Abstract
Lentiviral vectors deliver antigens to dendritic cells (DCs) in vivo, but they do not trigger DC maturation. We therefore expressed a viral protein that constitutively activates NF-κB, vFLIP from Kaposi's sarcoma-associated herpesvirus (KSHV), in a lentivector to mature DCs. vFLIP activated NF-κB in mouse bone marrow-derived DCs in vitro and matured these DCs to a similar extent as lipopolysaccharide; costimulatory markers CD80, CD86, CD40, and ICAM-1 were upregulated and tumor necrosis factor alpha and interleukin-12 secreted. The vFLIP-expressing lentivector also matured DCs in vivo. When we coexpressed vFLIP in a lentivector with ovalbumin (Ova), we found an increased immune response to Ova; up to 10 times more Ova-specific CD8+ T cells secreting gamma interferon were detected in the spleens of vFLIP_Ova-immunized mice than in the spleens of mice immunized with GFP_Ova. Furthermore, this increased CD8+ T-cell response correlated with improved tumor-free survival in a tumor therapy model. A single immunization with vFLIP_Ova also reduced the parasite load when mice were challenged with OVA-Leishmania donovani. In conclusion, vFLIP from KSHV is a DC activator, maturing DCs in vitro and in vivo. This demonstrates that NF-κB activation is sufficient to induce many aspects of DC maturation and that expression of a constitutive NF-κB activator can improve the efficacy of a vaccine vector.
Viral vectors carrying antigens are being developed as vaccines for cancer or infectious disease (reviewed in references 5 and 8), but as yet such vectors have shown limited success in clinical trials (see, e.g., references 42 and 53). One major difficulty has been in developing a vaccine vector that can mature dendritic cells (DCs) in vivo and direct a specific response to the antigen(s) expressed instead of to the vector. Often, antivector responses dominate over transgene-specific responses (e.g., with vectors based on adenovirus [43] or vaccinia virus [47]).
Lentivirus-based vaccines offer the advantage that they can express antigens in DCs in vivo (15, 37, 44), and they do not block DC maturation (as in the case of herpes simplex virus [45]) or express viral proteins (32, 54). The drawback is that they do not mature DCs (4, 16, 51); they only marginally affect DCs in vitro even at a multiplicity of infection (MOI) of 500 (50). Nevertheless, they can still stimulate transgene-specific CD4+ and CD8+ T-cell responses in mice (6, 12, 15, 20, 23, 37, 44), so presumably they exert some activation effects in vivo. Recent studies have shown that lentivectors can be targeted to DCs in vivo, improving their safety and efficacy (52) (27). Our present aim was to further improve the efficacy of lentivector vaccines by expressing an activator to supply an adjuvant. The use of adjuvants is particularly important in cancer vaccines, where it is necessary to break tolerance to self antigens (19). Aluminum salts are commonly used as an adjuvant in human vaccines, but this mainly directs antibody (Ab) responses. Cancer vaccines, however, require adjuvants that direct Th1 cell-mediated immunity.
We decided to target the NF-κB pathway because it is involved in maturation pathways such as the ICAM-1 and CD86 pathways and interleukin-12 (IL-12) secretion that promotes Th1 responses (24, 36, 41) and because it is critical for Toll-like receptor signaling (22). NF-κB subunits have distinct roles in DC maturation. Nuclear RelB is associated with efficient antigen-presenting cell function of DCs (38), RelA and p50 are required for the generation of DCs, and cRel and p50 are required for the survival of mature DCs and in IL-12 secretion (35). We chose to express the NF-κB activator vFLIP (viral FLICE-like inhibitory protein) from Kaposi's sarcoma-associated herpesvirus (KSHV) in the lentivector, as it persistently activates classical NF-κB (26) by associating with IKKγ in the IKK complex (17). vFLIP has also been shown to activate p52 through processing of NF-κB2/p100 (thereby releasing RelB) in the alternative NF-κB pathway (29).
We constructed a lentiviral vector expressing vFLIP and assessed its ability to mature bone marrow (BM)-derived DCs and to enhance the efficacy of a lentivector vaccine. Here, we show that vFLIP stably matures DCs in vitro and improves the lentivector vaccine in vivo by increasing the number of Ova-specific CD8+ T cells. This leads to improved survival of mice in a tumor therapy model and to a reduced parasite load after OVA-Leishmania donovani challenge. These data show that the efficacy of lentivirus-based vaccines (which lack the ability to intrinsically mature DCs) can be improved by including an NF-κB activator.
MATERIALS AND METHODS
Lentiviral vectors.
Dual-promoter human immunodeficiency virus type 1 vectors were derived from pHRSIN-CSGW (10, 14). Green fluorescent protein (GFP) was replaced by vFLIP or by Ova, which is a fusion between ovalbumin epitopes and the C terminus of the major histocompatibility complex (MHC) class II invariant chain (11, 44). Vectors were produced by cotransfection of the vector plasmid with pCMVR8.91 and pMDG as described previously (33, 54). Titers were measured by GFP expression and TaqMan PCR. Viral supernatants were concentrated by two ultracentrifugations to remove fetal calf serum and any contaminating OVA protein (as checked by Coomassie blue staining and immunoblotting, respectively, after sodium dodecyl sulfate-polyacrylamide gel electrophoresis of concentrated vector) as described previously (44). Each vector preparation contained approximately 104 GFP transducing units (105 genome transducing units) per ng p24.
Immunoblotting and nuclear fractionation.
Total cell extracts were prepared using radioimmunoprecipitation assay buffer, or nuclear and cytoplasmic fractionation was performed. In this case the cell pellet was resuspended in cold cytoplasmic buffer (10 mM HEPES [pH 7.6], 1 mM EDTA, 0.1 mM EGTA, 10 mM KCl, 1 mM dithiothreitol, 20 mM NaF, 5% glycerol, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitor mix [GE Healthcare]) and incubated on ice before addition of NP-40 (0.6%). The lysate was then underlayered with cytoplasmic buffer containing 30% sucrose and centrifuged for 5 min at 4°C before removal of the supernatant as the “cytoplasmic fraction.” The nuclear pellet was washed twice with cytoplasmic buffer and resuspended in nuclear lysis buffer (20 mM HEPES [pH 7.6], 0.2 mM EDTA, 0.1 mM EGTA, 25% glycerol, 0.42 mM NaCl, 1 mM dithiothreitol, 20 mM NaF, 1 mM Na4P2O2, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitor mix). Nuclear proteins were extracted by freeze-thaw cycles before the samples were centrifuged (10 min at 4°C) and the supernatant was harvested as the “nuclear fraction.” Samples were separated on a 10% denaturing sodium dodecyl sulfate-polyacrylamide gel, transferred to nitrocellulose, and probed with anti-OVA (rabbit) (made at UCL); anti-vFLIP (28); anti-β-actin (mouse) (from AbCam); anti-SP1 (rabbit) (from Santa Cruz); antitubulin (rabbit) (from Cell Signaling); anti-p65 (rabbit) (from Santa Cruz); and anti-JNK (rabbit), anti-phospho-JNK (mouse), anti-p38 (rabbit), and anti-phospho-p38 (rabbit) (all from Cell Signaling) plus anti-ERK and anti-phospho-ERK (from Promega). Horseradish peroxidase-conjugated secondary Abs (DakoCytomation) were added before developing blots with ECL reagents from Amersham Pharmacia Biotech.
NF-κB electrophoretic mobility shift assays.
DCs were harvested and nuclear fractions isolated. An NF-κB consensus oligonucleotide (AGTTGAGGGGACTTTCCCAGG) was labeled with 5 units of T4 polynucleotide kinase and polynucleotide kinase buffer (both from Promega) and 20 μCi [γ-32P]ATP for 30 min at 37°C. Then, 89 μl of TE (10 mM Tris [pH 8], 1 mM EDTA) was added and unincorporated [γ-32P]ATP removed using G-25 spin columns (Amersham Pharmacia Biotech). NF-κB DNA binding activities were determined by incubating 5 μg of nuclear extract with 1 μl of [γ-32P]ATP-labeled double-stranded NF-κB probe, 2 μl of binding buffer (Promega), and 7 μl of H2O for 30 min at room temperature. NF-κB/DNA complexes were resolved on a nondenaturing 5% polyacrylamide gel in 0.5% TBE (0.045 M Tris base, 0.045 M boric acid, 0.1 M EDTA). Gels were dried on to 3MM paper and retarded DNA protein complexes visualized using Hyperfilm MP (Amersham Pharmacia Biotech).
Transduction of DCs and flow cytometry.
Murine BM-derived DCs were prepared as previously described (21). Immature DCs were transduced on day 4 at an MOI of 20 as described previously (37, 44) and fed every 4 days with fresh medium containing granulocyte-macrophage colony-stimulating factor (50 ng/ml; from Peprotech). On day 5 posttransduction, DCs were harvested, washed, and blocked for Fc receptors before surface staining for maturation markers with the following biotin-conjugated Abs: anti-CD11c, anti-CD86, and anti-I-Ab (MHC class II) (all from BD Pharmingen); anti-CD40 (from Serotec); and anti-CD80, anti-ICAM-1, and anti-Kb (MHC class I) (all from eBioscience). A hamster isotype control Ab (biotin conjugated) was purchased from BD Pharmingen. Abs were then labeled with streptavidin RPE Cy-5 2o reagent (DakoCytomation) before flow cytometry. Lipopolysaccharide (LPS) (50 ng/ml) (Sigma) was added to untransduced DCs and left overnight as a positive control for maturation. Zymosan A (10 μg/ml) treatment (for 30 min at 37°C) was used as a control for ERK activation.
ELISA.
Culture supernatants were harvested from DCs plated at 5 × 105 cells per well (in 1.5 ml). IL-12p70 and tumor necrosis factor alpha (TNF-α) were detected by sandwich enzyme-linked immunosorbent assay (ELISA), using kits from eBioscience according to the manufacturer's guidelines. The substrate was tetramethylbenzidine, and the reaction was stopped with 1 M H3PO4. The optical density was measured at 450 nm and 570 nm, and the 570-nm values were subtracted from the 450-nm values before plotting the results.
DC purification from lymph nodes.
C57/BL6 mice (Harlan) were injected subcutaneously (s.c.) at the base of the tail with 1 × 108 infectious units (i.u.) lentivector. Six days later, lymph nodes (para-aortical and inguinal) were harvested (cells from mice in each group were pooled), incubated with collagenase CLS-4 (Worthington), and mashed to obtain single-cell suspensions. Fc receptors were blocked before CD11c-positive cells were selected using MACS beads (Miltenyi Biotec).
Pentamer staining.
One million splenocytes per sample were incubated with 10 μl of phycoerythrin-conjugated SIINFEKL/Kb pentamer or tetramer (Proimmune) for 12 min at room temperature. The cells were then washed and incubated on ice with biotin-conjugated anti-CD8 (Serotec) for 15 min before being washed and incubated with streptavidin-allophycocyanin (eBioscience) for 15 min. Samples were washed and acquired on a BD LSR machine using Cell-Quest software (BD Biosciences).
Intracellular cytokine staining.
Splenocytes were incubated overnight with or without OVA257-264 peptide. Monensin solution (eBiosciences; final concentration, 2 μM) was added and left for 3 h before surface staining cells for CD8. The cells were then fixed and permeabilized using a Cytofix/Cytoperm kit from BD Biosciences. An allophycocyanin-conjugated anti-gamma interferon (anti-IFN-γ) Ab (BD Pharmingen) was then added and left for 30 min before the cells were washed and samples were run on a BD LSR machine.
ELISPOT assay.
Enzyme-linked immunospot (ELISPOT) plates (Millipore) were coated overnight at 4°C with purified anti-IFN-γ (BD Pharmingen). Ex vivo ELISPOT assays were performed with serial dilutions of total splenocytes in triplicate with or without class I OVA257-264 peptide (Proimmune). Plates were cultured overnight and developed according to the manufacturer's directions. Spots were counted using an AID ELISPOT counter and software.
Tumor therapy.
EG7.OVA tumor cells were grown in RPMI plus 0.4 mg/ml G418 (Invitrogen). C57BL/6 mice were challenged with 2 ×106 tumor cells injected s.c. into the flank and then vaccinated as indicated in Results. Animals were killed once they had a tumor that reached a diameter of >15 mm. A χ2 test was used to determine the significance of differences between the controls and each vaccine group based on the numbers of tumor-free mice left at the end of the experiment. The Wilcoxon signed rank test (one tailed) was used to assess whether the results for the vFLIP vaccine were significantly different from those for the standard vaccine over time.
Vaccination against Leishmania donovani.
To test the efficacy of vFLIP_Ova in a parasite infection model, C57BL/6 mice were immunized s.c. with vFLIP_Ova or control vFLIP_GFP, and 7 days later tail blood was collected to determine the frequency of tetramer-positive CD8+ T cells. The following day, mice were infected with 2 × 107 Ova-transgenic L. donovani amastigotes (Ld18SrRNA::HASP1-18-OVA) (39). Mice were killed at day 28 postinfection and spleen parasite burdens determined from Giemsa-stained tissue impression smears (39).
RESULTS
Lentiviral vectors expressing vFLIP activate NF-κB in DCs.
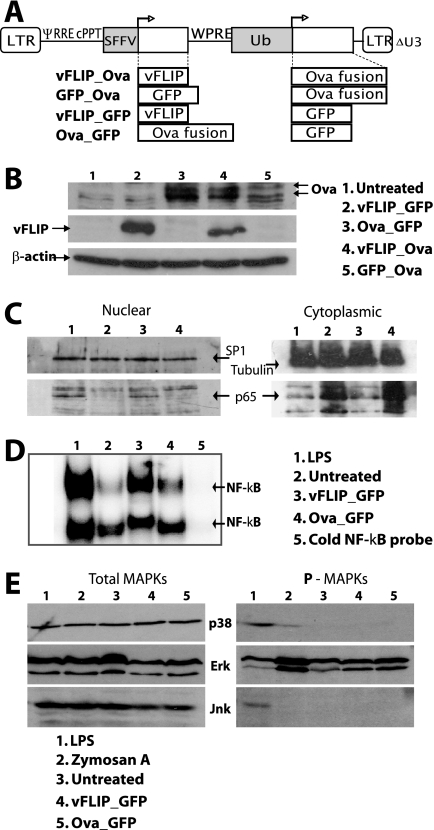
We used a dual-promoter lentiviral vector to coexpress vFLIP and Ova, which is a fusion of the C-terminal end of the invariant chain with amino acids 242 to 353 of ovalbumin and is highly immunogenic (44), or GFP (Fig. 1A). Expression of vFLIP and Ova was verified by immunoblotting after infection of 293T cells (Fig. 1B). We then infected mouse BM-derived DCs with the vFLIP lentivector to examine the effect of vFLIP on DC signaling pathways. p65 (RelA) was detected in the nuclei of the vFLIP DCs at a level similar to that in the LPS-treated DCs but not in the untreated or control vector DCs, in which the level of cytoplasmic p65 was higher (Fig. 1C). Increased nuclear NF-κB binding activity was also detected in vFLIP-transduced DCs (Fig. 1D). We have reported that expression of mitogen-activated protein kinase (MAPK) activators in DCs can regulate the immune response to lentivectors (14); however, vFLIP expression did not affect MAPK signaling, confirming that its effects are through NF-κB activation (Fig. 1E).
FIG. 1.
Lentiviral vectors, transgene expression, and cell signaling. (A) Dual-promoter lentivectors expressing vFLIP and an ovalbumin antigen (Ova, ovalbumin epitopes fused to the MHC class II invariant chain) with control vectors. LTR, long terminal repeat; ψ, packaging signal; RRE, Rev-responsive element; cPPT, central polypurine tract; SFFV, spleen focus-forming virus promoter; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element; Ub, human ubiquitin C promoter. (B) Immunoblot showing Ova and vFLIP expression 48 h after transduction of 293T cells at an MOI of 10. Twenty micrograms of protein was loaded per lane. Results from one representative experiment of three are shown. (C) BM-derived DCs were harvested 4 days after transduction. Immunoblots of nuclear samples (13 μg/lane) and cytoplasmic samples (26 μg/lane) were probed with Abs for tubulin, SP1, and p65. Tubulin was not detected in the nucleus, and SP1 was not detected in the cytoplasm (not shown). A repeat experiment showed similar results. (D) Electrophoretic mobility shift assay analysis with DC nuclear extracts made at 5 days; NF-κB binding activity in all DC groups was competed with cold probe (lane 5). Results from one representative experiment of three are shown. (E) Immunoblots for phosphorylated and total MAPKs using DC cell extracts prepared 5 days posttransduction. Samples (15 μg/lane) were probed for P-p38, P-ERK, and P-JNK and for total p38, ERK, and JNK. The experiment shown was repeated two or three times for each MAPK, confirming these results.
vFLIP matures DCs in culture and in vivo.
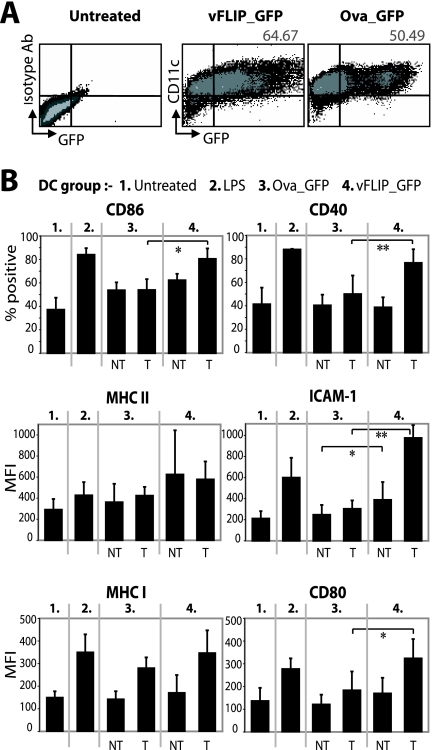
After transduction of BM-derived DCs with vFLIP_GFP, we analyzed maturation markers on the transduced or nontransduced cells defined by GFP expression (Fig. 2A). Results for CD86 and CD40 were plotted as the percentage of cells in each group positive for the marker, but for MHC class II, ICAM-1, MHC class I, and CD80, differences between groups were assessed by the mean fluorescence intensity, since over 80% of cells already expressed the marker. ICAM-1 lowers the required antigen dose for Th cell activation (3) and drives Th1 polarization (48), and CD40 is particularly important in DC persistence and T-cell immunity (18, 31, 34). CD86, CD40, ICAM-1, and CD80 were upregulated on vFLIP-expressing DCs compared to transduced DCs in the control vector group (Fig. 2B). Nontransduced DCs in the vFLIP group also slightly upregulated ICAM-1, perhaps induced by cytokines. Control vector-transduced DCs also exhibited MHC class I upregulation, perhaps caused by the lentiviral vector itself as observed in human DC cultures (50). Importantly, there was no significant difference in activation between the vFLIP-expressing DCs and the LPS treated DCs (Fig. 2B), demonstrating effective activation of DC costimulatory molecules by vFLIP.
FIG. 2.
vFLIP matures BM-derived DCs. (A) Flow cytometry 5 days after transduction of BM-derived DCs at an MOI of 20. The percentage of cells expressing CD11c and GFP after subtracting the background from the control plot is shown (80,000 events recorded per sample). CD11c-positive cells were considered DCs, since the majority (>95%) also expressed MHC class II (not shown). The mean percentages of transduced DCs in the cultures were 52.89 for the vFLIP group (standard deviation, 10.06) and 45.45 for the Ova group (standard deviation, 4.44), from three experiments. (B) Cells were divided into nontransduced (NT) and transduced (T) groups by GFP expression (results from three or four experiments are summarized). Paired t tests were used to calculate whether differences between the vFLIP group (T or NT cells) and the control vector group were significant, and any significant differences are shown. *, P < 0.05; **, P < 0.01. MFI, mean fluorescence intensity.
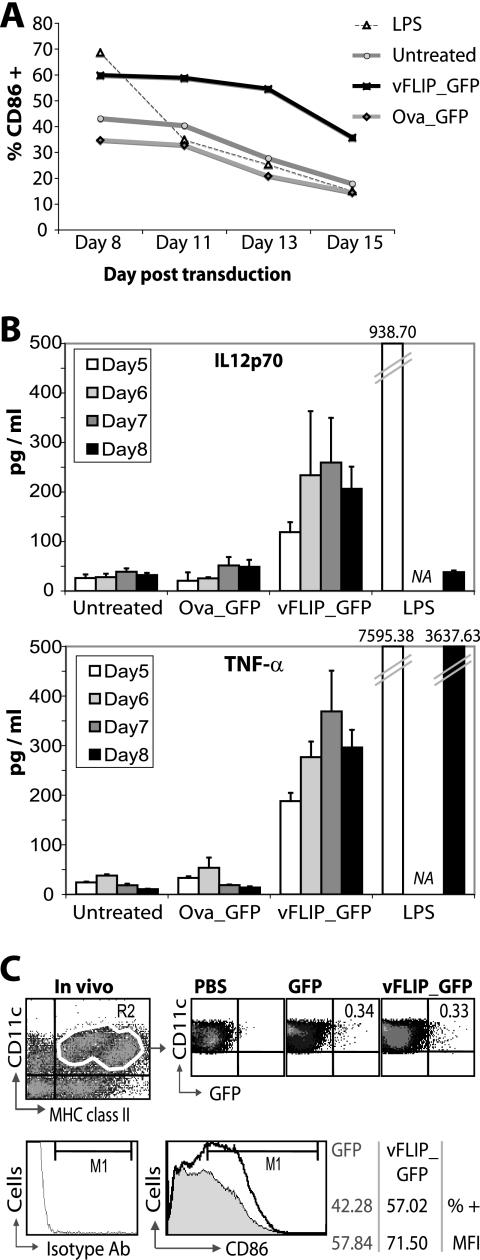
As vFLIP constitutively activates the IKK complex (17, 26), we examined whether vFLIP-transduced DCs could maintain their mature phenotype. DCs were therefore maintained in culture and their maturation state monitored over time. vFLIP-transduced DCs retained their upregulated CD86 for the duration of the culture, whereas the effect of LPS treatment (added on day 5) was short-lived (Fig. 3A).
FIG. 3.
The mature vFLIP DC phenotype in BM-derived DCs and in vivo. (A) BM-derived DCs were cultured with granulocyte-macrophage colony-stimulating factor, which was replaced every 4 days. Data show the percentage of cells positive for CD86 over time; results shown are for the transduced population. LPS was added on day 5. Results from one representative experiment of two performed are shown. (B) Supernatants were collected from DC cultures from day 2 to day 8 posttransduction and IL-12 and TNF-α measured by ELISA. TNF-α was not detected on day 2 or 3 and IL-12 was not detected on day 3 or 4 posttransduction in three experiments (data not shown). Each bar shows the mean and standard deviation for supernatants from three separate wells. NA, not assayed. LPS was added on day 4. Results shown for both cytokines are from one experiment, which was repeated twice with consistent results. (C) Mice (n = 3) were injected s.c. with the vFLIP_GFP lentivector or a GFP lentivector. Control mice (n = 4) received phosphate-buffered saline (PBS). Six days later, lymph nodes were collected and CD11c+ cells purified using MACS beads. The upper fluorescence-activated cell sorting plots show the percentage of DCs positive for GFP (≥40,000 events were recorded per sample). The lower plot shows the CD11c+ fractions from the GFP and vFLIP groups (there were too few cells in the PBS group) costained for CD11c and CD86 and gated on the CD11c+ cells (approximately 70% of cells). An isotype Ab was used to set the M1 gate (shown). As many events as possible were run: 470,000 for GFP versus 740,000 for vFLIP. The experiment shown is one of two experiments with similar results. MFI, mean fluorescence intensity.
We also measured secretion of IL-12p70 and TNF-α because of their importance in Th1 responses (46). vFLIP induced both IL-12p70 and TNF-α secretion (Fig. 3B). The amounts released were substantially smaller than those from DCs treated with LPS, which was added 1 day before analysis on day 4. However, IL-12p70 could be detected in vFLIP DC supernatant up to 15 days after transduction, when secretion from LPS-treated DCs had ceased (Fig. 3B and data not shown). These data are in contrast to our findings with selective p38 MAPK activation in DCs; in that case, costimulatory molecules were upregulated but IL-12p70 and TNF-α secretion was not induced (14).
Following s.c. lentivector injection, transduced DCs are detected in the draining lymph nodes (15, 20, 27). A similar percentage of lymph node DCs (CD11c+/MHC class II+) were transduced after s.c. injection with either the GFP or the vFLIP_GFP vector (Fig. 3C). Interestingly, we found that there was an upregulation of CD86 on DCs in the vFLIP-injected animals compared to the GFP-injected animals (Fig. 3C). This also included upregulation of CD86 on nontransduced DCs, perhaps as a result of cytokine secretion.
vFLIP enhances the in vivo CD8+ T-cell response to ovalbumin.
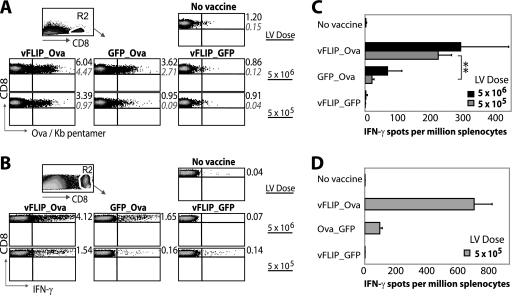
We next compared the vFLIP_Ova, GFP_Ova, and vFLIP_GFP vectors for their ability to induce an Ova-specific CD8+ T-cell response in mice after s.c. vaccination. The results were particularly striking when a vector dose of 5 × 105 i.u. was used. The vFLIP_Ova-vaccinated mice had 3 times more SIINFEKL/Kb pentamer-positive CD8+ T cells (Fig. 4A) and 10 times more IFN-γ-secreting CD8+ T cells as measured by intracellular fluorescence-activated cell sorting (Fig. 4B) or ELISPOT assay (Fig. 4C). The response was Ova specific, since it was not observed in mice vaccinated with the vFLIP_GFP vector. We noted that the expression of Ova was higher in the vFLIP_Ova vector than the GFP_Ova vector (Fig. 1B), and we therefore repeated the experiment using the control Ova_GFP, which expresses more Ova than vFLIP_Ova (Fig. 1B). Importantly, the results showed the vFLIP_Ova vector to be again superior (Fig. 4D), demonstrating that DC activation status rather than antigen expression level is critical.
FIG. 4.
vFLIP enhances the immune response to Ova. (A) Mice were immunized s.c. with the vFLIP_Ova, GFP_Ova, or vFLIP_GFP at 5 × 106 i.u. or 5 × 105 i.u. (n = 3) or were not immunized (no vaccine, n = 4). Twelve days later, spleens were collected and Ova-specific CD8+ T cells analyzed. The experiment shown here is representative of three performed. Splenocytes from individual mice were stained with SIINFEKL/Kb pentamer and CD8 Ab, and the CD8+ population was gated as shown. Numbers in black show the mean percentage of CD8+ T cells that are Ova specific (for the three mice) and the standard deviations are in gray italics (200,000 events were recorded per sample). (B) Spleens from mice in each group were pooled and cultured overnight with or without SIINFEKL peptide, and then intracellular staining for IFN-γ was performed. Numbers show the percentage of CD8+ T cells that were producing IFN-γ in each group; cells cultured in the absence of peptide did not release IFN-γ (not shown). A total of 200,000 events were recorded per sample. (C) Pooled splenocytes were cultured in an IFN-γ ELISPOT assay overnight with or without SIINFEKL peptide. Serial dilutions of splenocytes were plated in triplicate, and the results show the spot counts plus peptide (spots were not observed in the absence of peptide). Each bar shows the mean and standard deviation for triplicate wells. **, P < 0.01 (P = 0.001) by unpaired Student t test. (D) The experiment described in panel C was repeated using Ova_GFP as a control instead of GFP_Ova. Each bar shows the mean count from duplicate wells (plus peptide) with standard deviation (no spots were observed in the absence of peptide). Spot counts were off scale for the lentivector (LV) dose of 5 × 106 i.u. (not shown).
A stronger CD8+ response leads to improved immunity.
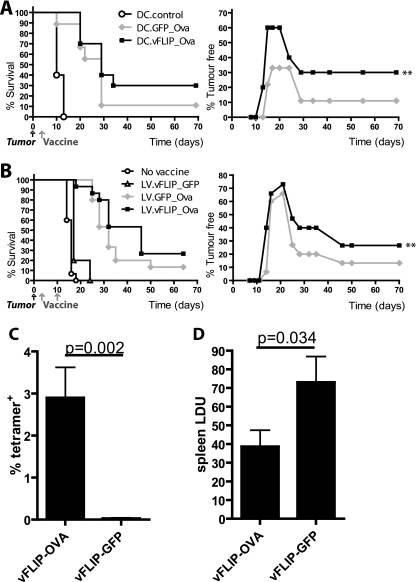
We have previously shown that a lentiviral vaccine expressing Ova can protect mice from tumors when they are vaccinated before tumor challenge (44). Therefore, we decided to test the novel vFLIP_Ova vector in a tumor therapy model, which is more challenging. A similar model showed that only 15% of mice survive by 70 days after tumor challenge when they are vaccinated with a standard lentivector expressing Ova (12). Here, we inoculated mice with a lethal dose of EG7.OVA tumor cells before vaccinating them either with transduced DCs or with the vectors directly (Fig. 5A and B, respectively). All mice developed tumors, some of which regressed over time (shown by the tumor-free curves in Fig. 5A and B). After either transduced DC injection (Fig. 5A) or direct lentivector injection (Fig. 5B), the number of tumor-free mice in the vFLIP group was significantly higher than that in the GFP group (for all time points after day 10). After direct vector injection (Fig. 5B), survival was also significantly improved in the vFLIP group. We also tested the efficacy of the vFLIP-Ova vector in a parasite protection model, using L. donovani expressing ovalbumin (39). Again we found a robust CD8+ T-cell response to Ova in the peripheral blood of mice after a single immunization with the vFLIP_Ova lentivector (Fig. 5C), and this was sufficient to give a significant reduction in parasite burden when the mice were subsequently challenged with OVA-L. donovani (Fig. 5D).
FIG. 5.
A vFLIP vaccine induces therapeutic antitumor immunity and protection against Leishmania infection. Mice were challenged with 2 × 106 EG7.OVA tumor cells before s.c. vaccination. Kaplan-Meier survival curves and percentages of tumor-free mice (plotted over time until day 70) are shown. **, P < 0.01. (A) The vaccine was 106 DCs, transduced with the stated vectors or nontransduced (DC.control), and administered 3 days after tumor challenge (n = 10 except for the DC.GFP_Ova group, where n = 9). (B) The vaccine was 1 × 107 i.u. of the lentivectors (LVs), injected at 3 days and 10 days after tumor challenge (n = 15 except in the vFLIP_GFP group, where n = 5). The significance of differences was analyzed using the Wilcoxon signed rank test and the χ2 test. After injection of transduced DCs, the number of tumor-free mice in the vFLIP group was significantly higher than that in the GFP group (P = 0.0078). After direct vector injection, the number of tumor-free mice was significantly higher in the vFLIP group than in the GFP group (P = 0.0039), and survival was significantly improved in the vFLIP group (χ2 = 4.62, P = 0.0316), whereas it was not in the GFP group (χ2 = 2.14, P = 0.1435). (C) Mice were immunized with 1 × 107 i.u. of the lentivectors and then bled after 7 days for tetramer staining of peripheral blood lymphocytes. (D) The same mice were infected with 2 × 107 amastigotes of OVA-L. donovani 8 days after lentivector immunization, and the spleen parasite burden was assessed after a further 28 days. Data were analyzed using a one-tailed t test (n = 5 mice per group). Error bars indicate standard deviations.
Taken together, these results show that the vFLIP_Ova vaccine is efficacious. Furthermore, direct injection of the virus was more effective than injection of transduced DCs (Fig. 5B), possibly because vFLIP can mature additional DCs in situ (Fig. 3C).
DISCUSSION
Recombinant lentivectors are promising vaccines for cancer or infectious disease because they can express antigens in DCs and direct antigen-specific T-helper and cytotoxic T-lymphocyte responses in mice (12, 15, 23, 37, 44). A recent study showed that lentivectors selectively transduce skin-derived DCs on s.c. injection, which then migrate to draining lymph nodes and present their lentivector-encoded antigen (20). However, the efficiency of such lentivector vaccines may be suboptimal, because they do not effectively mature DCs (4, 16, 51) and immature DCs presenting antigen in vivo may induce inferior T-cell responses or even tolerance (1, 18, 30).
The aim of this study was to find out if vaccine efficacy could be improved by expressing an NF-κB activator. The advantage of expressing a DC activator (as opposed to coinjecting an adjuvant) is that it will be active at the same time as the encoded antigen is presented and not during any initial presentation of viral proteins. We used vFLIP from KSHV in the vector because of its known ability to persistently activate NF-κB (17, 26, 29). We found that vFLIP induces DC maturation to a similar extent as LPS. The levels of the costimulatory markers CD80, CD86, CD40, and ICAM-1 were significantly higher on the vFLIP-expressing DCs.
The effects of vFLIP on DCs are likely to be due to activation of classical NF-κB, as p65 (RelA) was detected in the nuclei of vFLIP-transduced DCs. The genes encoding CD80, ICAM-1, MHC class I (Kb), and IL-12p40 all contain κB binding sites, explaining their induction (interestingly, it has been reported that ICAM-1 κB sites preferentially bind RelA homodimers [36]). We also showed that vFLIP does not activate MAPK pathways at the height of DC maturation. LPS, in comparison, clearly activated MAPKs in our cultures, which may explain the differences in the amounts of TNF-α and IL-12 released by the LPS- versus vFLIP-treated DCs.
Our in vivo results showed that immunization with a vector coexpressing vFLIP and Ova was superior to immunization with a vector expressing Ova without vFLIP; 10 times more Ova-specific T cells secreting IFN-γ were detected by both intracellular staining and ELISPOT assay. Our results support a recent study that showed that overexpression of the NF-κB activator NIK could enhance the efficacy of an adenovirus-based vaccine expressing GFP as an antigen (2). We have demonstrated the same principle here with a different antigen and vector. As the lentivector expresses transgenes at a relatively low level from a single-copy integrant, it was important to use vFLIP as an activator, as overexpression of a component of the NF-κB pathway was not possible. Additionally, because of the persistent NF-κB activation by vFLIP, DCs were stably matured and showed persistent cytokine secretion. vFLIP could also mature DCs in situ, with CD86 upregulated on most of the DC population in draining lymph nodes. We have shown that an NF-κB activator (vFLIP) improves survival in a tumor therapy model and that vFLIP vaccination can also reduce L. donovani parasite load.
Our results are relevant to KSHV pathogenesis, as it remains a possibility that the virus infects DCs in vivo; DCs have been found in KS lesions (13), and KSHV can infect myeloid DCs using DC-SIGN as a receptor (40). The effect of KSHV on DCs is unclear; experimental infection of human cord blood-derived stem cells with KSHV resulted in enhanced immunostimulatory properties of the progeny DCs (25), but KSHV infection of human myeloid DCs caused downregulation of MHC class I and reduced their immunostimulatory function (40). One study recently showed that DCs from KS patients secrete IL-10 (9). Our results show that vFLIP drives DCs to a mature phenotype and to secrete IL-12, so other KSHV proteins likely are responsible for downregulation of DC function. In lymphatic endothelial cells, vIRFs 1 and 2 can counteract vFLIP upregulation of antigen presentation by downregulating ICAM-1, CD86, and MHC class I (24). Interestingly, vFLIP has also been reported to extend cell survival (49), which could be valuable for DC vaccines, although there is a risk that such long-term activated DCs could induce tolerance to their target antigen or some nonspecific autoimmunity(7).
Acknowledgments
We thank Yasuhiro Ikeda for the dual-promoter vector, Michael Rowe for advice on statistics, and Claire Dodds for assistance with animal experiments.
This project was supported by a Programme Grant and a Ph.D. studentship from Cancer Research UK (to M.K.C.) and by grants from the Medical Research Council and Wellcome Trust (to P.M.K.).
The authors have no competing financial interests.
H.M.R., L.L., and N.B. designed and performed experiments. S.E., T.S., and S.K. performed experiments. M.K.C. and P.M.K. designed experiments. H.M.R. wrote the paper.
Footnotes
Published ahead of print on 26 November 2008.
REFERENCES
- 1.Albert, M. L., M. Jegathesan, and R. B. Darnell. 2001. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat. Immunol. 21010-1017. [DOI] [PubMed] [Google Scholar]
- 2.Andreakos, E., R. O. Williams, J. Wales, B. M. Foxwell, and M. Feldmann. 2006. Activation of NF-kappaB by the intracellular expression of NF-kappaB-inducing kinase acts as a powerful vaccine adjuvant. Proc. Natl. Acad. Sci. USA 10314459-14464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmann, M. F., K. McKall-Faienza, R. Schmits, D. Bouchard, J. Beach, D. E. Speiser, T. W. Mak, and P. S. Ohashi. 1997. Distinct roles for LFA-1 and CD28 during activation of naive T cells: adhesion versus costimulation. Immunity 7549-557. [DOI] [PubMed] [Google Scholar]
- 4.Breckpot, K., M. Dullaers, A. Bonehill, S. van Meirvenne, C. Heirman, C. de Greef, P. van der Bruggen, and K. Thielemans. 2003. Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. J. Gene Med. 5654-667. [DOI] [PubMed] [Google Scholar]
- 5.Breckpot, K., C. Heirman, B. Neyns, and K. Thielemans. 2004. Exploiting dendritic cells for cancer immunotherapy: genetic modification of dendritic cells. J. Gene Med. 61175-1188. [DOI] [PubMed] [Google Scholar]
- 6.Chapatte, L., S. Colombetti, J. C. Cerottini, and F. Levy. 2006. Efficient induction of tumor antigen-specific CD8+ memory T cells by recombinant lentivectors. Cancer Res. 661155-1160. [DOI] [PubMed] [Google Scholar]
- 7.Chen, M., Y. H. Wang, Y. Wang, L. Huang, H. Sandoval, Y. J. Liu, and J. Wang. 2006. Dendritic cell apoptosis in the maintenance of immune tolerance. Science 3111160-1164. [DOI] [PubMed] [Google Scholar]
- 8.Collins, M. K., and V. Cerundolo. 2004. Gene therapy meets vaccine development. Trends Biotechnol. 22623-626. [DOI] [PubMed] [Google Scholar]
- 9.Della Bella, S., S. Nicola, L. Brambilla, A. Riva, S. Ferrucci, P. Presicce, V. Boneschi, E. Berti, and M. L. Villa. 2006. Quantitative and functional defects of dendritic cells in classic Kaposi's sarcoma. Clin. Immunol. 119317-329. [DOI] [PubMed] [Google Scholar]
- 10.Demaison, C., K. Parsley, G. Brouns, M. Scherr, K. Battmer, C. Kinnon, M. Grez, and A. J. Thrasher. 2002. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum. Gene Ther. 13803-813. [DOI] [PubMed] [Google Scholar]
- 11.Diebold, S. S., M. Cotten, N. Koch, and M. Zenke. 2001. MHC class II presentation of endogenously expressed antigens by transfected dendritic cells. Gene Ther. 8487-493. [DOI] [PubMed] [Google Scholar]
- 12.Dullaers, M., S. Van Meirvenne, C. Heirman, L. Straetman, A. Bonehill, J. L. Aerts, K. Thielemans, and K. Breckpot. 2006. Induction of effective therapeutic antitumor immunity by direct in vivo administration of lentiviral vectors. Gene Ther. 13630-640. [DOI] [PubMed] [Google Scholar]
- 13.Ensoli, B., C. Sgadari, G. Barillari, M. C. Sirianni, M. Sturzl, and P. Monini. 2001. Biology of Kaposi's sarcoma. Eur. J. Cancer 371251-1269. [DOI] [PubMed] [Google Scholar]
- 14.Escors, D., L. Lopes, R. Lin, J. Hiscott, S. Akira, R. J. Davis, and M. K. Collins. 2008. Targeting dendritic cell signaling to regulate the response to immunization. Blood 1113050-3061. [DOI] [PubMed] [Google Scholar]
- 15.Esslinger, C., L. Chapatte, D. Finke, I. Miconnet, P. Guillaume, F. Levy, and H. R. MacDonald. 2003. In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8(+) T cell responses. J. Clin. Investig. 1111673-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esslinger, C., P. Romero, and H. R. MacDonald. 2002. Efficient transduction of dendritic cells and induction of a T-cell response by third-generation lentivectors. Hum. Gene Ther. 131091-1100. [DOI] [PubMed] [Google Scholar]
- 17.Field, N., W. Low, M. Daniels, S. Howell, L. Daviet, C. Boshoff, and M. Collins. 2003. KSHV vFLIP binds to IKK-gamma to activate IKK. J. Cell Sci. 1163721-3728. [DOI] [PubMed] [Google Scholar]
- 18.Fujii, S., K. Liu, C. Smith, A. J. Bonito, and R. M. Steinman. 2004. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J. Exp. Med. 1991607-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guy, B. 2007. The perfect mix: recent progress in adjuvant research. Nat. Rev. Microbiol. 5505-517. [DOI] [PubMed] [Google Scholar]
- 20.He, Y., J. Zhang, C. Donahue, and L. D. Falo, Jr. 2006. Skin-derived dendritic cells induce potent CD8(+) T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity 24643-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inaba, K., M. Inaba, M. Deguchi, K. Hagi, R. Yasumizu, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1993. Granulocytes, macrophages, and dendritic cells arise from a common major histocompatibility complex class II-negative progenitor in mouse bone marrow. Proc. Natl. Acad. Sci. USA 903038-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawai, T., and S. Akira. 2005. Pathogen recognition with Toll-like receptors. Curr. Opin. Immunol. 17338-344. [DOI] [PubMed] [Google Scholar]
- 23.Kim, J. H., N. Majumder, H. Lin, S. Watkins, L. D. Falo, Jr., and Z. You. 2005. Induction of therapeutic antitumor immunity by in vivo administration of a lentiviral vaccine. Hum. Gene Ther. 161255-1266. [DOI] [PubMed] [Google Scholar]
- 24.Lagos, D., M. W. Trotter, R. J. Vart, H. W. Wang, N. C. Matthews, A. Hansen, O. Flore, F. Gotch, and C. Boshoff. 2007. Kaposi sarcoma herpesvirus-encoded vFLIP and vIRF1 regulate antigen presentation in lymphatic endothelial cells. Blood 1091550-1558. [DOI] [PubMed] [Google Scholar]
- 25.Larcher, C., V. A. Nguyen, C. Furhapter, S. Ebner, E. Solder, H. Stossel, N. Romani, and N. Sepp. 2005. Human herpesvirus-8 infection of umbilical cord-blood-derived CD34+ stem cells enhances the immunostimulatory function of their dendritic cell progeny. Exp. Dermatol. 1441-49. [DOI] [PubMed] [Google Scholar]
- 26.Liu, L., M. T. Eby, N. Rathore, S. K. Sinha, A. Kumar, and P. M. Chaudhary. 2002. The human herpes virus 8-encoded viral FLICE inhibitory protein physically associates with and persistently activates the Ikappa B kinase complex. J. Biol. Chem. 27713745-13751. [DOI] [PubMed] [Google Scholar]
- 27.Lopes, L., M. Dewannieux, U. Gileadi, R. Bailey, Y. Ikeda, C. Whittaker, M. P. Collin, V. Cerundolo, M. Tomihari, K. Ariizumi, and M. K. Collins. 2008. Immunization with a lentivector that targets tumor antigen expression to dendritic cells induces potent CD8+ and CD4+ T-cell responses. J. Virol. 8286-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Low, W., M. Harries, H. Ye, M. Q. Du, C. Boshoff, and M. Collins. 2001. Internal ribosome entry site regulates translation of Kaposi's sarcoma-associated herpesvirus FLICE inhibitory protein. J. Virol. 752938-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matta, H., and P. M. Chaudhary. 2004. Activation of alternative NF-kappa B pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein (vFLIP). Proc. Natl. Acad. Sci. USA 1019399-9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menges, M., S. Rossner, C. Voigtlander, H. Schindler, N. A. Kukutsch, C. Bogdan, K. Erb, G. Schuler, and M. B. Lutz. 2002. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J. Exp. Med. 19515-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miga, A. J., S. R. Masters, B. G. Durell, M. Gonzalez, M. K. Jenkins, C. Maliszewski, H. Kikutani, W. F. Wade, and R. J. Noelle. 2001. Dendritic cell longevity and T cell persistence is controlled by CD154-CD40 interactions. Eur. J. Immunol. 31959-965. [DOI] [PubMed] [Google Scholar]
- 32.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272263-267. [DOI] [PubMed] [Google Scholar]
- 33.Neil, S., F. Martin, Y. Ikeda, and M. Collins. 2001. Postentry restriction to human immunodeficiency virus-based vector transduction in human monocytes. J. Virol. 755448-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Sullivan, B. J., and R. Thomas. 2002. CD40 ligation conditions dendritic cell antigen-presenting function through sustained activation of NF-kappaB. J. Immunol. 1685491-5498. [DOI] [PubMed] [Google Scholar]
- 35.Ouaaz, F., J. Arron, Y. Zheng, Y. Choi, and A. A. Beg. 2002. Dendritic cell development and survival require distinct NF-kappaB subunits. Immunity 16257-270. [DOI] [PubMed] [Google Scholar]
- 36.Pahl, H. L. 1999. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 186853-6866. [DOI] [PubMed] [Google Scholar]
- 37.Palmowski, M. J., L. Lopes, Y. Ikeda, M. Salio, V. Cerundolo, and M. K. Collins. 2004. Intravenous injection of a lentiviral vector encoding NY-ESO-1 induces an effective CTL response. J. Immunol. 1721582-1587. [DOI] [PubMed] [Google Scholar]
- 38.Pettit, A. R., C. Quinn, K. P. MacDonald, L. L. Cavanagh, G. Thomas, W. Townsend, M. Handel, and R. Thomas. 1997. Nuclear localization of RelB is associated with effective antigen-presenting cell function. J. Immunol. 1593681-3691. [PubMed] [Google Scholar]
- 39.Polley, R., S. Stager, S. Prickett, A. Maroof, S. Zubairi, D. F. Smith, and P. M. Kaye. 2006. Adoptive immunotherapy against experimental visceral leishmaniasis with CD8+ T cells requires the presence of cognate antigen. Infect. Immun. 74773-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rappocciolo, G., F. J. Jenkins, H. R. Hensler, P. Piazza, M. Jais, L. Borowski, S. C. Watkins, and C. R. Rinaldo, Jr. 2006. DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J. Immunol. 1761741-1749. [DOI] [PubMed] [Google Scholar]
- 41.Rescigno, M., M. Martino, C. L. Sutherland, M. R. Gold, and P. Ricciardi-Castagnoli. 1998. Dendritic cell survival and maturation are regulated by different signaling pathways. J. Exp. Med. 1882175-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg, S. A., J. C. Yang, D. J. Schwartzentruber, P. Hwu, S. L. Topalian, R. M. Sherry, N. P. Restifo, J. R. Wunderlich, C. A. Seipp, L. Rogers-Freezer, K. E. Morton, S. A. Mavroukakis, L. Gritz, D. L. Panicali, and D. E. White. 2003. Recombinant fowlpox viruses encoding the anchor-modified gp100 melanoma antigen can generate antitumor immune responses in patients with metastatic melanoma. Clin. Cancer Res. 92973-2980. [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenberg, S. A., Y. Zhai, J. C. Yang, D. J. Schwartzentruber, P. Hwu, F. M. Marincola, S. L. Topalian, N. P. Restifo, C. A. Seipp, J. H. Einhorn, B. Roberts, and D. E. White. 1998. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J. Natl. Cancer Inst. 901894-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowe, H. M., L. Lopes, Y. Ikeda, R. Bailey, I. Barde, M. Zenke, B. M. Chain, and M. K. Collins. 2006. Immunization with a lentiviral vector stimulates both CD4 and CD8 T cell responses to an ovalbumin transgene. Mol. Ther. 13310-319. [DOI] [PubMed] [Google Scholar]
- 45.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 293245-3253. [DOI] [PubMed] [Google Scholar]
- 46.Shibuya, K., D. Robinson, F. Zonin, S. B. Hartley, S. E. Macatonia, C. Somoza, C. A. Hunter, K. M. Murphy, and A. O'Garra. 1998. IL-1 alpha and TNF-alpha are required for IL-12-induced development of Th1 cells producing high levels of IFN-gamma in BALB/c but not C57BL/6 mice. J. Immunol. 1601708-1716. [PubMed] [Google Scholar]
- 47.Smith, C. L., F. Mirza, V. Pasquetto, D. C. Tscharke, M. J. Palmowski, P. R. Dunbar, A. Sette, A. L. Harris, and V. Cerundolo. 2005. Immunodominance of poxviral-specific CTL in a human trial of recombinant-modified vaccinia Ankara. J. Immunol. 1758431-8437. [DOI] [PubMed] [Google Scholar]
- 48.Smits, H. H., E. C. de Jong, J. H. Schuitemaker, T. B. Geijtenbeek, Y. van Kooyk, M. L. Kapsenberg, and E. A. Wierenga. 2002. Intercellular adhesion molecule-1/LFA-1 ligation favors human Th1 development. J. Immunol. 1681710-1716. [DOI] [PubMed] [Google Scholar]
- 49.Sun, Q., H. Matta, and P. M. Chaudhary. 2003. The human herpes virus 8-encoded viral FLICE inhibitory protein protects against growth factor withdrawal-induced apoptosis via NF-kappa B activation. Blood 1011956-1961. [DOI] [PubMed] [Google Scholar]
- 50.Tan, P. H., S. C. Beutelspacher, S. A. Xue, Y. H. Wang, P. Mitchell, J. C. McAlister, D. F. Larkin, M. O. McClure, H. J. Stauss, M. A. Ritter, G. Lombardi, and A. J. George. 2005. Modulation of human dendritic-cell function following transduction with viral vectors: implications for gene therapy. Blood 1053824-3832. [DOI] [PubMed] [Google Scholar]
- 51.Veron, P., S. Boutin, J. Bernard, O. Danos, J. Davoust, and C. Masurier. 2006. Efficient transduction of monocyte- and CD34+-derived Langerhans cells with lentiviral vectors in the absence of phenotypic and functional maturation. J. Gene Med. 8951-961. [DOI] [PubMed] [Google Scholar]
- 52.Yang, L., H. Yang, K. Rideout, T. Cho, K. I. Joo, L. Ziegler, A. Elliot, A. Walls, D. Yu, D. Baltimore, and P. Wang. 2008. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat. Biotechnol. 26326-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zajac, P., D. Oertli, W. Marti, M. Adamina, M. Bolli, U. Guller, C. Noppen, E. Padovan, E. Schultz-Thater, M. Heberer, and G. Spagnoli. 2003. Phase I/II clinical trial of a nonreplicative vaccinia virus expressing multiple HLA-A0201-restricted tumor-associated epitopes and costimulatory molecules in metastatic melanoma patients. Hum. Gene Ther. 141497-1510. [DOI] [PubMed] [Google Scholar]
- 54.Zufferey, R., D. Nagy, R. J. Mandel, L. Naldini, and D. Trono. 1997. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 15871-875. [DOI] [PubMed] [Google Scholar]