Abstract
Plasmacytoid dendritic cells (pDC) are an important component of the innate immune response, producing large amounts of alpha interferon in response to viral stimulation in vitro. Under noninflammatory conditions, pDC are not found in the skin and are restricted in location to the blood and lymph nodes. Therefore, their role in mucosal and cutaneous herpes simplex virus (HSV) infection has not been well-defined. In this study we show a role for human pDC in the immune response to HSV infection. First, by confocal microscopy we showed that pDC infiltrate the dermis of recurrent genital herpes simplex lesions at early and late phases, often at the dermo-epidermal junction. We then showed that pDC in vitro are resistant to HSV infection despite expressing the entry receptors CD111, CD112, and HVE-A. Within the lesions, pDC were found closely associated with CD3+ lymphocytes and NK cells, especially those which were activated (CD69+). Furthermore, these HSV-exposed pDC were able to stimulate virus-specific autologous T-lymphocyte proliferation. We conclude from this work that pDC may contribute to the immune control of recurrent herpes virus infection in vivo.
Herpes simplex diseases are caused by two different but closely related viruses. Herpes simplex virus type 1 (HSV-1) persistently infects 60 to 80% of regional populations, while the seroprevalence of HSV-2 varies from 7 to 80% depending on geographical location and sexual practices (11, 47). Oral cold sores are generally caused by HSV-1 infection and recurrent genital lesions are generally caused by HSV-2 infection.
In both initial and recurrent infections of human skin or mucosa, HSV is restricted to the epidermis, infecting keratinocytes. The virus then enters cutaneous sensory axons via a plexus of free nerve endings in the epidermis, is transported to the neurons, and establishes latent infection. Virus replication at the peripheral sites is controlled through innate and adaptive immune mechanisms, such as type I interferon (IFN) production by keratinocytes and by the actions of infiltrating macrophages, NK cells, and CD4 and CD8 lymphocytes. Macrophages can be infected by HSV; only immediate-early viral proteins are expressed, but there is no late protein expression and no virus assembly (37). Monocytes and lymphocytes are resistant to infection (2, 43), although lymphocytes become susceptible to infection upon activation (8). The model of immature monocyte-derived dendritic cells (MDDC) can be productively infected with HSV-1 and -2 (35); however, this infection leads to apoptosis (7). Similarly, HSV-2-infected rhesus macaque MDDC and murine bone marrow-derived DC undergo apoptosis (22, 38). An in vitro model of cross-presentation suggests that these virally infected apoptotic MDDC can be taken up by bystander MDDC and cross-presented to HSV-specific CD8 T lymphocytes (7). Consistent with this model, work in mice suggests Langerhans cells take up HSV antigen but transfer it to an intermediate CD8α+ DC in lymph nodes for presentation to (CD8) T lymphocytes (3).
Plasmacytoid DC (pDC) are an important component of both innate and adaptive immune responses, first by producing alpha IFN (IFN-α) to limit viral spread, followed by differentiation into antigen-presenting cells that initiate T-lymphocyte-mediated responses (28). Plasmacytoid DC are known to secrete IFN-α in response to stimulation with enveloped viruses, including human immunodeficiency virus (HIV) (6, 16, 30, 46), influenza virus (9, 15), and HSV (28, 45, 48), an effect partially mediated via TLR9 signaling (31). However, pDC from TLR9 knockout mice are still able to produce small amounts of IFN-α, suggesting that minor additional pathways of HSV recognition exist in the host (19). Whole virions are more efficient at inducing IFN-α than viral DNA alone (31), and an intact endolysosomal pathway is required for pDC-mediated responses to HSV (31). Many studies have utilized UV-inactivated HSV, indicating that viral replication is not required for IFN-α production by pDC (19, 28, 31).
Despite a large number of studies utilizing HSV as a potent stimulus for pDC activation, there is relatively little information on whether pDC are susceptible to viral infection. To our knowledge there has been no formal study of the infection of pDC with HSV, particularly at all stages of viral replication. One previous report mentioned a failure to detect green fluorescent protein-labeled HSV following infection of pDC (34); however, there were no data shown.
Following stimulation with viruses, pDC upregulate costimulatory molecules which, coupled with cytokine secretion, endow pDC with the capacity to stimulate T-lymphocyte proliferation and induce an adaptive immune response. Plasmacytoid DC display enormous plasticity in their ability to stimulate the adaptive immune response. HIV exposure induces maturation of pDC, and coculture with allogeneic T lymphocytes results in a T helper type 1 (Th1) phenotype (16). Alternatively, stimulating pDC with interleukin-3 (IL-3) and CD40L or HSV resulted in Th2 cytokine secretion by naïve CD4 T lymphocytes (23, 39). Others have shown a regulatory phenotype of T cells stimulated with HSV-activated pDC (24).
The role of pDC during an HSV infection has not been extensively studied. Cutaneous HSV is restricted to the epidermis, and pDC would not be expected to be present in normal skin. The large number of studies showing that HSV can activate pDC suggests, however, that pDC may play a role in viral immunity in vivo. Recent evidence suggests increased pDC in epidermal cell suspensions from patients with psoriasis vulgaris, contact dermatitis, and lupus (52). In addition, CD123+ CD45RA+ cells are recruited to the lamina propria of patients 1 to 7 days after experimentally induced nasal allergy (21). There was also a report of infiltration of pDC into the varicella skin lesion of one patient (17). In addition, wild-type mice showed enhanced recruitment of cells to the site of infection compared to pDC-depleted mice and these infiltrating cells produced higher amounts of IFN-α, suggesting that the cells were likely to be pDC (32). These limited studies on pDC infiltration into the skin during inflammation have not investigated what other cells the pDC may be interacting with or what role the pDC may be performing in the skin.
In this study we show for the first time infiltration of pDC into the dermis in herpetic lesions and in close association with activated T cells. The pDC do not express HSV glycoprotein antigens in lesions and are not infected with virus in vitro, but they are able to stimulate autologous T-lymphocyte proliferation. In this way pDC appear to play a role not only in the innate response to HSV infection, but also in initiating the adaptive immune response to the virus. As such, they may prove to be important in anti-HSV immunity, especially in local immune responses and in limiting spread of infection through IFN-α secretion.
MATERIALS AND METHODS
HSV-2 generation.
Stocks of HSV-2 (strain 186) were generated by passage in Vero cells cultured in Dulbecco's modified Eagle's medium supplemented with 5% fetal calf serum (JRH, Lenexa, KS). Virus stocks were obtained by three rounds of sonication of Vero cells in a cup sonicator (Branson, Danbury, CT) followed by the removal of cell debris by centrifugation at 800 × g for 10 min at 4°C. Virus titers in clarified supernatants were determined by plaque assay. Virus stocks were kept at −80°C until use. UV inactivation of HSV was performed as previously described (7). Briefly, virus was placed in a 20-mm petri dish within 10 cm of a 30-W UV light source for 10 min, with brief mixing every 3 min.
Sample collection and DC generation.
Whole-blood packs from anonymous donations were obtained from the Sydney Red Cross Blood Bank (Sydney, NSW, Australia). In Australia, 80% of individuals are seropositive for HSV-1 and 12% are HSV-2 positive, with a combined total of 85% of the population having one or the other or both (11). Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) gradient separation. Plasmacytoid DC were isolated by two-step magnetic bead separation. In the first step, T lymphocytes and monocytes were removed by magnetic bead depletion using CD3 and CD14 microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The eluted CD14− CD3− cells were incubated with BDCA-4 microbeads (Miltenyi Biotec), and BDCA-4+ cells were collected by removing the column from the magnetic field and flushing the cells out with a plunger. Isolated pDC were further enriched by passing the positive cells through a second column. The purity of isolated pDC was assessed by flow cytometry using an antibody cocktail of Lin-1, CD123, HLA-DR, and CD11c. The purity of isolated pDC was routinely greater than 85%.
Monocyte-derived DC were prepared as previously described (40). Briefly, CD14+ monocytes were obtained from PMBC by magnetic bead separation. These cells were seeded at 0.5 × 106/ml in RPMI 1640 supplemented with 10% fetal calf serum (RF-10) and 7.5 ng/ml of IL-4-granulocyte-macrophage colony-stimulating factor (GM-CSF; both from Prospec, Rehovot, Israel). Following 6-day culture, the cells were assessed by flow cytometry for expression of DC markers and were used as MDDC.
Antibodies.
Monoclonal antibodies (with conjugate labels indicated in parentheses) for CD123 (phycoerythrin [PE] or PE-Cy5), Lin-1 (fluorescein isothiocyanate [FITC]), HLA-DR (PE and peridinin chlorophyll protein), CD11c (allophycocyanin [APC]), CD14 (PE), CD1a (FITC and APC), CD3 (APC), and CD4 (PE-Cy7) were obtained from BD Pharmingen (San Jose, CA). HSV-2 culture identification/typing reagent (Trinity Biotech, Co. Wicklow, Ireland) was used to measure the level of productive infection in pDC and MDDC. Monoclonal TLR9 (FITC) antibody was obtained from Imgenex (San Diego, CA). ICP27 monoclonal antibody was purchased from Virostat (Portland, ME).
HSV entry receptor expression by pDC was assessed using unconjugated antibodies to CD111, CD112, and HVE-A (kind donation from Gary Cohen and Roslyn Eisenberg, University of Pennsylvania). Normal mouse immunoglobulin G was used as the isotype control and goat anti-mouse immunoglobulin-FITC (BD) was used as a secondary antibody.
Flow cytometry.
For surface staining, cells were incubated with relevant monoclonal antibodies for 30 min before fixing in 4% paraformaldehyde (Proscitech, Kirwan, QLD, Australia). When intracellular staining was required, the cells were surface stained first, followed by fixing in Cytofix/Cytoperm solution (BD Pharmingen) and washing in permeabilizing solution (phosphate-buffered saline supplemented with 0.5% saponin [Sigma-Aldrich, St. Louis, MO]). Cells were incubated at room temperature with antibodies for 15 min, washed in permeabilizing solution, and fixed in 4% paraformaldehyde.
Cells were acquired within 24 h using a FACSCalibur or FACSCanto flow cytometer (Becton Dickinson) and analyzed using CellQuest (Becton Dickinson) or FlowJo (TreeStar, Ashland, OR) software.
HSV infection of DC.
Dendritic cells (MDDC and pDC) were counted and infected with HSV-2 at a multiplicity of infection of 0.5 to 10. Virus was left to adsorb for 1 h at 37°C, after which time the cells were washed and then seeded at 1 × 106/ml in RPMI 1640 supplemented with 10% human AB serum (RH-10) for further incubation as required. For pDC cultures RH-10 was supplemented with IL-3 (20 ng/ml; R&D Systems, Minneapolis, MN). For MDDC cultures, RH-10 was supplemented with IL-4-GM-CSF (7.5 ng/ml). The levels of infection of pDC and MDDC were determined by flow cytometry and by quantitative PCR.
Type I IFN neutralization.
Isolated pDC were preincubated with neutralizing sheep polyclonal antibody to human IFN-α at 20,000 neutralizing U/ml (PBL, Piscataway, NJ) or with a combination of neutralizing antibody for IFN-α (20,000 nU/ml), sheep polyclonal neutralizing antibody to IFN-β (PBL; 2,000 U/ml), and mouse monoclonal antibody against IFN-α/β receptor (PBL; 20 μg/ml) for 10 min. Plasmacytoid DC were then exposed to virus as described above. After washing, pDC were cultured overnight in RH-10 and relevant neutralizing antibodies at the concentrations described above. Levels of infection of pDC were determined by flow cytometry. For IFN-α neutralizing experiments the supernatants were removed and stored at −80°C until analyzed by enzyme-linked immunosorbent assay (ELISA; PBL) according to the manufacturer's instructions to confirm efficient neutralization.
Quantitative PCR.
Total RNA was extracted using the RNAqueous kit (Ambion). RNA was DNase I treated (Invitrogen, Mount Waverley, VIC, Australia) and reverse transcribed using oligo(dT) and SuperScript III RNase H reverse transcriptase (Invitrogen) in the presence of RNase Out (Invitrogen). The cDNA was then subjected to quantitative PCR to determine the levels of expression of immediate-early genes ICP8 and ICP27, early gene UL30 (DNA polymerase), and late genes gB, gC, and gD in pDC and MDDC. The relative quantitation method (ΔΔCT) (29) was used to evaluate the expression of selected genes with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) amplicons used as an internal control to normalize all data (51). Primer pairs are shown in Table 1.
TABLE 1.
Primer pairs used for PCR
| Primer | Forward sequence | Reverse sequence |
|---|---|---|
| ICP27 | TGT CGG AGA TCG ACT ACA CG | GGT GCG TGT CCA GTA TTT CA |
| ICP8 | AAT CCG GCA TAA ACA TCT GC | AGC AAC ACC TTC CTG CAT CT |
| UL30 | GAC ACG GAC TCC ATT TTC GT | AGC AGC TTG GTG AAC GTT TT |
| gB | GTG GTG AAG GTG GTC GAG AT | GTC TGC ACC ATG ACC AAG TG |
| gC | AGA TCG TCG GGT TAC TGG TG | GGC TTC GTG GGT AGT AGC AT |
| gD | TAC TAC GCA GTG CTG GAA CG | CGA TGG TCA GGT TGT ACG TG |
| CD111 | GGA AAG CCT CAC TCT CAA CG | TGA TGG GTC CCT TGA AGA AG |
| CD112 | CCT CTG CAT CTC CAA AGA GG | CAC AGG TAT CAG GGC TGG TT |
| HVE-A | CCA CTG GGT ATG GTG GTT TC | TCA CCT TCT GCC TCC TGT CT |
PCR was performed using a MX3005 quantitative PCR machine (Stratagene, La Jolla, CA). Amplification conditions were as follows: initial cycle of 2 min at 50°C followed by 2 min at 95°C; then 50 cycles of PCR at 95°C for 15 s and 60°C for 45 s. The final cycle was 95°C for 60 s, 55°C for 30 s, and 95°C for 30 s.
In addition, the presence of mRNA for the HSV entry receptors CD111, CD112, and HVE-A in pDC and MDDC was determined using the amplification conditions described above.
Herpes lesion biopsies and examination by confocal microscopy.
Punch biopsies were taken from lesions from four patients with recurrent genital herpes 4 to 10 days after onset. Samples were obtained under a protocol approved by the ethics committees for the University of Texas or Sydney West Area Health Service. Written informed consent was obtained prior to sample donation. In the search for pDC in lesions, biopsies were selected according to the time after onset of lesion (4 to 10 days), presence of mononuclear infiltrate, and a clear view of the epidermis and dermis without necrotic cells obscuring the biopsy. The biopsies were taken such that the midpoint coincided with the edge of the lesion. The biopsies were snap-frozen in optimal cutting temperature compound, cut into 5-μm sections, and placed on slides. Slides were kept at −80°C until required for immunofluorescent staining.
Slides were fixed with ice-cold methanol-acetone prior to staining. Antibodies used for confocal microscopy include gC FITC (Virostat), CD3 (1:10; BD Pharmingen), CD69 (1:100; Biolegend, San Diego, CA), CD56 (1:200; Invitrogen, Carlsbad, CA), MxA (1:200; kind donation from Otto Haller, University of Freiburg), and polyclonal goat anti-human DLEC (BDCA-2; 1:25; R&D Systems). Where necessary, secondary antibodies used were Alexa Fluor 546 donkey anti-goat (1:400; Molecular Probes, Eugene, OR) and Alexa Fluor 488 donkey anti-mouse (1:400; Molecular Probes). Slides were incubated at 37°C with primary antibodies for 45 min. Following washing the secondary antibodies were added for 30 min at 37°C. Excess secondary antibody was washed and cells were counterstained using TO-PRO-3 (1:2,000; Molecular Probes). Following staining slides were mounted using Antifade Gold (Molecular Probes), coverslips were applied, and slides were refrigerated until analyzed by confocal microscopy (Leica Microsystems, Wetzlar, Germany).
CFSE dye dilution assay.
Prior to pDC isolation, approximately 10 × 106 PBMC were set aside and kept overnight at 4°C. pDC were exposed to HSV-2 for 1 h, washed three times, cultured overnight in RH-10 and 20 ng/ml IL-3, and then washed to remove IL-3 from the medium. PBMC were resuspended in serum-free medium and incubated at 37°C for 10 min with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes). Following incubation, cells were washed three times in ice-cold RH-10 and added to pDC at a ratio of 10 PBMC:1 pDC. Phytohemagglutinin (PHA) was added to one of the PBMC-pDC cocultures as a positive control. After 5 days of coculture at 37°C the cells were labeled using CD3 (APC) and CD4 (PE-Cy7), fixed in 4% paraformaldehyde, and analyzed for cell proliferation using a FACSCanto apparatus. The Student t test was performed to assess whether there was a statistical difference in proliferation between PBMC stimulated with pDC alone or with HSV-exposed pDC.
RESULTS
pDC in herpes lesion biopsies.
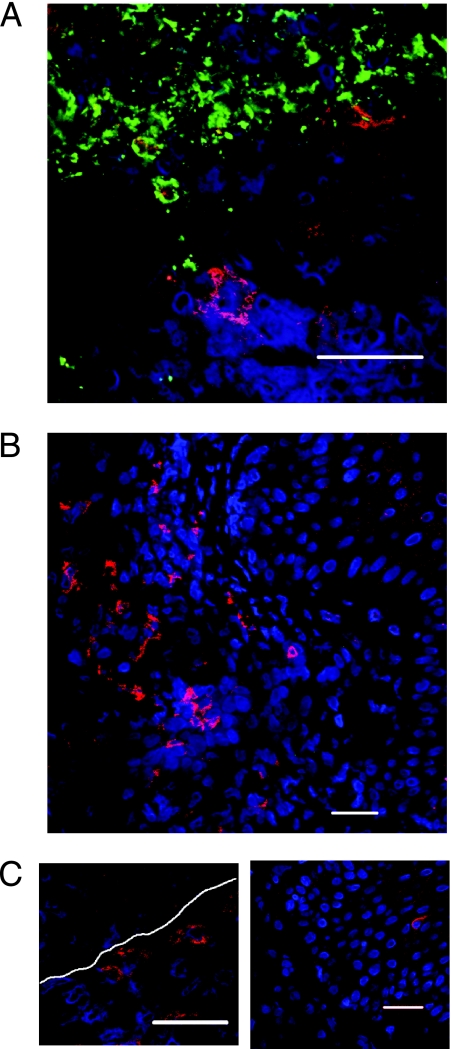
After obtaining informed consent, biopsies were taken from four patients with active recurrent HSV-2 lesions at 4 to 10 days after onset and frozen sections were stained with the following: antibody to BDCA-2 (DLEC), a pDC-specific antibody; TO-PRO-3, a nuclear stain and an antibody to HSV-1/-2 envelope; or CD123. Normal skin and tonsil biopsies were used as negative and positive controls, respectively. Initial studies showed colocalization of BDCA-2 and CD123 in skin biopsies (data not shown). As basophils also stain positive for CD123 (1), subsequent staining was with BDCA-2 alone. Plasmacytoid DC were found in four out of four lesion biopsies from different individuals (Fig. 1) but were not detected in normal skin (data not shown). One biopsy sample taken from normal skin adjacent to lesions did not show any signs of inflammatory histology, and there were no infiltrating cells present and no evidence of lesion. This sample was considered a normal control, and there were no pDC detected in this sample. In early lesion samples (day 4) where HSV was detected in keratinocytes of the epidermis, pDC were found nearby in the adjacent dermis (Fig. 1A). When detected in biopsies, the pDC were commonly observed singly or in clusters in the dermis (Fig. 1B and C) and occasionally singly in the epidermis (Fig. 1C, right panel). Within the dermis pDC were located mainly at or just beneath the epidermal-dermal junction (Fig. 1C, left panel). All sections showed a prominent mononuclear immune cell infiltrate in the dermis, as previously described (12).
FIG. 1.
Plasmacytoid pDC are detected in recurrent herpes simplex type 2 biopsies. Biopsies of herpes simplex type 2 lesions were stained for BDCA-2 (red), TO-PRO-3 (blue), and HSV-2 (green) and analyzed by confocal microscopy. (A) Biopsy of herpes simplex type 2 lesion stained with BDCA-2, TO-PRO-3, and HSV-2, indicating infiltration of pDC into virally infected lesions. (B) BDCA-2+ cells were commonly observed in the dermis. (C) BDCA-2+ cells were also found at the junction between the dermis and epidermis (left panel) and occasionally in the epidermis (right panel). White dotted lines indicate the dermo-epidermal junction as determined histologically. Bars, 10 μm.
pDC are resistant to infection with HSV-2 in vitro.
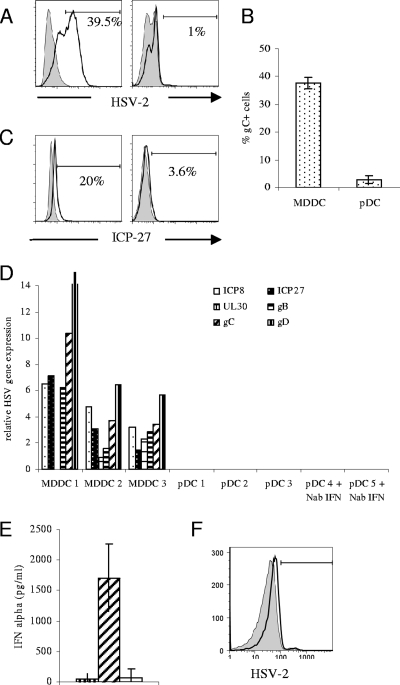
Having determined that pDC infiltrate dermis and accumulate near the dermo-epidermal junction, where they might contact HSV-infected keratinocytes during recurrent herpes lesions, the question of infectivity of pDC with HSV-2 in vitro was investigated. Previous work from our laboratory had shown productive HSV infection of MDDC, so these cells were used as a positive control. Monocyte-derived DC and pDC were exposed to HSV-2 at a multiplicity of infection of 5 for 1 h. The cells were washed three times and cultured for further times as described below. HSV-2 antigen expression was measured in pDC and MDDC after 16 h using a monoclonal antibody, flow cytometry-based HSV-2 diagnostic detection kit. These HSV-2 monoclonal antibodies recognize ribonucleotide reductase and glycoprotein B of HSV-2 expressed on infected cells. As expected MDDC were productively infected with 35 to 40% of live cells expressing HSV-2 glycoproteins on their cell surface (n = 3) (Fig. 2A, left panel). However, very low levels of glycoprotein were detected on the surface of isolated pDC (range, 0.7 to 4.8%; mean, 2.22%; n = 9) (Fig. 2A, right panel, and B).
FIG. 2.
Plasmacytoid DC exposed to HSV-2 in vitro are resistant to infection. MDDC and pDC were exposed to HSV-2 at a multiplicity of infection of 5. Also, pDC were cultured in the presence of neutralizing antibodies to IFN-α, IFN-β, and IFN-α/β receptor prior to exposure to HSV-2. (A and B) Surface HSV-2 gB expression was measured by flow cytometry 16 h postinfection. Representative data from one individual (A) (left panel, MDDC; right panel, pDC) and mean data from three MDDC and nine pDC donors (B) are shown. Error bars indicate standard deviations. (C) After 6 h of infection, intracellular ICP27 expression was measured by flow cytometry (left panel, MDDC; right panel, pDC). (D) At 16 h postinfection cells were lysed and quantitative PCR of six viral gene transcripts, representing immediate-early proteins (ICP8 and ICP27), early proteins (UL30), and late proteins (gB, gC, and gD) was performed. Expression of viral gene products relative to the housekeeping gene GAPDH are shown for each of three independent experiments. (E) IFN-α production by pDC 16 h postinfection. The first bar indicates mock-infected cells, the second shows results following HSV stimulation, and the third bar indicates ablation of IFN-α following culture in the presence of neutralizing antibodies to IFN-α, IFN-β, and IFN-α/β receptor. IFN-α was measured by ELISA, and the means of three separate experiments are shown. (F) Flow cytometric analysis of HSV-2 expression following culture in the presence of neutralizing antibodies to IFN-α, IFN-β, and IFN-α/β receptor indicates no increase in infectivity following treatment with neutralizing antibody. The gray histogram indicates infection of pDC with HSV (2.5%), and the black open histogram shows infection following addition of neutralizing antibodies to HSV (5%). Results shown are representative of three independent experiments.
An abortive viral infection has been documented in macrophages (37) where the replicative cycle ceased after transcription of immediate-early genes. To test whether this was a possibility in pDC, intracellular expression of the immediate-early HSV protein ICP27 was measured by flow cytometry 4 h after infection. Plasmacytoid DC did not express any ICP27, suggesting that pDC do not support even an abortive infection of HSV. In order to fully investigate this hypothesis a semiquantitative reverse transcriptase PCR was established in the laboratory to detect mRNA from six viral gene transcripts. A time course of infection of Vero cells and MDDC with HSV-2 was used to determine the optimal time of expression of the viral mRNA (data not shown). At 16 h postinfection all six gene transcripts were detected in both MDDC and in Vero cells, and this time point was used in subsequent studies for pDC infectivity. The viral RNAs measured in this assay were designed to cover the viral replication cycle cascade. There were two immediate-early genes (ICP8 and ICP27), an early gene (UL30), and three late genes (gB, gC, and gD) targeted in this approach. No viral transcripts were detected in pDC at 16 h postinfection, in contrast to MDDC, which showed high expression of all viral transcripts relative to cell numbers (detected using primers to GAPDH) (Fig. 2D). To rule out the possibility that RNA extraction of small numbers of pDC may skew the results, all infections of MDDC and pDC were carried out using 150,000 cells, and in two of three replicates both MDDC and pDC were obtained from the same donor.
The high levels of IFN-α in the cultures (Fig. 2E) were not responsible for the lack of infection, as addition of neutralizing antibody to IFN-α, IFN-β (sufficient to ablate IFN-α and IFN-β production), and the IFN-α/β receptor did not result in detection of any viral gene transcripts by quantitative PCR (Fig. 2D) or in any protein expression (Fig. 2F), suggesting that there was an alternative explanation for the lack of infection.
TLR9 expression of pDC is intracellular.
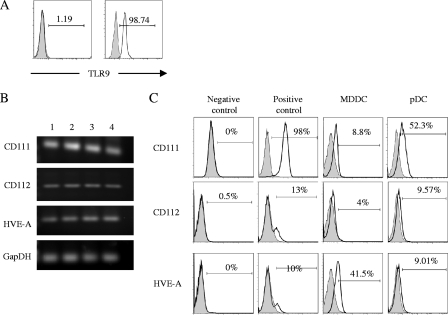
Since pDC produce IFN-α following HSV stimulation and this effect is mediated via TLR9, we examined the distribution of TLR9 on the surface and within the pDC. Consistent with previous reports of the intracellular location of TLR9 (27), little or no TLR9 was detected on the surface (mean, 2.96%; range, 0 to 8.4%; n = 6) (Fig. 3A, left panel) with 99% of cells expressing this pattern recognition receptor intracellularly (range, 98.74 to 99.54%; n = 3) (Fig. 3A, right panel), indicating HSV needs to enter endosomes for virus-TLR9 interactions.
FIG. 3.
Expression of TLR9 and HSV entry receptors by pDC. TLR9 expression by isolated pDC was measured by flow cytometry (A). Surface (left panel) and intracellular (right panel) staining were performed on freshly isolated pDC by using flow cytometry. Numbers indicate percentages of cells positive for TLR9. (B) PCR was performed using primers for the common HSV entry receptors. Both MDDC and pDC expressed all three HSV receptors. Results from three independent experiments are shown. Lane 1, pDC; lane 2, pDC + HSV; lane 3, MDDC; lane 4, MDDC + HSV. (C) Flow cytometric analysis of expression of HSV entry receptors on different cell types was performed using specific antibodies to CD111 (CK41), CD112 (MP9), and HVE-A (CW10). HeLa cells were used as negative control cells for expression of CD111 and positive controls for CD112 and HVE-A. Jurkat cells were negative controls for CD112 and HVE-A, and HL60 cells were positive controls for CD111. The first panel shows expression in negative control cell lines, the second is positive control expression, the third panel shows levels of expression on MDDC, and the fourth panel shows expression in pDC.
Expression of HSV entry receptors on pDC.
To further define the block in the HSV replication cycle, expression of HSV-specific receptors on pDC was examined. Specific cell surface receptors bind to HSV envelope glycoproteins to facilitate fusion and entry of virus into the cell (49). These receptors include heparan sulfate proteoglycans for gB and gC and herpes virus entry mediator A (HVE-A or HVEM), nectin 1 (CD111), and to a lesser extent nectin 2 (CD112) for gD. CD111 and CD112 are members of the immunoglobulin superfamily, while HVE-A is a member of the tumor necrosis factor receptor family (10, 36). Krummenacher et al. (26) have shown that both clinical and laboratory strains of HSV-1 utilize CD111 and HVE-A. However, CD112 is a weak receptor for HSV-2 or for gD mutant laboratory strains of HSV-1 (13).
We investigated whether pDC express the HSV entry receptors HVE-A and CD111 and CD112. Using a PCR approach we were able to show that pDC express mRNA for all three HSV entry receptors and that the level of expression was not altered by exposure to HSV (Fig. 3B). In addition, flow cytometric analysis of isolated pDC revealed moderate expression of CD111 (CK41; 28 to 60%; n = 3) and low levels of CD112 (MP9; 7.9 to 11%; n = 3) and HVE-A (CW10; 7.3 to 11%; n = 3) (Fig. 3C). The expression of CD111 and CD112 was higher on pDC than MDDC, but in contrast pDC had lower expression of HVE-A than MDDC. While both CD111 and HVE-A efficiently bind HSV strains, the strain used in this study (186) has been shown to use CD111 as its major receptor (26). In many cell lines the level of infection with HSV correlates with the level of expression of either CD111 or HVE-A. The relatively low expression of HVE-A on pDC would therefore be compensated for by the higher expression of CD111. The failure of pDC to become infected with HSV is therefore unlikely to result from a lack of HSV gD receptors. The expression of CD111 and HVE-A on MDDC was similar to that of Salio et al. (42); however, we found lower expression of CD112 on MDDC, which is probably due to the use of different antibodies.
Innate role of pDC in lesions.
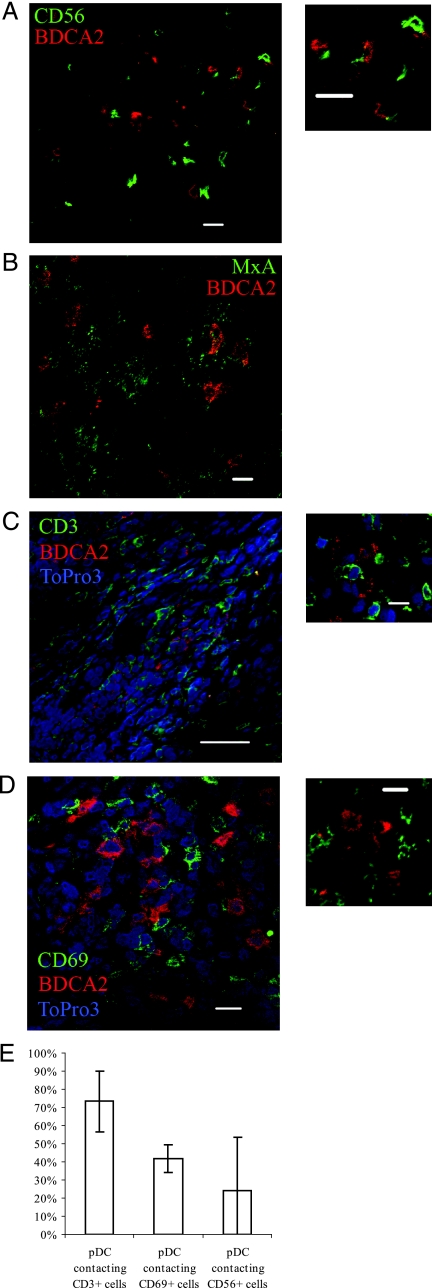
Plasmacytoid DC have been shown to interact with NK cells (18), and so to investigate whether NK cells and pDC interact in herpes lesions, biopsy slides were stained for BDCA-2 and CD56. CD56+ NK cells were detected in virally infected lesions (Fig. 4A and E). There was high variability in the numbers of pDC-NK interactions, which was related to the distance from the infected site. For example, close to the HSV-infected cells, approximately 60% of pDC were interacting with CD56+ NK cells, while deeper into the dermis the proportion of pDC interacting with NK cells fell to 18%.
FIG. 4.
Plasmacytoid DC interactions in herpes lesions. Biopsies of herpes simplex lesions were stained for BDCA-2 (red), TO-PRO-3 (blue), and (shown in green) for CD56 (A), MxA (B), CD3 (C), or CD69 (D) and analyzed by confocal microscopy. BDCA4+ pDC were found to interact with CD56+ NK cells, CD3+ T cells, and CD69+-activated cells, and they were also found in close proximity to MxA-producing cells. (E) Percentage of pDC contacting CD3, CD69, and CD56 cells in HSV lesions. Means of four fields are indicated. Bars, 10 μm.
MxA is a known interferon-stimulated protein, and the herpes lesion biopsies were found to express high levels of MxA, especially in the cells of the epidermis. The pDC themselves were not MxA antigen positive, but the cells in the dermis immediately surrounding them were MxA positive, suggesting that the IFN-α produced by pDC induces MxA production by neighboring cells (Fig. 4B).
Plasmacytoid DCs are associated with activated T lymphocytes in lesions.
Herpes lesion biopsies slides were stained for BDCA-2 and CD3 or CD69 to determine whether pDC were interacting with T lymphocytes in the lesions and whether these were activated. CD3+ cells were detected in abundance in the lesions (Fig. 4C and E), and often in association with pDC (Fig. 4C, insert). CD69 was used as a marker for activated T-cell status. As it was not possible to stain for CD3, CD69, and BDCA2 on the same slide, it was deduced that most CD69+ cells were T lymphocytes, as the numbers of CD56+ cells that were infiltrating the lesions was not sufficient to account for all the CD69+ cells present (Fig. 4A). BDCA-2+ pDC were frequently associated with CD69+ cells (Fig. 4D and E), with an estimated 42% ± 9.25% of all pDC in the lesions found to be in contact with a CD69+ cell (Fig. 4E).
HSV-2 exposed pDC stimulate T-lymphocyte proliferation.
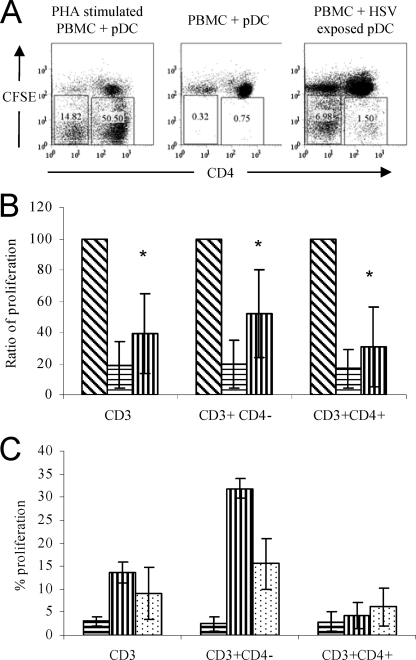
We postulated that these pDC might be able to induce HSV-specific autologous T-lymphocyte proliferation in HSV-1-seropositive subjects as pDC upregulate their costimulatory molecule expression and produce IFN-α in response to stimulation with live HSV (Fig. 2E and data not shown) (23). In our assay, pDC were exposed to HSV-2 for 1 h, washed, and cultured overnight. Stimulated pDC were washed and cocultured with autologous PBMC for a further 5 days before analysis of CFSE dilution by flow cytometry. There were interindividual differences in the capacity of T cells to proliferate. In order to normalize this variable mitogenic stimulation, PHA was used as an indicator of maximum T-cell response in each individual and the pDC-induced T-cell proliferation was then compared to this as a percentage of maximum proliferation. Plasmacytoid DC, exposed to HSV-2, were able to induce more (HSV-1/2 cross-reactive) proliferation of CD3 lymphocytes than unexposed pDC (normalized means of 38.8 and 18.9, respectively; n = 7; P < 0.05 [paired t test]). When divided into CD4+ and CD4- (i.e., CD8+) T lymphocytes there was a significant increase in proliferation induced by HSV-2 stimulated pDC in both T lymphocyte subsets compared to controls (Fig. 5), although this was most pronounced in the CD8 T-lymphocyte population.
FIG. 5.
Isolated pDC exposed to HSV-2 stimulate autologous T-lymphocyte proliferation. Isolated pDC were exposed to HSV-2 for 1 h, washed three times, and cultured for a further 16 h. pDC were cultured with autologous CFSE-labeled PBMC for 5 days, and the proliferation induced in CD3+ cells was determined by CFSE dilution by flow cytometry. CD3+ cells were further divided into CD3+ CD4+ and CD3+ CD4− fractions, and proliferation in each population was measured. (A) Representative flow cytometric data from one donor. The numbers in the boxes indicate percentages of CD3+ cells that proliferated. (B) Mean proliferation from seven independent individuals. Data were normalized against the proliferation induced by PHA stimulation (100%). Bars indicate the relative proliferation of autologous PBMC when cocultured with PHA (diagonal stripes), unstimulated pDC (horizontal stripes), and HSV-exposed pDC (vertical stripes). (C) pDC exposed to UV-inactivated HSV stimulate CD3 T-cell proliferation. Bars indicate percentages of CD3+, CD3+ CD4−, and CD3+ CD4+ cells in the proliferating gate (i.e., those cells that had a reduction in the expression of CFSE) following exposure to unstimulated pDC (horizontal stripes), HSV-exposed pDC (vertical stripes), and UV-inactivated HSV-exposed pDC (dots). Results are the means of two independent experiments. Error bars indicate standard deviations. *, P < 0.05, determined by paired t test, between unstimulated pDC and HSV-exposed pDC.
In dose-response experiments where pDC were exposed to differing MOIs of HSV, the levels of T-lymphocyte proliferation induced by pDC plateaued above an MOI of 1 (data not shown). This probably reflects the high responsiveness of pDC to stimulation with HSV and may be due to the presence of noninfectious virions within the HSV inoculum. UV inactivation of virus still induces IFN-α production by pDC. Plasmacytoid DCs exposed to UV-inactivated virus were able to induce HSV-specific T-cell proliferation, although at lower levels than the live virus (Fig. 5c) and again predominantly in the CD8 T-lymphocyte population. The reduced capacity to induce proliferation may be due to the UV inactivation of the viral DNA partially inhibiting TLR9 activation and consequent pDC maturation.
DISCUSSION
In this study we show for the first time that pDC infiltrate the dermis of recurrent genital herpes lesions. Data presented here indicate a role for these cells in the control of HSV spread in such lesions. The pDC were identified unequivocally by dual staining with BDCA-2 and CD123. Their distribution was interesting; although rare in the epidermis, they were found in the dermis and tended to accumulate selectively at the dermo-epidermal junction, where contact with HSV from infected keratinocytes is likely. The presence of pDC at the dermo-epidermal junction may contribute to the inability of HSV to spread into the dermis, by producing IFN-α and protecting dermal fibroblasts from infection with this virus. The production of MxA in cells surrounding pDC in the dermis supports such a role. Perhaps the observed presence of NK cells interacting with pDC contributes by augmenting IFN-α production and cytotoxic elimination of any cells which become infected with HSV. In vivo localization of pDC in herpes lesions provides an explanation for the recent findings in mouse models demonstrating that knockout of TLR9 (and of pDC) leads to enhanced spread of genital infection in mouse models (32). Our results are also supported by evidence of migration of pDC to the skin during allergy (21, 52) and more recently by a case report of pDC in lesions of a varicella patient (17). It is not clear from our data whether the pDC that infiltrate the lesion will migrate to the draining lymph nodes once they have taken up antigen in the skin. Plasmacytoid DC are potent inducers of cutaneous lymphocyte-associated antigen on HSV-reactive CD4 lymphocytes (25), and their presence in HSV lesions may enhance CD4 T-lymphocyte trafficking to the skin or enable them to remain in the lesion longer. In an elegant study by Zhu et al., HSV-2-specific CD8 T lymphocytes were found to persist at the dermo-epidermal junction even after the lesion had healed, suggesting CD8 T lymphocytes may play a role in local containment of viral replication after subsequent reactivation and shedding from intracutaneous nerve endings (54). Our data indicate that pDC preferentially induce CD8 T-lymphocyte proliferation and as such pDC may contribute to this local containment. As pDC were found in lesions up to 10 days after onset, we propose that pDC might play an additional role in the local containment of viral replication. Further evidence of a role of pDC in the control of HSV infection comes from a study that showed pDC-depleted mice were less able to control intravaginal HSV infection than wild-type mice (32). Delivery of CpGs to murine vaginal mucosa results in recruitment of inflammatory cells to the mucosa and protection from subsequent challenge with HSV (5). In this model, the antiviral effects of CpG were mediated through TLR9. Another study found low levels of pDC in noninflamed vaginal tissue, with elevated numbers following intravaginal treatment with CpG ODN (44).
Having found pDC in herpes simplex biopsy lesions, we proceeded to investigate the role that these cells may play during a recurrence. Our laboratory has previously shown that MDDC are infected by HSV and undergo apoptosis and that these apoptotic cells were then taken up by bystander MDDC which presented HSV antigen to CD8 T lymphocytes (7). In light of this, we investigated whether pDC were infected with HSV in vitro, and we found no viral transcripts or protein expression 16 to 24 h after inoculation. There have been relatively few studies of HSV infection of pDC, with one study mentioning a failure to detect GFP-HSV-1 after 24 h of culture (34), but to our knowledge no data have been shown. The focus has been more on blood-borne viruses, especially HIV-1. Plasmacytoid DC have been shown to be infected with HIV-1 both in vitro and in vivo (14, 16), but there is a high viral load in the blood and lymph nodes that provides the opportunity for circulating pDC to encounter and become infected with virus.
Plasmacytoid DC express receptors used by HSV-1 and -2 to infect cells via gD but are not productively infected and do not support viral gene replication, at least as demonstrated by production of early and late viral protein transcripts. Paradoxically, however, these pDC are able to respond to inactivated or live virus through the abundant production of IFN-α (28, 45, 48) through TLR9, most of which is found in endosomes (27). TLR9 signaling can be triggered by unmethylated CpG motifs, commonly found in viral and bacterial but not in mammalian DNA. HSV DNA has been shown to contain many CpG motifs (33). In unstimulated pDC, the TLR9 was shown to be colocalized with the endoplasmic reticulum marker calnexin (27). However, pDC rapidly internalized purified CpGs into a subcellular compartment and TLR9 was transported from the endoplasmic reticulum to these compartments (27). Furthermore, TLR9-CpG DNA interactions have been shown to occur in compartments in which the pH is 6.5 to 5, consistent with lysosomes/late endosomes (41). The intracellular location of TLR9 suggests that HSV-2 does bind and enter the endosomes of pDC but that there is a block of productive viral replication at a stage after binding to the gD receptors. Future studies will investigate the mechanisms of binding and uptake of HSV into endosomes and a possible role for the C-type lectin receptor BDCA-2 in HSV-2 binding, like DC-SIGN concentrating virus on MDDCs. Presumably, the HSV is rapidly destroyed within endosomes producing insufficient viral proteins for detection by our intracellular staining assays. The stage and mechanisms of the block in virus entry and replication will need to be further defined but they do not appear to be IFN-α dependent, as shown by the failure of neutralization of the majority of IFN-α to enhance HSV infection of pDC.
As pDC are mature after exposure to HSV, in terms of upregulating costimulatory molecule expression and cytokine secretion, they may be able to stimulate T-lymphocyte proliferation when skin DC (Langerhans cells and/or dermal dendritic cells) are functionally impaired due to viral infection. Therefore, pDC may provide an auxiliary antigen presentation capacity. In mice, activation of pDC following exposure to HSV is delayed until pDC enter the lymph nodes (53), but due to constraints of obtaining lymph nodes from humans, there are currently no human data to support this. By visualizing pDC in lesions in close proximity to CD69+ T lymphocytes in recurrent herpes lesions we conclude that in humans, antigen presentation by pDC to T lymphocytes could also be occurring locally in vivo where antigen taken up at the dermo-epidermal junction is presented by pDC to locally infiltrating T lymphocytes, initially CD4 lymphocytes and then CD8 lymphocytes. Infected Langerhans cells are also probably migrating to the draining lymph nodes where HSV antigen is passed on to resident DC in lymph nodes (equivalent to murine CD8α+ DC) or subsequent stimulation of CD8 lymphocytes as described recently in mouse models (3, 4).
The ability of pDC to induce CD8 T-lymphocyte proliferation in the absence of any detectable infection indicates a capacity of these cells to cross-present antigens. This function of pDC is somewhat controversial with mouse models suggesting an inability of pDC to cross-present antigen (reviewed in reference 50). However, our data and another study (20) in humans clearly indicate that pDC are able to cross-present antigens and stimulate CD8 T-lymphocyte proliferation.
The presence of pDC in recurrent herpetic lesions should lead to further definition of their function and importance in the immune response, particularly their role in the early phases of lesions and whether they play a different role in initial as well as recurrent herpes infection.
Acknowledgments
We thank Gary Cohen, Roslyn Eisenberg, and Claude Krummenacher at the University of Pennsylvania for supplying CD111, CD112, and HVE-A antibodies. We also thank Otto Haller at the Department of Virology of the University of Freiburg for supplying MxA antibody.
Footnotes
Published ahead of print on 10 December 2008.
REFERENCES
- 1.Agis, H., W. Fureder, H. C. Bankl, M. Kundi, W. R. Sperr, M. Willheim, G. Boltz-Nitulescu, J. H. Butterfield, K. Kishi, K. Lechner, and P. Valent. 1996. Comparative immunophenotypic analysis of human mast cells, blood basophils and monocytes. Immunology 87535-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albers, I., H. Kirchner, and I. Domke-Opitz. 1989. Resistance of human blood monocytes to infection with herpes simplex virus. Virology 169466-469. [DOI] [PubMed] [Google Scholar]
- 3.Allan, R. S., C. M. Smith, G. T. Belz, A. L. van Lint, L. M. Wakim, W. R. Heath, and F. R. Carbone. 2003. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science 3011925-1928. [DOI] [PubMed] [Google Scholar]
- 4.Allan, R. S., J. Waithman, S. Bedoui, C. M. Jones, J. A. Villadangos, Y. Zhan, A. M. Lew, K. Shortman, W. R. Heath, and F. R. Carbone. 2006. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25153-162. [DOI] [PubMed] [Google Scholar]
- 5.Ashkar, A. A., S. Bauer, W. J. Mitchell, J. Vieira, and K. L. Rosenthal. 2003. Local delivery of CpG oligodeoxynucleotides induces rapid changes in the genital mucosa and inhibits replication, but not entry, of herpes simplex virus type 2. J. Virol. 778948-8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beignon, A.-S., K. McKenna, M. Skoberne, O. Manches, I. DaSilva, D. G. Kavanagh, M. Larsson, R. J. Gorelick, J. D. Lifson, and N. Bhardwaj. 2005. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Investig. 1153265-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosnjak, L., M. Miranda-Saksena, D. M. Koelle, R. A. Boadle, C. A. Jones, and A. L. Cunningham. 2005. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J. Immunol. 1742220-2227. [DOI] [PubMed] [Google Scholar]
- 8.Braun, R., H. Teute, H. Kirchner, and K. Munk. 1984. Replication of herpes simplex virus in human T lymphocytes: characterization of the viral target cell. J. Immunol. 132914-919. [PubMed] [Google Scholar]
- 9.Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1305-310. [DOI] [PubMed] [Google Scholar]
- 10.Cocchi, F., L. Menotti, P. Mirandola, M. Lopez, and G. Campadelli-Fiume. 1998. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 729992-10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham, A. L., R. Taylor, J. Taylor, C. Marks, J. Shaw, and A. Mindel. 2006. Prevalence of infection with herpes simplex virus types 1 and 2 in Australia: a nationwide population based survey. Sex. Transm. Infect. 82164-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham, A. L., R. R. Turner, A. C. Miller, M. F. Para, and T. C. Merigan. 1985. Evolution of recurrent herpes simplex lesions. An immunohistologic study. J. Clin. Investig. 75226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean, H. J., S. S. Terhune, M. T. Shieh, N. Susmarski, and P. G. Spear. 1994. Single amino acid substitutions in gD of herpes simplex virus 1 confer resistance to gD-mediated interference and cause cell-type-dependent alterations in infectivity. Virology 19967-80. [DOI] [PubMed] [Google Scholar]
- 14.Donaghy, H., B. Gazzard, F. Gotch, and S. Patterson. 2003. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood 1014505-4511. [DOI] [PubMed] [Google Scholar]
- 15.Fonteneau, J.-F., M. Gilliet, M. Larsson, I. Dasilva, C. Munz, Y.-J. Liu, and N. Bhardwaj. 2003. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood 1013520-3526. [DOI] [PubMed] [Google Scholar]
- 16.Fonteneau, J.-F., M. Larsson, A.-S. Beignon, K. McKenna, I. Dasilva, A. Amara, Y.-J. Liu, J. D. Lifson, D. R. Littman, and N. Bhardwaj. 2004. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J. Virol. 785223-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerlini, G., G. Mariotti, B. Bianchi, and N. Pimpinelli. 2005. Massive recruitment of type I interferon producing plasmacytoid dendritic cells in varicella skin lesions. J. Investig. Dermatol. 126507-509. [DOI] [PubMed] [Google Scholar]
- 18.Gerosa, F., A. Gobbi, P. Zorzi, S. Burg, F. Briere, G. Carra, and G. Trinchieri. 2005. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J. Immunol. 174727-734. [DOI] [PubMed] [Google Scholar]
- 19.Hochrein, H., B. Schlatter, M. O'Keeffe, C. Wagner, F. Schmitz, M. Schiemann, S. Bauer, M. Suter, and H. Wagner. 2004. Herpes simplex virus type-1 induces IFN-α production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 10111416-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoeffel, G., A.-C. Ripoche, D. Matheoud, M. Nascimbeni, N. Escriou, P. Lebon, F. Heshmati, J.-G. Guillet, M. Gannage, S. Caillat-Zucman, N. Casartelli, O. Schwartz, H. De la Salle, D. Hanau, A. Hosmalin, and C. Maranon. 2007. Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity 27481-492. [DOI] [PubMed] [Google Scholar]
- 21.Jahnsen, F. L., F. Lund-Johansen, J. F. Dunne, L. Farkas, R. Haye, and P. Brandtzaeg. 2000. Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J. Immunol. 1654062-4068. [DOI] [PubMed] [Google Scholar]
- 22.Jones, C. A., M. Fernandez, K. Herc, L. Bosnjak, M. Miranda-Saksena, R. A. Boadle, and A. Cunningham. 2003. Herpes simplex virus type 2 induces rapid cell death and functional impairment of murine dendritic cells in vitro. J. Virol. 7711139-11149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadowaki, N., S. Antonenko, J. Y.-N. Lau, and Y.-J. Liu. 2000. Natural interferon α/β-producing cells link innate and adaptive immunity. J. Exp. Med. 192219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawamura, K., N. Kadowaki, T. Kitawaki, and T. Uchiyama. 2006. Virus-stimulated plasmacytoid dendritic cells induce CD4+ cytotoxic regulatory T cells. Blood 1071031-1038. [DOI] [PubMed] [Google Scholar]
- 25.Koelle, D. M., J. Huang, M. T. Hensel, and C. L. McClurkan. 2006. Innate immune responses to herpes simplex virus type 2 influence skin homing molecule expression by memory CD4+ lymphocytes. J. Virol. 802863-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krummenacher, C., F. Baribaud, M. Ponce de Leon, I. Baribaud, J. C. Whitbeck, R. Xu, G. H. Cohen, and R. J. Eisenberg. 2004. Comparative usage of herpesvirus entry mediator A and nectin-1 by laboratory strains and clinical isolates of herpes simplex virus. Virology 322286-299. [DOI] [PubMed] [Google Scholar]
- 27.Latz, E., A. Schoenemeyer, A. Visintin, K. A. Fitzgerald, B. G. Monks, C. F. Knetter, E. Lien, N. J. Nilsen, T. Espevik, and D. T. Golenbock. 2004. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5190-198. [DOI] [PubMed] [Google Scholar]
- 28.Liu, Y.-J. 2005. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23275-306. [DOI] [PubMed] [Google Scholar]
- 29.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 30.Lore, K., A. Smed-Sorensen, J. Vasudevan, J. R. Mascola, and R. A. Koup. 2005. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J. Exp. Med. 2012023-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lund, J. M., M. M. Linehan, N. Iijima, and A. Iwasaki. 2006. Cutting edge: plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J. Immunol. 1777510-7514. [DOI] [PubMed] [Google Scholar]
- 33.Lundberg, P., P. Welander, X. Han, and E. Cantin. 2003. Herpes simplex virus type 1 DNA is immunostimulatory in vitro and in vivo. J. Virol. 7711158-11169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Megjugorac, N. J., E. S. Jacobs, A. G. Izaguirre, T. C. George, G. Gupta, and P. Fitzgerald-Bocarsly. 2007. Image-based study of interferongenic interactions between plasmacytoid dendritic cells and HSV-infected monocyte-derived dendritic cells. Immunol. Investig. 36739-761. [DOI] [PubMed] [Google Scholar]
- 35.Mikloska, Z., L. Bosnjak, and A. L. Cunningham. 2001. Immature monocyte-derived dendritic cells are productively infected with herpes simplex virus type 1. J. Virol. 755958-5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87427-436. [DOI] [PubMed] [Google Scholar]
- 37.Morahan, P. S., S. Mama, F. Anaraki, and K. Leary. 1989. Molecular localization of abortive infection of resident peritoneal macrophages by herpes simplex virus type 1. J. Virol. 632300-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peretti, S., A. Shaw, J. Blanchard, R. Bohm, G. Morrow, J. D. Lifson, A. Gettie, and M. Pope. 2005. Immunomodulatory effects of HSV-2 infection on immature macaque dendritic cells modify innate and adaptive responses. Blood 1061305-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rissoan, M. C., V. Soumelis, N. Kadowaki, G. Grouard, F. Briere, R. de-Waal-Malefyt, and Y. J. Liu. 1999. Reciprocal control of T helper cell and dendritic cell differentiation. Science 2831183-1186. [DOI] [PubMed] [Google Scholar]
- 40.Romani, N., S. Gruner, D. Brang, E. Kampgen, A. Lenz, B. Trockenbacher, G. Konwalinka, P. Fritsch, R. Steinman, and G. Schuler. 1994. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 18083-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rutz, M., J. Metzger, T. Gellert, P. Luppa, G. B. Lipford, H. Wagner, and S. Bauer. 2004. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. Eur. J. Immunol. 342541-2550. [DOI] [PubMed] [Google Scholar]
- 42.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 293245-3253. [DOI] [PubMed] [Google Scholar]
- 43.Sarmiento, M., and E. S. Kleinerman. 1990. Innate resistance to herpes simplex virus infection. Human lymphocyte and monocyte inhibition of viral replication. J. Immunol. 1441942-1953. [PubMed] [Google Scholar]
- 44.Shen, H., and A. Iwasaki. 2006. A crucial role for plasmacytoid dendritic cells in antiviral protection by CpG ODN-based vaginal microbicide. J. Clin. Investig. 1162237-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siegal, F. P., N. Kadowaki, M. Shodell, B. P. Fitzgerald, K. Shah, S. Ho, S. Antonenko, and Y. J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 2841835-1837. [DOI] [PubMed] [Google Scholar]
- 46.Smed-Sorensen, A., K. Lore, J. Vasudevan, M. K. Louder, J. Andersson, J. R. Mascola, A.-L. Spetz, and R. A. Koup. 2005. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J. Virol. 798861-8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith, J. S., and N. J. Robinson. 2002. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J. Infect. Dis. 186(Suppl. 1)S3-S28. [DOI] [PubMed] [Google Scholar]
- 48.Soumelis, V., I. Scott, F. Gheyas, D. Bouhour, G. Cozon, L. Cotte, L. Huang, J. A. Levy, and Y. J. Liu. 2001. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood 98906-912. [DOI] [PubMed] [Google Scholar]
- 49.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 2751-8. [DOI] [PubMed] [Google Scholar]
- 50.Villadangos, J. A., and L. Young. 2007. Antigen presentation properties of plasmacytoid dendritic cells. Immunity 29352-361. [DOI] [PubMed] [Google Scholar]
- 51.Watson, S., S. Mercier, C. Bye, J. Wilkinson, A. Cunningham, and A. Harman. 2007. Determination of suitable housekeeping genes for normalisation of quantitative real time PCR analysis of cells infected with human immunodeficiency virus and herpes viruses. Virology J. 4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wollenberg, A., M. Wagner, S. Gunther, A. Towarowski, E. Tuma, M. Moderer, S. Rothenfusser, S. Wetzel, S. Endres, and G. Hartmann. 2002. Plasmacytoid dendritic cells: a new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J. Investig. Dermatol. 1191096-1102. [DOI] [PubMed] [Google Scholar]
- 53.Yoneyama, H., K. Matsuno, Y. Zhang, T. Nishiwaki, M. Kitabatake, S. Ueha, S. Narumi, S. Morikawa, T. Ezaki, B. Lu, C. Gerard, S. Ishikawa, and K. Matsushima. 2004. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int. Immunol. 16915-928. [DOI] [PubMed] [Google Scholar]
- 54.Zhu, J., D. M. Koelle, J. Cao, J. Vazquez, M. L. Huang, F. Hladik, A. Wald, and L. Corey. 2007. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J. Exp. Med. 204595-603. [DOI] [PMC free article] [PubMed] [Google Scholar]