Abstract
Kaposi's sarcoma (KS) is an angioproliferative inflammatory disorder induced by endothelial cell infection with the KS-associated herpesvirus (KSHV). ORFK13/vFLIP, one of the KSHV genes expressed in KS, encodes a 188-amino-acid protein which binds to the Iκb kinase (IKK) complex to activate NF-κB. We examined ORFK13/vFLIP contribution to KS phenotype and potential for therapeutic targeting. Retroviral transduction of ORFK13/vFLIP into primary human endothelial cells induces the spindle morphology distinctive of KS cells and promotes the formation of abnormal vascular networks typical of KS vasculature; upregulates the expression of proinflammatory cytokines, chemokines, and interferon-responsive genes; and stimulates the adhesion of inflammatory cells characteristic of KS lesions. Thymidine phosphorylase, a cellular enzyme markedly induced by ORFK13/vFLIP, can metabolize the prodrug 5-fluoro-5-deoxyuridine (5-dFUrd) to 5-fluouridine (5-FU), a potent thymidine synthase inhibitor, which blocks DNA and RNA synthesis. When tested for cytotoxicity, 5-dFUrd (0.1 to 1 μM) selectively killed ORFK13/vFLIP-expressing endothelial cells while sparing control cells. These results demonstrate that ORFK13/vFLIP directly and indirectly contributes to the inflammatory and vascular phenotype of KS and identify 5-dFUrd as a potential new drug that targets KSHV latency for the treatment of KS and other KSHV-associated malignancies.
Kaposi's sarcoma-associated herpesvirus (KSHV/human herpesvirus 8) is the etiological agent of Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), and a subset of multicentric Castleman's diseases. KS typically presents as a multicentric angioproliferative tumor characterized by multiple nodular or macular lesions often on the skin, and less frequently in the gastrointestinal tract and the lung. Histologically, the lesions consist of spindle cells infected with KSHV, inflammatory infiltrates of monocytes/macrophages, lymphocytes and other cells, and “vascular slits” replete of red blood cells (8). KS spindle cells are likely to be of endothelial lineage (19).
In KS tissues, KSHV establishes a mostly latent infection characterized by expression of a limited number of viral genes that are likely important to the disease pathogenesis (30). ORFK13 is one such KSHV latent gene. Its gene product, called vFLIP (for viral Flice-like inhibitory protein) or K-FLIP, comprises two tandem death-effector domains that are often found in apoptotic signaling mediators such as cellular FLICE inhibitory protein (cFLIP) and caspase-8/FLICE. Consistent with its sequence similarity with cFLIP, vFLIP was found to inhibit caspase activation and prevent apoptotic cell death (39). Silencing ORFK13/vFLIP expression by RNA interference stopped PEL growth in vitro and in vivo, providing evidence of the essential role of K13/vFLIP in PEL pathogenesis (16). Transgenic mice of K13/vFLIP in lymphoid cells developed more lymphomas than controls (11). Similar to the viral proteins of many other lymphogenic viruses, K13/vFLIP activates NF-κB (1, 10, 25, 27, 42). By activating NF-κB and inhibiting the AP-1 pathway, K13/vFLIP was recently reported to promote viral latency (49).
Recent studies have characterized selected effects of K13/vFLIP expression in primary endothelial cells transduced with ORFK13/vFLIP (15, 20, 29), providing important insights into its function. Here, we broadly investigated K13/vFLIP function in endothelial cells. By establishing stable retrovirus-mediated transduction of ORFK13/vFLIP in primary human endothelial cells, we have extensively characterized the biochemical and functional consequences of K13/vFLIP expression in these cells.
MATERIALS AND METHODS
Cells.
Human umbilical vein endothelial cells (HUVEC), isolated by collagenase digestion of umbilical veins, were cultured in HUVEC culture medium (M199 medium, 10% [vol/vol] fetal bovine serum [FBS]), human AB serum (5% [vol/vol]), heparin (25 μg/ml [Sigma-Aldrich, St. Louis, MO]), ascorbic acid (50 μg/ml [Sigma-Aldrich]), endothelial growth supplement from bovine neural tissue (15 μg/ml [Sigma-Aldrich]), l-glutamine (1.6 mM [Invitrogen, Carlsbad, CA]) and 1% (vol/vol) penicillin-streptomycin liquid (Invitrogen), as described previously (31). The human monocytic cell lines U937 and THP-1, the Burkitt's lymphoma cell line BL41, and the PEL cell line BCBL1 were cultured in RPMI 1640 GlutaMax-I medium (Invitrogen) with 10% heat-inactivated FBS. Phoenix, the retroviral packaging cell line (gifted from G. Nolan, Stanford University) was maintained in Dulbecco modified Eagle medium (high glucose)-GlutaMax-I medium (Invitrogen) with 10% FBS. Peripheral blood mononuclear cells were obtained from leukocyte-enriched peripheral blood (National Institute of Health, Clinical Center Blood Bank). Monocyte-enriched mononuclear cells were obtained by positive selection with CD11b magnetic beads (Militenyi Biotec, Bergisch Gladbach, Germany).
Plasmids.
The DNA fragment for the K13 open reading frame was amplified from KSHV DNA (BC-1) by PCR with primers designing an amino-terminal FLAG epitope (DYKDDDDK) and inserted into the LZRSpBMN-IRES-GFP retroviral vector (G. Nolan) at the BamHI and EcoRI sites. A point mutation (A57L) was introduced in K13/vFLIP by conventional PCR techniques; nucleotide 169GCG171(Ala) in ORFK13 was changed to 169CTA171 (Leu). DNA sequencing confirmed accuracy of amplification and insertion (Applied Biosystems, Foster City, CA; DNA Sequencing Facility at National Cancer Institute, Bethesda, MD).
Retroviral infection.
Plasmid transfection was performed with Lipofectamine 2000 (Invitrogen) as described by the manufacturer. Two days later, the supernatant was filtered (0.45-mm-pore-size filter) and Polybrene (hexadimethrine bromide [Sigma-Aldrich]) was added at a final concentration of 4 μg/ml. The viral supernatant was added to HUVEC (∼80% confluence) grown on gelatin-coated 60-mm plastic plate, and the cells were spun (2,500 rpm, 30°C for 60 min). Immediately after the centrifugation, supernatant was replaced with fresh HUVEC culture medium, and cells were placed in incubator. Infection was evaluated based on green fluorescent protein (GFP) expression microscopically by Olympus IX51 (Olympus Optical, Melville NY) and/or by flow cytometry (FACSCalibur; BD Bioscience, San Diego, CA).
Matrigel cord formation assay.
Cord formation on Matrigel was carried out as described previously (33). In brief, Matrigel (BD Bioscience, Bedford, MA) was solidified onto 48-well plates at 37°C for 30 min; 15,000 cells were seeded onto the gel. After 18 h of incubation, HUVEC formed a network of cord-like structure, observed by using bright-field microscopy. The number of angles was counted at low magnification (10× objective); five fields/well were counted, and the mean number of angles were averaged, as described previously (33).
Proliferation assay.
HUVEC (1,500 cells/well) were cultured for 3 days in 96-well tissue culture plates (Corning Incorporated, Corning, NY) in M199 containing 18% FBS and 25 μg of heparin/ml with bFGF (25 ng/ml; R&D Systems, Minneapolis, MN), vascular endothelial growth factor (VEGF) (25 ng/ml; R&D Systems), or growth factor supplement from bovine neural tissue (15 μg/ml; Sigma Aldrich). Proliferation was measured by evaluating [methyl-3H]thymidine uptake (0.6 μCi/well [0.022 MBq/well]; New England Nuclear, Beverly, MA) during the last 16 to 18 h of culture. Each assay was done in triplicate and repeated at least three times.
RNA preparation, microarray analysis, and RT-PCR.
Total RNA was prepared with TRIzol (Invitrogen) according to the manufacturer's protocol. cRNA synthesis, hybridization with Affymetrix HG133A, and analyses were performed by the Virus Tumor Biology Section of Laboratory of Cellular Oncology, Center for Cancer Research, National Cancer Institute. For conventional reverse transcriptase PCR (RT-PCR), RNA was treated with DNase I (Invitrogen), and cDNA was synthesized by using avian myeloblastosis virus reverse transcriptase (Roche, Basel, Switzerland) with poly(dT) primer (Invitrogen) according to the manufacturer's protocol. One-twentieth of the reaction solution was used for PCR with Taq DNA polymerase (Invitrogen). Each reaction was loaded onto 1.5% agarose-TAE gel with ethidium bromide. For quantitative RT-PCR (qRT-PCR), cDNA was measured with SYBR green master mix kit (Applied Biosystems) by 7900HT fast real-time PCR system (Applied Biosystems). Each cycle threshold (CT) was obtained from SDS2.3 software (Applied Biosystems) and normalized by the CT of the housekeeping gene (Gapdh or β-actin). qRT-PCR experiments were performed in duplicate and repeated at least three times. The sequences of the primer sets were as follows: Bcl2A1 (5′-CATTCTCAGCACATTGCCTCAACAG-3′ and 5′-CCAGCCTCCGTTTTGCCTTATC-3′); Cox2 (5′-GCATTCTTTGCCCAGCACTTC-3′ and 5′-CATCGCATACTCTGTTGTGTTCCC-3′); Cxadr (5′-AAAGCCAAAGGGGAAACTGC-3′ and 5′-GGCACATCTTCCCTGATATC-3′); Emcn (5′-CAAGCACTTCAGCAACCAGCC-3′ and 5′-TGTGAGAGAACAGGAGAGCCCC-3′); Iap2 (5′-TGCCAAGTGGTTTCCAAGGTG-3′ and 5′-GTTGCTCTTTCTCTCTCCTCTTCCC-3′); Icam1 (5′-TTGAACCCCACAGTCACCTATGG-3′ and 5′-TCCCTTCTGAGACCTCTGGCTTC-3′); Il-6 (5′-GGAGAAGATTCCAAAGATGTAGCCG-3′ and 5′-TGGGTCAGGGGTGGTTATTGC-3′); Ip-10 (5′-TGAAAGCAGTTAGCAAGGAAAGGTC-3′ and 5′-TGAAGCAGGGTCAGAACATCCAC-3′); Isg15 (5′-ATGCTGGCGGGCAACGAATTCCAGGTG-3′ and 5′-GCCGCCTCCCCGCAGGCGCAGATTCAT-3′); Mcp-2 (5′-GGAGAGATGGGTCAGGGATTCC-3′ and 5′-CAGACAGGTAGGAGGGAGAACAATG-3′); Mip1A (5′-CGGTGTCATCTTCCTAACCAAGC-3′ and 5′-CAGCCCTGAACAAAAGCATCC-3′); Mx1 (5′-AAGATGGTTGTTTCCGAAGTGGAC-3′ and 5′-TCCTGGTAACTGACCTTGCCTCTC-3′); Lif (5′-TCACCATCTGTGCCTTTGCTGC-3′ and 5′-CGGGGAAGAGAACGAAGAACCTAC-3′); Lox-1 (5′-TGAAGGACCAGCCTGATGAGAAGTC-3′ and 5′-TGAGCCCGAGGAAAATAGGTAACAG-3′); Pd-ecgf (5′-TTCAATGTCATCCAGAGCCCAG-3′ and 5′-AGCCCCTCCACGAGTTTCTTAC-3′); Rantes (5′-CTCGCTGTCATCCTCATTGCTAC-3′ and 5′-CCTGGGGAAGGTTTTTGTAACTG-3′); Sele (5′-CCAGGTGAACCCAACAATAGGC-3′ and 5′-GCACTCCATTCTCCAGAGGACATAC-3′); Sema3C (5′-TCAGCCTTTCCCACCATCCTTTAG-3′ and 5′-GCAGCGTCCTTTTCCAGATTCAC-3′); Stat1 (5′-AGAACAGAGAACACGAGACCAATGG-3′ and 5′-GCTGGAAAAGACTGAAGGTGCG-3′); Vcam1 (5′-CACTTTATGTCAATGTTGCCCCC-3′ and 5′-TCCTGTCTCCGCTTTTTTCTTCAG-3′); Xaf1 (5′-TTTTGATGTCAGAGCCCAAGCC-3′ and 5′-TCACCTTTCACAAGACCACCACAG-3′); and ORKF13 (5′-CCCTGTTAGCGGAATGTCTGTTTC-3′ and 5′-GTAAGAATGTCTGTGGTGTGCTGC-3′).
ELISA.
Enzyme-linked immunosorbent assays (ELISAs) for IP-10, I-TAC, and MCP-2 were carried out by using ELISA kits from R&D Systems. Conditioned media were prepared in M199 medium with 1% FBS and 25 μg of heparin/ml from 3-day cultures of 80% confluent cells incubated in 12-well plates.
Indirect fluorescent staining.
HUVEC infected with either control or K13/vFLIP-expressing retrovirus were seeded onto the fibronectin-coated four-chamber glass slides (BD Biosciences, San Jose, CA). Cells were washed with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde, and permeabilized with 1% Triton-PBS. Fixed cells were incubated with Alexa 546-conjugated phalloidin (Invitrogen) and DAPI (4′,6′-diamidino-2-phenylindole) for 10 min at room temperature. After a washing step, glass slides were mounted with Dako fluorescent mounting medium (Dako, Glostrup, Denmark).
AIDS-KS tissue specimens were fixed in paraformaldehyde and incubated in 15% sucrose in PBS overnight, followed by additional incubation in 30% sucrose in PBS for 1 to 2 days at 4°C. Tissue samples were embedded in OCT compound (Sakura Finetek, Tokyo, Japan), frozen in a dry ice-methyl butanol bath, and kept at −80°C. Frozen tissues were sliced, placed on glass slides, and stained with hematoxylin and eosin (Histoserv, Inc., Germantown, MD). For immunostaining, slides were fixed with 4% paraformaldehyde for 5 min at room temperature, washed in PBS, and then soaked in PBSTB (PBS plus 0.1% Triton, 1% bovine serum albumin, and 5% FBS) for 1 h at room temperature. Primary antibodies (1:200 dilution in PBSTB) were added to slides for 1 h at room temperature; slides were washed in PBS for 20 min, followed by additional incubation with secondary antibodies (1:500 dilution) for 1 h at room temperature. After being washed, the samples were mounted (mounting solution from Dako) and observed through a laser scanning confocal microscope LSM510 equipped with an objective lens (Plan Neofluar ×10/0.3; Carl Zeiss MicroImaging, Thornwood, NY). The pseudocolored images were converted into tiff files and exported into Adobe Photoshop (Adobe System, San Jose, CA).
Monocyte adhesion assay.
THP-1, U937, and human CD11b+ cells isolated from the peripheral blood were suspended in PBS at 107/ml with red fluorescent dye PKH26 (Sigma-Aldrich) for 10 min at room temperature. FBS was added to stop RKH26 uptake, and cells were washed with PBS. Labeled cells (30,000 cells/well) were added to HUVEC monolayers established in 48-well plate and incubated for 1 h. After washing to remove detached cells, adherent cells were counted under a fluorescence microscope Olympus IX51 phase-contrast microscope equipped with a ×4/0.13 PhC objective lens and a ×10 eyepiece (Olympus Optical, Melville, NY). The results reflect the counting of five replicate cultures/experiment.
Antibodies.
For immunostaining: mouse anti-CD68 monoclonal antibody (clone Ki-M7; AbD Serotec, Raleigh, NC), goat anti-CD31 polyclonal antibody (BD Biosciences), and rabbit anti-VEGF antibody (Santa Cruz Biotechnology, Santa Cruz, CA). For Western blotting: mouse monoclonal anti-p65 antibody (BD Biosciences), goat anti-COX2 polyclonal antibody (Santa Cruz Biotechnology), rabbit anti-hIL-6 polyclonal antibody (Pepro Tech, Rocky Hill, NJ), goat anti-actin antibody (Santa Cruz Biotechnology), rabbit polyclonal antibodies to p38, phospho-p38 (Thr180/Tyr182), phospho-JNK (Thr183/Tyr185), phospho-AKT (Ser473), and anti-IkB mouse monoclonal antibody (Cell Signaling Technology, Danvers, MA).
Chemicals.
Bay11-7082 {(E)3-[(4-methylphenyl)sulfonyl]-2-propenenitrile [Calbiochem, San Diego, CA]}, SB202190 [4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)-1H-imidazole], and 5-dFUrd were purchased from Sigma-Aldrich. KIN59 (5′-O-trityl-inosine) was kindly provided by J. Balzarini (Rega Institute for Medical Research, Leuven, Belgium) (24).
Cytotoxicity assay.
On a gelatin-coated 96-well plate, either control or K13/vFLIP retrovirus-infected HUVEC was seeded (1,500 to 2,000 cells/well) and incubated for 2 h to allow cell attachment to the plate. Serial dilutions of 5-dFUrd in M199 complete HUVEC medium were added. In some experiments, the thymidine phosphorylase inhibitor, KIN59 (1 to 10 μM) or the same volume (0.1% [vol/vol]) of control dimethyl sulfoxide (DMSO) was added. Samples were tested in triplicate. After 48 h of culture, the cytotoxicity was evaluated by measuring the conversion rate of WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] to WST-8 Formazan (Cell Counting Kit 8 [Dojindo Molecular Technologies, Gaithersburg, MD]) according to the manufacturer's protocol. The absorbance at 450 nm was measured by using a VersaMax turnable microplate reader (Molecular Devices, Sunnyvale, CA). The numbers of live cells were calculated from a standard curve generated with HUVEC. The results were then expressed as percentages of living cell numbers.
Statistical analysis.
Results are expressed as means ± the standard deviation (SD). A Student t test was applied to evaluate group differences; a P value of <0.05 was considered significant.
RESULTS
Retroviral induction of KSHV ORFK13/vFLIP.
We selected primary HUVEC for these studies because of their susceptibility to KSHV infection. HUVEC (from two separately derived populations; passage 2) were transduced with the K13/vFLIP gene by infection with a Moloney murine leukemia virus-derived retroviral vector carrying the FlagORFK13-IRES-Gfp gene cassette. As a control, we used HUVEC transduced with the empty retroviral vector. By day 3, more than 70% of control and K13-transduced HUVEC were GFP positive as measured by flow cytometry (Fig. 1A). At this time (day 3 after infection), K13/vFLIP retrovirus-infected HUVEC exhibited a dramatic change in shape to become elongated and resembling KS spindle cells (Fig. 1A and B). This change in cell morphology, not seen in control cells, persisted over subsequent propagation in culture over 3 weeks. When stained with fluorescent phalloidin, most K13/vFLIP-HUVEC displayed a prominent bipolar elongation of filamentous actin, which was not seen in control cells (Fig. 1B), a finding indicative of rearrangement of the actin cytoskeleton. By qRT-PCR, K13/vFLIP mRNA was specifically amplified from K13/vFLIP-HUVEC but not from control cells, and the level of K13/vFLIP expression was comparable to that found in BCBL-1 cells that are naturally infected with KSHV (Fig. 1C). Functional evidence for the presence of K13/vFLIP protein in K13/vFLIP-HUVEC was derived by analysis of IκBα, which is known to undergo ubiquitin-dependent degradation following K13/vFLIP binding to IKKγ and activation of IKK kinase activity (25). By Western blotting, we found that IκBα protein levels were markedly reduced in K13/vFLIP-HUVEC compared to control cells, whereas levels of the p65 subunit of NF-κB were unchanged (Fig. 1D).
FIG. 1.
Morphological changes in HUVEC transduced with K13/vFLIP retrovirus. (A) Two independent isolates of HUVEC were infected with ORFK13/vFLIP-IRES-Gfp retrovirus or IRES-Gfp retrovirus to yield K13#1 and K13#2 cultures. On day 3 after infection, living cells were observed under phase-contrast microscopy (original magnification, ×100, upper panels). Infectivity was evaluated by flow cytometric evaluation of GFP fluorescence (lower graphs). (B) Actin fibers stained by phalloidin in K13 (right)- and vector (left)-transduced cells growing on fibronectin-coated glass chamber slides observed by laser confocal microscopy (original magnification, ×63). (C) K13/vFLIP mRNA expression in BCBL1 cells and retrovirus-infected HUVEC measured by qRT-PCR. Burkitt's lymphoma BL41 cells were used as a negative control. (D) Western blot analysis of IκBα and NFκB p65 levels in cell lysates of K13/vFLIP- and control vector-infected HUVEC.
Since K13/vFLIP activates the NF-κB pathway through its interaction with IKKγ and/or through TRAFs and NF-κB2/p100 (p52) (17, 28), and this pathway upregulates transcription of growth stimulatory and prosurvival genes, we tested the effect of K13/vFLIP expression on the growth and survival of HUVEC. Using tritium-labeled thymidine, we found that K13/vFLIP-HUVEC proliferated less vigorously than control cells in response to bFGF/FGF2, VEGF165, and endothelial cell growth supplement (ECGF, a crude mixture of endothelial growth factor supplements from bovine neural tissue [Sigma-Aldrich]) (Fig. 2A). This reduced proliferation to ECGF by K13/vFLIP-HUVEC was associated with a reduction in the number of viable cells compared to control cells (Fig. 2B). K13/vFLIP-HUVEC did not undergo long-term outgrowth and did not display a prolongation in life span compared to control cells (not shown).
FIG. 2.
Effects of K13/vFLIP transduction on HUVEC proliferation and ECM-dependent cord formation. (A) Proliferation assay. HUVEC transduced with either vector or K13/vFLIP-retrovirus were cultured (96-well plate) overnight without growth factors and then incubated for additional an 48 h in medium alone or medium supplemented with VEGF (25 ng/ml), bFGF (25 ng/ml), or endothelial cell growth supplement (ECGF [Sigma-Aldrich]). Proliferation was measured by [3H]thymidine deoxyribose uptake during the last 16 h of culture. (B) Growth curve. Cells were seeded on gelatin-coated 48-well plates (10,000 cells/well). At each time point, cells were treated with trypsin and counted. Control and K13/vFLIP cells reached 100% confluence on days 4 and 5, respectively. (C) HUVEC (15,000 cells/well) were seeded onto 48-well plates coated with solidified Matrigel (BD Bioscience) and incubated at 37°C overnight. Cord angles were counted under phase-contrast microscopy (original magnification, ×40 [top graph]). Representative images of cord formation by control and K13-expressing HUVEC are shown (bottom panels). The results reflect the means (± SDs) of triplicate counts from three experiments.
In additional analyses of the effects of K13/vFLIP in HUVEC function, we tested the extracellular matrix (ECM)-dependent formation of cordlike structures, an in vitro assay that recapitulates important steps of endothelial cell assembly into vascular structures, including cell attachment to the ECM, the generation of chemokine gradients, polarization, the formation of filopodia, cell migration, cell-to-cell attachment, and the formation of an orderly cord network (37). This is a tightly regulated, multistep morphogenic process, which involves dynamic change in the endothelial cells and is orchestrated by a variety of molecules. K13/vFLIP-HUVEC was consistently defective in its ability to form the characteristic network of cordlike structures produced by control cells. Microscopically, K13/vFLIP-HUVEC displayed reduced formation of cytoplasmic extensions connecting cells to each other and an increased tendency to form regularly distributed cell aggregates rather than connecting cords onto ECM (Fig. 2C). As a result, we documented a significant (P < 0.01) reduction in the number of intersecting angles formed by K13/vFLIP-HUVEC compared to control (Fig. 2C). Collectively, these results indicate that K13/vFLIP expression induces marked phenotypic and functional alterations in primary endothelial cells.
Analysis of gene expression regulated by K13/vFLIP.
For a comprehensive analysis of changes in gene transcription induced by K13/vFLIP in HUVEC, Affymetrix GeneChip arrays were used. Two-independent experiments originated from different donor-derived HUVECs were carried out. After retroviral infection, each HUVEC was expanded under standard culture conditions for 7 to 10 days. Analysis of array results indicated that expression of a large number of genes was higher in K13/vFLIP-HUVEC than in control cells. Of 22,000 probe sets (14,500 genes), 499 probes detected a >2-fold increased expression (log ratio ≥ 1, signal strength > 150; see Table S1 in the supplemental material). The results for the most induced genes (log ratio ≥4) grouped by gene family are shown in Table 1.
TABLE 1.
mRNAs strongly increased in K13/vFLIP-HUVEC compared to control HUVEC (log ratio ≥ 4)
| Category and gene (abbreviation) | Probe set name | Gene accession no. | Ratio (log2)a of K13/vFLIP to control cells in expt:
|
|
|---|---|---|---|---|
| 1 | 2 | |||
| Chemokines | ||||
| CCL3/MIP1α | 205114_s_at | NM_002983.1 | 9.3 | 6.5 |
| CCL5/RANTES | 1405_i_at | M21121 | 9.7 | 9.4 |
| 204653_at | NM_002985.1 | 5.1 | 4.4 | |
| CCL20/MIP3α | 205476_at | NM_004591.1 | 8.9 | 6.6 |
| CCL8/MCP2 | 214038_at | AI984980 | 7.5 | 7.6 |
| CXCL2/MIP2α | 209774_x_at | M57731.1 | 5.0 | 3.0 |
| CXCL3/MIP2β/GRO3 | 207850_at | NM_002090.1 | 6.6 | 5.4 |
| CXCL5/ENA78 | 215101_s_at | BG166705 | 8.2 | 6.5 |
| 214974_x_at | AK026546.1 | 8.0 | 5.8 | |
| CXCL6/GCP2 | 206336_at | NM_002993.1 | 6.0 | 5.6 |
| CXCL10/IP10 | 204533_at | NM_001565.1 | 7.4 | 9.1 |
| CXCL11/I-TAC | 210163_at | AF030514.1 | 5.9 | 7.0 |
| 211122_s_at | AF002985.1 | 6.1 | 6.7 | |
| CX3CL1/fractalkine | 823_at | U84487 | 6.8 | 5.2 |
| 203687_at | NM_002996.1 | 5.9 | 5.6 | |
| Cytokines, growth factors, and secreted ligands | ||||
| CSF2/GM-CSF | 210229_s_at | M11734.1 | 8.2 | 5.5 |
| CSF3/G-CSF | 207442_at | NM_000759.1 | 7.3 | 4.9 |
| IL-1α | 210118_s_at | M15329.1 | 5.4 | 4.1 |
| IL-1β | 39402_at | M15330 | 7.7 | 4.4 |
| 205067_at | NM_000576.1 | 6.7 | 4.5 | |
| IL-6 | 205207_at | NM_000600.1 | 5.3 | 5.8 |
| IL-11 | 206924_at | NM_000641.1 | 5.4 | 3.2 |
| IL-27b | 219424_at | NM_005755.1 | 7.9 | 8.5 |
| Leukemia inhibitory factor (LIF) | 205266_at | NM_002309.2 | 5.3 | 4.1 |
| Platelet-derived endothelial cell growth factor (PD-ECGF) | 204858_s_at | NM_001953.2 | 2.9 | 5.8 |
| SEMA3C/semaphorin E | 203789_s_at | NM_006379.1 | 4.9 | 7.3 |
| Complement component 3 (C3) | 217767_at | NM_000064.1 | 4.6 | 4.3 |
| Complement factor B (CFB) | 202357_s_at | NM_001710.1 | 6.9 | 5.7 |
| Complement C1s subcomponent (C1S) | 208747_s_at | M18767.1 | 7.4 | 8.8 |
| Receptors and other cell surface proteins | ||||
| Bone marrow stromal cell antigen 2 (BST2) | 201641_at | NM_004335.2 | 4.6 | 4.5 |
| HLA class II gamma chain | 209619_at | K01144.1 | 3.2 | 5.2 |
| Integrin α1 | 214660_at | X68742.1 | 3.9 | 4.4 |
| Lectinlike ox-LDL receptor (LOX1) | 210004_at | AF035776.1 | 6.9 | 9.5 |
| TNF-α-induced protein 6 (TNFAIP6) | 206025_s_at | AW188198 | 7.1 | 7.7 |
| TNFR superfamily 9 (TNFRSF9/4-1BB) | 207536_s_at | NM_001561.2 | 5.8 | 4.4 |
| TNFR superfamily 11b (TNFSF11B/OPG) | 204933_s_at | NM_002546.1 | 9.1 | 7.8 |
| 204932_at | BF433902 | 7.1 | 8.3 | |
| VCAM1 | 203868_s_at | NM_001078.1 | 6.6 | 5.5 |
| Apoptosis-related genes | ||||
| BCL2-related protein A1 (BCL2A1)/BFL1 | 205681_at | NM_004049.1 | 4.2 | 5.8 |
| IAP2 | 210538_s_at | U37546.1 | 6.1 | 4.8 |
| Superoxide dismutase 2 (SOD2) | 215078_at | AL050388.1 | 3.6 | 4.7 |
| 215223_s_at | W46388 | 4.6 | 3.6 | |
| TNFR-associated factor 1 (TRAF1) | 205599_at | NM_005658.1 | 4.0 | 4.5 |
| TNF-α-induced protein 3 (TNFAIP3)/A20 | 202644_s_at | NM_006290.1 | 4.1 | 4.8 |
| 202643_s_at | AI738896 | 4.2 | 4.4 | |
| XIAP associated factor-1 (XAF1) | 206026_s_at | NM_017523.1 | 8.7 | 7.5 |
| 206133_at | NM_017523.1 | 4.1 | 5.1 | |
| Anti-virus and IFN-induced genes | ||||
| 2′5′Oligoadenylate synthetase 1 (OAS1) | 205552_s_at | NM_002534.1 | 3.9 | 5.6 |
| 202869_at | NM_016816.1 | 3.6 | 5.6 | |
| 204972_at | NM_016817.1 | 5.5 | 7.0 | |
| 2′5′Oligoadenylate synthetase 1 (OAS2) | 206553_at | NM_002535.1 | 4.7 | 4.2 |
| 2′5′Oligoadenylate synthetase 3 (OAS3) | 218400_at | NM_006187.1 | 3.6 | 4.6 |
| 2′5′Oligoadenylate synthetase-like (OASL) | 210797_s_at | AF063612.1 | 4.0 | 5.4 |
| 205660_at | NM_003733.1 | 3.1 | 5.2 | |
| APOBEC3G | 204205_at | NM_021822.1 | 3.8 | 4.7 |
| Diubiquitin (UBD) | 205890_s_at | NM_006398.1 | 11.8 | 8.4 |
| Hect domain and RLD 6 (HERC6) | 219352_at | NM_017912.1 | 4.3 | 5.5 |
| Interferon alpha-inducible protein 6 (IFI6) | 204415_at | NM_022873.1 | 5.8 | 6.1 |
| Interferon gamma-inducible protein 30 (IFI30) | 201422_at | NM_006332.1 | 4.8 | 5.0 |
| Interferon-induced protein (IFI44) | 214453_s_at | NM_006417.1 | 3.6 | 5.3 |
| 214059_at | BE049439 | 3.8 | 4.7 | |
| Interferon-induced protein 35 (IFI35) | 209417_s_at | BC001356.1 | 4.1 | 5.8 |
| Interferon-induced protein with TRP 1 (IFIT1) | 203153_at | NM_001548.1 | 9.3 | 12.1 |
| Interferon-induced protein with TRP 4 (IFIT4) | 204747_at | NM_001549.1 | 4.6 | 6.4 |
| Interferon-induced transmembrane protein 2 (IFITM2) | 201601_x_at | NM_003641.1 | 4.8 | 4.7 |
| 214022_s_at | AA749101 | 4.5 | 5.0 | |
| Interferon-inducible protein 44-like | 204439_at | NM_006820.1 | 6.8 | 8.4 |
| Interferon-stimulated gene, 15 kDa (ISG15) | 205483_s_at | NM_005101.1 | 4.9 | 3.8 |
| Interferon-stimulated gene, 20 kDa (ISG20) | 204655_at | NM_002985.1 | 5.6 | 8.1 |
| 204698_at | NM_002201.2 | 5.1 | 5.2 | |
| 33304_at | U88964 | 4.3 | 4.2 | |
| Melanoma differentiation-associated protein 5 (MDA5) | 219209_at | NM_022168.1 | 4.4 | 4.7 |
| Myxovirus resistance 1 (MX1) | 202086_at | NM_002462.1 | 6.1 | 7.0 |
| Myxovirus resistance 2 (MX2) | 204994_at | NM_002463.1 | 6.7 | 8.0 |
| Retinoic acid- and interferon-inducible protein, 58 kDa | 203595_s_at | N47725 | 3.3 | 5.7 |
| 203596_s_at | NM_012420.1 | 3.2 | 4.9 | |
| Viperin/cig5 | 213797_at | AI337069 | 6.2 | 9.5 |
| Transcription | ||||
| ATF-like 3 (B-ATF3) | 220358_at | NM_018664.1 | 4.8 | 3.9 |
| C/EBP delta | 203973_s_at | NM_005195.1 | 4.6 | 4.2 |
| NKX3.1 | 209706_at | AF247704.1 | 5.0 | 4.9 |
| mSin3B | 222095_s_at | AW450345 | 5.7 | 6.6 |
| Similar to mSin3B | 209353_s_at | BC001205.1 | 5.6 | 4.4 |
| Others | ||||
| Carbonic anhydrase VIII (CA8) | 220234_at | NM_004056.2 | 4.2 | 4.7 |
| Caspase-1 (CASP1) | 211368_s_at | U13700.1 | 3.5 | 4.6 |
| Galectin 3-binding protein (LGALS3BP) | 200923_at | NM_005567.2 | 4.9 | 5.4 |
| Kynureninase (l-kynurenine hydrolase) | 210663_s_at | BC000879.1 | 7.0 | 8.6 |
| 217388_s_at | D55639.1 | 7.1 | 8.3 | |
| 204385_at | NM_003937.1 | 5.2 | 5.6 | |
| Laminin γ2 (LAMC2) | 202267_at | NM_005562.1 | 6.6 | 5.8 |
| 207517_at | NM_018891.1 | 6.8 | 4.1 | |
| MMP7 | 204259_at | NM_002423.2 | 2.4 | 6.3 |
| Plasminogen activator inhibitor 2 (PAI2) | 204614_at | NM_002575.1 | 5.6 | 5.2 |
| Pregnancy-associated plasma protein A (PAPPA) | 201981_at | AA148534 | 5.4 | 5.8 |
| Proteasome subunit beta 9 (PSMB9) | 204279_at | NM_002800.1 | 5.0 | 6.6 |
| Receptor transporter protein 4 (RTP4) | 219684_at | NM_022147.1 | 5.1 | 6.2 |
| Similar to differential display and activated by p53 (DDA3) | 201645_at | BC001425.1 | 7.4 | 5.2 |
| Solute carrier family 15 A3 (SLC15A3) | 219593_at | NM_016582.1 | 5.4 | 7.4 |
| TNFAIP3 interacting protein 3 (TNIP3) | 220655_at | NM_024873.1 | 4.5 | 4.4 |
The results are expressed as ratios of K13/control (log2) in two experiments.
The expression of chemokine genes known to stimulate chemotaxis in monocyte/macrophages and lymphocytes was markedly increased by K13/vFLIP, including Ccl3/Mip1Α, Ccl5/ Rantes, Ccl20/Mip3Α, Ccl8/Mcp-2, Cxcl2/Mip2Α, Cxcl3/Mip2Β, Cxcl5/Ena78, Cxcl6/Gcp-2, Cxcl10/Ip-10, and Cxcl11/I-tac. Cytokine genes with proinflammatory and/or growth- stimulatory activities that were highly induced by K13/vFLIP included Csf2/Gm-csf, IL-1A, IL-1B, IL-6, IL-11, IL-27b/Ebi3 (EBV-inducible3), and Lif (leukemia inhibitory factor). Of these gene products, secreted interleukin-1 (IL-1), IP-10, and I-TAC have been reported to variously affect endothelial cells (2, 36, 47). The IL-1 and IP-10 receptors are expressed in HUVEC (36, 47), and we found that CXCR7/RDC1, a receptor for I-TAC and SDF-1(9), is highly expressed in control HUVEC (data not shown) and is moderately (ca. 3.2- to 4.0-fold) augmented by K13/vFLIP (see Table S1 in the supplemental material).
Among genes found to be common targets of tumor necrosis factor alpha (TNF-α) stimulation (45, 46), Traf1, Iap2, and Tnfaip3/A20 were highly induced by K13/vFLIP in HUVEC, whereas TNF-α was not. These genes, together with Tnip3 (Table 1), have been linked to initiation of a negative-feedback loop for NF-κB, occurring late in the course of inflammatory responses (21, 48). A number of IFN-inducible genes were also highly induced in K13/vFLIP-expressing HUVEC. A previous study (45) found that endothelial cells treated with TNF-α display increased expression of the IFN-inducible genes 2′5′ oligoadenylate synthetase 1 (Oas1), diubiquitin (Ubd), interferon-induced protein 44 (Ifi44), interferon-stimulated gene 20 kD (Isg20), melanoma differential associated protein 5 (Mda5), and myxovirus resistance 1 (Mx1). We found these same genes to be induced by K13/vFLIP expression in HUVEC. Other IFN-inducible genes that we found induced by K13/vFLIP in HUVEC include Apobec3G, Isg15, [scapi]l-kynurenine hydrolase (Kynu) (18), and receptor (chemosensory) transporter protein 4 (Rtp4) (14).
In contrast to its activity as a broad inducer of transcription, K13/vFLIP substantially diminished the expression of only few genes in HUVEC (Table 2 and see Table S2 in the supplemental material), including Coxackievirus and adenovirus receptor (Cxadr) and G-protein-coupled receptor 126 (Gpr126), whose repression was previously noted in TNF-α-treated endothelial cells (45).
TABLE 2.
mRNAs decreased in K13-expressing HUVEC compared to control HUVEC (log ratio ≥ 2)
| Gene (abbreviation) | Probe set name | Gene accession no. | Ratio (log2)a of K13/control in expt:
|
|
|---|---|---|---|---|
| 1 | 2 | |||
| BMP 4 | 211518_s_at | D30751.1 | -2.1 | -5.2 |
| Clusterin | 222043_at | AI982754 | -1.8 | -3.8 |
| 208791_at | M25915.1 | -1.9 | -3.1 | |
| 208792_s_at | M25915.1 | -1.7 | -3.3 | |
| Coxsackievirus and adenovirus receptor (CXADR) | 203917_at | NM_001338.1 | -1.6 | -5.5 |
| Endomucin | 219436_s_at | NM_016242.1 | -2.4 | -4.6 |
| Furry homolog (FRY) | 214319_at | W58342 | -1.7 | -3.4 |
| G protein-coupled receptor 126 | 213094_at | AL033377 | -1.8 | -4.0 |
| IMAGE135460 | 214920_at | R33964 | -1.2 | -4.1 |
| Integrin alpha 6 | 215177_s_at | AV733308 | -1.8 | -3.3 |
| 201656_at | NM_000210.1 | -1.7 | -3.4 | |
| LIM domain binding 2 (LDB2) | 206481_s_at | NM_001290.1 | -1.3 | -3.8 |
| Matrix Gla protein (MGP) | 202291_s_at | NM_000900.1 | -2.0 | -2.9 |
| Multimerin (MMRN) | 205612_at | NM_007351.1 | -1.9 | -3.2 |
| Paraneoplastic antigen 2 (PNMA2) | 209598_at | AB020690.1 | -2.3 | -3.2 |
The results are expressed as ratios of K13 to control in two experiments.
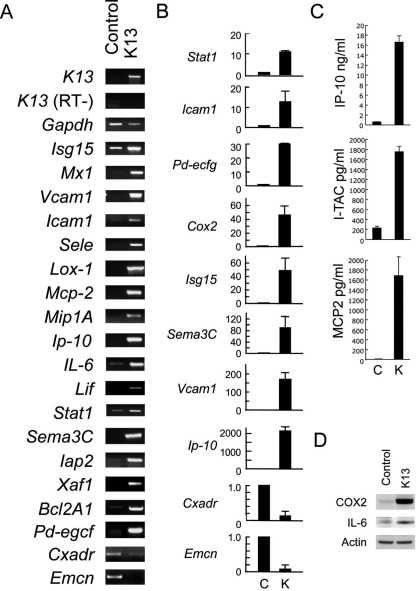
We performed RT-PCR, ELISA, and immunoblotting to confirm selected microarray results. By conventional RT-PCR, we confirmed increased expression of a number of genes (Fig. 3A). Although Isg15, IL-6, Stat1, Iap2, and Bcl2A were detected in the control cells, most of the other mRNAs were only detected in the K13/vFLIP-HUVEC in conventional RT-PCR, suggesting that expression of these genes was switched on by a K13/vFLIP-mediated pathway. By qRT-PCR (Fig. 3B), we documented a 10- to 40-fold increase in the expression of the Stat1, Icam1, Pd-ecgf, Cox2, and Isg15 genes in K13/vFLIP-HUVEC (day 10 after transduction) compared to controls. We also found that Ip-10 mRNA was strongly induced (as much as 2,000-fold) and that Cxadr and Emcn mRNAs were reduced (by ∼90%) in HUVEC by K13/vFLIP. By ELISA (Fig. 3, C), we detected increased levels of IP-10 (∼20-fold increase), I-TAC (∼8-fold increase), and MCP-2 (not detectable in the control conditional medium) in the culture supernatant of K13/vFLIP-HUVEC compared to the control. It is worth noting that K13/vFLIP-HUVEC culture supernatants contained IP-10 at sufficiently high levels (16.5 ± 1.3 ng/ml) to negatively regulate endothelial cell growth and ECM-dependent cord formation (2). By Western blotting (Fig. 3D), we confirmed increased expression of IL-6 and COX2 (cycloexogenase2) in K13/vFLIP-HUVEC compared to the control.
FIG. 3.
Gene regulation by K13/vFLIP in HUVEC. (A) RT-PCR for selected genes. Amplified DNAs were loaded onto 1.5% agarose-TAE gels and visualized by ethidium bromide. (B) Fold change in expression levels of selected genes evaluated by qRT-PCR. Expression levels were normalized by Gapdh and expressed as the fold change compared to control. (C) Secretion of IP-10, I-TAC, and MCP-2 evaluated by ELISA. Each supernatant was harvested from 3-day cultures of K13/vFLIP-expressing or control HUVEC in 12-well plates. The results reflect the mean concentrations (± the SD) from two or three independent experiments. (D) Western blotting for COX2, IL-6, and actin. Equal amounts of cell lysates were immunoblotted with specific antibodies.
Involvement of the NF-κB pathway in the regulation of gene expression by K13/vFLIP.
To examine whether K13/vFLIP modulates gene expression through the NF-κB pathway, we used Bay11-7082, an inhibitor of NF-κB activity (35). On day 5 after retrovirus infection, 5 μM Bay11-7085 or vehicle (dimethyl sulfate [DMSO]) was added to K13/vFLIP-transduced HUVEC, and on day 7 the cells were harvested (Fig.4Aa). At the time of harvest, the spindle cell morphology induced by K13/vFLIP was slightly reversed by Bay11-7085, but the drug-containing cell cultures were sparse (Fig.4Ab), suggesting drug toxicity. By qRT-PCR, Bay11-7085 reduced by at least 50% expression of Icam1, Vcam1, Cox2, and Ip-10, which was upregulated in K13/vFLIP-HUVEC treated with DMSO compared to control HUVEC (Fig.4Ac). These results suggested a role for the NF-κB pathway in gene regulation by K13/vFLIP, but the presence of drug toxicity that may differently affect the stability of distinct mRNAs prompted additional experiments.
FIG. 4.
The IKK/IκB/NF-κB pathway is critical for altered gene expression by K13/vFLIP. (A) Effects of the NF-κB inhibitor Bay11-7082. (a) Schematic representation of the experiment. (b) Representative microscopic morphology of K13/vFLIP-HUVEC treated with DMSO or Bay11-7082 for 2 days (original magnification, ×200). (c) qRT-PCR for Icam1, Cox2, Vcam1, and Ip-10 in K13/vFLIP-HUVEC treated with DMSO or Bay11-7082 for 2 days; the results are shown as the fold change compared to control HUVEC treated with DMSO (0.1% [vol/vol]). (B) Loss-of-function mutation at A57 in K13/vFLIP affected on morphological and transcription changes. (a) Representative microscopic morphology of HUVEC transduced with control, wild-type K13/vFLIP and its mutant A57L retrovirus 3 days after infection (original magnification, ×100). (b) qRT-PCR for ICAM1, Stat1, Cox2, Vcam1, Ip-10, and Emcn in HUVEC transduced with control, wild-type K13/vFLIP and its mutant A57L retrovirus 3 days after infection. The results are shown as the fold change compared to control HUVEC.
Bagneris et al. reported the crystal structure of the K13/vFLIP-IKKγ complex, revealing that the molecular cleft on the death-effector domain of K13/vFLIP represents a binding platform for IKKγ helices (amino acids 150 to 270) (4). Ala57 located in one of two clefts of K13/vFLIP was found to be critical for IKKγ binding because A57L substitution caused K13/vFLIP to both lose physical interaction with IKKγ in vitro and the ability to activate NF-κB in a transient reporter assay (4). To overcome the limitations of the experiments with Bay11-7082, we engineered an A57L mutation into the retroviral expression vector for K13/vFLIP. Unlike wild-type K13/vFLIP, mutant K13/vFLIPA57L transduced into HUVEC did not change the cell morphology (Fig.4Ba). This difference could not be attributed to reduced HUVEC infection by the mutant K13/vFLIPA57L, as assessed by microscopic evaluation of GFP expression. On day 5 after infection, cells were harvested for RNA extraction. Compared to wild-type K13/vFLIP, which clearly induced expression of the Icam1, Stat1, Cox2, Vcam1, and Ip-10 genes (albeit to a somewhat lower degree than that observed in cells on day 7 or 10 after infection), the mutant K13/vFLIPA57L failed to induce the expression of the Icam1, Stat1, Cox2, and Vcam1 genes and stimulated very little Ip-10 expression (Fig.4Bb). Emcn expression, which is suppressed by K13/vFLIP transduction, showed no reduction by K13/vFLIPA57L. Thus, we conclude that the K13/vFLIP-IKKγ axis is critical to K13/vFLIP modulation of gene expression in HUVEC. Other studies will be required to establish whether K13/vFLIP can recruit additional pathways for regulation of selected genes.
SAPK activation in K13/vFLIP-expressing HUVEC.
The pattern of gene and protein expression in K13/vFLIP-HUVEC resembled, at least in part, the pattern induced in HUVEC by inflammatory-type cytokines. We found the expression of two such cytokines, IL-1α and IL-1β to be highly induced by K13/vFLIP in HUVEC (Table 1), which express the cognate surface receptors for these cytokines. Since IL-1α and IL-1β, and other inflammatory cytokines are known to induce activation of the mitogen-activated protein kinases (MAPKs) p38/SAPK2 and JNK/SAPK (12), we examined their phosphorylation status in K13/vFLIP-HUVEC. As shown in Fig. 4A, JNK/SAPK was markedly more phosphorylated in K13-HUVEC compared to the control, and p38 was slightly more phosphorylated in K13-HUVEC compared to controls. STAT1 (for signal transducer and activator of transcription 1) is a transcription factor activated by a variety of signals, including signals from a TNF-induced autocrine loop mediated by interferon regulatory factors (IRF1/3) (49). Interestingly, phosphorylated STAT1 (tyrosine-701) was also markedly increased in K13/vFLIP- HUVEC compared to control (Fig. 5A). It may be related to increased Stat1 expression (Fig. 3A and B) or be the result of activation by signals indirectly induced by K13/vFLIP expression. Phosphorylated STAT3 was not detected (not shown), and the kinases ERK1/2 and AKT (Ser473) displayed similar degrees of phosphorylation in K13/vFLIP and control HUVEC (Fig. 5A).
FIG. 5.
Activation of STAT1, JNK, and p38 in K13/vFLIP-expressing HUVEC; p38 activation is required for COX2 expression. (A) Western blot analysis of cell lysates from HUVEC infected with K13/vFLIP or control retrovirus. (B) RT-PCR analysis of Gapdh, Il-6, Cox2, and K13/vFLIP expression in untreated HUVEC and HUVEC treated with SB202190. The bar graph depicts the relative expression of Cox2 measured by qRT-PCR (normalized by Gapdh). (C) Western blot analysis of COX2 levels in HUVEC after 2-day incubation with or without 10 μM SB202190.
Previously, TNF-α-induced Cox2 expression in endothelial cells was reported to be p38 MAPK/SAPK dependent (44). We now tested whether the p38 MAPK/SAPK pathway is involved in K13-induced activation of Cox2 in HUVEC. The p38 MAPK/SAPK inhibitor SB202190 (10 μM), selectively reduced Cox2 expression in K13/vFLIP-HUVEC as detected by RT-PCR (Fig. 5B) and Western blotting (Fig. 5C). In contrast, expression of K13/vFLIP and expression of IL-6, which was markedly induced by K13/vFLIP in HUVEC (Fig. 3C and Table 1), was not significantly altered by the p38 MAPK/SAPK inhibitor (Fig. 5B). These results provide evidence that the p38 MAPK/SAPK pathway is required for COX2 stimulation in K13/vFLIP-HUVEC.
Monocyte/macrophage attachment to K13/vFLIP-expressing HUVEC, infiltration of KS tissue, and VEGF expression.
K13/vFLIP induced an “inflammatory-type” phenotype in HUVEC by promoting the secretion of inflammatory-type cytokines/chemokines and stimulating expression of Icam1 and Vcam1 (Table 1). Since inflammatory capillary endothelium is known to promote the recruitment of inflammatory leukocytes to sites of inflammation (22), we examined leukocyte attachment to K13/vFLIP-HUVEC. CD11b+ mononuclear cells purified from peripheral blood and monocytic lineage THP-1 and U937 cell lines were fluorescein labeled and incubated (1 h, 37°C) on monolayers of either control or K13/vFLIP-expressing HUVEC. After removal of the nonadherent cells, cell attachment was measured. As shown in Fig. 6, we found that all cells tested displayed a significantly (P < 0.01) greater attachment to K13/vFLIP-HUVEC compared to control HUVEC. This indicates that K13/vFLIP expression in endothelial cells promotes monocyte/macrophage adhesion to these cells.
FIG. 6.
Enhanced monocyte attachment to HUVEC induced by K13/vFLIP. Red fluorescence-labeled THP1 cells, U937, and purified peripheral CD11b+ cells were incubated onto monolayers of HUVEC transduced with K13/vFLIP or empty retrovirus. After 2 h of incubation, the wells were washed twice with PBS, and attached cells were counted under fluorescence microscopy. Each sample was counted five times, and the average numbers (± the SD) are shown. Representative results from three experiments are shown.
Monocyte/macrophages often infiltrate KS tissues (8), an observation confirmed here by CD68 immunostaining of a representative KS tissue from an untreated patient (Fig. 7). KS tissues are rich in VEGF (38), an observation confirmed here by VEGF immunostaining (Fig. 7B). Since K13/vFLIP did not promote VEGF mRNA expression in HUVEC (Table 1 and see Fig. S1 in the supplemental material), and VEGF was not detectable by ELISA in the culture supernatants of K13/vFLIP-HUVEC (not shown), we examined the possibility that K13/vFLIP might indirectly contribute to VEGF expression in KS tissues. Double staining for monocyte/macrophages (CD68, red) and VEGF (green) showed colocalization (yellow) in a proportion of cells (Fig. 7C), demonstrating that inflammatory cells of monocyte/macrophage lineage are a source for VEGF in KS lesions. Additional cells besides CD68+ cells expressed VEGF, including some vascular mural cells surrounding CD31+ cells (Fig. 7B). These observations provide evidence that KSHV through K13/vFLIP contributes to the recruitment of inflammatory cells and to VEGF accumulation in KS tissues.
FIG. 7.
CD68+ monocyte/macrophages infiltrate KS tissue. Frozen sections of KS biopsy tissue on glass slides were observed by laser confocal microscopy. Control antibodies (mouse, rabbit, and goat immunoglobulins) were stained with DAPI (A), goat anti-human CD31 antibody plus rabbit anti-VEGF antibody (B), and mouse anti-human CD68 monoclonal antibody plus rabbit anti-VEGF antibody (C). As secondary antibodies, Alexa 488-conjugated anti-rabbit, Alexa 546-cojugated anti-mouse, and Alexa 546-cojugated anti-goat antibodies were used. DNA was stained by using DAPI. (D) Enlargement of the area outlined by the dotted white line in panel C. Arrows point to the cells expressing both CD68 and VEGF (yellow, right panel).
Enhanced biological activity of 5-dFUrd induced by K13/vFLIP.
ORFK13 transduction in HUVEC greatly stimulated the expression of Pd-Ecgf (for platelet-derived endothelial cell growth factor) (Table 1 and Fig. 3A). PD-ECGF is also a thymidine phosphorylase (TP) (34), which can catalyze the conversion of thymidine + phosphate into thymine + 2-deoxy-d-ribose-1-phosphate (7). A substrate for TP is 5-dFUrd, a pyrimidine nucleoside with antineoplastic activity, which is largely dependent upon its enzymatic conversion to 5-FU by PD-ECGF/TP (3, 6) (Fig. 8A). Increased expression of PD-ECGF/TP in K13/vFLIP-HUVEC would be expected to cause an increased conversion of 5-dFUrd prodrug into 5-FU. We compared the cytotoxic effects of 5-dFUrd on K13/vFLIP- HUVEC and control HUVEC. After 48 h of incubation with 5-dFUrd at concentrations ranging between 0.01 nM and 1 mM, we found that K13-expressing HUVEC were significantly more sensitive than control to 5-dFUrd (Fig. 8B): 50% of the K13-HUVEC (black dots) were killed at a drug concentration as low as 1 μM, a drug concentration that minimally affected the viability of control cells (open dots in Fig. 8B). A 5-dFUrd concentration of 10 μM was required to kill ∼60% of the control cells, and a drug concentration of 100 μM killed most control and K13/vFLIP-HUVEC (Fig. 8B). We determined that the 50% effective dose for 5-dFUrd is 13 μM for control HUVEC and 1.1 μM for K13/vFLIP-HUVEC, an ∼10-fold difference. These experiments demonstrate that K13/vFLIP-HUVEC is significantly more sensitive to 5-dFUrd than control cells.
FIG. 8.
Concentration-dependent cytotoxicity of 5-dFUrd for K13/vFLIP-transduced and control HUVEC. (A) Chemical structure of 5-dFUrd and its metabolite 5-FU. PD-ECGF/TP converts 5-dFUrd into 5-F (Pi = inorganic phosphate). (B) Viability of HUVEC infected with either control or K13-coding retrovirus after 48-hour incubation with 5-dFUrd-containing medium. Cell viability was measured by using a Cell Counting Kit-8 (Dojindo). The results reflect the percent viability (± the SD). Representative results of three experiments performed are shown. (C) HUVEC viability after 48 h of incubation with 0.01, 0.1, and 1 μM 5-dFUrd. The results are expressed as the percent viability in medium only and reflect the means (± the SD) of three experiments. (D) KIN59 reverses 5-dFUrd-induced cytotoxicity for K13/vFLIP-HUVEC. Control and K13/vFLIP-HUVEC were treated with 5-dFUrf (1 μM) with or without KIN59 (1, 3, or 10 μM) for 48 h. The results reflect % viability in medium only and represent the means (± the SD) of three experiments.
Finally, to address whether PD-ECGF/TP contributed to the increased 5-dFUrd susceptibility of K13/vFLIP-HUVEC, we used a chemical inhibitor of PD-ECGF/TP, KIN59 (5′-O-trityl-inosine) (24). KIN59 inhibits the conversion of thymidine to thymine by E. coli TP and human PD-ECGF/TP in vitro and in vivo as determined by the chick chorioallantoic membrane assay (23). Because we found that 80 μM KIN59 killed 50% of HUVEC (data not shown), we tested KIN59 at concentrations ranging between 1 μM and 10 μM. As shown in Fig. 8D, the cytotoxicity of 5-dFUrd for K13/vFLIP-HUVEC was significantly reduced by KIN59 (black bars), which displayed no effects on the viability of control cells (white bars). These results indicate that the increased Pd-ecgf expression in K13/vFLIP-HUVEC is responsible for the augmented cytotoxicity of 5-dFUrd.
DISCUSSION
We show that expression of the KSHV ORFK13/vFLIP gene in endothelial cells contributes to the proinflammatory phenotype, the spindle cell morphology, and the characteristic vascular lesions of KS. We find that K13/vFLIP does not directly promote expression of genes linked to increased cells growth while inducing selected antiapoptotic proteins such as Iap2 and Bcl2-related A1 (Table 1). However, by indirectly stimulating the expression of VEGF and other growth factors, K13/vFLIP may ultimately contribute to the proliferative phenotype of KS lesions.
The proteins IL-6, granulocyte-macrophage colony-stimulating factor, IL-8, CCL5/RANTES, CCL20/MIP3A, HLA class I, and VCAM1 were previously reported to be K13/vFLIP-induced proteins, as Cox2 and Cxcr7/Rdc1 mRNAs (15, 20, 29). We additionally found considerable induction of other chemokines for attraction of monocyte/macrophages, lymphocytes, neutrophils, dendritic cells, and activated T and NK cells; of other proinflammatory cytokines, including IL-1α and IL-1β; and of other growth factors, including G-csf and Lif (Table 1). Consistent with K13/vFLIP promoting a proinflammatory, proadhesive, and chemotactic phenotype, we found that K13/vFLIP significantly enhances the attachment of monocytic cells to endothelial monolayers (Fig. 5), and immunohistochemistry of a typical KS lesion confirmed prominent infiltration with CD68+ monocytic cells (Fig. 6). Thus, our results confirmed that K13/vFLIP is a driving force for the recruitment of the inflammatory cell infiltrate that contributes to KS progression (32).
Activation of the NF-κB pathway is an essential component of the pathogenicity of gammaherpesviruses. K13/vFLIP physically associates with IKKγ, and this interaction activates the canonical NF-κB pathway (25). Consistent with its ability to activate the canonical NF-κB pathway, we found that K13/vFLIP stimulates expression of many genes for chemokines, cytokines, growth factors, and surface receptors known to be targets of NF-κB. A chemical inhibitor of NF-κB reduced expression of selected genes induced by K13/vFLIP, and endothelial cells transduced with a mutant K13/vFLIP (K13/vFLIPA57L) that no longer binds to IKKγ failed to exhibit increased expression of index genes normally induced by wild-type K13/vFLIP. According to a previous report, K13/vFLIP can also activate the noncanonical NF-κB pathway by physically binding NF-κB2/p100 (27), but the importance of this pathway could not be confirmed in the present study.
K13/vFLIP activated the stress-activated protein kinases p38 and JNK/SAPK in endothelial cells, but not ERK1/2 (Fig. 5A). A potential mechanism for JNK/SAPK activation is through a physical interaction of K13/vFLIP with TRAFs receptor-associated factors) (10, 17), similar to the well-described pathway initiated by TNF receptor (TNFR) engagement. Another possibility is that these kinases are simply activated as a result of autocrine signaling by inflammatory cytokines such as IL-1β, which is markedly induced by K13/vFLIP in endothelial cells. Regardless of the mechanism for its activation, we found that the p38 kinase is clearly upstream of Cox2, since the p38MAPK inhibitor SB202190 selectively reduced expression of COX2.
Erythrocyte-replete vascular slits, edema, and neovascularization are characteristic features of KS histology, which give the lesions a red or purple color. The vascular slits are believed to reflect rudimentary attempts at forming vascular structures by the virally infected cells, likely of endothelial cell lineage. We show that K13/vFLIP disrupts the ability of endothelial cells to form ECM-dependent vascular structures and may thus contribute to formation of a discontinuous vascular system, leading to KS vascular slits. Many factors likely contribute to this defect, but K13/vFLIP stimulates secretion of IP-10, which alone can disrupt ECM-dependent cord formation (2). K13/vFLIP can also enhance expression of SEMA3C and CXCR7/RDC1. Semaphorins are guidance molecules critical to neuronal and vascular development (40). CXCR7/RDC1, a coreceptor for CXCL12/SDF-1, forms heterodimers with CXCR4, a chemokine receptor with essential functions in vascular development and angiogenesis (26, 37, 41). Cox2, through prostaglandin E2, may provide proangiogenic activity to KS lesions (5).
A most interesting observation we made is that endothelial cells expressing K13/vFLIP are significantly more sensitive to treatment with 5-dFUrd compared to control endothelial cells and are 10 to 100 times more sensitive to 5-dFUrd than cancer cell lines (3, 43). This increased drug sensitivity of endothelial cells is attributable to K13/vFLIP inducing endothelial cell expression of PD-ECGF/TP, an enzyme that converts 5-dFUrd into the active pyrimidine nucleoside 5-FU, which interferes with DNA and RNA synthesis and generate anticancer activity (3, 7). A chemical inhibitor of PD-ECGF/TP specifically reduced 5-dFUrd cytotoxicity for K13/vFLIP-HUVEC, indicating the involvement of PD-ECGF/TP enzymatic activity. 5-dFUrd is an oral drug that has already been tested in several clinical cancer trials (13) and would be a promising compound for the treatment of KS that could be readily tested in KS patients.
Supplementary Material
Acknowledgments
This study was supported by the intramural research program of the National Institute of Health, National Cancer Institute, Center for Cancer Research.
We thank Robert Yarchoan, Kathryn Wyvill, and Karen Alteman (CCR, NCI, NIH) for providing KS specimens; G. Nolan (Stanford University, Stanford, CA) for the retroviral vector; Jan Balzarini (Rega Institute for Medical Research, Rega, Belgium) for the KIN59; Susan Garfield and Poonam Mannan (Laboratory of Experimental Carcinogenesis, CCR, NCI, NIH) for the laser confocal microscopy; the Laboratory of Human Carcinogenesis (CCR, NCI, NIH) for providing ABI7900HT; and Lucy Sierra, Paola Gasperini, and Ombretta Salvucci for their help on various aspects of this work.
Footnotes
Published ahead of print on 17 December 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.An, J., Y. Sun, R. Sun, and M. B. Rettig. 2003. Kaposi's sarcoma-associated herpesvirus encoded vFLIP induces cellular IL-6 expression: the role of the NF-κB and JNK/AP1 pathways. Oncogene 223371-3385. [DOI] [PubMed] [Google Scholar]
- 2.Angiolillo, A. L., C. Sgadari, D. D. Taub, F. Liao, J. M. Farber, S. Maheshwari, H. K. Kleinman, G. H. Reaman, and G. Tosato. 1995. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J. Exp. Med. 182155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong, R. D., and R. B. Diasio. 1980. Metabolism and biological activity of 5′-deoxy-5-fluorouridine, a novel fluoropyrimidine. Cancer Res. 403333-3338. [PubMed] [Google Scholar]
- 4.Bagneris, C., A. V. Ageichik, N. Cronin, B. Wallace, M. Collins, C. Boshoff, G. Waksman, and T. Barrett. 2008. Crystal structure of a vFlip-IKKγ complex: insights into viral activation of the IKK signalosome. Mol. Cell 30620-631. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Av, P., L. J. Crofford, R. L. Wilder, and T. Hla. 1995. Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-1: a potential mechanism for inflammatory angiogenesis. FEBS Lett. 37283-87. [DOI] [PubMed] [Google Scholar]
- 6.Birnie, G. D., H. Kroeger, and C. Heidelberger. 1963. Studies of fluorinated pyrimidines. XVIII. The degradation of 5-fluoro-2′-deoxyuridine and related compounds by nucleoside phosphorylase. Biochemistry 2566-572. [DOI] [PubMed] [Google Scholar]
- 7.Bose, R., and E. W. Yamada. 1974. Uridine phosphorylase, molecular properties and mechanism of catalysis. Biochemistry 132051-2056. [DOI] [PubMed] [Google Scholar]
- 8.Boshoff, C., and R. Weiss. 2002. AIDS-related malignancies. Nat. Rev. Cancer 2373-382. [DOI] [PubMed] [Google Scholar]
- 9.Burns, J. M., B. C. Summers, Y. Wang, A. Melikian, R. Berahovich, Z. Miao, M. E. Penfold, M. J. Sunshine, D. R. Littman, C. J. Kuo, K. Wei, B. E. McMaster, K. Wright, M. C. Howard, and T. J. Schall. 2006. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J. Exp. Med. 2032201-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhary, P. M., A. Jasmin, M. T. Eby, and L. Hood. 1999. Modulation of the NF-κB pathway by virally encoded death effector domains-containing proteins. Oncogene 185738-5746. [DOI] [PubMed] [Google Scholar]
- 11.Chugh, P., H. Matta, S. Schamus, S. Zachariah, A. Kumar, J. A. Richardson, A. L. Smith, and P. M. Chaudhary. 2005. Constitutive NF-κB activation, normal Fas-induced apoptosis, and increased incidence of lymphoma in human herpesvirus 8 K13 transgenic mice. Proc. Natl. Acad. Sci. USA 10212885-12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103239-252. [DOI] [PubMed] [Google Scholar]
- 13.Fossa, S. D., A. Flokkmann, M. Heier, M. Aas, B. Moe, R. Heintz, and S. Linder-Ciccolunghi. 1986. Phase I/II tolerability/pharmacokinetic study with one-hour intravenous infusion of doxifluiridine (5′-dFUrd) 3 g/m2 VS 5 g/m2 QD × 5 per month. Cancer Chemother. Pharmacol. 18252-256. [DOI] [PubMed] [Google Scholar]
- 14.Gifford, C. A., A. M. Assiri, M. C. Satterfield, T. E. Spencer, and T. L. Ott. 2008. Receptor transporter protein 4 (RTP4) in endometrium, ovary, and peripheral blood leukocytes of pregnant and cyclic ewes. Biol. Reprod. 79518-524. [DOI] [PubMed] [Google Scholar]
- 15.Grossmann, C., S. Podgrabinska, M. Skobe, and D. Ganem. 2006. Activation of NF-κB by the latent vFLIP gene of Kaposi's sarcoma-associated herpesvirus is required for the spindle shape of virus-infected endothelial cells and contributes to their proinflammatory phenotype. J. Virol. 807179-7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guasparri, I., S. A. Keller, and E. Cesarman. 2004. KSHV vFLIP is essential for the survival of infected lymphoma cells. J. Exp. Med. 199993-1003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Guasparri, I., H. Wu, and E. Cesarman. 2006. The KSHV oncoprotein vFLIP contains a TRAF-interacting motif and requires TRAF2 and TRAF3 for signalling. EMBO Rep. 7114-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heyes, M. P., K. Saito, E. O. Major, S. Milstien, S. P. Markey, and J. H. Vickers. 1993. A mechanism of quinolinic acid formation by brain in inflammatory neurological disease. Attenuation of synthesis from l-tryptophan by 6-chlorotryptophan and 4-chloro-3-hydroxyanthranilate. Brain 116(Pt. 6)1425-1450. [DOI] [PubMed] [Google Scholar]
- 19.Hong, Y. K., K. Foreman, J. W. Shin, S. Hirakawa, C. L. Curry, D. R. Sage, T. Libermann, B. J. Dezube, J. D. Fingeroth, and M. Detmar. 2004. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat. Genet. 36683-685. [DOI] [PubMed] [Google Scholar]
- 20.Lagos, D., M. W. Trotter, R. J. Vart, H. W. Wang, N. C. Matthews, A. Hansen, O. Flore, F. Gotch, and C. Boshoff. 2007. Kaposi sarcoma herpesvirus-encoded vFLIP and vIRF1 regulate antigen presentation in lymphatic endothelial cells. Blood 1091550-1558. [DOI] [PubMed] [Google Scholar]
- 21.Lee, E. G., D. L. Boone, S. Chai, S. L. Libby, M. Chien, J. P. Lodolce, and A. Ma. 2000. Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science 2892350-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ley, K. 2002. Integration of inflammatory signals by rolling neutrophils. Immunol. Rev. 1868-18. [DOI] [PubMed] [Google Scholar]
- 23.Liekens, S., A. Bronckaers, A. I. Hernandez, E. M. Priego, E. Casanova, M. J. Camarasa, M. J. Perez-Perez, and J. Balzarini. 2006. 5′-O-tritylated nucleoside derivatives: inhibition of thymidine phosphorylase and angiogenesis. Mol. Pharmacol. 70501-509. [DOI] [PubMed] [Google Scholar]
- 24.Liekens, S., A. I. Hernandez, D. Ribatti, E. De Clercq, M. J. Camarasa, M. J. Perez-Perez, and J. Balzarini. 2004. The nucleoside derivative 5′-O-trityl-inosine (KIN59) suppresses thymidine phosphorylase-triggered angiogenesis via a noncompetitive mechanism of action. J. Biol. Chem. 27929598-29605. [DOI] [PubMed] [Google Scholar]
- 25.Liu, L., M. T. Eby, N. Rathore, S. K. Sinha, A. Kumar, and P. M. Chaudhary. 2002. The human herpesvirus 8-encoded viral FLICE inhibitory protein physically associates with and persistently activates the IκB kinase complex. J. Biol. Chem. 27713745-13751. [DOI] [PubMed] [Google Scholar]
- 26.Ma, Q., D. Jones, P. R. Borghesani, R. A. Segal, T. Nagasawa, T. Kishimoto, R. T. Bronson, and T. A. Springer. 1998. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc. Natl. Acad. Sci. USA 959448-9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matta, H., and P. M. Chaudhary. 2004. Activation of alternative NF-κB pathway by human herpesvirus 8-encoded Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein (vFLIP). Proc. Natl. Acad. Sci. USA 1019399-9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matta, H., L. Mazzacurati, S. Schamus, T. Yang, Q. Sun, and P. M. Chaudhary. 2007. KSHV oncoprotein K13 bypasses TRAFs and directly interacts with the IκB kinase complex to selectively activate NF-κB without JNK activation. J. Biol. Chem. 28224858-24865. [DOI] [PubMed] [Google Scholar]
- 29.Matta, H., R. M. Surabhi, J. Zhao, V. Punj, Q. Sun, S. Schamus, L. Mazzacurati, and P. M. Chaudhary. 2007. Induction of spindle cell morphology in human vascular endothelial cells by human herpesvirus 8-encoded viral FLICE inhibitory protein K13. Oncogene 261656-1660. [DOI] [PubMed] [Google Scholar]
- 30.Moore, P. S., and Y. Chan. 2001. Kaposi's sarcoma-associated herpesvirus, p. 2803-2833. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 31.Murga, M., O. Fernandez-Capetillo, and G. Tosato. 2005. Neuropilin-1 regulates attachment in human endothelial cells independently of vascular endothelial growth factor receptor-2. Blood 1051992-1999. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura, S., S. Z. Salahuddin, P. Biberfeld, B. Ensoli, P. D. Markham, F. Wong-Staal, and R. C. Gallo. 1988. Kaposi's sarcoma cells: long-term culture with growth factor from retrovirus-infected CD4+ T cells. Science 242426-430. [DOI] [PubMed] [Google Scholar]
- 33.Narazaki, M., and G. Tosato. 2006. Ligand-induced internalization selects use of common receptor neuropilin-1 by VEGF165 and semaphorin3A. Blood 1073892-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishino, I., A. Spinazzola, and M. Hirano. 1999. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science 283689-692. [DOI] [PubMed] [Google Scholar]
- 35.Pierce, J. W., R. Schoenleber, G. Jesmok, J. Best, S. A. Moore, T. Collins, and M. E. Gerritsen. 1997. Novel inhibitors of cytokine-induced IκBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 27221096-21103. [DOI] [PubMed] [Google Scholar]
- 36.Salcedo, R., J. H. Resau, D. Halverson, E. A. Hudson, M. Dambach, D. Powell, K. Wasserman, and J. J. Oppenheim. 2000. Differential expression and responsiveness of chemokine receptors (CXCR1-3) by human microvascular endothelial cells and umbilical vein endothelial cells. FASEB J. 142055-2064. [DOI] [PubMed] [Google Scholar]
- 37.Salvucci, O., L. Yao, S. Villalba, A. Sajewicz, S. Pittaluga, and G. Tosato. 2002. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood 992703-2711. [DOI] [PubMed] [Google Scholar]
- 38.Samaniego, F., P. D. Markham, R. Gendelman, Y. Watanabe, V. Kao, K. Kowalski, J. A. Sonnabend, A. Pintus, R. C. Gallo, and B. Ensoli. 1998. Vascular endothelial growth factor and basic fibroblast growth factor present in Kaposi's sarcoma (KS) are induced by inflammatory cytokines and synergize to promote vascular permeability and KS lesion development. Am. J. Pathol. 1521433-1443. [PMC free article] [PubMed] [Google Scholar]
- 39.Sarid, R., T. Ben-Moshe, G. Kazimirsky, S. Weisberg, E. Appel, D. Kobiler, S. Lustig, and C. Brodie. 2001. vFLIP protects PC-12 cells from apoptosis induced by Sindbis virus: implications for the role of TNF-α. Cell Death Differ. 81224-1231. [DOI] [PubMed] [Google Scholar]
- 40.Serini, G., D. Valdembri, S. Zanivan, G. Morterra, C. Burkhardt, F. Caccavari, L. Zammataro, L. Primo, L. Tamagnone, M. Logan, M. Tessier-Lavigne, M. Taniguchi, A. W. Puschel, and F. Bussolino. 2003. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 424391-397. [DOI] [PubMed] [Google Scholar]
- 41.Sierro, F., C. Biben, L. Martinez-Munoz, M. Mellado, R. M. Ransohoff, M. Li, B. Woehl, H. Leung, J. Groom, M. Batten, R. P. Harvey, A. C. Martinez, C. R. Mackay, and F. Mackay. 2007. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc. Natl. Acad. Sci. USA 10414759-14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun, Q., S. Zachariah, and P. M. Chaudhary. 2003. The human herpesvirus 8-encoded viral FLICE-inhibitory protein induces cellular transformation via NF-κB activation. J. Biol. Chem. 27852437-52445. [DOI] [PubMed] [Google Scholar]
- 43.Tevaearai, H. T., P. L. Laurent, L. Suardet, J. F. Eliason, J. C. Givel, and N. Odartchenko. 1992. Interactions of interferon-alpha 2a with 5′-deoxy-5-fluorouridine in colorectal cancer cells in vitro. Eur. J. Cancer 28368-372. [DOI] [PubMed] [Google Scholar]
- 44.Viemann, D., M. Goebeler, S. Schmid, K. Klimmek, C. Sorg, S. Ludwig, and J. Roth. 2004. Transcriptional profiling of IKK2/NF-κB- and p38 MAP kinase-dependent gene expression in TNF-alpha-stimulated primary human endothelial cells. Blood 1033365-3373. [DOI] [PubMed] [Google Scholar]
- 45.Viemann, D., M. Goebeler, S. Schmid, U. Nordhues, K. Klimmek, C. Sorg, and J. Roth. 2006. TNF induces distinct gene expression programs in microvascular and macrovascular human endothelial cells. J. Leukoc. Biol. 80174-185. [DOI] [PubMed] [Google Scholar]
- 46.Wang, C. Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 2811680-1683. [DOI] [PubMed] [Google Scholar]
- 47.Williams, M. R., N. Kataoka, Y. Sakurai, C. M. Powers, S. G. Eskin, and L. V. McIntire. 2008. Gene expression of endothelial cells due to interleukin-1 beta stimulation and neutrophil transmigration. Endothelium 1573-165. [DOI] [PubMed] [Google Scholar]
- 48.Wullaert, A., L. Verstrepen, S. Van Huffel, M. Adib-Conquy, S. Cornelis, M. Kreike, M. Haegman, K. El Bakkouri, M. Sanders, K. Verhelst, I. Carpentier, J. M. Cavaillon, K. Heyninck, and R. Beyaert. 2007. LIND/ABIN-3 is a novel lipopolysaccharide-inducible inhibitor of NF-κB activation. J. Biol. Chem. 28281-90. [DOI] [PubMed] [Google Scholar]
- 49.Yarilina, A., K. H. Park-Min, T. Antoniv, X. Hu, and L. B. Ivashkiv. 2008. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat. Immunol. 9378-387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.