Abstract
Woodchuck hepatitis virus (WHV) is an established model for human hepatitis B virus. The kinetics of virus and host responses in serum and liver during acute, self-limited WHV infection in adult woodchucks were studied. Serum WHV DNA and surface antigen (WHsAg) were detected as early as 1 to 3 weeks following experimental infection and peaked between 1 and 5 weeks postinfection. Thereafter, serum WHsAg levels declined rapidly and became undetectable, while WHV DNA levels became undetectable much later, between 4 and 20 weeks postinfection. Decreasing viremia correlated with transient liver injury marked by an increase in serum sorbitol dehydrogenase (SDH) levels. Clearance of WHV DNA from serum was associated with the normalization of serum SDH. Circulating immune complexes (CICs) of WHsAg and antibodies against WHsAg (anti-WHs) that correlated temporarily with the peaks in serum viremia and WHs antigenemia were detected. CICs were no longer detected in serum once free anti-WHs became detectable. The detection of CICs around the peak in serum viremia and WHs antigenemia in resolving woodchucks suggests a critical role for the humoral immune response against WHsAg in the early elimination of viral and subviral particles from the peripheral blood. Individual kinetic variation during WHV infections in resolving woodchucks infected with the same WHV inoculum and dose is likely due to the outbred nature of the animals, indicating that the onset and magnitude of the individual immune response determine the intensity of virus inhibition and the timing of virus elimination from serum.
Infection of adult humans with the hepatitis B virus (HBV) often results in acute hepatitis followed by recovery based on serological and clinical parameters (2). Progression to chronic HBV infection and associated diseases later in life, including liver cirrhosis and hepatocellular carcinoma (HCC), occur infrequently in infected immunocompetent adults, but HBV infection often persists in unvaccinated infants born to HBV carrier mothers or infected horizontally (2). Self-limited HBV infection usually involves a robust primary immune response, acute hepatitis with limited liver injury, and a substantial clearance of virus and viral antigens from the peripheral blood and liver (3, 49). Several studies indicated that the clearance of acute HBV infection relies on the development of an adequate immune response against HBV, including protective, virus-neutralizing antibodies against HBV surface antigen (HBsAg), virus antigen-specific responses of T helper (Th) cells and cytolytic T lymphocytes, and the expression of antiviral cytokines in liver, such as gamma interferon and tumor necrosis factor alpha (1, 9, 13, 20, 24, 26, 40). In contrast, patients with established chronic HBV infection involving persistent viral replication frequently exhibit weak and inefficient humoral and cellular immune responses, resulting in continual virus replication and HBs antigenemia (1, 9, 13, 20, 24, 26, 40).
The kinetic development of appropriate immune responses (or the failure thereof) during the earliest stages of adult and neonatal HBV infection, and their differential influence on the onset and outcome of infection, has been characterized to some extent using animal models (see below) but not humans. This is mainly because patients usually present with clinical symptoms several weeks after the HBV transmission event (except for a few rare cases involving known exposure times) (e.g., see reference 49), and chronicity is a less frequent outcome in these cases. Moreover, neonates born to chronic carrier mothers have not been subjected to detailed kinetic studies, so the contribution of the earliest immune responses promoting viral elimination versus persistence in humans is not fully understood.
Woodchuck hepatitis virus (WHV) is like HBV, an orthohepadnavirus of the Eastern woodchuck (Marmota monax) with essentially identical biological properties, genomic organizations, and replicative strategies (10). Experimental infection of woodchucks with WHV is a well-accepted animal model for many aspects of the pathogenesis of human HBV infection including viral and host factors involved in the outcome of acute infection, disease progression, immune response, and antiviral therapy (22, 29, 33, 34, 43, 44). Infection of neonatal or adult woodchucks with a standardized inoculum of WHV produces predictable proportions of acute, self-limited (i.e., resolved) infections versus chronic infections (6). This mimics the effects of age on the outcome of HBV infection in humans (2). WHV infections of adult woodchucks resolve in more than 95% of the cases, whereas 60 to 75% of neonatal woodchucks develop chronic infections (6). In neonatal and adult woodchucks, the resolution of WHV infection is associated with seroconversion to protective, virus-neutralizing antibodies to WHV surface antigen (anti-WHs) (5, 6, 8, 30-32). Clearance of WHV during the acute phase of infection is associated further with strong and frequently detectable Th-cell responses to WHV antigens in the peripheral blood and also with strong Th1 cytokine expression in the liver (5, 8, 30-32, 38, 47, 48). In contrast, the persistence of WHV replication and the onset and subsequent progression to chronicity as an outcome of experimental neonatal WHV infection are associated with deficiencies in virus-specific B- and Th-cell responses in the periphery, deficiencies in Th1 cytokine expression in the liver, and reduced immune-mediated liver injury (5, 8, 30, 32, 38, 47, 48). These results indicate a crucial role of humoral and cellular immune responses in the outcome of WHV infection. Detailed kinetic studies of WHV viremia and WHs antigenemia in correlation with acute liver injury and resolution are not available. Likewise, the presence and possible role of circulating immune complexes (CICs) involving the viral surface antigen (WHsAg) and its antibodies (anti-WHs) are not characterized sufficiently for woodchucks (or humans) in order to better understand the process of recovery or its failure, leading to chronicity.
To investigate these issues in greater detail, the kinetics of WHV viremia, WHs antigenemia, CICs, and the “free” antibody response against WHsAg were determined using experimentally infected adult woodchucks. For WHs antigenemia, the ratios of the different surface proteins and their glycosylation patterns within subviral particles were compared. In addition to typical anti-WHs responses (detected as free antibody), CICs were measured in resolving woodchucks and correlated subsequently with other virus and host response markers, including the severity of acute liver injury. For additional comparisons, attempts were made to detect CICs in woodchucks with established chronic WHV infection, which lack detectable free anti-WHs.
MATERIALS AND METHODS
Experimental animals and serum samples.
Woodchucks were born to WHV-negative females and reared in environmentally controlled laboratory animal facilities at Cornell University. All experiments involving woodchucks were performed under protocols approved by the Cornell University Institutional Animal Care and Use Committee. Six adult woodchucks of both sexes, 1 to 6 years of age, all seronegative for markers of WHV infection, were experimentally infected with WHV to study virus-host kinetics (see below). Stored serial serum samples from three additional experimentally infected adult woodchucks, 3 to 5 years of age, with self-limited WHV infection were also available. These woodchucks had been infected experimentally as adults by intravenous inoculation with 1 × 104 50% woodchuck infectious doses (WID50) of WHV of a standardized WHV inoculum (cWHV7P2) (6). Diagnosis of resolution was based on the loss of detectable levels of WHV DNA and WHsAg in serum and on the detection of serum antibodies to WHV core antigen (anti-WHc) and WHsAg (anti-WHs) following infection. Stored serum samples from nine adult chronic WHV carrier woodchucks, approximately 1 to 2 years of age, were also available. These woodchucks had been infected experimentally as neonates at 3 days of age by subcutaneous inoculation with 5 × 106 WID50 of WHV of a standardized WHV inoculum (WHV7P1) (6). Diagnosis of persistent WHV infection was based on the consecutive detection of WHV DNA and WHsAg in serum from 3 months of age. At the time of sample collection, woodchucks had minimal chronic hepatitis based on serum liver enzyme profiles. All animals were free of HCC at the time of sampling, as determined by hepatic ultrasound examination and normal serum activity of γ-glutamyl-transferase (GGT).
Experimental WHV infection kinetics.
Six adult woodchucks were inoculated intravenously with 1 × 107 WID50 of WHV7P1 (6). Blood samples were obtained under general anesthesia (50 mg of ketamine/kg of body weight and 5 mg/kg xylazine intramuscularly) 2 weeks before experimental WHV infection prior to inoculation at “week 0” (time zero) and thereafter at weekly or biweekly intervals until the end of the study at week 16 or 28 postinfection. For determining markers of WHV infection within the liver, hepatic specimens were obtained by percutaneous needle biopsy starting 2 weeks prior to infection. Following the infection, one additional biopsy was obtained from some of the woodchucks at week 8 or 10 postinfection. For other woodchucks, liver biopsies were obtained more frequently, at weeks 4, 8, 12, 16, 20, 24, and 28 postinfection. Biopsies were performed while animals were under general anesthesia (50 mg/kg ketamine and 5 mg/kg xylazine intramuscularly) with 16-gauge Bard Biopty-Cut (C. R. Bard Inc., Covington, GA) disposable biopsy needles directed by ultrasound imaging (39, 42).
Serum WHV nucleic acids.
Serum WHV DNA was determined by real-time PCR (assay sensitivity, ≥1 × 102 WHV genome equivalents [WHVge]/ml) with primers located within the S region of the envelope protein, which recognized all orthohepadnaviral genomes, as described previously (41). Briefly, WHV DNA from 200 μl of serum was extracted in 50 μl of buffer using the High Pure viral nucleic acid kit (Roche, Mannheim, Germany). PCRs were normalized as described previously (41) by using a WHV reference serum and WHV plasmid pW8, which contains a full-length WHV genome (6).
Serum WHsAg.
WHsAg in serum was measured quantitatively by the electroimmunodiffusion technique (Laurell electrophoresis) using a polyclonal antiserum against this antigen (46), as described previously (11). Briefly, 0.5% agarose containing a 1:25 dilution of rabbit anti-WHs antiserum was layered onto a glass slide. Thereafter, 10 μl of serum or appropriate dilutions of serum were transferred to the wells on the agarose-layered glass slide and separated by electrophoresis. The migrating antigen within the immunoagar forms immune precipitates until the antigen is used up, resulting in precipitation “arches.” The length of the “arches” in this assay is proportional to the amount of the applied antigen. Reactions were normalized by using serum from a chronic WHV carrier woodchuck (woodchuck F5413) with known WHsAg concentrations. This serum was calibrated in μg of WHsAg protein using purified WHsAg (46). The detection limit for WHsAg is approximately 2 μg/ml (11).
CICs.
WHsAg-containing CICs in serum were detected by polyethylene glycol (PEG) precipitation followed by Western blotting of WHs proteins as described previously (25). Briefly, immune complexes of 100 μl of cleared sera were precipitated by adding 50 μl of 7.5% PEG 6000 (wt/wt). After incubation at 4°C for 12 h, the immune complexes were pelleted at 6,800 × g in a V vial for 5 min at 4°C. The precipitate was washed two times with 200 μl of cold 2.5% (wt/wt) PEG 6000, and the resulting pellet was resuspended in 30 μl phosphate-buffered saline (PBS). WHsAg within the precipitated immune complexes was detected after sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) by Western blotting (see below).
Isolation of subviral particles from serum.
Subviral particles in serum of WHV-infected woodchucks were isolated as described previously (46). Briefly, 3 to 6 ml of serum from individual woodchucks was pelleted through two layers of 10% and 15% sucrose at 25,000 rpm for 16 h at 10°C using an SW41 swing-out rotor from Beckmann (Munich, Germany). The pellet was dissolved in PBS, adjusted with solid cesium chloride (CsCl) to a buoyant density of 1.30 g/ml, and layered within a CsCl gradient ranging from 1.16 to 1.35 g/ml. After centrifugation at 25,000 rpm for 36 h at 10°C with the SW41 rotor (Beckmann), the gradient was fractionized and tested for the presence of WHsAg by SDS-PAGE and silver staining as described previously (46). WHsAg-containing fractions were pooled and concentrated using an ultrafiltration device (Vivascience, Sartorius, Germany). The concentration of WHsAg was estimated from the optical density at 280 nm, with an optical density at 280 nm value of 5.1 equaling 1.0 mg WHsAg per ml (46).
SDS-PAGE and immunoblotting of WHsAg.
Purified subviral particles or resuspended CICs were treated with Laemmli buffer containing 8% dithiothreitol for 15 min at 70°C and analyzed on 12% precast polyacrylamide gels (Invitrogen, Karlsruhe, Germany). Following SDS-PAGE, the gel was blotted onto a polyvinylidene difluoride membrane (Millipore, Eschborn, Germany). The membrane was then blocked in 3% low-fat milk powder in PBS and incubated with the same polyclonal rabbit anti-WHs antiserum as described above, diluted 1:1,000 in PBS with 1% low-fat milk powder for 1 h at 37°C as described previously (46). This was followed by incubation with a peroxidase-conjugated donkey anti-rabbit antibody (Dianova, Hamburg, Germany) at a dilution of 1:10,000 in PBS. WHsAg bands were visualized with the ECL detection kit (Roche, Mannheim, Germany). In immunoblots with reduced and SDS-denatured WHsAg, this antiserum reacts strongly with the glycans of the M protein and weakly with deglycosylated M protein, L protein, and glycosylated S protein. Unglycosylated denatured S protein does not react (46).
WHV antibodies.
Anti-WHc and anti-WHs were measured by qualitative enzyme-linked immunosorbent assay (ELISA) using a 1:10 dilution of serum as described previously (7). The cutoff of these assays was defined as ≥0.05 optical density units (ODU).
Serum biochemistry.
Serum biochemical measurements included serum GGT, alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and sorbitol dehydrogenase (SDH) (39, 42). Serum activities of these liver enzymes were quantified at the New York State Diagnostic Laboratory at Cornell University using a Hitachi autoanalyzer. Serum activities of AST, ALT, and SDH are markers of hepatocellular injury in woodchucks. Serum GGT is a marker of HCC.
Histopathology and immunohistochemistry.
Aliquots of liver biopsy specimens were fixed in phosphate-buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin for histopathological analysis (i.e., portal hepatitis, lobular hepatitis, bile duct proliferation, and liver cell dysplasia), as described previously (23, 39, 42). According to their severity and distribution, individual histological changes associated with WHV infection were scored on a scale of 0 (not present) to 4 (most pronounced). Sections of these tissues were also stained for the intrahepatic expression of WHV core antigen (WHcAg) and WHsAg using immunohistochemical methods and polyclonal rabbit antibodies for the respective antigens (23, 39, 42). Other sections of these tissues were stained for proliferating cell nuclear antigen (PCNA) using a cross-species reactive monoclonal mouse anti-human PCNA antibody (Roche Diagnostics Corp., Indianapolis, IN) (23, 39, 42). Apoptotic activity in liver tissues was determined by the detection of DNA strand cleavage in hepatocytes using terminal deoxynucleotidyl transferase-mediated uridine deoxynucleotide nick end-labeling technique (Roche Diagnostics Corp.) (23, 39, 42). CD3-positive lymphocytes in liver were stained with a cross-reacting polyclonal rabbit anti-human CD3 antibody (Dako Inc., Carpinteria, CA) (39, 42). Macrophages in liver were stained with a cross-reacting monoclonal mouse antibody (Dako Inc.) (6, 38).
RESULTS
Kinetic study of self-limited experimental WHV infection in adult woodchucks.
Markers of WHV infection and host immune responses to WHV antigens were characterized in two repeat experiments, each with three adult animals. All six woodchucks were inoculated intravenously with 1 × 107 WID50 of WHV7P1. Following inoculation, woodchucks of group 1 (woodchucks F3524, F3538, and M3540) were monitored weekly until the end of the study in week 16. Woodchucks of group 2 (woodchucks F3391, F3539, and M3553) were monitored for a longer period, and monitoring was performed weekly during the initial 12 weeks and then every other week until the end of the study in week 28. Within the differential detection limits of assays used, these close monitoring intervals enabled a detailed characterization of both the uniformity and variation in the responses of viral markers, CICs, and antibody responses in the peripheral blood from the early to late acute stages of resolving WHV infection, as described below.
(i) Serum WHV DNA.
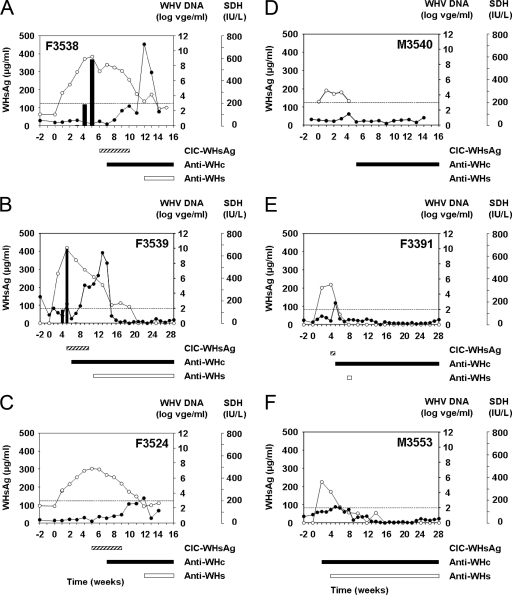
All six woodchucks resolved their infection based on the inability to detect WHV DNA in serum at the end of the study, as determined by real-time PCR. Individual variation in the kinetics of WHV DNA was noted during the course of infection. WHV viremia was high and long-lasting in woodchucks F3538 and F3539, intermediate in woodchuck F3524, and low and of short duration in woodchucks F3391, M3540, and M3553 (Fig. 1). All woodchucks became positive by PCR for WHV DNA in serum by 1 to 2 weeks following inoculation. The initially detectable WHV DNA concentrations ranged between 1 × 104 WHVge/ml in woodchuck M3540 and 4 × 106 WHVge/ml in woodchuck F3539, with all concentrations clearly detectable above the respective assay cutoff values.
FIG. 1.
Course of acute, self-limited WHV infection in adult woodchucks following experimental WHV infection. (A) Woodchuck F3538. (B) Woodchuck F3539. (C) Woodchuck F3524. (D) Woodchuck M3540. (E) Woodchuck F3391. (F) Woodchuck M3553. All woodchucks were infected with 1 × 107 WID50 of the WHV7P1 inoculum at week zero. The kinetics of WHV DNA, free WHsAg, and SDH in serum are presented in the graphs. Bars below the graphs indicate the appearance and duration of CICs, anti-WHc, and free anti-WHs in serum. Serum WHV DNA was quantitated by a real-time PCR-based assay with cutoff values of 1 × 103 WHVge/ml for woodchucks F3524, F3538, and M3540 and 1 × 102 WHVge/ml for woodchucks F3391, F3539, and M3553, as indicated by the dotted line. Free WHsAg was quantitated by electroimmunodiffusion with a detection limit of 2 μg/ml. SDH was quantitated using a Hitachi autoanalyzer. The baseline value for SDH observed in healthy adult WHV-negative woodchucks is 40 IU/liter. CICs were detected qualitatively by PEG precipitation followed by Western blotting. Anti-WHc and anti-WHs were measured qualitatively by ELISA with an assay cutoff value of ≥0.05 ODU. ○, serum WHV DNA (WHVge/ml); ▪, serum WHsAg (μg/ml); •, serum SDH activity (IU/liter).
In woodchucks F3524, F3538, and F3539, WHV DNA concentrations increased over time and peaked between 4 and 5 weeks postinfection. In woodchucks M3540, M3553, and F3391, WHV DNA was also detected initially at 1 or 2 weeks postinfection, but those levels corresponded to (or were very near) the maximal observed WHV DNA concentrations. In these woodchucks, maximal WHV DNA concentrations were considered low viremic, ranging from between 1 × 104 and 4 × 105 WHVge/ml. In contrast, in woodchuck F3524, the maximum WHV DNA concentration was considered intermediate viremic at 3 × 107 WHVge/ml (week 5), and in woodchucks F3538 and F3539, maximal WHV DNA concentrations were considered high viremic at 2 × 109 and 1 × 1010 WHVge/ml (weeks 5 and 4, respectively).
The times of WHV DNA elimination from serum (i.e., durations until WHV DNA concentrations were below the assay cutoff) varied among the woodchucks but generally correlated with the level of viral replication: in woodchucks F3391, M3540, and M3553 (low viremic), WHV DNA became undetectable between 5 and 6 weeks postinfection. In woodchuck F3524 (intermediate viremic), WHV DNA became undetectable by week 12, while in woodchucks F3538 and F3539 (high viremic), WHV DNA became undetectable by weeks 14 and 20, respectively. Overall, the half-life of serum WHV DNA after the peak in viremia was estimated to be 2 to 3 days for woodchucks with high, intermediate, or low viremia, with the exception of woodchuck F3391, which had a shorter half-life.
The influence of animal age on the magnitude and course of viremia was negligible because rapid or prolonged clearances of WHV from serum were equally observed among younger and older woodchucks; i.e., the high-viremic woodchucks F3538 and F3539 were 2 and 3 years of age, the intermediate-viremic woodchuck F3524 was 3 years of age, and the low-viremic woodchucks F3391, M3540, and M3553 were 6, 1, and 2 years of age at the time of WHV inoculation.
(ii) Serum WHsAg.
Using electroimmunodiffusion, free serum WHsAg was detected in the high-viremic woodchucks F3538 and F3539 (shown for woodchuck F3538 in Fig. 2). Free WHsAg was detected for a shorter duration in sera of both woodchucks compared to the respective durations for serum WHV DNA (i.e., detected for 3 to 4 weeks, starting at 3 weeks postinfection) (Fig. 1 and 2). In woodchuck F3538, the concentration of serum WHsAg increased within 2 weeks, from an initial concentration of 3 μg/ml at 3 weeks postinfection to a maximum concentration of 367 μg/ml at week 5 postinfection; i.e., the doubling time was approximately 2 days. Somewhat higher levels were detected initially in woodchuck F3539 at week 3 (74 μg/ml), suggesting an even shorter doubling time (from below 2 μg/ml to 74 μg/ml within 7 days), and the maximal concentration of 414 μg/ml at week 4 was quite remarkable as well. In both woodchucks, the timing of the observed peak in WHs antigenemia correlated with the peak in serum WHV viremia. Serum WHsAg concentrations decreased much more rapidly than did WHV viremia in both woodchucks; it was undetectable in woodchuck F3538 1 week after the peak (i.e., 6 weeks postinfection) (Fig. 2) and by 1 to 2 weeks after the peak in woodchuck F3539 (i.e., 5 to 6 weeks postinfection). This corresponds to a half-life of less than 1 day.
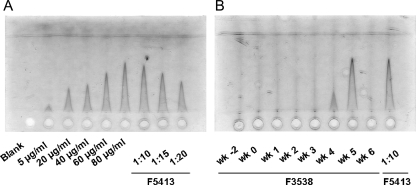
FIG. 2.
Detection of free serum WHsAg during the course of acute, self-limited WHV infection in adult woodchucks. (A) Standard dilutions of purified WHsAg and reference serum from chronic WHV carrier woodchuck F5413. Free WHsAg circulating in serum was quantitated by Laurell electrophoresis (electroimmunodiffusion). Using endpoint titration, it was estimated that the reference serum contained 729 μg WHsAg per ml. (B) Dilutions (1:5) of serum from woodchuck F3538 obtained at different time points during the course of WHV infection. Values were compared against values for a 1:10 dilution of the reference serum from woodchuck F5413. The cutoff of the assay was defined as 2 μg/ml.
(iii) CICs.
Using PEG precipitation followed by Western blotting, CICs consisting of WHsAg “complexed” with anti-WHs were detected in four of the six woodchucks (Fig. 3). CICs in woodchucks F3391, F3539, and F3524 were detected initially between 4 and 5 weeks postinfection, and their appearance correlated with the peaks in WHV viremia and WHs antigenemia (Fig. 1). Woodchuck F3538 tested positive for serum CICs for the first time 1 week following maximum viral replication, at 6 weeks postinfection. With the exception of woodchuck F3391, in which serum CICs were detected for only 1 week and at a relatively low level, CICs in the other three woodchucks were observed for 5 to 6 weeks. In all woodchucks, the levels of CICs appeared to be highest during the initial 2 to 4 weeks of detection and then declined thereafter during the remaining 1 to 2 weeks. The disappearance of CICs from serum in woodchuck F3539 correlated with the first detection of free anti-WHs (Fig. 1). In the other three woodchucks, serum anti-WHs became detectable between 2 and 4 weeks following the disappearance of CICs.
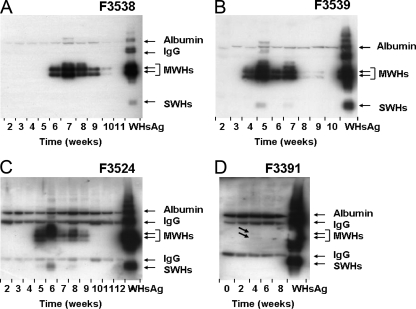
FIG. 3.
Detection of circulating immune complexes during the course of acute, self-limited WHV infection in adult woodchucks. (A) Woodchuck F3538. (B) Woodchuck F3539. (C) Woodchuck 3524. (D) Woodchuck 3391. CICs in serum were detected by PEG precipitation followed by Western blotting with rabbit anti-WHs antiserum. Purified serum WHsAg (0.5 μg) was used as a standard. IgG, light (25 kDa) and heavy (50 kDa) chains of IgG; MWHs, WHV middle (pre-S2) surface protein; SWHs, WHV small/major (S) surface protein. Arrows in D indicate CICs detected in the serum of woodchuck F3391 at 4 weeks postinfection.
(iv) Antibody responses to WHcAg and WHsAg.
All WHV-infected woodchucks developed serum anti-WHc between 1 and 2 weeks after the peak in WHV viremia and WHs antigenemia (i.e., between 5 and 7 weeks postinfection). Anti-WHc in M3553 appeared at the time of maximum viral replication, at 2 weeks postinfection. Another notable exception was woodchuck M3540, in which serum anti-WHc was detected starting 4 weeks after the peak in WHV DNA and WHsAg (i.e., at 7 weeks postinfection). In all woodchucks, anti-WHc was present in serum until the end of the study.
The appearance of conventional “free” serum anti-WHs (i.e., not “complexed” with WHsAg) varied between individual woodchucks, but in most woodchucks, it became detectable between 2 and 5 weeks following the first appearance of anti-WHc, between 3 and 6 weeks following the disappearance of WHsAg, and immediately or between 2 and 4 weeks following the disappearance of CICs. No anti-WHs was detected in serum of woodchuck M3540; this woodchuck also had no detectable free serum WHsAg and no CICs involving WHsAg and anti-WHs. Woodchuck M3553 had a long-lasting anti-WHs response, with no detectable serum WHsAg or CICs. Free anti-WHs was present in serum from most of the woodchucks until the end of the study, with the exception of woodchuck F3391, where anti-WHs was detected once at 8 weeks postinfection.
(v) Liver injury response.
Serum SDH activity was determined for all woodchucks as a marker of hepatocellular injury (Fig. 1) and then correlated with elevations of other liver enzymes in serum and also with the expression of WHV antigens and histopathological changes in hepatic biopsy specimens (Tables 1, 2, and 3). The level of serum SDH increased remarkably starting at 8 weeks postinfection in the two high-viremic woodchucks (woodchucks F3538 and F3539) to peak activities at 12 weeks postinfection of 725 and 632 IU/liter. Serum SDH activities were less pronounced in the other four woodchucks, with maximum activities between 5 and 10 weeks postinfection ranging between 90 IU/liter (woodchuck M3540) and 222 IU/liter (woodchuck F3524). It is important that peaks in serum SDH occurred after serum WHV DNA and WHsAg levels had already started to decline and after anti-WHc became detectable. Also, at peak SDH activity, most woodchucks already had detectable anti-WHs. The SDH level declined relatively rapidly following the peak in all woodchucks and normalized within 1 to 4 weeks, including the high-viremic woodchucks F3538 and F3539.
TABLE 1.
Comparison of serum liver enzyme activities prior to experimental WHV infection and at the time of maximum acute liver injury following infection
| Woodchuck | Level of viremia | Serum liver enzyme activity (IU/liter)a
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ALP | ALT | AST | GGT | ||||||
| F3538 | High | 4 | 7 | 4 | 39 | 46 | 296 | 3 | 3 |
| F3539 | High | 11 | 4 | 5 | 62 | 30 | 317 | 5 | 62 |
| F3524 | Intermediate | 3 | 6 | 4 | 62 | 70 | 498 | 5 | 6 |
| M3540 | Low | 6 | 5 | 4 | 4 | 41 | 50 | 3 | 3 |
| F3991 | Low | 6 | 5 | 4 | 13 | 14 | 41 | 3 | 3 |
| M3553 | Low | 17 | 30 | 4 | 9 | 24 | 56 | 3 | 3 |
The first value in each column was determined prior to experimental WHV infection at week zero. The second value was determined at the time of maximum serum SDH activity as a marker of acute liver injury. Peak serum SDH activities varied between woodchucks and were detected at 4 weeks postinfection in woodchuck M3540, at 5 weeks postinfection in woodchucks F3991 and M3553, at 10 weeks postinfection in woodchuck F3524, and at 12 weeks postinfection in woodchucks F3538 and F3539 (Fig. 1).
TABLE 2.
Comparison of WHV antigen expression and histological changes in liver prior to experimental WHV infection and at the time of maximum acute liver injury following infection
| Woodchuck | Level of viremia | WHV antigen expression (%)
|
Histological changes (scores)a
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHcAg
|
WHsAg
|
Portal hepatitis
|
Lobular hepatitis
|
Bile duct proliferation
|
Liver cell dysplasia
|
||||||||
| 2 wk preinfection | Max serum SDH activity | 2 wk preinfection | Max serum SDH activity | 2 wk preinfection | Max serum SDH activity | 2 wk preinfection | Max serum SDH activity | 2 wk preinfection | Max serum SDH activity | 2 wk preinfection | Max serum SDH activity | ||
| F3538 | High | 0 | 75 | 0 | 0.5 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 0 |
| F3539 | High | 0 | 50 | 0 | 0.5 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| F3524 | Intermediate | 0 | 25 | 0 | 0.5 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 2 |
| M3540b | Low | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| F3991 | Low | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 2 | 0 | 0 | 1 | 1 |
| M3553 | Low | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
The first value in each column was determined using hepatic specimens obtained 2 weeks prior to experimental WHV infection. The second value was determined using hepatic specimens obtained at or around the time of maximum serum SDH activity (see Table 1 for details). Liver biopsies were obtained from woodchucks F3991 and M3553 at 4 weeks postinfection, from woodchuck M3540 at 8 weeks postinfection, from woodchucks F3538 and F3524 at 10 weeks postinfection, and from woodchuck F3539 at 12 weeks postinfection.
Maximum SDH activity for woodchuck M3540 was at week 4 postinfection, whereas the presented data were derived from data for liver tissue obtained 4 weeks after the SDH peak (i.e., at 8 weeks postinfection).
TABLE 3.
Comparison of hepatocyte proliferation, apoptosis in hepatocytes, and liver inflammation prior to experimental WHV infection and at the time of maximum acute liver injury following infection
| Woodchuck | Level of viremia | Liver proliferation, apoptosis, and inflammation (%)a
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PCNA
|
Apoptosis
|
CD3+ cells
|
Macrophages
|
||||||
| 2 wk preinfection | Max serum SDH activity | 2 wk preinfection | Max serum SDH activity | 2 wk preinfection | Max serum SDH activity | 2 wk preinfection | Max serum SDH activity | ||
| F3538 | High | 0.9 | 11.0 | 0.5 | 2.0 | 9.7 | 85.0 | 0.6 | 13.7 |
| F3539 | High | 0.9 | 2.6 | 0 | 0.3 | 8.8 | 67.7 | 5.3 | 8.8 |
| F3524 | Intermediate | 0.5 | 8.2 | 0.5 | 1.4 | 10.7 | 48.9 | 2.3 | 16.7 |
| M3540b | Low | 0.8 | 1.7 | 0.5 | 0.8 | 11.7 | 9.7 | 5.1 | 2.1 |
| F3991 | Low | 0 | 0.2 | 0 | 0 | 35.5 | 46.0 | 26.4 | 10.0 |
| M3553 | Low | 0.5 | 0.8 | 0 | 0.2 | 40.0 | 36.5 | 12.9 | 16.3 |
The first value in each column was determined for hepatic specimens obtained 2 weeks prior to experimental WHV infection. The second value was determined for hepatic specimens obtained at or around the time of maximum serum SDH activity (see Tables 1 and 2 for details).
Maximum SDH activity for woodchuck M3540 was at 4 weeks postinfection, whereas the presented data were derived from using liver tissue obtained 4 weeks later (i.e., at 8 weeks postinfection).
As with SDH, the serum activities of ALT and AST became transiently increased in most woodchucks (Table 1). Peak ALT and AST activities were observed at the same time as peak SDH activity. High- or intermediate-viremic woodchucks (woodchucks F3524, F3538, and F3539) had greater increases in serum ALT and AST activity than did low-viremic woodchucks (woodchucks F3391, M3553, and M3540). These markers are generally less sensitive than SDH as markers for the severity of liver injury in woodchucks. At the time of maximum liver injury (indicated by SDH, ALT, and AST), the activities of other enzyme markers like ALP and GGT remained unchanged from preinfection levels in most woodchucks, except for woodchucks F3539 and M3553, where marked increases in levels of these two enzymes were noted.
During peak liver injury, high- and intermediate-viremic woodchucks (woodchucks F3524, F3538, and F3539) had increased hepatic expression of WHcAg and cytoplasmic WHsAg, which were not evident in the livers of low-viremic woodchucks (woodchucks F3391, M3553, and M3540) (Table 2). Most woodchucks had histological changes including portal and lobular hepatitis that were scored as mild to more severe for this species (Table 2). No liver changes were observed in woodchuck M3540, where the liver biopsy was obtained 4 weeks after the peak in SDH activity (i.e., at 8 weeks postinfection), at a time when hepatic injury had already abated. No liver changes were observed in the low-viremic woodchuck M3553. Most woodchucks were clear of histological changes in bile duct proliferation and liver cell dysplasia at the time of biopsy, except for woodchucks F3524 and F3538, which each had a mild change in one or both markers, respectively (Table 2).
Liver cell proliferation and apoptosis in hepatocytes became increased in woodchucks at the time of maximum acute liver injury, except in the low-viremic woodchuck F3991, where proliferation but no apoptosis was noted (Table 3). High- and intermediate-viremic woodchucks (woodchucks F3524, F3538, and F3539) demonstrated greater increases in liver regeneration and/or elimination of hepatocytes than did low-viremic woodchucks (woodchucks F3391, M3553, and M3540). Consistent with the patterns of liver injury indicated above, the numbers of CD3+ lymphocytes and macrophages within the liver were increased in most woodchucks (Table 3). Hepatic inflammation by these cell types was more remarkable in the high- and intermediate-viremic woodchucks than in low-viremic woodchucks. Interestingly, however, in woodchucks F3991 and M3553, the respective numbers for macrophages versus CD3+ lymphocytes were actually less at postinfection than at preinfection even though the overall degrees of inflammation and liver injury were increased postinfection. A lower number of these cell types was also observed in the biopsy from woodchuck M3540, for which hepatic injury had already abated.
Detection of CICs in sera of woodchucks with self-limited versus chronic WHV infection.
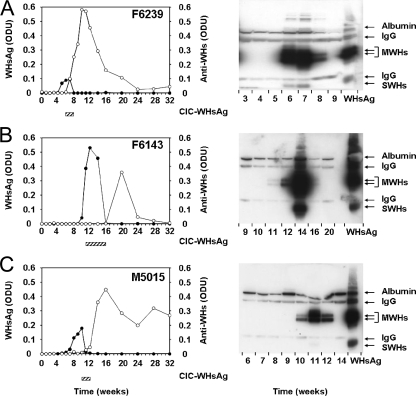
For further correlation of the appearance and duration of free WHsAg, CICs, and free anti-WHs in relation to outcome of infection, serum samples from woodchucks previously infected with WHV (three adult woodchucks with self-limited WHV infection and nine neonatally infected woodchucks with chronic WHV infection) were assayed. Woodchucks F6143, F6239, and M5015 had been experimentally infected with 104 WID50 of cloned WHV7P2, which has the same biological outcome properties as the parental uncloned WHV7P1 inoculum used in the above-described kinetic study (6). These woodchucks were monitored for a total of 32 weeks through their recovery from acute WHV infection and hepatitis. CICs were detected in sera of all three woodchucks for a duration of 3 to 6 weeks but with variable timing in the responses (Fig. 4). In woodchuck F6143, CICs were detected at 11 weeks postinfection, and their appearance correlated with the first increase in free serum WHsAg concentrations (i.e., with the beginning of WHs antigenemia). CICs in woodchucks F6239 and M5015 were detected at 6 and 10 weeks postinfection, respectively, and their appearance correlated with the peak of WHs antigenemia. This was associated further with the initial detection of free anti-WHs. In woodchuck F6143, free anti-WHs became detectable only after CICs had disappeared from serum. Relative to the signal for the antigen standard (0.5 μg WHsAg), CIC levels in woodchucks F6143 and F6239 were higher than those in woodchuck M5015. The variation in CIC levels was unexpected because woodchucks F6239 and M5015 had no remarkable differences in their levels of free WHsAg and free anti-WHs. Comparison of the appearance and duration of WHsAg, CICs, and anti-WHs in these three woodchucks with those in the six woodchucks from the above-described kinetic study indicated that the inoculum dose used for experimental WHV infection had no effect on the kinetic of standard viral and host markers.
FIG. 4.
Correlation of free WHsAg, CICs, and anti-WHs during the course of acute, self-limited WHV infection in adult woodchucks. (A) Woodchuck F6239. (B) Woodchuck F6143. (C) Woodchuck M5015. Woodchucks were infected with 1 × 104 WID50 of the cWHV7P2 inoculum at week zero. The kinetics of WHsAg and anti-WHs in serum are presented in the graphs. Bars below the graphs indicate the appearance and duration of CICs in serum. WHsAg and anti-WHs were measured qualitatively by ELISA, with an assay cutoff value of ≥0.05 ODU. CICs in serum were detected by PEG precipitation followed by Western blotting with rabbit anti-WHs antiserum. Purified serum WHsAg (0.5 μg) was used as a standard. IgG, light (25 kDa) and heavy (50 kDa) chains of IgG; MWHs, WHV middle (pre-S2) surface protein; SWHs, WHV small/major (S) surface protein. •, serum WHsAg (ODU); ○, serum anti-WHs (ODU).
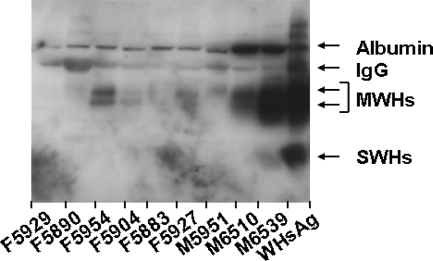
Individual serum samples were also analyzed for CICs from nine woodchucks during the chronic phase of experimental neonatal infection with uncloned WHV7P1 inoculum. The age of the woodchucks at the time of sampling was either 15 months (woodchucks M6510 and M6539) or 22 months (woodchucks F5883, F5890, F5904, F5927, F5929, M5951, and F5954). In five of the nine woodchucks, CICs were detected in serum at variable levels (Fig. 5). Compared to the antigen standard (0.5 μg WHsAg), woodchuck M6539 had the most remarkable levels of CICs. CIC levels in woodchucks F5954 and M6510 were intermediate, whereas the levels were low in woodchucks F5904 and F5927. CICs were not detected in the sample from each of the remaining chronic WHV carrier woodchucks. Comparison of CIC levels with concentrations of serum WHV DNA, free WHsAg, and anti-WHc (Table 4) indicated that the presence of CICs in chronic WHV carrier woodchucks does not correlate with the magnitude of WHs antigenemia, WHV viremia, and antibody response to WHcAg. However, a possible direct correlation between CICs and free WHsAg in serum (similar to woodchucks with self-limited WHV infection) was observed, with woodchuck M6539 having the highest levels of CICs and WHsAg, followed by those for woodchucks F5954, F5927, and F5904. In contrast, woodchuck M6510 had intermediate CIC levels with relatively low levels of free WHsAg, and woodchucks F5883 and M5951 had relatively high levels of free WHsAg with no CICs. The apparent lack of correlation in these animals, however, may relate more to the immune status of the carrier (e.g., immune tolerant versus partial immune response).
FIG. 5.
Detection of circulating immune complexes in adult woodchucks with established chronic WHV infection. CICs in serum were detected by PEG precipitation followed by Western blotting with rabbit anti-WHs antiserum. Purified serum WHsAg (0.5 μg) was used as a standard. IgG, light (25 kDa) and heavy chains (50 kDa) of IgG; MWHs, WHV middle (pre-S2) surface protein; SWHs, WHV small/major (S) surface protein.
TABLE 4.
Comparison of circulating immune complexes with WHs antigenemia, WHV viremia, and anti-WHc response in woodchucks with established chronic WHV infection
| Woodchucka | CIC (signal strength)b | WHsAg (ODU) | WHV DNA (WHVge/ml) | Anti-WHc (ODU) |
|---|---|---|---|---|
| M6539 | +++ | 0.319 | 7.5 × 1010 | 0.295 |
| F5954 | ++ | 0.228 | 6.7 × 1010 | 0.122 |
| M6510 | ++ | 0.177 | 2.6 × 1010 | 0.195 |
| F5904 | + | 0.218 | 7.7 × 109 | 0.226 |
| F5927 | + | 0.183 | 9.2 × 109 | 0.152 |
| M5951 | − | 0.205 | 4.0 × 1010 | 0.122 |
| F5883 | − | 0.196 | 1.8 × 1010 | 0.077 |
| F5890 | − | 0.172 | 7.6 × 109 | 0.097 |
| F5929 | − | 0.154 | 7.2 × 109 | 0.131 |
Woodchucks were infected experimentally as neonates at 3 days of age with 5 × 106 WID50 of the WHV7P1 inoculum.
+++, high; ++, intermediate; +, low; −, no signal.
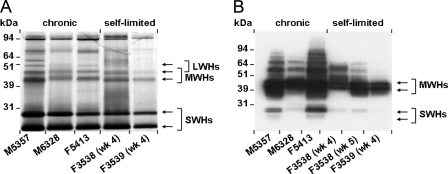
Comparison of S, M, and L surface proteins of WHsAg in acute and chronic WHV infections.
Subviral WHs particles representing primarily excess surface antigens of WHV were isolated from serum of woodchucks with acute, self-limited or established chronic WHV infection. The purified particles were separated by SDS-PAGE, and the L, M, and S proteins of WHsAg were analyzed by silver staining and/or Western blotting (Fig. 6).
FIG. 6.
Comparison of the S, M, and L surface proteins of WHsAg within subviral particles isolated from sera of adult woodchucks with acute, self-limited WHV infection and woodchucks with established chronic WHV infection. Subviral particles isolated from serum were separated by SDS-PAGE, and the S, M, and L proteins of WHsAg were detected by silver staining using 0.5 μg of subviral particles (A) or by Western blotting with a polyclonal rabbit anti-WHs antiserum using 1.0 μg of subviral particles (B). LWHs, WHV large (pre-S1) surface protein; MWHs, WHV middle (pre-S2) surface protein; SWHs, WHV small/major (S) surface protein.
In both acute, self-limited and chronic WHV infections, silver staining indicated two strong protein bands in all woodchucks, with molecular masses below 31 kDa (Fig. 6A). These represent the nonglycosylated (smaller) and the single-N-glycosylated (larger) forms of the S protein, with molecular masses of 24 and 27 kDa, respectively. Two weaker bands above the S protein with molecular masses of 41 and 45 kDa were also observed for all woodchucks, which represent multiple-N- and -O-glycosylated forms of the M protein. A comparison of S and M proteins between woodchucks with acute, self-limited or established chronic WHV infections demonstrated no differences in the ratios of individual proteins within subviral particles, with the S protein as the predominant protein. Because of the weak signals for the L protein, a comparison of this protein between woodchucks with different outcomes of experimental WHV infections was not possible.
In further comparisons of the S and M proteins by Western blotting using a polyclonal rabbit anti-WHs antiserum that can react with denatured WHsAg, all woodchucks demonstrated multiple-N- and -O-glycosylated forms of the M protein and the nonglycosylated and single-N-glycosylated forms of the S protein (Fig. 6B). The polyclonal antibody reacted much better with the sequential epitopes in the pre-S domains of the M and L proteins than with the S protein, which contains mainly conformational, disulfide-dependent epitopes. Again, no differences in the protein ratios and glycosylation patterns within subviral particles were observed between woodchucks in different outcome settings or times postinfection, but some microheterogeneity of the M and L proteins between the different samples was present.
DISCUSSION
Adult, WHV-susceptible woodchucks were infected experimentally with WHV to investigate the dynamically changing relationships between WHV viremia and antigenemia, humoral immune responses also involving CICs, and acute liver injury. Although all six woodchucks received the same relatively high inoculum and dose, the course of acute, self-limited WHV infection varied remarkably among animals. Individual differences based on the markers were minimal immediately after inoculation. WHV DNA was already detectable at 1 or 2 weeks postinfection. Thereafter, the kinetics of serum WHV viremia differed remarkably among individual woodchucks. The peak of viral replication had already occurred after 2 weeks in two woodchucks at a low level, whereas WHV viremia in the other woodchucks continued to increase, and a maximum viral replication of up to 1 × 1010 WHVge/ml was observed between 4 and 5 weeks postinfection. The peak concentration and duration of WHV DNA in serum suggested a classification of viremia as being high in two woodchucks, intermediate in one, and low in three. With the PCR assay used, it was not possible to differentiate whether WHV DNA detected in sera of low-viremic woodchucks following inoculation was part of the inoculum or progeny WHV DNA. The fact that low-viremic woodchucks had a productive WHV infection was demonstrated by the detection of anti-WHc in all animals, in addition to CICs, anti-WHs, and acute liver injury in some. Furthermore, a comparison of the half-life for the clearance of WHV DNA following the peak in WHV viremia indicated a similar viral clearance rate in low-, intermediate-, and high-viremic woodchucks during the recovery phase, thus strongly suggesting that new WHV was still being produced in face of the developing host response.
Free WHsAg was detectable by electroimmunodiffusion only in sera of the high-viremic woodchucks, and the peak in WHs antigenemia correlated with the peak in serum WHV viremia (Fig. 2). Due to the low sensitivity of the assay, WHsAg was probably not detected in the remaining woodchucks or in the high-viremic woodchucks at later time points. Free WHsAg in sera of both high-viremic woodchucks was present for only 2 to 3 weeks. WHsAg was cleared more rapidly from serum than serum WHV DNA in both woodchucks. This difference in kinetics may be explained by the fact that WHsAg was detected only as free antigen, whereas WHV DNA could be extracted both from free and anti-WHs-complexed WHV. Furthermore, the antigenicity of WHV particles may be different from that of spherical WHsAg subviral particles. The envelope of HBV and HBsAg filaments contain a higher proportion of L protein than HBsAg spherical particles. The pre-S domains partially mask the S domain, which becomes more accessible to monoclonal antibodies after the removal of pre-S by trypsin (16). The WHsAg filaments also contain a higher proportion of WHs L protein than spherical WHs particles (46). Thus, WHV may escape WHsAg S antibody recognition easier than the subviral particles.
Following the peak in WHV viremia, serum WHV DNA concentrations declined continuously over 9 weeks (woodchuck F3538) or 14 weeks (woodchuck F3539) before becoming undetectable (Fig. 1). The length of time of the presence of WHV DNA in sera of the remaining woodchucks was shorter, and levels of WHV DNA declined to undetectable levels over 2 weeks (woodchuck M3540) to 7 weeks (woodchuck F3524) following the peak in WHV viremia. Obviously, viral clearance rates (i.e., half-lives of serum WHV DNA) were similar between woodchucks irrespective of peak viremia.
The magnitude of maximum WHV DNA concentrations and duration of viral clearance in individual woodchucks are dependent on several factors. Viral factors that influence the course of acute, self-limited WHV infection include the inoculum dose, viral strain within the inoculum, and route of inoculation (6). The age and status of the immune system of an individual woodchuck are host factors that determine the outcome and course of WHV infection (6, 8, 32, 38, 47, 48). Studies of humans and woodchucks suggested that the fine balance between viral load and quality of the individual antiviral immune response is important for an efficient suppression of viral replication resulting in the eradication of the virus from the host (3, 8, 32, 38, 47, 48). The quality of the antiviral immune response appears to be the main determining factor for the observed differences in the course of WHV infection in the present study, because all group 1 and group 2 adult woodchucks had been infected with the same inoculum and dose.
The protein composition of subviral surface antigen particles was determined in resolving and chronic carrier woodchucks in order to explore whether differential host immune responses or replication-dependent processes might skew the ratios of viral and subviral particles or their component proteins. A comparison of the subviral protein compositions by silver staining revealed no significant differences between woodchucks with different outcomes of WHV infection (Fig. 6) and confirmed previous results obtained with chronic WHV carriers (46). Furthermore, minor differences in the migration patterns of the M proteins were detected by Western blotting in subviral particles from resolving woodchucks and chronic WHV carrier woodchucks or from woodchuck F3538 during the acute phase of WHV infection (Fig. 6). These are probably caused by slight differences in glycosylation (42).
Host responses correlated temporally with viral markers as expected. Antibody responses against WHV antigens were elicited in individual woodchucks and, as with Th-cell responses that develop first to WHsAg and then to WHcAg (5, 30, 31), the earliest antibody responses (detected as CICs) were directed against WHsAg. CICs containing WHsAg and anti-WHs were detected around the time of peak WHV viremia and WHs antigenemia (Fig. 3 and 4). Due to the preferential reactivity of the CICs detecting antiserum with the WHsAg M protein in immunoblots, we cannot exclude the possibility that a subfraction of WHs-containing CICs with a very low or absent proportion of the M protein was missed, but as shown in Fig. 6B, there is no indication for such a heterogeneity. Although not tested in this study, it is possible that anti-WHs within CICs represents the immunoglobulin M IgM class, whereas the later-appearing free anti-WHs represents the IgG class. However, in contrast to the core antigen, which readily elicits measureable IgM antibodies in a T-cell-independent manner (35), the surface antigen of hepadnaviruses is a strictly T-cell-dependent antigen and poorly immunogenic directly for B cells in vitro.
The rapid decline of levels of free WHsAg and the presence of CICs demonstrate directly that antibodies against WHsAg are involved in the clearance of viral and subviral particles from peripheral blood in resolving woodchucks. This finding is comparable to findings for acute, self-limited HBV infection in humans in which the neutralization of virus is clearly associated with the presence of antibodies against HBsAg (2, 3). Anti-HBs, however, appears not to play as important a role in the early clearance of subviral particles during resolving HBV infection (see below).
CICs in patients with chronic HBV infection were described previously (25). In contrast to chronic WHV carrier woodchucks, amounts of CICs were high in chronic HBV carriers and low or absent in acute HBV infection (27, 28). In chronic WHV carrier woodchucks with comparable levels of free WHsAg, smaller amounts of CICs were present than during acute infection (Fig. 5 and Table 4). The reason for this difference between WHV and HBV is unknown but may relate to the immune system of woodchucks and/or the structural differences between HBV and WHV. Furthermore, the sensitivity of the assay used and the immune status of woodchucks at the time of serum sample collection (e.g., normal versus elevated liver enzyme activities indicating exacerbations of liver injury) may have been different.
One to two weeks following the initial appearance of CICs, antibodies against WHcAg became detectable (Fig. 1), and their presence lasted until the end of the study (i.e., up to 7 months in some woodchucks). The constitutive presence of anti-WHc in all resolving woodchucks is in contrast to the much shorter detection of anti-WHs in woodchucks with WHs seroconversion (Fig. 1 and 4). The differences in the humoral response against both WHV antigens may be explained by the higher immunogenicity of WHcAg over WHsAg. Free anti-WHs, as a marker of the more favorable outcome of WHV infection, appeared only during the later stages of infection and was associated with almost undetectable levels of serum WHV DNA and normalized serum activity of the liver enzyme SDH. Besides elimination of viral particles from blood, anti-WHs is also important for the protection of hepatocytes from WHV reinfection within the regenerating liver (15). In this respect, the roles of the antibody responses are similar in woodchucks and humans.
All woodchucks in the experimental infections described here resolved their WHV infection based on undetectable levels of serum WHV DNA at the end of the study. Comparable to adult chimpanzees with acute, self-limited HBV infection (37), levels of serum WHV DNA in woodchucks started to decline substantially before the peak in acute liver injury was observed, as determined by increases in serum SDH activity (Fig. 1). The reduction in serum WHV DNA levels before liver injury suggests that noncytotoxic immune mechanisms are mainly responsible for the initial decrease of WHV replication. As demonstrated in HBV-transgenic mice and HBV-infected chimpanzees, a massive production of the cytokine gamma interferon by HBV-specific CD8+ T cells followed by tumor necrosis factor alpha from macrophages and hepatic Kupffer cells is responsible for the initial reduction in levels of HBV DNA and was also described previously for the self-limited outcome of acute WHV infection in woodchucks (12, 14, 15, 19, 21, 37, 38, 48). The inhibition of HBV replication by these cytokines is a result of destabilized HBV mRNA and HBV covalently closed circular DNA in infected hepatocytes (17, 37) and, by analogy, in WHV-infected hepatocytes of woodchucks.
One of the later steps in terminating acute WHV infection in woodchucks is the induction of necrosis and apoptosis of WHV-infected hepatocytes by cytotoxic T lymphocytes, resulting in the eradication of virus-infected cells from the liver. All six woodchucks had increases in the serum activity of SDH (Fig. 1) and/or other liver enzymes (Table 1) as markers of hepatocellular injury, although peak SDH concentrations varied among animals and across the time interval. Correlating with the magnitude of serum SDH activity, differences between individual woodchucks were also observed for the hepatic expression of WHcAg and WHsAg (Table 2), for histological changes in liver (Table 2), for apoptosis of hepatocytes and hepatic cell proliferation (Table 3), and for hepatic inflammation by CD3+ cells and macrophages (Table 3). The termination of viral infection was associated with killing of (infected) hepatocytes and the subsequent regeneration of the liver via the proliferation of (uninfected) hepatocytes as described previously for HBV-infected chimpanzees and WHV-infected woodchucks (8, 15, 18, 21, 37, 45, 47, 51).
Comparisons of the above-described parameters demonstrated that acute liver injury, as determined by the peak in serum SDH activity, was more pronounced in woodchucks with higher levels of peak viremia than in woodchucks with lower levels of peak viremia. Serum SDH concentrations declined rapidly following the peak in enzyme activity and became normal thereafter, suggesting that the duration of acute liver injury is relatively short. This suggests further that woodchuck liver has a high potential to regenerate and that rapid regeneration may compensate for the severity of liver damage in resolving woodchucks. This assumption is supported by the results for the proliferation and apoptosis of hepatocytes (Table 3), with a much higher percentage of hepatocytes undergoing cell division than apoptosis at the peak of serum SDH activity. The above-described results indicate furthermore that the suppression of WHV replication in the liver is caused mainly by noncytotoxic rather than cytotoxic mechanisms as also described previously in other studies of resolving WHV infection in woodchucks (15, 21, 38, 47, 48). One possible explanation for the different course of acute, self-limited WHV infection in individual woodchucks may relate to the individual immune response genes leading to different antigen recognition on virus-infected hepatocytes and different regulation, level, and action of cytokine production. Inbred woodchucks are not available, and no studies that measure cytokine levels in blood during the peak of WHV viremia and acute liver injury have been undertaken so far.
The course of acute, self-limited WHV infection in adult woodchucks is generally comparable to that of HBV infection in adult humans and chimpanzees and remarkably variable in individuals from the three species (4, 37, 49). In patients with resolving HBV infection, viremia at the onset of clinical symptoms ranged between 103 and 108 genome equivalents/ml (4), which is similar to the level of WHV viremia observed at the onset of strong elevations in the SDH concentration (Fig. 1). Furthermore, the apparent half-life of HBV DNA during the elimination phase is 1.6 to 4 days (4, 50) and, thus, similar to the apparent WHV DNA half-life of 2 to 3 days. The apparent half-life of HBV or WHV measured in our and previous studies (4, 46) is the sum of eliminated and newly exported virus and does not reflect the true half-life due to elimination (33). The true half-life of HBV DNA was found to be much shorter (4 h) in chimpanzees with acute HBV infection (36). We do not know whether this also applies to acute WHV infection, because we have not quantitatively determined the intrahepatic WHV DNA concentration. Major differences in the course of WHV and HBV infection concern the appearance, concentration, and elimination of subviral particles from the peripheral blood. Ninety percent of all patients with acute resolving HBV infection had 10 to 100 μg/ml free HBsAg in the first available serum sample at the onset of disease symptoms (i.e., during a rather late stage of infection) (4), whereas only about half of the resolving woodchucks were WHsAg positive (>2 μg/ml) by serum samples obtained during the entire observation period (Fig. 1 and 4). However, the maximum WHsAg concentration in the sera of two woodchucks reached up to 400 μg/ml and was 4- to 40-fold higher than those in patients with 10 to 100 μg HBsAg per ml of serum (4, 11).
Free HBsAg appears to be more resistant to elimination from peripheral blood than does free WHsAg because subviral particles in sera of patients that eventually resolved were detectable over several weeks following inoculation and before acute hepatitis was observed (49). Using the same type of assay (i.e., electroimmunodiffusion) (11), the half-life time of HBsAg during acute hepatitis was determined to be 6 to 8 days (4), whereas the half-life time of free WHsAg was less than 1 day despite much higher antigen concentrations. Results of this study suggest that the rapid development of an antibody response against WHsAg is mainly responsible for the fast elimination of subviral particles from serum, as demonstrated by the appearance of large amounts of CICs around the peak in WHs antigenemia, followed by the appearance of free anti-WHs around the time of undetectable serum WHV DNA. The generation of CICs during or even before the “anticore window” (i.e., the time between the loss of free WHsAg and the appearance of anti-WHs, during which anti-WHc is the only detectable serological host response marker) suggests a more rapid elicitation of a humoral response during WHV infection in woodchucks than during HBV infection in patients in which anti-HBs become detectable mainly during the end stages of resolving infection and sometimes much later after complete reconvalescence (2, 3).
In summary, this study of woodchucks shows that the resolution of acute WHV infection involves a process with variable but appropriate virus-specific immune responses in the peripheral blood and liver. The rapid development of a humoral response against free WHsAg in form of CICs contributes to the early elimination of subviral and viral particles from the periphery. Marked reductions in levels of WHV from peak viremia begin before the peak in acute liver injury is detected by serum biochemical criteria. The eventual elimination of WHV DNA from the periphery is seen with the appearance of free anti-WHs and following the removal of WHV-infected hepatocytes from liver and replenishment with uninfected hepatocytes. Thus, the onset and magnitude of host control are important determinants for the eradication of WHV infection. An understanding of the kinetics of virological and immunological responses that are involved in the resolution of WHV infection will reveal the mechanisms for the persistence of WHV in chronic infection and thus facilitate the development of strategies for effective antiviral therapy against established chronic HBV infection and its sequelae.
Acknowledgments
This work was supported by grant SFB535/A2 from the German Research Foundation DFG to D.G. and W.H.G. and by contract number N01-AI-05399 from the National Institute of Allergy and Infectious Diseases to the College of Veterinary Medicine at Cornell University.
We gratefully acknowledge the expert assistance of Betty Baldwin, Lou Ann Graham, Erin Graham, David Dietterich, and Chris Bellezza of Cornell University. We thank Sigrun Broehl and Ulrike Wend from the Institute of Medical Virology, Justus Liebig University, for excellent technical assistance.
Footnotes
Published ahead of print on 3 December 2008.
REFERENCES
- 1.Bertoletti, A., and C. Ferrari. 2003. Kinetics of the immune response during HBV and HCV infection. Hepatology 384-13. [DOI] [PubMed] [Google Scholar]
- 2.Chisari, F. V. 2000. Rous-Whipple Award Lecture. Viruses, immunity, and cancer: lessons from hepatitis B. Am. J. Pathol. 1561117-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chisari, F. V., and C. Ferrari. 1995. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 1329-60. [DOI] [PubMed] [Google Scholar]
- 4.Chulanov, V. P., G. A. Shipulin, S. Schaefer, and W. H. Gerlich. 2003. Kinetics of HBV DNA and HBsAg in acute hepatitis B patients with and without coinfection by other hepatitis viruses. J. Med. Virol. 69313-323. [DOI] [PubMed] [Google Scholar]
- 5.Cote, P. J., and J. L. Gerin. 1995. In vitro activation of woodchuck lymphocytes measured by radiopurine incorporation and interleukin-2 production: implications for modeling immunity and therapy in hepatitis B virus infection. Hepatology 22687-699. [PubMed] [Google Scholar]
- 6.Cote, P. J., B. E. Korba, R. H. Miller, J. R. Jacob, B. H. Baldwin, W. E. Hornbuckle, R. H. Purcell, B. C. Tennant, and J. L. Gerin. 2000. Effects of age and viral determinants on chronicity as an outcome of experimental woodchuck hepatitis virus infection. Hepatology 31190-200. [DOI] [PubMed] [Google Scholar]
- 7.Cote, P. J., C. Roneker, K. Cass, F. Schodel, D. Peterson, B. Tennant, F. De Noronha, and J. Gerin. 1993. New enzyme immunoassays for the serologic detection of woodchuck hepatitis virus infection. Viral Immunol. 6161-169. [DOI] [PubMed] [Google Scholar]
- 8.Cote, P. J., I. Toshkov, C. Bellezza, M. Ascenzi, C. Roneker, L. A. Graham, B. H. Baldwin, K. Gaye, I. Nakamura, B. E. Korba, B. C. Tennant, and J. L. Gerin. 2000. Temporal pathogenesis of experimental neonatal woodchuck hepatitis virus infection: increased initial viral load and decreased severity of acute hepatitis during the development of chronic viral infection. Hepatology 32807-817. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari, C., A. Penna, A. Bertoletti, A. Valli, A. D. Antoni, T. Giuberti, A. Cavalli, M. A. Petit, and F. Fiaccadori. 1990. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J. Immunol. 1453442-3449. [PubMed] [Google Scholar]
- 10.Ganem, D. 1996. Hepadnaviridae: the virus and their replication, p. 2703-2737. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 11.Gerlich, W. H., U. Wend, and D. Glebe. 2004. Quantitative assay of hepatitis B surface antigen in serum or plasma using Laurell electrophoresis. Methods Mol. Med. 9557-63. [DOI] [PubMed] [Google Scholar]
- 12.Guidotti, L. G., K. Ando, M. V. Hobbs, T. Ishikawa, L. Runkel, R. D. Schreiber, and F. V. Chisari. 1994. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc. Natl. Acad. Sci. USA 913764-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 1965-91. [DOI] [PubMed] [Google Scholar]
- 14.Guidotti, L. G., R. Rochford, J. Chung, M. Shapiro, R. Purcell, and F. V. Chisari. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science 284825-829. [DOI] [PubMed] [Google Scholar]
- 15.Guo, J. T., H. Zhou, C. Liu, C. Aldrich, J. Saputelli, T. Whitaker, M. I. Barrasa, W. S. Mason, and C. Seeger. 2000. Apoptosis and regeneration of hepatocytes during recovery from transient hepadnavirus infections. J. Virol. 741495-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heermann, K. H., U. Goldmann, W. Schwartz, T. Seyffarth, H. Baumgarten, and W. H. Gerlich. 1984. Large surface proteins of hepatitis B virus containing the pre-s sequence. J. Virol. 52396-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heise, T., L. G. Guidotti, and F. V. Chisari. 2001. Characterization of nuclear RNases that cleave hepatitis B virus RNA near the La protein binding site. J. Virol. 756874-6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodgson, P. D., M. D. Grant, and T. I. Michalak. 1999. Perforin and Fas/Fas ligand-mediated cytotoxicity in acute and chronic woodchuck viral hepatitis. Clin. Exp. Immunol. 11863-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodgson, P. D., and T. I. Michalak. 2001. Augmented hepatic interferon gamma expression and T-cell influx characterize acute hepatitis progressing to recovery and residual lifelong virus persistence in experimental adult woodchuck hepatitis virus infection. Hepatology 341049-1059. [DOI] [PubMed] [Google Scholar]
- 20.Jung, M. C., H. M. Diepolder, U. Spengler, E. A. Wierenga, R. Zachoval, R. M. Hoffmann, D. Eichenlaub, G. Frosner, H. Will, and G. R. Pape. 1995. Activation of a heterogeneous hepatitis B (HB) core and e antigen-specific CD4+ T-cell population during seroconversion to anti-HBe and anti-HBs in hepatitis B virus infection. J. Virol. 693358-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kajino, K., A. R. Jilbert, J. Saputelli, C. E. Aldrich, J. Cullen, and W. S. Mason. 1994. Woodchuck hepatitis virus infections: very rapid recovery after a prolonged viremia and infection of virtually every hepatocyte. J. Virol. 685792-5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korba, B. E., P. Cote, W. Hornbuckle, B. C. Tennant, and J. L. Gerin. 2000. Treatment of chronic woodchuck hepatitis virus infection in the Eastern woodchuck (Marmota monax) with nucleoside analogues is predictive of therapy for chronic hepatitis B virus infection in humans. Hepatology 311165-1175. [DOI] [PubMed] [Google Scholar]
- 23.Korba, B. E., P. J. Cote, S. Menne, I. Toshkov, B. H. Baldwin, F. V. Wells, B. C. Tennant, and J. L. Gerin. 2004. Clevudine therapy with vaccine inhibits progression of chronic hepatitis and delays onset of hepatocellular carcinoma in chronic woodchuck hepatitis virus infection. Antivir. Ther. 9937-952. [PubMed] [Google Scholar]
- 24.Lohr, H. F., W. Weber, J. Schlaak, B. Goergen, K. H. M. zum Buschenfelde, and G. Gerken. 1995. Proliferative response of CD4+ T cells and hepatitis B virus clearance in chronic hepatitis with or without hepatitis B e-minus hepatitis B virus mutants. Hepatology 2261-68. [DOI] [PubMed] [Google Scholar]
- 25.Madalinski, K., B. Burczynska, K. H. Heermann, A. Uy, and W. H. Gerlich. 1991. Analysis of viral proteins in circulating immune complexes from chronic carriers of hepatitis B virus. Clin. Exp. Immunol. 84493-500. [PMC free article] [PubMed] [Google Scholar]
- 26.Marinos, G., F. Torre, S. Chokshi, M. Hussain, B. E. Clarke, D. J. Rowlands, A. L. Eddleston, N. V. Naoumov, and R. Williams. 1995. Induction of T-helper cell response to hepatitis B core antigen in chronic hepatitis B: a major factor in activation of the host immune response to the hepatitis B virus. Hepatology 221040-1049. [DOI] [PubMed] [Google Scholar]
- 27.Maruyama, T., A. McLachlan, S. Iino, K. Koike, K. Kurokawa, and D. R. Milich. 1993. The serology of chronic hepatitis B infection revisited. J. Clin. Investig. 912586-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maruyama, T., F. Schodel, S. Iino, K. Koike, K. Yasuda, D. Peterson, and D. R. Milich. 1994. Distinguishing between acute and symptomatic chronic hepatitis B virus infection. Gastroenterology 1061006-1015. [DOI] [PubMed] [Google Scholar]
- 29.Menne, S., and P. J. Cote. 2007. The woodchuck as an animal model for pathogenesis and therapy of chronic hepatitis B virus infection. World J. Gastroenterol. 13104-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menne, S., J. Maschke, M. Lu, H. Grosse-Wilde, and M. Roggendorf. 1998. T-cell response to woodchuck hepatitis virus (WHV) antigens during acute self-limited WHV infection and convalescence and after viral challenge. J. Virol. 726083-6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menne, S., J. Maschke, T. K. Tolle, M. Lu, and M. Roggendorf. 1997. Characterization of T-cell response to woodchuck hepatitis virus core protein and protection of woodchucks from infection by immunization with peptides containing a T-cell epitope. J. Virol. 7165-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menne, S., C. A. Roneker, M. Roggendorf, J. L. Gerin, P. J. Cote, and B. C. Tennant. 2002. Deficiencies in the acute-phase cell-mediated immune response to viral antigens are associated with development of chronic woodchuck hepatitis virus infection following neonatal inoculation. J. Virol. 761769-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menne, S., and B. C. Tennant. 1999. Unraveling hepatitis B virus infection of mice and men (and woodchucks and ducks). Nat. Med. 51125-1126. [DOI] [PubMed] [Google Scholar]
- 34.Michalak, T. I. 2000. Occult persistence and lymphotropism of hepadnaviral infection: insights from the woodchuck viral hepatitis model. Immunol. Rev. 17498-111. [DOI] [PubMed] [Google Scholar]
- 35.Milich, D. R., and A. McLachlan. 1986. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science 2341398-1401. [DOI] [PubMed] [Google Scholar]
- 36.Murray, J. M., R. H. Purcell, and S. F. Wieland. 2006. The half-life of hepatitis B virions. Hepatology 441117-1121. [DOI] [PubMed] [Google Scholar]
- 37.Murray, J. M., S. F. Wieland, R. H. Purcell, and F. V. Chisari. 2005. Dynamics of hepatitis B virus clearance in chimpanzees. Proc. Natl. Acad. Sci. USA 10217780-17785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura, I., J. T. Nupp, M. Cowlen, W. C. Hall, B. C. Tennant, J. L. Casey, J. L. Gerin, and P. J. Cote. 2001. Pathogenesis of experimental neonatal woodchuck hepatitis virus infection: chronicity as an outcome of infection is associated with a diminished acute hepatitis that is temporally deficient for the expression of interferon gamma and tumor necrosis factor-alpha messenger RNAs. Hepatology 33439-447. [DOI] [PubMed] [Google Scholar]
- 39.Peek, S. F., P. J. Cote, J. R. Jacob, I. A. Toshkov, W. E. Hornbuckle, B. H. Baldwin, F. V. Wells, C. K. Chu, J. L. Gerin, B. C. Tennant, and B. E. Korba. 2001. Antiviral activity of clevudine [L-FMAU, (1-(2-fluoro-5-methyl-beta, L-arabinofuranosyl) uracil)] against woodchuck hepatitis virus replication and gene expression in chronically infected woodchucks (Marmota monax). Hepatology 33254-266. [DOI] [PubMed] [Google Scholar]
- 40.Rehermann, B. 2000. Intrahepatic T cells in hepatitis B: viral control versus liver cell injury. J. Exp. Med. 1911263-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaefer, S., D. Glebe, U. C. Wend, J. Oyunbileg, and W. H. Gerlich. 2003. Universal primers for real-time amplification of DNA from all known Orthohepadnavirus species. J. Clin. Virol. 2730-37. [DOI] [PubMed] [Google Scholar]
- 42.Tennant, B. C., B. H. Baldwin, L. A. Graham, M. A. Ascenzi, W. E. Hornbuckle, P. H. Rowland, I. A. Tochkov, A. E. Yeager, H. N. Erb, J. M. Colacino, C. Lopez, J. A. Engelhardt, R. R. Bowsher, F. C. Richardson, W. Lewis, P. J. Cote, B. E. Korba, and J. L. Gerin. 1998. Antiviral activity and toxicity of fialuridine in the woodchuck model of hepatitis B virus infection. Hepatology 28179-191. [DOI] [PubMed] [Google Scholar]
- 43.Tennant, B. C., and J. L. Gerin. 2001. The woodchuck model of hepatitis B virus infection. Ilar. J. 4289-102. [DOI] [PubMed] [Google Scholar]
- 44.Tennant, B. C., I. A. Toshkov, S. F. Peek, J. R. Jacob, S. Menne, W. E. Hornbuckle, R. D. Schinazi, B. E. Korba, P. J. Cote, and J. L. Gerin. 2004. Hepatocellular carcinoma in the woodchuck model of hepatitis B virus infection. Gastroenterology 127S283-S293. [DOI] [PubMed] [Google Scholar]
- 45.Thimme, R., S. Wieland, C. Steiger, J. Ghrayeb, K. A. Reimann, R. H. Purcell, and F. V. Chisari. 2003. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 7768-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tolle, T. K., D. Glebe, M. Linder, D. Linder, S. Schmitt, R. Geyer, and W. H. Gerlich. 1998. Structure and glycosylation patterns of surface proteins from woodchuck hepatitis virus. J. Virol. 729978-9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, Y., S. Menne, B. H. Baldwin, B. C. Tennant, J. L. Gerin, and P. J. Cote. 2004. Kinetics of viremia and acute liver injury in relation to outcome of neonatal woodchuck hepatitis virus infection. J. Med. Virol. 72406-415. [DOI] [PubMed] [Google Scholar]
- 48.Wang, Y., S. Menne, J. R. Jacob, B. C. Tennant, J. L. Gerin, and P. J. Cote. 2003. Role of type 1 versus type 2 immune responses in liver during the onset of chronic woodchuck hepatitis virus infection. Hepatology 37771-780. [DOI] [PubMed] [Google Scholar]
- 49.Webster, G. J., S. Reignat, M. K. Maini, S. A. Whalley, G. S. Ogg, A. King, D. Brown, P. L. Amlot, R. Williams, D. Vergani, G. M. Dusheiko, and A. Bertoletti. 2000. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology 321117-1124. [DOI] [PubMed] [Google Scholar]
- 50.Whalley, S. A., J. M. Murray, D. Brown, G. J. Webster, V. C. Emery, G. M. Dusheiko, and A. S. Perelson. 2001. Kinetics of acute hepatitis B virus infection in humans. J. Exp. Med. 193847-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wieland, S. F., H. C. Spangenberg, R. Thimme, R. H. Purcell, and F. V. Chisari. 2004. Expansion and contraction of the hepatitis B virus transcriptional template in infected chimpanzees. Proc. Natl. Acad. Sci. USA 1012129-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]