Abstract
Immune responses against adenovirus (Ad) vectors pose a possible concern for the outcome of treatment efficacy. To address the role of preexisting immunity in oncolytic Ad vector antitumor efficacy following intratumoral injection of vector as well as tumor-to-tissue spread of the vector, we employed the Syrian hamster model. These animals are immunocompetent, and their tumors and tissues are permissive for replication of Ad type 5 (Ad5). We used the adenovirus death protein-overexpressing Ad5-based vector INGN 007. Subcutaneous tumors were established in groups of hamsters that were or were not immunized with Ad5. Half of the hamsters in these groups were immunosuppressed with cyclophosphamide. For all groups, tumors injected with INGN 007 grew significantly more slowly than those injected with buffer. Under immunocompetent conditions, there was no significant effect of preexisting immunity on vector antitumor efficacy. Soon after the tumors in naïve animals were injected with vector, the hamsters developed neutralizing antibody (NAb) and the difference in NAb titers between the naïve and immunized groups diminished. Under immunosuppressed conditions, preexisting NAb did significantly reduce vector efficacy. Thus, NAb do reduce vector efficacy to some extent, but immunosuppression is required to observe the effect. Regarding vector toxicity, there was spillover of vector from the tumor to the liver and lungs in naïve immunocompetent hamsters, and this was nearly eliminated in the immunized hamsters. Thus, preexisting immunity to Ad5 does not affect INGN 007 antitumor efficacy following intratumoral injection, but immunity prevents vector spillover from the tumor to the liver and lungs.
Oncolytic (replication-competent) viral vectors are being investigated as a treatment for cancer (2, 19, 25, 27). Recently, an oncolytic adenovirus serotype 5 (Ad5)-based vector was approved for cancer therapy in humans for the first time (14, 42). Oncolytic vectors based on Ad, reovirus, herpes simplex virus type 1 (HSV-1), poxvirus, poliovirus, Newcastle disease virus, measles virus, and vesicular stomatitis virus (VSV) are being studied extensively in both preclinical and clinical settings (16, 20, 24). Oncolytic Ad vectors are popular due to the Ad safety profile and ease of manipulation and handling (6, 13, 18, 23).
Oncolytic Ad vectors infect and kill cancer cells as a result of the normal Ad life cycle by replicating in cells and releasing progeny viruses. These vectors rely on replication and spread through the tumor to achieve efficacy. A majority of the human population is seropositive for Ad5, which is acquired as a childhood infection (4, 15, 39). Elimination of the vector by preexisting immunity to Ad or vector elimination by the adaptive immune response generated after administration of the vector poses a possible concern with respect to achieving significant antitumor efficacy. A key question is whether the oncolytic Ad vector can efficiently eliminate tumor cells faster than its own clearance by the immune system. Several studies show that suppressing the immune system enhances the efficacy of oncolytic vectors (10, 12, 31).
Alternatively, studies show that activation of the adaptive immune system by the vector might increase tumor cell killing, thereby increasing vector antitumor efficacy (11, 21, 27, 34). Studies with oncolytic HSV and VSV show that these vectors induce long-term antitumor immunity (11, 21, 27, 34). Therefore, apart from direct cell lysis, oncolytic vectors may be able to achieve antitumor efficacy by activating the antitumor immune response. Therefore, induced or preexisting immunity to the vector can be either a hurdle or beneficial for vector efficacy.
Most efforts to address the effect of preexisting immunity were performed by gene transfer studies with replication-defective Ad vectors (28, 41). These studies showed that preexisting immunity significantly reduces gene transfer and expression in the target organ. In contrast, other studies showed that preexisting immunity does not prevent gene transfer (26) and does not affect vector antitumor efficacy (1). Little work has been done to address the role of induced or preexisting immunity on the efficacy and toxicity of oncolytic Ad vectors (3, 39). Studies with these vectors have been difficult because of a lack of immunocompetent and permissive animal models. Ad replication is generally species specific, and human Ads replicate poorly in cells from most nonhuman species. Consequently, Ad vectors are commonly evaluated in immunodeficient mice bearing human tumor xenografts. However, this model cannot adequately address the effect of the host immune system on the vector-infected tumor or the toxicity of the vector in normal tissues.
We recently developed a novel Syrian hamster model for the study of oncolytic Ad5-based vectors (30). These animals are both replication permissive for Ad5 and immunocompetent. In the present study, we modeled the effect of preexisting immunity to Ad5 on the efficacy of an oncolytic Ad vector, INGN 007, and the spillover of the vector from the site of injection to the liver and lungs.
MATERIALS AND METHODS
Cell culture.
The Syrian hamster renal cell line HaK and the human lung carcinoma cell line A549 (American Type Culture Collection [ATCC], Manassas, VA) were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS) as previously described (30, 33).
Viruses and vectors.
The oncolytic Ad vector INGN 007 is identical to wild-type human Ad5, except that INGN 007 lacks most of the E3 genes and overexpresses the E3-11.6K adenovirus death protein (ADP) (8, 22, 36). Stocks of INGN 007 and Ad5 were obtained from Introgen Therapeutics, Inc. (Houston, TX). Virus stocks were grown in HEK 293 cells and purified by column chromatography. Virus particle titers were determined by high-performance liquid chromatography. Infectious titers were determined in our laboratory by plaque assays and 50% tissue culture infectious dose (TCID50) assays on A549 cells (35).
Animals.
Syrian (golden) hamsters (Mesocricetus auratus; 4 to 5 weeks old) were obtained from Harlan Sprague Dawley (Indianapolis, IN). Studies were approved by the Institutional Animal Care and Use Committee of Saint Louis University and were conducted in accordance with institutional and federal regulations.
Immunosuppression.
Cyclophosphamide (CP) (Sigma-Aldrich, St. Louis, MO) was dissolved in 0.9% NaCl to a final concentration of 21 mg/ml and then filter sterilized. Drug solutions were stored at 4°C in the dark. CP was administered twice every week by intraperitoneal injection for the duration of the study. The initial dose was 140 mg/kg body weight, and the subsequent doses were 100 mg/kg. The doses and schedule were based on our previous studies (31). Immunosuppressed hamsters were housed in sterile caging and fed irradiated chow. The antibiotic Baytril (Bayer HealthCare, Shawnee Mission, KS) was added to sterile drinking water at a final concentration of 100 mg/liter (31).
Immunization and boosting.
Preexisting immunity was generated by a single intramuscular injection of Ad5 (1 × 1010 PFU). For boosting, the same dose of Ad5 was injected intramuscularly at 14 or 15 days postimmunization. The dose of the immunization and booster was based on the amount of vector that would be used for intratumoral (i.t.) injection. When a naïve immunocompetent animal is treated with the vector, it mounts an immune response against the input vector, and the immunity of an already immunized immunocompetent animal will be boosted with the vector treatment.
Antitumor efficacy.
Subcutaneous HaK tumors were established in the hind flanks of hamsters by injecting 2 × 107 cells in 200 μl of serum-free Dulbecco's modified Eagle's medium. Animals were randomized based on tumor volume prior to initiation of i.t. virus injections. Established HaK tumors were injected i.t. with 1 × 1010 PFU of INGN 007 or with vehicle for six consecutive days. Tumor volumes were measured with digital calipers twice every week.
Establishing virus quantities in tissues.
Animals were sacrificed and organs were harvested. The whole tumor and part of the right lateral lobe of the liver were collected in sterile tubes, and blood was collected in anticoagulant tubes. All of the tissues were frozen immediately in liquid nitrogen and stored at −80°C. The net weight of the solid tissues was determined, and tissues were homogenized in phosphate-buffered saline (PBS) with a single tungsten carbide bead, using a TissueLyser instrument (Qiagen, Valencia, CA). For real-time PCR, DNA was isolated from each homogenate with a Magtration system (PSS BioInstruments, Livermore, CA) and quantified with a PicoGreen assay (Invitrogen, Carlsbad, CA). Real-time PCR was performed with 1 μg total DNA per reaction, when possible, with an ABI 7500 system (Applied Biosystems, Foster City, CA) (31, 38). For determinations of infectious virus titers, tissue homogenates were freeze-thawed three times, sonicated for 7 min, centrifuged, and titrated by TCID50 assays with A549 cells (31, 32, 38). Each sample was titrated in a single 96-well plate. Some wells were spiked with a known amount of Ad5 as a positive control to test for inhibition of infection by the extract. The plates were incubated for 14 days, the wells were scored (positive or negative) for cytopathic effect (CPE), and titers were calculated according to the Reed-Muench method (5, 32).
Neutralization assay.
One day prior to the assay, A549 cells were plated in 96-well plates at 8 × 105 cells per plate in a volume of 100 μl per well. Serum samples were incubated at 56°C for 30 min to inactivate complement. Two serum samples were assayed per plate. Serum samples (in four replicate wells) were diluted twofold across a round-bottomed 96-well plate in medium containing 20% FBS to normalize the total serum concentration across the plate (31). One row contained no serum samples in order to observe the effect of virus only. Dilutions of sera were incubated with 100 PFU per well of INGN 007 for 1 h at 37°C. After 1 h of incubation, the serum-virus mixtures (100-μl total volume) were transferred to the 96-well plate containing A549 cells. After infection for 1 h at 37°C, the medium was removed and replaced with fresh Dulbecco's modified Eagle's medium containing 5% FBS. Wells were individually scored (positive or negative) for CPE at 7 days postinfection. Neutralizing antibody (NAb) titers were determined by the highest dilution of serum that resulted in at least 50% inhibition of CPE (≤2 of 4 wells positive for CPE). For NAb assay of the tumors, the tumors were collected and processed in the same way as that described for the TCID50 assay and the supernatant was used to perform the neutralization assay.
Western blots.
A549 cells were infected at 50 PFU/cell with INGN 007 or were mock infected. At 24 h postinfection, cells were extracted in Laemmli buffer (50 mM Tris-Cl, pH 6.8, 100 mM dithiothreitol, 2% sodium dodecyl sulfate, 0.1% bromophenol blue, and 10% glycerol). Samples were electrophoresed by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the proteins were electrophoretically transferred to an Immobilon-P membrane (Millipore, Billerica, MA). The membrane was blocked with 5% nonfat milk and cut into strips. The strips were probed with either immune hamster serum (1:500 dilution), hamster tumor extract (1:500 dilution), or rabbit serum against Ad5 capsid proteins (1:1,600 dilution) (ATCC) (37). Subsequently, the membrane was incubated with horseradish peroxidase-conjugated affinity-purified goat anti-hamster immunoglobulin G (IgG) (1:500; Cappel, MP Biomedicals, Solon, OH) or goat anti-rabbit IgG (1:4,000; Cappel, MP Biomedicals). The strips were aligned properly, and a LumiGLO peroxidase chemiluminescent substrate kit (KPL Inc., Gaithersburg, MD) was used to visualize the bands.
Immunofluorescence.
A549 cells were plated on glass coverslips 1 to 2 days before injection. Cells were infected with INGN 007 at a multiplicity of 20 PFU/cell. At 24 h postinfection, cells were rinsed with PBS, fixed in 3.7% paraformaldehyde in PBS for 10 min, and subsequently permeabilized with methanol containing 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) at −20°C for 6 min. After rehydration in PBS, cells were immunostained with either hamster tumor extracts (1:100) or immune hamster serum (1:100) diluted in PBS-1% bovine serum albumin-0.1% sodium azide. The secondary Ab was affinity-purified goat anti-hamster IgG (conjugated with Alexa Fluor 488 [Molecular Probes]; Invitrogen Corp., Carlsbad, CA). Images were taken on a Nikon Optiphot microscope (Nikon, Melville, NY) equipped with a Nikon DXM1200 digital camera and ACT-1 software (Nikon).
Statistical analysis.
The data from multiple groups were compared by one-way analysis of variance, with a Tukey post hoc test to evaluate differences between groups. In the event that a significant difference was detected with the test of homogeneity of variances (i.e., analysis of variance was not valid), a Kruskal-Wallis test was followed by pairwise comparison of groups with the Mann-Whitney U test. Significant differences were defined as those with P values of ≤0.05. For assays where undetectable levels of virus or NAb were encountered, a value just below the detection limit was assigned for performance of the statistical analyses.
RESULTS
Evaluation of Ab response after immunization with Ad5.
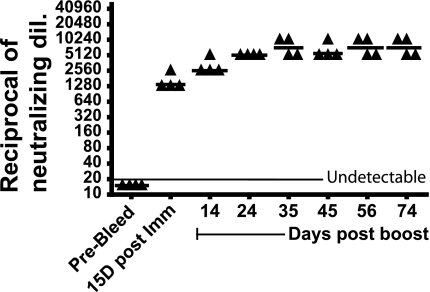
As mentioned above, the majority of the human population has preexisting Ab to Ad due to natural infection (4, 15, 39), and even if patients do not have preexisting Ab against the virus, they develop Ab soon after the vector is injected into a tumor. We have shown previously that Syrian hamsters are permissive to human Ad5 and develop an Ab response against an oncolytic Ad5-based vector when the vector is injected i.t. (30). In order to model preexisting immunity to Ad5 in the human population, hamsters were immunized intramuscularly with Ad5 (1 × 1010 PFU) and then boosted with the same dose of Ad5 15 days later. The dose used for immunization was a single therapeutic dose of the vector INGN 007 (which was used in subsequent experiments). Serum was collected before immunization and at multiple times postimmunization, and a virus neutralization assay was performed. As shown in Fig. 1, we observed a strong NAb response following primary immunization (median of 1,280 [i.e., 1:1,280]) and boosting (median of 2,560). The NAb levels remained consistently high throughout the experiment (90 days postimmunization), perhaps because of continued low levels of replication of the Ad5 that was used for immunization.
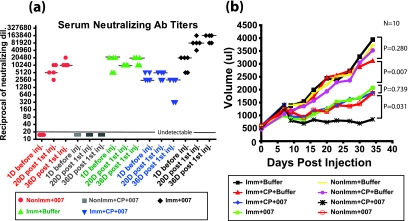
FIG. 1.
Anti-Ad5 NAb develop after immunization and boosting with Ad5. Syrian hamsters were immunized intramuscularly with Ad5 (1 × 1010 PFU) and then boosted after 15 days with the same amount of virus. Serum was collected by retro-orbital bleeding at different time points. NAb titers were determined and were plotted as the reciprocal of the highest dilution of serum that resulted in at least 50% inhibition of CPE when incubated with 100 PFU of INGN 007.
Effect of preexisting immunity to Ad5 on i.t. vector efficacy in immunocompetent hamsters.
To address whether preexisting immunity to Ad5 will limit the use of oncolytic Ad5-based vectors for cancer gene therapy, we compared the effectiveness of INGN 007 in naïve and Ad5-immune hamsters. Three groups of hamsters were established. One group was immunized by intramuscular injection of Ad5 (1 × 1010 PFU), and the remaining two groups remained naïve. At 14 days postimmunization, HaK cells were injected subcutaneously to form tumors. When the tumor volumes reached ∼0.2 to 0.3 ml, the tumors from the immunized group and one nonimmunized group were injected with INGN 007 (six consecutive injections of 1 × 1010 PFU); the other nonimmunized group was injected with buffer.
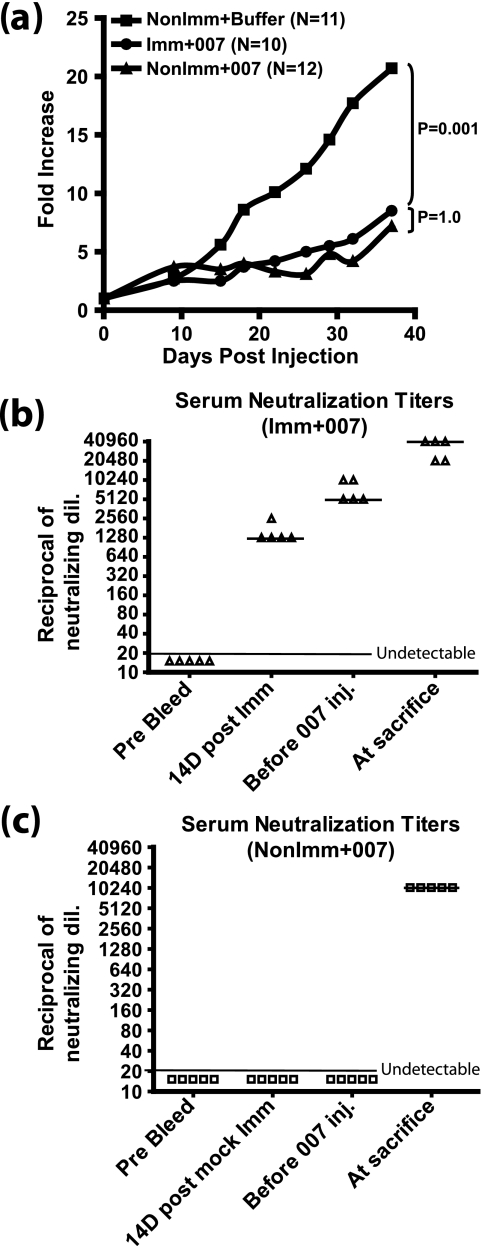
As shown in Fig. 2a, the buffer-injected tumors (NonImm+Buffer) grew well, increasing in size about 20-fold after 38 days. With the immunized and nonimmunized groups that received i.t. injections of INGN 007 (Imm+007 and NonImm+007), there was significant suppression of tumor growth compared to the buffer-injected controls (NonImm+Buffer) (P = 0.001) (Fig. 2a). Interestingly, the growth of tumors in the immunized (Imm+007) and nonimmunized (NonImm+007) groups was not significantly different (P = 1.0). Therefore, the i.t. vector efficacy was not affected by preexisting anti-Ad immunity.
FIG. 2.
Preexisting immunity does not affect vector antitumor efficacy in immunocompetent hamsters. Established HaK tumors were injected for six consecutive days with 1 × 1010 PFU of INGN 007 or buffer. (a) Vector antitumor efficacy. Tumors were measured using digital calipers, and the mean tumor growth of each group is shown. The growth of INGN 007-treated tumors was significantly suppressed in both immunized (Imm+007) and nonimmunized (NonImm+007) groups compared to that of the buffer-injected tumors (P = 0.001). No statistically significant differences were found between immunized (Imm+007) and nonimmunized (NonImm+007) groups treated with INGN 007 (P = 1.0). (b and c) Serum NAb titers. A neutralization assay was performed to determine circulating anti-Ad NAb levels at different time points, as indicated, for immunized (b) and nonimmunized (c) groups. NAb titers were determined by the highest dilution of serum that resulted in at least 50% inhibition of CPE when incubated with 100 PFU of INGN 007. The bar through the triangles indicates the median NAb value. Anti-Ad Ab present in the tumors in this experiment are shown in Fig. 3.
As part of the same experiment shown in Fig. 2a, a neutralization assay was performed with sera isolated at different time points to monitor the level of anti-Ad NAb titers. In the immunized hamsters, the NAb titers reached a median value of 1,280 at 14 days postimmunization (Fig. 2b). This was the point at which HaK cells were injected to form tumors. At ∼5 weeks post-HaK injection, just before the tumors were injected with INGN 007, the NAb titers had reached a median of 5,120. After the tumors were injected with INGN 007 (six consecutive injections), the serum NAb titers increased to a median of ≥40,960 at the termination of the experiment. In contrast, in the nonimmunized hamsters, the median NAb titer was 10,240 at sacrifice (Fig. 2c). These results show that there is a good NAb response to the vector following i.t. injection and that there is a strong boost in NAb in preimmunized hamsters.
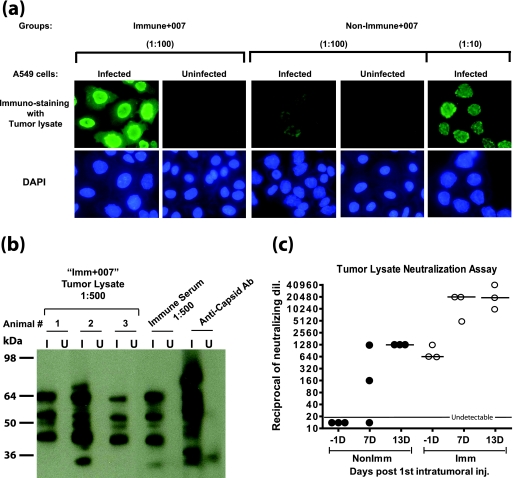
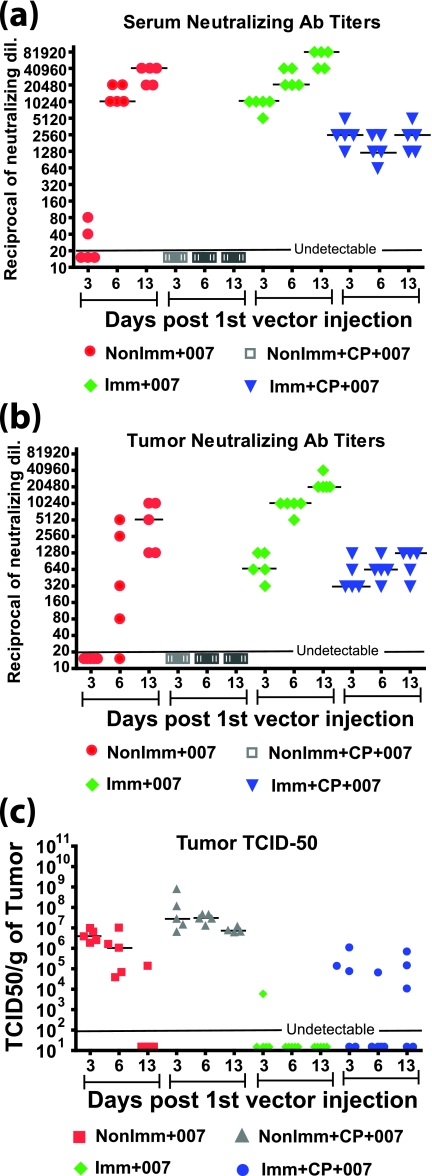
The serum Ab concentration may not necessarily reflect the Ab levels inside tumors. Therefore, we examined the anti-Ad Ab levels in the tumors from the experiment in Fig. 2. Tumor extracts from the Imm+007 group 7 days after the first vector injection were diluted 1:100 and those from the NonImm+007 group were diluted 1:10 and 1:100, and the extracts were then assayed by immunofluorescence on INGN 007-infected A549 cells. As shown in Fig. 3a (left panels), the tumor extracts from immunized hamsters had high Ab levels against the vector. The extracts from nonimmunized hamsters also showed moderate Ab levels (Fig. 3a, right panels).
FIG. 3.
Anti-Ad5 Ab are present inside tumors. Tumors were isolated from immunized and nonimmunized hamsters from the experiments in Fig. 2 at 7 and 13 days after the first i.t. injection of vector. The tumors were homogenized in PBS, and the supernatants were used for the assays. (a) Immunofluorescence. INGN 007-infected (20 PFU/cell for 24 h) or uninfected A549 cells were used for an immunofluorescence assay with tumor lysates (isolated 7 days following the first injection of INGN 007). Alexa Fluor 488-conjugated goat anti-hamster IgG was used to detect the hamster Ab against the vector. (b) Western blotting. INGN 007-infected (I) or uninfected (U) A549 cell lysates (multiplicity of infection of 50 PFU/cell) were electrophoresed and transferred to a membrane. The membrane was cut into strips, and a Western blot was performed with 1:500 diluted tumor lysates (isolated 7 days after the first INGN 007 injection) from three different animals in the Imm+007 group, a serum from the immunized group, and a rabbit anti-capsid Ab as indicated. (c) Ad neutralization assay. The tumor lysate was assayed for the presence of anti-Ad NAb.
To determine whether the Ab in the tumor lysates and immune sera from the immunized group reacted to similar or different Ad proteins, a Western blot was performed on INGN 007-infected or uninfected A549 cell extracts. As shown in Fig. 3b, the tumor lysates and the sera reacted to similar sets of Ad proteins. In this Western blot as well as in the immunofluorescence assay shown in Fig. 3a, the secondary Ab was against hamster IgG; it is interesting that a strong and complex anti-Ad IgG Ab response had developed by only 7 days following the initial injection of INGN 007 (Fig. 3a and b).
As another part of the experiment shown in Fig. 2 and 3a and b, we determined the anti-Ad NAb levels in the tumor extracts. As shown in Fig. 3c, the tumors from the nonimmunized hamsters had undetectable NAb levels before vector injection. Various amounts of NAb were detected 7 days after the first i.t. vector injection, which further increased to a median of 1,280 at 13 days. Thus, some of the naïve hamsters mounted a NAb response, within 7 days after the vector was injected into the tumor, that resulted in the infiltration of NAb into the tumor. The immunized hamsters, however, had a median NAb titer of 640 inside the tumor before vector injection, which further increased to a median of 20,480 7 and 13 days after the first i.t. vector injection (Fig. 3c).
From the experiment shown in Fig. 2 and 3, we concluded that although the NAb in the tumors were much higher in the preimmunized hamsters than in the naïve hamsters, nevertheless there was no significant difference in INGN 007 antitumor efficacy in the immunized and naïve hamsters (Fig. 2a). We know that an immune response reduces oncolytic Ad vector antitumor efficacy (see reference 31 and below). Therefore, it appears that once the NAb in the tumor reach a certain level, as seen in the naïve animals, additional NAb have little effect on vector antitumor efficacy.
Effect of preexisting circulating anti-Ad5 NAb on i.t. vector efficacy.
As discussed above, the experiment shown in Fig. 2 and 3 indicates that preexisting immunity to Ad5 does not affect vector efficacy in suppressing tumors following i.t. injection of vector into immunocompetent hamsters. However, we know that immunity to the vector does reduce vector efficacy, as indicated by our previous studies showing that immunosuppression of hamsters with the alkylating drug CP increases vector antitumor efficacy (31). In our CP immunosuppression studies, all immunity based on immune cells, e.g., natural killer cells, T lymphocytes, and B lymphocytes, was eliminated. However, in the experiment described for Fig. 2 and 3, the entire immune response resulting from immunization and subsequent vector administration is intact. Thus, to understand the specific role of anti-Ad Ab in vector efficacy, we immunized the hamsters with Ad5 to generate Ab, established HaK tumors, treated the hamsters with CP to eliminate immune cells, injected the vector into the tumors, and determined vector antitumor efficacy.
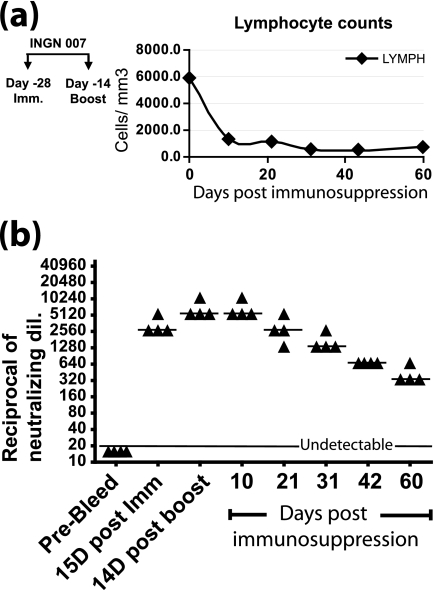
Prior to conducting this experiment, we determined the stability of anti-Ad5 NAb in immunosuppressed hamsters. Hamsters were immunized with Ad5, boosted with Ad5 after 14 days, and then immunosuppressed with CP 14 days after the booster. The animals were kept immunosuppressed throughout the experiment by administering CP twice every week (31). The total leukocyte counts and differential cell counts were determined, and these confirmed that the animals were immunosuppressed. Lymphocyte counts are plotted in Fig. 4a. A neutralization assay with the sera revealed that the NAb titer in circulation decayed by one-half almost every 10 days and that there was still a significant amount (titer of 320) of NAb present at 60 days postimmunosuppression (Fig. 4b).
FIG. 4.
CP nearly eliminates lymphocytes, and preexisting anti-Ad NAb decline by half every ∼10 days. (a) Lymphocyte counts. Hamsters were immunized with Ad5 (1 × 1010 PFU) and then boosted with the same amount of virus after 15 days. Fourteen days after the boost, the hamsters were immunosuppressed throughout the length of the experiment with CP, starting at day 0. Differential blood counts were performed, and the lymphocyte count was plotted. (b) Serum NAb titers. Methods are described in the legend to Fig. 1.
In the follow-up experiment, in which we evaluated the role of preexisting NAb on vector antitumor efficacy, we included not only immunosuppressed hamsters but also, as controls, hamsters that were not immunosuppressed. Eight groups of hamsters were established (Table 1). Four groups were immunized by intramuscular injection of Ad5 (1 × 1010 PFU), and the remaining four groups remained naïve. At 14 days postimmunization, HaK cells were injected subcutaneously to form tumors. When the tumors were ready to be injected with vector, two groups within the immunized and nonimmunized cohorts were immunosuppressed with CP, while the other two groups remained immunocompetent (Table 1). The hamsters were immunosuppressed with CP for a week before injection of the vector to eliminate nearly all immune cells prior to vector administration. Tumors from each of the subgroups were injected with INGN 007 or buffer. Hematology analysis of blood at multiple time points proved that the lymphocytes, monocytes, neutrophils, and eosinophils remained depressed throughout the study (data not shown).
TABLE 1.
| Immune status | Addition of CP | Group name
|
|
|---|---|---|---|
| Buffer groups | INGN 007 groups | ||
| Immune | + | Imm+CP+Buffer | Imm+CP+007 |
| − | Imm+Buffer | Imm+007 | |
| Nonimmune | + | NonImm+CP+Buffer | NonImm+CP+007 |
| − | NonImm+Buffer | NonImm+007 | |
The NAb levels in the groups and the amounts of tumor growth are shown in Fig. 5a and b, respectively. As expected, there was no NAb response in the NonImm+CP+007 group (Fig. 5a). In the Imm+CP+007 group, there was a median NAb titer of 2,560 before vector i.t. injection, and the NAb titer was maintained at the same level at 36 days postinjection (Fig. 5a). In the NonImm+007 group, 20 days after the first vector injection, the median NAb titer was 5,120, which further increased to 10,240 at the time of sacrifice (Fig. 5a). In the Imm+007 group, the median NAb titer was 20,480 before the i.t. vector injection and, remarkably, was boosted to 81,920 at day 20 and to 163,840 at day 36 after the first i.t. vector treatment (Fig. 5a). The Imm+Buffer group had steady levels of NAb, with a median of 20,480 (Fig. 5a).
FIG. 5.
Serum NAb titers and vector antitumor efficacy in naïve and immunized hamsters. (a) Neutralization assay. A neutralization assay was performed to determine circulating anti-Ad NAb in different groups at different time points (1 day before the first i.t. injection, 20 days after the first i.t. injection, and at the time of sacrifice). NAb titers were determined by the highest dilution of serum that resulted in at least 50% inhibition of CPE when incubated with 100 PFU of INGN 007. (b) INGN 007 antitumor efficacy. Established HaK tumors (mean volume, 500 μl) were injected for six consecutive days with 1 × 1010 PFU of INGN 007 or buffer. Tumors were measured using digital calipers, and the mean tumor volume for 10 hamsters in each group is shown.
The growth of tumors for both the immunized and nonimmunized groups injected with buffer was not inhibited (Fig. 5b). CP treatment alone (Imm+CP+Buffer and NonImm+CP+Buffer) did not significantly affect tumor growth. Also, immunization with Ad5 alone (Imm+Buffer and Imm+CP+Buffer) did not affect tumor growth (Fig. 5b). INGN 007 suppressed tumor growth compared to that in the buffer controls. Importantly, tumor suppression was less in the Imm+CP+007 group than in the NonImm+CP+007 group (P = 0.031) (Fig. 5b). Thus, in immunosuppressed hamsters, preexisting circulating NAb do reduce vector i.t. efficacy.
Regarding the role of preexisting immunity in antitumor efficacy in immunocompetent control animals, the results of the experiment in Fig. 5 were similar to those in Fig. 2a., i.e., tumor suppression in the Imm+007 and NonImm+007 groups was not significantly different (P = 0.739) (Fig. 5b). Therefore, it seems that in immunocompetent animals, preexisting immunity does not affect the i.t. efficacy of the vector.
Kinetics of i.t. anti-Ad NAb levels and recovery of infectious vectors from tumors.
In a separate arm of the study described for Fig. 5, we compared the levels of anti-Ad5 NAb in sera (Fig. 6a) and inside tumors (Fig. 6b) to the infectious virus titers inside tumors at various times post-vector administration (Fig. 6c). Sera and tumors from immunized or nonimmunized hamsters with and without immunosuppression were collected 3, 6, and 13 days following the first day of i.t. vector injection (there were six daily injections of 1 × 1010 PFU). As expected, no NAb were detected in the NonImm+CP+007 group at all time points for both sera and tumors (Fig. 6a and b). A TCID50 assay with the tumor lysates showed that the vector titers remained consistently high for this group (with about a 10-fold drop from 3 to 13 days, which might be due to vector leakage from the tumor) (Fig. 6c). In the Imm+CP+007 group, the NAb titers stayed steady at all three time points, with a median of 2,560 for sera (Fig. 6a) versus 640 to 1,280 for tumors (Fig. 6b). The tumors had moderate amounts of vector in three of five hamsters on days 3 and 13; on day 6, one of five hamsters had detectable vector in the tumor (Fig. 6c). Regarding the groups that were not immunosuppressed, for the NonImm+007 group neither the serum (Fig. 6a) nor tumor (Fig. 6b) extracts had detectable NAb for most of the hamsters on day 3. On day 6, the median NAb level increased to 10,240 for sera versus various amounts (≤5,120) for the tumors. By 13 days, the NAb titers further increased to 40,960 for sera and 5,120 for tumors. These data indicate that NAb tended to increase in both the serum and the tumor, but like the case in the Imm+CP+007 group, the amount of NAb in the tumor was considerably less than that in the serum. In the NonImm+007 group, the amount of vector recovered from the tumor dropped considerably from day 3 to day 13, when only one of five hamsters had detectable vector (Fig. 6c). This trend was also true for the Imm+007 group. On day 3, the median NAb titer was 10,240 in the serum (Fig. 6a) versus 640 in the tumors (Fig. 6b). On day 6, the NAb were boosted to 20,480 in sera versus 10,240 in the tumors, and they were boosted to 81,920 in sera versus 20,480 in tumors at day 13 post-vector injection (Fig. 6a and b). For this group, the vector was undetectable in four of five hamsters on day 3, and only a very low level of the vector was recovered from one hamster (Fig. 6c). At other time points, no vector was detected in this group.
FIG. 6.
Amount of infectious vector in the tumor is inversely related to the NAb titer. The data shown are from a separate arm of the experiment described for Fig. 5. Sera and tumors were collected from different groups of hamsters 3, 6, and 13 days after the first vector injection. (a) Neutralization assays were performed with sera, and the NAb titers were plotted. The tumor lysates from the corresponding animals were used to determine tumor NAb titers (b) and tumor TCID50 titers (c).
Comparing Fig. 6b and c, we concluded that the amount of virus in the tumor is inversely related to the amount of NAb present in the tumor. The decrease in vector titers over time in the tumors of nonimmunized immunocompetent hamsters and consistently low vector levels in immunized groups (both with and without immunosuppression) (Fig. 6c) resulted in decreased vector efficacy (Fig. 5b) compared to that in the nonimmunized immunosuppressed group, which had high vector levels at all time points (Fig. 6c). It is interesting that even though very little or no vector was recovered from the Imm+007 group at all time points (Fig. 6c), the efficacy of the vector in this group was similar to that observed for the NonImm+007 group (Fig. 5b).
Statistical analyses were performed on the data in Fig. 6a, b, and c, and the results are provided in Table S1 in the supplemental material. A statistical analysis of Fig. 6a and b showed that in the NonImm+007 and Imm+007 groups, the serum NAb and i.t. NAb increased significantly from day 3 to day 13. In the Imm+CP+007 group, both the serum and i.t. NAb levels stayed steady, without any significant difference in the day 3, 6, and 13 NAb titers. The statistical analysis performed for Fig. 6c showed that the TCID50 titers in the NonImm+007 group dropped significantly from day 3 to day 13. For the NonImm+CP+007 group, the drop in virus titers from day 3 to day 13 was not statistically significant. Also, the levels of virus in the Imm+007 and Imm+CP+007 groups were not statistically different on days 3, 6, and 13.
Role of preexisting immunity in vector spread to other organs.
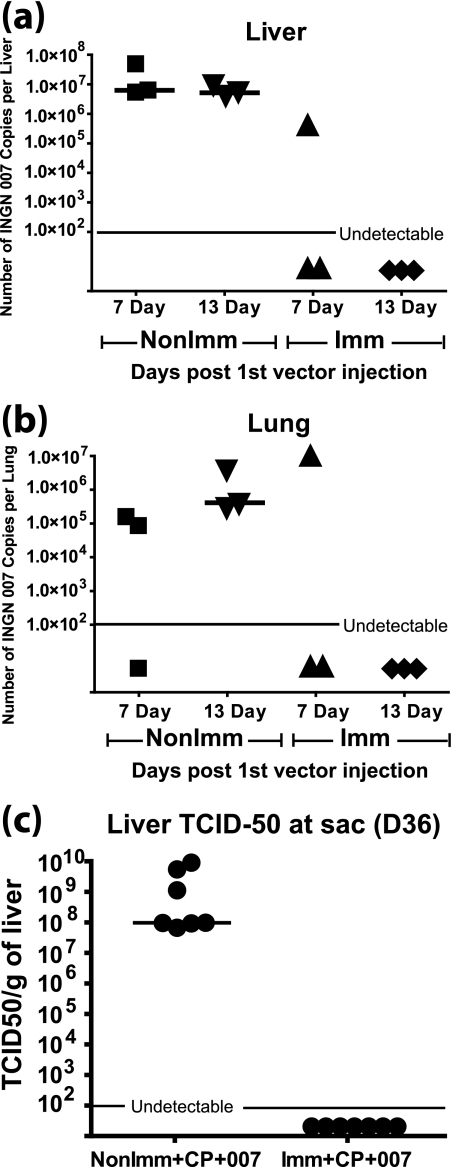
We showed previously that when INGN 007 is injected into tumors, it replicates inside the tumors and spreads to other organs, such as the liver and lungs, where it also replicates (30). This spread might lead to hepatotoxicity and healthy tissue damage, which might limit the use of such vectors in humans. As mentioned before, much of the human population has preexisting immunity to Ad5. The experiments described for Fig. 2, 3, 5, and 6 addressed the role of preexisting immunity in vector efficacy, but an equally interesting question is the role of immunity in vector spread from the site of injection. Therefore, using some of the same hamsters from the two large experiments described for Fig. 2, 3, 5, and 6, we asked whether preexisting immunity to Ad5 can prevent vector spread and toxicity to other organs. Following injection of the tumors in preimmunized and nonimmunized immunocompetent hamsters with INGN 007 (six consecutive injections), as described for Fig. 2 and 3, the liver and lungs were collected 7 and 13 days after the first i.t. injection. When the liver and lung extracts were examined by TCID50 assay, all of the samples were negative (data not shown). When these extracts were assayed for INGN 007 by quantitative real-time PCR, some viral DNA was found in the nonimmunized hamsters on both day 7 and day 13. Importantly, though, vector DNA was detected in the liver and lungs of only one of three hamsters in the immunized group (tissues were from the same animal) on day 7 and in none on day 13 (Fig. 7a and b). Because the quantitative PCR assay is able to detect even a short segment of vector DNA, and considering that the livers and lungs were negative for vector by TCID50 assay, the positive PCR signal may not represent infectious vector but rather Ad DNA (intact or degraded) from infected cells that had undergone lysis. In any case, our results indicate that preexisting immunity greatly prevents the dissemination of vector from the tumor (site of injection) to other organs, thereby reducing the toxicity associated with the vector.
FIG. 7.
Preexisting Ab against Ad effectively prevent vector spillover from the site of injection in the tumor to the liver and lungs. Livers (a) and lungs (b) were collected from immunized and nonimmunized hamsters 7 and 13 days after the first i.t. injection. The tissues were homogenized in PBS, and DNA was isolated and quantified by PicoGreen assay. Real-time PCR with primers against INGN 007 was performed. This subexperiment is an arm of the experiment shown in Fig. 2 and 3. (c) Liver TCID50 titers 36 days after the first i.t. injection. This subexperiment is an arm of the experiment shown in Fig. 5 and 6. P = 0.0006 for NonImm+CP+007 versus Imm+CP+007 groups. sac, sacrifice.
In the experiment for Fig. 5, at the termination of the study (day 36 after the first i.t. vector injection), the livers were collected from the Imm+CP+007 and NonImm+CP+007 groups. A TCID50 assay was performed with the liver homogenates. As shown in Fig. 7c, there were very high levels of infectious vector (median of ∼108 TCID50/g) in the livers of the hamsters in the NonImm+CP+007 group. No serum NAb was detected in this group (Fig. 5a). On the other hand, no infectious vector was recovered from the livers of the animals in the Imm+CP+007 group (Fig. 7c). This group had quite high levels of NAb (median of 2,560) at day 36 (Fig. 5a). This result, together with the results in Fig. 7a and b, underscores the importance of circulating NAb in preventing vector spillover from the site of injection to the normal tissues.
DISCUSSION
Few studies have addressed the role of preexisting immunity in the antitumor efficacy of oncolytic Ad5-based vectors. In the studies that have been conducted (3, 39), immunodeficient mice were passively immunized to mimic the effect of preexisting immunity, which does not reflect the scenario encountered in patients. Most studies involving immunocompetent animals addressed the effects of preexisting immunity on gene transfer, expression, and toxicity caused by replication-defective Ad vectors (1, 28, 40). A lack of an immunocompetent animal model was a major obstacle for these kinds of studies with oncolytic Ad vectors.
In the present study, we modeled preexisting immunity in the Syrian hamster, which is both immunocompetent and permissive for Ad5 replication (30). This model allowed us to address the effects of preexisting immunity and newly acquired immunity in naïve animals on the antitumor efficacy and tumor-to-tissue spread of the oncolytic Ad vector INGN 007. This model resembles what may occur in patients undergoing oncolytic Ad vector cancer gene therapy. We found that with both of the immunocompetent groups (immunized and naïve), INGN 007 injected into tumors suppressed tumor growth significantly compared to that in the buffer-injected groups. Even though NAb were present inside the tumors of immunized animals when the vector was injected for the first time, the NAb did not eliminate vector efficacy. One reason for this might be that when the vector was injected multiple times at a high concentration, the vector may have overcome the local concentration of NAb at the exact site of injection inside the tumor, thereby allowing the vector to infect cells at that site.
When we compared the INGN 007 antitumor efficacies in immunocompetent hamsters that had or had not been immunized, we found that preexisting immunity did not have a significant effect on vector antitumor efficacy. Observations similar to ours have been made with VSV, where NAb appeared within 3 days after VSV injection and virus replication was significantly reduced but significant tumor suppression was still observed (9). Studies with oncolytic HSV also showed that NAb were unable to prevent the i.t. spread of HSV when the vector was administered i.t. (7, 29). It is notable that in our experiments the naïve hamsters developed a strong immune response soon after the vector was injected into the tumor, and NAb were detectable inside the tumor as early as 6 days after the first vector administration (Fig. 3c and 6b). Therefore, the difference between an immunized and naïve animal is diminished within 6 days of the first i.t. vector injection. This may explain, at least in part, why we did not see any difference in vector efficacy between immunized and naïve immunocompetent animals. We know from the experiment in which we immunized the hamsters and then immunosuppressed them that NAb did reduce vector antitumor efficacy compared to that in nonimmunized immunosuppressed hamsters (Fig. 5b). Therefore, it seems that in immunocompetent hamsters, antivector immunity (NAb, cell-mediated, adaptive, and innate immunity) can quickly reach a level where it can impede vector antitumor efficacy, and the level of preexisting immunity does not make a difference.
Still, considering that the immunized hamsters had much higher levels of NAb in sera and tumors throughout the course of the experiment than did the nonimmunized hamsters, it is surprising that there were no differences in vector antitumor efficacy. The amount of vector in the tumor detectable by TCID50 assay correspondingly falls as the NAb levels in the serum and tumor rise (Fig. 6). In fact, in the immunized immunocompetent hamsters, very little vector was detected in tumors 3 days following the first i.t. injection of vector, and no vector was found by 6 and 13 days. In the nonimmunized immunocompetent hamsters, the vector became nearly undetectable by 13 days. Nevertheless, tumor growth was suppressed compared to that of the buffer controls, and there was no statistical difference in tumor suppression between the immunized and nonimmunized immunocompetent groups. If there was little vector present in the tumors throughout the latter course of the experiment, then what accounts for the tumor suppression? We know that the vector can infect and replicate in tumor cells and presumably can spread from cell to cell, as shown in the immunosuppressed hamsters. One possibility is that many tumor cells are infected and subsequently destroyed by the initially injected vector and that more cells are infected and destroyed by the released virus during the period of six vector injections and in the days soon following the last injection, before the vector is eliminated by the immune response. For example, if half of the cells in the tumor were destroyed during this period, then the overall growth of the tumor would be slowed, as shown in Fig. 2a and 5b. Another possibility is that the immune response against the vector activates an immune response against the tumor and that it is this response that contributes to tumor growth suppression. In support of this idea, Hu et al. (17) showed that immunization of mice with a replication-defective Ad vector expressing LacZ followed by i.t. injection with the vector redirected the antivector immunity to the tumor, resulting in suppression of tumor growth.
Our results and those with other types of vectors do not mean that the immune response/preexisting immunity to the vector has no effect on vector efficacy, because we have shown before (31) that vector antitumor efficacy is greater in naïve hamsters that have been immunosuppressed than in naïve immunocompetent animals. Also, in the present study, when we compared vector efficacy in immunosuppressed hamsters that were previously immunized versus that in naïve immunosuppressed hamsters, we found that preexisting immunity did significantly reduce vector efficacy (Fig. 5). Since treatment with CP eliminates leukocytes that mediate innate and adaptive immune responses, the reduction in efficacy most likely is caused by the previously induced anti-Ad NAb. This indicates that preexisting NAb do reduce vector efficacy. For some reason, perhaps as discussed earlier, this reduction in efficacy is masked in immunocompetent animals.
To our knowledge, our study is the first to quantitate anti-Ad Ab, including NAb, inside tumors (i.e., in tumor extracts). Significant levels of NAb were observed in tumors and were detectable as soon as serum NAb were detectable. As shown by Western blotting, the Ab in the tumor and the serum detected more or less similar Ad proteins. An interesting continuation of our studies would be to analyze whether the residual NAb inside the tumor of an immunosuppressed hamster can be overcome by additional i.t. injections to achieve better tumor efficacy.
We (30) and others (1) have shown that when a vector is injected into a tumor, the vector spills over to other organs; if the vector is replication competent and the organs are permissive, then the vector can replicate, particularly under immunosuppressed conditions (31). In the present study, we were able to address the role of preexisting NAb in the possible toxicity caused by spillover of the vector from the tumor and replication in other organs. We found that preexisting NAb eliminated vector spillover to the liver and lungs, as determined by TCID50 assay. We did detect vector DNA in liver and lung extracts by quantitative PCR, but it is not known if this represents vector that spread to the tissues but is not infectious, whether it is DNA fragments from such a vector, or whether it is neutralized vector present in these tissues, e.g., in the blood in the tissues.
In summary, in immunocompetent animals, preexisting immunity to the vector did not affect vector antitumor efficacy following i.t. injection of the vector, but it markedly reduced spillover of the vector to the liver and lungs. Thus, when human cancer patients are treated by i.t. injection of an oncolytic Ad vector, it may be useful to immunize the patients prior to vector administration, e.g., by using a replication-defective Ad vector.
Our present study using CP to immunosuppress the hamsters confirmed our earlier study (31) showing that if immunity is completely eliminated, then the vector is more effective in suppressing tumors. This result raises the possibility that some type of immunosuppression protocol could be used to treat cancer patients with an oncolytic Ad vector. Immunity could not be eliminated completely, of course, because this would lead to dissemination of the vector from the tumor to other tissues, as well as to other problems. However, the patients could perhaps be immunized with Ad prior to immunosuppression or passively immunized during immunosuppression. The immunity, in particular NAb, as we have shown in our studies, might reduce but not eliminate vector antitumor efficacy, and it could effectively reduce vector dissemination from the tumor to other tissues.
Supplementary Material
Acknowledgments
This research was supported by grants CA118022, CA108335, and CA81829 to W.S.M.W. from the National Institutes of Health.
We thank Maria Thomas, Ann Tollefson, Drew Lichtenstein, and Baoling Ying for helpful discussions. We also thank Introgen Therapeutics for kindly providing the purified INGN 007 and Ad5 stocks.
Footnotes
Published ahead of print on 10 December 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Bramson, J. L., M. Hitt, J. Gauldie, and F. L Graham. 1997. Preexisting immunity to adenovirus does not prevent tumor regression following intratumoral administration of a vector expressing IL-12 but inhibits virus dissemination. Gene Ther. 41069-1076. [DOI] [PubMed] [Google Scholar]
- 2.Cattaneo, R., T. Miest, E. V. Shashkova, and M. A. Barry. 2008. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat. Rev. Microbiol. 6529-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, Y., D. C. Yu, D. Charlton, and D. R. Henderson. 2000. Pre-existent adenovirus antibody inhibits systemic toxicity and antitumor activity of CN706 in the nude mouse LNCaP xenograft model: implications and proposals for human therapy. Hum. Gene Ther. 111553-1567. [DOI] [PubMed] [Google Scholar]
- 4.Chirmule, N., K. J. Propert, S. A. Magosin, Y. Qian, R. Qian, and J. M. Wilson. 1999. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 61574-1583. [DOI] [PubMed] [Google Scholar]
- 5.Condit, R. C. 2001. Principles of virology, p. 19-51. In D. M. Knipe, P. M. Howley, D. E. Griffin, M. A. Martin, R. A. Lamb, B. Roizman, and S. E. Straus (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, PA.
- 6.Crompton, A. M., and D. H. Kirn. 2007. From ONYX-015 to armed vaccinia viruses: the education and evolution of oncolytic virus development. Curr. Cancer Drug Targets 7133-139. [DOI] [PubMed] [Google Scholar]
- 7.Delman, K. A., J. J. Bennett, J. S. Zager, B. M. Burt, P. F. McAuliffe, H. Petrowsky, D. A. Kooby, W. G. Hawkins, B. C. Horsburgh, P. Johnson, and Y. Fong. 2000. Effects of preexisting immunity on the response to herpes simplex-based oncolytic viral therapy. Hum. Gene Ther. 112465-2472. [DOI] [PubMed] [Google Scholar]
- 8.Doronin, K., K. Toth, M. Kuppuswamy, P. Krajcsi, A. E. Tollefson, and W. S. M. Wold. 2003. Overexpression of the ADP (E3-11.6K) protein increases cell lysis and spread of adenovirus. Virology 305378-387. [DOI] [PubMed] [Google Scholar]
- 9.Ebert, O., K. Shinozaki, T. G. Huang, M. J. Savontaus, A. Garcia-Sastre, and S. L. C. Woo. 2003. Oncolytic vesicular stomatitis virus for treatment of orthotopic hepatocellular carcinoma in immune-competent rats. Cancer Res. 633605-3611. [PubMed] [Google Scholar]
- 10.Friedman, A., J. J. P. Tian, G. Fulci, E. A. Chiocca, and J. Wang. 2006. Glioma virotherapy: effects of innate immune suppression and increased viral replication capacity. Cancer Res. 662314-2319. [DOI] [PubMed] [Google Scholar]
- 11.Fukuhara, H., and T. Todo. 2007. Oncolytic herpes simplex virus type 1 and host immune responses. Curr. Cancer Drug Targets 7149-155. [DOI] [PubMed] [Google Scholar]
- 12.Fulci, G., L. Breymann, D. Gianni, K. Kurozomi, S. S. Rhee, J. H. Yu, B. Kaur, D. N. Louis, R. Weissleder, M. A. Caligiuri, and E. A. Chiocca. 2006. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc. Natl. Acad. Sci. USA 10312873-12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galanis, E., S. H. Okuno, A. G. Nascimento, B. D. Lewis, R. A. Lee, A. M. Oliveira, J. A. Slaon, P. Atherton, J. H. Edmonson, C. Erlichman, B. Randley, Q. Wang, S. Freeman, and J. Rubin. 2005. Phase I-II trial of ONYX-015 in combination with MAP chemotherapy in patients with advanced sarcomas. Gene Ther. 12437-445. [DOI] [PubMed] [Google Scholar]
- 14.Garber, K. 2006. China approves world's first oncolytic virus therapy for cancer treatment. J. Natl. Cancer Inst. 98298-300. [DOI] [PubMed] [Google Scholar]
- 15.Harvey, B. G., N. R. Hackett, T. El Sawy, T. K. Rosengart, E. A. Hirschowitz, M. D. Lieberman, M. L. Lesser, and R. G. Crystal. 1999. Variability of human systemic humoral immune responses to adenovirus gene transfer vectors administered to different organs. J. Virol. 736729-6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermiston, T. W., and I. Kuhn. 2002. Armed therapeutic viruses: strategies and challenges to arming oncolytic viruses with therapeutic genes. Cancer Gene Ther. 91022-1035. [DOI] [PubMed] [Google Scholar]
- 17.Hu, W. X., J. J. Davis, H. B. Zhu, F. Q. Dong, W. Guo, J. Ang, H. Peng, Z. S. Guo, D. L. Bartlett, S. G. Swisher, and B. L. Fang. 2007. Redirecting adaptive immunity against foreign antigens to tumors for cancer therapy. Cancer Biol. Ther. 61773-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan, J. M. 2005. Adenovirus-based cancer gene therapy. Curr. Gene Ther. 5595-605. [DOI] [PubMed] [Google Scholar]
- 19.Kelly, E., and S. J. Russell. 2007. History of oncolytic viruses: genesis to genetic engineering. Mol. Ther. 15651-659. [DOI] [PubMed] [Google Scholar]
- 20.Kirn, D., R. L. Martuza, and J. Zwiebel. 2001. Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat. Med. 7781-787. [DOI] [PubMed] [Google Scholar]
- 21.Li, H., A. Dutuor, L. Tao, X. P. Fu, and X. L. Zhang. 2007. Virotherapy with a type 2 herpes simplex virus-derived oncolytic virus induces potent antitumor immunity against neuroblastoma. Clin. Cancer Res. 13316-322. [DOI] [PubMed] [Google Scholar]
- 22.Lichtenstein, D. L., K. Toth, K. Doronin, A. E. Tollefson, and W. S. M. Wold. 2004. Functions and mechanisms of action of the adenovirus E3 proteins. Int. Rev. Immunol. 2375-111. [DOI] [PubMed] [Google Scholar]
- 23.Lichtenstein, D. L., and W. S. M. Wold. 2004. Experimental infections of humans with wild-type adenoviruses and with replication-competent adenovirus vectors: replication, safety, and transmission. Cancer Gene Ther. 11819-829. [DOI] [PubMed] [Google Scholar]
- 24.Lin, E., and J. Nemunaitis. 2004. Oncolytic viral therapies. Cancer Gene Ther. 11643-664. [DOI] [PubMed] [Google Scholar]
- 25.Liu, T. C., T. H. Hwang, J. C. Bell, and D. H. Kirn. 2008. Translation of targeted oncolytic virotherapeutics from the lab into the clinic, and back again: a high-value iterative loop. Mol. Ther. 161006-1008. [DOI] [PubMed] [Google Scholar]
- 26.Molnar-Kimber, K., D. H. Sterman, Y. Chang, J. Kang, M. ElBash, M. Lanuti, A. Elshami, K. Gelfand, J. M. Wilson, L. R. Kaiser, and S. M. Albelda. 1998. Impact of preexisting and induced humoral and cellular immune responses in an adenovirus-based gene therapy phase I clinical trial for localized mesothelioma. Hum. Gene Ther. 92121-2133. [DOI] [PubMed] [Google Scholar]
- 27.Parato, K. A., D. Senger, P. A. J. Forsyth, and J. C. Bell. 2005. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. Cancer 5965-976. [DOI] [PubMed] [Google Scholar]
- 28.Schulick, A. H., G. Vassalli, P. F. Dunn, G. Dong, J. J. Rade, C. Zamarron, and D. A. Dichek. 1997. Established immunity precludes adenovirus-mediated gene transfer in rat carotid arteries—potential for immunosuppression and vector engineering to overcome barriers of immunity. J. Clin. Investig. 99209-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simmons, A., and A. A. Nash. 1985. Role of antibody in primary and recurrent herpes simplex virus infection. J. Virol. 53944-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas, M. A., J. F. Spencer, M. C. La Regina, D. Dhar, A. E. Tollefson, K. Toth, and W. S. M. Wold. 2006. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 661270-1276. [DOI] [PubMed] [Google Scholar]
- 31.Thomas, M. A., J. F. Spencer, K. Toth, J. E. Sagartz, N. Phillips, and W. S. M. Wold. 2008. Immunosuppression enhances oncolytic adenovirus replication and anti tumor efficacy in the Syrian hamster model. Mol. Ther. 161665-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas, M. A., J. F. Spencer, and W. S. M. Wold. 2007. Use of the Syrian hamster as an animal model for oncolytic adenovirus vectors. Methods Mol. Med. 130169-183. [DOI] [PubMed] [Google Scholar]
- 33.Thomas, M. A., J. F. Spencer, and W. S. M. Wold. 2007. The use of the Syrian hamster as an animal model for oncolytic adenovirus vectors, p. 169-183. In A. E. Tollefson and W. S. M. Wold (ed.), Adenovirus methods and protocols. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 34.Todo, T., S. D. Rabkin, P. Sundaresan, A. G. Wu, K. R. Meehan, H. B. Herscowitz, and R. L. Martuza. 1999. Systemic antitumor immunity in experimental brain tumor therapy using a multimutated, replication-competent herpes simplex virus. Hum. Gene Ther. 102741-2755. [DOI] [PubMed] [Google Scholar]
- 35.Tollefson, A. E., T. W. Hermiston, and W. S. M. Wold. 1998. Preparation and titration of CsCl-banded adenovirus stocks, p. 1-9. In W. S. M. Wold (ed.), Adenovirus methods and protocols. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 36.Tollefson, A. E., A. Scaria, T. W. Hermiston, J. S. Ryerse, L. J. Wold, and W. S. M. Wold. 1996. The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J. Virol. 702296-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toth, K., J. F. Spencer, A. E. Tollefson, M. Kuppuswamy, K. Doronin, D. L. Lichtenstein, M. C. La Regina, G. A. Prince, and W. S. M. Wold. 2005. Cotton rat tumor model for the evaluation of oncolytic adenoviruses. Hum. Gene Ther. 16139-146. [DOI] [PubMed] [Google Scholar]
- 38.Toth, K., J. F. Spencer, D. Dhar, J. E. Sagartz, R. M. Buller, G. R. Painter, and W. S. M. Wold. 2008. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus-induced mortality in a permissive, immunosuppressed animal model. Proc. Natl. Acad. Sci. USA 1057293-7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai, V., D. E. Johnson, A. Rahman, S. F. Wen, D. LaFace, J. Philopena, J. Nery, M. Zepeda, D. C. Maneval, G. W. Demers, and R. Ralston. 2004. Impact of human neutralizing antibodies on antitumor efficacy of an oncolytic adenovirus in a murine model. Clin. Cancer Res. 107199-7206. [DOI] [PubMed] [Google Scholar]
- 40.Varnavski, A. N., Y. Zhang, M. Schnell, J. Tazelaar, J. P. Louboutin, Q. C. Yu, A. Bagg, G. P. Gao, and J. M. Wilson. 2002. Preexisting immunity to adenovirus in rhesus monkeys fails to prevent vector-induced toxicity. J. Virol. 765711-5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlachaki, M. T., A. Hernandez-Garcia, M. Ittmann, M. Chhikara, L. K. Aguilar, X. H. Zhu, B. S. The, E. B. Butler, S. Woo, T. C. Thompson, H. Barrera-Saldana, and E. Aguilar-Cordova. 2002. Impact of preimmunization on adenoviral vector expression and toxicity in a subcutaneous mouse cancer model. Mol. Ther. 6342-348. [DOI] [PubMed] [Google Scholar]
- 42.Yu, W., and H. Fang. 2007. Clinical trials with oncolytic adenovirus in China. Curr. Cancer Drug Targets 7141-148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.