Abstract
NKT cells are a specialized population of T lymphocytes that have an increasingly recognized role in immunoregulation, including controlling the response to viral infections. The characteristics of NKT cells in the peripheral blood of macaques during simian immunodeficiency virus (SIV) or chimeric simian/human immunodeficiency virus (HIV) (SHIV) infection were assessed. NKT cells comprised a mean of 0.19% of peripheral blood lymphocytes across the 64 uninfected macaques studied. Although the range in the percentages of NKT cells was large (0 to 2.2%), levels were stable over time within individual macaques without SIV/SHIV infection. The majority of NKT cells in macaques were CD4+ (on average 67%) with smaller populations being CD8+ (21%) and CD4/CD8 double positive (13%). A precipitous decline in CD4+ NKT cells occurred in all six macaques infected with CXCR4-tropic SHIVmn229 early after infection, with a concomitant rise in CD8+ NKT cells in some animals. The depletion of CD4+ NKT cells was tightly correlated with the depletion of total CD4+ T cells. R5-tropic SIVmac251 infection of macaques resulted in a slower and more variable decline in CD4+ NKT cells, with animals that were able to control SIV virus levels maintaining higher levels of CD4+ NKT cells. An inverse correlation between the depletion of total and CD4+ NKT cells and SIV viral load during chronic infection was observed. Our results demonstrate the infection-driven depletion of peripheral CD4+ NKT cells during both SHIV and SIV infection of macaques. Further studies of the implications of the loss of NKT cell subsets in the pathogenesis of HIV disease are needed.
Natural killer T (NKT) cells are important regulators of immunity for infectious diseases, tumor surveillance, allergy, and autoimmunity (2, 17, 36). NKT cells have a semi-invariant T-cell receptor consisting of an invariant T-cell receptor α (TCR-α) chain (Vα14-Ja18 in mice and Va24-Ja18 in humans), paired with a limited array of TCR-β chains (comprised of Vβ8.2, Vβ7, or Vβ2 in mice and Vβ11 in humans), that facilitates the recognition of lipid-based antigens presented by the major histocompatibility complex-like molecule CD1d. NKT cells are a potent source of cytokines and facilitate several aspects of adaptive immunity in both mice and humans. NKT cells are typically a small subset of lymphocytes within lymphoid tissues and peripheral blood (16).
Human NKT cells can express CD4 and CD8 molecules, and these cells are susceptible to human immunodeficiency virus type 1 (HIV-1) infection in vitro (28). Several studies have demonstrated reduced numbers of NKT cells in the peripheral blood of HIV-infected humans (28, 31, 35), which is at least partially reversed following effective antiretroviral therapy (34, 37). However, the highly variable numbers of NKT cells in the blood of healthy human subjects, and the inability to compare NKT cells before and after HIV infection within an individual, complicate analyses of the impact of HIV infection on NKT cells. The degree to which NKT cell depletion contributes to the immunodeficiency of HIV infection in humans is not known, although a higher rate of malignancies has been observed in HIV-1-infected subjects with lower NKT cell levels (29).
Asian macaques such as rhesus, cynomolgus, and pigtail macaques are well-accepted primate models for the study of HIV (22). Several viruses including simian immunodeficiency virus (SIV) and chimeric simian/HIV (SHIV) cause an AIDS-like disease in macaques including pigtail macaques (1). SIV and SHIV viruses, like HIV-1 strains, vary in their uses of either CXCR4 or CCR5 chemokines to enter CD4-bearing cells. There have been only a limited number of studies of NKT cells in macaques (14, 15, 20, 27), often studying NKT cells within lymphoid organs such as the spleen. We undertook a comprehensive kinetic study of NKT cells in pigtail macaques using an α-galactosylceramide (α-GalCer)-loaded CD1d tetramer prior to, and throughout, both CCR5-using SIVmac251 infection and CXCR4-using SHIVmn229 infection.
MATERIALS AND METHODS
Animals and viruses.
Pigtail macaques (Macaca nemestrina) were obtained from the National Health and Medical Research Council-supported macaque breeding facility in Australia. All macaques were juveniles, aged 3 to 5 years of age, during the studies. Macaques were studied as part of groups participating in SIV infection and SHIV infection studies that were previously reported (12, 21). Our institutional animal ethics committee approved all animal use protocols. Serial fresh blood samples or frozen peripheral blood mononuclear cells were analyzed for each macaque. For SIV infection, we studied macaques infected with the CCR5-tropic SIVmac251 as previously reported (21, 33). For SHIV, we studied macaques infected with the CXCR4-tropic SHIVmn229 as previously reported (11, 12). We observed typical patterns of infection of pigtail macaques with these viruses as was previously reported (1). Plasma SIV or SHIV viral RNAs were studied by real-time reverse transcription-PCR and depletion of total CD4 T cells followed by flow cytometry as previously described (11).
NKT cell analyses.
NKT cells were defined as CD3+ lymphocytes stained with the αGalCer-loaded mouse CD1d tetramer (CD1d/α-GalCer tetramer, produced in house from a baculovirus construct originally provided by Mitchell Kronenberg) (25). NKT cell subsets were studied by counterstaining with CD3, CD4, CD8, CD45RA, and CD95 monoclonal antibodies as previously described (24, 32). Intracellular gamma interferon (IFN-γ) expression by peripheral blood NKT cells was assessed by flow cytometry following phorbol myristate acetate (50 ng/ml) and ionomycin (3 μg/ml) stimulation of peripheral blood ex vivo for 6 h as previously described (8). The intracellular expression of IFN-γ was studied as previously described for T-cell studies of pigtail macaques (13).
RESULTS
NKT cells in peripheral blood of pigtail macaques.
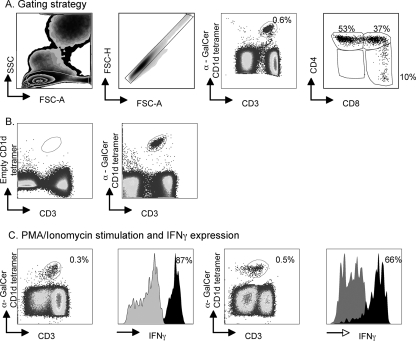
The study of NKT cells has become technically simpler in recent years with the use of CD1d tetramers bound to α-GalCer: these tetramers have been used with success on mouse, macaque, and human samples (3, 18, 25, 27). NKT cells were defined by gating on CD3 lymphocytes that bound to the CD1d/α-GalCer tetramer (Fig. 1A). We counterstained cells with CD4 and CD8 staining to identify NKT cell subsets. We validated the staining with the CD1d/α-GalCer tetramer by using an empty CD1d tetramer and saw no nonspecific staining (Fig. 1b). The NKT cells also expressed CD45RA (44 to 90% of NKT cells) and CD95 (92 to 99% of NKT cells) but did not express CCR7, CD56, or CD25 (not shown). NKT cells are typically a potent source of cytokines, and, confirming this activity in the pigtail macaques that we were studying, most of the NKT cells expressed high levels of IFN-γ when stimulated in vitro (Fig. 1C).
FIG. 1.
NKT cells in pigtail macaques. (A) Gating strategy to define NKT cells in peripheral blood and proportions of CD4+ and CD8+ subsets. SSC, side scatter; FSC, forward scatter. (B) An empty CD1d tetramer (without α-GalCer) is shown to validate the staining of the α-GalCer/CD1d tetramer. (C) Expression of IFN-γ following in vitro stimulation is shown for two animals. PMA, phorbol myristate acetate.
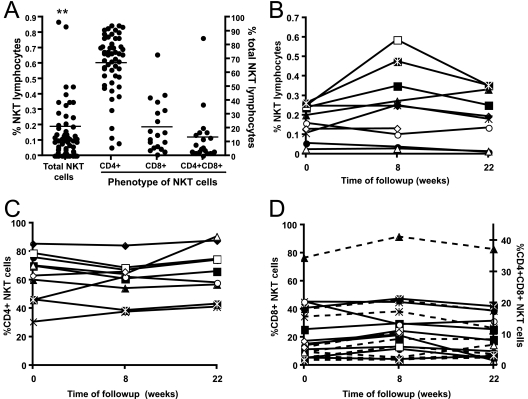
NKT cell frequencies in the peripheral blood of humans are highly variable (3). Thus, the lack of predisease blood samples can be problematic when ascribing the depletion of NKT cells to particular diseases. An advantage of macaque models in studying NKT cells over mouse and human systems is the ability to monitor NKT cells over time through the repeated sampling of blood and knowledge of baseline NKT cell levels prior to a disease process. We first studied NKT cell levels in peripheral blood samples of 64 naïve pigtail macaques. A large range of peripheral blood NKT cell levels in healthy pigtail macaques was observed, ranging from 0 to 2.2% of lymphocytes, with a mean level of 0.19% (Fig. 2A). Eleven of the 64 macaques had <0.01% NKT cells out of all lymphocytes. Although the CD4+ NKT cell subset was almost always the largest (in 93% of samples), there was also variability in the proportion of NKT cell populations that were CD4+, CD8+, or CD4+ CD8+ (Fig. 2A). Consistent with some previous reports of studies using rhesus macaques (14, 20), very few NKT cells in the animals tested carried the CD4− CD8− phenotype that is common for mice and humans.
FIG. 2.
Levels of NKT cells in naïve pigtail macaques. (A) Levels of CD1d tetramer-positive NKT cells from 64 macaques are shown in the left dot plot. The two asterisks reflect two macaques with levels of 1 to 2%. The line represents the mean. The proportions of CD4+ CD8−, CD8+ CD4−, and CD4+ CD8+ NKT cells are shown in the three right-hand dot plots, expressed as a percentage of total NKT cells. (B, C, and D) Proportions of total, CD4+ CD8−, CD8+ CD4−, and CD4+ CD8+ (dotted lines) NKT cells over time in naïve macaques.
Given the significant variability of NKT cell frequencies between macaques, NKT cell percentages in 10 macaques were examined over a 5-month period of time and found to be stable (within twofold) throughout this period. (Fig. 2B). Similarly, the proportions of CD4+ CD8−, CD8+ CD4−, and CD4+ CD8+ NKT cell subsets were relatively stable over time within each animal (Fig. 2C and D).
NKT cell frequencies are frequently assessed in blood and lymphoid organs in mouse studies where NKT cells typically comprise 0.2 to 0.5% of lymphocytes in the thymus and blood, 1% in the spleen, and 20 to 40% in the liver (16). The availability of various organs at autopsy of macaques allowed us to perform a direct comparison of NKT cell levels in blood taken both prior to infection and at autopsy to that in lymph nodes, spleen, bone marrow, and liver samples in five SIV- or SHIV-infected pigtail macaques (see Fig. S1 in the supplemental material). Marginally lower numbers of NKT cells, as a proportion of lymphocytes, were present in lymph node, spleen, and bone marrow samples compared to peripheral blood taken at the time of autopsy. In one animal, there was a twofold increase in NKT cells within the lymphocyte population from the liver, although this was not a consistent finding. We also studied one animal (animal 9532) that had virtually no NKT cells present in peripheral blood at autopsy and found that NKT cells were also absent in the other organs studied.
Effect of SHIVmn229 infection on NKT cell populations over time.
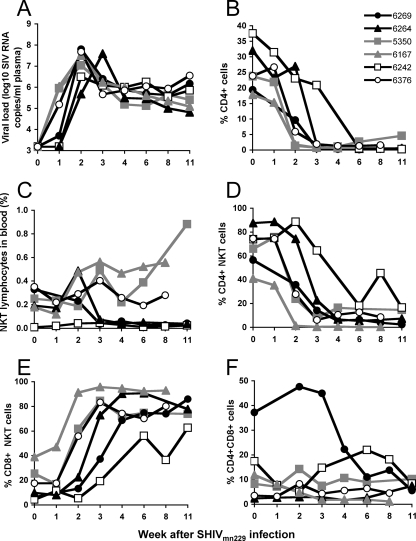
In vitro, human NKT cells are susceptible to HIV-1 infection (28), and in vivo, HIV-1 infection of humans correlates with reduced numbers of peripheral blood NKT cells (31, 35). We first asked whether reductions in NKT cell numbers are observed in six SHIVmn229-infected macaques prospectively monitored over time with a range of baseline NKT cell levels. SHIVmn229 is CXCR4 tropic, and infection of pigtail macaques with this virus resulted in a rapid, profound, and irreversible loss of peripheral CD4 T cells in all animals studied (Fig. 3A and B) (11). We observed a variable pattern of total NKT cell levels over time following SHIVmn229 infection. Two of the animals (animals 6269 and 6167) (Fig. 3C) had a rapid and sustained loss of NKT cells at 3 weeks after SHIVmn229 infection. Both the proportion of NKT cells within the lymphocyte gate and the absolute number of NKT cells declined in these animals (see Table S1 in the supplemental material). Of the other four animals studied, two had minimal changes and two had a two- to threefold rise in total numbers of NKT cells, as a proportion of lymphocytes, over time.
FIG. 3.
NKT cells following X4-tropic SHIV infection. (A and B) Viral load (A) and total peripheral CD4 T cells (B) following SHIVmn229 infection of six pigtail macaques. Animal identification numbers are shown in the legend. (C) Levels of total NKT cells over time after infection. Data for animals with total NKT cell depletion are shown as black closed symbols, and those with increased numbers of NKT cells are shown as gray symbols. (D, E, and F) Proportions of CD4+ CD8−, CD8+ CD4−, and CD4+ CD8+ NKT cells after infection.
Since SHIV infections target CD4-expressing cells, we then analyzed the effect of SHIVmn229 infection on the proportion of defined CD4/CD8 NKT cell subsets over time (Fig. 3D and E). Despite the variable total levels of NKT cells, we consistently observed an abrupt loss of the proportion, and absolute number, of CD4+ CD8− NKT cells over the first 1 to 4 weeks of infection that was sustained over time (Fig. 3D) (see Table S1 in the supplemental material). A concomitant rise in the proportion of CD8+ NKT cells was observed in all six animals over the same time period (Fig. 3E). In most animals, this did not represent more than a twofold rise in absolute numbers of CD8+ NKT cells; however, in two animals (animals 5350 and 6167), there were large (7- to 10-fold) absolute rises in the numbers of CD8+ NKT cells (0.06% to 0.65% and 0.07% to 0.51% of lymphocytes), reflecting the overall rise in numbers of NKT cells after SHIVmn229 infection in these animals (Fig. 3C). Levels of CD4+ CD8+ NKT cells in these animals generally fell or remained at low levels (Fig. 3F).
NKT cell populations following SIVmac251 infection of macaques.
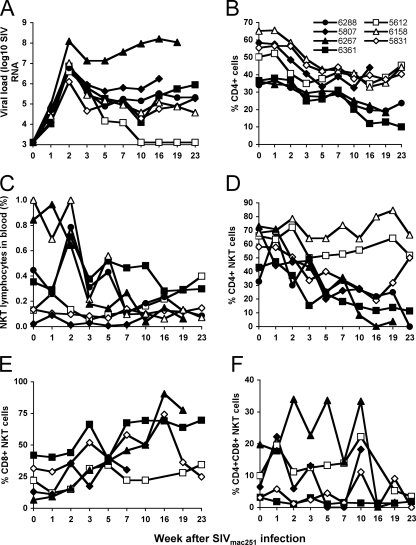
SIVmac251 is a CCR5-tropic virus that, in comparison to CXCR4-tropic viruses, results in slow and more variable depletion of total CD4 T cells, more analogous to human HIV-1 infection. Furthermore, most human infections result from viruses tropic for CCR5, and CCR5-tropic SIV infections of macaques are generally viewed as being more appropriate models of human HIV-1 infection (38). We evaluated the effect on peripheral NKT cell populations following SIVmac251 infection of seven pigtail macaques with variable numbers of baseline NKT cells in peripheral blood. All infected animals developed high levels of SIV viremia during acute infection, but, as expected, there were variable levels of viremia and CD4 T-cell depletion during chronic infection (Fig. 4A and B). We again observed a variable pattern of total NKT cell changes over time, with three animals (animals 6158, 6267, and 6288) having a sustained loss of NKT cells both as a proportion of lymphocytes (to ≤20% of baseline levels) (Fig. 4C) and in their absolute numbers (see Table S1 in the supplemental material). The other four animals maintained more stable levels of NKT cells over time (Fig. 4C) (see Table S1 in the supplemental material). Interestingly, those animals with the highest baseline levels of NKT cells experienced the greatest declines. Four animals experienced reductions in numbers of CD4+ NKT cells following SIVmac251 infection, expressed both as a proportion of NKT cells (Fig. 4D) and in absolute numbers (see Table S1 in the supplemental material). In five of the seven SIVmac239-infected animals, we also monitored numbers of CD8+ and CD4+ CD8+ NKT cells over time. There was a rise in the proportion of CD8+ CD4− NKT cells over time in the two animals (animals 6267 and 6361) with depleted CD4+ NKT cells monitored 19 to 23 weeks after infection (Fig. 4E). In these animals, the number of CD8+ NKT cells expressed as a proportion of total lymphocytes remained constant during infection (changing from 0.055% to 0.048% and from 0.15% to 0.18%, respectively) rather than reflecting a large absolute increase in CD8+ NKT cells. Again, CD4+ CD8+ NKT cells did not show any consistent pattern over time but generally remained at a low frequency (Fig. 4F).
FIG. 4.
NKT cells following R5-tropic SIV infection. (A and B) Viral load (A) and total peripheral CD4 T cells (B) following SIVmac251 infection of seven pigtail macaques. Animal identification numbers are shown in the legend. (C) Levels of total NKT cells over time after infection. (D) Levels of CD4+ CD8− NKT cells after infection. Animals with CD4+ NKT cell depletion are shown in black closed symbols. (E and F) Levels of CD8+ CD4− and CD4+ CD8+ NKT cells after infection.
Associations between NKT cell levels, peripheral CD4 T-cell depletion, and SIV viral load.
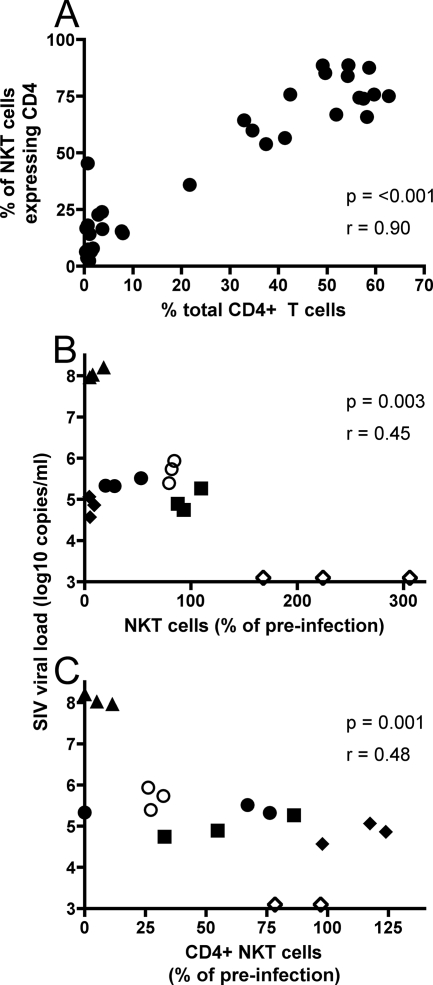
The dramatic loss of CD4+ NKT cells following SHIVmn229 infection mirrored the loss of total CD4+ T cells (Fig. 3B and D). Indeed, we observed that levels of CD4+ NKT cells and total peripheral CD4+ T cells were tightly correlated (Fig. 5A), suggesting a common mechanism of depletion. SHIVmn229 is, however, uniformly pathogenic, with high viral loads, and there was no relationship between the depletion of NKT cells and viral load in the SHIVmn229 study (not shown). We therefore studied the relationship between viral load and NKT cells in the macaques infected with SIVmac251, since infection with this virus results in a wide range of subsequent set point viral loads. The relationship between SIV viral levels and total, or CD4+ CD8−, NKT cell levels was assessed (Fig. 5B and C). We chose the last three viral load samples available prior to week 23 in all animals to study, since these levels most closely reflect the chronic viremia that more accurately predicts disease both for macaques with SIV infection and for humans with HIV-1 infection (30). We expressed NKT cell frequencies as a proportion of baseline levels, since we showed baseline NKT cell numbers varied widely (Fig. 2A) but remain relatively uniform within individual animals (Fig. 2B). The proportions of both total NKT cells and CD4+ CD8− NKT cells were inversely proportional to SIV viral load during chronic SIVmac251 infection (Fig. 5B and C). There was no relationship between CD4− CD8+ NKT cells and viral load (not shown).
FIG. 5.
Relationship between CD4 depletion, viral load, and NKT cell levels. (A) Correlation between depletion of total CD4 T cells (expressed as a proportion of total lymphocytes) and CD4+ CD8− NKT cells (expressed as a proportion of baseline levels) following SHIVmn229 infection. Dots represent all values from the six animals shown in Fig. 3B and D. (B) Correlation between SIV viral load and total NKT cells (expressed as a proportion of the baseline) following SIVmac251 infection. (C) Correlation between SIV viral load and CD4+ CD8− NKT cells (expressed as a proportion of baseline) following SIVmac251 infection. Dot symbols (B and C) represent the last three measurements during chronic infection for each individual animal.
DISCUSSION
This study comprehensively analyzed peripheral blood NKT cells in healthy pigtail macaques and then monitored levels of NKT cells and defined CD4/CD8 NKT cell subsets over time after either CXCR4-tropic SHIV infection or CCR5-tropic SIV infection. The depletion of total NKT cell populations after infection was variable, but the depletion of CD4+ CD8− NKT cells was uniform in six of six macaques early after SHIV infection and occurred in four of seven macaques within 5 months after SIV infection. The loss of CD4+ CD8− NKT cells was tightly correlated with the depletion of conventional CD4+ T cells, suggesting a common mechanism. The destruction of both total and CD4+ NKT cells correlated with viral load during chronic SIV infection. Our results suggest that SIV and SHIV infections preferentially deplete CD4+ CD8− NKT cells, likely through direct infection. This is consistent with a previous study of NKT cells isolated from rhesus macaques where CD4+ but not CD4− NKT cells were susceptible to infection with SIV in vitro (27).
Levels of NKT cells in pigtail macaques shared several similarities with NKT cells in humans, with a large range of total NKT cells, although generally, they are up to 1% of peripheral lymphocytes (3). Similar to macaques, a given frequency of NKT cells within individual healthy humans is typically stable over time. The relative stability in blood within each macaque allows some confidence that sustained changes over time can be ascribed to SIV or SHIV infection.
The majority of NKT cells in peripheral blood from the large number of naïve pigtail macaques that we studied expressed CD4+, but there were also significant portions of NKT cells that expressed both CD4 and CD8 or CD8 alone, while few NKT cells were CD4− CD8−. This is in contrast with some previous studies of NKT cells from rhesus macaques, where there seems to be little agreement in the cell surface phenotype of NKT cells with regard to CD4 and CD8 expression. In one study (15), NKT cells defined as being Vα24+ 6B11+ (an antibody specific for the invariant NKT cell TCR-α chain) were nearly all CD4−, and approximately half were CD8α+ CD8β−. However, these cells were expanded in vitro, which may have impacted their cell surface phenotype. Another study, again using in vitro-expanded NKT cells derived from peripheral blood mononuclear cells, reported NKT cells to be nearly all CD8+, with few CD4+ or double-negative NKT cells. A previous paper by Motsinger et al. (27) examined fresh ex vivo spleen-derived NKT cells (defined by Vα24 expression and CD1d/α-GalCer tetramer binding) from rhesus macaques. They showed that the majority (average 65%) of NKT cells were CD8+ and that approximately 20% were CD4+. It was possible that spleen-derived NKT cells examined in the latter study (27) are different from blood-derived NKT cells. A recent study (14) directly compared rhesus macaque blood and spleen-derived NKT cells and found that most spleen NKT cells were CD4+ CD8+, compared to blood, where most NKT cells were CD4− CD8+, although even in blood, 20% were reported to be CD4+ CD8+. Our results are not exactly consistent with any of the previous studies, but this may be due to the fact that we have used pigtailed macaques versus the use of rhesus macaques in all previous studies. Nonetheless, it is clear that NKT cell subsets exist in macaques, and the different subsets of NKT cells in humans and mice are known to have clear functional consequences (6, 7, 9, 19, 23). The role that these different subsets play in the immune system is unknown, but we maintain that this is an important consideration when studying NKT cells.
The kinetics of depletion of CD4+ CD8− NKT cells closely mimicked those of total CD4 T-cell depletion in SHIV-infected pigtail macaques, and in general, a similar relationship has been observed in HIV-infected humans (26, 34, 35, 37). Our results are consistent with the expression of both CXCR4 and CCR5 by macaque NKT cells (27). In contrast with humans infected with HIV-1 (26), we observed a large and sustained concomitant increase in absolute levels of CD4− CD8+ NKT cells in two macaques infected with the rapidly virulent CXCR4-using SHIVmn229. An increase in CD8+ NKT cells was not observed following infection with the CCR5-tropic SIVmac251 virus, consistent with suggestions that infections with SIVmac251 and related CCR5-tropic strains are the more appropriate models of HIV pathogenesis (38).
Our studies do not yet address the implications of NKT cell depletion for HIV/SIV disease. Given the close relationship between CD4+ T-cell depletion and CD4+ NKT cell depletion that we observed, it could prove difficult to tease out the specific role of NKT cell depletion. Emerging work does, however, suggest that the depletion of NKT cells correlates with an increased risk of cancer in HIV-infected subjects (29). This is consistent with a role for NKT cells in tumor surveillance in mice (10). Given the potent ability of NKT cells to produce multiple cytokines and coordinate innate and adaptive immune responses, it is likely that NKT cell depletion has multiple functional consequences. There is also increasing interest in stimulating NKT cells to enhance immunity, for example, using α-GalCer as a vaccine adjuvant (4, 5). Such studies may ultimately define the utility of NKT cells and reveal strategies to specifically enhance their function and prevent consequences of NKT cell depletion during HIV infection.
Supplementary Material
Acknowledgments
We thank Sheilajen Alcantara, Roberta Goli, Kellie Frost, and Stuart Berzins for expert assistance and advice.
This work was supported by Australian National Health and Medical Research Council awards 454363, 454569, and 299907.
Footnotes
Published ahead of print on 3 December 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Batten, C. J., R. D. Rose, K. M. Wilson, M. B. Agy, S. Chea, I. Stratov, D. C. Montefiori, and S. J. Kent. 2006. Comparative evaluation of simian, simian-human, and human immunodeficiency virus infections in the pigtail macaque (Macaca nemestrina) model. AIDS Res. Hum. Retrovir. 22580-588. [DOI] [PubMed] [Google Scholar]
- 2.Bendelac, A., P. B. Savage, and L. Teyton. 2007. The biology of NKT cells. Annu. Rev. Immunol. 25297-336. [DOI] [PubMed] [Google Scholar]
- 3.Berzins, S. P., A. D. Cochrane, D. G. Pellicci, M. J. Smyth, and D. I. Godfrey. 2005. Limited correlation between human thymus and blood NKT cell content revealed by an ontogeny study of paired tissue samples. Eur. J. Immunol. 351399-1407. [DOI] [PubMed] [Google Scholar]
- 4.Cerundolo, V., and M. Salio. 2007. Harnessing NKT cells for therapeutic applications. Curr. Top. Microbiol. Immunol. 314325-340. [DOI] [PubMed] [Google Scholar]
- 5.Chang, D. H., K. Osman, J. Connolly, A. Kukreja, J. Krasovsky, M. Pack, A. Hutchinson, M. Geller, N. Liu, R. Annable, J. Shay, K. Kirchhoff, N. Nishi, Y. Ando, K. Hayashi, H. Hassoun, R. M. Steinman, and M. V. Dhodapkar. 2005. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J. Exp. Med. 2011503-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, X., X. Wang, G. S. Besra, and J. E. Gumperz. 2007. Modulation of CD1d-restricted NKT cell responses by CD4. J. Leukoc. Biol. 821455-1465. [DOI] [PubMed] [Google Scholar]
- 7.Coquet, J. M., S. Chakravarti, K. Kyparissoudis, F. W. McNab, L. A. Pitt, B. S. McKenzie, S. P. Berzins, M. J. Smyth, and D. I. Godfrey. 2008. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4− NK1.1− NKT cell population. Proc. Natl. Acad. Sci. USA 10511287-11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coquet, J. M., K. Kyparissoudis, D. G. Pellicci, G. Besra, S. P. Berzins, M. J. Smyth, and D. I. Godfrey. 2007. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J. Immunol. 1782827-2834. [DOI] [PubMed] [Google Scholar]
- 9.Crowe, N. Y., J. M. Coquet, S. P. Berzins, K. Kyparissoudis, R. Keating, D. G. Pellicci, Y. Hayakawa, D. I. Godfrey, and M. J. Smyth. 2005. Differential antitumor immunity mediated by NKT cell subsets in vivo. J. Exp. Med. 2021279-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowe, N. Y., M. J. Smyth, and D. I. Godfrey. 2002. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J. Exp. Med. 196119-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dale, C. J., R. De Rose, I. Stratov, S. Chea, D. Montefiori, S. A. Thomson, I. A. Ramshaw, B. E. Coupar, D. B. Boyle, M. Law, and S. J. Kent. 2004. Efficacy of DNA and fowlpoxvirus prime/boost vaccines for simian/human immunodeficiency virus. J. Virol. 7813819-13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Rose, R., C. J. Batten, M. Z. Smith, C. S. Fernandez, V. Peut, S. Thomson, I. A. Ramshaw, B. E. Coupar, D. B. Boyle, V. Venturi, M. P. Davenport, and S. J. Kent. 2007. Comparative efficacy of subtype AE simian-human immunodeficiency virus priming and boosting vaccines in pigtail macaques. J. Virol. 81292-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Rose, R., C. S. Fernandez, L. Loh, V. Peut, R. D. Mason, S. Alcantara, J. Reece, and S. J. Kent. 2008. Delivery of immunotherapy with peptide-pulsed blood in macaques. Virology 187204-210. [DOI] [PubMed] [Google Scholar]
- 14.Gansuvd, B., J. Goodwin, C. K. Asiedu, X. L. Jiang, U. Jargal, P. Andrades, M. A. Exley, and J. M. Thomas. 2008. Invariant natural killer T cells from rhesus macaque spleen and peripheral blood are phenotypically and functionally distinct populations. J. Med. Primatol. 371-11. [DOI] [PubMed] [Google Scholar]
- 15.Gansuvd, B., W. J. Hubbard, A. Hutchings, F. T. Thomas, J. Goodwin, S. B. Wilson, M. A. Exley, and J. M. Thomas. 2003. Phenotypic and functional characterization of long-term cultured rhesus macaque spleen-derived NKT cells. J. Immunol. 1712904-2911. [DOI] [PubMed] [Google Scholar]
- 16.Godfrey, D. I., and S. P. Berzins. 2007. Control points in NKT-cell development. Nat. Rev. Immunol. 7505-518. [DOI] [PubMed] [Google Scholar]
- 17.Godfrey, D. I., and M. Kronenberg. 2004. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Investig. 1141379-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godfrey, D. I., H. R. MacDonald, M. Kronenberg, M. J. Smyth, and L. Van Kaer. 2004. NKT cells: what's in a name? Nat. Rev. Immunol. 4231-237. [DOI] [PubMed] [Google Scholar]
- 19.Gumperz, J. E., S. Miyake, T. Yamamura, and M. B. Brenner. 2002. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 195625-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kashiwase, K., A. Kikuchi, Y. Ando, A. Nicol, S. A. Porcelli, K. Tokunaga, M. Omine, M. Satake, T. Juji, M. Nieda, and Y. Koezuka. 2003. The CD1d natural killer T-cell antigen presentation pathway is highly conserved between humans and rhesus macaques. Immunogenetics 54776-781. [DOI] [PubMed] [Google Scholar]
- 21.Kent, S. J., R. De Rose, V. V. Mokhonov, E. A. Mohkonova, C. S. Fernandez, S. Alcantara, E. Rollman, R. D. Mason, L. Loh, V. Peut, J. Reece, X. J. Wang, K. M. Wilson, A. Suhrbier, and A. A. Khromykh. 2008. Evaluation of recombinant Kunjin replicon SIV vaccines for protective efficacy in macaques. Virology 374528-534. [DOI] [PubMed] [Google Scholar]
- 22.Lackner, A. A., and R. S. Veazey. 2007. Current concepts in AIDS pathogenesis: insights from the SIV/macaque model. Annu. Rev. Med. 58461-476. [DOI] [PubMed] [Google Scholar]
- 23.Lee, P. T., K. Benlagha, L. Teyton, and A. Bendelac. 2002. Distinct functional lineages of human V(alpha)24 natural killer T cells. J. Exp. Med. 195637-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason, R. D., R. De Rose, N. Seddiki, A. Kelleher, and S. J. Kent. 2008. Low pre-infection levels and loss of central memory CD4+ T cells may predict rapid progression in SIV-infected pigtail macaques. Virology 38111-15. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda, J. L., O. V. Naidenko, L. Gapin, T. Nakayama, M. Taniguchi, C. R. Wang, Y. Koezuka, and M. Kronenberg. 2000. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192741-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montoya, C. J., J. C. Catano, Z. Ramirez, M. T. Rugeles, S. B. Wilson, and A. L. Landay. 2008. Invariant NKT cells from HIV-1 or Mycobacterium tuberculosis-infected patients express an activated phenotype. Clin. Immunol. 1271-6. [DOI] [PubMed] [Google Scholar]
- 27.Motsinger, A., A. Azimzadeh, A. K. Stanic, R. P. Johnson, L. Van Kaer, S. Joyce, and D. Unutmaz. 2003. Identification and simian immunodeficiency virus infection of CD1d-restricted macaque natural killer T cells. J. Virol. 778153-8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motsinger, A., D. W. Haas, A. K. Stanic, L. Van Kaer, S. Joyce, and D. Unutmaz. 2002. CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J. Exp. Med. 195869-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nowicki, M. J., C. Vigen, W. J. Mack, E. Seaberg, A. Landay, K. Anastos, M. Young, H. Minkoff, R. Greenblatt, and A. M. Levine. 2008. Association of cells with natural killer (NK) and NKT immunophenotype with incident cancers in HIV-infected women. AIDS Res. Hum. Retrovir. 24163-168. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien, W. A., P. M. Hartigan, D. Martin, J. Esinhart, A. Hill, S. Benoit, M. Rubin, M. S. Simberkoff, J. D. Hamilton, et al. 1996. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. N. Engl. J. Med. 334426-431. [DOI] [PubMed] [Google Scholar]
- 31.Sandberg, J. K., N. M. Fast, E. H. Palacios, G. Fennelly, J. Dobroszycki, P. Palumbo, A. Wiznia, R. M. Grant, N. Bhardwaj, M. G. Rosenberg, and D. F. Nixon. 2002. Selective loss of innate CD4+ Vα24 natural killer T cells in human immunodeficiency virus infection. J. Virol. 767528-7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, M. Z., T. E. Asher, V. Venturi, M. P. Davenport, D. C. Douek, D. A. Price, and S. J. Kent. 2008. Limited maintenance of vaccine-induced simian immunodeficiency virus-specific CD8 T-cell receptor clonotypes after virus challenge. J. Virol. 827357-7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith, M. Z., C. J. Dale, R. De Rose, I. Stratov, C. S. Fernandez, A. G. Brooks, J. T. Weinfurter, K. Krebs, C. Riek, D. I. Watkins, D. H. O'Connor, and S. J. Kent. 2005. Analysis of pigtail macaque major histocompatibility complex class I molecules presenting immunodominant simian immunodeficiency virus epitopes. J. Virol. 79684-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Vliet, H. J., M. G. van Vonderen, J. W. Molling, H. J. Bontkes, M. Reijm, P. Reiss, M. A. van Agtmael, S. A. Danner, A. J. van den Eertwegh, B. M. von Blomberg, and R. J. Scheper. 2006. Rapid recovery of NKT cells upon institution of highly active antiretroviral therapy for HIV-1 infection. J. Immunol. 1775775-5778. [DOI] [PubMed] [Google Scholar]
- 35.van der Vliet, H. J., B. M. von Blomberg, M. D. Hazenberg, N. Nishi, S. A. Otto, B. H. van Benthem, M. Prins, F. A. Claessen, A. J. van den Eertwegh, G. Giaccone, F. Miedema, R. J. Scheper, and H. M. Pinedo. 2002. Selective decrease in circulating V alpha 24+V beta 11+ NKT cells during HIV type 1 infection. J. Immunol. 1681490-1495. [DOI] [PubMed] [Google Scholar]
- 36.Van Kaer, L. 2007. NKT cells: T lymphocytes with innate effector functions. Curr. Opin. Immunol. 19354-364. [DOI] [PubMed] [Google Scholar]
- 37.Vasan, S., M. A. Poles, A. Horowitz, E. E. Siladji, M. Markowitz, and M. Tsuji. 2007. Function of NKT cells, potential anti-HIV effector cells, are improved by beginning HAART during acute HIV-1 infection. Int. Immunol. 19943-951. [DOI] [PubMed] [Google Scholar]
- 38.Watkins, D. I., D. R. Burton, E. G. Kallas, J. P. Moore, and W. C. Koff. 2008. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat. Med. 14617-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.