Abstract
Varicella-zoster virus (VZV) glycoprotein H (gH) is the major neutralization target of VZV, and its neutralizing epitope is conformational. Ten neutralizing human monoclonal antibodies to gH were used to map the epitopes by immunohistochemical analysis and were categorized into seven epitope groups. The combinational neutralization efficacy of two epitope groups was not synergistic. Each epitope was partially or completely resistant to concanavalin A blocking of the glycomoiety of gH, and their antibodies inhibited the cell-to-cell spread of infection. The neutralization epitope comprised at least seven independent protein portions of gH that served as the target to inhibit cell-to-cell spread.
Varicella-zoster virus (VZV) glycoprotein H (gH) is the major target for neutralization (4, 5, 7, 9, 18), and it plays an important role in viral entry and cell-to-cell spread of infection (1, 3, 11, 12, 15). We isolated human monoclonal antibodies (MAb) to gH using an antibody library called AIMS4 constructed from B-lymphocyte-rich tissues of several dozen people (6, 19). Nine clones were selected for their neutralizing ability and their Fab sequences of heavy (H) and light (L) chains and used, in addition to TI-57, an anti-gH human MAb from a hybridoma, to characterize the neutralization epitopes of gH (18). Our system makes it possible to use the Fab form, which has about one-third the molecular weight of immunoglobulin G (IgG), in order to eliminate the spatial interaction between the Fc or other unreacted Fab of IgG molecules on one gH molecule. The neutralizing epitopes of gH are conformational, making gH hardly detectable by Western blot or enzyme-linked immunosorbent assay, and therefore, the conformational epitopes were mapped immunohistochemically. The combinational neutralizing activity between two species of Fab protein A (Fab-pp) forms and the inhibition of cell-to-cell infection were characterized, and the neutralization domain of gH was found to comprise a cluster of the seven neutralization epitopes and to prevent cell-to-cell infection.
Human embryonic lung cells were used to propagate Oka varicella vaccine, and cell-free virus was obtained by sonication of infected cells in SPGC medium (phosphate-buffered saline [PBS] containing 0.1% sodium glutamate, 5% sucrose, and 10% fetal bovine serum) followed by centrifugation (13, 14, 16).
Except for TI-57, each MAb was expressed in two forms: Fab-pp and Fab with an avidin tag (Fab-Avi-tag). Fab-pp corresponds to an Fab molecule fused with two domains of the Fc-binding protein A from Staphylococcus aureus (8) and purified on an IgG-conjugated column (19). Fab-Avi-tag is composed of an Fab bearing a 23-amino-acid-long peptide tag that can be biotinylated by the bacterial BirA biotin ligase (1). Fab-Avi-tag antibodies were purified by using SoftLink soft release avidin resin (Promega, Madison, WI).
To map the neutralizing epitope by Fab-pp, VZV-infected cells in 24-well plates were fixed by air-drying and then with 50% methanol and 50% acetone. The Fab-pp form (5 μg/ml in 0.5 ml of PBS with 3% skim milk) was used to block gH epitopes for 24 h at 4°C, and then 0.1 ml containing 1 to 10 μg Fab-Avi-tag was added and incubated at 4°C overnight. After incubation with streptavidin conjugated with peroxidase, competition for the gH epitope by the first Fab-pp and the challenging Fab-Avi-tag reaction was visualized by using a Dako liquid diaminobenzidine substrate chromogen detection system (17).
To assess the relationship between the glycomoiety and epitope, VZV-infected cells in eight-chamber culture slides were fixed by air drying and 50% methanol and 50% acetone. Then, the cells were treated with 0.5 ml/well of 200 μg/ml concanavalin A (ConA) (Wako Pure Chemical Industries Ltd., Osaka, Japan) in PBS for 1 h and with bovine serum for 1 h. After being washed with PBS, the cells were incubated with 1 μg/ml Fab-pp from each clone or 1:50-diluted zoster serum at 37°C for 1 h, washed with PBS, and incubated with fluorescein isothiocyanate (FITC)-conjugated anti-human IgG (H+L) rabbit serum (Wako) at 37°C for 1 h. The cells were observed under a fluorescence microscope.
The cells in six-well plates were infected with 50 PFU/0.05 ml of cell-free virus for 1 h and incubated for 1 h without antibody after washing the cells and then in the medium containing 500 μg/ml of the Fab-pp of clones 10, 11, 24, 36, 60, or 94 for 4 days without a change of medium (19). After fixation with 5% formalin, the cells were stained with methylene blue.
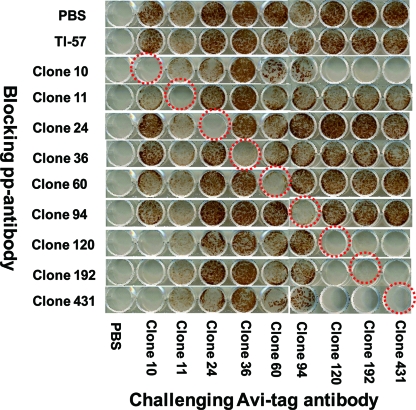
Blocking with PBS failed to inhibit the staining with each Avi-tag antibody, and all the infected cells were positively stained (Fig. 1). Blocking with a homologous Fab-pp blocked the immunostaining with Avi-tag antibody, as shown by the red circles. The Fab-pp of clones 11, 24, 36, 60, and 94 failed to block binding by the clone 10 Avi-tag antibody, and the Fab-pp of clones 120, 192, and 431 blocked binding by the clone 10 Avi-tag antibody, indicating that the epitope of clone 10 was similar to those of clones 120, 192, and 431 but different from those of clones 11, 24, 36, 60, and 94. The Fab-pp of clones 24, 36, 60, and 94 blocked only homologous combinations with each Avi-tag antibody and failed to block binding with the other Avi-tag antibodies. Altogether, epitope mapping of clones indicated the presence of six epitope groups, 10, 120, 192, and 431; 11; 24; 36; 60; and 94, in the neutralization domain of gH.
FIG. 1.
Epitope mapping of gH by competitive immunostaining. Infected cells were first blocked by Fab-pp and then challenged by Fab-Avi-tag for visualization of the reaction of gH and Fab-Avi-tag. When PBS was used as the blocking agent, the challenging Fab-Avi-tag recognized the gH epitope, resulting in positive staining of infected cells, as shown in the top lane. When an Fab-pp successfully blocked the reaction with Fab-Avi-tag, the staining was blocked, as marked by dotted red circles around cultures with the homologous antibody combinations. When the Fab-pp and Fab-Avi-tag recognized different epitopes, infected cells were stained. Clones 10, 120, 192, and 431 showed identical reaction profiles, indicating that they belonged to the same epitope group. TI-57 blocking allowed staining by all kinds of Fab-Avi-tag, indicating that TI-57 recognized different epitopes than the other Fab-pp.
TI-57 antibody did not block any reaction of Avi-tag antibodies with clones 10, 120, 192, 431, 11, 24, 36, 60, or 94, even when used at 20 μg/ml to block the epitope of gH. This suggested that the epitope recognized by TI-57 was different from those recognized by the nine clones. TI-57 is not produced any more, and the amount of TI-57 was not sufficient to perform further work with TI-57. The target epitopes of gH for neutralization were defined by the 7 groups of gH antibodies, 10, 120, 192, and 431; 11; 24; 36; 60; 94; and TI-57.
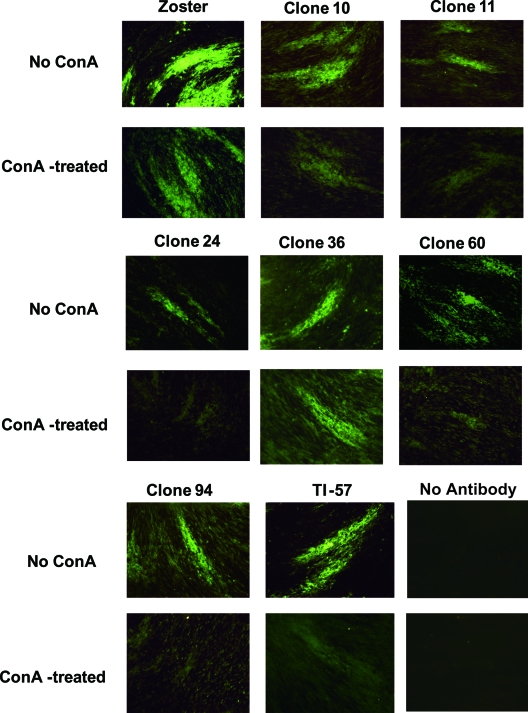
ConA presents as a tetramer with a molecular mass of approximately 108,000 Da, while the molecular masses of Fab-pp and gH are 50,000 and 120,000 Da, respectively. Tetrameric ConA efficiently inactivates viral infectivity (8) and may interfere with the interaction between Fab-pp and the epitope because of its spatial bulkiness when it reacts with the glycomoiety near the target epitope (20). Figure 2 shows the specificity of immunofluorescence and a comparison of the immunofluorescent staining by each antibody clone with and without ConA treatment. The intensity of staining by zoster serum was reduced by ConA treatment, possibly due to blocking of the interaction of the antibody with viral glycoproteins. Staining of infected cells by clone 36 with and without ConA and the contrast between infected and uninfected cells were not affected by ConA treatment, while those parameters in the other clones were reduced slightly or greatly by ConA treatment. Six epitopes were located near the glycomoiety of gH and not in the glycomoiety itself, and the epitope recognized by clone 36 was remote enough to evade spatial blocking by the tetrameric ConA bound to the glycomoiety. This indicated that the epitopes recognized by anti-gH neutralizing MAbs were protein portions or at least not glycomoieties of gH that interact with ConA.
FIG. 2.
Interference with epitope recognition by binding of ConA to gH glycomoieties. The cells on eight-chamber glass slides were treated with 0.5 ml/well of 200 μg/ml ConA or PBS and then with Fab-pp, followed by staining with FITC-labeled anti-human IgG (H+L) rabbit serum. “No Antibody” indicates that infected cells were directly stained with FITC-labeled anti-human IgG (H+L) rabbit serum to determine the specificity of anti-gH MAb. The FITC staining of infected cells without and with ConA treatment is shown. The specificity of FITC staining of infected cells among surrounding uninfected cells and the FITC staining contrast with and without ConA treatment illustrate the effects of ConA treatment on the interaction of anti-gH MAb with infected cells.
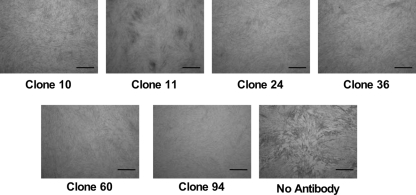
Figure 3 shows the successful inhibition of plaque formation by the six clones representing six epitope groups. The infected culture without antibody showed extensive cytopathology, but the typical cytopathology did not develop in the infected cultures treated with each Fab-pp.
FIG. 3.
Inhibition of cell-to-cell spread of infection and plaque formation by Fab-pp treatment. The cells were infected and incubated for 1 h without antibody and then treated with 500 μg/ml of Fab-pp of clone 10, 11, 24, 36, 60, or 94 for 4 days. The cells were fixed with formalin and stained with methylene blue. Plaques with extensive cytopathology were observed in infected cultures without antibody treatment, while treatment with 500 μg/ml of Fab-pp inhibited the spread of cytopathology. Bars indicate 1 mm.
Table 1 shows the genetic characterization of the H chains of each clone. The variable H chain (VH) gene sequences from framework region 1 to framework region 3 of the seven antibody clones were compared with the germ line sequences listed in VBASE (VBASE Directory of Human V Gene Sequences; http://vbase.mrc-cpe.cam.ac.uk/). The original germ lines for clones 10 and 431, which recognize one epitope, were DP51 and DP50, respectively, and their complementarity-determining region 3 sequences were different in size and amino acids (19). Tyrosine-tyrosine in clone 10 and phenylalanine-tyrosine in clone 431 may form a hydrophobic core and recognize the same antigenic structure. Antibody clone 11, with no mutation in its VH gene, showed low neutralizing activity (50% inhibitory concentration, 8,000 nM), indicating a naïve antibody. The other clones had 18 to 29 nucleotide mutations in the VH genes, which may have contributed to efficient neutralizing activity by reacting with different epitopes.
TABLE 1.
Genetic characterization of the variable H chains compared with germ line sequences in VBASEa
| Clone | Germ line | No. of mutated nt/total no. of ntb | No. of mutated nt in:
|
Neutralization titerc (IC50 nM) | ||||
|---|---|---|---|---|---|---|---|---|
| FR1 | CDR1 | FR2 | CDR2 | FR3 | ||||
| 010 | DP-51 | 24/291 | 9 | 6 | 2 | 1 | 6 | 40 |
| 011 | DP-10 | 0/296 | 0 | 0 | 0 | 0 | 0 | 8,000 |
| 024 | DP-35 | 29/296 | 3 | 5 | 4 | 9 | 8 | 3.0 |
| 036 | DP-67 | 27/294 | 9 | 2 | 2 | 6 | 8 | 400 |
| 060 | DP-49 | 28/294 | 3 | 3 | 4 | 11 | 7 | 25 |
| 094 | DP-46 | 21/294 | 2 | 2 | 1 | 8 | 8 | 0.12 |
| 431 | DP-50 | 18/296 | 1 | 2 | 1 | 10 | 4 | 200 |
nt, nucleotides; FR; framework region, CDR; complementarity-determining region.
The regions covered by the primers used for PCR were excluded.
Neutralization titers are from Suzuki et al. (19). IC50, 50% inhibitory concentration.
Because these clones recognized multiple epitopes, we examined the combinational neutralizing activity of different Fab-pp forms by using a plaque reduction assay (14, 15, 19, 23). Eight kinds of Fab-pp clones were mixed in all possible combinations of two different clones, 36 in total, and the mean neutralizing efficacy was determined seven times. No combination of two clones reduced the number of plaques more than expected (data not shown), indicating that no combination of two clones had synergism. In a different virus system, the addition of MAbs to different functional domains, the V2, V3, or CD4 binding site of human immunodeficiency virus glycoprotein gp120, produced synergistic neutralization (21, 22). In contrast, the multiple neutralizing epitopes of gH might have recognized one functional domain, resulting in no synergism.
Murine MAb 206 to gH neutralizes VZV and inhibits cell-to-cell fusion in gH+gL-transfected cells (2, 3). Prior reports indicate that gH can endocytose on its own, without gE (10), and that interaction with gE may lead to trans-Golgi network targeting. gE can increase endocytosis of gH lacking a YNKI endocytosis motif (11). The inhibitory mechanism of gH in the virus-to-cell or cell-to-cell interaction by neutralizing anti-gH MAbs is not clear. Some combinations of our MAbs that recognized six epitopes might be antagonistic, and further analysis of the relationship between neutralization and cell-to-cell infection among these MAbs might elucidate the gE-gH interaction in the virus-cell interaction and cell-to-cell infection.
All the anti-gH MAbs that had neutralizing activity against VZV blocked entry and egress of the viruses (19), suggesting that both infection by viruses and syncytium formation after infection would be mediated by the same single functional domain on the gH molecule (3-5, 7, 9, 11, 12, 15, 18). In conclusion, the neutralizing domain comprises at least seven independent protein portions of gH.
Nucleotide sequence accession numbers.
The accession numbers of the H and L chains for clones 10, 24, 36, 60, 94, 120, 192, and 431 are AB063700 and AB064076, AB063703 and AB064219, AB063705 and AB064116, AB063707 and AB063990, AB063708 and AB064045, AB063700 and AB063929, AB063700 and AB063932, and AB355876 and AB355875, respectively.
Acknowledgments
We thank Katherine Ono for editing the manuscript.
This study was supported in part by a grant for Research on Pharmaceutical and Medical Safety from the Ministry of Health, Labor, and Welfare of Japan, a grant for Research Promotion of Emerging and Re-emerging Infectious Diseases (H18-Shinko-013) from the Ministry of Health, Labor, and Welfare of Japan, and a Grant-in-Aid (no. 135508094) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Published ahead of print on 10 December 2008.
REFERENCES
- 1.Cole, N. L., and C. Grose. 2003. Membrane fusion mediated by herpesvirus glycoproteins: the paradigm of varicella-zoster virus. Rev. Med. Virol. 13207-222. [DOI] [PubMed] [Google Scholar]
- 2.Drew, P. D., M. T. Moss, T. J. Pasieka, C. Grose, W. J. Harris, and A. J. Porter. 2001. Multimeric humanized varicella-zoster virus antibody fragments to gH neutralize virus while monomeric fragments do not. J. Gen. Virol. 821959-1963. [DOI] [PubMed] [Google Scholar]
- 3.Duus, K. M., C. Hatfield, and C. Grose. 1995. Cell surface expression and fusion by the varicella-zoster virus gH:gL glycoprotein complex: analysis by laser scanning confocal microscopy. Virology 210429-440. [DOI] [PubMed] [Google Scholar]
- 4.Forghani, B., L. Ni, and C. Grose. 1994. Neutralization epitope of the varicella-zoster virus gH:gL glycoprotein complex. Virology 199458-462. [DOI] [PubMed] [Google Scholar]
- 5.Grose, C., D. P. Edwards, W. E. Friedrichs, K. A. Weigle, and W. L. McGuire. 1983. Monoclonal antibodies against three major glycoproteins of varicella-zoster virus. Infect. Immun. 40381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higo-Moriguchi, K., Y. Akahori, Y. Iba, Y. Kurosawa, and K. Taniguchi. 2004. Isolation of human monoclonal antibodies that neutralize human rotavirus. J. Virol. 783325-3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keller, P. M., B. J. Neff, and R. W. Ellis. 1984. Three major glycoprotein genes of varicella-zoster virus whose products have neutralization epitopes. J. Virol. 52293-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsui, S., T. Okuno, and K. Shiraki. 1994. Functional roles of terminal glycomoieties in varicella-zoster virus infection. Virology 19850-58. [DOI] [PubMed] [Google Scholar]
- 9.Montalvo, E. A., and C. Grose. 1986. Neutralization epitope of varicella zoster virus on native viral glycoprotein gp118 (VZV glycoprotein gpIII). Virology 149230-241. [DOI] [PubMed] [Google Scholar]
- 10.Pasieka, T. J., L. Maresova, and C. Grose. 2003. A functional YNKI motif in the short cytoplasmic tail of varicella-zoster virus glycoprotein gH mediates clathrin-dependent and antibody-independent endocytosis. J. Virol. 774191-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasieka, T. J., L. Maresova, K. Shiraki, and C. Grose. 2004. Regulation of varicella-zoster virus-induced cell-to-cell fusion by the endocytosis-competent glycoproteins gH and gE. J. Virol. 782884-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez, J. E., T. Moninger, and C. Grose. 1993. Entry and egress of varicella virus blocked by same anti-gH monoclonal antibody. Virology 196840-844. [DOI] [PubMed] [Google Scholar]
- 13.Shiraki, K., Y. Hayakawa, H. Mori, J. Namazue, A. Takamizawa, I. Yoshida, K. Yamanishi, and M. Takahashi. 1991. Development of immunogenic recombinant Oka varicella vaccine expressing hepatitis B virus surface antigen. J. Gen. Virol. 721393-1399. [DOI] [PubMed] [Google Scholar]
- 14.Shiraki, K., T. Okuno, K. Yamanishi, and M. Takahashi. 1982. Polypeptides of varicella-zoster virus (VZV) and immunological relationship of VZV and herpes simplex virus (HSV). J. Gen. Virol. 61255-269. [DOI] [PubMed] [Google Scholar]
- 15.Shiraki, K., H. Sato, J. Yamamura, Z. H. Li, T. Yokoyama, T. Hasegawa, T. Okuno, M. Kurokawa, and S. Kageyama. 1997. Functions of purified gB, gE:gI, and gH:gL, and their sialyl residues in varicella-zoster virus infection. Arch. Virol. 1422295-2301. [DOI] [PubMed] [Google Scholar]
- 16.Shiraki, K., and M. Takahashi. 1982. Virus particles and glycoprotein excreted from cultured cells infected with varicella-zoster virus (VZV). J. Gen. Virol. 61271-275. [DOI] [PubMed] [Google Scholar]
- 17.Shiraki, K., Y. Yoshida, Y. Asano, K. Yamanishi, and M. Takahashi. 2003. Pathogenetic tropism of varicella-zoster virus to primary human hepatocytes and attenuating tropism of Oka varicella vaccine strain to neonatal dermal fibroblasts. J. Infect. Dis. 1881875-1877. [DOI] [PubMed] [Google Scholar]
- 18.Sugano, T., T. Tomiyama, Y. Matsumoto, S. Sasaki, T. Kimura, B. Forghani, and Y. Masuho. 1991. A human monoclonal antibody against varicella-zoster virus glycoprotein III. J. Gen. Virol. 722065-2073. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki, K., Y. Akahori, Y. Asano, Y. Kurosawa, and K. Shiraki. 2007. Isolation of therapeutic human monoclonal antibodies for varicella-zoster virus and the effect of light chains on the neutralizing activity. J. Med. Virol. 79852-862. [DOI] [PubMed] [Google Scholar]
- 20.Taketa, K., K. Kamakura, S. Satomura, and H. Taga. 1998. Lectin-dependent modulation of interaction between human alpha-fetoprotein and its monoclonal antibodies. Epitope mapping. Tumour Biol. 19318-328. [DOI] [PubMed] [Google Scholar]
- 21.Tilley, S. A., W. J. Honnen, M. E. Racho, T. C. Chou, and A. Pinter. 1992. Synergistic neutralization of HIV-1 by human monoclonal antibodies against the V3 loop and the CD4-binding site of gp120. AIDS Res. Hum. Retrovir. 8461-467. [DOI] [PubMed] [Google Scholar]
- 22.Vijh-Warrier, S., A. Pinter, W. J. Honnen, and S. A. Tilley. 1996. Synergistic neutralization of human immunodeficiency virus type 1 by a chimpanzee monoclonal antibody against the V2 domain of gp120 in combination with monoclonal antibodies against the V3 loop and the CD4-binding site. J. Virol. 704466-4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoyama, T., S. Ayabe, H. Miyagi, T. Sugano, A. Otsu, H. Sato, S. Kageyama, T. Fujii, and K. Shiraki. 2001. Varicella-zoster virus gH:gL contains a structure reactive with the anti-human gamma chain of IgG near the glycosylation site. J. Gen. Virol. 82331-334. [DOI] [PubMed] [Google Scholar]