Abstract
Cyclophilin A (CypA) is an important human immunodeficiency virus type 1 (HIV-1) cofactor in human cells. HIV-1 A92E and G94D capsid escape mutants arise during CypA inhibition and in certain cell lines are dependent on CypA inhibition. Here we show that dependence on CypA inhibition is due to high CypA levels. Restricted HIV-1 is stable, and remarkably, restriction is augmented by arresting cell division. Nuclear entry is not inhibited. We propose that high CypA levels and capsid mutations combine to disturb uncoating, leading to poor infectivity, particularly in arrested cells. Our data suggest a role for CypA in uncoating the core of HIV-1 to facilitate integration.
Cyclophilin A (CypA) is an important species-specific cofactor for human immunodeficiency virus type 1 (HIV-1). CypA-HIV-1 gag interaction leads to incorporation of CypA into viral particles, although efficient recruitment of CypA to incoming HIV-1 cores in target cells renders virion-associated CypA largely redundant (14, 20, 21). The CypA binding site is a conserved glycine-proline motif in an exposed surface loop structure on the HIV-1 capsid (CA) protein, and CypA catalyzes cis-trans isomerization of the proline peptide bond, leading to conformational change (3, 4). Preventing CypA-CA interaction, using competitive CypA inhibitors, e.g., cyclosporine (Cs), or by mutating the CypA binding site, leads to reduced HIV-1 infectivity in most human cells (8, 10, 11, 14, 18, 20, 21).
Passage of HIV-1 in HeLa cells, during CypA inhibition, leads to CA escape mutants A92E and G94D (1). These mutants tolerate CypA inhibition, but remarkably, their replication becomes dependent on the presence of the inhibitor in HeLa or H9 cells but not Jurkat, TE671, or HOS cells (10, 17, 23). Here we show that restriction of A92E and G94D in HeLa cells is due to high levels of CypA and that this restriction is enhanced when the restrictive target cells are arrested. We hypothesize that CypA prevents appropriate uncoating of the mutant viruses, leading to a block to infectivity and poor infection of arrested cells.
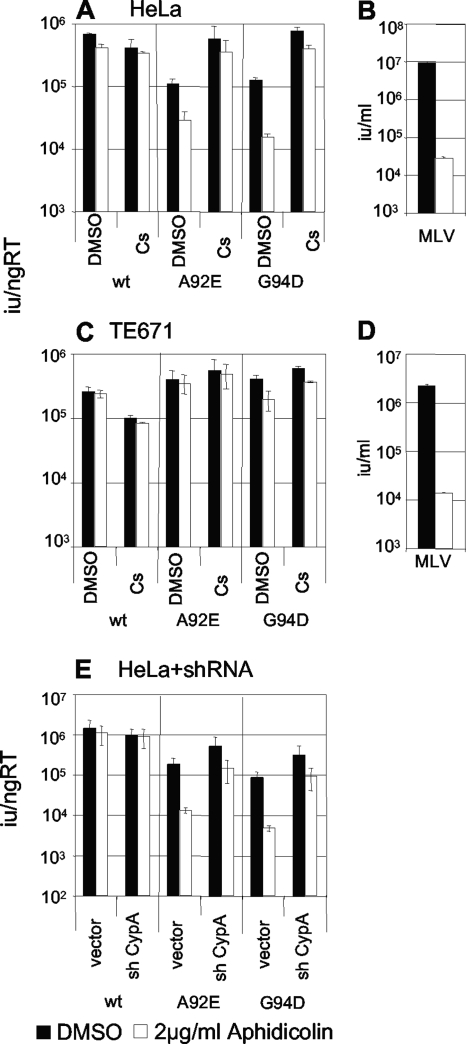
In HeLa cells HIV-1 A92E or G94D infectivity is restricted to around 10% of wild-type infectivity but is completely restored by inhibiting CypA (5, 10, 18). The recent demonstration that HIV-1 CA has a role in infecting nondividing cells suggested a role for CypA in this process (22). We therefore tested whether arresting HeLa cells with aphidicolin impacts on restriction of A92E and G94D HIV-1 CA mutants. HeLa cells (Fig. 1A) and permissive TE671 cells (Fig. 1C) were arrested with aphidicolin, an inhibitor of DNA polymerase II, which blocks cell division in early S phase. The arrested cells were then infected with wild-type or mutant HIV-1 green fluorescent protein (GFP) in the presence and absence of Cs as previously described (16) (Fig. 1A and C). The wild-type HIV-1 titer was not significantly reduced by arresting HeLa or TE671 cells (Fig. 1A and C). Conversely, capsid mutants A92E and G94D showed a significant decrease in infectivity upon aphidicolin treatment in HeLa cells (Fig. 1A) but not TE671 cells (Fig. 1C). Importantly, Cs rescued infectivity of the mutants in HeLa cells to the level of the wild-type virus in untreated cells, suggesting that the block to infection of arrested HeLa cells is dependent on CypA. To confirm that CypA is essential for the aphidicolin-induced restriction, we demonstrated that in HeLa cells expressing CypA-specific short hairpin RNA, and therefore reduced CypA levels, the inhibition of the mutant viruses by aphidicolin is almost completely ablated (Fig. 1E). As a positive control, we demonstrated that aphidicolin treatment of HeLa or TE671 caused a 500-fold reduction in murine leukemia virus (MLV) GFP titer (Fig. 1B and D).
FIG. 1.
Restriction of CA mutants A92E and G94D is strengthened by arresting HeLa cells with aphidicolin. HeLa cells (A and B) or TE671 cells (C and D) were infected with wild-type or mutant virus in the presence (white bars) and absence (black bars) of aphidicolin. Cells were also treated with 5 μM Cs or vehicle (dimethyl sulfoxide) control as shown. HeLa (B) and TE671 (D) cells were infected with MLV as a control with or without aphidicolin, as shown. (E) HeLa cells transduced with empty vector (vector) or vector encoding a short hairpin RNA targeting CypA (sh CypA) were also infected with wild-type or mutant HIV-1 A92E or G94D in the presence (white bars) and absence (black bars) of aphidicolin, as shown. Infected cells were counted by fluorescence-activated cell sorting 48 h after infection, and infectious titers were calculated. HIV-1 infectivity is expressed as infectious units per ng reverse transcriptase activity. MLV titers are expressed as infectious units per milliliter. Errors are standard deviations of titers determined at two doses. Results are representative of at least two experiments performed using independent preparations of virus. wt, wild type; RT, reverse transcriptase; DMSO, dimethyl sulfoxide.
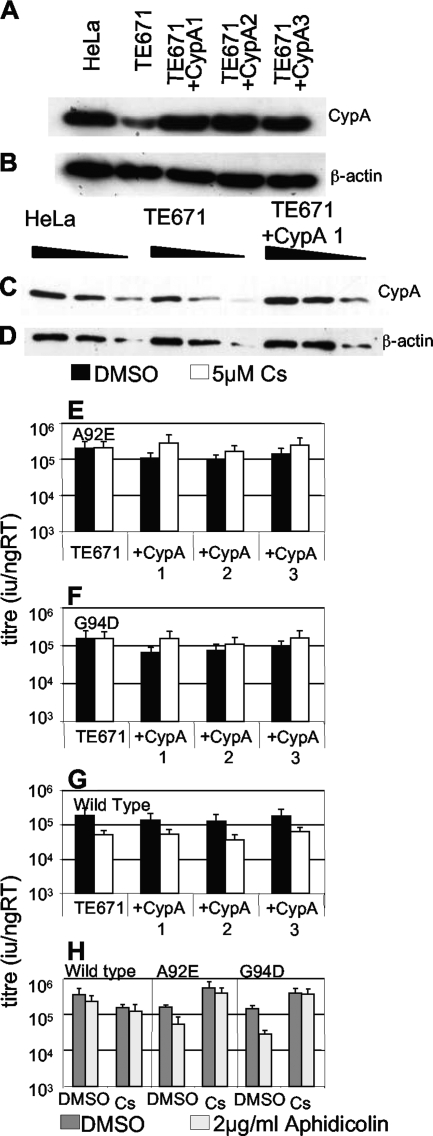
We next sought to establish why the two HIV-1 CA mutants are specifically restricted in HeLa but not TE671 cells. We reasoned that the simplest explanation is that the reported higher CypA levels in HeLa cells (10, 23) (Fig. 2) directly cause specific restriction of the A92E and G94D mutants. We therefore overexpressed CypA in TE671 cells and tested them for their ability to restrict A92E, G94D, or wild-type HIV-1 GFP, in the presence and absence of Cs. Indeed, increasing CypA levels in TE671 cells restricted A92E and G94D in a CypA-dependent way (Fig. 2E and F). Wild-type virus titer was only marginally affected. In fact CypA expression slightly reduced sensitivity to Cs treatment (Fig. 2G). Importantly, arresting the modified TE671 cells with aphidicolin caused a further inhibition of HIV-1 mutant, but not wild-type, titer as predicted. Moreover, the aphidicolin-mediated block for the mutant titer was completely rescued by inhibition of CypA with Cs (Fig. 2H). We have therefore reproduced the specific restriction of HIV-1 A92E and G94D and their aphidicolin sensitivity by increasing CypA protein levels in TE671 cells.
FIG. 2.
Overexpression of CypA in TE671 causes restriction of HIV-1 CA mutants. HeLa cells, parental TE671 cells, and three clones of TE671 transduced with a lentiviral CypA expression vector were analyzed by Western blotting for CypA expression (A and C) or β-actin as a loading control (B and D). Samples were equalized by assay of protein content (Bradford assay). Threefold serial dilutions of samples were loaded in panels C and D. Parental TE671 cells or three CypA-overexpressing TE671 clones were infected with A92E (E), G94D (F), or wild-type (G) HIV-1 GFP, in the presence (white bars) or absence (black bars) of 5 μM Cs. (H) Titers of wild-type, A92E, or G94D HIV-1 GFP were determined on TE671 cells overexpressing CypA (clone 1) in the presence (light gray bars) or absence (dark gray bars) of aphidicolin or the presence or absence of Cs (as shown). The cells were analyzed for infection by fluorescence-activated cell sorting 48 h later, and infectious titers were calculated. Titers are expressed as infectious units per nanogram reverse transcriptase, and errors are standard deviations of titers determined at three doses. The data are representative of two experiments using independent virus preparations. RT, reverse transcriptase; DMSO, dimethyl sulfoxide.
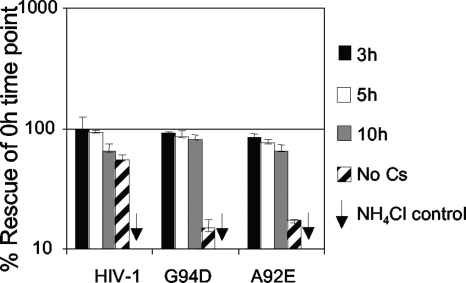
We next examined whether higher levels of CypA in HeLa cells caused irreversible inhibition of the HIV-1 mutants. We infected HeLa cells with HIV-1 GFP A92E or G94D, or wild type, and incubated the cells for specific periods before adding Cs to rescue infection (Fig. 3). We bound virus at 4°C for 1 h and warmed the cells to 37°C for 10 min to synchronize entry, blocking further entry with 20 μM ammonium chloride. This generates a 10-min pulse of infection, which we followed with Cs treatment. We thereby measured for how long the 10-min pulse of virus was sensitive to rescue by Cs. In fact, the mutant viruses can be almost completely rescued by addition of Cs for up to 10 hours after infection, suggesting that HeLa-restricted mutant HIV-1 is stably sequestered.
FIG. 3.
Restricted HIV-1 CA A92E or G94D is stable and can be rescued 10 hours after infection. HeLa cells were infected with wild-type or CA mutant virus as shown for 10 min, after which entry was stopped by addition of NH4Cl. Cs (5 μM) was added at the time of infection or at 3 (black bars), 5 (white bars), or 10 (gray bars) hours postinfection or not at all (striped bars). As a control for the NH4Cl-mediated block, cells with a parallel infection were treated with NH4Cl at the time at which the virus was added (arrows). The cells were analyzed for GFP expression by fluorescence-activated cell sorting 48 h postinfection. The data are plotted as infection relative to the sample in which Cs was added together with the virus, thus of the maximal rescue by Cs treatment. Maximal rescue equated to a 10-fold increase in infectivity, and the doses of virus used were equivalent to a multiplicity of infection between 0.01 and 0.2. Errors are the standard deviations calculated from two independent experiments.
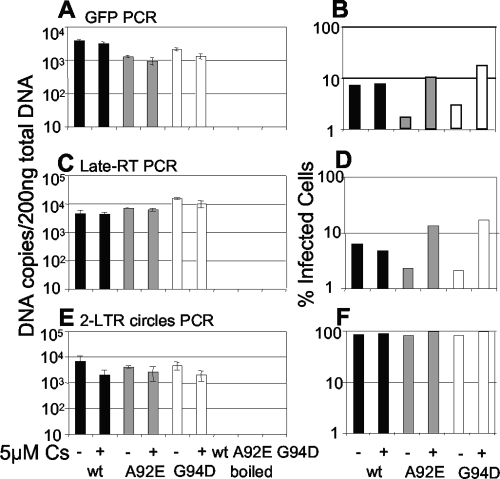
We next tested whether restricted A92E and G94D CA mutants had reduced viral DNA synthesis. We measured early (Fig. 4A) and late (Fig. 4C) reverse transcription product, 6 h after infection as described previously (13), and measured the number of GFP-infected cells in parallel samples 48 h later (Fig. 4B and D). We also measured two-long-terminal-repeat (2-LTR) DNA circles by quantitative PCR (6) (Fig. 4E and F). 2-LTR circles are consistently found in the nucleus and are therefore used as a marker for nuclear entry (9). 2-LTR circles represent approximately 10% of the unintegrated nuclear HIV-1 DNA (6), and to achieve measurable levels, we were obliged to infect the cells at a multiplicity of at least 2.
FIG. 4.
Restricted HIV-1 CA mutant A92E or G94D synthesizes normal levels of DNA, but circle formation is increased. HeLa cells were infected with HIV-1 wild type (black bars) or mutant A92E (gray bars) or G94D (white bars). As a negative control, cells were infected in parallel with an equal dose of boiled virus. Total DNA was prepared from cells at 6 h (A), 12 h (C), or 18 h (E) postinfection. Synthesis of viral DNA was measured by TaqMan quantitative PCR, and DNA synthesis per 200 ng total DNA was calculated. Cells were treated with 5 μM Cs or vehicle (dimethyl sulfoxide) control, as shown. The TaqMan amplicon encodes GFP (A); LTR-gag, a late reverse transcription product (C); or 2-LTR circles (E). The percentage of infection (B, D, and F) was measured in a parallel infection 48 h postinfection by fluorescence-activated cell sorting. Errors are standard deviations of quantitative PCR values from duplicate infections. The results are representative of at least two experiments using independent virus preparations. wt, wild type; RT, reverse transcription.
There was no significant reduction in HIV-1 CA mutant viral DNA synthesis in HeLa cells, indicating that reverse transcription proceeds normally, despite the block to infectivity (Fig. 4A to D). Importantly, the number of circles formed is slightly increased during mutant-restricted infection (Fig. 4E). This observation is reminiscent of the effect of integrase inhibitors, which increase circle formation presumably by increasing the concentration of nuclear unintegrated linear DNA products, the substrate for circle formation (6). These observations suggest that the mutant HIV-1 viruses are able to access the nucleus but that their ability to integrate is inhibited. Cs treatment also reduces wild-type HIV-1 circle formation, consistent with its weak impact on HIV-1 infectivity in HeLa cells (1).
It is striking that restriction of the HIV-1 A92E and G94D CA mutations in HeLa cells is likely due to two- to threefold-higher CypA levels (Fig. 2). We hypothesized that sensitivity to higher CypA levels might be due to increased affinity for CypA. However, we found that recombinant mutant and wild-type capsids bound recombinant CypA with similar Kd (dissociation constant) values of between 5 and 7 μM as demonstrated by isothermal titration calorimetry, performed as described previously (13) (data not shown). We suspect that CypA activity impacts on mutant core uncoating and/or stability. Indeed, recombinant A92E and G94D capsid mutants have been shown to form longer and more regular capsid assemblies in vitro (15), suggesting that they form more stable capsid structures than does wild-type HIV-1. An impact on stability is also inferred from the long period after which mutant virus can be rescued by Cs addition after infection (Fig. 3). Restricted virus can be fully rescued for up to 10 h after infection, suggesting that the effect of CypA on the mutant capsids is reversible and that the CypA does not simply uncoat the mutant virus. Rather, this finding implies that the CypA stabilizes the mutant HIV-1 CA core, preventing appropriate uncoating and reducing its ability to infect nondividing cells but not to access the nucleus as shown by measurement of DNA circles (Fig. 4). In this respect the restricted HIV-1 mutants behave like MLV and are inhibited when target cells are not dividing. Measuring infection of macrophages from six independent donors suggests that macrophages do not commonly restrict HIV-1 A92E or G94D (data not shown). We imagine that this is due to relatively lower levels of CypA in macrophages than in HeLa cells. It remains unclear whether peptidyl prolyl isomerase activity on CA P90 underlies CypA-mediated restriction. It is tempting to speculate that it does, but unfortunately no CypA mutants have been defined which are able to bind HIV-1 CA but are deficient in isomerization.
In Old World monkey cells, inhibiting CypA also leads to an increase in HIV-1 infectivity (2, 12, 19). This is due to the fact that CypA recruitment to HIV-1 CA sensitizes it to recognition by simian TRIM5α presumably driven by prolyl isomerization-mediated conformational change. It is also possible that the effect of CypA is subtle and that CypA impacts on CA stability and uncoating, and it is this that impacts TRIM5α sensitivity. In support of this notion human TRIM5α is more efficiently saturated by HIV-1 when CypA is inhibited (21). Moreover, mutations that alter CA stability also impact on HIV-1's ability to saturate TRIMCyp (7).
Human TRIM5α has been shown not to restrict A92E or G94D in HeLa cells (17), and the fact that the late restriction does not have the characteristics of a TRIM5α-mediated block suggests that it does not in the modified TE671 cells. In human cells CypA does not impact on the inability of TRIM5α to restrict HIV-1, and the mechanism by which it increases HIV-1 titer remains incompletely characterized (12, 17). We hypothesize that HIV-1 has evolved to utilize CypA to appropriately influence uncoating for optimal infectivity in the chimpanzee/human lineage. Given the species-specific and cell-type-specific impact of CypA activity on HIV-1 replication, it seems likely that optimal uncoating is dependent on the specific positive and negative cellular factors present, including restriction factors such as TRIM5α, as well as other as-yet-undefined host factors.
Acknowledgments
We thank Chris Aiken and Ariberto Fassati for helpful discussion.
This work was supported by Wellcome Trust fellowships to G.J.T. (WT076608) and M.N. (WT077161) and the Medical Research Council.
Footnotes
Published ahead of print on 10 December 2008.
REFERENCES
- 1.Aberham, C., S. Weber, and W. Phares. 1996. Spontaneous mutations in the human immunodeficiency virus type 1 gag gene that affect viral replication in the presence of cyclosporins. J. Virol. 703536-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berthoux, L., S. Sebastian, E. Sokolskaja, and J. Luban. 2005. Cyclophilin A is required for TRIM5{alpha}-mediated resistance to HIV-1 in Old World monkey cells. Proc. Natl. Acad. Sci. USA 10214849-14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosco, D. A., E. Z. Eisenmesser, S. Pochapsky, W. I. Sundquist, and D. Kern. 2002. Catalysis of cis/trans isomerization in native HIV-1 capsid by human cyclophilin A. Proc. Natl. Acad. Sci. USA 995247-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosco, D. A., and D. Kern. 2004. Catalysis and binding of cyclophilin A with different HIV-1 capsid constructs. Biochemistry 436110-6119. [DOI] [PubMed] [Google Scholar]
- 5.Braaten, D., C. Aberham, E. K. Franke, L. Yin, W. Phares, and J. Luban. 1996. Cyclosporine A-resistant human immunodeficiency virus type 1 mutants demonstrate that Gag encodes the functional target of cyclophilin A. J. Virol. 705170-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler, S. L., M. S. Hansen, and F. D. Bushman. 2001. A quantitative assay for HIV DNA integration in vivo. Nat. Med. 7631-634. [DOI] [PubMed] [Google Scholar]
- 7.Forshey, B. M., J. Shi, and C. Aiken. 2005. Structural requirements for recognition of the human immunodeficiency virus type 1 core during host restriction in owl monkey cells. J. Virol. 79869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franke, E. K., H. E. Yuan, and J. Luban. 1994. Specific incorporation of cyclophilin A into HIV-1 virions. Nature 372359-362. [DOI] [PubMed] [Google Scholar]
- 9.Fritsch, E., and H. M. Temin. 1977. Formation and structure of infectious DNA of spleen necrosis virus. J. Virol. 21119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatziioannou, T., D. Perez-Caballero, S. Cowan, and P. D. Bieniasz. 2005. Cyclophilin interactions with incoming human immunodeficiency virus type 1 capsids with opposing effects on infectivity in human cells. J. Virol. 79176-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda, Y., L. Ylinen, M. Kahar-Bador, and G. J. Towers. 2004. Influence of gag on human immunodeficiency virus type 1 species-specific tropism. J. Virol. 7811816-11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keckesova, Z., L. Ylinen, and G. J. Towers. 2006. Cyclophilin A renders human immunodeficiency virus type 1 sensitive to Old World monkey but not human TRIM5α antiviral activity. J. Virol. 804683-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keeble, A. H., Z. Khan, A. Forster, and L. C. James. 2008. TRIM21 is an IgG receptor that is structurally, thermodynamically, and kinetically conserved. Proc. Natl. Acad. Sci. USA 1056045-6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kootstra, N. A., C. Munk, N. Tonnu, N. R. Landau, and I. M. Verma. 2003. Abrogation of postentry restriction of HIV-1-based lentiviral vector transduction in simian cells. Proc. Natl. Acad. Sci. USA 1001298-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, S., C. P. Hill, W. I. Sundquist, and J. T. Finch. 2000. Image reconstructions of helical assemblies of the HIV-1 CA protein. Nature 407409-413. [DOI] [PubMed] [Google Scholar]
- 16.Passerini, L. D., Z. Keckesova, and G. J. Towers. 2006. Retroviral restriction factors Fv1 and TRIM5α act independently and can compete for incoming virus before reverse transcription. J. Virol. 802100-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sokolskaja, E., L. Berthoux, and J. Luban. 2006. Cyclophilin A and TRIM5α independently regulate human immunodeficiency virus type 1 infectivity in human cells. J. Virol. 802855-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sokolskaja, E., D. M. Sayah, and J. Luban. 2004. Target cell cyclophilin A modulates human immunodeficiency virus type 1 infectivity. J. Virol. 7812800-12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stremlau, M., B. Song, H. Javanbakht, M. Perron, and J. Sodroski. 2006. Cyclophilin A: an auxiliary but not necessary cofactor for TRIM5alpha restriction of HIV-1. Virology 351112-120. [DOI] [PubMed] [Google Scholar]
- 20.Thali, M., A. Bukovsky, E. Kondo, B. Rosenwirth, C. T. Walsh, J. Sodroski, and H. G. Gottlinger. 1994. Functional association of cyclophilin A with HIV-1 virions. Nature 372363-365. [DOI] [PubMed] [Google Scholar]
- 21.Towers, G. J., T. Hatziioannou, S. Cowan, S. P. Goff, J. Luban, and P. D. Bieniasz. 2003. Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat. Med. 91138-1143. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita, M., O. Perez, T. J. Hope, and M. Emerman. 2007. Evidence for direct involvement of the capsid protein in HIV infection of nondividing cells. PLoS Pathog. 31502-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin, L., D. Braaten, and J. Luban. 1998. Human immunodeficiency virus type 1 replication is modulated by host cyclophilin A expression levels. J. Virol. 726430-6436. [DOI] [PMC free article] [PubMed] [Google Scholar]