conditions. The fact that OVA257-specific CD8 T cells infiltrate the TG only following exposure to cognate antigen suggests that infiltration is restricted to CD8 T cells that recently experienced antigen. This observation combined with the activated phenotype of both the gB498-specific and gB498-nonspecific CD8 T cells in the TG suggests that both populations experienced cognate antigen during primary HSV-1 infection.
In the absence of simultaneous OVA257 peptide immunization, OT-1 CD8 T cells were minimally expanded and activated in the DLN of HSV-1 infected mice, and few if any infiltrated the infected TG. Thus, bystander activation of CD8 T cells of irrelevant specificity within LN draining sites of HSV-1 infection appears to be minimal and to contribute little if at all to CD8 T-cell infiltration of the TG. During primary HSV-1 infection of mice, antigen presentation in the lymphoid organs is likely restricted largely to HSV-1 antigens, promoting selective infiltration of the TG by HSV-specific CD8 T cells. It is noteworthy that gB498-nonspecific CD8 T cells consistently infiltrate the infected TG 1 day earlier than the gB498-specific cells. This suggests that these cells might be expanded earlier within the DLN and is consistent with the observation that gB498-nonspecific CD8 T cells are preferentially expanded in ex vivo TG cultures.
The antigen specificity of the gB498-nonspecific CD8 T cells remains undefined. They were not stimulated to produce IFN-γ by syngeneic cells that were transfected with plasmids that express full-length gB, suggesting that they are not specific for other gB epitopes. However, this conclusion is rendered tentative by the observation that the gB-transfected cells were less efficient than HSV-1-infected or gB498-pulsed targets. The observations that these cells were expanded in TG cultures that did not reactivate and that they can block HSV-1 reactivation from latency in sensory neurons suggest that they recognize a viral protein that is expressed early enough in reactivation to permit a response that prevents formation of infectious virions. The sequence of HSV-1 gene expression during reactivation from latency remains unresolved, but identification of the antigen specificity of CD8 T cells that can prevent reactivation might shed light on those viral proteins that are expressed early in the reactivation process.
Our data establish that simultaneous exposure to non-HSV-1 immunogens during primary HSV-1 infection will result in infiltration of the infected TG by CD8 T cells of irrelevant specificity. However, we show that even if CD8 T cells of irrelevant specificity infiltrate the TG during acute HSV-1 infection, those cells will be selectively lost over time during latency. This conclusion is based on the marked diminution of the OVA257-specific OT-1 CD8 T-cell population within the TG between 8 and 40 dpi. A similar pattern of OT-1 CD8 T-cell loss from the dorsal root ganglion was seen in a previous study, although the shorter follow-up resulted in a less dramatic reduction (24). It is likely that this decline in the OT-1 CD8 T-cell population reflects normal attrition that is overcome in the gB498-specific and gB498-nonspecific CD8 T-cell populations by exposure to HSV-1 antigens.
Our previous study demonstrated that the gB498-specific CD8 T-cell population is maintained in latently infected TG of IL-15−/− mice, whereas their counterparts in noninfected tissue were lost (18). Interestingly, the gB498-nonspecific CD8 T-cell population was also maintained in the TG of these mice, suggesting either that they too can respond to HSV-1 antigens or that the TG provides a nonspecific signal that permits their survival in the absence of homeostatic signals. Our current observation that OT-1 cells are not maintained in the TG argues against a nonspecific survival signal and supports the view that both the gB498-specific and gB498-nonspecific CD8 T cells experience cognate antigen within latently infected TG. This interpretation is consistent with our observation that gB498-nonspecific CD8 T cells maintain an activated phenotype within TG during the course of viral latency.
Our findings suggest that CD8 T cells employ a broader TCR repertoire in controlling latent HSV-1 in sensory neurons than was previously appreciated. Although gB498-nonspecific CD8 T cells in infected TG exhibit more TCR diversity than their gB498-specific counterparts, use of Vβ5.1/5.2 and Vβ8.1/8.2 is dominant among the gB498-nonspecific CD8 T cells. A more accurate assessment of the TCR diversity among HSV-specific cells within the gB498-nonspecific CD8 T-cell population will await identification of the specific epitopes recognized by these cells. Since our data demonstrate that both gB498-specific and gB498-nonspecific CD8 T cells persistently express an activation phenotype within latently infected TG, identification of the antigen specificity of these cells will provide candidate genes that may be expressed in latently infected neurons.
Acknowledgments
Support for this work was provided by NIH grants R01-EY05945 (to R.L.H.), P30-EY08098 (to R.L.H.), K23-AI064396 (to T.L.C.), and T32-AI060525 (to B.S.S.); an unrestricted grant from Research to Prevent Blindness (New York, NY); and the Eye and Ear Foundation of Pittsburgh.
We thank Dawn Maker and Jessica Spehar for technical assistance, Nancy Zurowski for flow cytometry acquisition, and the National Institute of Allergy and Infectious Diseases Tetramer Core Facility (Emory University Vaccine Center, Atlanta, GA) for supplying tetramers.
The authors have no conflicting financial interests.
Footnotes
Published ahead of print on 10 December 2008.
REFERENCES
- 1.Allan, R. S., J. Waithman, S. Bedoui, C. M. Jones, J. A. Villadangos, Y. Zhan, A. M. Lew, K. Shortman, W. R. Heath, and F. R. Carbone. 2006. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25153-162. [DOI] [PubMed] [Google Scholar]
- 2.Baron, C., M. C. Meunier, E. Caron, C. Cote, M. J. Cameron, D. J. Kelvin, R. LeBlanc, V. Rineau, and C. Perreault. 2006. Asynchronous differentiation of CD8 T cells that recognize dominant and cryptic antigens. J. Immunol. 1778466-8475. [DOI] [PubMed] [Google Scholar]
- 3.Coles, R. M., S. N. Mueller, W. R. Heath, F. R. Carbone, and A. G. Brooks. 2002. Progression of armed CTL from draining lymph node to spleen shortly after localized infection with herpes simplex virus 1. J. Immunol. 168834-838. [DOI] [PubMed] [Google Scholar]
- 4.Coulombe, M., and R. G. Gill. 1996. T lymphocyte indifference to extrathymic islet allografts. J. Immunol. 1561998-2003. [PubMed] [Google Scholar]
- 5.Decman, V., M. L. Freeman, P. R. Kinchington, and R. L. Hendricks. 2005. Immune control of HSV-1 latency. Viral Immunol. 18466-473. [DOI] [PubMed] [Google Scholar]
- 6.Decman, V., P. R. Kinchington, S. A. Harvey, and R. L. Hendricks. 2005. Gamma interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J. Virol. 7910339-10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galea, I., M. Bernardes-Silva, P. A. Forse, N. van Rooijen, R. S. Liblau, and V. H. Perry. 2007. An antigen-specific pathway for CD8 T cells across the blood-brain barrier. J. Exp. Med. 2042023-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hand, T. W., M. Morre, and S. M. Kaech. 2007. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc. Natl. Acad. Sci. USA 10411730-11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones, C. M., S. C. Cose, R. M. Coles, A. C. Winterhalter, A. G. Brooks, W. R. Heath, and F. R. Carbone. 2000. Herpes simplex virus type 1-specific cytotoxic T-lymphocyte arming occurs within lymph nodes draining the site of cutaneous infection. J. Virol. 742414-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joshi, N. S., W. Cui, A. Chandele, H. K. Lee, D. R. Urso, J. Hagman, L. Gapin, and S. M. Kaech. 2007. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27281-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khanna, K. M., R. H. Bonneau, P. R. Kinchington, and R. L. Hendricks. 2003. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 18593-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khanna, K. M., A. J. Lepisto, and R. L. Hendricks. 2004. Immunity to latent viral infection: many skirmishes but few fatalities. Trends Immunol. 25230-234. [DOI] [PubMed] [Google Scholar]
- 13.Knipe, D. M., P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.). 2001. Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 14.Liu, T., K. M. Khanna, X. Chen, D. J. Fink, and R. L. Hendricks. 2000. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 1911459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamoto, Y., T. Suda, T. Momoi, and S. Kaneko. 2004. Different procarcinogenic potentials of lymphocyte subsets in a transgenic mouse model of chronic hepatitis B. Cancer Res. 643326-3333. [DOI] [PubMed] [Google Scholar]
- 16.Salvucci, L. A., R. H. Bonneau, and S. S. Tevethia. 1995. Polymorphism within the herpes simplex virus (HSV) ribonucleotide reductase large subunit (ICP6) confers type specificity for recognition by HSV type 1-specific cytotoxic T lymphocytes. J. Virol. 691122-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sercarz, E. E., P. V. Lehmann, A. Ametani, G. Benichou, A. Miller, and K. Moudgil. 1993. Dominance and crypticity of T cell antigenic determinants. Annu. Rev. Immunol. 11729-766. [DOI] [PubMed] [Google Scholar]
- 18.Sheridan, B. S., K. M. Khanna, G. M. Frank, and R. L. Hendricks. 2006. Latent virus influences the generation and maintenance of CD8+ T cell memory. J. Immunol. 1778356-8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheridan, B. S., J. E. Knickelbein, and R. L. Hendricks. 2007. CD8 T cells and latent herpes simplex virus type 1: keeping the peace in sensory ganglia. Expert Opin. Biol. Ther. 71323-1331. [DOI] [PubMed] [Google Scholar]
- 20.Son, Y. I., S. Egawa, T. Tatsumi, R. E. Redlinger, Jr., P. Kalinski, and T. Kanto. 2002. A novel bulk-culture method for generating mature dendritic cells from mouse bone marrow cells. J. Immunol. Methods 262145-157. [DOI] [PubMed] [Google Scholar]
- 21.Stock, A. T., C. M. Jones, W. R. Heath, and F. R. Carbone. 2006. CTL response compensation for the loss of an immunodominant class I-restricted HSV-1 determinant. Immunol. Cell Biol. 84543-550. [DOI] [PubMed] [Google Scholar]
- 22.Suvas, S., A. K. Azkur, and B. T. Rouse. 2006. Qa-1b and CD94-NKG2a interaction regulate cytolytic activity of herpes simplex virus-specific memory CD8+ T cells in the latently infected trigeminal ganglia. J. Immunol. 1761703-1711. [DOI] [PubMed] [Google Scholar]
- 23.Turner, S. J., and F. R. Carbone. 1998. A dominant V beta bias in the CTL response after HSV-1 infection is determined by peptide residues predicted to also interact with the TCR beta-chain CDR3. Mol. Immunol. 35307-316. [DOI] [PubMed] [Google Scholar]
- 24.van Lint, A. L., L. Kleinert, S. R. Clarke, A. Stock, W. R. Heath, and F. R. Carbone. 2005. Latent infection with herpes simplex virus is associated with ongoing CD8+ T-cell stimulation by parenchymal cells within sensory ganglia. J. Virol. 7914843-14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakim, L. M., J. Waithman, N. van Rooijen, W. R. Heath, and F. R. Carbone. 2008. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 319198-202. [DOI] [PubMed] [Google Scholar]
- 26.Wallace, M. E., M. Bryden, S. C. Cose, R. M. Coles, T. N. Schumacher, A. Brooks, and F. R. Carbone. 2000. Junctional biases in the naive TCR repertoire control the CTL response to an immunodominant determinant of HSV-1. Immunity 12547-556. [DOI] [PubMed] [Google Scholar]
- 27.Wallace, M. E., R. Keating, W. R. Heath, and F. R. Carbone. 1999. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J. Virol. 737619-7626. [DOI] [PMC free article] [PubMed] [Google Scholar]