Abstract
The human immunodeficiency virus type 1 (HIV-1) envelope spike is a heavily glycosylated trimeric structure in which protein surfaces conserved between different HIV-1 isolates are particularly well hidden from antibody recognition. However, even variable regions on the spike tend to be less antigenic and immunogenic than one might have anticipated for external structures. Here we show that the envelope spike of primary viruses has an ability to restrict antibody recognition of variable regions. We show that access to an artificial epitope, introduced at multiple positions across the spike, is frequently limited, even though the epitope has been inserted at surface-exposed regions on the spike. Based on the data, we posit that restricted antibody access may be the result, at least in part, of a rigidification of the epitope sequence in the context of the spike and/or a highly effective flexible arrangement of the glycan shield on primary viruses. Evolution of the HIV envelope structure to incorporate extra polypeptide sequences into nominally accessible regions with limited antibody recognition may contribute to reducing the magnitude of antibody responses during infection and allow the virus to replicate unhindered by antibody pressure for longer periods.
The efficacy of most viral vaccines is a result of their ability to elicit neutralizing antibodies (NAbs) (57). However, efforts to develop human immunodeficiency virus (HIV) vaccine antigens that can elicit NAbs with broad activity have not been successful so far and are frustrated particularly by the apparent resistance of many HIV type 1 (HIV-1) isolates to the potential neutralizing activity of antibodies elicited by current immunogens.
The primary target for NAbs on HIV-1 is the envelope spike, a noncovalently linked heterotrimeric complex of the surface unit glycoprotein gp120 and the transmembrane unit gp41. In this study we focused our attention on the gp120 subunit. gp120 harbors several features that hide highly conserved sites from antibody recognition, for example, dense glycosylation and sequence-variable loops (28, 31, 39, 40, 56, 59, 73, 75). Furthermore, some conserved sites are only transiently exposed or are not fully formed until the virus has attached to the target cell, which greatly reduces the chance of antibody recognition (13, 35, 76). More recently it has been postulated that the functional trimer on primary viral isolates may be protected also by a mechanism dubbed conformational masking (32, 79), which suggests that nonneutralizing antibodies may be unable to bind their epitopes in the context of the viral spike due to restraints on conformational changes required for the binding of these antibodies.
Most of the features outlined above are likely the result of viral evolution in response to specific NAb selection pressure during infection and help explain why antibodies might fail to effectively recognize most conserved sites on the spike. However, the general resistance of even relatively closely related primary HIV isolates to the abundance of anti-gp120 and anti-gp41 monoclonal antibodies (MAbs) and immune sera described to date is remarkable. We postulated that this general resistance may be due to features of the envelope spike not previously appreciated.
We have therefore examined the antigenicity of a defined antibody epitope within the context of the functional envelope spike. We generated a panel of chimeric viruses engrafted at different individual positions with the well-characterized hemagglutinin (HA) epitope tag YPYDVPDYA. The feasibility of HIV epitope tagging has been demonstrated recently in elegant studies using HIV and simian immunodeficiency virus (SIV) engrafted with a FLAG epitope tag (DYKDDDDK). These studies showed that antibody binding to envelope spikes is necessary and sufficient for virus neutralization in vitro (36, 60, 77, 78). However, given that only a single anti-epitope tag MAb was utilized in all of these studies, they provided relatively limited information on accessibility of the target epitope to different antibodies. The work described here complements and expands on those previous studies. Here, we used two high-affinity MAbs and a high-titer serum to the epitope tag, aiming to obtain greater resolution into epitope antigenicity in the context of the viral spike. The HA tag was inserted at various positions throughout the gp120 subunits of the envelope spikes from two viruses: HXB2, a T-cell line-adapted virus that is generally sensitive to neutralization by most antibodies, and JRCSF, a primary isolate that in general is moderately resistant to anti-HIV MAbs and HIV-positive sera (2, 41, 44). The chosen positions are expected to be surface exposed based on current models, and we expected that introduction of the foreign epitope at selected sites would result in viruses that were generally susceptible to anti-HA antibody neutralizing activity. However, contrary to our expectations, we observed antibody neutralization to be dependent upon a combination of the specific antibody used and the location of the epitope. For the primary isolate JRCSF in particular, recognition of the HA tag by different HA antibodies was substantially suppressed compared to the much greater access in the context of HXB2. We believe that our results are indicative of a “built-in” ability of the envelope spike, in particular on primary viruses, to limit access of polypeptide sequences to antibody and thus avoid antibody-mediated neutralization.
MATERIALS AND METHODS
Antibodies.
The anti-HA rat MAb 3F10 was purchased from Roche. The anti-HA mouse MAb 16B12 and rabbit polyclonal antiserum HA.11 were purchased from Covance Research Products. Human MAbs 17b and 48d, against epitopes overlapping the coreceptor binding site of gp120 (71), were kindly provided by James Robinson (Tulane University). Human MAb A32, against a discontinuous epitope involving the C1, C2, and C4 regions of gp120 (47), and murine MAb D50, to an epitope within the C-heptad repeat region of gp41 (15), were obtained through the IAVI Neutralizing Antibody Consortium. MAb b6, against the CD4 binding site (CD4bs) of gp120 (62), was from our laboratory.
Fab′ 16B12 and 3F10 were generated as described elsewhere for MAb F425-B4e8 (1), by first digesting the respective immunoglobulin Gs (IgGs) with agarose-bound pepsin (Pierce) (2 h at 37°C in 20 mM sodium acetate buffer, pH 4.5). The solutions were then adjusted to pH 7 to 8, after which dithiothreitol was added (1.5 mM final concentration) so as to cleave the F(ab′)2 products to Fab′ (3 h at 37°C). The preparations were dialyzed against phosphate-buffered saline, and purity was ascertained by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue-based staining.
Plasmid constructs.
Plasmid pSVIIIexE7pA-JRCSF (81), carrying the full-length env of the primary virus JRCSF, was used here as a template for mutagenesis and for the generation of pseudovirions (see below). The reporter plasmid pNL4-3.Luc.R−E− (10, 20), encoding HIV-1 structural proteins and a luciferase enzyme, was obtained from the NIH AIDS Research and Reference Reagent Program (contributed by Nathaniel Landau).
Mutagenesis.
Insertion of HA tag sequences into gp120 was performed by the site-directed mutagenesis approach described by Zheng et al. (80), except that a mixture of Taq (2.5 U; Roche) and Pfu Ultra (2.5 U; Stratagene) DNA polymerases was used in the reactions (50-μl mixtures). The HA tag sequence was inserted without any additional alterations to the parental env sequence. Single-residue substitutions in HA epitope grafts were generated by QuikChange XL mutagenesis reactions (Stratagene) using the plasmids with the HA epitope insertions as templates (50 to 100 ng). All mutant plasmids were sequenced to ensure that only the desired mutations had been introduced.
Pseudovirions and solubilized gp120.
Pseudotyped viruses were generated in 293T cells as described previously (81), by transient transfection of wild-type or mutant env plasmids and the luciferase reporter plasmid pNL4-3.Luc.R−E−. Solubilized monomeric gp120 was obtained by adding detergent (Empigen [Calbiochem]; 1% [vol/vol] final concentration) to the harvested culture supernatants to lyse virions. Detergent-treated supernatants were stored at −20°C until needed.
Neutralization assays.
Neutralization assays with single-round infectious pseudovirus were performed essentially as described elsewhere (81), using U87.CD4+CCR5+ and U87.CD4+CXCR4+ target cells obtained from the NIH AIDS Research and Reference Reagent Program (contributed by HongKui Deng and Dan Littman) (3). Briefly, 2 to 5 × 104 targets cells were seeded into 96-well plates (Corning) and incubated overnight at 37°C. Virus (120 μl) was mixed with an equal volume of serially diluted antibody and incubated for 1 h at 37°C. An aliquot of this mixture (200 μl) was then added to the targets cells, and the cells were incubated for a further 2 to 3 days, as recommended (43). To measure luciferase activity, the tissue culture medium was removed, the wells washed once with Ca2+/Mg2+-free phosphate-buffered saline, and 50 μl of appropriately diluted luciferase cell culture lysis reagent (Promega) added. The lysate was mixed by pipetting vigorously up and down. Aliquots (20 μl) were transferred to opaque 96-well assay plates (Corning), and luciferase activity was measured on a luminometer (EG&G Berthold LB 96V; Perkin-Elmer) using luciferase assay substrate (Promega). The percentage of virus neutralization at a given antibody concentration was determined by calculating the reduction in luciferase activity in the presence of antibody relative to virus-only wells.
ELISA.
Capture enzyme-linked immunosorbent assays (ELISAs) for the detection of gp120 in detergent-treated viral lysates were performed as described previously, using Fc-specific secondary antibody and 3,3′,5,5′-tetramethylbenzidine (Pierce) for detection (52). Detergent-treated culture supernatants were equalized for gp120 concentration using a mixture of purified IgGs from HIV-1-infected individuals with broad neutralizing activity. ELISAs were performed in duplicate.
RESULTS
HA epitope tag insertions are permissive in the context of the functional HIV envelope spike.
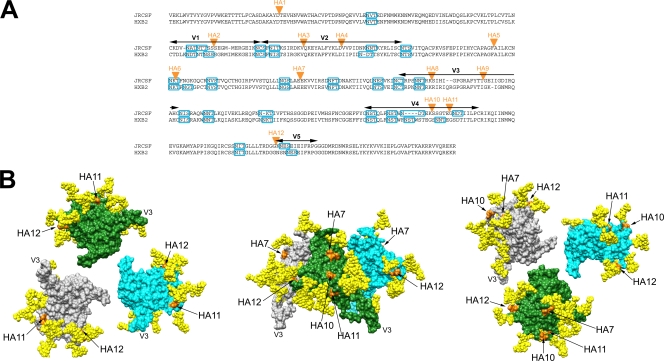
The HA epitope tag has been used extensively for investigating the topology of membrane proteins (reviewed in references 16 and 23). Here, we utilized the HA tag to investigate the antigenicity of the HIV-1 envelope spike in the biologically relevant context of virus neutralization. We first inserted the HA tag at 12 different locations (numbered 1 to 12) within the gp120 sequence of a plasmid encoding the envelope of the primary isolate JRCSF (see Fig. 2A), so as to generate pseudotyped viruses. Epitope insertions were made in all five variable-loop regions on gp120, which are generally thought to be relatively surface exposed on the trimer structure, given the often-observed accumulation of mutations in these regions and elicitation of NAbs to them during infection (19, 49, 63, 68, 69). Insertions were also made in the C1 and C2 constant segments. The C1 and C2 insertion sites were selected on the basis of early oligomeric models of the HIV-1 envelope spike, which suggested that some of the selected sites might be exposed on the viral spike (34). We did not target the C3, C4, and C5 constant regions, given that these regions are considered mostly hidden from antibody within the gp120 core (46, 48).
FIG. 2.
Locations of HA epitope tag insertions in JRCSF and HXB2. (A) Protein sequence alignment of JRCSF and HXB2 gp120s, denoting the locations of HA epitope tag insertions (orange triangles). The sequence alignments were made with ClustalW2 (http://www.ebi.ac.uk/Tools/clustalw2/index.html). Sections of variable regions in gp120 are marked by arrows (black), and potential N-glycosylation sequons (PNGS) are denoted by rectangular boxes (blue). JRCSF contains 23 PNGS and HXB2 24 PNGS. The locations of the HA tag insertions are as follows: HA1, C1 region; HA2, V1 region; HA3 and HA4, V2 region; HA5, HA6, and HA7, C2 region; HA8 and HA9, V3 region; HA10 and HA11, V4 region; HA12, V5 region. (B) Surface representation of an unliganded gp120 spike from three different perspectives, illustrating the locations of the HA tag insertions. Left panel, from the perspective of the target cell, with V3 projecting toward the viewer. Middle panel, side view of the unliganded trimer, with the V3 loops pointing downward to the target cell and the virus surface at the top of the image. Right panel, from the perspective of gp41/virion surface, with V3 projecting away from the viewer. Each gp120 protomer (derived from gp120coreJRFL+V3; PDB ID 2B4C) is depicted in a different color (gray, green, or cyan). The oligomer configuration is based on cryo-electron microscopy images of an unliganded gp120 trimer fitted with X-ray coordinates of the gp120core+V3 structure (37). The GlcNAc2Man3 pentose cores of glycans attached to the Asn glycosylation site in PNGS are shown as space-filling models (yellow). The GlcNAc2Man3 cores were modeled using GlyProt (4). We modeled only the pentose core, given that it is the same in high-mannose, complex, and hybrid glycans that occur naturally on gp120. The two gp120 residues between which the HA epitope tag was inserted are colored orange; the locations of HA2 and HA3 cannot be depicted because the V1/V2 loop is truncated in this and all gp120 crystal structures. The molecular graphic images were generated with the UCSF Chimera package (54) and then labeled and compiled in Adobe Photoshop CS (version 8.0).
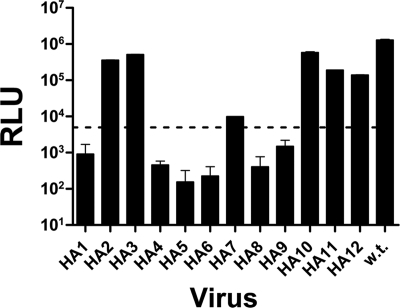
Infectivity of recombinant pseudovirions carrying the HA tags was tested by a single-round entry assay using U87.CD4+CCR5+ target cells (81). Six of the 12 JRCSF HA-tagged pseudoviruses (HA2, HA3, HA7, HA10, HA11, and HA12) exhibited infectivity levels above the recommended (43) cutoff value of 5,000 relative luciferase units (RLU) (Fig. 1). With the exception of mutant HA7, which had an infectivity level of ∼104 RLU, infectivity levels of all tagged viruses were in the same range as that of wild-type virus (105 to 106 RLU). Thus, insertion of the HA tags did not substantially diminish the basal ability of the envelope glycoproteins to support virus entry. The observed frequency (50%) of epitope tag insertions that yielded infectious virus is comparable to the average frequency observed for functional epitope-tagged proteins when tags are inserted at internal sites (23). Given that we wished to focus here specifically on those constructs that were able to yield infectious virus, we did not examine the nonfunctional constructs further for the potential basis of their lack of infectivity. However, we do note that mutations in the C1 region near the HA1 insertion site can disrupt gp120-gp41 association (21), and point mutations in or near the location of epitope tag insertion in mutant HA4 are known to affect the efficiency of CD4 binding to gp120 (51, 52, 62). Mutations near the HA5 and HA6 sites may have affected gp160 processing (51), and insertion in V3 (mutants HA8 and HA9) may have disrupted interactions with coreceptor molecules on target cells (8).
FIG. 1.
Infectivities of HA-tagged JRCSF pseudoviruses. Infectivity levels were determined by measuring luciferase activity in target cell lysates, expressed as RLU. Values are the means and standard deviations from duplicate wells. Wild-type (w.t.) and tagged viruses are denoted on the x axis. The dashed line marks the cutoff value (5,000 RLU) for viral infectivity.
Having established the locations of sites in JRCSF that yielded acceptable levels of infectious virions (i.e., HA2, HA3, HA7, HA10, HA11, and HA12), we generated the six equivalent HA grafts in the HXB2 gp120 sequence background (Fig. 2A). We found that, with the exception of the HA3 insertion, all HXB2 HA-tagged viruses exhibited acceptable infectivity levels (i.e., >5,000 RLU) (data not shown). HXB2-HA3 pseudovirions exhibited very low infectivity levels, and therefore this construct was not utilized further.
We used a model of an unliganded gp120 trimer spike, derived from recent cryo-electron tomography imaging (37), to map the locations of the HA tag insertions that yielded infectious pseudotyped viruses (Fig. 2B). The model utilized here is based on the coordinates for the structure of the V3-containing gp120 core of virus JRFL (22), and it differs from earlier oligomeric models of the trimer spike (34) (data not shown). JRFL has 91% amino acid sequence homology to JRCSF, meaning that the core structures are highly similar. This notion is supported by the high similarity between the core structures of YU2 and HXB2, which share only 84% amino acid sequence homology (33). The V1/V2 loops, containing HA2 and HA3, are not present in any of the gp120 structures reported so far, and thus the locations of the tags for HA2 and HA3 cannot be modeled. Mapping of the remaining locations onto the unliganded trimer model suggests that the HA tag insertions should be exposed to solvent (Fig. 2B). Although some of the HA locations, e.g., HA11 and HA12, are bounded by glycans, it should be noted that none of the HA tag locations, in the context of the trimeric model, are occluded by glycans from a neighboring gp120 protomer, nor are they close to gp120-gp41 interfaces.
Anti-HA MAbs recognize tagged monomeric gp120.
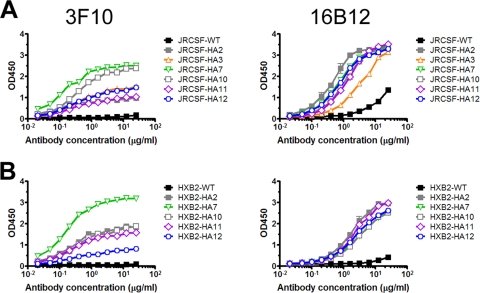
To ensure that the tag was presented in an environment that would minimally allow antibody recognition in the context of monomeric gp120, we performed a series of ELISAs with the two anti-HA MAbs 3F10 and 16B12. We also sought to perform similar ELISAs with the anti-HA polyclonal rabbit serum HA.11. 3F10 is a high-affinity rat anti-HA MAb, obtained by immunization with a keyhole limpet hemocyanin-coupled polypeptide derived from the influenza virus HA1 subunit that contains the HA tag sequence described elsewhere (50). Murine MAb 16B12 and rabbit serum HA.11, both of which are reported to have high affinity for the HA-tagged proteins, were obtained by immunizing the corresponding animals with the peptide CYPYDVPDYASL, conjugated via the N-terminal Cys residue onto keyhole limpet hemocyanin. These antibody preparations were selected for this study because they are reported to bind to the HA tag when inserted internally in a protein sequence, based on information provided by the respective manufacturers and published studies.
MAb 16B12 bound each group of HA-tagged gp120s at high levels and with largely similar apparent affinities (half-maximal binding concentrations). Most JRCSF-HA-tagged gp120s, with the exception of JRCSF-HA3 gp120, were bound by MAb 16B12 with an average apparent affinity of ∼0.7 μg/ml (Fig. 3A), and all HA-tagged HXB2 gp120s were bound at an average apparent affinity of 2 μg/ml (Fig. 3B).
FIG. 3.
Anti-HA MAbs recognize tagged monomeric gp120. Solubilized monomeric gp120 from viral lysates was captured onto ELISA plate wells using an anti-gp120 sheep antibody to the C5 region and probed with MAbs 3F10 and 16B12, starting at a concentration of 25 μg/ml. (A) JRCSF gp120. (B) HXB2 gp120. All values are the means from replicate wells.
In contrast to MAb 16B12, MAb 3F10 bound the monomeric gp120s at various plateau levels; JRCSF-HA7, JRCSF-HA10, and HXB2-HA7 were bound at higher levels than the other HA-tagged gp120s (Fig. 3). However, careful examination revealed that MAb 3F10 bound all tagged gp120s with similar apparent binding affinities (∼0.1 μg/ml). We were, however, unable to adequately perform binding experiments with serum HA.11 due to unexpectedly high ELISA signals with wild-type gp120, which was not resolved by the use of different blocking buffers.
HA epitope tag insertions do not result in substantial changes to the overall quaternary organization of the functional envelope spike.
To determine whether the permissive HA epitope insertions had grossly affected the tertiary and/or quaternary conformation of the envelope spike, we first determined the sensitivity to three anti-gp120 MAbs (A32, b6, and 17b) and to an anti-gp41 MAb (D50) with no reported neutralizing activity against wild-type JRCSF. The reasoning was that if insertion of the tag had resulted in an envelope spike structure where protein surfaces sequestered on the wild-type trimer were now better exposed, then the corresponding antibodies would gain neutralizing activity. The antibodies were selected because of their broad reactivity and recognition of different conformational epitopes: MAb A32 binds a complex epitope involving C1/C2/C4 adjacent to the CD4bs (5, 45), MAb b6 binds an epitope overlapping the CD4bs (62), MAb 17b binds an epitope overlapping the coreceptor binding site (71), and MAb D50 binds an epitope located immediately upstream of the membrane-proximal region recognized by the broadly neutralizing MAb 2F5 (14, 15).
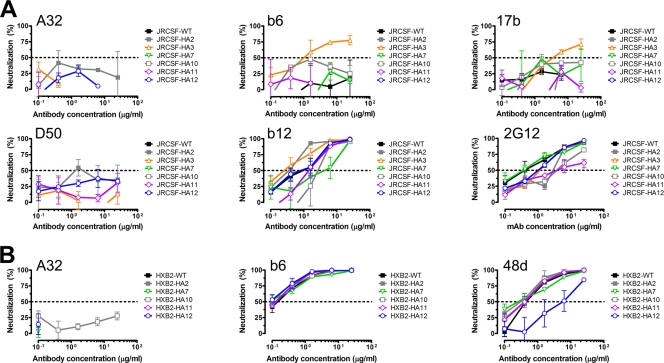
We found that only virus mutant HA3, which has an HA tag insertion in the V2 loop, was sensitive to neutralization by MAbs b6 and 17b compared to wild-type virus (50% inhibitory concentration [IC50], <25 μg/ml) (Fig. 4A). The binding affinities of MAbs b6 and 17b are known to be influenced by mutations in V2 (52, 62, 71), which may explain the enhanced neutralizing activity against mutant HA3. The increased sensitivity of HA3 to neutralization by 17b and b6, but not to MAbs A32 and D50, suggested that HA tag insertion had likely resulted in a relatively localized alteration in gp120. The five remaining HA-tagged viruses (HA2, HA7, HA10, HA11, and HA12) were neutralized relatively poorly by all four MAbs (IC50, >25 μg/m);(Fig. 4A). These results suggested that the quaternary organization of the spikes on these mutant viruses, though altered somewhat by the HA tag insertions, was not grossly deformed relative to the quaternary structure of the spikes on wild-type virus.
FIG. 4.
HA tag insertions do not result in gross conformational changes in the envelope spike. (A) Sensitivity of JRCSF wild-type (WT) virus and HA-tagged mutants to nonneutralizing anti-gp120 MAbs A32, b6, and 17b; anti-gp41 MAb D50; and the broadly neutralizing MAbs b12 and 2G12. (B) Sensitivity of HXB2-WT virus and HA-tagged mutants to anti-gp120 MAbs A32, b6, and 48d. Dotted lines indicate 50% neutralization; neutralization of <50% is considered weak. For clarity, viral infectivity enhancement effects are not shown (i.e., those portions of the curves below the x axis). Error bars denote the signal ranges from replicate wells.
We characterized the mutant viruses further by determining their sensitivity to neutralization by the broadly neutralizing MAb b12, which recognizes an epitope overlapping the CD4bs (6, 62), and the broadly neutralizing MAb 2G12, which recognizes a cluster of terminal carbohydrate residues on the gp120 “silent face” (64, 65, 72). We found that the sensitivities were generally not very different from that of wild-type virus (Fig. 4A); only JRCSF-HA2, -HA10, and -HA11 were noticeably more resistant to 2G12 than wild-type virus (∼8-fold) (Fig. 4A). The increased resistance of mutant viruses JRCSF-HA10 and -HA11 relative to wild-type virus is likely due to the insertions in V4; mutations in V4 have been previously associated with changes in 2G12 binding affinity (65).
We then examined the sensitivity of HXB2-HA-tagged viruses to neutralization by anti-gp120 MAbs b6, A32, and 48d (Fig. 4B). MAb 48d was used instead of MAb 17b for reasons of availability; it binds an epitope overlapping the coreceptor binding site that is highly similar to that of MAb 17b (71). The majority of the HXB2 HA-tagged viruses exhibited a level of neutralization sensitivity similar to the neutralization sensitivity of wild-type virus to these three antibodies (Fig. 4B). Two exceptions were observed: the HXB2-HA12 mutant was ∼10-fold more resistant to neutralization by MAb 48d than wild-type virus, and the HXB2-HA10 mutant was somewhat more sensitive to neutralization by MAb A32 than the wild type. However, in the case of MAb A32, the level of neutralization was modest (<30%) at the highest antibody concentration tested (25 μg/ml) (Fig. 4B).
HA-tagged viruses are selectively neutralized by anti-HA antibodies.
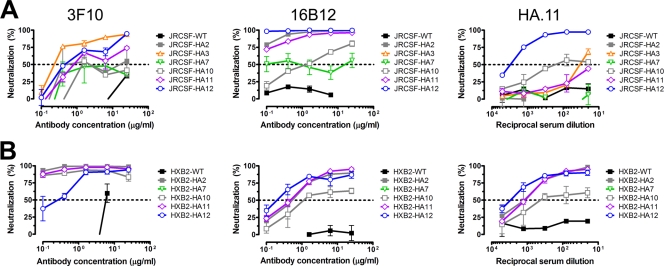
We next investigated the neutralization sensitivities of the HA-tagged viruses to MAbs 3F10 and 16B12 and the anti-HA rabbit serum HA.11. Figure 5A reveals a mosaic of neutralizing sensitivities defined by each antibody-virus combination, with a surprisingly large number of combinations for tagged JRCSF viruses showing some degree of neutralization resistance. JRCSF-HA7 and HXB2-HA7 are resistant to all three antibody preparations, suggesting that the inserted tag in the C2 region, though accessible on monomeric gp120, becomes occluded on primary and T-cell line-adapted virus envelope spikes. JRCSF-HA12 and HXB2-HA12 are highly sensitive to all three preparations (IC50s of <1 μg/ml for the MAbs and <1:1,000 for the serum), indicating that the tag on V5 is readily accessible on envelope spikes.
FIG. 5.
HA-tagged viruses are sensitive to neutralization by anti-HA antibodies. (A) Neutralization sensitivity of pseudotyped JRCSF wild-type (WT) and HA-tagged viruses to anti-HA MAbs 3F10 and 16B12 and anti-HA polyclonal serum HA.11. The data are the mean values from two or three independent experiments performed in duplicate. Error bars denote the signal range from replicate wells. MAb 16B12 enhanced the infectivity of mutant JRCSF-HA3 (graph not shown). (B) Neutralization sensitivity of HXB2 WT viruses and HA-tagged mutants to the three anti-HA antibody preparations.
In contrast, remarkably, the polyclonal anti-HA serum does not significantly neutralize any of the other JRCSF-tagged viruses while neutralizing most of the HXB2-tagged viruses, suggesting poor recognition of the tag in the context of the primary envelope spike by the immunodominant HA specificities in the serum. MAb 3F10 follows the pattern of the polyclonal anti-HA serum by neutralizing JRCSF tagged viruses considerably less well than HXB2; two viruses, JRCSF-HA10 (V4) and JRCSF-HA2 (V2) are not effectively neutralized by this MAb. Thus, there appear to be restrictions placed on antibody recognition of these variable loops in the context of the primary envelope. In contrast to MAb 3F10, MAb 16B12 neutralizes both JRCSF and HXB2 tagged viruses effectively (Fig. 5). The somewhat lower apparent affinity of MAb 16B12 for the HA-tagged HXB2 gp120s might explain the overall lower neutralization potency of MAb 16B12 against the HA-tagged HXB2 mutants than against the HA-tagged JRCSF variants (Fig. 3).
In comparing MAbs 3F10 and 16B12, the most striking difference was observed for JRCSF-HA2 and -HA3, which have the HA tag inserted at the center of V1 and in the N-terminal segment of V2, respectively. MAb 3F10 neutralized JRCSF-HA3 relatively strongly (IC50 of ∼0.2 μg/ml), whereas MAb 16B12 did not neutralize this mutant even up to the highest antibody concentration tested (25 μg/ml). This result was unexpected, given that JRCSF-HA3 was sensitive to neutralization by the otherwise nonneutralizing MAbs b6 and 17b (Fig. 4A) and, thus, that the envelope spike might be somewhat more accessible to antibody than the wild-type spike. The reverse was observed with JRCSF-HA2, which was neutralized strongly (IC50 of <0.1 μg/ml) by MAb 16B12, whereas MAb 3F10 did not achieve >50% neutralization at the highest antibody concentration tested (Fig. 5).
Access of anti-HA MAbs to their epitopes on the viral spike is not restricted due to antibody size.
It has been shown that antibodies to the coreceptor site on gp120 may be sterically hindered from interacting with their epitopes once the virus has engaged CD4 on target cells and that this steric restriction can be bypassed using antibody fragments, such as Fabs or scFvs (35, 76). Therefore, we also considered the possibility that some of the insertions may be located at positions of limited accessibility to full-size antibodies on the spike. 3F10 and 16B12 IgGs were therefore fragmented to Fab′ with pepsin and dithiothreitol and tested for neutralization efficacy against the JRCSF-HA tagged viruses tested as described above with IgG. The JRCSF-HA mutants were chosen because they exhibited the largest degree of neutralization resistance. We found that neither Fab′ 16B12 nor Fab′ 3F10 neutralized any of the HA-tagged viruses more efficiently than the corresponding IgG at equimolar concentrations (data not shown). Rather, diminished neutralization potency was observed with all the viruses. These observations support models suggesting that the large size of IgG molecules is often beneficial to neutralization efficiency (7, 27). Based on these findings, it seems unlikely that the differences in neutralization profiles were due to steric hindrance of bulky IgG molecules.
Minor differences in fine specificity are sufficient to affect the neutralizing capacity of anti-HA antibodies.
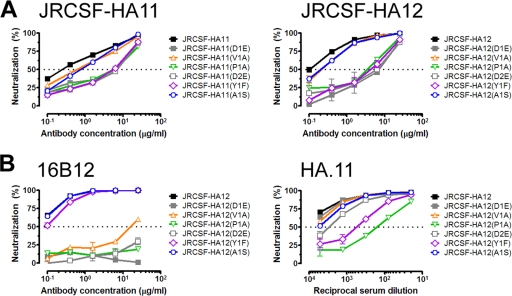
We next determined the fine specificities of the anti-HA antibody preparations (Fig. 6), reasoning that differences in specificity might provide insight into the contrasting capacities of the MAbs and serum to neutralize the HA-tagged viruses. We again focused on the JRCSF-HA tagged viruses, given that the greatest differences in neutralization were observed in the JRCSF background. To fine map the specificities, conservative single point substitutions were made in the DVPDYA portion of the HA tag in JRCSF-HA11 and JRCSF-HA12; the selected substitutions in this portion of the tag were based on a past study showing that anti-HA MAbs are exquisitely sensitive to such mutations (9). Testing of MAb 3F10 against both sets of point mutants showed that this antibody requires the Asp residues, the Pro, and the Tyr for efficient neutralization (Fig. 6A). The observed changes in neutralization profiles were the same for mutations generated in the HA11 and HA12 virus mutant backgrounds, indicating that the effects are likely an accurate map of the residues critical for binding of MAb 3F10.
FIG. 6.
Anti-HA MAbs and serum differ slightly in their fine specificities for the HA epitope tag. (A) MAb 3F10 neutralization of JRCSF-HA11 and -HA12 and their corresponding mutants containing conservative point substitutions in the DVPDYA portion of the HA tags. (B) Neutralization of JRCSF-HA12 and -HA12 point mutants by MAb 16B12 and serum HA.11. The HA tag point mutations were the same as used previously to map the fine specificity of other anti-HA MAbs (9); Asp residues were mutated to Glu (first and second Asps are denoted D1 and D2, respectively), Val and Pro residues were mutated to Ala, Tyr was mutated to Phe, and Ala was mutated to Ser.
We mapped the specificities of MAb 16B12 and serum HA.11 using the variants generated in the JRCSF-HA12 background, given that both of these antibody preparations neutralized JRCSF-HA12. We found the fine specificity of MAb 16B12 to be highly similar to that of MAb 3F10. Both MAbs require both Asp residues and the Pro residue in the mutated DVPDYA sequence for efficient neutralization (Fig. 6B). However, in contrast to MAb 3F10, MAb 16B12 does not require the presence of the Tyr residue for efficient binding; instead, the Val is critical. Like MAb 3F10, serum HA.11 requires both the Pro and Tyr for efficient neutralization (Fig. 6B). However, in contrast to the case for the MAbs, none of the additional mutations had a significant effect on neutralization efficiency. The dominant specificity observed with serum HA.11 was not batch dependent, as the same results were obtained with pools of different batches obtained from the manufacturer (data not shown). Based on these results, we inferred that the slight variations in fine specificity affect the ability of the anti-HA antibodies to recognize the HA epitope tag in the context of the functional viral spike, particularly in the context of the primary virus JRCSF.
It might be argued that the HA tag has limited conformational flexibility due to the presence of the two Pro residues, thus resulting in the different neutralization profiles observed here. Although this possibility cannot be excluded fully, results from other work suggest that the Pro residues do not impose a large restriction on the flexibility of the tag. For example, the HA tag adopts an extended conformation in the context of the native HA protein (74), whereas other conformations of the tag when bound by antibody have been observed (9, 67).
DISCUSSION
The results described here show that the 9-amino-acid HA tag can be introduced at a number of positions in the variable loops V1, V2, V4, and V5 of HIV-1 gp120 with substantial retention of viral infectivity. As such, these results mirror those reported by Laird and Desrosiers, who also observed that recombinant SIVmac239 engrafted with a FLAG epitope tag in V1, V2, or V4 replicates with kinetics similar to those of the parental virus (36). We found that the HA tag was generally well recognized in the context of monomeric gp120 and in the context of the envelope spike of a lab-adapted virus, as indicated by neutralization sensitivity of mutant viruses. However, recognition in the context of a primary isolate envelope spike was much more restricted, as indicated again by neutralization sensitivity. Remarkably, only the V5-tagged primary virus was neutralized effectively by the anti=HA tag serum, although the same serum neutralized nearly all of the tagged lab-adapted viruses.
Based on the most recent models of the envelope spike (37), loss of HA tag recognition is not thought to be due to concealment of the tag at monomer interfaces in the trimer. One possible explanation for the differences in neutralization profiles between the anti-HA MAbs and the serum may be that the MAbs and serum antibodies interact with the epitope tag from different angles; in some instances the engrafted tag is presented in a manner that allows for broad antibody recognition, whereas in other instances the tag is presented such that only limited recognition is possible. The fine specificity of the MAbs differed notably from that of the anti-HA serum, suggesting indeed possible differences in the angle of antibody interaction. If so, our results illustrate how serum antibodies to a linear target epitope on the HIV spike might fail to neutralize virus as a result of not having the “correct” fine specificity, even though they bind the bona fide target epitope.
Although the above-mentioned rationale may explain differences in neutralization between the MAbs and serum at certain positions on the primary isolate envelope spike, the striking differences in neutralizing activity overall suggest that other factors, perhaps inherent to the viral spike, may play a role in restricting antibody access. Furthermore, the above-mentioned rationale does not fully explain the differences in neutralizing activity between the two MAbs; their close similarity in fine specificity would suggest that they interact from similar angles with the epitope tag, a notion we base on complex structures of two other anti-HA MAbs exhibiting a major overlap in fine specificity (9). Taking these observations into consideration, we tentatively propose two additional, not mutually exclusive, hypotheses to explain the differences in neutralizing activities observed here.
The first hypothesis is limited flexibility or rigidification of epitope sequences within the framework of the functional spike, meaning an intrinsic constraint on the flexibility of certain gp120 segments in the context of the oligomeric spike. The level of constraint manifested within the spike structure from a given virus may relate to the physical stability of the oligomeric spike, which has been shown to vary between virus strains (M. B. Zwick et al., unpublished observations). Our data would suggest that on primary viruses such as JRCSF, there may be greater rigidification of antibody epitopes than on T-cell line-adapted viruses such as HXB2. This might explain how the two anti-HA MAbs exhibit very different levels of neutralizing activity against some of the HA-tagged viruses; limited flexibility of the target epitope at certain sites would reduce conformational adjustments in the fine contacts with each antibody upon binding (12), thus reducing optimal interaction. Clearly, determination of the three-dimensional structures of the two anti-HA MAbs tested here, alone and in complex with peptide, would be required to provide insight into how these antibodies interact with the HA tag sequence and how conformational changes shape this interaction. The rigidification hypothesis proposed here relates somewhat to the concept of gp120 conformational masking reviewed recently (55), which suggests that many of the antibodies that are unable to neutralize HIV may require relatively large conformational changes to effectively bind their epitopes. It is hypothesized that such large changes may not be possible in the context of the functional spike due to conformational restrictions that limit optimal antibody-epitope interactions (32).
The second hypothesis that we propose to possibly explain the observed differences is an intrinsic (re)arrangement of glycans upon gp120 oligomerization. This “built-in” glycan shielding mechanism would naturally limit any antibody attack against the primary virus spike. Glycans in V1 have been shown to occlude portions of the V3 variable region in oligomeric gp120 but not the monomer, and the inaccessibility of antibody epitopes caused by V3 glycans is generally manifested only in the functional oligomer (38, 66). Furthermore, the combined introduction of N-glycosylation sites derived from neutralization-resistant viruses into neutralization-sensitive isolates has been shown to cause an increase in resistance to antibody that is greater than the sum of incremental increases in resistance observed upon insertion of the individual glycans (58, 73). Although we cannot fully exclude the possibility that introduction of the HA tag may have affected the spatial organization of glycans on the viral spike, our results from testing with various anti-gp120 antibodies suggest little difference between the oligomeric structure of the mutant viral spikes and that of wild-type virus (Fig. 4).
To determine whether glycans may be limiting antibody access, we attempted to deglycosylate virion-associated gp120 in pseudovirus preparations, similar to previous work with SIV (42). However, these attempts were unsuccessful, as incubation in deglycosylation buffer severely reduced the infectivity of pseudovirus preparations (data not shown). Site-directed mutagenesis to remove select glycans was not pursued, as it is well established that removal of glycosylation sites can sometimes result in a generalized increase in virus sensitivity to antibody neutralization (24, 28-30, 39); increased neutralization resistance upon removal of glycans has also been reported (18). Given these caveats, it is likely that a large set of mutant viruses would have to be generated with various combinations of mutated glycosylation sites to define, with high confidence, which glycans might be limiting anti-HA antibody access. Given the complexity of such an analysis, we refrained from pursuing it here.
Irrespective of the precise mechanism(s) involved in limiting antibody access, it is clear that the HIV-1 primary envelope spike can incorporate a considerable stretch of extra sequence into several variable regions with limited antibody recognition. How would this feature be advantageous for the virus? In the first instance, reducing the antigenicity of variable regions may reduce immunogenicity and slow the development of NAb responses in primary infection. Rigidification in the trimer would lower the ability of antibodies raised against variable regions in the context of monomer or other forms of viral debris (53) to react with trimer and neutralize virus. Antibodies raised against rigidified trimer would still be expected to neutralize virus. Glycan rearrangement would lower the ability of antibodies raised against viral debris to recognize trimer and neutralize virus and would reduce the likelihood of raising antibodies directly against trimer. Indeed, NAb responses to HIV-1 are often not detectable until 4 to 10 weeks after infection (19, 61, 73) whereas, for example, NAb responses can be measured within 2 weeks for influenza virus (11, 26) and by 3 weeks for severe acute respiratory syndrome coronavirus (70). Furthermore, the ability to restrict NAb responses may favor the outgrowth of escape variants from the prevailing NAb response (61).
The data presented here parallel and expand on work from previous studies investigating the neutralization sensitivity of SIV and HIV isolates engrafted with a FLAG epitope tag to an anti-FLAG antibody (36, 60, 77). Laird and Desrosiers showed that the primary isolate SIVmac239 engrafted with a FLAG tag in the V1 loop was highly sensitive to neutralization, whereas grafting in V2 or V4 did not yield viruses that were sensitive (36). Those results roughly mirror the results obtained here for the primary HIV-1 isolate JRCSF. However, in contrast to the SIVmac239 results, V4-tagged viruses were sensitive to MAb neutralization, and insertion of the tag in V1 did not result in mutants that are globally more sensitive to antibody neutralization than the parental viruses. These differences may be reflective of differences between the conformation and organization of the spike on HIV-1 versus SIV.
A notable contrast between this study and previous work with FLAG-tagged viruses is the use of multiple antibody preparations here compared to use of a single antitag antibody in previous studies. The use of multiple antibodies provides additional insight into the overall antigenicity of the tag. For example, Yang et al. previously observed that the sensitivity of primary isolates engrafted at V4 to neutralization was similar to that of a lab-adapted virus tagged at the equivalent position (77). The use of additional antibody preparations here shows that not all antitag antibodies are able to effectively neutralize the V4-tagged primary virus JRCSF, though all neutralized the equivalent lab-adapted strain HXB2.
It should also be remarked that the V5 region was found here to be very receptive to HA tag insertions, as reflected in the sensitivity of the HXB2-HA12 and JRCSF-HA12 mutant viruses to neutralization by the HA MAbs and the anti-HA serum preparation. This high antigenicity of the HA tag suggests that V5 may be generally useful as a site for inserting foreign sequences (for example, linear B-cell epitopes) to elicit antibodies, as has been done with antigenic loop regions on influenza virus HA1 (17, 25). It may perhaps also prove possible to use V5 loop epitope-tagged viruses to study the antigenicity and immunogenicity of the envelope spike in vivo.
In sum, we present here an unanticipated twist in HIV resistance to the neutralizing activity of antibodies. Introduction of the foreign HA tag at locations on gp120 that are considered relatively surface accessible on the virus spike would be expected to render the virus susceptible to anti-HA antibody neutralization, at least when introduced at certain locations. However, instead neutralization by antibody is found to be greatly influenced by a combination of epitope location and the antibody preparation used. This “innate” mechanism of restricted epitope recognition requires the oligomeric state of the HIV envelope spike, but this alone is not sufficient. The contrasting neutralization sensitivity profiles of the HA-tagged primary virus JRCSF and the corresponding lab-adapted strain HXB2 shows that the access-restrictive mechanism is dominant in the former. How this resistance is modulated at the molecular level is not entirely clear and will certainly require further investigation. In any case, the data provide further evidence of the highly successful evolutionary adaptation of HIV-1 to resist antibody recognition.
Acknowledgments
We thank James Robinson (Tulane University) for kindly providing MAbs 17b and 48d; Sriram Subramaniam (National Cancer Institute, NIH) for providing models of the unliganded gp120 trimer; and Pascal Poignard, Ian Wilson, and Robyn Stanfield (The Scripps Research Institute) for helpful discussions. Molecular graphics images were produced using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco.
This work was supported by the International AIDS Vaccine Initiative (IAVI) through the Neutralizing Antibody Consortium and by NIH grant AI33292 (to D.R.B.). The Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, is supported by NIH grant P41 RR-01081.
Footnotes
Published ahead of print on 26 November 2008.
REFERENCES
- 1.Bell, C. H., R. Pantophlet, A. Schiefner, L. A. Cavacini, R. L. Stanfield, D. R. Burton, and I. A. Wilson. 2008. Structure of antibody F425-B4e8 in complex with a V3 peptide reveals a new binding mode for HIV-1 neutralization. J. Mol. Biol. 375969-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 7813232-13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjorndal, A., H. Deng, M. Jansson, J. R. Fiore, C. Colognesi, A. Karlsson, J. Albert, G. Scarlatti, D. R. Littman, and E. M. Fenyo. 1997. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J. Virol. 717478-7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohne-Lang, A., and C.-W. von der Lieth. 2005. GlyProt: in silico glycosylation of proteins. Nucleic Acids Res. 33W214-W219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boots, L. J., P. M. McKenna, B. A. Arnold, P. M. Keller, M. K. Gorny, S. Zolla-Pazner, J. E. Robinson, and A. J. Conley. 1997. Anti-human immunodeficiency virus type 1 human monoclonal antibodies that bind discontinuous epitopes in the viral glycoproteins can identify mimotopes from recombinant phage peptide display libraries. AIDS Res. Hum. Retroviruses 131549-1559. [DOI] [PubMed] [Google Scholar]
- 6.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, et al. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 2661024-1027. [DOI] [PubMed] [Google Scholar]
- 7.Burton, D. R., E. O. Saphire, and P. W. Parren. 2001. A model for neutralization of viruses based on antibody coating of the virion surface. Curr. Top. Microbiol. Immunol. 260109-143. [DOI] [PubMed] [Google Scholar]
- 8.Cardozo, T., T. Kimura, S. Philpott, B. Weiser, H. Burger, and S. Zolla-Pazner. 2007. Structural basis for coreceptor selectivity by the HIV type 1 V3 loop. AIDS Res. Hum. Retroviruses 23415-426. [DOI] [PubMed] [Google Scholar]
- 9.Churchill, M. E., E. A. Stura, C. Pinilla, J. R. Appel, R. A. Houghten, D. H. Kono, R. S. Balderas, G. G. Fieser, U. Schulze-Gahmen, and I. A. Wilson. 1994. Crystal structure of a peptide complex of anti-influenza peptide antibody Fab 26/9. Comparison of two different antibodies bound to the same peptide antigen. J. Mol. Biol. 241534-556. [DOI] [PubMed] [Google Scholar]
- 10.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206935-944. [DOI] [PubMed] [Google Scholar]
- 11.Couch, R. B., and J. A. Kasel. 1983. Immunity to influenza in man. Annu. Rev. Microbiol. 37529-549. [DOI] [PubMed] [Google Scholar]
- 12.Davies, D. R., and G. H. Cohen. 1996. Interactions of protein antigens with antibodies. Proc. Natl. Acad. Sci. USA 937-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decker, J. M., F. Bibollet-Ruche, X. Wei, S. Wang, D. N. Levy, W. Wang, E. Delaporte, M. Peeters, C. A. Derdeyn, S. Allen, E. Hunter, M. S. Saag, J. A. Hoxie, B. H. Hahn, P. D. Kwong, J. E. Robinson, and G. M. Shaw. 2005. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J. Exp. Med. 2011407-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Rosny, E., R. Vassell, S. Jiang, R. Kunert, and C. D. Weiss. 2004. Binding of the 2F5 monoclonal antibody to native and fusion-intermediate forms of human immunodeficiency virus type 1 gp41: implications for fusion-inducing conformational changes. J. Virol. 782627-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Earl, P. L., C. C. Broder, R. W. Doms, and B. Moss. 1997. Epitope map of human immunodeficiency virus type 1 gp41 derived from 47 monoclonal antibodies produced by immunization with oligomeric envelope protein. J. Virol. 712674-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fritze, C. E., and T. R. Anderson. 2000. Epitope tagging: general method for tracking recombinant proteins. Methods Enzymol. 3273-16. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Sastre, A., and P. Palese. 1995. Influenza virus vectors. Biologicals 23171-178. [DOI] [PubMed] [Google Scholar]
- 18.Gram, G. J., A. Hemming, A. Bolmstedt, B. Jansson, S. Olofsson, L. Akerblom, J. O. Nielsen, and J. E. Hansen. 1994. Identification of an N-linked glycan in the V1-loop of HIV-1 gp120 influencing neutralization by anti-V3 antibodies and soluble CD4. Arch. Virol. 139253-261. [DOI] [PubMed] [Google Scholar]
- 19.Gray, E. S., P. L. Moore, I. A. Choge, J. M. Decker, F. Bibollet-Ruche, H. Li, N. Leseka, F. Treurnicht, K. Mlisana, G. M. Shaw, S. S. Karim, C. Williamson, and L. Morris. 2007. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. J. Virol. 816187-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 696705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helseth, E., U. Olshevsky, C. Furman, and J. Sodroski. 1991. Human immunodeficiency virus type 1 gp120 envelope glycoprotein regions important for association with the gp41 transmembrane glycoprotein. J. Virol. 652119-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang, C. C., M. Tang, M. Y. Zhang, S. Majeed, E. Montabana, R. L. Stanfield, D. S. Dimitrov, B. Korber, J. Sodroski, I. A. Wilson, R. Wyatt, and P. D. Kwong. 2005. Structure of a V3-containing HIV-1 gp120 core. Science 3101025-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarvik, J. W., and C. A. Telmer. 1998. Epitope tagging. Annu. Rev. Genet. 32601-618. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, W. E., H. Sanford, L. Schwall, D. R. Burton, P. W. Parren, J. E. Robinson, and R. C. Desrosiers. 2003. Assorted mutations in the envelope gene of simian immunodeficiency virus lead to loss of neutralization resistance against antibodies representing a broad spectrum of specificities. J. Virol. 779993-10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalyan, N. K., S. G. Lee, J. Wilhelm, M. R. Pisano, W. T. Hum, C. L. Hsiao, A. R. Davis, J. W. Eichberg, M. Robert-Guroff, and P. P. Hung. 1994. Immunogenicity of recombinant influenza virus haemagglutinin carrying peptides from the envelope protein of human immunodeficiency virus type 1. Vaccine 12753-760. [DOI] [PubMed] [Google Scholar]
- 26.Katz, J. M., W. Lim, C. B. Bridges, T. Rowe, J. Hu-Primmer, X. Lu, R. A. Abernathy, M. Clarke, L. Conn, H. Kwong, M. Lee, G. Au, Y. Y. Ho, K. H. Mak, N. J. Cox, and K. Fukuda. 1999. Antibody response in individuals infected with avian influenza A (H5N1) viruses and detection of anti-H5 antibody among household and social contacts. J. Infect. Dis. 1801763-1770. [DOI] [PubMed] [Google Scholar]
- 27.Klasse, P. J., and Q. J. Sattentau. 2002. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J. Gen. Virol. 832091-2108. [DOI] [PubMed] [Google Scholar]
- 28.Koch, M., M. Pancera, P. D. Kwong, P. Kolchinsky, C. Grundner, L. Wang, W. A. Hendrickson, J. Sodroski, and R. Wyatt. 2003. Structure-based, targeted deglycosylation of HIV-1 gp120 and effects on neutralization sensitivity and antibody recognition. Virology 313387-400. [DOI] [PubMed] [Google Scholar]
- 29.Kolchinsky, P., E. Kiprilov, P. Bartley, R. Rubinstein, and J. Sodroski. 2001. Loss of a single N-linked glycan allows CD4-independent human immunodeficiency virus type 1 infection by altering the position of the gp120 V1/V2 variable loops. J. Virol. 753435-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolchinsky, P., E. Kiprilov, and J. Sodroski. 2001. Increased neutralization sensitivity of CD4-independent human immunodeficiency virus variants. J. Virol. 752041-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krachmarov, C., A. Pinter, W. J. Honnen, M. K. Gorny, P. N. Nyambi, S. Zolla-Pazner, and S. C. Kayman. 2005. Antibodies that are cross-reactive for human immunodeficiency virus type 1 clade A and clade B V3 domains are common in patient sera from Cameroon, but their neutralization activity is usually restricted by epitope masking. J. Virol. 79780-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwong, P. D., M. L. Doyle, D. J. Casper, C. Cicala, S. A. Leavitt, S. Majeed, T. D. Steenbeke, M. Venturi, I. Chaiken, M. Fung, H. Katinger, P. W. Parren, J. Robinson, D. Van Ryk, L. Wang, D. R. Burton, E. Freire, R. Wyatt, J. Sodroski, W. A. Hendrickson, and J. Arthos. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420678-682. [DOI] [PubMed] [Google Scholar]
- 33.Kwong, P. D., R. Wyatt, S. Majeed, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 2000. Structures of HIV-1 gp120 envelope glycoproteins from laboratory-adapted and primary isolates. Structure Fold Des. 81329-1339. [DOI] [PubMed] [Google Scholar]
- 34.Kwong, P. D., R. Wyatt, Q. J. Sattentau, J. Sodroski, and W. A. Hendrickson. 2000. Oligomeric modeling and electrostatic analysis of the gp120 envelope glycoprotein of human immunodeficiency virus. J. Virol. 741961-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labrijn, A. F., P. Poignard, A. Raja, M. B. Zwick, K. Delgado, M. Franti, J. Binley, V. Vivona, C. Grundner, C. C. Huang, M. Venturi, C. J. Petropoulos, T. Wrin, D. S. Dimitrov, J. Robinson, P. D. Kwong, R. T. Wyatt, J. Sodroski, and D. R. Burton. 2003. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J. Virol. 7710557-10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laird, M. E., and R. C. Desrosiers. 2007. Infectivity and neutralization of simian immunodeficiency virus with FLAG epitope insertion in gp120 variable loops. J. Virol. 8110838-10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu, J., A. Bartesaghi, M. J. Borgnia, G. Sapiro, and S. Subramaniam. 2008. Molecular architecture of native HIV-1 gp120 trimers. Nature 455109-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Losman, B., A. Bolmstedt, K. Schonning, A. Bjorndal, C. Westin, E. M. Fenyo, and S. Olofsson. 2001. Protection of neutralization epitopes in the V3 loop of oligomeric human immunodeficiency virus type 1 glycoprotein 120 by N-linked oligosaccharides in the V1 region. AIDS Res. Hum. Retroviruses 171067-1076. [DOI] [PubMed] [Google Scholar]
- 39.Malenbaum, S. E., D. Yang, L. Cavacini, M. Posner, J. Robinson, and C. Cheng-Mayer. 2000. The N-terminal V3 loop glycan modulates the interaction of clade A and B human immunodeficiency virus type 1 envelopes with CD4 and chemokine receptors. J. Virol. 7411008-11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCaffrey, R. A., C. Saunders, M. Hensel, and L. Stamatatos. 2004. N-linked glycosylation of the V3 loop and the immunologically silent face of gp120 protects human immunodeficiency virus type 1 SF162 from neutralization by anti-gp120 and anti-gp41 antibodies. J. Virol. 783279-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKeating, J. A., Y. J. Zhang, C. Arnold, R. Frederiksson, E. M. Fenyo, and P. Balfe. 1996. Chimeric viruses expressing primary envelope glycoproteins of human immunodeficiency virus type I show increased sensitivity to neutralization by human sera. Virology 220450-460. [DOI] [PubMed] [Google Scholar]
- 42.Means, R. E., and R. C. Desrosiers. 2000. Resistance of native, oligomeric envelope on simian immunodeficiency virus to digestion by glycosidases. J. Virol. 7411181-11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montefiori, D. 2004. Evaluating neutralizing antibodies against HIV, SIV and SHIV in luciferase reporter gene assays, p. 12.11.1-12.11.15. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, W. Strober, and R. Coico (ed.), Current protocols in immunology. John Wiley & Sons, New York, NY. [DOI] [PubMed]
- 44.Moore, J. P., Y. Cao, L. Qing, Q. J. Sattentau, J. Pyati, R. Koduri, J. Robinson, C. F. Barbas III, D. R. Burton, and D. D. Ho. 1995. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J. Virol. 69101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore, J. P., and D. D. Ho. 1995. HIV-1 neutralization: the consequences of viral adaptation to growth on transformed T cells. AIDS 9(Suppl. A)S117-S136. [PubMed] [Google Scholar]
- 46.Moore, J. P., B. A. Jameson, Q. J. Sattentau, R. Willey, and J. Sodroski. 1993. Towards a structure of the HIV-1 envelope glycoprotein gp120: an immunochemical approach. Philos. Trans. R. Soc. London B 34283-88. [DOI] [PubMed] [Google Scholar]
- 47.Moore, J. P., F. E. McCutchan, S. W. Poon, J. Mascola, J. Liu, Y. Cao, and D. D. Ho. 1994. Exploration of antigenic variation in gp120 from clades A through F of human immunodeficiency virus type 1 by using monoclonal antibodies. J. Virol. 688350-8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore, J. P., and J. Sodroski. 1996. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J. Virol. 701863-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore, P. L., E. S. Gray, I. A. Choge, N. Ranchobe, K. Mlisana, S. S. Abdool Karim, C. Williamson, and L. Morris. 2008. The C3-V4 region is a major target of autologous neutralizing antibodies in human immunodeficiency virus type 1 subtype C infection. J. Virol. 821860-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niman, H. L., R. A. Houghten, L. E. Walker, R. A. Reisfeld, I. A. Wilson, J. M. Hogle, and R. A. Lerner. 1983. Generation of protein-reactive antibodies by short peptides is an event of high frequency: implications for the structural basis of immune recognition. Proc. Natl. Acad. Sci. USA 804949-4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olshevsky, U., E. Helseth, C. Furman, J. Li, W. Haseltine, and J. Sodroski. 1990. Identification of individual human immunodeficiency virus type 1 gp120 amino acids important for CD4 receptor binding. J. Virol. 645701-5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pantophlet, R., E. Ollmann Saphire, P. Poignard, P. W. Parren, I. A. Wilson, and D. R. Burton. 2003. Fine mapping of the interaction of neutralizing and nonneutralizing monoclonal antibodies with the CD4 binding site of human immunodeficiency virus type 1 gp120. J. Virol. 77642-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parren, P. W., D. R. Burton, and Q. J. Sattentau. 1997. HIV-1 antibody—debris or virion? Nat. Med. 3366-367. [DOI] [PubMed] [Google Scholar]
- 54.Pettersen, E. F., T. D. Goddard, C. C. Huang, G. S. Couch, D. M. Greenblatt, E. C. Meng, and T. E. Ferrin. 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 251605-1612. [DOI] [PubMed] [Google Scholar]
- 55.Phogat, S., and R. Wyatt. 2007. Rational modifications of HIV-1 envelope glycoproteins for immunogen design. Curr. Pharm. Des. 13213-227. [DOI] [PubMed] [Google Scholar]
- 56.Pinter, A., W. J. Honnen, Y. He, M. K. Gorny, S. Zolla-Pazner, and S. C. Kayman. 2004. The V1/V2 domain of gp120 is a global regulator of the sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J. Virol. 785205-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plotkin, S. A. 2001. Immunologic correlates of protection induced by vaccination. Pediatr. Infect. Dis. J. 2063-75. [DOI] [PubMed] [Google Scholar]
- 58.Poon, A. F., F. I. Lewis, S. L. Pond, and S. D. Frost. 2007. Evolutionary interactions between N-linked glycosylation sites in the HIV-1 envelope. PLoS Comput. Biol. 3e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reitter, J. N., R. E. Means, and R. C. Desrosiers. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4679-684. [DOI] [PubMed] [Google Scholar]
- 60.Ren, X., J. Sodroski, and X. Yang. 2005. An unrelated monoclonal antibody neutralizes human immunodeficiency virus type 1 by binding to an artificial epitope engineered in a functionally neutral region of the viral envelope glycoproteins. J. Virol. 795616-5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 1004144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roben, P., J. P. Moore, M. Thali, J. Sodroski, C. F. Barbas III, and D. R. Burton. 1994. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J. Virol. 684821-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sagar, M., X. Wu, S. Lee, and J. Overbaugh. 2006. Human immunodeficiency virus type 1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. J. Virol. 809586-9598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanders, R. W., M. Venturi, L. Schiffner, R. Kalyanaraman, H. Katinger, K. O. Lloyd, P. D. Kwong, and J. P. Moore. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 767293-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scanlan, C. N., R. Pantophlet, M. R. Wormald, E. Ollmann Saphire, R. Stanfield, I. A. Wilson, H. Katinger, R. A. Dwek, P. M. Rudd, and D. R. Burton. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of α1→2 mannose residues on the outer face of gp120. J. Virol. 767306-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schonning, K., B. Jansson, S. Olofsson, J. O. Nielsen, and J. S. Hansen. 1996. Resistance to V3-directed neutralization caused by an N-linked oligosaccharide depends on the quaternary structure of the HIV-1 envelope oligomer. Virology 218134-140. [DOI] [PubMed] [Google Scholar]
- 67.Schulze-Gahmen, U., J. M. Rini, and I. A. Wilson. 1993. Detailed analysis of the free and bound conformations of an antibody. X-ray structures of Fab 17/9 and three different Fab-peptide complexes. J. Mol. Biol. 2341098-1118. [DOI] [PubMed] [Google Scholar]
- 68.Simmonds, P., P. Balfe, C. A. Ludlam, J. O. Bishop, and A. J. Brown. 1990. Analysis of sequence diversity in hypervariable regions of the external glycoprotein of human immunodeficiency virus type 1. J. Virol. 645840-5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stephens, D. M., J. W. Eichberg, N. L. Haigwood, K. S. Steimer, D. Davis, and P. J. Lachmann. 1992. Antibodies are produced to the variable regions of the external envelope glycoprotein of human immunodeficiency virus type 1 in chimpanzees infected with the virus and baboons immunized with a candidate recombinant vaccine. J. Gen. Virol. 731099-1106. [DOI] [PubMed] [Google Scholar]
- 70.Temperton, N. J., P. K. Chan, G. Simmons, M. C. Zambon, R. S. Tedder, Y. Takeuchi, and R. A. Weiss. 2005. Longitudinally profiling neutralizing antibody response to SARS coronavirus with pseudotypes. Emerg. Infect. Dis. 11411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thali, M., J. P. Moore, C. Furman, M. Charles, D. D. Ho, J. Robinson, and J. Sodroski. 1993. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J. Virol. 673978-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 701100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422307-312. [DOI] [PubMed] [Google Scholar]
- 74.Wilson, I. A., J. J. Skehel, and D. C. Wiley. 1981. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature 289366-373. [DOI] [PubMed] [Google Scholar]
- 75.Wyatt, R., N. Sullivan, M. Thali, H. Repke, D. Ho, J. Robinson, M. Posner, and J. Sodroski. 1993. Functional and immunologic characterization of human immunodeficiency virus type 1 envelope glycoproteins containing deletions of the major variable regions. J. Virol. 674557-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiang, S. H., L. Wang, M. Abreu, C. C. Huang, P. D. Kwong, E. Rosenberg, J. E. Robinson, and J. Sodroski. 2003. Epitope mapping and characterization of a novel CD4-induced human monoclonal antibody capable of neutralizing primary HIV-1 strains. Virology 315124-134. [DOI] [PubMed] [Google Scholar]
- 77.Yang, X., I. Lipchina, S. Cocklin, I. Chaiken, and J. Sodroski. 2006. Antibody binding is a dominant determinant of the efficiency of human immunodeficiency virus type 1 neutralization. J. Virol. 8011404-11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang, X., I. Lipchina, M. Lifton, L. Wang, and J. Sodroski. 2007. Antibody binding in proximity to the receptor/glycoprotein complex leads to a basal level of virus neutralization. J. Virol. 818809-8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuan, W., J. Bazick, and J. Sodroski. 2006. Characterization of the multiple conformational states of free monomeric and trimeric human immunodeficiency virus envelope glycoproteins after fixation by cross-linker. J. Virol. 806725-6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng, L., U. Baumann, and J. L. Reymond. 2004. An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res. 32e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zwick, M. B., M. Wang, P. Poignard, G. Stiegler, H. Katinger, D. R. Burton, and P. W. Parren. 2001. Neutralization synergy of human immunodeficiency virus type 1 primary isolates by cocktails of broadly neutralizing antibodies. J. Virol. 7512198-12208. [DOI] [PMC free article] [PubMed] [Google Scholar]