Abstract
Several studies have recently demonstrated the existence of human T-cell leukemia virus type 1 (HTLV-1) antisense transcripts, which allow the synthesis of the newly described HBZ protein. Although previous reports have been aimed at understanding the potential role of the HBZ protein in HTLV-1 pathogenesis, little is known as to how this viral gene is regulated. Here, using our K30-3′asLuc reporter construct, we show that the viral Tax protein upregulates antisense transcription through its action on the TRE sequences located in the 3′ long terminal repeat. Generation of stable clones in 293T cells demonstrated that Tax-induced HBZ expression is importantly influenced by the integration site in the host genome. The cellular DNA context could thus affect the level of HBZ mRNA expression in infected cells.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia/lymphoma (ATLL) and HTLV-1-associated myelopathy, also known as tropical spastic paraparesis (13, 29, 34, 35, 37, 46), leading to disease development in less than 5% of infected individuals (42). The HTLV-1 Tax protein is generally considered to play a central role in the viral pathogenesis, although its precise function remains to be determined. Tax transactivates the viral promoter, mainly through three Tax responsive element 1 (TRE1) repeats located in the U3 region of the 5′ long terminal repeat (LTR) (14, 15, 17, 19, 44). Each TRE1 repeat contains an imperfect cyclic AMP response element (CRE), and they are crucial for both basal and Tax-induced promoter activity (14, 15, 17, 19, 44). Tax associates with TRE1-bound ATF/CREB transcription factors and recruits the transcriptional coactivator CBP/p300, which results in a strong transcriptional activation (12, 22). Tax also upregulates the expression of cellular genes through the activation of several transcription factors, such as CREB-1, NF-κB and SRF (1, 30, 45).
Recently, we (6) and others (31, 39) have clearly demonstrated the existence of antisense transcripts in HTLV-1, which initiate from the 3′ LTR. These transcripts are alternatively spliced and encode the newly described HTLV-1 bZIP factor (HBZ) through an ATG initiation codon being present in exon 1 located in the 3′ LTR. Interestingly, HBZ downregulates the Tax-dependent transactivation of viral gene expression through the formation of heterodimers with CREB-1 and CREB-2. In addition, HBZ interacts with c-Jun, JunB, and JunD and modulates their transcriptional activities (4, 24, 41). Recent studies have shown that HBZ is expressed in all ATL cell lines, suggesting that it might play an unsuspected role in the biological process leading to ATLL development (28, 43).
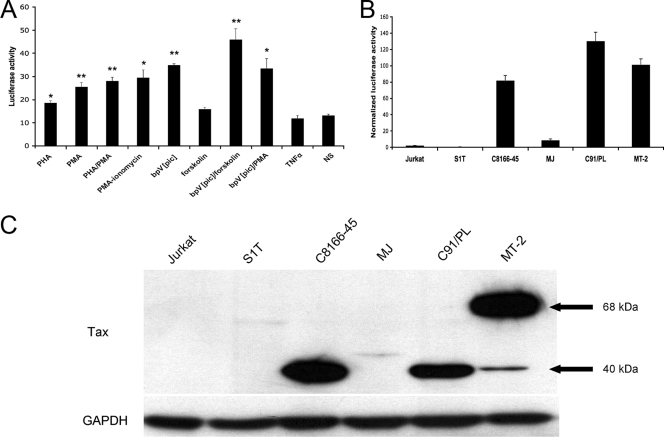
Despite the increasing number of studies aimed at understanding the role of HBZ in viral pathogenesis, little is known about the promoter region regulating its expression. In order to clarify this issue, we have performed several transfection experiments using our previously described K30-3′asLuc construct (6). In this construct, a cassette containing the luciferase reporter gene and the simian virus 40 poly(A) signal was cloned in the antisense orientation in a 5′ LTR-deleted version of the full-length HTLV-1 K30 proviral DNA. More precisely, the luciferase reporter gene lies downstream of the 5′ end of HBZ exon 2 while remaining in frame of the HBZ open reading frame. Jurkat cells were transiently transfected with K30-3′asLuc by electroporation (250 V, 950 μF) and stimulated or left untreated for 8 h with different T-cell activators. At 16 h posttransfection, luciferase activity was measured as previously described (20). The antisense promoter was shown to be inducible in stimulated Jurkat cells by phytohemagglutinin and phorbol myristate acetate but also, more importantly, by a combination of the protein tyrosine phosphatase inhibitor bpV[pic] and forskolin (Fig. 1A) (20). Since bpV[pic]/forskolin treatment has been previously shown to upregulate HTLV-1 sense gene expression by activating CREB transcription factors (32, 36), these results suggested a possible role for this transcription factor in antisense promoter activity. We next transfected various HTLV-1-infected T-cell lines with K30-3′asLuc along with the β-galactosidase-expressing plasmid pRc-actin-LacZ by using the same electroporation conditions as those described above. The β-galactosidase activity was measured using the Galacto-Light kit (Applied Biosystems, Bedford, MS). Interestingly, the results suggested that antisense promoter activity was higher in HTLV-1-infected cells than in noninfected Jurkat cells, with the exception of the Tax-nonexpressing S1T cell line (23). A strong luciferase signal was obtained for virion-producing cell lines MT-2 and C91/PL, while MJ cells, which produce less viral particles (unpublished data), showed lower luciferase activity. In addition, non-HTLV-1-producing Tax-expressing C8166-45 cells (5) showed a signal comparable to the one obtained with the MT-2 and C91/PL cells (Fig. 1B). Hence, these results suggested a possible implication of the Tax protein on the regulation of antisense promoter activity. To test this potential implication of Tax, levels of Tax protein were evaluated. Cell extract preparation and Western blot analysis were conducted as previously described (6). Using our anti-Tax antibody (dilution, 1/100) (2) along with a horseradish peroxidase-coupled anti-mouse immunoglobulin G (1/10,000) (Santa Cruz Biotechnology Inc., Santa Cruz, CA), Tax was detected in C8166-45, C91/PL, and MT-2 cells (Fig. 1C). In MT-2 cells, a 68-kDa protein was detected and corresponded to a previously identified Tax-Env fusion protein, which has been suggested to be also functionally active (7, 18). No specific band could be detected for the MJ, S1T, and Jurkat cell lines. Equal loads of extracts were confirmed with GAPDH (glyceraldehyde-2-phosphate dehydrogenase) by using a mouse anti-GAPDH antibody (1/1,000; Santa Cruz Biotechnology Inc.). The absence of a Tax-specific band in MJ cells was most likely due to low expression level, since reverse transcriptase PCR (RT-PCR) confirmed the presence of Tax mRNA in this cell line (data not shown).
FIG. 1.
HTLV-1 antisense promoter activity in stimulated Jurkat cells and HTLV-1-infected cells. (A) Jurkat cells were transfected with 15 μg of K30-3′asLuc and stimulated for 8 h with various T-cell-activating agents (3 μg/ml phytohemagglutinin, 20 ng/ml phorbol myristate acetate, 1 μM ionomycin, 15 μM bpV[pic], 10 μM forskolin, 20 ng/ml tumor necrosis factor alpha [TNF-α]) at 16 h posttransfection or left untreated (no stimulation [NS]). Cells were monitored for luciferase activity. Luciferase activity values are presented as the means ± standard deviations (SD) of the results for triplicates. Student's t tests were performed to determine significant differences between samples and the unstimulated control. *, P < 0.05; **, P < 0.01. (B) Jurkat cells and various HTLV-1-infected cells were transfected with 15 μg K30-3′asLuc along with 4 μg pRc-actin-LacZ. Cells were lysed 24 h posttransfection and monitored for luciferase and β-galactosidase activities. Luciferase values are presented as the means ± SD of the results for triplicates and are expressed as normalized RLU (RLU/β-galactosidase). (C) The amount of Tax protein expressed in each cell line was assessed by Western blotting. Detection of GAPDH is shown as a loading control.
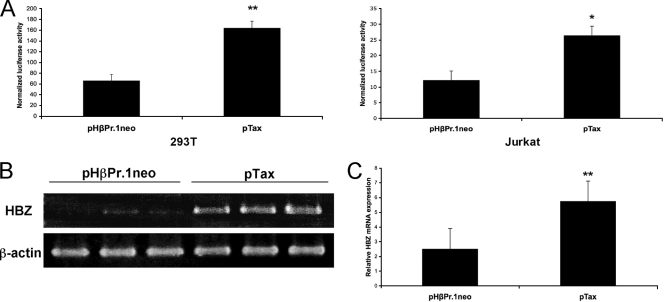
As the presence of Tax correlated with high levels of antisense transcription-driven luciferase activity, we then performed several cotransfection experiments with 293T and Jurkat cells (as outlined in reference 6). In both cell types, cotransfection of a Tax expression vector, pTax (25), and K30-3′asLuc resulted in a statistically significant increase in luciferase activity compared to the control (Fig. 2A). Because LTR-driven transcription is differently regulated in a plasmidic context in comparison to the chromatin context (27, 33), the effect of Tax expression on antisense promoter activity was further evaluated in an integrated proviral DNA. As initial RT-PCR experiments indicated the presence of HBZ spliced mRNA in Tax-nonproducing S1T cells, S1T cells were thus electroporated (see conditions described above) with a Tax-expressing vector (versus pHβPr.1neo) and analyzed for the presence of the major HBZ spliced transcript by RT-PCR. PCR amplifications were conducted on oligo(dT)-primed cDNAs with a forward primer spanning the HBZ mRNA splice junction (5′-ATGGCGGCCTCAGGGCTGT-3′) and a reverse primer located in the HBZ coding region (5′-TGGAGGGCCCCGTCGCAG-3′) and with β-actin-specific primers (5′-CGTGACATTAAGGAGAAGCTG-3′ and 5′-CTCAGGAGGAGCAATGATCTT-3′). PCR conditions were as follows: a first step of denaturation at 95°C for 10 s, followed by 35 (standard) or 50 (real-time) cycles of denaturation (94°C for 3 s), annealing (60°C for 15 s), and elongation (72°C for 12 s). Real-time RT-PCR experiments were performed under these conditions using the SYBR Premix Ex Taq (perfect real time) kit. The HPRT-1 gene served as the internal control with the following forward and reverse primers: 5′-AAGCTTGCGACCTTGACC-3′ and 5′-GACCAGTCAACAGGGGACATAA-3′. Both real-time and standard semiquantitative RT-PCR experiments showed a significant increase in HBZ mRNA level in the Tax-transfected S1T cells, thus confirming the results obtained with our luciferase reporter construct (Fig. 2B and C).
FIG. 2.
Upregulation of antisense transcription by the viral Tax protein. (A) 293T and Jurkat cells were transfected with 500 ng of K30-3′asLuc, 200 ng of pRc-actin-LacZ, and 300 ng of pTax or the empty vector pHβPr.1neo. Cells were lysed 48 h posttransfection and monitored for luciferase and β-galactosidase activities. Data for luciferase activity represent the mean values ± SD of the results for three measured samples and are expressed as normalized RLU (RLU/β-galactosidase). (B and C) S1T cells were electroporated with 15 μg of pTax or pHβPr.1neo. Total RNA was isolated 48 h posttransfection, and the HBZ spliced mRNA was amplified by standard (B) or real-time (C) RT-PCR, using β-actin or HPRT-1 as an internal control, respectively. These results are representative of three independent experiments and are presented in independently transfected triplicates. Student's t tests were performed to determine significant differences between the samples (*, P < 0.05; **, P < 0.01).
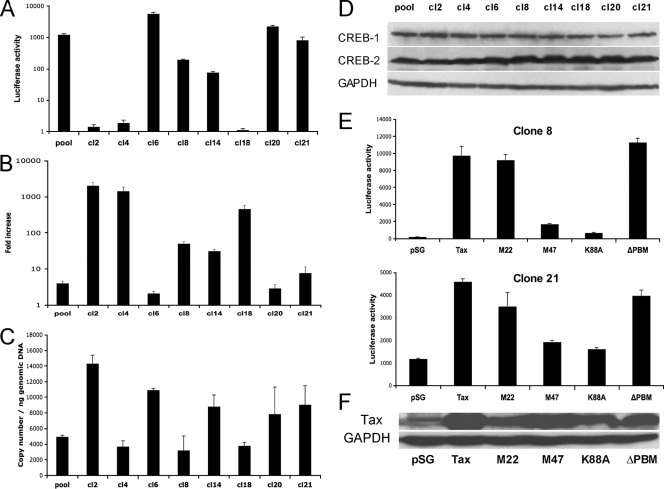
Since electroporation of S1T cells resulted in about 5 to 10% of transfected cells (data not shown) and might account for the low level of induction, 293T cells were next stably transfected with K30-3′asLuc and pCMV-Hyg at a 10:1 ratio using Lipofectamine 2000 (Invitrogen). Individual clones were selected in the presence of 200 μg/ml hygromycin B and then tested for luciferase activity. As shown in Fig. 3 A, we first noted important variations in basal luciferase activities in the different clones (from 1 to 5,564 relative light units [RLU]), suggesting that the integration sites importantly influenced the basal activity of the HBZ promoter. Transfection of the 293T cell clones with pTax resulted in an important induction of luciferase activity, reaching activity levels increased up to 2,000-fold for certain clones (Fig. 3B). The observed Tax inductions were generally inversely correlated with basal luciferase activity levels of the different clones.
FIG. 3.
Tax-induced antisense promoter activity is influenced by the integration site. (A) 293T cells were stably transfected with 4 μg K30-3′asLuc and 400 ng pCMV-Hyg. Different clones and a pool of clones were measured for luciferase activity. Data for luciferase activity represent the mean value ± SD of the results for three measured samples. (B) Pooled clones and different 293T cell clones were transfected with 800 ng of pTax or the empty vector and monitored for luciferase activity 48 h after transfection. Results are presented as the amount of increase in normalized luciferase activity measured for Tax-expressing cells over the activity of cells transfected with the control vector. (C) Real-time PCR amplification of the luciferase gene was conducted in order to quantify the integrated plasmid copy number in each clone. Results were normalized with the HPRT-1 gene and are shown as the number of copies per nanogram of genomic DNA. (D) Western blot analysis for CREB-1, CREB-2, and GAPDH (loading control) was performed on 50 μg of total protein extracts from each 293T cell clone. (E) Two stable K30-3′asLuc clones were transfected with 800 ng pSG-Tax, pSG-Tax M22, pSG-Tax M47, pSG-Tax K88A, pSG-Tax ΔPBM, or the empty pSG-5 vector. Cells were lysed 48 h posttransfection and monitored for luciferase activity. Data for luciferase activity represent the mean values ± SD of the results for three measured samples. (F) Tax levels from each expression vector were assessed by Western blot analysis using Tax antiserum. GAPDH levels were analyzed as a control.
Assessment of integrated plasmidic DNA copy numbers was then conducted by real-time PCR on 100 ng of genomic DNA of the different clones using luciferase-specific forward (5′-TGTTGTTCCATTCCATCACG-3′) and reverse (5′-TGGCGAAGAAGGAGAATAGG-3′) primers (Fig. 3C). Each DNA sample was normalized with the HPRT-1 control gene amplification. A standard curve was similarly amplified in parallel and consisted of 4.7 × 103 to 4.7 × 107 copies of pGL3basic DNA diluted in 100 ng of nontransfected 293T genomic DNA. Results showed variations in the normalized copy numbers among the different clones, ranging from 3,200 to 14,300 copies/ng of genomic DNA, but these variations did not correlate with basal or Tax-induced levels of luciferase activity, indicating that the number of integrated plasmids was not responsible for the observed differences in luciferase activity levels. In parallel, Western blot analyses were carried out using mouse anti-CREB-1 and anti-CREB-2 antibodies (1/500; Santa Cruz Biotechnology Inc.). Results showed weak variations in the expression of these transcription factors in the different clones (Fig. 3D).
To better understand this Tax-dependent upregulation of HBZ gene expression, two representative clones (8 and 21) were transfected with vectors expressing various Tax mutants (11, 38). Compared to the Tax-mediated increase in luciferase gene expression, Tax M47 and Tax K88A mutants (deficient for CREB-dependent transcriptional activation and CBP/p300 recruitment, respectively) resulted in a more limited increase in the activation of luciferase activity. In contrast, an NF-κB activation-defective Tax (Tax M22) or a PDZ binding-motif-mutated Tax (Tax ΔPBM) led to levels of induction comparable to those of wild-type Tax-transfected cells (Fig. 3E). Detection of Tax protein levels using a Tax antiserum (1/1,000; NIH AIDS Research and Reference Reagent Program, Germantown, MD) and horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (1/10,000; Santa Cruz Biotechnology Inc.) demonstrated that the low induction levels observed for the M47 and K88A mutants could not be attributed to a lower protein expression (Fig. 3F). These results therefore suggested that Tax induction of the antisense promoter requires the participation of CREB.
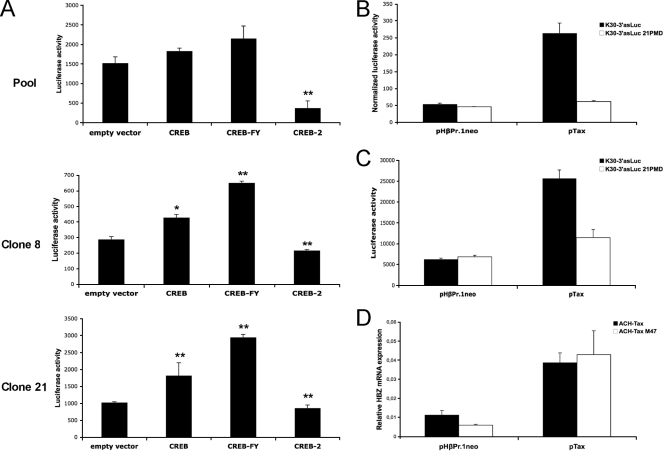
In order to confirm that CREB can directly modulate HBZ promoter activity, expression vectors for wild-type CREB, a constitutively active CREB mutant (CREB-FY) (9) and CREB-2 (10) were transfected into stable 293T cell clones 8 and 21 or into pooled, stably transfected 293T cells. Results demonstrated that both wild-type CREB and CREB-FY could potently increase luciferase activity, albeit at a lower level than the one observed when Tax is expressed. On the other hand, CREB-2 repressed luciferase gene expression to levels lower than those of the basal activity (Fig. 4A). The negative effect of CREB-2 on the antisense promoter is not surprising, as this transcriptional factor has been reported to repress the expression of cellular genes containing CREs in their promoter (16). It is likely that CREB-2 on its own downregulates antisense transcription by interacting with CBP/p300, thus preventing it from binding transcription factors positioned on the 3′ LTR, although it might have a positive role in the presence of Tax, as previously reported (10).
FIG. 4.
Positive regulation of the antisense promoter by CREB. (A) Two 293T cells clones stably transfected with K30-3′asLuc and the isolated pool were transfected with 800 ng of expression vectors for CREB, CREB-FY, or CREB-2 or the empty vector. Cells were lysed 48 h posttransfection and monitored for luciferase activity. Data for luciferase activity represent the mean values ± SD of the results for three measured samples. (B) 293T cells were transfected with 500 ng of K30-3′asLuc or K30-3′asLuc 21PMD, 200 ng pRc-actin-LacZ, and 300 ng of pTax (versus pHβPr.1neo). Cells were lysed 48 h posttransfection and monitored for luciferase and β-galactosidase activities. Data for luciferase activity represent the mean values ± SD of the results for three measured samples and are expressed as normalized RLU (RLU/β-galactosidase). (C) 293T cells stably transfected with K30-3′asLuc or K30-3′asLuc 21PMD were transfected with 800 ng of pTax or pHβPr.1neo. Cells were lysed 48 h posttransfection and monitored for luciferase activity. Data for luciferase activity represent the mean values ± SD of the results for three measured samples. (D) Wild type- or Tax M47-expressing ACH proviral DNA (500 ng) was transfected into 293T cells with 200 ng of pRc-actin-LacZ and 300 ng of pTax or pHβPr.1neo. HBZ mRNA expression levels were quantified by real-time RT-PCR, using the HPRT-1 gene as a reference control. *, P < 0.05; **, P < 0.01.
Based on the above-described CREB-1-dependent upregulation of luciferase expression in our stably transfected clones, the 3′ LTR sequence of K30-3′asLuc was replaced with an LTR mutated in the TRE1 repeats to be unable to bind to CREB (3), thereby generating K30-3′asLuc 21PMD. These constructs were transiently cotransfected into 293T cells with our Tax expression vector (Fig. 4B). While no major differences were observed in basal luciferase activity between the samples, the K30-3′asLuc 21PMD-transfected cells showed a dramatically reduced response to Tax compared to those transfected with the wild-type construct. 293T cells were then stably transfected with the wild-type or TRE1-mutated K30-3′asLuc constructs (Fig. 4C). Tax expression in both cell populations demonstrated results similar to those presented above, further suggesting that TRE1 repeats were required for optimal Tax-mediated activation of HBZ expression. These results are thus in agreement with a previous study that demonstrated the nearly equal capacities of the 5′ LTR and the 3′ LTR to bind transcription factors and coactivators in HTLV-1-infected cell lines and ATL cells (21).
To further confirm the role of CREB in Tax-induced HBZ expression in the proviral DNA context, ACH proviral DNA expressing either wild-type Tax (ACH-Tax) or the Tax M47 mutant (ACH-Tax M47) were cotransfected into 293T cells along with pTax or the empty vector (Fig. 4D). The relative level of HBZ mRNA was then analyzed by real-time RT-PCR. In the absence of pTax, the mutated ACH-Tax M47 proviral DNA showed a significantly reduced level of HBZ mRNA compared to wild-type ACH-Tax. On the other hand, Tax expression resulted in an important increase in HBZ mRNA levels in both ACH-Tax and ACH-Tax M47 samples, confirming the modulator role of Tax in antisense transcription. No significant difference could be observed between the ACH-Tax and ACH-Tax M47 samples when a Tax expression vector was cotransfected, most likely because the level of Tax protein produced from the expression vector importantly exceeds the one expressed from the proviral DNA.
The results presented in this study are important information for the understanding of HTLV-1 replication and pathogenesis. First, we provide evidence that Tax can control the expression of its viral repressor, HBZ, which is in agreement with a recent study (47). The existence of such a retroaction loop controlling the TAX/HBZ expression balance should prevent exaggerated Tax expression, which could ultimately lead to a strong and unfavorable anti-Tax immune response. In addition, our results show that the Tax-dependent induction of antisense promoter activity importantly depends on the integration site. The more pronounced Tax-dependent induction in 293T cell clones with low basal promoter activity might be consequential to the recently described capacity of Tax to induce the disassembly of nucleosomes at the viral promoter (40). In contrast, strong basal HBZ promoter activity would probably be associated with a favorable chromatin context, which would further explain the reduced upregulation mediated by Tax.
Recent studies have shown that HTLV-1 integrates randomly in the host genome (8), although integration in transcriptionally active units could be associated with HTLV-1-associated myelopathy/tropical spastic paraparesis development (26). One could equally hypothesize that integration into a transcriptionally active site of the genome would affect the sense/antisense transcriptional balance in favor of HBZ expression, a feature that appears to be characteristic of ATL cells, according to another recent study (43). In conclusion, our results demonstrate that Tax plays an important role in regulating HBZ expression. Further analyses are warranted to better understand the regulation of HBZ expression and how this might act upon transformation of HTLV-1-infected cells and consequently ATLL development.
(This work was performed by S.L. in partial fulfillment of a Ph.D. degree from the Microbiology-Immunology Program, Laval University.)
Acknowledgments
We thank D. Branch (The Institute of Medical Science, University of Toronto, Toronto, Canada) for the generous gift of the S1T cell line. We are also thankful to Lee Ratner (Washington University, St. Louis, MO) for providing the pACH Tax wild type and pACH M47 vectors.
This work was supported by The Cancer Research Society Inc. (B.B.) and by the Centre National de la Recherche Scientifique (CNRS)-Université Montpellier I and the Association pour la Recherche sur le Cancer (ARC no. 3606) (J.-M.M.). S.L. was supported by a CIHR Ph.D. scholarship, and B.B. holds a Canada Research Chair, tier 2.
Footnotes
Published ahead of print on 10 December 2008.
REFERENCES
- 1.Armstrong, A. P., A. A. Franklin, M. N. Uittenbogaard, H. A. Giebler, and J. K. Nyborg. 1993. Pleiotropic effect of the human T-cell leukemia virus Tax protein on the DNA binding activity of eukaryotic transcription factors. Proc. Natl. Acad. Sci. USA 907303-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arpin-André, C., and J. M. Mesnard. 2007. The PDZ domain-binding motif of the human T cell leukemia virus type 1 Tax protein induces mislocalization of the tumor suppressor hScrib in T cells. J. Biol. Chem. 28233132-33141. [DOI] [PubMed] [Google Scholar]
- 3.Barnhart, M. K., L. M. Connor, and S. J. Marriott. 1997. Function of the human T-cell leukemia virus type 1 21-base-pair repeats in basal transcription. J. Virol. 71337-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basbous, J., C. Arpin, G. Gaudray, M. Piechaczyk, C. Devaux, and J. M. Mesnard. 2003. The HBZ factor of human T-cell leukemia virus type I dimerizes with transcription factors JunB and c-Jun and modulates their transcriptional activity. J. Biol. Chem. 27843620-43627. [DOI] [PubMed] [Google Scholar]
- 5.Bhat, N. K., Y. Adachi, K. P. Samuel, and D. Derse. 1993. HTLV-1 gene expression by defective proviruses in an infected T-cell line. Virology 19615-24. [DOI] [PubMed] [Google Scholar]
- 6.Cavanagh, M. H., S. Landry, B. Audet, C. Arpin-Andre, P. Hivin, M. E. Pare, J. Thete, E. Wattel, S. J. Marriott, J. M. Mesnard, and B. Barbeau. 2006. HTLV-I antisense transcripts initiating in the 3′LTR are alternatively spliced and polyadenylated. Retrovirology 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datta, S., N. H. Kothari, and H. Fan. 2000. In vivo genomic footprinting of the human T-cell leukemia virus type 1 (HTLV-1) long terminal repeat enhancer sequences in HTLV-1-infected human T-cell lines with different levels of Tax I activity. J. Virol. 748277-8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derse, D., B. Crise, Y. Li, G. Princler, N. Lum, C. Stewart, C. F. McGrath, S. H. Hughes, D. J. Munroe, and X. Wu. 2007. Human T-cell leukemia virus type 1 integration target sites in the human genome: comparison with those of other retroviruses. J. Virol. 816731-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du, K., H. Asahara, U. S. Jhala, B. L. Wagner, and M. Montminy. 2000. Characterization of a CREB gain-of-function mutant with constitutive transcriptional activity in vivo. Mol. Cell. Biol. 204320-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gachon, F., A. Peleraux, S. Thebault, J. Dick, I. Lemasson, C. Devaux, and J. M. Mesnard. 1998. CREB-2, a cellular CRE-dependent transcription repressor, functions in association with Tax as an activator of the human T-cell leukemia virus type 1 promoter. J. Virol. 728332-8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gachon, F., S. Thebault, A. Peleraux, C. Devaux, and J. M. Mesnard. 2000. Molecular interactions involved in the transactivation of the human T-cell leukemia virus type 1 promoter mediated by Tax and CREB-2 (ATF-4). Mol. Cell. Biol. 203470-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georges, S. A., H. A. Giebler, P. A. Cole, K. Luger, P. J. Laybourn, and J. K. Nyborg. 2003. Tax recruitment of CBP/p300, via the KIX domain, reveals a potent requirement for acetyltransferase activity that is chromatin dependent and histone tail independent. Mol. Cell. Biol. 233392-3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii407-410. [DOI] [PubMed] [Google Scholar]
- 14.Giebler, H. A., J. E. Loring, K. van Orden, M. A. Colgin, J. E. Garrus, K. W. Escudero, A. Brauweiler, and J. K. Nyborg. 1997. Anchoring of CREB binding protein to the human T-cell leukemia virus type 1 promoter: a molecular mechanism of Tax transactivation. Mol. Cell. Biol. 175156-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrod, R., Y. Tang, C. Nicot, H. S. Lu, A. Vassilev, Y. Nakatani, and C. Z. Giam. 1998. An exposed KID-like domain in human T-cell lymphotropic virus type 1 Tax is responsible for the recruitment of coactivators CBP/p300. Mol. Cell. Biol. 185052-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karpinski, B. A., G. D. Morle, J. Huggenvik, M. D. Uhler, and J. M. Leiden. 1992. Molecular cloning of human CREB-2: an ATF/CREB transcription factor that can negatively regulate transcription from the cAMP response element. Proc. Natl. Acad. Sci. USA 894820-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashanchi, F., J. F. Duvall, R. P. Kwok, J. R. Lundblad, R. H. Goodman, and J. N. Brady. 1998. The coactivator CBP stimulates human T-cell lymphotrophic virus type I Tax transactivation in vitro. J. Biol. Chem. 27334646-34652. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi, N., N. Yamamoto, Y. Koyanagi, J. Schneider, G. Hunsmann, and M. Hatanaka. 1984. Translation of HTLV (human T-cell leukemia virus) RNA in a nuclease-treated rabbit reticulocyte system. EMBO J. 3321-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwok, R. P., M. E. Laurance, J. R. Lundblad, P. S. Goldman, H. Shih, L. M. Connor, S. J. Marriott, and R. H. Goodman. 1996. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature 380642-646. [DOI] [PubMed] [Google Scholar]
- 20.Langlois, M., B. Audet, E. Legault, M. E. Pare, M. Ouellet, J. Roy, N. Dumais, J. M. Mesnard, D. M. Rothstein, S. J. Marriott, M. J. Tremblay, and B. Barbeau. 2004. Activation of HTLV-I gene transcription by protein tyrosine phosphatase inhibitors. Virology 329395-411. [DOI] [PubMed] [Google Scholar]
- 21.Lemasson, I., N. J. Polakowski, P. J. Laybourn, and J. K. Nyborg. 2004. Transcription regulatory complexes bind the human T-cell leukemia virus 5′ and 3′ long terminal repeats to control gene expression. Mol. Cell. Biol. 246117-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu, H., C. A. Pise-Masison, T. M. Fletcher, R. L. Schiltz, A. K. Nagaich, M. Radonovich, G. Hager, P. A. Cole, and J. N. Brady. 2002. Acetylation of nucleosomal histones by p300 facilitates transcription from Tax-responsive human T-cell leukemia virus type 1 chromatin template. Mol. Cell. Biol. 224450-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeda, M., N. Arima, Y. Daitoku, M. Kashihara, H. Okamoto, T. Uchiyama, K. Shirono, M. Matsuoka, T. Hattori, K. Takatsuki, et al. 1987. Evidence for the interleukin-2 dependent expansion of leukemic cells in adult T cell leukemia. Blood 701407-1411. [PubMed] [Google Scholar]
- 24.Matsumoto, J., T. Ohshima, O. Isono, and K. Shimotohno. 2005. HTLV-1 HBZ suppresses AP-1 activity by impairing both the DNA-binding ability and the stability of c-Jun protein. Oncogene 241001-1010. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto, K., H. Shibata, J. I. Fujisawa, H. Inoue, A. Hakura, T. Tsukahara, and M. Fujii. 1997. Human T-cell leukemia virus type 1 Tax protein transforms rat fibroblasts via two distinct pathways. J. Virol. 714445-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meekings, K. N., J. Leipzig, F. D. Bushman, G. P. Taylor, and C. R. Bangham. 2008. HTLV-1 integration into transcriptionally active genomic regions is associated with proviral expression and with HAM/TSP. PLoS Pathog. 4e1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller, S. C., A. Taylor, K. Watanabe, K. Mok, and F. M. Torti. 1997. Regulation of NF-kappaB and HIV-1 LTR activity in mouse L cells by ultraviolet radiation: LTR trans-activation in a nonirradiated genome in heterokaryons. Exp. Cell Res. 2309-21. [DOI] [PubMed] [Google Scholar]
- 28.Miyazaki, M., J. Yasunaga, Y. Taniguchi, S. Tamiya, T. Nakahata, and M. Matsuoka. 2007. Preferential selection of human T-cell leukemia virus type 1 provirus lacking the 5′ long terminal repeat during oncogenesis. J. Virol. 815714-5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyoshi, I., I. Kubonishi, S. Yoshimoto, T. Akagi, Y. Ohtsuki, Y. Shiraishi, K. Nagata, and Y. Hinuma. 1981. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature 294770-771. [DOI] [PubMed] [Google Scholar]
- 30.Munoz, E., and A. Israel. 1995. Activation of NF-kappa B by the Tax protein of HTLV-1. Immunobiology 193128-136. [DOI] [PubMed] [Google Scholar]
- 31.Murata, K., T. Hayashibara, K. Sugahara, A. Uemura, T. Yamaguchi, H. Harasawa, H. Hasegawa, K. Tsuruda, T. Okazaki, T. Koji, T. Miyanishi, Y. Yamada, and S. Kamihira. 2006. A novel alternative splicing isoform of human T-cell leukemia virus type 1 bZIP factor (HBZ-SI) targets distinct subnuclear localization. J. Virol. 802495-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura, M., M. Niki, K. Ohtani, and K. Sugamura. 1989. Differential activation of the 21-base-pair enhancer element of human T-cell leukemia virus type I by its own trans-activator and cyclic AMP. Nucleic Acids Res. 175207-5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada, M., and K. T. Jeang. 2002. Differential requirements for activation of integrated and transiently transfected human T-cell leukemia virus type 1 long terminal repeat. J. Virol. 7612564-12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet i1031-1032. [DOI] [PubMed] [Google Scholar]
- 35.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 777415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poteat, H. T., P. Kadison, K. McGuire, L. Park, R. E. Park, J. G. Sodroski, and W. A. Haseltine. 1989. Response of the human T-cell leukemia virus type 1 long terminal repeat to cyclic AMP. J. Virol. 631604-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodgers-Johnson, P., D. C. Gajdusek, O. S. Morgan, V. Zaninovic, P. S. Sarin, and D. S. Graham. 1985. HTLV-I and HTLV-III antibodies and tropical spastic paraparesis. Lancet ii1247-1248. [DOI] [PubMed] [Google Scholar]
- 38.Rousset, R., C. Desbois, F. Bantignies, and P. Jalinot. 1996. Effects on NF-kappa B1/p105 processing of the interaction between the HTLV-1 transactivator Tax and the proteasome. Nature 381328-331. [DOI] [PubMed] [Google Scholar]
- 39.Satou, Y., J. Yasunaga, M. Yoshida, and M. Matsuoka. 2006. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc. Natl. Acad. Sci. USA 103720-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma, N., and J. K. Nyborg. 2008. The coactivators CBP/p300 and the histone chaperone NAP1 promote transcription-independent nucleosome eviction at the HTLV-1 promoter. Proc. Natl. Acad. Sci. USA 1057959-7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thébault, S., J. Basbous, P. Hivin, C. Devaux, and J. M. Mesnard. 2004. HBZ interacts with JunD and stimulates its transcriptional activity. FEBS Lett. 562165-170. [DOI] [PubMed] [Google Scholar]
- 42.Uchiyama, T. 1997. Human T cell leukemia virus type I (HTLV-I) and human diseases. Annu. Rev. Immunol. 1515-37. [DOI] [PubMed] [Google Scholar]
- 43.Usui, T., K. Yanagihara, K. Tsukasaki, K. Murata, H. Hasegawa, Y. Yamada, and S. Kamihira. 2008. Characteristic expression of HTLV-1 basic zipper factor (HBZ) transcripts in HTLV-1 provirus-positive cells. Retrovirology 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan, J. P., J. E. Garrus, H. A. Giebler, L. A. Stargell, and J. K. Nyborg. 1998. Molecular interactions between the coactivator CBP and the human T-cell leukemia virus Tax protein. J. Mol. Biol. 281395-400. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida, M. 1994. Mechanism of transcriptional activation of viral and cellular genes by oncogenic protein of HTLV-1. Leukemia 8(Suppl. 1)S51-S53. [PubMed] [Google Scholar]
- 46.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 792031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida, M., Y. Satou, J. Yasunaga, J. Fujisawa, and M. Matsuoka. 2008. Transcriptional control of spliced and unspliced human T-cell leukemia virus type 1 bZIP factor (HBZ) gene. J. Virol. 829359-9368. [DOI] [PMC free article] [PubMed] [Google Scholar]