Abstract
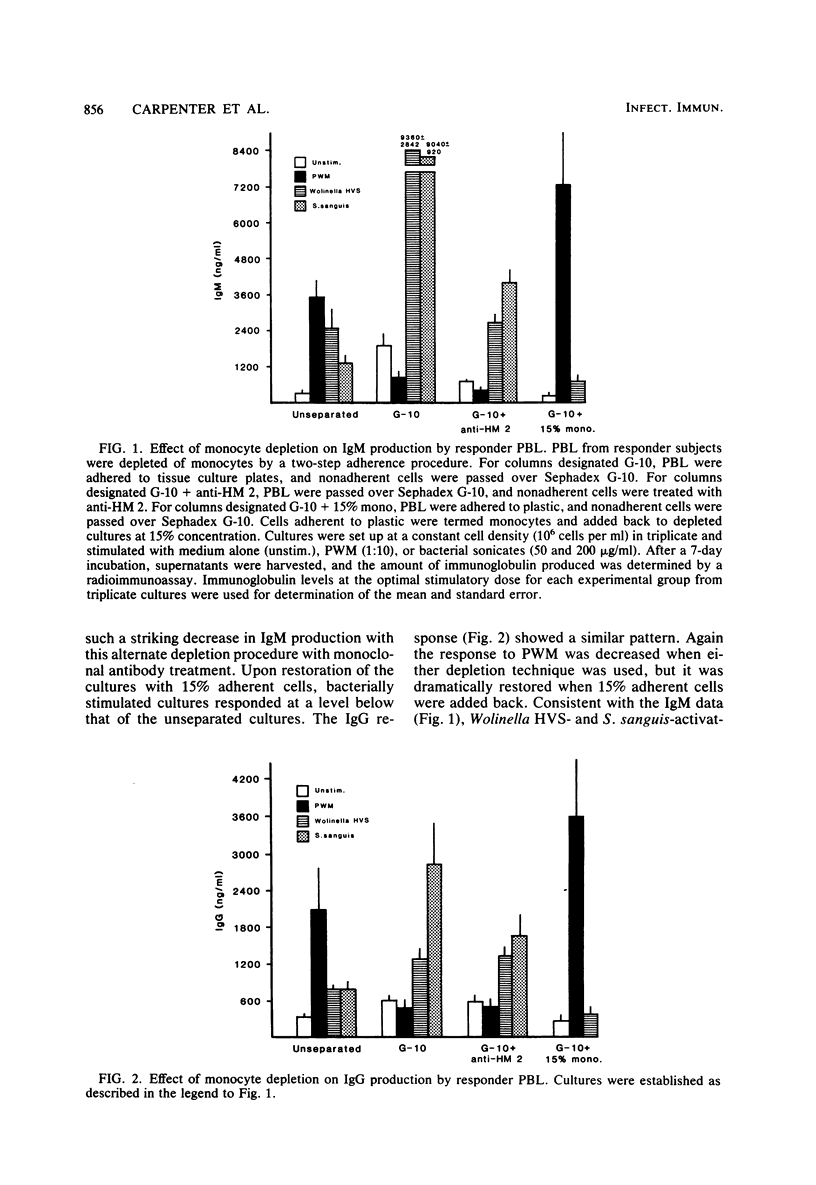
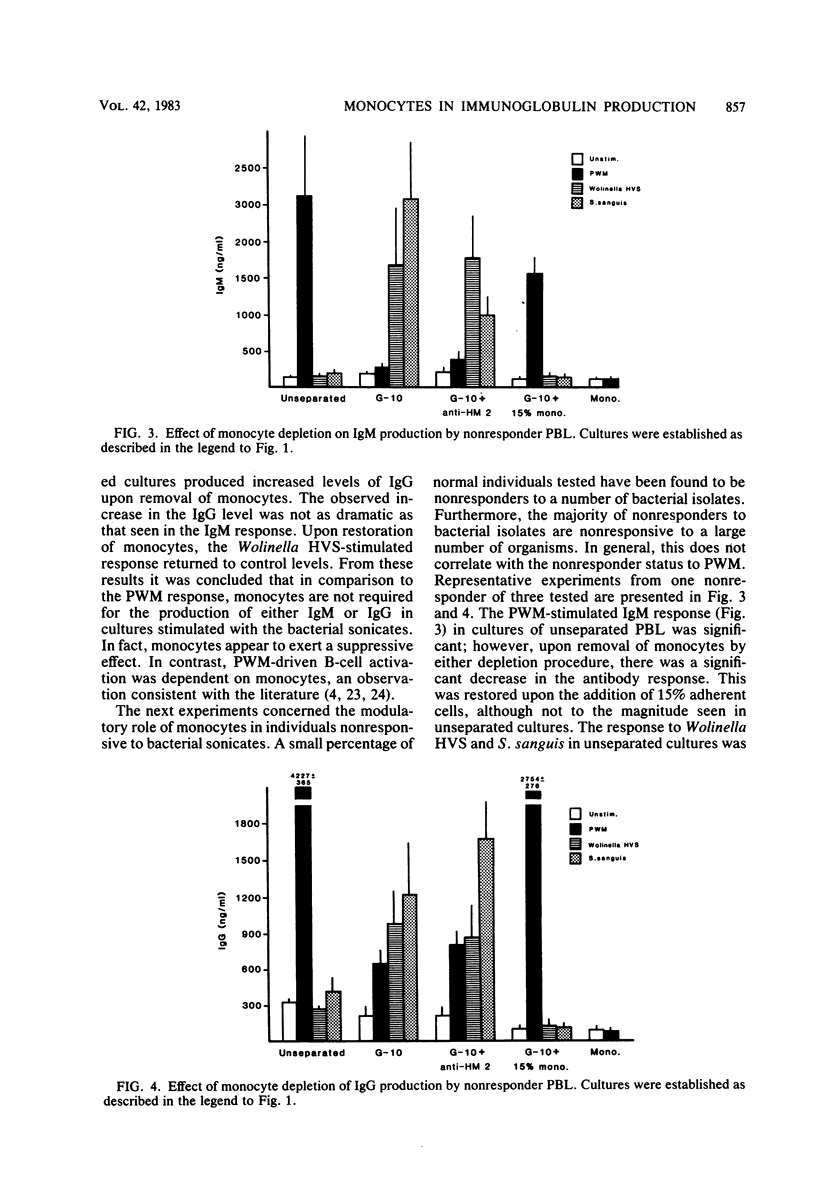
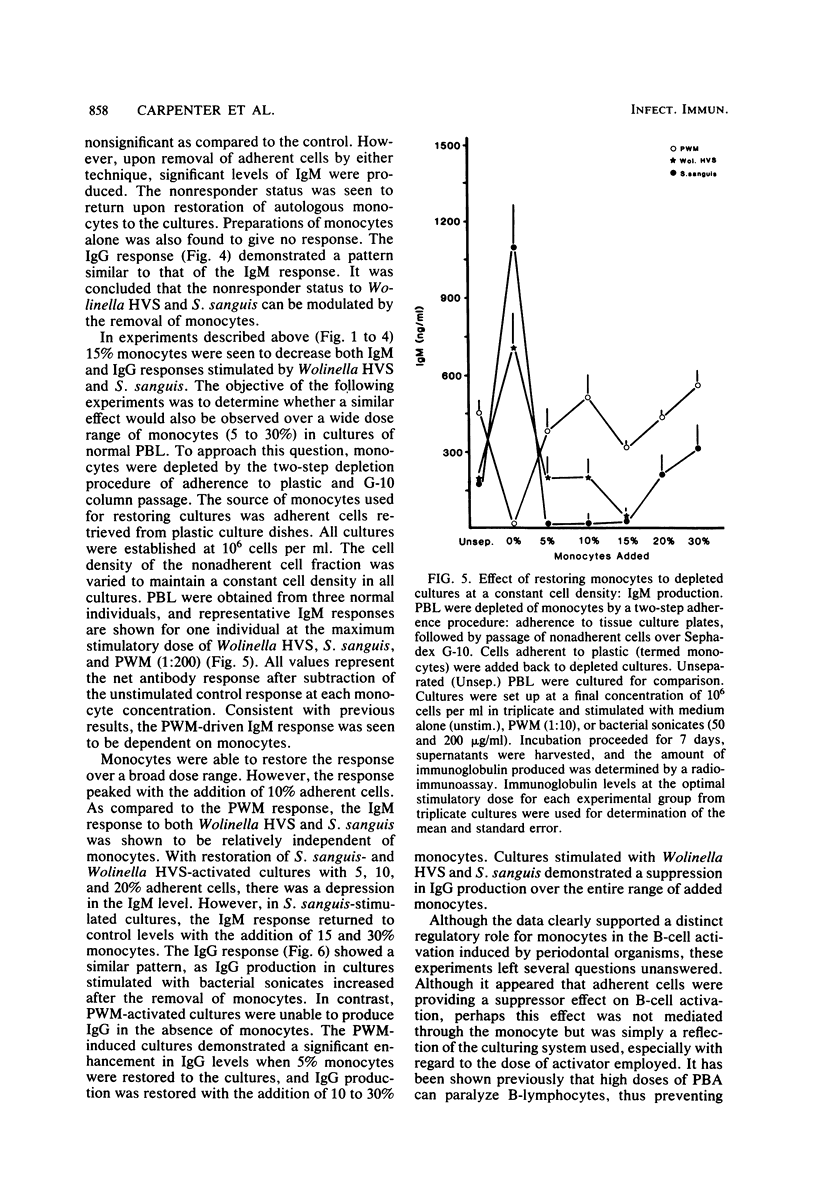
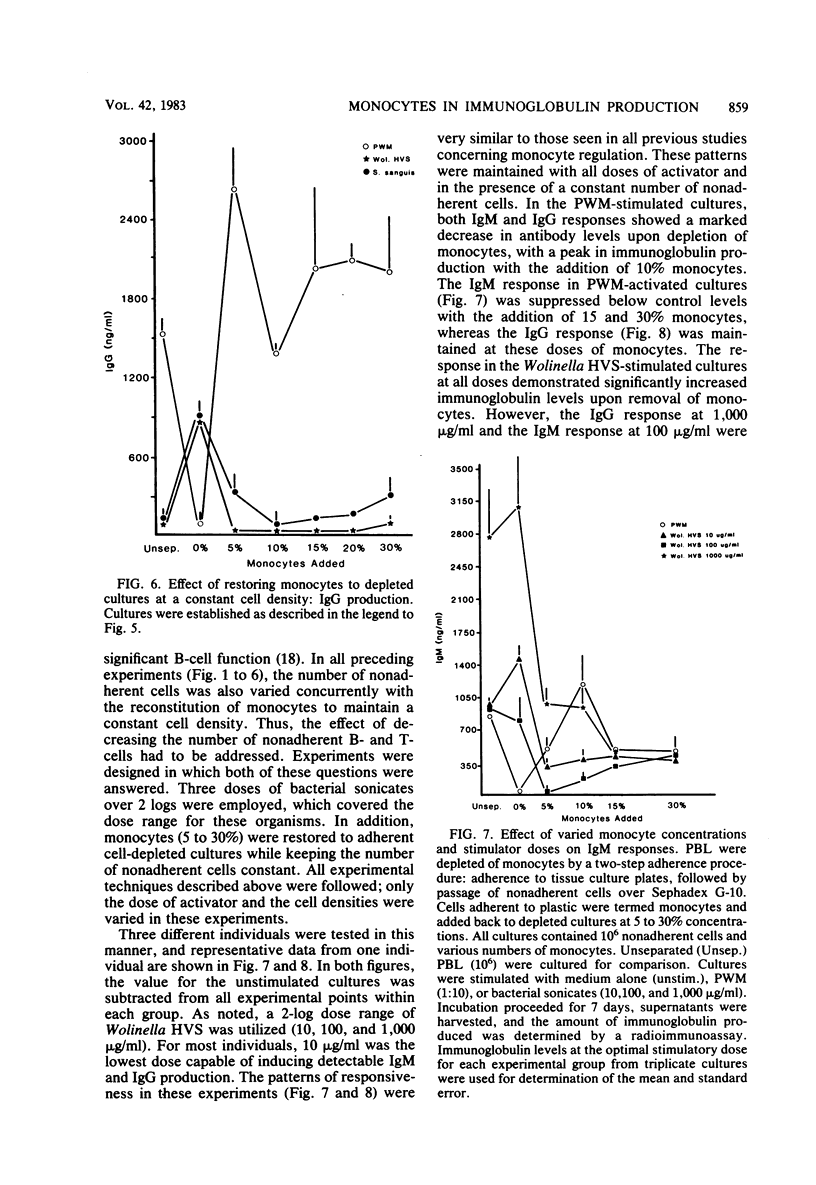
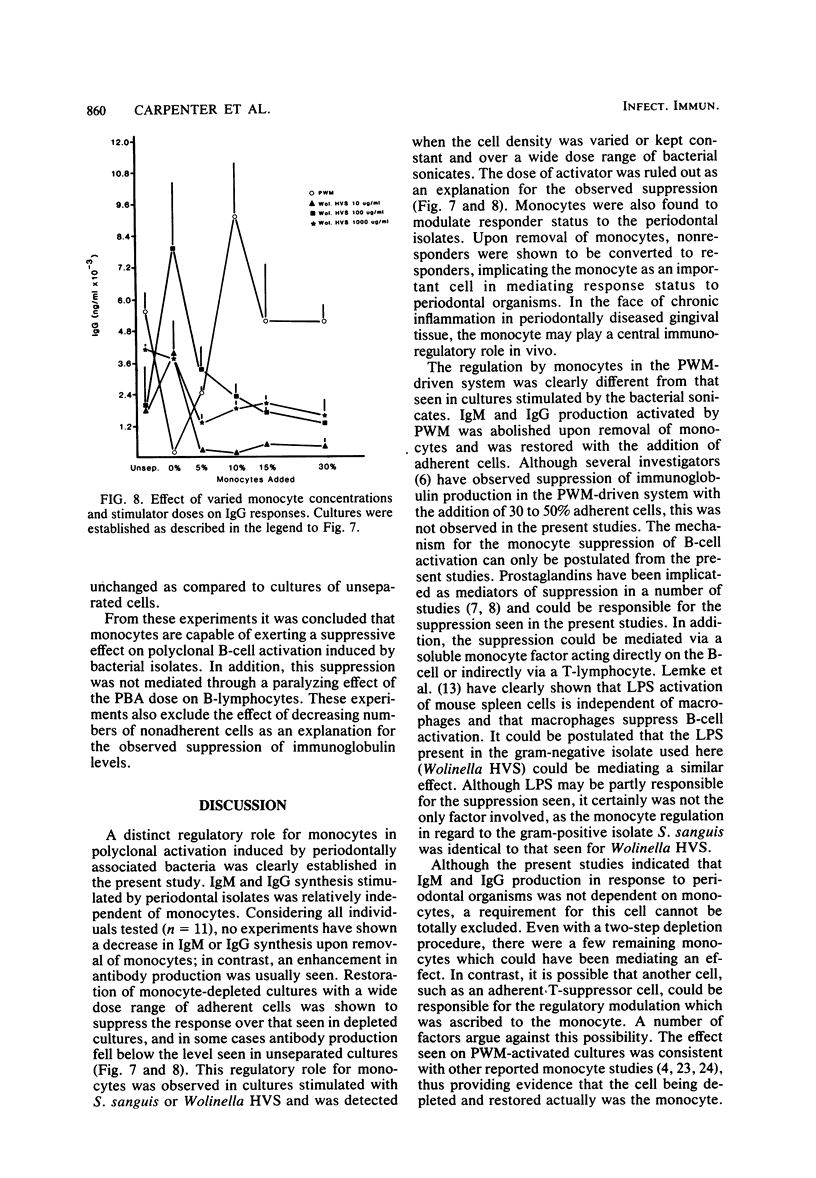
These studies were initiated to investigate monocyte regulation of polyclonal antibody responses of human peripheral blood lymphocytes stimulated by sonicates of periodontally associated bacteria. With pokeweed mitogen (PWM) as a positive reference, the role of monocytes in the peripheral blood lymphocyte response to Streptococcus sanguis and Wolinella HVS was examined by manipulating the number of monocytes and lymphocytes in culture. In comparison to PWM, optimal responses to the bacterial sonicates required very few monocytes (0.3% of the total cultured cells). Restoration of monocytes to physiological levels resulted in suppression of the response. PWM-stimulated responses were optimal at 5 to 15% monocyte content and were abolished after monocyte depletion. Individuals who were low responders or nonresponders to bacterial sonicates responded at normal levels after manipulation of monocyte concentration. Nonresponders produced normal levels of antibody when the monocyte concentration was reduced to 0.3% but were inhibited after monocyte reconstitution. The effects of monocyte concentration were tested over a wide dose range of bacterial sonicate and found to conform to the observed pattern throughout the dose range tested (10 to 1,000 micrograms/ml). The contrasting monocyte requirement of peripheral blood lymphocytes stimulated with PWM versus bacterial sonicates may reflect a quantitative difference in optimal macrophage concentration or may be due to a qualitative difference in lymphocyte-monocyte interactions in response to these activators.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. J., Chan S. P., Socransky S. S., Oppenheim J. J., Mergenhagen S. E. Importance of Actinomyces and certain gram-negative anaerobic organisms in the transformation of lymphocytes from patients with periodontal disease. Infect Immun. 1976 May;13(5):1363–1368. doi: 10.1128/iai.13.5.1363-1368.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick P. H., Carpenter A. B., Holdeman L. V., Miller G. A., Ranney R. R., Palcanis K. G., Tew J. G. Polyclonal B-cell activation induced by extracts of Gram-negative bacteria isolated from periodontally diseased sites. Infect Immun. 1981 Oct;34(1):43–49. doi: 10.1128/iai.34.1.43-49.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona C., Broder S., Dimitriu A., Waldmann T. A. Polyclonal activation of human B lymphocytes by Nocardia water soluble mitogen (NWSM). Immunol Rev. 1979;45:69–92. doi: 10.1111/j.1600-065x.1979.tb00273.x. [DOI] [PubMed] [Google Scholar]
- Gerrard T. L., Fauci A. S. Activation and immunoregulation of antigen-specific human b lymphocyte responses: multifaceted role of the monocyte. J Immunol. 1982 May;128(5):2367–2372. [PubMed] [Google Scholar]
- Gmelig-Meyling F., Waldmann T. A. Human B cell activation in vitro: augmentation and suppression by monocytes of the immunoglobulin production induced by various B cell stimulants. J Immunol. 1981 Feb;126(2):529–537. [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Dean J. H., Richardson G. L., Herberman R. B. Depletion of monocytes from human peripheral blood mononuclear leukocytes: comparison of the sephadex G-10 column method with other commonly used techniques. J Immunol Methods. 1980;32(1):11–29. doi: 10.1016/0022-1759(80)90113-1. [DOI] [PubMed] [Google Scholar]
- Knapp W., Baumgartner G. Monocyte-mediated suppression of human B lymphocyte differentiation in vitro. J Immunol. 1978 Sep;121(3):1177–1183. [PubMed] [Google Scholar]
- Koller C. A., King G. W., Hurtubise P. E., Sagone A. L., LoBuglio A. F. Characterization of glass adherent human mononuclear cells. J Immunol. 1973 Nov;111(5):1610–1612. [PubMed] [Google Scholar]
- Lemke H., Coutinho A., Opitz H. G., Gronowicz E. Macrophages suppress direct B-cell activation by lipopolysaccharide. Scand J Immunol. 1975;4(7):707–720. doi: 10.1111/j.1365-3083.1975.tb02679.x. [DOI] [PubMed] [Google Scholar]
- Levitt D., Duber-Stull D., Lawton A. R. T-cell-dependent and independent plasma cell differentiation induced by Escherichia coli Lipopolysaccharide in human peripheral blood lymphocytes. Clin Immunol Immunopathol. 1981 Mar;18(3):309–321. doi: 10.1016/0090-1229(81)90124-0. [DOI] [PubMed] [Google Scholar]
- Levy P. C., Shaw G. M., LoBuglio A. F. Human monocyte, lymphocyte, and granulocyte antibody-dependent cell-mediated cytotoxicity toward tumor cells. I. General characteristics of cytolysis. J Immunol. 1979 Aug;123(2):594–599. [PubMed] [Google Scholar]
- Mangan D. F., Lopatin D. E. In vitro stimulation of immunoglobulin production from human peripheral blood lymphocytes by a soluble preparation of Actinomyces viscosus. Infect Immun. 1981 Jan;31(1):236–244. doi: 10.1128/iai.31.1.236-244.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E., Coppleson L. W. A THREE-CELL INTERACTION REQUIRED FOR THE INDUCTION OF THE PRIMARY IMMUNE RESPONSE in vitro. Proc Natl Acad Sci U S A. 1968 Oct;61(2):542–547. doi: 10.1073/pnas.61.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G., Coutinho A. Factors influencing activation of B-cells in immunity. Ann N Y Acad Sci. 1975 Feb 28;249:68–88. doi: 10.1111/j.1749-6632.1975.tb29059.x. [DOI] [PubMed] [Google Scholar]
- Persson U., Hammarström L., Möller E., Möller G., Smith C. I. The role of adherent cells in B and T lymphocyte activation. Immunol Rev. 1978;40:78–101. doi: 10.1111/j.1600-065x.1978.tb00402.x. [DOI] [PubMed] [Google Scholar]
- Ranney R. R., Ruddy S., Tew J. G., Welshimer H. J., Palcanis K. G., Segreti A. Immunological studies of young adults with severe periodontitis. I. Medical evaluation and humoral factors. J Periodontal Res. 1981 Jul;16(4):390–402. doi: 10.1111/j.1600-0765.1981.tb00990.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lipsky P. E. Monocyte dependence of pokeweed mitogen-induced differentiation of immunoglobulin-secreting cells from human peripheral blood mononuclear cells. J Immunol. 1979 Mar;122(3):926–931. [PubMed] [Google Scholar]
- Shortman K., Palmer J. The requirement for macrophages in the in vitro immune response. Cell Immunol. 1971 Oct;2(5):399–410. doi: 10.1016/0008-8749(71)90051-7. [DOI] [PubMed] [Google Scholar]
- Sjöberg O., Andersson J., Möller G. Requirement for adherent cells in the primary and secondary immune response in vitro. Eur J Immunol. 1972 Apr;2(2):123–126. doi: 10.1002/eji.1830020206. [DOI] [PubMed] [Google Scholar]
- Smith S., Bick P. H., Miller G. A., Ranney R. R., Rice P. L., Lalor J. H., Tew J. G. Polyclonal B-cell activation: severe periodontal disease in young adults. Clin Immunol Immunopathol. 1980 Jul;16(3):354–366. doi: 10.1016/0090-1229(80)90141-5. [DOI] [PubMed] [Google Scholar]
- Stashenko P. Regulatory effect of monocytes on T cell proliferative responses to oral microbial antigens. Infect Immun. 1982 Dec;38(3):938–947. doi: 10.1128/iai.38.3.938-947.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Broder S. Polyclonal B-cell activators in the study of the regulation of immunoglobulin synthesis in the human system. Adv Immunol. 1982;32:1–63. doi: 10.1016/s0065-2776(08)60720-8. [DOI] [PubMed] [Google Scholar]



