Abstract
Myeloid differentiation factor 88 (MyD88) is an essential adaptor protein in the Toll-like receptor-mediated innate signaling pathway, as well as in interleukin-1 receptor (IL-1R) and IL-18R signaling. The importance of MyD88 in the regulation of innate immunity to microbial pathogens has been well demonstrated. However, its role in regulating acquired immunity to viral pathogens and neuropathogenesis is not entirely clear. In the present study, we examine the role of MyD88 in the CD4+ T-cell response following lymphocytic choriomeningitis virus (LCMV) infection. We demonstrate that wild-type (WT) mice developed a CD4+ T-cell-mediated wasting disease after intracranial infection with LCMV. In contrast, MyD88 knockout (KO) mice did not develop wasting disease in response to the same infection. This effect was not the result of MyD88 regulation of IL-1 or IL-18 responses since IL-1R1 KO and IL-18R KO mice were not protected from weight loss. In the absence of MyD88, naïve CD4+ T cells failed to differentiate to LCMV-specific CD4 T cells. We demonstrated that MyD88 KO antigen-presenting cells are capable of activating WT CD4+ T cells. Importantly, when MyD88 KO CD4+ T cells were reconstituted with an MyD88-expressing lentivirus, the rescued CD4+ T cells were able to respond to LCMV infection and support IgG2a antibody production. Overall, these studies reveal a previously unknown role of MyD88-dependent signaling in CD4+ T cells in the regulation of the virus-specific CD4+ T-cell response and in viral infection-induced immunopathology in the central nervous system.
Myeloid differentiation factor (MyD88) is an essential adaptor molecule in all Toll-like receptor (TLR) signaling pathways except TLR3. In addition, MyD88 is also important for interleukin-1 receptor (IL-1R)/IL18R-mediated signaling. Many studies have demonstrated the importance of MyD88-dependent signaling in the regulation of innate as well as acquired immunity, in particular, T-cell responses, to various microbial pathogens (1, 2, 37, 39, 44). Although it is not entirely clear how innate MyD88-dependent signaling regulates the activation of T-cell responses, it has been suggested that MyD88 expression in antigen-presenting cells (APCs), including dendritic cells (DCs), plays a key role in the activation of T-cell responses (2, 15, 27, 39). Additionally, very recent studies have revealed an important role of MyD88 expression in T cells in regulating T-cell activation and pathogenesis in response to model antigens or parasites (11, 26). However, it is not known whether MyD88 expression in T cells plays a similar role in the activation of T cells and regulation of pathogenesis in response to a virus.
Lymphocytic choriomeningitis virus (LCMV) is a noncytolytic virus, and most of the diseases associated with LCMV infection in mice are mediated by either innate or acquired immune responses. It has been well demonstrated that both CD8+ and CD4+ T cells play a role in LCMV-associated diseases, i.e., CD8+ T-cell-mediated meningitis (3, 8) or CD4+ T-cell-mediated weight loss (14, 21). In the absence of both CD8+ and CD4+ T cells, mice do not develop symptoms following intracranial LCMV infection (29, 51). Thus, LCMV is a suitable model to evaluate the contribution of a variety of signaling molecules in the activation of CD4+ as well as CD8+ T-cell responses to virus infection and viral pathogenesis (3, 8).
Studies from our group and others have shown that MyD88 is critical for the induction of the LCMV-specific CD8+ T-cell response (18, 40, 49). Moreover, we have also shown that the defective CD8+ T-cell response to LCMV infection in MyD88 knockout (KO) mice is not due to a total failure of the APC system because adoptively transferred P14 T-cell receptor (TCR) transgenic CD8+ T cells (expressing a TCR transgene specific for the LCMV glycoprotein consisting of residues 33 to 41 [GP33-41] epitope) expand and function comparably in both MyD88 KO and wild-type (WT) mice (49). In the present study, we used LCMV infection as a model to test whether the MyD88 signaling pathway plays a role in the activation of CD4+ T cells in response to a natural viral pathogen. Surprisingly, we found that the MyD88 signaling pathway functions within the CD4+ T cells themselves and is essential for normal CD4+ T-cell function, and we further demonstrated that this MyD88 signaling is independent of IL-1R- and IL-18R-mediated signaling.
MATERIALS AND METHODS
Reagents.
The Armstrong strain of LCMV was kindly provided by Liisa K. Selin (University of Massachusetts Medical School, Worcester, MA) and was propagated on BHK-21 cells (ATCC) at a low multiplicity of infection ([MOI] 0.01). Viral titers were determined with an immunological focus assay (4). Rat anti-LCMV nucleoprotein antibody was kindly provided by Demetrius Moskophidis (Medical College of Georgia, Augusta, GA) (4). The DC line DC2.4 was kindly provided by Kenneth L. Rock (University of Massachusetts Medical School, Worcester, MA). The CD8α T-cell depletion antibody, clone 2.43 hybridoma (rat immunoglobulin G1 [IgG1]) (41), was obtained from ATCC. CD4+ CD25+ regulatory T (Treg) cell-depletion antibody, anti-mouse CD25 hybridoma (clone PC61; rat IgG1 isotype), was obtained from ATCC. Culture supernatants were collected and purified using protein G columns. To deplete Treg cells, a single dose of 500 μg of purified PC61 or an equivalent amount of a rat IgG isotype antibody was injected into CD8+ T-cell-depleted MyD88 KO mice via tail vein on day −5 before intracranial LCMV challenge (12, 38, 39, 43). The efficacy of CD4+ CD25+ Treg-cell depletion was confirmed by flow cytometry using allophycocyanin- or phycoerythrin-conjugated anti-mouse CD4 and fluorescein isothiocyanate-conjugated anti-CD25 antibody (clone 7D4) (BD PharMingen), anti-CD3 and anti-CD28 (clones 145-2C11 and 37.51, respectively; BD PharMingen).
Mice.
TLR2 and MyD88 KO mice were the gift of S. Akira (Osaka University, Japan). MyD88 KO mice were backcrossed with C57BL/6 mice at least six generations. The genotypes of the mice were determined by PCR of tail DNA. Mice were bred and maintained under specific-pathogen-free conditions. Age-matched C57BL/6 mice (WT control mice), gamma interferon (IFN-γ) KO mice, CD8α+ T-cell-deficient mice, IL-1R1 KO mice, IL-18 KO mice, and TCR-β/δ-deficient mice were purchased from the Jackson Laboratories (Bar Harbor, ME). All mice used were 6 to 10 weeks old.
In order to determine whether the TLR-MyD88 pathway plays a role in CD4+ T-cell-mediated central nervous system immunopathology, CD8+ T cells were depleted before intracranial infection with LCMV by intraperitoneal injection with a rat monoclonal antibody against mouse Lyt2, antibody 2.43 (ATCC) (17, 21, 41), on day −5, −2, 0, +2, and +5 relative to intracranial LCMV infection and then at weekly intervals. The efficacy of CD8 T-cell depletion was confirmed by flow cytometry using the anti-CD8β antibody (BD PharMingen). For intracranial infection, mice were lightly anesthetized with isoflurane and injected with 30 μl of LCMV Armstrong (200 PFU) diluted with phosphate-buffered saline (PBS). Mice were monitored and weighed daily. The weight loss was calculated as follows: [(weight after infection − weight before infection)/weight before infection] × 100. For intravenous infection, mice were infected with 200 μl of LCMV Armstrong (5 × 105 PFU) diluted with minimal essential medium-2% fetal calf serum (FCS). Animals were housed and experiments were performed in accordance with animal welfare guidelines.
Viral peptide.
The LCMV-specific CD4+ T-cell epitope peptide used in this study was major histocompatibility complex (MHC) class II I-Ab restricted GP61-80 (34). The peptide was synthesized by the Tufts University peptide core facility and purified by high-performance liquid chromatography.
Quantitation of CD4+ T-cell response by intracellular staining for IFN-γ and tumor necrosis factor alpha (TNF-α).
Methods used to isolate mononuclear cells (MNC) from the brain have been described previously (50). Briefly, mice were perfused through the right ventricle with 10 ml of PBS prior to tissue removal. Brain tissues were mashed through a 70-μm-pore-size strainer in RPMI medium with 10% FCS. The resulting cell suspensions were centrifuged, and the pellet was resuspended in a 38% Percoll solution (Pharmacia), layered onto a 68% Percoll solution, and centrifuged at 600 × g for 20 min at 4°C. MNC were recovered from the gradient interface and washed twice with complete RPMI medium before use.
To prepare conventional DCs ([cDCs] CD11c+ DCs), both WT and MyD88 KO mice were subcutaneously injected with 2.5 × 105 B16-Flt3L melanoma cells in 200 μl of PBS to increase the frequencies of cDCs. On day 10 after injection, spleens were collected and CD11c+ DCs were enriched using anti-CD11c magnetic beads (Miltenyi Biotec). Enriched cDCs, together with a control DC line, DC2.4, were infected with LCMV WE strain at a multiplicity of infection of 0.5 (32, 50) because DCs are not sensitive to the infection of LCMV Armstrong (13). At 48 h postinfection, DCs were collected, and 5 × 104 cDCs were cocultured with 5 × 105 purified LCMV-immune (day 10 postinfection) CD4+ T cells for 5 h in the presence of brefeldin A (1 μg/ml; BD PharMingen). The expression of IFN-γ was examined by intracellular cytokine staining (ICS).
ICS was performed as described previously (33). Briefly, cells were cultured in 96-well flat-bottom plates at a density of 106 cells/well in 200 μl of RPMI 1640 medium supplemented with 10% FCS, recombinant human IL-2 (hIL-2; 20 U/ml; Roche), and brefeldin A (1 μg/ml; BD PharMingen) in the presence or absence of the LCMV-specific CD4 epitope peptide GP61-80 (GLKGPDIYKGVYQFKSVEFD) at a concentration of 4 μg/ml. Cells were also cultured with phorbol myristate acetate (100 ng/ml) and ionomycin (500 ng/ml). After 5 h of culture, the cells were harvested, washed once in PBS, and surface stained with allophycocyanin-conjugated monoclonal rat antibody specific to mouse CD4 (clone RM4-5; BD PharMingen). After being washed, the cells were stained for intracellular cytokines by using a Cytofix/Cytoperm kit (BD PharMingen) according to manufacturer's instructions. Phycoerythrin-conjugated monoclonal rat antibodies specific to murine IFN-γ (clone XMG1.2; BD PharMingen) or fluorescein isothiocyanate-conjugated monoclonal rat antibody to murine TNF-α (clone MP6-XT22; BD PharMingen) and the isotype control (rat IgG1) were used to identify cytokine-positive cells. Samples were acquired on a BD-LSR-II flow cytometer (Becton Dickinson). Data were analyzed with FlowJo software.
To test whether the MyD88 signaling pathway affects TCR-dependent CD4 T-cell activation, splenocytes were stimulated with immobilized anti-CD3 (20 μg/ml) and soluble anti-CD28 (100 ng/ml). After incubation for 72 h, the levels of IFN-γ in the supernatants were determined using an enzyme-linked immunosorbent assay (BD PharMingen) according to the manufacturer's instructions.
Quantitative analysis of virus-specific CD4+ T cells by tetramer staining.
I-Ab-restricted tetramer complexed with LCMV CD4 epitope peptide GP66-77 (DIYKGVYQFKSV) or control peptide were kindly provided by the NIH Tetramer Core Facility (Emory Vaccine Center, Atlanta, GA). Single-cell suspensions prepared from spleen and brain were stained with I-Ab tetramer. After the samples were stained at 37°C for 3 h in RPMI medium-2% FCS (48), cells were washed once with PBS and stained with anti-CD4 allophycocyanin-conjugated rat monoclonal antibody with 1% bovine serum albumin and 0.2% sodium azide. After being stained for 30 min at 4°C, cells were washed twice with PBS, fixed in PBS containing 4% formaldehyde, and analyzed on a BD-LSR-II flow cytometer (Becton Dickinson). Data were analyzed with FlowJo software.
Purification of CD4+ T cells and adoptive transfer experiment.
Spleens and mesenteric lymph nodes collected from naïve mice were pooled, and a single-cell suspension was prepared. Erythrocytes were lysed with red blood cell lysis buffer (Sigma). CD4+ T cells were purified using a CD4+ T-cell isolation kit (mouse CD4+ T cells are isolated by depletion of non-CD4+ T cells) following the instructions supplied by manufacturer (Miltenyi Biotec, Auburn, CA). The purity of CD4+ T cells was always >90% as assessed by flow cytometry and with no detectable CD8+ T cells. A total of 5 ×106 purified CD4+ T cells were adoptively transferred into recipient mice via tail vein injection. One day after adoptive transfer, recipients were infected with LCMV Armstrong.
Construction of mouse MyD88 lentivirus.
The gene coding for mouse MyD88 was amplified by using reverse transcription-PCR. Total RNA was prepared from normal C57BL/6 mouse splenocytes using a Qiagen RNeasy Mini kit. The primers were designed according to the sequence deposited in the GenBank database (accession no. NM_010851): forward primer, 5′-AGCACCGGTGCCTGCCATGTCTGCGGGAGAC-3′ (underlined AgeI restriction site); reverse primer, 5′-ATTGCCCTTGGCTCTAGAGGGTCATCTGTAGGGCAGGG-3′ (underlined XbaI restriction site). The PCR product was first ligated directly into pcDNA3-V5/His-TOPO as described by the manufacturer (Invitrogen). Plasmid DNA containing MyD88 sequence was isolated, and the insert DNA was excised by AgeI and XbaI digestion. Enhanced green fluorescent protein (EGFP) in lentiviral vector pUb-EGFP-Thy1.1 (kindly provided by Zhibin Chen, University of Miami Miller School of Medicine) was excised by AgeI/XbaI digestion and replaced with the mouse MyD88 gene. This construct was transformed into Stbl2 competent cells. The final MyD88 lentiviral construct, named pUb-MyD88/Thy1.1, was confirmed by restriction analysis and DNA sequencing (Tufts University DNA Core Facility). Expression of MyD88 is under the control of ubiquitin-C promoter. Additional plasmids necessary for generating lentivirus including cytomegalovirus-Eco (rodent specific) and Rous sarcoma virus-Rev were the gift of Z. Chen. All plasmids were extracted using a Qiagen Hi-Speed Maxi kit.
Production and quantification of MyD88 lentivirus.
Protocols provided by Chen and also described by Dull et al. (9) were followed, with some modifications. Briefly, 5 × 106 of the GP2-293 packaging cells (BD) were plated into 10-cm2 petri dishes. GP2-293 is an HEK-293-based packaging cell line that stably expresses the viral gag and pol genes. When cells were 90% to 95% confluent, they were transfected using a calcium chloride transfection protocol. The following amounts of plasmids were used per dish: 20 μg of pUb-MyD88/Thy1.1 or control pUb-EGFP/Thy1.1 and 10 μg of both Rous sarcoma virus-Rev and cytomegalovirus-Eco. At 4 h after transfection, medium was removed, cells were washed with PBS, and 10 ml of Dulbecco's modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum was added. At 36 to 48 h after transfection, culture supernatants were collected and spun at low speed (2,000 rpm for 10 min). The titers of the MyD88 lentivirus and control lentivirus were determined by transducing NIH 3T3 fibroblasts seeded in a 12-well plate (5 × 104 cells per well) with serially diluted lentivirus-containing supernatants supplemented with 6.0 μg/ml polybrene (Sigma). Thy1.1 was used as a marker to determine the concentration of the lentivirus. After incubation for 48 h, the expression of Thy1.1 was analyzed by FACS staining with anti-Thy1.1 antibody (BD). The concentration of lentivirus was calculated on the basis of the number of Thy1.1-positive cells and expressed as infectious virus particle (titer is defined as the number of infectious virus particles/ml).
Purification and transduction of MyD88 KO CD4+ T cells with MyD88 lentivirus and adoptive transfer and analysis of MyD88 lentivirus-transduced MyD88 KO CD4+ T cells in vivo.
Naïve MyD88 KO CD4+ T cells were purified from MyD88 KO female mice using a CD4 T-cell purification kit as described above (Miltenyi Biotec, Auburn, CA). Cells were activated and transduced with either MyD88 lentivirus pUb-MyD88/Thy1.1 or control lentivirus pUb-EGFP/Thy1.1 following the protocol kindly provided by Laurence Turka and colleagues. Briefly, purified naïve MyD88 KO CD4+ T cells (>93% purity as checked by FACS staining) were plated into 24-well plates at 2 × 106 cells per well and activated with 1 μM ionomycin, 3 ng/ml of phorbol myristate acetate, and 10 units/ml of recombinant hIL-2 (Roche) for 24 h. After activation, medium was removed, and 1.0 ml of lentivirus supernatants together with 6 μg/ml of polybrene and 10 units/ml of hIL-2 was added, and cells were spun at 2,200 rpm for 90 min at 22°C. After centrifugal infection, cells were washed once with DMEM-10% FCS, and then 1.0 ml of DMEM-10% FCS and 10 units/ml of hIL-2 were added, and cells were incubated an additional 48 h. The efficiency of transduction of CD4+ T cells was determined by the expression of Thy1.1. A total of 5 × 106 of the transduced CD4+ T cells were adoptively transferred into TCR-β/δ-deficient female mice via tail vein injection. After 24 h, mice were challenged intravenously with 5 × 105 PFU of LCMV Armstrong. At day 9 postinfection, the CD4 T-cell responses in recipients were examined by restimulation of donor CD4+ T cells in vitro with LCMV-specific CD4 epitope peptide GP61-80, and the expression of IFN-γ was analyzed by ICS as described above.
Histopathology study.
On day 12 after intracranial infection with LCMV, two mice from each group (WT, IL-1R1 KO, and MyD88 KO mice) were euthanized, and their brains were fixed with Bouin's fixative reagent. The brain tissues were routinely embedded in paraffin and sectioned at 6 μm. Brain sections were stained with hematoxylin and eosin.
Statistical analysis.
Data were evaluated using a two-tailed Student's t test. Results were expressed as means ± standard deviation. P values of <0.05 were regarded as significant.
RESULTS
LCMV-induced weight loss is MyD88 dependent but is IL-1R1/IL-18R independent.
Intracranial infection of immunocompetent mice with LCMV leads to fatal CD8+ T-cell-mediated LCM while intracranial infection of CD8+ T-cell-deficient mice with LCMV causes wasting disease, characterized by CD4+ T cell-mediated weight loss (7, 10, 14, 29). Our previous studies have demonstrated that CD8+ T cells are abnormal in MyD88 KO mice (49). Our initial experiments showed that intracranial LCMV infection in MyD88 KO mice induced a delayed lethal meningitis compared to WT mice (data not shown), suggesting that, although impaired, the CD8+ T-cell response could still be detrimental to the host in an intracranial infection model. In the present study, we eliminated CD8+ T cells and then determined the role of the MyD88 adaptor molecule in the CD4+ T-cell response to LCMV infection. Interestingly, after depletion of CD8+ T cells and intracranial infection with LCMV, WT mice started losing weight at about day 7 postinfection, and at peak they lost up to 30% of their body weight (Fig. 1A). In contrast, CD8+ T-cell-depleted MyD88 KO mice did not show any symptoms of disease.
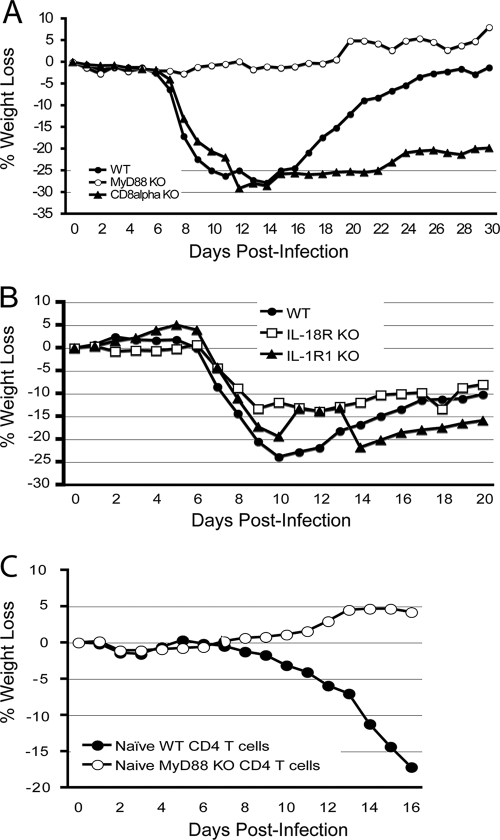
FIG. 1.
LCMV-induced weight loss is MyD88 dependent but IL-1R1 or IL-18R independent. All mice, except CD8α T-cell and TCR-β/δ KO mice, were injected with monoclonal antibody 2.43 to deplete CD8+ T cells, as described in Materials and Methods. (A) Groups of WT (n = 29), MyD88 KO (n = 24), and CD8α T-cell-deficient (n = 15) mice were intracranially infected with LCMV Armstrong as described above. Body weight loss was compared. In total, 1 of 29 WT mice died on day 12 postinfection, and 3 of 15 CD8α KO mice died between day 10 and day 13 postinfection. (B) WT (n = 10), MyD88 KO mice (n = 5), IL-1R1 KO (n = 15), and IL-18R KO mice (n = 18) were intracranially infected with LCMV Armstrong. Body weight loss was compared. A representative of three to five experiments is shown. (C) A total of 5 × 106 purified naïve WT or MyD88 KO CD4+ T cells were adoptively transferred into TCR-β/δ KO recipients; the next day, recipients were followed by intracranial LCMV infection. The weight loss was recorded and compared.
We used CD8α+ T-cell-deficient mice to validate our observations. These mice lack CD8+ T cells but have normal CD4+ T cells. After intracranial infection with LCMV, CD8α+ T-cell KO mice steadily lost weight (Fig. 1A), which is consistent with previous findings that CD4+ T cells are responsible for weight loss (10). Taken together, these studies suggested that MyD88 is involved in LCMV-induced CD4+ T-cell responses and the CD4+ T-cell-dependent wasting disease.
MyD88 is an essential adaptor molecule for several receptors including all TLRs (except TLR3) and both the IL-1R and the IL-18R-mediated signaling pathways (1, 44). To determine whether IL-1R and IL-18R rather than TLRs were responsible for MyD88-dependent wasting disease, IL-1R1 KO and IL-18R KO mice were depleted of CD8+ T cells and infected intracranially with LCMV. CD8+ T-cell-depleted IL-1R1 KO and IL-18R KO mice exhibited weight loss compared to CD8+ T-cell-depleted MyD88 KO mice (Fig. 1B). Thus, these studies demonstrated that neither IL-1R1 nor IL-18R alone played a major role in LCMV-induced MyD88-dependent wasting disease.
Intracranial LCMV infection induced severe inflammation in the brains of WT and IL-1R1 KO mice but not in MyD88 KO mice.
To determine whether the observed clinical symptoms were related to inflammation in the brain, histopathologic analysis was performed. After depletion of CD8+ T cells and intracranial infection with LCMV, the brains of WT and IL-1R1 KO mice demonstrated a prominent infiltration of MNC compared to the brains of uninfected WT mice. The severity of the meningitis was assessed based on the accumulation of inflammatory cells in the meninges of the brains. The meninges of intracranially infected WT and IL-1R1 KO mice exhibited prominent signs of inflammation (Fig. 2). In marked contrast, there was little, if any, inflammation in the brains of MyD88 KO mice (Fig. 2).
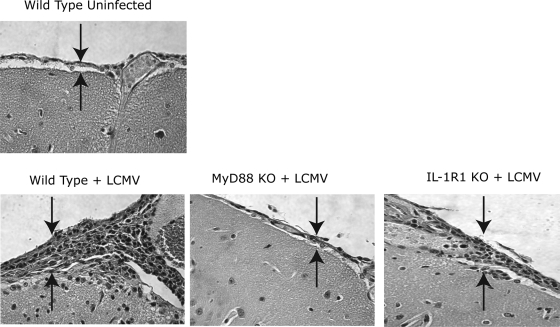
FIG. 2.
LCMV-induced brain histological change is MyD88 dependent but IL-1R1 or IL-18R independent. Mice were depleted of CD8+ T cells with monoclonal antibody 2.43 and were intracranially infected with LCMV Armstrong as described in Materials and Methods. Two mice from each of WT, MyD88 KO, and IL-1R1 KO groups were sacrificed on day 12 postinfection; brains were sectioned and stained with hematoxylin and eosin. WT mouse brain injected intracranially with PBS was a negative control. The severity of the meningitis was assessed based on the accumulation of the inflammatory cells in the meninges of the brains (two arrows in each image point to the thickness of the meninges, representing the amount of the accumulated inflammatory cells). Representative sections of cerebral cortex, with a focus on meninges/meningitis, are shown. The relative severity of the meningitis was as follows: uninfected WT mice (−), LCMV-infected WT mice (++), IL-1R1 KO mice (++), and MyD88 KO mice (+).
LCMV Armstrong is usually cleared within 2 weeks postinfection in WT mice (28, 31, 33), and depletion of CD8+ T cells leads to persistent LCMV infection (20, 47). We have measured the virus titers in mice depleted of CD8 (see Fig. 4F). Consistent with previous studies (25), our CD8-depleted WT mice were unable to clear LCMV Armstrong infection for up to 30 days postinfection. MyD88 KO CD8-depleted mice had even higher levels of virus.
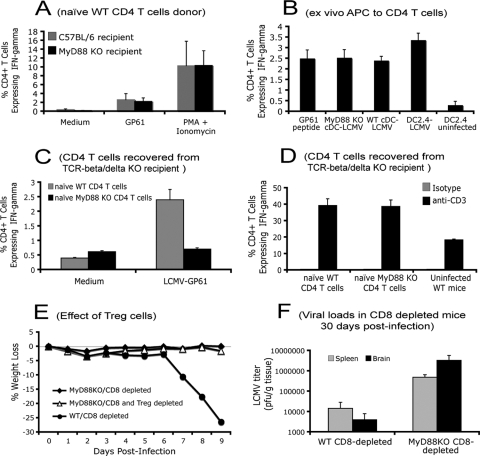
FIG. 4.
MyD88 signaling pathway intrinsically regulates the activation of naive CD4+ T cells. (A) A total of 5 × 105 purified naïve B6/Thy1.1 CD4+ T cells were adoptively transferred into CD8+ T-cell-depleted WT and MyD88 KO recipients (n = 3 for each group), followed by intravenous infection with LCMV. At day 10 postinfection, CD4+ T-cell responses (IFN-γ expression) in the spleens were analyzed by ICS after restimulation with LCMV CD4 epitope peptide GP61-80 or phorbol myristate acetate (PMA) and ionomycin. Cells were gated on CD4+ Thy1.1+ T cells (donor origin for both MyD88 KO and WT recipients). (B) LCMV-infected CD11c+ cDCs or DC2.4 cells were cocultured with purified LCMV-immune (day 10 postinfection) CD4+ T cells. The expression of IFN-γ was analyzed using ICS. (C and D) A total of 5 × 106 purified naïve WT or MyD88 KO CD4+ T cells were adoptively transferred into TCR-β/δ KO mice, followed by intracranial LCMV infection. At day 20 postinfection, the function of CD4+ T cells in spleen was examined by restimulating CD4+ T cells with LCMV GP61-80 peptide for 5 h (C) or with immobilized anti-CD3 antibody for 72 h (D). The expression of IFN-γ was measured by intracellular IFN-γ staining. Results shown are the average from three mice per group. (E) Both MyD88 KO and WT mice were depleted of CD8+ T cells with monoclonal antibody 2.43 as described above. CD4+ CD25+ regulatory Treg cells in MyD88 KO mice were depleted with PC61 antibody or an equivalent amount of a rat IgG isotype antibody. Mice were intracranially infected with LCMV Armstrong as described above. Body weight loss was compared for up to 9 days postinfection. (F) CD8-depleted mice failed to clear LCMV Armstrong infection. Mice were depleted of CD8 T cells as described in Material and Methods and intracranially infected with LCMV Armstrong. At day 30 postinfection, virus titers in spleens and brains were determined. Results shown are the average from three to five mice per group.
CD4+ T-cell activation in response to intracranial LCMV infection is MyD88 dependent but IL-1R1-/IL-18R independent.
It has been demonstrated that the intracranially LCMV-induced wasting disease is mediated by LCMV-specific CD4+ T cells that infiltrate the central nervous system (7, 14, 21). LCMV-specific CD4+ T cells express both IFN-γ and TNF-α (17, 36), which we used as functional indicators to assess the CD4+ T-cell response. To determine whether the functionality of CD4+ T cells correlates with the clinical symptoms (Fig. 1A and B) and the pathological changes in the brain (Fig. 2), WT, MyD88 KO, IL-1R1 KO, and IL-18R KO mice were depleted of CD8+ T cells and infected intracranially with LCMV. The state of CD4+ T-cell activation in the brain as well as in the spleen and peripheral blood was determined by intracellular staining of IFN-γ and FACS analysis after restimulation in vitro with the LCMV-specific dominant CD4 epitope peptide, GP61-80 (5, 46). In MyD88 KO mice, the LCMV-specific CD4+ T-cell IFN-γ response in all the tissues tested was severely impaired compared to WT mice (Fig. 3A to D). In contrast, LCMV infection induced comparable CD4+ T-cell IFN-γ and TNF-α responses in WT, IL-1R1 KO, and IL-18R KO mice (Fig. 3A to D and data not shown). Taken together, these results correlated with the clinical symptoms and demonstrated that an IL-1R/IL-18R-independent but MyD88-dependent signaling pathway is essential for activation of the CD4+ T cells in response to LCMV infection.
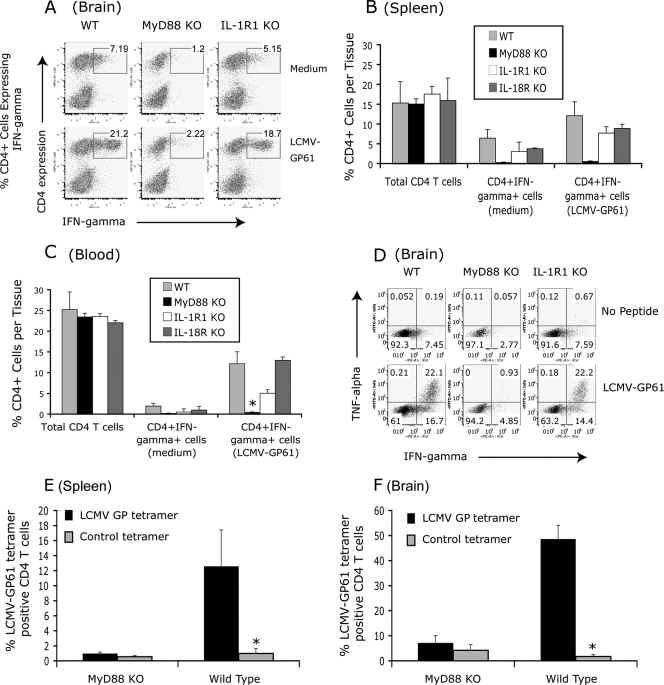
FIG. 3.
LCMV-induced CD4+ T-cell activation is MyD88 dependent but IL-1R1 and IL-18R independent. WT (n = 9), MyD88 KO (n = 9), IL-1R1 KO (n = 6), and IL-18R KO (n = 5) mice were intracranially infected with LCMV as described in Materials and Methods. Between days 13 to 15 postinfection, CD4+ T-cell responses in the brain, spleen, and peripheral blood lymphocytes were examined by restimulation in vitro with LCMV CD4 T-cell epitope peptide GP61-80 and intracellular staining for IFN-γ (A to C) or TNF-α (D). The average percentages of the total CD4+ T cells and CD4+ T cells expressing IFN-γ (CD4+ IFN-γ+ T cells) in brain, spleen, and peripheral blood lymphocytes are shown in panels A to C. Results are representative of at least four experiments for WT and MyD88 KO mice and two experiments for IL-1R1 and IL-18R KO mice. (E and F) WT (n = 3) and MyD88 KO mice (n = 3) were intracranially infected with LCMV. At day 9 postinfection, spleen and brain were collected. LCMV-specific CD4 T cells in spleen and brain were directly visualized using I-Ab-restricted CD4 tetramer complexed with LCMV GP66-77 peptide or a control peptide. Results shown are the average from three mice per group. *, P < 0.05.
To determine if the MyD88 molecule is involved in the development of LCMV-specific CD4+ T-cell responses, LCMV-specific CD4+ T cells were directly visualized using LCMV-specific MHC class II-restricted tetramer reagent (NIH Tetramer Core Facility). LCMV infection in WT mice induced a robust CD4+ T-cell response (Fig. 3E and F). Surprisingly, LCMV infection in MyD88 KO mice did not induce LCMV-specific CD4+ T-cell responses. Therefore, these results demonstrated that the MyD88 protein is essential for the development of LCMV-specific CD4+ T-cell responses.
MyD88 KO mice have normal APC function in response to LCMV infection.
After being engaged by antigen-presenting cells (APCs), naïve CD4+ T cells undergo clonal expansion and then differentiate into functional subsets of either Th1 or Th2 cells (15, 42). Several studies have demonstrated that the MyD88 adaptor protein is involved in the activation of APCs (15). To determine how MyD88 is involved in the regulation of CD4+ T-cell responses to LCMV, the following experiments were conducted. First, we wanted to determine whether the impaired CD4+ T-cell response in MyD88 KO mice to LCMV infection is due to the failure of the APC system. Purified naive B6/Thy1.1 CD4+ T cells were transferred into CD8+ T-cell-depleted MyD88 KO and C57BL/6 WT mice followed by intravenous infection with LCMV Armstrong (Fig. 4A). At day 8 postinfection, the function of transferred CD4+ T cells was determined by ICS. Thy1.1 was used as a marker for the transferred T cells. We found, surprisingly, that in response to LCMV infection, WT donor CD4+ (Thy1.1+) T cells produced IFN-γ regardless of the expression of MyD88 in the recipient APC system (Fig. 4A). These results suggested that MyD88 KO mice have a competent APC system.
To further examine if MyD88 expression in APCs could affect APC function in terms of activation of CD4+ T cells, we directly compared the antigen presentation capacity of both WT and MyD88 KO APCs ex vivo. To prepare APCs, cDCs were isolated from both WT and MyD88 KO mice. cDCs were infected with the WE strain of LCMV, and at 48 h postinfection, LCMV-infected cDCs were cocultured for 5 h with WT CD4+ T cells (isolated from day 10 LCMV-infected WT mice). The LCMV-infected DC2.4 DC line was included as a positive control and prepared as described previously (32, 50). The expression of IFN-γ on the cocultured CD4+ T cells was monitored by ICS. Interestingly, both WT and MyD88 KO cDCs were able to present LCMV-specific CD4 epitopes to activate WT CD4+ T cells (Fig. 4B). The same experiment was also conducted with cDCs infected in vivo and isolated from day 2.5 LCMV-infected WT and MyD88 KO mice. Similarly, MyD88 KO cDCs were capable of activating WT CD4+ T cells compared to their WT control cDCs (data not shown). Collectively, using both in vivo and ex vivo methods, we demonstrated that APCs in MyD88 KO mice are comparable to APCs from WT mice in their ability to activate WT CD4+ T cells. Thus, these studies demonstrated that the absence of MyD88 signaling in APCs does not account for the impaired CD4+ T-cell function in MyD88 KO mice in response to LCMV infection. These results suggested that MyD88 might be intrinsically involved in CD4+ T-cell functional maturation.
Expression of MyD88 in CD4+ T cells is critical to their functional maturation and to inducing weight loss.
To determine how MyD88 is involved in the regulation of CD4+ T cells in response to LCMV, naïve CD4+ T cells were purified from both MyD88 KO and WT mice. A total of 5 × 106 purified naïve CD4+ T cells from both strains were adoptively transferred into TCR-β/δ KO recipients (deficient of both CD4+ and CD8+ T cells) via tail vein injection (Fig. 4C). The following day, TCR-β/δ KO recipients were challenged by intracranial LCMV infection. Consistent with results from the LCMV-infected WT mice (Fig. 1A and B), CD4+ T cells from WT naïve mice mediated weight loss (Fig. 1C). The function of transferred CD4+ T cells was examined by ICS. WT CD4+ T cells had normal function; i.e., they responded to stimulation with LCMV GP61-80 peptide by producing IFN-γ (Fig. 4C). In contrast, MyD88 KO CD4+ T cells failed to produce IFN-γ in response to LCMV GP61-80 peptide restimulation. Both transferred WT and MyD88 KO CD4+ T cells responded to anti-CD3 stimulation (Fig. 4D), suggesting that MyD88 KO CD4+ T cells were capable of IFN-γ expression when their TCR was cross-linked ex vivo although these cells failed to respond to LCMV infection in vivo. Thus, we conclude that the incompetent CD4+ T-cell response in MyD88 KO mice to LCMV is not due to the defective antigen presentation capacity of MyD88 KO APCs. Their defect is the inability to produce IFN-γ and TNF-α in response to LCMV.
In addition, it has been reported that CD4+ CD25+ Treg cells play a role in the regulation of MyD88-dependent CD4+ T-cell activation to model antigens (38, 39). To further determine whether Treg cells are responsible for the defective CD4+ T-cell response in MyD88 KO mice following acute LCMV infection, Treg cells in MyD88 KO mice were depleted before LCMV infection with the commonly used monoclonal antibody PC61 (12, 38, 39, 43). Treg-cell depletion was confirmed by flow cytometry. The percentage of CD25+ CD4+ T cells significantly decreased compared to their counterpart in the isotype control antibody-treated MyD88 KO mice (0.76% ± 0.03% versus 8.19% ± 1.27%, respectively). Depletion of CD4+ CD25+ Treg cells did not induce the wasting disease in MyD88 KO mice following intracranial LCMV infection (Fig. 4E), suggesting that Treg cells do not play a decisive role in the defective CD4+ T-cell response in MyD88 KO mice.
Together, these observations suggested that the MyD88 signaling pathway is intrinsically involved in the functional maturation of CD4+ T cells and independent of Treg cells.
Expression of MyD88 in MyD88 KO CD4+ T cells restores CD4+ T-cell function.
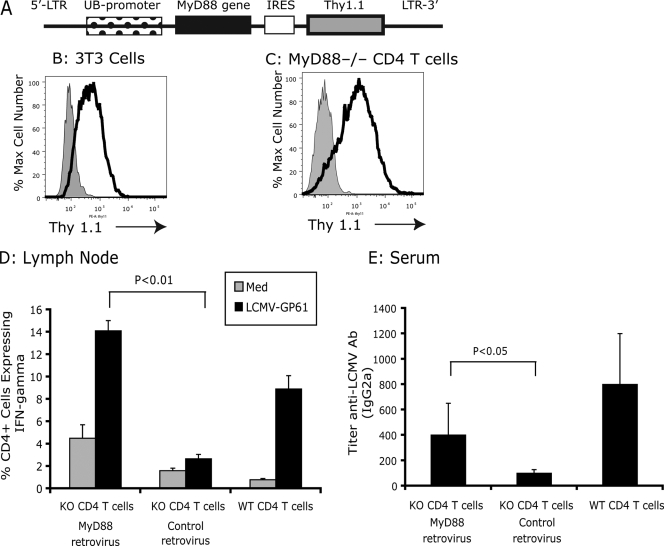
Having shown that MyD88 could be intrinsically involved in the responsiveness of CD4+ T cells, we next determined if reconstitution of MyD88 KO CD4+ T cells with the MyD88 gene could restore function. We constructed an MyD88-expressing lentivirus, pUb-MyD88/Thy1.1 (Fig. 5A). MyD88 and control lentivirus efficiently transduced both NIH 3T3 cells and MyD88 KO CD4+ T cells (Fig. 5B and C). When MyD88 KO CD4+ T cells were transduced with the MyD88-expressing lentivirus to rescue MyD88 activity, the CD4+ T cells were able to respond to LCMV infection by expression of IFN-γ (Fig. 5D). In contrast, when MyD88 KO CD4+ T cells were transduced with a control lentivirus, MyD88 KO CD4+ T cells remained unable to respond to LCMV infection (Fig. 5D). Furthermore, when TCR-β/δ KO recipients received MyD88-reconstituted (lentiviral rescue) MyD88 KO CD4+ T cells, they produced significantly more IgG2a anti-LCMV antibody (Fig. 5E) than TCR-β/δ KO recipients that received control lentivirus-transduced KO CD4+ T cells in response to LCMV. Therefore, lentiviral gene transfer of mouse MyD88 into MyD88 KO CD4+ T cells was sufficient to restore function for both IFN-γ production and T helper function for the antibody response, demonstrating the critical role of MyD88 in T cells in the response to a viral infection.
FIG. 5.
Reconstituted MyD88 KO CD4+ T cells function with MyD88-expressing lentivirus. (A) Diagram of mouse MyD88-expressing lentivirus. (B) Viral titer was determined by transduction of NIH 3T3 cells for 48 h and flow cytometry analysis of the expression of Thy1.1. Cells were infected with lentivirus and stained with anti-Thy1.1 antibody (black line) or with anti-Thy1.1 isotype control antibody (gray fill). (C) Purified MyD88 CD4+ T cells were transduced with lentivirus for 48 h, and the expression of Thy1.1 was examined. Cells were infected with lentivirus and were doubly stained with anti-Thy1.1 and anti-CD4 antibodies (black line) or with anti-Thy1.1 isotype control antibody and anti-CD4 antibodies (gray fill). (D) A total of 5 × 106 of lentivirus-transduced MyD88 KO CD4 T cells were adoptively transferred into TCR-β/δ-deficient mice, and recipients were challenged with LCMV Armstrong (intravenously). At day 9 postinfection, mesenteric lymph nodes were collected, and the function of CD4+ T cells was analyzed by in vitro restimulation with either medium or LCMV-specific CD4 epitope peptide GP61-80. The expression of IFN-γ was measured by intracellular IFN-γ staining. (E) Serum was collected at day 9 postinfection, and the levels of LCMV-specific antibody were measured by using an enzyme-linked immunosorbent assay. Serum collected from LCMV-infected (day 9) WT mice was used as the control.
DISCUSSION
A number of recent studies have highlighted the importance of MyD88 in the regulation of the inflammatory responses in innate immune cells, but its role in the regulation of the adaptive immune response and immunopathology is poorly defined (6, 23, 24). How do MyD88-dependent signals affect T-cell responses? The current paradigm for explaining the regulation of T cells by MyD88 is that TLR-dependent activation of MyD88 in DCs leads to their maturation as APCs with the secretion of immune-modulated chemokines and cytokines and upregulation of the MHC and other costimulatory molecules and, in turn, plays a central role in stimulation of the T-cell responses (15, 16). Our present study reveals an unappreciated role of MyD88 signaling in the regulation of the CD4+ T-cell response to virus infection. We demonstrate that the MyD88 molecule expressed in the CD4+ T cells themselves controls the development of virus-specific CD4+ T cells following challenge with a natural murine viral pathogen, LCMV.
The MyD88 adaptor protein is not only essential for TLR signaling (with the exception of TLR3) but is also a critical adaptor protein in IL-1R- and IL-18R-dependent signaling (1, 2, 44). By directly comparing the impact of the MyD88-, IL-1R1-, and IL-18R-mediated signaling pathways on the activation of a virus-specific CD4+ T-cell response, we demonstrated that the defect is likely TLR specific since the IL-1R1- and IL-18R-dependent signaling pathways do not play a major role in the activation and differentiation of CD4+ T cells to LCMV infection. A recent publication also indicated that the IL-18-dependent signaling pathway is not involved in the LCMV-specific CD4+ T-cell response (30). Thus, our study outlines the importance of the MyD88 signaling pathway in the regulation of CD4+ T-cell responses to a natural murine viral pathogen. Our studies are consistent with a recent publication by Larosa et al. (26), in which the authors demonstrated that the expression of MyD88 in CD4+ T cells is essential for the production of IFN-γ in CD4+ T cells and for the protection of mice from Toxoplasma gondii infection.
Although MyD88 is critically involved in the induction of chemokines and cytokines from APCs or other cells (2, 15, 37, 44, 49), our study has demonstrated that naïve WT CD4+ T cells have comparable function when transferred into MyD88 KO recipients compared to those in WT recipients (Fig. 4A). Furthermore, our ex vivo study has shown that MyD88 KO APCs (cDCs) are comparable to WT APCs in their ability to present LCMV-specific CD4 epitopes to activate LCMV-immune WT CD4+ T cells. Collectively, these results suggest that the defective CD4+ responses to LCMV infection in MyD88 KO mice are not due to a failure of the APC system (Fig. 4A to C).
Our adoptive transfer experiments with purified CD4+ T cells indicate that the defective CD4+ T-cell response is related to an intrinsic defect in CD4+ T cells. When WT or MyD88 KO naïve CD4+ T cells were transferred into TCR-β/δ KO recipients, only WT CD4+ T cells had the capacity to mediate weight loss and to express IFN-γ (Fig. 1C, 4D). Furthermore, TCR-β/δ KO recipients that received WT CD4+ T cells produced significantly more IgG2a isotype anti-LCMV antibody, a characteristic of a Th1-type immune response, in comparison to TCR-β/δ KO recipients receiving MyD88 KO CD4+ T cells. Importantly, we provided evidence that the function of MyD88 KO CD4+ T cells can be restored by transducing MyD88 KO CD4+ T cells with an MyD88-expressing lentivirus (Fig. 5). Therefore, our studies demonstrate that MyD88 in T cells is responsible for activation of CD4+ T cells in response to LCMV infection.
How MyD88 in CD4+ T cells regulates virus-specific CD4+ T-cell responses is currently not clear. TLRs are expressed in T cells, including conventional CD4+ T cells (CD4+ αβ T cells), at least at the mRNA level (19, 22). Certain types of TLR ligands can directly activate T cells in the absence of APCs, suggesting that TLRs, including TLR2, play a role in the activation of T cells (11, 19). A recent publication has provided direct evidence that MyD88 is responsible for CpG DNA-induced direct activation of naïve CD4+ T cells and that phosphatidylinositol 3-kinase is involved in this MyD88-dependent activation pathway (11). While our observations have some similarities to these studies, there are some fundamental differences between our model and other models that make our findings regarding the TLR adaptor protein, MyD88, and its role in the regulation of the virus-specific CD4+ T-cell responses distinct. Previous studies have used nonreplicating antigens or TLR ligands to evaluate the activation of CD4+ T cells. In our study, we used a natural murine viral pathogen, LCMV. After infection of the target cells with LCMV, endogenously synthesized LCMV GP (which contains the dominant CD4+ T-cell epitope GP61-80) needs to be processed by the APCs to form a complex with MHC class II I-Ab molecules and to be presented on the APC surface in order to stimulate a CD4+ T-cell response (35). Moreover, virus-induced activation of T cells involves the dynamic interaction of APCs and T cells in the immunological synapse, which plays a major role in the activation of CD4+ T cells. In contrast, other TLR ligands, including tripalmitoyl-Cys-Ser-Lys-Lys-Lys-Lys (TLR1/TLR2 ligand) and CpG (TLR9 ligand), activate CD4+ T cells in a ligand receptor-specific but antigen-unspecific interaction manner (11, 19). Thus, viruses like LCMV could use a distinctive mechanism to activate MyD88-dependent CD4+ T-cell activation.
The role of the Treg cells in a virus-induced immune response has recently attracted increased attention (38, 39). It has been demonstrated that Treg cells suppress both expansion and functional maturation of the effector T-cell response, and it has been reported that Treg cells repress both the primary and secondary virus-induced immune responses (45). However, the role of Treg cells in the LCMV-induced immune responses is uncertain (43). Pasare et al. showed that depletion of Treg cells with anti-CD25-depleting monoclonal antibody (clone PC61) restored CD4+ T-cell responses to ovalbumin in MyD88 KO mice, suggesting that Treg cells contribute to the defective CD4+ T-cell response to model antigens in MyD88 KO mice (39). In the present study, depletion of Treg cells had little effect on LCMV-induced CD4+ T-cell responses and weight loss in MyD88 KO mice (Fig. 4E). Thus, our study suggests that the impaired CD4+ T-cell responses in MyD88 KO mice in response to LCMV infection are unlikely to be due to the action of Treg cells.
In summary, these studies reveal for the first time that the expression of MyD88 in CD4+ T cells is essential for the activation of CD4+ T cells in response to a natural murine viral pathogen. Our studies and studies of others demonstrated that the effects of MyD88 on T-cell responses are independent of APCs and Treg cells (11, 26). Furthermore, we demonstrated that MyD88 expression in CD4+ T cells is required for the production of both IFN-γ and TNF-α as well as helper cell activity for antibody production in response to virus.
Acknowledgments
We are grateful to S. Akira (Osaka University, Japan) for generously providing a breeding pair of MyD88 knockout mice. We thank Raymond M. Welsh and Rafi Ahmed for critical reading of the manuscript, Leslie Berg for helpful discussions, and Laurence Turka and Zhibin Chen for kindly providing plasmids and protocols. We thank Matthew Murawski for careful reading of the manuscript and helpful discussions and Junko Kato for excellent secretarial assistance.
This work was supported by NIAID Regional Center of Excellence Grant AI 057159, NIH grants R01 AI 49309, P01 AI 0577484, and JDRF 24-2008-950 (R.W.F.) and NIH grant AI 51415 (E.A.K.-J.).
We declare that we have no competing financial interests.
Footnotes
Published ahead of print on 3 December 2008.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9143-150. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S. 2003. Mammalian Toll-like receptors. Curr. Opin. Immunol. 155-11. [DOI] [PubMed] [Google Scholar]
- 3.Allan, J. E., J. E. Dixon, and P. C. Doherty. 1987. Nature of the inflammatory process in the central nervous system of mice infected with lymphocytic choriomeningitis virus. Curr. Top. Microbiol. Immunol. 134131-143. [DOI] [PubMed] [Google Scholar]
- 4.Battegay, M., S. Cooper, A. Althage, J. Banziger, H. Hengartner, and R. M. Zinkernagel. 1991. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J. Virol. Methods 33191-198. [DOI] [PubMed] [Google Scholar]
- 5.Brooks, D. G., L. Teyton, M. B. Oldstone, and D. B. McGavern. 2005. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J. Virol. 7910514-10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coban, C., K. J. Ishii, S. Uematsu, N. Arisue, S. Sato, M. Yamamoto, T. Kawai, O. Takeuchi, H. Hisaeda, T. Horii, and S. Akira. 2007. Pathological role of Toll-like receptor signaling in cerebral malaria. Int. Immunol. 1967-79. [DOI] [PubMed] [Google Scholar]
- 7.Doherty, P. C., S. Hou, and P. J. Southern. 1993. Lymphocytic choriomeningitis virus induces a chronic wasting disease in mice lacking class I major histocompatibility complex glycoproteins. J. Neuroimmunol. 4611-17. [DOI] [PubMed] [Google Scholar]
- 8.Doherty, P. C., and R. M. Zinkernagel. 1974. T-cell-mediated immunopathology in viral infections. Transplant Rev. 1989-120. [DOI] [PubMed] [Google Scholar]
- 9.Dull, T., R. Zufferey, M. Kelly, R. J. Mandel, M. Nguyen, D. Trono, and L. Naldini. 1998. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 728463-8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung-Leung, W. P., T. M. Kundig, R. M. Zinkernagel, and T. W. Mak. 1991. Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J. Exp. Med. 1741425-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gelman, A. E., D. F. LaRosa, J. Zhang, P. T. Walsh, Y. Choi, J. O. Sunyer, and L. A. Turka. 2006. The adaptor molecule MyD88 activates PI-3 kinase signaling in CD4+ T cells and enables CpG oligodeoxynucleotide-mediated costimulation. Immunity 25783-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haeryfar, S. M., R. J. DiPaolo, D. C. Tscharke, J. R. Bennink, and J. W. Yewdell. 2005. Regulatory T cells suppress CD8+ T cell responses induced by direct priming and cross-priming and moderate immunodominance disparities. J. Immunol. 1743344-3351. [DOI] [PubMed] [Google Scholar]
- 13.Hahm, B., M. J. Trifilo, E. I. Zuniga, and M. B. Oldstone. 2005. Viruses evade the immune system through type I interferon-mediated STAT2-dependent, but STAT1-independent, signaling. Immunity 22247-257. [DOI] [PubMed] [Google Scholar]
- 14.Hildeman, D., D. Yanez, K. Pederson, T. Havighurst, and D. Muller. 1997. Vaccination against persistent viral infection exacerbates CD4+ T-cell-mediated immunopathological disease. J. Virol. 719672-9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5987-995. [DOI] [PubMed] [Google Scholar]
- 16.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 17.Jellison, E. R., S. K. Kim, and R. M. Welsh. 2005. Cutting edge: MHC class II-restricted killing in vivo during viral infection. J. Immunol. 174614-618. [DOI] [PubMed] [Google Scholar]
- 18.Jung, A., H. Kato, Y. Kumagai, H. Kumar, T. Kawai, O. Takeuchi, and S. Akira. 2008. Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces a cytotoxic T-cell response via MyD88. J. Virol. 82196-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabelitz, D. 2007. Expression and function of Toll-like receptors in T lymphocytes. Curr. Opin. Immunol. 1939-45. [DOI] [PubMed] [Google Scholar]
- 20.Kagi, D., B. Ledermann, K. Burki, R. M. Zinkernagel, and H. Hengartner. 1995. Lymphocyte-mediated cytotoxicity in vitro and in vivo: mechanisms and significance. Immunol. Rev. 14695-115. [DOI] [PubMed] [Google Scholar]
- 21.Kamperschroer, C., and D. G. Quinn. 2002. The role of proinflammatory cytokines in wasting disease during lymphocytic choriomeningitis virus infection. J. Immunol. 169340-349. [DOI] [PubMed] [Google Scholar]
- 22.Komai-Koma, M., L. Jones, G. S. Ogg, D. Xu, and F. Y. Liew. 2004. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc. Natl. Acad. Sci. USA 1013029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurt-Jones, E. A., M. Chan, S. Zhou, J. Wang, G. Reed, R. Bronson, M. M. Arnold, D. M. Knipe, and R. W. Finberg. 2004. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. USA 1011315-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang, K. S., P. Georgiev, M. Recher, A. A. Navarini, A. Bergthaler, M. Heikenwalder, N. L. Harris, T. Junt, B. Odermatt, P. A. Clavien, H. Pircher, S. Akira, H. Hengartner, and R. M. Zinkernagel. 2006. Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling. J. Clin. Investig. 1162456-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang, K. S., A. N. Hegazy, P. A. Lang, B. Eschli, M. Lohning, H. Hengartner, R. M. Zinkernagel, and M. Recher. 2007. “Negative vaccination” by specific CD4 T cell tolerisation enhances virus-specific protective antibody responses. PLoS ONE 2e1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larosa, D. F., J. S. Stumhofer, A. E. Gelman, A. H. Rahman, D. K. Taylor, C. A. Hunter, and L. A. Turka. 2008. T cell expression of MyD88 is required for resistance to Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 1053855-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1135-145. [DOI] [PubMed] [Google Scholar]
- 28.Mueller, S. N., M. Matloubian, D. M. Clemens, A. H. Sharpe, G. J. Freeman, S. Gangappa, C. P. Larsen, and R. Ahmed. 2007. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc. Natl. Acad. Sci. USA 10415430-15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller, D., B. H. Koller, J. L. Whitton, K. E. LaPan, K. K. Brigman, and J. A. Frelinger. 1992. LCMV-specific, class II-restricted cytotoxic T cells in beta 2-microglobulin-deficient mice. Science 2551576-1578. [DOI] [PubMed] [Google Scholar]
- 30.Nembrini, C., B. Abel, M. Kopf, and B. J. Marsland. 2006. Strong TCR signaling, TLR ligands, and cytokine redundancies ensure robust development of type 1 effector T cells. J. Immunol. 1767180-7188. [DOI] [PubMed] [Google Scholar]
- 31.Oldstone, M. B. 2002. Biology and pathogenesis of lymphocytic choriomeningitis virus infection. Curr. Top. Microbiol. Immunol. 26383-117. [DOI] [PubMed] [Google Scholar]
- 32.Ou, R., M. Zhang, L. Huang, and D. Moskophidis. 2008. Control of virus-specific CD8 T cell exhaustion and immune-mediated pathology by E3 ubiquitin ligase Cbl-b during chronic viral infection. J. Virol. 823353-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ou, R., S. Zhou, L. Huang, and D. Moskophidis. 2001. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J. Virol. 758407-8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oxenius, A., M. F. Bachmann, P. G. Ashton-Rickardt, S. Tonegawa, R. M. Zinkernagel, and H. Hengartner. 1995. Presentation of endogenous viral proteins in association with major histocompatibility complex class II: on the role of intracellular compartmentalization, invariant chain and the TAP transporter system. Eur. J. Immunol. 253402-3411. [DOI] [PubMed] [Google Scholar]
- 35.Oxenius, A., M. F. Bachmann, D. Mathis, C. Benoist, R. M. Zinkernagel, and H. Hengartner. 1997. Functional in vivo MHC class II loading by endogenously synthesized glycoprotein during viral infection. J. Immunol. 1585717-5726. [PubMed] [Google Scholar]
- 36.Oxenius, A., R. M. Zinkernagel, and H. Hengartner. 1998. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity 9449-457. [DOI] [PubMed] [Google Scholar]
- 37.Palliser, D., H. Ploegh, and M. Boes. 2004. Myeloid differentiation factor 88 is required for cross-priming in vivo. J. Immunol. 1723415-3421. [DOI] [PubMed] [Google Scholar]
- 38.Pasare, C., and R. Medzhitov. 2003. Toll pathway-dependent blockade of CD4+ CD25+ T cell-mediated suppression by dendritic cells. Science 2991033-1036. [DOI] [PubMed] [Google Scholar]
- 39.Pasare, C., and R. Medzhitov. 2004. Toll-dependent control mechanisms of CD4 T cell activation. Immunity 21733-741. [DOI] [PubMed] [Google Scholar]
- 40.Rahman, A. H., W. Cui, D. F. Larosa, D. K. Taylor, J. Zhang, D. R. Goldstein, E. J. Wherry, S. M. Kaech, and L. A. Turka. 2008. MyD88 plays a critical T cell-intrinsic role in supporting CD8 T cell expansion during acute lymphocytic choriomeningitis virus infection. J. Immunol. 1813804-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarmiento, M., A. L. Glasebrook, and F. W. Fitch. 1980. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J. Immunol. 1252665-2672. [PubMed] [Google Scholar]
- 42.Seder, R. A., and R. Ahmed. 2003. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 4835-842. [DOI] [PubMed] [Google Scholar]
- 43.Suvas, S., and B. T. Rouse. 2006. Treg control of antimicrobial T cell responses. Curr. Opin. Immunol. 18344-348. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi, O., and S. Akira. 2002. MyD88 as a bottleneck in Toll/IL-1 signaling. Curr. Top. Microbiol. Immunol. 270155-167. [DOI] [PubMed] [Google Scholar]
- 45.Toka, F. N., S. Suvas, and B. T. Rouse. 2004. CD4+ CD25+ T cells regulate vaccine-generated primary and memory CD8+ T-cell responses against herpes simplex virus type 1. J. Virol. 7813082-13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varga, S. M., and R. M. Welsh. 2000. High frequency of virus-specific interleukin-2-producing CD4+ T cells and Th1 dominance during lymphocytic choriomeningitis virus infection. J. Virol. 744429-4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh, C. M., M. Matloubian, C. C. Liu, R. Ueda, C. G. Kurahara, J. L. Christensen, M. T. Huang, J. D. Young, R. Ahmed, and W. R. Clark. 1994. Immune function in mice lacking the perforin gene. Proc. Natl. Acad. Sci. USA 9110854-10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams, M. A., E. V. Ravkov, and M. J. Bevan. 2008. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity 28533-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou, S., E. A. Kurt-Jones, L. Mandell, A. Cerny, M. Chan, D. T. Golenbock, and R. W. Finberg. 2005. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur. J. Immunol. 35822-830. [DOI] [PubMed] [Google Scholar]
- 50.Zhou, S., R. Ou, L. Huang, G. E. Price, and D. Moskophidis. 2004. Differential tissue-specific regulation of antiviral CD8+ T-cell immune responses during chronic viral infection. J. Virol. 783578-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zinkernagel, R. M., C. J. Pfau, H. Hengartner, and A. Althage. 1985. Susceptibility to murine lymphocytic choriomeningitis maps to class I MHC genes—a model for MHC/disease associations. Nature 316814-817. [DOI] [PubMed] [Google Scholar]