Abstract
The herpes simplex virus type 1 (HSV-1) protein ICP27 has been implicated in a variety of functions important for viral replication including host shutoff, viral gene expression, activation of mitogen-activated protein kinases p38 and Jun N-terminal protein kinase (JNK), and apoptosis inhibition. In the present study we sought to examine the functions of ICP27 in the absence of viral infection by creating stable HeLa cell lines that inducibly express ICP27. Here, we characterize two such cell lines and show that ICP27 expression is associated with a cellular growth defect. The observed defect is caused at least in part by the induction of apoptosis as indicated by caspase-3 activation, annexin V staining, and characteristic changes in cellular morphology. In an effort to identify the function of ICP27 responsible for inducing apoptosis, we show that ICP27 expression is sufficient to activate p38 signaling to a level that is similar to that observed during wild-type HSV-1 infection. However, ICP27 expression alone is unable to lead to a strong activation of JNK signaling. Using chemical inhibitors, we show that the ICP27-mediated activation of p38 signaling is responsible for the observed induction of apoptosis in the induced cell lines. Our findings suggest that during viral infection, ICP27 activates p38 and JNK signaling pathways via two distinct mechanisms. ICP27 directly activates p38 signaling, leading to stimulation of the host cell apoptotic pathways. In contrast, robust activation of JNK signaling by ICP27 requires one or more delayed early or late viral gene products and may be associated with the inhibition of apoptosis.
Herpes simplex virus type 1 (HSV-1) is a common human pathogen that can replicate lytically in a variety of cell types during acute infection and can establish latency in peripheral neurons. During acute infection, gene expression occurs in a tightly regulated temporal cascade separated into immediate-early (IE), delayed-early (DE), and late (L) gene expression. The viral tegument protein VP16 transactivates the expression of the five IE genes. Four of the viral IE proteins, infected cell protein 0 (ICP0), ICP4, ICP22, and ICP27, have been implicated in controlling further viral gene expression and affecting normal host cell function.
ICP27 is an essential multifunctional viral regulatory protein that has been shown to affect both viral and cellular gene expression (44). It is required for efficient expression of a subset of DE and L genes (34, 41, 53). Previous research has demonstrated that ICP27 can alter both the transcription of viral genes (29) and translation of their mRNAs (14, 32). Additionally, ICP27 has been shown to bind RNA (37) and shuttle between the cytoplasm and nucleus (36, 38, 45, 50). These activities are consistent with data that indicate that ICP27 serves as an mRNA export factor for intronless viral transcripts (10, 11, 31). Furthermore, ICP27 inhibits normal cellular gene expression by inhibiting mRNA splicing as well as the transcription of some host genes (21, 22, 46, 51). ICP27 expression can also lead to increased expression of some cellular mRNAs due to stabilization of transcripts containing AU-rich elements (7, 12).
Several studies have shown that ICP27 is also involved in activating p38 and Jun N-terminal protein kinase (JNK) mitogen-activated protein kinase (MAPK) signaling pathways during HSV-1 infection. Both of these MAPK pathways are known as stress-activated protein kinases (SAPKs) due their involvement in controlling cellular responses to various types of stress. Initial studies showed that p38 and JNK signaling pathways become activated during the initial stages of infection, as early as 3 h postinfection (hpi), with a peak of activation at 6 to 8 hpi (56). The activation of SAPK signaling pathways appears to be important for normal viral replication since the presence of pharmacological inhibitors of p38 and JNK signaling leads to a significant drop in viral yields (30, 35). The mechanism behind the activation of the SAPK signaling pathways by HSV-1 has yet to be fully understood. It is known that the activation occurs downstream of Ras (35). The timing of the activation pointed toward an IE gene product as being responsible for initial induction since IE proteins are present at high levels at 3 hpi. Consistent with this, studies showed that ICP27 is required in the context of viral infection for activation of the SAPK pathways (12, 23, 24). The N-terminal end of the ICP27 protein, which plays a functional role in nuclear export, is required for SAPK activation (12, 23, 33). Additionally, it was shown that ICP27-mediated JNK signaling leads to the activation of NF-κB during viral infection (24). Interestingly, activation of NF-κB during HSV-1 infections has been associated with the inhibition of apoptosis, which is activated by very early events in the infection (18). ICP27 is also required during viral infection to prevent activation of apoptosis associated with p38-mediated destabilization of Bcl-2 (57).
MAPKs play diverse roles as regulators of normal cellular functions. In addition to p38 and JNK, extracellular signal-regulated kinase 1 (ERK1), ERK2, and ERK5 belong to this group of serine/threonine kinases. The SAPKs, p38 and JNK, are responsible for controlling cellular responses to environmental stress (e.g., UV irradiation, osmotic shock, and oxidative stress), cellular damage (e.g., DNA and RNA damage), and cytokine signaling (1, 2, 28, 42, 52). Signaling from these diverse sources then leads to the phosphorylation and activation of MAPK kinase kinases. MAPK kinase kinases then phosphorylate the MAPK kinases (MKKs) that are responsible for phosphorylating MAPKs, which in the case of p38 and JNK are MKK3/MKK6 and MKK4/MKK7, respectively. In addition to this classic kinase cascade of activation, p38 can also be autophosphorylated by interacting with TAB1 (17). SAPK signaling has been shown to play fundamental roles in a variety of cell functions, including apoptosis, oncogenesis, cell division, differentiation, immune activation, and inflammation (reviewed in references 43 and 52).
It is currently unclear whether the requirement for ICP27 in SAPK activation during viral infection is a direct effect of the protein or an indirect downstream effect dependent on other viral factors, which are themselves ICP27 dependent. In order to look at the direct effects of ICP27 on SAPK signaling and other host cell functions we sought to develop a system that will allow inducible expression of ICP27 in uninfected cells and in the absence of transient transfection or viral transduction, both of which could potentially activate SAPKs. For this we utilized a doxycycline-inducible promoter system in HeLa cells to express ICP27. Using this system, we demonstrate that ICP27 expression is sufficient to induce p38 signaling to levels similar to those seen during wild-type HSV-1 infection. Furthermore, we show that ICP27-induced p38 signaling inhibits normal cellular growth and leads to apoptosis. In contrast, ICP27 expression alone appears to be insufficient for the full activation of JNK signaling. Therefore, it appears that ICP27 activates the p38 and JNK signaling pathways by two distinct mechanisms.
MATERIALS AND METHODS
Cells.
HeLa Tet-On (HTO) cells were obtained from Clontech. HTO cells stably express a modified tetracycline (Tet) transactivator from Escherichia coli fused to the HSV-1 VP16 activation domain, which induces expression of genes controlled by the Tet operator in the presence of doxycycline (20). Vero cells (African green monkey kidney cells) were obtained from the American Type Culture Collection. V27 (41) cells are Vero cells that contain a stably transfected copy of the HSV-1 ICP27 gene. The ICP27 gene is not constitutively expressed by V27 cells but is induced by HSV-1 infection. HTO and Vero cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented to contain 10% and 5% heat-inactivated fetal bovine serum (FBS), respectively, 50 U of penicillin/ml, and 50 μg of streptomycin/ml. The medium for the V27 cells was the same as that for Vero cells except that it also contained 300 μg/ml G418. The construction of the HTO-H, HTO-L, TE12, and TE51 cell lines is described below. They were maintained in DMEM supplemented to contain 10% Tet-free fetal bovine serum, 50 U of penicillin/ml, 50 μg of streptomycin/ml, 400 μg/ml G418, and 100 μg/ml hygromycin B.
Stable cell line generation.
TE12 and TE51 are HeLa cell lines that conditionally express ICP27 via a doxycycline-regulated system (19, 20). They were made by stably transfecting HTO cells with plasmid pTE27. The pTE27 plasmid was created by cloning the ICP27 gene from pBH27 (40) into the pTRE-Tight vector purchased from Clontech. Briefly, pBH27 was cleaved at an AgeI restriction site 75 bp downstream of the ICP27 gene transcriptional start site and at the HindIII restriction site. The AgeI-generated DNA ends were blunted using the Klenow fragment of E. coli DNA polymerase. The ICP27 gene fragment was ligated into the multiple cloning site of the pTRE-Tight plasmid using the PvuII (blunted as above) and HindIII restriction sites. This created an ICP27 gene under the control of the minimal cytomegalovirus IE gene promoter and seven repeats of a modified Tet operator site. HTO cells were cotransfected with either pTE27 and the linear hygromycin selection marker (BD Biosciences) to generate the TE12 and TE51 cell lines or with pTRE-Tight-Luc (BD Biosciences) and the linear hygromycin selection marker (BD Biosciences) to generate the HTO-L cell line. Additionally, the selection marker alone was transfected in to create the HTO-H cell line. Transfections were performed using Lipofectamine 2000 (Invitrogen) at a 20:1 ratio (wt/wt) of plasmid to linear selection marker following the manufacturer's recommended protocol. HTO-H cells were isolated as a pooled population of hygromycin B-resistant cells, while clones of potential luciferase and ICP27 cell lines were selected in the presence of hygromycin B (200 μg/ml; BD Biosciences). Drug-resistant colonies were isolated using cloning cylinders and screened for the inducible expression of luciferase or functional ICP27 using immunoblotting assays, plaque assays, and immunofluorescence.
Viruses and infections.
The wild-type (WT) strain of HSV-1 used in these studies was KOS1.1 (27). Viral ICP4 (d120) (13) and ICP27 (d27-1) (41) deletion mutants have been described previously. Infections were carried out at a multiplicity of infection (MOI) of 10 or 1, as indicated, in phosphate-buffered saline containing 0.9 mM CaCl2, 0.5 mM MgCl2, 0.1% glucose, and 0.1% heat-inactivated newborn calf serum. Virus absorption was carried out for 1 h at 37°C, at which time the viral inoculum was replaced. The viral inoculum was replaced with 199 medium containing 2% heat-inactivated newborn calf serum, 50 U of penicillin/ml, and 50 μg of streptomycin/ml. In experiments examining SAPK activity, the inoculum was replaced with spent medium from duplicate uninfected wells. Following medium replacement, all infections were incubated at 37°C.
Virus plaque assays were performed using the same medium as for normal virus infections except that 1% heat-inactivated normal pooled human serum was added. Plaque assay mixtures were incubated at 37°C for 4 days to allow plaques to develop. Virus yield experiments used monolayers infected at an MOI of 1 that were treated at 2 hpi with an acid-glycine wash (pH 3.0) to reduce background due to virus particles that had not entered cells by this time (9). The infections were stopped after 24 h, and virus was harvested by the addition of 5 ml of sterile milk to each flask, followed by three cycles of freeze-thawing at −80°C. The amount of infectious virus was determined by plaque assay of the infected cell lysate on complementing V27 cells. Confluent monolayers were infected with 1-ml aliquots of 100-fold and higher dilutions to test for plaque formation. Therefore, the limit of detection of virus replication for each infection was 1 × 102 PFU.
Cell growth assays.
To measure effects on cell growth over time due to ICP27 expression, 30,000 HTO-H, TE12, and TE51 cells were plated in 12.5-cm2 tissue culture flasks. Half of the flasks were immediately treated with 2 μg/ml of doxycycline. At each time point, six flasks per cell line were trypsinized (three induced and three uninduced). The cells were resuspended in DMEM and counted using a hemacytometer.
Activator and inhibitor treatment.
Sorbitol was used to induce SAPK signaling by treating cells with a final concentration of 0.5 M sorbitol (Invitrogen) for 30 min (6). Apoptosis was induced by treating cells with staurosporine (ICN Biomedicals) dissolved in dimethyl sulfoxide (DMSO) at final concentration of 1 μM for 1 and 3 h. Pharmacological inhibitors of SAPK signaling SB203580 (p38 inhibitor; Promega) and SP600125 (JNK inhibitor; Biosource) were added 30 min prior to infection or sorbitol induction at final concentrations of 5 μM and 1 μM, respectively.
Immunoblotting assays.
Fifty-percent confluent monolayers of HTO-H, TE12, and TE51 cells were induced with 2 μg/ml doxycycline for 24 h. Analysis of ICP27 protein expression by immunoblotting was carried out as described previously (41). Cell lysates for the analysis of SAPK activation were prepared first by washing cells with cold phosphate-buffered saline supplemented with TLCK (Nα-p-tosyl-l-lysine chloromethyl ketone) and phenylmethylsulfonyl fluoride. Cells were then lysed directly in PSB buffer (40 mM Tris, pH 6.8, 1% sodium dodecyl sulfate, 10% glycerol, 2% β-mercaptoethanol, and 0.01% bromophenol blue). The lysate was then boiled for 5 min and frozen overnight at −80°C. For sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the samples were boiled again for 5 min and loaded onto 10% acrylamide gels. Proteins were transferred to nitrocellulose membranes and probed for SAPKs and ICP27. The antibody for ICP27, H1119 (mouse monoclonal used at a dilution of 1:5,400), was purchased from the Rumbaugh-Goodwin Institute for Cancer Research (Plantation, FL). Cellular p38 and phospho-p38 were detected using rabbit polyclonal antibodies 9212 and 9211, respectively (Cell Signaling Danvers, MA), at a dilution of 1:1,000. Polyclonal rabbit antibodies against cellular JNK1 (44-690G) and phospho-JNK (44-682G) were purchased from Biosource (Camarillo, CA) and used at dilutions of 1:10,000 and 1:1000, respectively. The secondary antibodies used for immunoblot detection were horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G and horseradish peroxidase goat anti-rabbit, purchased from Jackson ImmunoResearch (West Grove, PA), and were both diluted 1:5,000. Secondary antibodies were detected with enhanced chemiluminescence Western blotting detection reagents (Amersham).
Indirect immunofluorescence.
For infection and induction experiments to examine ICP27 expression and JNK signaling, cells were fixed in 3.7% formaldehyde in phosphate-buffered saline for 10 min, followed by acetone permeabilization for 2 min (39). Cells used for caspase-3 immunofluorescence experiments were fixed and permeabilized in methanol at −20°C for 10 min. For fluorescent staining, coverslips were incubated at 37°C for 1 h with various primary antibodies. The primary antibodies were mouse monoclonal anti-ICP27 (H1119) at a 1:1,000 dilution, rabbit polyclonal anti-ICP27 Clu38 (a kind gift from Saul Silverstein, Columbia University) at a dilution of 1:800, rabbit polyclonal anti-JNK1 at a 1:1,000 dilution (Biosource 44-690G), rabbit polyclonal anti-phospho-JNK at a 1:1,000 dilution (Biosource 44-682G), goat polyclonal anti-caspase-3 p11 (K19) at a 1:100 dilution (Santa Cruz sc-1224), and goat polyclonal anti-luciferase at a 1:500 dilution (Promega G7451). After primary incubation, cells underwent secondary staining for 1 h at 37°C. For ICP27 immunofluorescence alone, a 1:1,000 dilution of Cy3-conjugated goat anti-mouse immunoglobulin G was used. For ICP27 and JNK costaining in HTO-H, TE12, and TE51 cell lines, a combination of mouse and rabbit primary antibodies was used; secondary staining was done with a 1:1,000 dilution of Cy3-conjugated goat anti-rabbit immunoglobulin G and a 1:200 dilution of Cy2-conjugated goat anti-mouse immunoglobulin G. Luciferase expression and JNK phosphorylation in HTO-L cells were visualized by using a combination of goat and rabbit primary antibodies, respectively. HTO-L cells were then costained with Cy2-conjugated donkey anti-rabbit immunoglobulin G diluted 1:200 and a 1:1,000 dilution of Cy3-conjugated donkey anti-goat immunoglobulin G. When cells were costained for ICP27 and caspase-3, secondary staining was done sequentially starting with a 1:1,000 dilution of Cy3-conjugated donkey anti-goat immunoglobulin G antibody, followed by a 1:200 dilution of Cy2-conjugated goat anti-mouse immunoglobulin G antibody. All secondary antibodies were purchased from Jackson ImmunoResearch. Nuclei of all cells were costained using Hoechst stain at a 1:1,000 dilution.
Flow cytometry analysis.
Approximately 25% confluent monolayers of HTO-L, TE12, and TE51 cells were left uninduced or induced with 2 μg/ml doxycycline for 72 h. Cells were then trypsinized and pelleted. The level of apoptosis in the cells was assayed using an APOAF Annexin V-FITC Apoptosis Detection Kit (Sigma). The stained cells were then analyzed using a FACSCalibur flow cytometer (BD Biosciences), and the collected data were analyzed using FlowJo, version 8.7 (Tree Star), software. Samples were gated using forward scatter and side scatter to exclude any cell debris. Apoptotic cells were identified as cells that were annexin V-fluorescein isothiocyanate (FITC) positive.
RESULTS
Isolation and initial characterization of cell lines that inducibly express ICP27.
Most prior attempts to study the effects of ICP27 on host cell function have been conducted in the context of viral infection. Such studies have identified a host of potential functions that affect cellular gene expression and physiology. However, analyses in infected cells do not clearly separate the primary functions of ICP27 from secondary effects that are mediated by viral gene products whose expression is ICP27 dependent. Therefore, in an effort to identify the primary effects of ICP27 on cell function, we sought to engineer human cell lines that express ICP27 in an inducible manner. For this purpose, we first engineered a doxycycline-regulatable ICP27 gene contained on a plasmid (pTE27). We then transfected G418-resistant HTO cells, which are HeLa cells stably expressing the Tet-on transactivator (20), with pTE27 and a linear hygromycin resistance gene. Sixty hygromycin- and G418-resistant clones were selected and screened for ICP27 expression in both the absence and presence of doxycycline (2 μg/ml for 24 h). The level of ICP27 expression was then compared to that in virally infected cells and uninduced cells using immunoblotting assays of cell lysates. Hygromycin-resistant HTO cells, HTO-H, were included as a control. Two clones, TE12 and TE51, showed no detectable background expression of ICP27 and high levels of inducible ICP27 that were equal to or exceeded the expression levels seen during HSV-1 infection (Fig. 1A).
FIG. 1.
TE12 and TE51 cell lines are able to inducibly express functional ICP27. (A) Immunoblot assay comparing HTO-H, TE12, and TE51 ICP27 expression levels. Cells were infected with WT HSV-1 at an MOI of 5 and harvested at 8 hpi. For induction samples, cells were untreated or treated with 2 μg/ml doxycycline (Dox) for 24 h. (B to E) Immunofluorescence analysis of ICP27 expression in the TE12 and TE51 cell lines in the presence (w/ Dox) or absence of doxycycline (w/o Dox) (24 h posttreatment). The red signal indicates ICP27 expression, and the blue signal is due to Hoechst staining of nuclei. (F) Single-cycle yield assays. Replicate cultures of HTO-H, TE12, and TE51 cells were infected in duplicate at an MOI of 1 with WT HSV-1 or the ICP27 null mutant d27-1 in the presence or absence of doxycycline. After 24 h, the infections were terminated by freezing. Virus was released from the cells by three cycles of freeze-thawing, and the total infectious virus in the lysate was determining by a plaque assay on V27 cells.
To determine if these cell lines show uniform expression of ICP27 after induction, we used immunofluorescence microcopy. This analysis indicated that there was a very low level of ICP27 expression in uninduced cells on a per cell basis (Fig. 1B and D) as well as a high percentage of cells expressing ICP27 after induction (Fig. 1C and E). Both TE12 and TE51 cell lines showed less than 1% of cells that were ICP27 positive in the absence of doxycycline. After 24 h of induction, approximately 50% of the cells were ICP27 positive, a result that was consistently seen over several experiments (data not shown). Subcloning of the two lines did not result in an increased percentage of cells expressing ICP27 following induction. Moreover, following infection with the HSV-1 ICP27 deletion mutant d27-1, nearly 100% of TE12 and TE51 cells expressed ICP27 (data not shown). This indicates that the vast majority of TE12 and TE51 cells carry the ICP27 gene, which can be activated by infection (15, 25), but that only approximately 50% of them express it to detectable levels following induction. At present, we do not understand why a subpopulation of cells is refractory to induction.
In order to ensure that the ICP27 expressed by TE12 and TE51 is still functional, we infected these cells with d27-1 in the presence and absence of doxycycline to determine if the ICP27 expressed by the cells is able to complement the ICP27 defect of d27-1. Both cell lines were able to restore growth to the d27−1 mutant to a level similar to the WT virus KOS1.1 (Fig. 1F). It is important to note that infection with HSV-1 has been shown to induce the expression of stably transfected genes, including genes controlled by Tet-regulatable elements (15, 25), likely accounting for the high level of virus growth observed in TE12 and TE51 cells in both the absence and presence of doxycycline. Consistent with this, and as stated above, nearly 100% of TE12 and TE51 cells expressed ICP27 following infection with d27-1 (data not shown).
After ensuring that both inducible cell lines expressed functional ICP27, we next sought to characterize the expression kinetics of ICP27 following doxycycline induction. By immunoblot analysis and immunofluorescent microscopy, we found that ICP27 expression was detectable by 8 h postinduction and was highly expressed at a constant level from 12 h to 24 h postinduction (data not shown). It was observed that at early time points (6 to 8 h), ICP27 is initially localized to the nucleus. However, over time (12 to 24 h), the localization of ICP27 became increasingly cytoplasmic. After 24 h, expression was slowly lost in the cell population over the course of several days until approximately only 5% of the cells remained ICP27 positive at 4 days postinduction, as assayed by immunofluorescence microcopy (data not shown). This loss of expression was typically associated with cytopathology in the cultures, suggesting that ICP27 expression was toxic to cells.
To explore the possible effects of ICP27 on cell growth and viability, equal numbers of HTO-H, TE12, and TE51 cells were plated, and the total number of cells was followed for 4 days in the presence or absence of doxycycline induction. Doxycycline had no discernible effect on the growth of control HTO-H cells (Fig. 2A). However, doxycycline treatment of both TE12 and TE51 cells led to a significant reduction in cell number that was clearly evident by day 2 and dramatic by day 4 (Fig. 2B and C).
FIG. 2.
Doxycycline-induced expression of ICP27 reduces cellular growth. Equal numbers (3 × 104) of HTO-H, TE12, and TE51 cells were plated in triplicate in the presence (Dox+) or absence of doxycycline (2 μg/ml). Cells were harvested at 1, 2, 3, and 4 days postplating and counted using a hemacytometer.
ICP27 expression activates apoptosis.
The apparent growth inhibition seen above, coupled with the cytopathic effects observed, suggested that ICP27 might be inducing apoptosis in the TE12 and TE51 cells. In order to determine if this is the case, we used immunofluorescence microscopy to monitor caspase-3 activation over time following the induction of ICP27 expression. Little to no activated caspase-3 was seen in control HTO-H or HTO-L cells at any time following addition of doxycycline, indicating that neither doxycycline treatment nor induced protein expression is sufficient to trigger apoptosis (data not shown). Additionally, little activation was observed in uninduced TE51 cells (Fig. 3A) or in the TE51 cells during the first 2 days of doxycycline induction (Fig. 3B and C). However, after three days of induced expression of ICP27, caspase-3 activation indicative of apoptosis was observed, predominantly in cells expressing ICP27 (Fig. 3D). Similar results were observed in TE12 cells (data not shown).
FIG. 3.
ICP27 expression activates apoptosis. Induction of apoptosis by ICP27. Immunofluorescence analysis of caspase-3 activation in cells expressing ICP27. TE51 cells were incubated in the absence (−Dox; A) or presence (+Dox; B to D) of doxycycline (2 μg/ml). Cells were fixed and processed at 1, 2, or 3 days postinduction, as indicated, using antibodies specific for activated caspase-3 and ICP27. (E and F) Effect of ICP27 on staurosporine (Staur)-induced apoptosis. Untreated TE12 cells (E) or TE12 cells following 24 h of doxycycline-induced ICP27 expression (F) were treated with staurosporine (2 μM). Cells were fixed and processed 3 h after staurosporine treatment using antibodies specific for activated caspase-3 and ICP27. White triangles denote cells displaying chromatin condensation. Representative figures are shown.
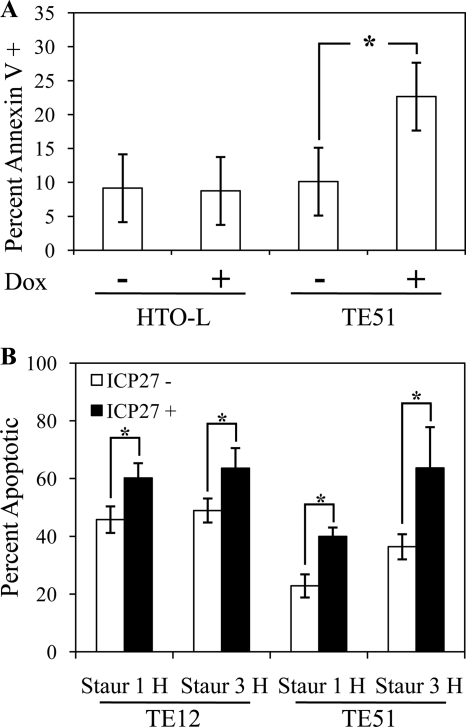
As an independent measure of apoptosis, we used flow cytometry to measure the number of annexin V-positive cells following induction of either luciferase expression in HTO-L cells or ICP27 expression in TE51 cells. While there was no change in the percentage of apoptotic cells following 3 days of luciferase expression compared to uninduced HTO-L cells, there was a significant increase in the number of annexin V-positive cells following 3 days of ICP27 expression (Fig. 4A). Interestingly, the approximately 12% increase in apoptotic cells observed in cells expressing ICP27 correlates to the relative ICP27 expression percentage following 3 days of expression (data not shown). These results coupled with the caspase-3 studies demonstrate that ICP27 expression in HeLa cells leads to apoptosis, likely explaining the observed defect in cellular growth that is associated with ICP27 expression in the TE12 and TE51 cells.
FIG. 4.
Quantitation of ICP27-induced apoptosis. (A) Flow cytometric analysis of annexin V staining in HTO-L and TE51 cells. Cells were left untreated or treated with doxycycline ([Dox] 2 μg/ml) for 3 days to induce either luciferase or ICP27 expression, as indicated. Cells were stained using annexin V conjugated to FITC and counted using a FACSCalibur flow cytometer. The data were analyzed using FlowJo software. *, P < 0.05. (B) Quantitation of apoptosis in staurosporine-treated cells. TE12 and TE51 cells were induced with doxycycline for 24 h and then treated with 2 μM staurosporine (Staur). Cells were fixed 1 or 3 h after staurosporine addition and stained for ICP27 and activated caspase-3. Cells were classified as apoptotic based on the presence of activated caspase-3 or chromatin condensation. The level of apoptosis in ICP27-negative cells (white bars) versus ICP27-positive cells (black bars) is shown. More than 1,000 cells were counted per time point per cell line. *, P < 0.05.
The above results were somewhat surprising because, as mentioned in the introduction, prior research has indicated that ICP27 inhibits apoptosis during viral infection (3). Therefore, we asked whether ICP27 could inhibit staurosporine-induced apoptosis in TE12 and TE51 cells following 24 h of doxycycline-induced expression. Staurosporine treatment induced apoptosis in uninduced cells at 1 and 3 h posttreatment, as has been previously shown (Fig. 3E; also data not shown). ICP27 had no obvious inhibitory effect on the induction of apoptosis since ICP27-expressing cells did not appear to be protected from apoptosis (Fig. 3F; also data not shown). In fact, caspase-3 activation and chromatin condensation at these time points appeared to preferentially occur in cells expressing ICP27, suggesting that ICP27 sensitizes cells to staurosporine-induced apoptosis. To examine this further, we analyzed the level of ICP27-negative versus ICP27-positive staurosporine-treated cells by counting results of three separate experiments. Cells were identified as apoptotic based on staining for activated caspase-3 or chromatin condensation (Fig. 3E and F). As shown in Fig. 4B, there is a significant increase in the percentage of apoptotic cells in cells expressing ICP27 compared to nonexpressing cells at both 1 and 3 h after staurosporine treatment. These results combined with those above demonstrate that, in the absence of infection, ICP27 has a proapoptotic effect in HeLa cells.
ICP27-mediated activation of SAPKs.
We speculated that the activation of apoptosis in cells expressing ICP27 could be due to ICP27-mediated SAPK signaling since both p38 and JNK signaling have been shown to be proapoptotic in some cases (52). To see whether ICP27 activates SAPK signaling pathways in TE12 and TE51 cells, lysates of control or induced cells were probed for both total and phosphorylated p38 and JNK. The results of the experiment showed that both sorbitol, an inducer of SAPK signaling through osmotic stress, and viral infection (MOI of 10 at 8 hpi) led to the phosphorylation of p38 and JNK in HTO-H, TE12, and TE51 cells (Fig. 5A lanes, 1, 5, and 9 and lanes 2, 6, and 10, respectively) although in general sorbitol appeared to be a more effective activator at the time points examined. No major changes in levels of total JNK1 or p38 were seen under any of the tested conditions. As expected, untreated cells (Fig. 5A, lanes 3, 7, and 11) showed little to no SAPK signaling, nor did HTO-H control cells treated with doxycycline (Fig. 5A, lane 4). However, TE12 and TE51 cells that had been treated with doxycycline for 24 h showed a clear increase in p38 phosphorylation that was comparable to that seen in virally infected cells (Fig. 5A, lanes 8 and 12). In contrast, JNK phosphorylation was not observed in TE12 and TE51 cells following either 12 h or 24 h of ICP27 induction (Fig. 5A, lanes 8 and 12; also data not shown). In an effort to ensure that the activation of p38 was due to the biochemical effects of ICP27 expression and not simply due to the overexpression of an induced protein, we compared p38 phosphorylation in induced HTO-L and TE51 cells. While both cell lines showed strong p38 phosphorylation following sorbitol treatment, only TE51 cells showed p38 phosphorylation after doxycycline induction (Fig. 5B). These results show that ICP27 expression in HeLa cells is sufficient for the phosphorylation of p38 MAPK.
FIG. 5.
ICP27 activates p38 signaling in the absence of viral infection. (A) Immunoblot analysis of SAPK signaling activation in HTO-H, TE12, and TE51 cells under the following conditions: after 30 min of sorbitol (Sorb) treatment, after infection with HSV-1 WT strain KOS1.1 (MOI of 10; 8 hpi), without treatment (NT), or with doxycycline induction (Dox+) (2 μg/ml for 24 h) as indicated. (B) Immunoblot analysis of p38 phosphorylation in HTO-L and TE51 cells under the following conditions: after 30 min of sorbitol (Sorb) treatment, without treatment (NT), or with doxycycline induction (Dox+) (2 μg/ml for 24 h) as indicated. Protein extracts were analyzed by immunoblotting for p38, phosphorylated p38 (P-p38), JNK1, and phosphorylated JNK1/JNK2.
The observation that ICP27 expression alone activates p38 signaling but is apparently insufficient to activate JNK signaling is interesting in light of the results of Hargett et al. (23, 24), who showed that ICP27 is required for JNK activation during infection. However, it is possible that the JNK immunoblotting assay is not sensitive enough to detect low levels of JNK phosphorylation in a subset of the total cell population. Therefore, to further investigate the effect of ICP27 expression on JNK activation, we used immunofluorescence microscopy to look at JNK phosphorylation in cells expressing ICP27. Little or no phospho-JNK was seen in untreated TE12 and TE51 cells or in control HTO-H cells in the presence of doxycycline (Fig. 6A and data not shown). Consistent with the immunoblotting results, sorbitol induction and WT HSV-1 infection led to intense nuclear staining for phospho-JNK, indicative of JNK phosphorylation and translocation to the nucleus (Fig. 6B and C). Interestingly, some phosphorylated JNK was seen in TE12 and TE51 cells that had been treated with doxycycline, and its presence correlated with ICP27 expression (Fig. 6D and data not shown). However, the overall level of staining was lower than in sorbitol-treated or infected cells, and the staining pattern showed nuclear speckles of phosphorylated JNK instead of the intense diffuse staining pattern. Immunofluorescent staining with an anti-PML antibody indicated that these speckles did not correspond to ND10 domains (data not shown). Thus, it appears that ICP27 expression alone is sufficient to induce some JNK phosphorylation, but the levels are reduced compared to the level seen during viral infection. This suggests that additional HSV-1 factors may be required for the full phosphorylation observed during infection.
FIG. 6.
ICP27 expression leads to phosphorylation of JNK. Immunofluorescence analysis of JNK activation in cells expressing ICP27. TE12 cells were left untreated (A) or treated with sorbitol for 30 min (B), infected for 8 h with WT HSV-1 (MOI of 10) (C), or treated with doxycycline (2 μg/ml for 24 h) (D). TE12 cells were also infected for 8 h with d27-1 (E) or infected for 8 h with d120 (F). Infections were performed at an MOI of 10. Following treatment, cells were fixed and processed using antibodies specific for phosphorylated JNK1/JNK2 (red signal) and ICP27 (green signal).
To further investigate whether factors other than ICP27 contribute to JNK phosphorylation during infection, we infected HTO-H, TE12, and TE51 cells with KOS 1.1, d27-1, and d120 (an ICP4 deletion mutant) and compared phospho-JNK levels at 8 hpi using immunofluorescence microscopy. As expected, HTO-H cells infected with d27-1 virus show little if any JNK phosphorylation (data not shown), consistent with prior results that indicated that ICP27 is required for JNK activation (23, 24). In contrast, high levels of nuclear phosphorylated JNK were seen in both KOS and d27-1-infected TE51 cells (Fig. 6E; also data not shown). Cells infected with d120 virus, however, which lacks the ICP4 gene required for DE and L gene expression, displayed less intense nuclear staining of phosphorylated JNK (Fig. 6F). Interestingly, the d120-infected cells showed a speckling pattern similar to that seen in cells expressing ICP27 (Fig. 6D). Similar results were seen in TE51 cells (data not shown). These results suggest that, in addition to ICP27, at least one other viral factor (ICP4 or a DE/L gene product) is required for full induction of JNK phosphorylation. This experiment further strengthens the conclusion that ICP27 is only partially sufficient for the activation of JNK signaling during infection.
ICP27-mediated activation of p38 signaling leads to apoptosis.
As mentioned above, previous research has associated HSV-1-induced JNK signaling with NF-κB activation during infection (24). Additionally, other work has shown the importance of NF-κB activation in preventing apoptosis during infection (18). Therefore, we hypothesized that in TE12 and TE51 cells, ICP27-mediated activation of p38 signaling in the absence of strong JNK activation results in apoptosis. To test this, we conducted a series of experiments using pharmacological inhibitors of p38 and JNK signaling. First, we verified the activity of the inhibitors, SB203580 and SP600125 (p38 and JNK inhibitors, respectively) by adding them to cells 30 min prior to sorbitol-induced stress. Cells were then lysed after 30 min of sorbitol exposure, and SAPK activation was examined by immunoblot analysis. Figure 7A shows significant and specific reductions in SAPK activation although the p38 inhibition did not completely prevent phosphorylation of p38. We next asked whether treatment with JNK and p38 inhibitors could suppress the apoptosis induced by ICP27 expression. We induced expression of ICP27 in the TE12 and TE51 cell lines in the presence or absence of SB203580 and SP600125 for 3 days. The cells were then fixed and stained for activated caspase-3 and examined using immunofluorescence microscopy. In uninduced TE51 cells, where no ICP27 was expressed, vehicle control treatment with DMSO as well as p38 and JNK inhibitor treatment led to little or no activation of caspase-3 (Fig. 7B to D). As expected, induction of ICP27 by doxycycline led to significant apoptosis in the vehicle control-treated cells (Fig. 7E). Treatment of induced cells with JNK inhibitor also had little or no effect on caspase-3 activation (Fig. 7G and H). Cells treated with p38 inhibitor, however, showed a significant reduction in apoptosis based on caspase-3 activation and nuclear morphology (Fig. 7F and H). Similar overall results were observed in TE12 cells (data not shown). Thus, p38 activation appears to be necessary for ICP27-induced apoptosis in HeLa cells.
FIG. 7.
Treatment with p38 inhibitors prevents ICP27 induced apoptosis. (A) Immunoblot analysis of SAPK phosphorylation in HTO-H cells. Cells were either left untreated or treated with sorbitol for 30 min. Cells were then treated with vehicle control (V/C; DMSO), p38 inhibitor (SB203580 at 5 μM), or JNK inhibitor (SP600125 at 1 μM) for 30 min as indicated. Following treatment, protein extracts were collected and analyzed by immunoblotting for p38, phosphorylated p38 (P-p38), JNK1, and phosphorylated JNK1 and JNK2 (P-JNK1 and P-JNK2, respectively). (B to G) Immunofluorescence analysis of caspase-3 activation in cells expressing ICP27 treated with inhibitors. Uninduced TE51 cells were treated with vehicle control (V/C; DMSO) (B), p38 inhibitor (C), and JNK inhibitor (D). TE51 cells induced with doxycycline (2 μg/ml) were treated with DMSO (E), p38 inhibitor (F), and JNK inhibitor (G). Cells were fixed and processed 3 h posttreatment using antibodies specific for activated caspase-3 (red-Cy3) and ICP27 (green-Cy2). (H) Enumeration of the percentage of ICP27-positive cells staining positive for activated caspase-3 following treatment with vehicle control, p38 inhibitor, and JNK inhibitor (>1,200 cells counted for each condition). *, P < 0.05. Dox+, with doxycycline; Dox−, without doxycycline.
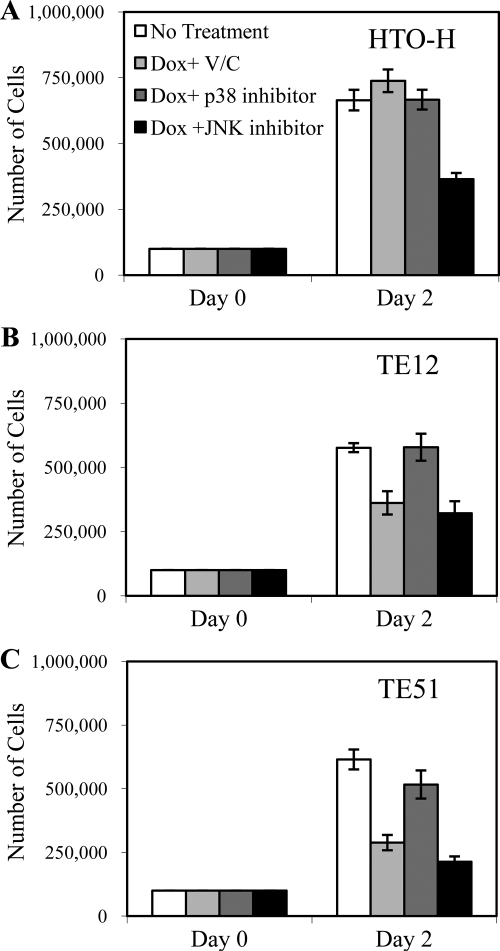
The observation that p38 inhibitor treatment prevents apoptosis induced by ICP27 led us to ask if p38 inhibition could also restore normal growth to TE12 and TE51 cells after induction of ICP27 expression. To examine this, we conducted a cell growth assay in the presence and absence of SAPK inhibitors. Equal numbers of HTO-H, TE12, and TE51 cells were plated, and inhibitors were added to the medium prior to the induction of ICP27 expression. Cells were counted 2 days postinduction and compared to uninduced and induced cells that had not been treated with inhibitors. We noted that the JNK inhibitor had a negative effect on all cell lines including HTO-H cells, consistent with data indicating that JNK signaling is required for HeLa cell proliferation (26). While treatment of HTO-H cells with p38 inhibitor had little or no effect on cellular growth (Fig. 8A), it almost fully restored growth to TE12 and TE51 cells following 2 days of induced expression (Fig. 8B and C). These data suggest that ICP27-mediated p38 activation leads to apoptosis and is responsible for the defect in cellular growth that we observe in TE12 and TE51 cells following ICP27 induction.
FIG. 8.
Cell growth following SAPK inhibitor treatment. Equal numbers of HTO-H, TE12, and TE51 cells were plated in triplicate in the presence or absence of doxycycline (2 μg/ml). Cells were treated upon induction with vehicle control (V/C; DMSO), p38 inhibitor (SB203580 at 5 μM), or JNK inhibitor (SP600125 at 1 μM). Cells were harvested and counted 2 days postinduction/treatment using a hemacytometer.
DISCUSSION
ICP27 expression activates p38 signaling.
Previous reports using viral deletion mutants have shown that ICP27 expression is required during HSV-1 infection for p38 activation (12, 23, 24). The study presented in this paper, however, is the first to show that ICP27 expression alone is sufficient for the activation of p38 signaling. The importance of p38 activation during infection has been shown by previous research, which demonstrated that inhibition of p38 signaling leads to a significant defect in HSV-1 replication (30). Signaling through p38 could potentially have a host of effects on viral replication and cellular function. Research from Corcoran et al. has shown that HSV-1-mediated p38 signaling is required for stabilization of certain cellular transcripts which have AU-rich instability elements in their 3′ untranslated regions (12). Additionally, p38 signaling can lead to global upregulation of translation by activating MAPK-interacting kinase 1 (Mnk1)/Mnk2 (17, 55). In fact, Walsh and Mohr have shown that HSV-1-mediated activation of p38 leads to Mnk1 activation and the stimulation of viral protein synthesis (54). Activated p38 can also translocate to the nucleus, where it is able to activate a range of transcription factors leading to increased cytokine expression, apoptosis, cell growth, or differentiation (52). Interestingly, a recent study has shown that p38 signaling during HSV-1 infection of T cells is required for interleukin-10 synthesis, which could help lead to the induction of immunological tolerance (49).
The mechanism behind ICP27-mediated activation of p38 remains to be characterized. Previous studies have shown that the activation of p38 and JNK in HSV-1-infected cells appears to occur downstream of Ras (35). Additional studies have identified regions of ICP27 that are required for p38 activation (12, 23), but little is known about the method of activation. The differential activation of JNK and p38 observed in this paper argues against a shared mechanism of signaling activation. Therefore, it is not likely that the activation is occurring through cellular receptors for oxidative stress, UV exposure, or surface receptors for cytokine signaling, e.g., Fas, since these signal receptors activate both pathways, as occurs following sorbitol treatment (52). This could point toward a potentially novel mechanism for p38 activation by ICP27. It is possible that the known effects of ICP27 on cellular gene expression could play a role, including its reported ability to inhibit pre-mRNA splicing (8, 12, 21, 22, 46, 51). Further research, however, will be required to identify the mechanism involved. The inducible cell lines described here could be useful tools in such studies.
ICP27 expression does not fully activate JNK signaling.
In our experiments, we observed some activation of the JNK pathway by ICP27 based on the levels of phospho-JNK. However, it did not appear to be as robust as the activation seen during HSV-1 infection. Interestingly the phosphorylated JNK induced by ICP27 accumulated in faint nuclear speckles, a pattern that is quite distinct from the robust nuclear staining seen during normal HSV-1 infection. The speckles are reminiscent of the activated JNK speckles that have been reported during varicella-zoster virus infection (58). Our findings are significant since prior reports have shown that during infection ICP27 is required for robust JNK activation (23, 24). Together, the data suggest that an additional DE or L gene is required to fully activate JNK signaling. The incomplete activation of JNK we describe is consistent with several previous observations. Most recently, Hargett et al. showed that during infection ICP27 expression initiated NF-κB activation and that ICP4 expression further increased it (24). In the same study, evidence was provided that activation of NF-κB is due in part to ICP27-mediated activation of JNK signaling. Furthermore, Goodkin et al. have previously shown that a DE or leaky-late gene expressed between 3 and 6 hpi is required for NF-κB activation (18). Finally, research has shown that the regions of ICP27 that are responsible for nuclear export and RNA binding are required for JNK activation, which suggests that ICP27's role may be at least in part to transactivate the expression of a key viral gene product (12, 23). These previous findings along with the results of this study strongly argue that there are one or more ICP27-dependent DE or L genes that together with ICP27 lead to full JNK activation during infection. Further research is needed to identify the relevant viral gene or genes responsible for full activation.
ICP27-mediated activation of p38 signaling leads to apoptosis.
Our studies indicate that in HeLa cells ICP27-mediated activation of p38 signaling leads to activation of the cellular apoptotic response. This finding not only explains the loss of expression observed in our cell lines over extended periods of ICP27 expression but also may help explain why ICP27 genes introduced into cells by stable transfections are generally silenced (S. Rice, unpublished data). The findings of this study also shed light on the complex regulation of apoptosis by HSV-1 factors. Research has shown that HSV-1 blocks apoptosis and alters p38 signaling during infection (57). Sanfilippo et al. showed that IE gene expression is required for apoptosis induction (47), and further work by these investigators implicated the ICP0 mRNA (48). In this work, we found that expression of ICP27 can induce apoptosis as well as sensitize cells to staurosporine-induced apoptosis. Thus, it appears that ICP27 is also a proapoptotic factor. This is in contrast to previous reports which have shown that ICP27 prevents apoptosis during HSV-1 infection (3). However, previous work was carried out in the context of infection and thus could not differentiate between direct and indirect effects of ICP27. Our finding that ICP27 expression is not sufficient for inhibition of induced apoptosis agrees with work by Aubert et al. that showed that ICP27 expressed from transiently transfected plasmids is unable to block staurosporine-induced apoptosis (5).
In our HeLa cell system, ICP27 expression is induced after 8 h of doxycycline treatment, but the resulting ICP27-dependent cytopathology and apoptosis are not evident for 2 to 3 days. These somewhat slow kinetics presumably reflect both the need for ICP27 to build up to critical levels and the specific mechanism, currently unknown, by which it stimulates p38 signaling and apoptosis. Regarding the induction of apoptosis, it is possible that effects on Bcl-2 are involved, as Zachos et al. have shown that activation of p38 signaling leads to destabilization of Bcl-2, resulting in apoptosis during infection with ICP27 mutant viruses (57). Additionally, work by Galvan et al. showed that overexpression of Bcl-2 is able to prevent apoptosis associated with the expression of IE proteins (16). Thus, in our system, expression of ICP27 could activate p38 and lead to loss of Bcl-2, resulting in sensitization to or induction of apoptosis. Obviously, further work is required to test this and other models of how ICP27 induces apoptosis.
NF-κB activation has been shown to play a critical role in inhibiting cellular apoptotic pathways during HSV-1 infection (18). Interestingly, NF-κB activation during infection has been associated with ICP27-mediated JNK signaling (24), which our work suggests involves the expression of DE or L gene(s) along with ICP27. This fits well with findings from Aubert et al., who showed that DE and L gene expression are required for the prevention of apoptosis (4). Thus, it seems likely that during HSV-1 infection, ICP27 directly induces proapoptotic signaling early during infection, along with other IE factors such as the ICP0 mRNA (48). However, this induction is later suppressed by the effects of one or more ICP27-dependent DE or L gene products.
Based on our work and previous studies, we propose the following model to explain the relationship between ICP27-mediated SAPK signaling and the regulation of apoptosis during HSV-1 infection. First, ICP27 directly leads to activation of p38 through an as yet uncharacterized interaction with cellular signaling components. The p38 signaling triggers the host cell apoptotic response through an as yet unknown mechanism. Second, ICP27, along with ICP4, mediates the expression of one or more DE or L gene products that are responsible for the activation of robust JNK signaling. The activation of JNK signaling subsequently leads to NF-κB activation. Finally, NF-κB activation leads to the expression of host cell antiapoptotic factors that serve to block host cell death. Further work is certainly needed to test the various aspects of this model. A central feature of this model is that ICP27, through its direct and indirect effects on SAPK signaling pathways, is a key regulator of cell fate during HSV-1 infection.
Acknowledgments
We acknowledge the assistance of the Flow Cytometry Core Facility of the University of Minnesota Cancer Center, a comprehensive cancer center designated by the National Cancer Institute, supported in part by P30 CA77598. This research was supported by a grant to S.A.R. from the NIH (R01-AI42737).
We also thank Saul Silverstein for the generous gift of ICP27 antiserum and Wade Bresnahan for helpful comments on the manuscript.
Footnotes
Published ahead of print on 10 December 2008.
REFERENCES
- 1.Adler, V., S. Y. Fuchs, J. Kim, A. Kraft, M. P. King, J. Pelling, and Z. Ronai. 1995. Jun-NH2-terminal kinase activation mediated by UV-induced DNA lesions in melanoma and fibroblast cells. Cell Growth Differ. 61437-1446. [PubMed] [Google Scholar]
- 2.Assefa, Z., M. Garmyn, R. Bouillon, W. Merlevede, J. R. Vandenheede, and P. Agostinis. 1997. Differential stimulation of ERK and JNK activities by ultraviolet B irradiation and epidermal growth factor in human keratinocytes. J. Investig. Dermatol. 108886-891. [DOI] [PubMed] [Google Scholar]
- 3.Aubert, M., and J. A. Blaho. 1999. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J. Virol. 732803-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubert, M., S. A. Rice, and J. A. Blaho. 2001. Accumulation of herpes simplex virus type 1 early and leaky-late proteins correlates with apoptosis prevention in infected human HEp-2 cells. J. Virol. 751013-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aubert, M., L. E. Pomeranz, and J. A. Blaho. 2007. Herpes simplex virus blocks apoptosis by precluding mitochondrial cytochrome c release independent of caspase activation in infected human epithelial cells. Apoptosis 1219-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogoyevitch, M. A., A. J. Ketterman, and P. H. Sugden. 1995. Cellular stresses differentially activate c-Jun N-terminal protein kinases and extracellular signal-regulated protein kinases in cultured ventricular myocytes. J. Biol. Chem. 27029710-29717. [DOI] [PubMed] [Google Scholar]
- 7.Brown, C. R., M. S. Nakamura, J. D. Mosca, G. S. Hayward, S. E. Straus, and L. P. Perera. 1995. Herpes simplex virus trans-regulatory protein ICP27 stabilizes and binds to 3′ ends of labile mRNA. J. Virol. 697187-7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryant, H. E., S. E. Wadd, A. I. Lamond, S. J. Silverstein, and J. B. Clements. 2001. Herpes simplex virus IE63 (ICP27) protein interacts with spliceosome-associated protein 145 and inhibits splicing prior to the first catalytic step. J. Virol. 754376-4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai, W., S. Person, C. DebRoy, and B. Gu. 1988. Functional regions and structural features of the gB glycoprotein of herpes simplex virus type 1: an analysis of linker insertion mutants. J. Mol. Biol. 201575-588. [DOI] [PubMed] [Google Scholar]
- 10.Chen, I.-H. B., K. S. Sciabica, and R. M. Sandri-Goldin. 2002. ICP27 interacts with the RNA export factor Aly/REF to direct herpes simplex virus type 1 intronless mRNAs to the TAP export pathway. J. Virol. 7612877-12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, I.-H. B., L. Li, L. Silva, and R. M. Sandri-Goldin. 2005. ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites although TAP/NXF1 is required for ICP27 export. J. Virol. 793949-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corcoran, J. A., W. L. Hsu, and J. R. Smiley. 2006. Herpes simplex virus ICP27 is required for virus-induced stabilization of the ARE-containing IEX-1 mRNA encoded by the human IER3 gene. J. Virol. 809720-9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLuca, N. A., A. M. McCarthy, and P. A. Schaffer. 1985. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J. Virol. 56558-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellison, K. S., R. A. Maranchuk, K. L. Mottet, and J. R. Smiley. 2005. Control of VP16 translation by the herpes simplex virus type 1 immediate-early protein ICP27. J. Virol. 794120-4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everett, R. D. 1985. Activation of cellular promoters during herpes virus infection of biochemically transformed cells. EMBO J. 41973-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galvan, V., R. Brandimarti, J. Munger, and B. Roizman. 2000. Bcl-2 blocks a caspase-dependent pathway of apoptosis activated by herpes simplex virus 1 infection in HEp-2 cells. J. Virol. 741931-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge, B., H. Gram, F. Di Padova, B. Huang, L. New, R. J. Ulevith, Y. Luo, and J. Han. 2002. MAPKK-independent activation of p38 mediated by TAB1-dependent autophosphorylation of p38 α. Science 2951291-1294. [DOI] [PubMed] [Google Scholar]
- 18.Goodkin, M. L., A. T. Ting, and J. A. Blaho. 2003. NF-κB is required for apoptosis prevention during herpes simplex virus type 1 infection. J. Virol. 777261-7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gossen, M., and H. Bujard. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 895541-5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gossen, M., S. Freundlieb, G. Bender, G. Muller, W. Hillen, and H. Bujard. 1995. Transcriptional activation by tetracycline in mammalian cells. Science 2681766-1769. [DOI] [PubMed] [Google Scholar]
- 21.Hardwicke, M. A., and R. M. Sandri-Goldin. 1994. The herpes simplex virus regulatory protein ICP27 contributes to the decrease in cellular mRNA levels during infection. J. Virol. 684797-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardy, W. R., and R. M. Sandri-Goldin. 1994. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J. Virol. 687790-7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hargett, D., T. McLean, and S. L. Bachenheimer. 2005. Herpes simplex virus ICP27 activation of stress kinases JNK and p38. J. Virol. 798348-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hargett, D., S. Rice, and S. L. Bachenheimer. 2006. Herpes simplex virus type 1 ICP27-dependent activation of NF-κB. J. Virol. 8010565-10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrlinger, U., P. A. Pechan, A. H. Jacobs, C. Woiciechowski, N. G. Rainov, C. Fraefel, W. Paulus, and S. A. Reeves. 2000. HSV-1 infected cell proteins influence tetracycline-regulated transgene expression. J. Gene Med. 2379-389. [DOI] [PubMed] [Google Scholar]
- 26.Holzberg, D., C. G. Knight, O. Dittrich-Breiholz, H. Schneider, A. Dörrie, E. Hoffmann, K. Resch, and M. Kracht. 2003. Disruption of the c-JUN-JNK complex by a cell-permeable peptide containing the c-JUN delta domain induces apoptosis and affects a distinct set of interleukin-1-induced inflammatory genes. J. Biol. Chem. 27840213-40223. [DOI] [PubMed] [Google Scholar]
- 27.Hughes, R. G., Jr., and W. H. Munyon. 1975. Temperature-sensitive mutants of herpes simplex virus type 1 defective in lysis but not in transformation. J. Virol. 16275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iordanov, M. S., D. Pribnow, J. L. Magun, T. H. Dinh, J. A Pearson, and B. E. Magun. 1998. Ultraviolet radiation triggers the ribotoxic stress response in mammalian cells. J. Biol. Chem. 27315794-157803. [DOI] [PubMed] [Google Scholar]
- 29.Jean, S., K. M. LeVan, B. Song, M. Levine, and D. M. Knipe. 2001. Herpes simplex virus 1 ICP27 is required for transcription of two viral late (gamma 2) genes in infected cells. Virology 283273-284. [DOI] [PubMed] [Google Scholar]
- 30.Karaca, G., D. Hargett, T. I. McLean, J. S. Aguilar, P. Ghazal, E. K. Wagner, and S. L. Bachenheimer. 2004. Inhibition of the stress-activated kinase, p38, does not affect the virus transcriptional program of herpes simplex virus type 1. Virology 329142-156. [DOI] [PubMed] [Google Scholar]
- 31.Koffa, M. D., J. B. Clements, E. Izaurralde, S. Wadd, S. A. Wilson, I. W. Mattaj, and S. Kuersten. 2001. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO J. 205769-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larralde, O., R. W. Smith, G. S. Wilkie, P. Malik, N. K. Gray, and J. B. Clements. 2006. Direct stimulation of translation by the multifunctional herpesvirus ICP27 protein. J. Virol. 801588-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lengyel, J., C. Guy, V. Leong, S. Borge, and S. A. Rice. 2002. Mapping of functional regions in the amino-terminal portion of the herpes simplex virus ICP27 regulatory protein: importance of the leucine-rich nuclear export signal and RGG box RNA-binding domain. J. Virol. 7611866-11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCarthy, A. M., L. McMahan, and P. A. Schaffer. 1989. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J. Virol. 6318-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLean, T. I., and S. L. Bachenheimer. 1999. Activation of c-Jun N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J. Virol. 738415-8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mears, W. E., and S. A. Rice. 1998. The herpes simplex virus immediate-early protein ICP27 shuttles between nucleus and cytoplasm. Virology 242128-137. [DOI] [PubMed] [Google Scholar]
- 37.Mears, W. E., and S. A. Rice. 1996. The RGG box motif of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation. J. Virol. 707445-7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phelan, A., and J. B. Clements. 1997. Herpes simplex virus type 1 immediate early protein IE63 shuttles between nuclear compartments and the cytoplasm. J. Gen. Virol. 783327-3331. [DOI] [PubMed] [Google Scholar]
- 39.Quinlan, M. P., L. B. Chen, and D. M. Knipe. 1984. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell 36857-868. [DOI] [PubMed] [Google Scholar]
- 40.Rice, S. A., and D. M. Knipe. 1988. Gene-specific transactivation by herpes simplex virus type 1 alpha protein ICP27. J. Virol. 623814-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rice, S. A., and D. M. Knipe. 1990. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J. Virol. 641704-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosette, C., and M. Karin. 1996. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science 2741194-1197. [DOI] [PubMed] [Google Scholar]
- 43.Roux, P. P., and J. Blenis. 2004. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68320-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sacks, W. R., C. C. Greene, D. P. Aschman, and P. A. Schaffer. 1985. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J. Virol. 55796-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandri-Goldin, R. M. 1998. ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 12868-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandri-Goldin, R. M., and G. E. Mendoza. 1992. A herpesvirus regulatory protein appears to act post-transcriptionally by affecting mRNA processing. Genes Dev. 6848-863. [DOI] [PubMed] [Google Scholar]
- 47.Sanfilippo, C. M., F. N. W. Chirimuuta, and J. A. Blaho. 2004. Herpes simplex virus type 1 immediate-early gene expression is required for the induction of apoptosis in human epithelial HEp-2 cells. J. Virol. 78224-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanfilippo C. M., and J. A. Blaho. 2006. ICP0 gene expression is a herpes simplex virus type 1 apoptotic trigger. J. Virol. 806810-6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sloan, D. D., and K. R. Jerome. 2007. Herpes simplex virus remodels T cell receptor signaling resulting in p38-dependent selective synthesis of interleukin-10. J. Virol. 8112504-12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soliman, T. M., R. M. Sandri-Goldin, and S. J. Silverstein. 1997. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J. Virol. 719188-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spencer, C. A., M. E. Dahmus, and S. A. Rice. 1997. Repression of host RNA polymerase II transcription by herpes simplex virus type 1. J. Virol. 712031-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tibbles, L. A., and J. R. Woodgett. 1999. The stress-activated protein kinase pathways. Cell Mol. Life Sci. 551230-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uprichard, S. L., and D. M. Knipe. 1996. Herpes simplex ICP27 mutant viruses exhibit reduced expression of specific DNA replication genes. J. Virol. 701969-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walsh, D., and I. Mohr. 2004. Phosphorylation of eIF4E by Mnk-1 enhances HSV-1 translation and replication in quiescent cells. Genes Dev. 18660-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waskiewicz, A. J., A. Flynn, C. G. Proud, and J. A. Cooper. 1997. Mitogen-activated protein kinases activate the serine/ threonine kinases Mnk1 and Mnk2. EMBO J. 161909-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zachos, G., J. B. Clements, and J. Conner. 1999. Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1. J. Biol. Chem. 2745097-5103. [DOI] [PubMed] [Google Scholar]
- 57.Zachos, G., M. Koffa, C. M. Preston, J. B. Clements, and J. Connor. 2001. Herpes simplex virus type 1 blocks the apoptotic host cell defense mechanisms that target Bcl-2 and manipulates activation of p38 mitogen-activated protein kinase to improve viral replication. J. Virol. 752710-2728. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Zapata, H. J., M. Nakatsugawa, and J. F. Moffat. 2007. Varicella-Zoster Virus infection of human fibroblast cells activates the c-Jun N-terminal kinase pathway. J. Virol. 81977-990. [DOI] [PMC free article] [PubMed] [Google Scholar]