Abstract
To address the initiation of virus infection in the respiratory tract, we established two culture systems for differentiated bovine airway epithelial cells (BAEC). Filter-grown BAEC differentiated under air-liquid interface (ALI) conditions to generate a pseudo-stratified mucociliary epithelium. Alternatively, precision-cut lung slices (PCLS) from the bovine airways were generated that retained the original composition and distribution of differentiated epithelial cells. With both systems, epithelial cells were readily infected by bovine parainfluenza virus 3 (BPIV3). Ciliated cells were the most prominent cell type affected by BPIV3. Surprisingly, differentiated BAEC were resistant to infection by bovine respiratory syncytial virus (BRSV), when the virus was applied at the same multiplicity of infection that was sufficient for infection by BPIV3. In the case of PCLS, infection by BRSV was observed in cells located in lower cell layers but not in epithelial cells facing the lumen of the airways. The identity of the infected cells could not be determined because of a lack of specific antibodies. Increasing the virus titer 30-fold resulted in infection of the ALI cultures of BAEC, whereas in PCLS the ciliated epithelium was still refractory to infection by BRSV. These results indicate that differentiated BAEC are readily infected by BPIV3 but rather resistant to infection by BRSV. Disease caused by BRSV may require that calves encounter environmental stimuli that render BAEC susceptible to infection.
Immortalized cell lines have been and still are an invaluable tool in the elucidation of the replication cycle of animal viruses. However, the natural target cells of viruses often are differentiated cells, which have characteristic features that are absent from immortalized cells. Therefore, not all aspects of the viral pathogenesis can be addressed by experimental infection of continuous cell lines. In recent years, well-differentiated epithelial cells derived from airway tissue have been used to analyze respiratory virus infections. When cultured under air-liquid interface (ALI) conditions, these cells grow to establish a multilayered, polarized, and differentiated tissue culture that closely resembles the airway epithelium in vivo with regard to morphology and functions, including mucus production and ciliary motion (6). They have been applied, for example, to study infection by human respiratory syncytial virus (HRSV) and human parainfluenza virus 3 (HPIV3). In both cases, only the ciliated epithelial cells were found to be sensitive to infection (21, 22).
RSV and PIV3 are classified in different genera, Pneumovirus and Respirovirus, and subfamilies, Paramyxovirinae and Pneumovirinae, within the family Paramyxoviridae, order Mononegavirales. HRSV and HPIV3 are the most important viral agents responsible for serious pediatric respiratory disease worldwide. By the age of 2 years, the majority of children have been infected by these viruses. In severe cases, infection results in bronchiolitis and pneumonia. Reinfections are common, although subsequent infections are partially restricted, and the disease severity is reduced. For both viruses, there are bovine counterparts, BRSV and BPIV3, that cause similar diseases in calves (3, 12, 17).
RSV and PIV3 are enveloped viruses that contain surface glycoproteins for the interaction with target cells. Primary attachment of RSV is mediated by the G protein, which recognizes heparinlike glycosaminoglycans (7). Internalization of the viral genome occurs by fusion of the viral envelope with the plasma membrane of the host cell. This process is mediated by the fusion protein F, which has to be cleaved into the subunits F1 and F2 to become fusion active. As reported for several other paramyxoviruses, the proteolytic activation of the F protein is accomplished by the action of cellular proteases, furin or furinlike enzymes. A unique feature of RSV is that the F protein contains two furin cleavage sites (5, 23). Therefore, proteolytic activation of the F protein results not only in the generation of the subunits F1 and F2 but also in the release of a small peptide, which in the case of BRSV has been shown to be converted into a bioactive peptide (24). Attachment of PIV3 to host cells is mediated by the HN protein, which has a sialic acid-binding activity for the interaction with sialylated cell surface molecules (10). The neuraminidase activity of the HN protein resembles that of the NA protein of influenza viruses and may facilitate the spread of infection. Internalization of the PIV3 genome is mediated by the F protein, which induces the fusion of the viral and the cellular membrane. Virus entry may require—in addition to sialic acid-containing receptors—the interaction of F with nucleolin (2). Similar to RSV, the F protein of PIV3 is fusion active only after generation of the subunits F1 and F2 by proteolytic cleavage (9).
To analyze the infection of the target cells of BRSV and BPIV3, we established ALI cultures for bovine airway epithelial cells (BAEC). Furthermore, we prepared precision-cut lung slices (PCLS), which contain the well-differentiated respiratory epithelial cells in the original setting. These culture systems have been applied for the first time to analyze infection by bovine respiratory viruses. Cells of both cultures were readily infected by BPIV3, whereas they were rather refractory to infection by BRSV. Our results suggest that these two viruses have developed different strategies to infect their host.
(Part of this work was performed by K.G. in partial fulfillment of the requirements for a Ph.D. from Tieraerztliche Hochschule Hannover, Hannover, Germany.)
MATERIALS AND METHODS
Culture of continuous cell lines.
MDBK cells (Madin-Darby bovine kidney cells; kindly provided by Wolfgang Garten, Philipps-Universität Marburg, Marburg, Germany), as well as Vero cells (African green monkey cells; ATCC, CCL-81), were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with either 10 or 5% fetal bovine serum (FBS). HBE cells (human bronchial epithelial cells, 16HBE14o−) were kindly provided by D. C. Gruenert (University of Vermont). For cultivation, the cells were routinely passaged in a mixture of DMEM and Ham F-12 (1:1) containing 5% FBS. Cell cultures were seeded on filter supports (2.5 × 105 cells) and incubated at 37°C in a humidified atmosphere containing 5% CO2, with medium in the upper and lower chamber. KOP-R cells (bovine oropharynx tissue, RIE 244; Friedrich-Loeffler-Institut, Insel Riems, Germany) were incubated in 75-cm2 culture flasks under same conditions in Eagle minimum essential medium supplemented with 10% FBS.
ALI cultures.
Primary BAEC were obtained from bovine bronchi of 6- to 8-month-old calves, and epithelial cells were isolated as described by Bals et al. (1). After BAEC had grown in tissue culture flasks with supplemented airway epithelial growth medium (Promocell, 10% FBS) to 70 to 80% confluence, cells were seeded on collagen type I-coated, semipermeable membrane supports (Greiner, 24-well, 0.4-μm pore size) at a density of 2.5 × 105 cells per filter support. Well-differentiated BAEC cultures were established by using ALI conditions. Briefly, after BAEC had become confluent, the airway epithelial growth medium of the apical chamber was removed, and the medium of the basal chamber was replaced by ALI medium (DMEM and Ham F-12 at a ratio of 1:1, including antibiotics and antimycotics) supplemented with 2% Ultroser G and retinoic acid (15 ng/ml). Culturing the cells under ALI conditions for at least 2 to 3 weeks resulted in differentiated epithelial cells resembling a pseudostratified mucociliary epithelium. The electrical resistance was determined with a voltohmmeter (Millipore) according to the manufacturer's instructions.
PCLS.
PCLS were obtained from the lungs of cattle of different ages and prepared after having filled the lobus accessorius with low-melting-point agarose (agarose LM GQT; GERBU, Gaiberg, Germany) as previously described (18). After stamping cylindrical parts out of the tissue (8-mm tissue coring tool), tissue slices 400 to 500 μm thick were produced by using a Krumdieck tissue slicer MD 4000-01 with a cycle speed of 60 slices/min. Each PCLS was incubated in 1 ml of RPMI 1640 medium (Invitrogen/Gibco, Germany) in 24-well plates at 37°C and 5% CO2. To remove the agarose, medium was changed every half hour during the first 4 h and once after 24 h.
The viability of the epithelial cells was verified by screening for the ciliary activity using a light microscope (Zeiss Axiovert 35) equipped with an ORCA C4742-80 digital camera (Hamamatsu) and SIMPLE-PCI analysis software (Compix Imaging Systems). In selected samples, the slices were analyzed for reversible bronchoconstriction after the addition or removal of methacholine, respectively, as described previously (18). The viability of the cells was also determined by using a Live/Dead viability/cytotoxicity assay kit (Fluo Probes, FP-BE4710). For this purpose, the slices were washed with phosphate-buffered saline (PBS) and incubated with Calcein AM (1 μM) and EthD-1 (2 μM) for 30 min. After the removal of the incubation solution and a further washing step with PBS, the slices were embedded in Mowiol resin and visualized by using a Leica DM IRB2 confocal laser scanning microscope.
Virus.
For the construction of recombinant BRSV expressing green fluorescent protein (BRSV-GFP), a cloning cassette containing the open reading frame (ORF) of GFP was generated via PCR. The GFP cassette was inserted into a NotI restriction site into the BRSV antigenome (BRSV ATue51908; kindly provided by Karl-Klaus Conzelmann, Max-von-Pettenkofer-Institut, Munich, Germany) upstream of the NS1 gene at the 5′-terminal end. The correct sequences of the GFP ORF and the cloning site of the virus antigenome were confirmed by sequencing. Primer sequences are available upon request. Stocks of BRSV-GFP were prepared using MDBK cells. For this purpose, the cells were seeded into culture 75-cm2 flasks and incubated for 1 day at 37°C and 5% CO2 till they were nearly confluent. The cells were washed with PBS and inoculated with BRSV-GFP (multiplicity of infection [MOI] = 0.1) for 2.5 h at 37°C and 5% CO2, followed by the addition of DMEM and incubation for 6 to 7 days, until a distinct cytopathogenic effect was visible.
The Snook strain of BRSV (14) was prepared in fetal calf kidney cells inoculated at an MOI of 0.01 with virus in bronchoalveolar lavage from a gnotobiotic calf inoculated 6 days previously with virus that had been passaged on two previous occasions in the lungs of gnotobiotic calves (15). The bronchoalveolar lavage was shown to be free from contamination with bovine viral diarrhea virus, as determined by immunofluorescence after staining of acetone-fixed, virus-infected fetal calf kidney cells with a polyclonal anti-bovine viral diarrhea virus serum and fluorescein isothiocyanate (FITC) anti-bovine immunoglobulin G, and free from mycoplasmas, as determined by staining with Hoechst reagent (Sigma, Gillingham, United Kingdom).
BPIV3 was obtained from the Friedrich-Loeffler-Institut (Insel Riems, Germany) and grown on KOP-R cells. After growth in culture flasks till 80% confluence, the cells were infected at an MOI of 0.1 for 2 h. After 3 to 4 days of incubation at 37°C and 5% CO2, 50 to 60% of the cells showed a cytopathogenic effect.
Virus-containing supernatants of MDBK and KOP-R cells, respectively, were collected and centrifuged at 2,000 × g for 15 min to remove cell debris. Virus stocks were frozen in liquid nitrogen and stored at −80°C. For infection frozen aliquots of either virus were thawed and diluted with DMEM.
Virus infection.
Cultures of continuous cells were washed three times with PBS prior to infection at an MOI of 0.1. With filter-grown cells, virus suspensions were administered to the upper chamber for 2 h at 37°C. The viral inoculum was replaced by 150 μl of DMEM, and the cells were further incubated for 2 days, followed by fixation with 3% paraformaldehyde.
Prior to infection of BAEC, the cells were washed thoroughly with PBS to remove secreted mucus from the apical surface. For infection, 150 μl of viral suspension were applied to the apical compartment for 2 to 3 h (37°C). After removal of the inoculum the cultures were incubated for 2 or 3 days under ALI conditions. Staining was performed after fixation with 3% paraformaldehyde.
PCLS were washed with medium and infected with 300 μl of viral suspension. At 2 h postinfection, medium was added to a final volume of 1 ml. The next day the medium was changed, and slices were incubated for 2 to 5 days at 37°C, followed by fixation with 3% paraformaldehyde.
Immunostaining.
For permeabilization, cells were treated with 0.2% Triton X-100. Permeabilized and nonpermeabilized cells were subjected to immunostaining by sequential incubation with the respective antibodies; in the case of filter cultures, the reagents were added to the apical filter chamber.
As an epithelial cell marker, a monoclonal antibody against human cytokeratin (DakoCytomation) was used followed by an appropriate FITC-labeled second antibody. For cilium staining, cells were treated with a Cy3-labeled monoclonal antibody recognizing β-tubulin (Sigma). The goblet cells were stained indirectly by using the mucin-5AC antibody (gastric; Acris), followed by a FITC-labeled secondary antibody.
BPIV3-infected cells were visualized using a polyclonal antiviral antiserum (VMRD, caprine origin) and an FITC-labeled secondary antibody.
Cell nuclei were stained by incubation with DAPI (4′,6′-diamidino-2-phenylindole). For this purpose, DAPI was added to the apical surface of the cells and removed after incubation for 15 min (37°C), before the cells were washed three times with PBS.
Photomicrographs of immunostained and GFP-expressing cells were obtained by using the Leica DM IRB2 laser scanning microscope, an inverted microscope connected to a TCS SP2 AOBS scanhead, and the imaging software Imaris 6.0.
RESULTS
Infection of immortalized cells by BRSV and BPI3.
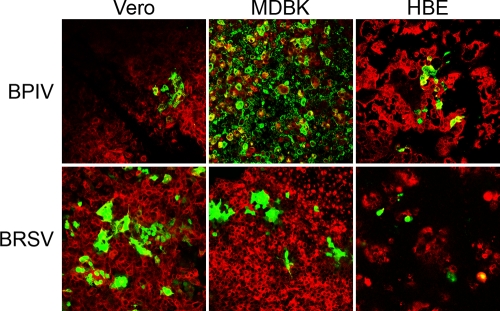
Prior to infection of primary respiratory epithelial cells, we determined the sensitivity of continuous cell lines to infection by BRSV-GFP and BPIV3. Cells derived from different species (Vero, MDBK, and HBE cells) were infected at an MOI of 0.1 and analyzed at 48 h postinfection for the presence of infected cells by fluorescence microscopy. As shown in Fig. 1, simian, bovine, and human cells were sensitive to infection by either virus. BPIV3 was most efficient in the infection of MDBK cells. In contrast, BRSV-GFP-infected Vero cells and MDBK cells with equal efficiency and induced the formation of syncytia. Applying the BRSV-GFP inoculum to HBE cells resulted in a lower number of infected cells and in no syncytium formation.
FIG. 1.
Infection of continuous cell lines (Vero, MDBK, and HBE) by BPIV3 and BRSV. Confluent cells grown on collagenized filter supports were inoculated with virus at an MOI of 0.1 from the apical side. At 2 dpi, cultures were fixed and stained with an antibody for β-tubulin (red). Virus-infected cells are visualized by the expression of GFP (green).
Preparation and infection of well-differentiated epithelial cells derived from the bovine airway.
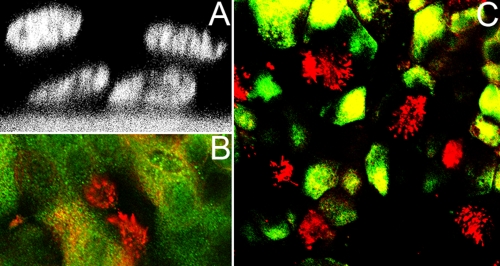
Since respiratory epithelial cells have been reported to be the primary target cells of BRSV and BPIV3, we established an ALI system for culturing well-differentiated BAEC. The cells were seeded on collagenized semipermeable membrane supports and cultivated to complete confluence. When the medium was removed from the apical filter chamber and cells were kept under ALI conditions for more than 2 weeks, BAEC differentiated into a pseudo-stratified respiratory epithelium composed of different cell types. Figure 2A shows the characteristic distribution of nuclei within the layer of epithelial cells. Mucociliary activity started usually on day 10 and was associated with cilium development and the appearance of mucus-secreting cells. The epithelial character of the cells was verified by cytokeratin staining (Fig. 2C). Among the cells facing the lumen there were both ciliated cells, as identified by a Cy3-labeled antibody directed against β-tubulin (Fig. 2C), and cells covered by mucus, as determined by mucin-5AC staining (Fig. 2B). These well-differentiated ALI cultures maintained the described properties for up to 6 weeks. When the cells had become confluent on filters, they had developed a high electrical resistance of ∼2.9 kΩcm2. This value decreased somewhat after the application of ALI conditions and then remained in the range of 1.5 to 2.0 kΩcm2 for more than 2 weeks.
FIG. 2.
ALI cultures of BAEC. (A) Pseudostratified epithelium of BAEC grown for 2 weeks under ALI conditions and visualized by DAPI staining and confocal laser scanning microscopy. (B) Mucociliary differentiation of 2-week-old ALI cultures of BAEC. Cilia were stained by an antibody recognizing β-tubulin (red). Mucus-producing cells were detected by an antibody specific for mucin-5AC. (C) Distribution of ciliated cells within the respiratory epithelium. At 2 weeks after ALI culturing, BAEC were stained for cytoceratin (green), an epithelial cell marker, and for β-tubulin to detect cilia (red).
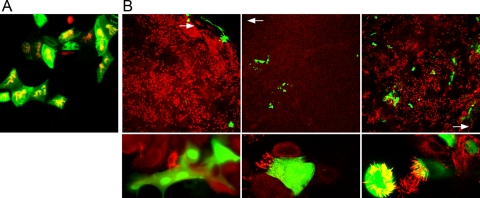
To investigate the infection by BPIV3 and to identify primary target cells within the respiratory epithelium, we infected well-differentiated BAEC from the apical surface. For this purpose, we inoculated 2- to 6-week-old ALI cultures of BAEC with BPIV3 and fixed the cells at 2 days postinfection (dpi). After double immunostaining of the cultures for the presence of cilia and virus antigen, infected cells were visualized by fluorescence microscopy. As shown in Fig. 3A, infection by BPIV3 predominantly involved ciliated cells. In this culture system, viral spread beyond ciliated cells, for instance to mucus-producing cells, was only detected rarely. These data suggest that ciliated cells of the respiratory epithelium are the primary target cells for BPIV3.
FIG. 3.
Well-differentiated BAEC infected by BPIV3 (A) or BRSV (B). (A) BPIV3 was applied to the apical surface at an MOI of 0.1 for 2 h. At 2 dpi, cultures were fixed and cilia were visualized by staining for β-tubulin (red). Virus-infected cells were detected by immunostaining (green). (B) BRSV infection of pseudostratified epithelia of BAEC. Infected cells were fixed 3 (left and right panels) and 10 (middle panels) dpi, respectively, and stained with an antibody recognizing β-tubulin (red). Virus-infected cells were visualized by GFP expression (green). BRSV applied at an MOI of 0.1 (left and middle panels) predominantly infected cells in the border area of the filter, where differentiation was less developed (white arrow, top panels). The infected cells were not ciliated (bottom panels, higher magnification). When virus was applied at an MOI of 3.5 (right panels), BRSV-infected well-differentiated BAEC cells were distributed all over the filter (top panel, lower magnification), including ciliated cells (bottom panel, higher magnification).
BRSV infection of well-differentiated BAEC.
In spite of the species-specific infection of the respective host, HRSV and BRSV show no difference in the infection of continuous cell cultures. To analyze the species specificity of BRSV infection, we established the ALI system for BAEC. Differentiated BAEC were infected by BRSV-GFP at an MOI of 0.1 that was also used in the experiments described above. After an incubation of 3 h, the cells were cultured for 3 days under ALI conditions and then subjected to an analysis by confocal fluorescence microscopy. Infected cells were visualized by BRSV-mediated GFP-expression; ciliated cells were detected using antibodies directed against β-tubulin (Fig. 3B, left panels). Under these conditions, very few GFP-expressing cells were observed, and these cells were predominantly found in the border area of the filter, where differentiation of the cells was less developed (Fig. 3B, upper left panel). Furthermore, BRSV infection was detectable in nonciliated cells exclusively (Fig. 3B, lower left panel). If the incubation time was extended to 10 days, infection was still restricted to few cells (Fig. 3B, upper middle panel) which were not ciliated (Fig. 3B, lower middle panel). Occasionally, syncytium formation was detected. These results indicate that BRSV is not able to establish an infection in well-differentiated BAEC cells when applied at an MOI of 0.1.
To investigate BRSV infection at a higher multiplicity, we inoculated the apical surface of the cells with BRSV-GFP at an MOI of 3.5 and analyzed the cells at 3 dpi for GFP expression. In this case, the GFP-expressing cells were distributed all over the pseudostratified epithelium (Fig. 3B, upper right panel). Unlike the infection at a low MOI, the appearance of GFP-expressing cells was not restricted to the border area of the filter. Under these conditions, ciliated cells were sensitive to infection (Fig. 3B, lower right panel). These observations provide evidence that BRSV is able to infect well-differentiated BAEC, but only when applied at a higher MOI than that required for BPIV3 infection. As discussed below, the resistance to infection cannot be explained by restriction of the entry site to the basolateral plasma membrane.
Analysis of PCLS.
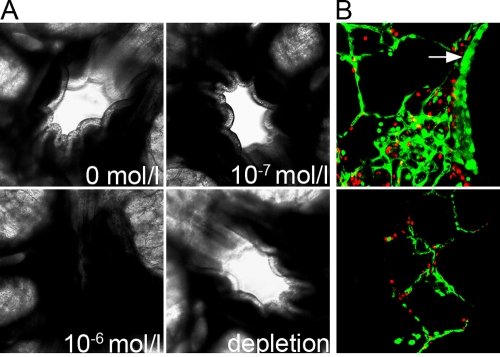
To confirm the results obtained with the ALI cultures, we established another model to investigate the infection of BAEC by BPIV3 and BRSV. For this purpose, PCLS were prepared from bovine lungs. Using this culture system, it is possible to investigate BAEC in the original tissue organization. The viability of the PCLS was determined by different criteria. During the whole experiment, the ciliary activity of the bronchial epithelium was detectable upon microscopic inspection. Although this criterion of viability is reported to be a reliable parameter (4, 20), two other methods were applied to verify that the cells of the PCLS functioned properly. Addition of methacholine (10−6 mol/liter) induced bronchoconstriction (Fig. 4A). This effect was dose dependent; no bronchoconstriction was observed at concentrations of 10−7 mol/liter or lower. Removal of the pharmacological agent resulted in a reversion of the contraction (Fig. 4A, lower right panel). In addition, we applied a staining procedure to visualize live and dead cells. Slices that were tested by this method showed that cells of the respiratory epithelium, of neighboring tissues and of the alveoli were vital at 24 h after preparation (Fig. 4B). Some dead cells were detected in the lower cell layers. The high vitality of the cells decreased only slowly, so that 6 days after preparation, the PCLS still were in adequate condition and appropriate for infection studies.
FIG. 4.
Vitality of PCLS 1 day after preparation. (A) Bronchoconstriction. PCLS prepared from the bronchioli and cultured for 1 day were treated with different concentrations of methacholine. The addition of methacholine at a concentration of 10−6 mol/liter induced bronchoconstriction within seconds (lower left panel). The removal of methacholine resulted in a reversion of this effect (lower right panel). (B) Live/dead viability assay. Live cells are shown in green; dead cells are shown in red. The cells of the respiratory epithelium facing the airway are indicated by a white arrow (top panel). The bottom panel shows alveoli.
Virus infection of PCLS.
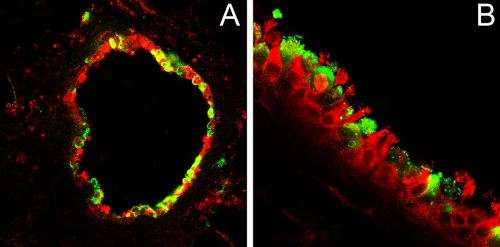
To analyze whether PCLS are suitable for infection, we inoculated them with BPIV3 (105 PFU/ml). At 48 h postinfection, microscopic investigation of infected slices revealed some destruction of the respiratory epithelium, as indicated by detached cells released into the lumen of the bronchioli. This was not the case in uninfected PCLS, indicating that BPIV3 infection has a cytopathogenic effect. By day 5 postinfection, the epithelium was completely destroyed. When the slices were stained for virus antigen and β-tubulin, cells of the respiratory epithelium derived from bronchioli were found to be the main site of infection (Fig. 5), although infected cells were also present in other areas of the lung, e.g., the alveoli or around blood vessels. Taken together, these findings indicate that ciliated respiratory epithelial cells are target cells for infection by BPIV3, which results in a severe cytopathogenic effect.
FIG. 5.
BPIV3 infection of PCLS. At 2 dpi, the slices were stained for virus antigen (green) and β-tubulin (red). Pictures were taken at lower (A) and higher (B) magnifications.
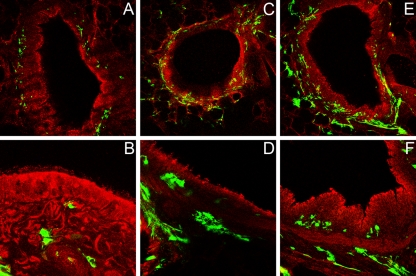
PCLS were also analyzed as to whether they are sensitive to infection by BRSV. Slices were infected with BRSV-GFP, and at 2, 5, or 10 dpi the cells were prepared for confocal fluorescence microscopy. Cells were stained using an antibody directed against β-tubulin; virus-infected cells were detected by determining GFP expression. As shown in Fig. 6A, when PCLS from bronchioli were infected with 105 PFU/ml, no BRSV-mediated GFP expression was detectable in the ciliated epithelium at 2 days after inoculation. BRSV-infected cells were present only in lower cell layers. When the incubation time was extended to 5 dpi, virus infection spread to neighboring cells of the connective tissue but not to the ciliated epithelial cells (Fig. 6B). At 10 dpi, infection had further spread (Fig. 6C), but there was still no GFP expression in the ciliated epithelium (Fig. 6D). In slices obtained from alveoli, infection was less prominent compared to PCLS from bronchioli (data not shown). When the titer of the inoculum was increased 100-fold (107 PFU/ml) and PCLS were analyzed at 2 dpi (Fig. 6E) or 5 dpi (Fig. 6F), a similar picture was obtained, with infected cells being present in lower cell layers but not in epithelial cells facing the lumen of the airways. These observations are in agreement with the findings of BRSV infection in ALI cultures of BAEC. In contrast to infection by BPIV3, BAEC appear to be rather insensitive to infection by BRSV.
FIG. 6.
BRSV infection of PCLS. One day after preparation, PCLS were inoculated with BRSV-GFP. Infected cells are shown in green. For comparison, cells were stained for β-tubulin (red). PCLS infected with 105 PFU/ml were stained at 2 (A), 5 (B) or 10 (C and D) dpi. PCLS infected with 107 PFU/ml were stained at 2 (E) or 5 (F) dpi. For clarity, the images in panels B, D, and F are shown at a higher magnification.
DISCUSSION
In this study, we have reported for the first time the establishment of cultures of differentiated BAEC to analyze the infection by respiratory viruses. We focused on two important etiological agents of respiratory disease in cattle, BRSV and BPIV3. The human counterparts of these viruses, HRSV and HPIV3, have been analyzed in ALI cultures of human airway epithelial cells and found to preferentially infect ciliated cells. A similar result was obtained in the present study for BPIV3, whereas BRSV was found to be rather inefficient in infecting differentiated BAEC. In ALI cultures, ciliated epithelial cells were sensitive to BRSV only when the virus was applied at an MOI of 3.5; on the other hand, BPIV3 was able to initiate infection even at an MOI as low as 0.1. The resistance of the ciliated epithelial cells to BRSV infection was also observed when PCLS were analyzed. Cells facing the lumen of the airways remained uninfected even when the virus inoculum contained a titer of 107 PFU/ml. Infected cells were detected only in lower cell layers. Infection of these cells is probably the result of direct inoculation of the tissue bypassing the epithelium. Upon longer incubation, infection spread to neighboring cells but never reached the surface of the epithelium. The identity of the infected cells was not determined because of a lack of specific antibodies. The morphological appearance of the infected cells suggests that at least some of them may be dendritic cells. The finding that the surface layer of the respiratory epithelium is resistant to BRSV infection was surprising as these cells have been reported to be major target cells for BRSV (17). To explain this unexpected result, we considered different possibilities. Since BRSV-GFP is an attenuated virus that does not cause disease symptoms in calves, we also applied the virulent Snook strain that is used for animal experiments (16). This virus had only been passed once in tissues culture from calf lung wash material and, therefore, will not be as adapted to tissue cell culture as the BRSV-GFP virus. However, BAEC were as refractory to the Snook strain of BRSV as they were to BRSV-GFP (data not shown). The same was true when the parental virus of BRSV-GFP (without the ORF of GFP) was used (data not shown). Our experiments were started with cells from adult animals. However, switching to the lung cells from calves that were about 6 months old did not result in different results. Therefore, the resistance of the differentiated epithelial cells to BRSV infection appears not to be an age-dependent property of BAEC.
The inability of BRSV to infect BAEC may be related to the availability of appropriate receptors on the cell surface. Although glycosaminoglycans are sufficient for attachment of RSV to the cell surface, virus entry is assumed to require the interaction with a specific protein receptor (13). This protein appears to be a common protein because many cell lines from different species are sensitive to infection by both BRSV and HRSV. However, the infection of differentiated respiratory epithelial cells has been reported to be species specific (11). In fact, it has been shown that the F2 subunit of the viral fusion protein is the major determinant for this host cell specificity. In a comparative analysis of different recombinant viruses, only chimeric viruses that contained the F2 protein of HRSV were able to infect differentiated human respiratory epithelial cells (11). The fact that cultures of many continuous cells—even from different species—are sensitive to BRSV infection while differentiated airway epithelial cells are refractory even to the homologous virus may be explained by a different level of surface expression of the receptor required for virus entry. The tight packing of the cells in a differentiated epithelium may allow virus infection only when the number of receptors exceeds a certain threshold value that is higher than in the case of immortalized cells which grow to a less densely packed monolayer. An alternative explanation is that the receptor is downregulated during the differentiation process of BAEC. Either of these two possibilities is consistent with our finding that in ALI cultures BRSV infection was detected in the border area of the filter where cells are not yet fully differentiated. In the case of adenoviruses and measles virus, the resistance of airway epithelial cells to apical infection has been attributed to the fact that the cellular receptor is located on the basolateral membrane (8, 19). However, it has been shown for HRSV that infection of HAEC occurs predominantly via the apical membrane (22). Furthermore, in PCLS, we observed the spread of BRSV infection in deeper cell layers of the epithelium, but the virus appeared not to be able to infect the surface layers via the basolateral membrane.
This explanation of the resistance of BAEC to BRSV infection by downregulation of the cellular receptor raises the possibility that the receptor can be upregulated by environmental stimuli. Such inductors of upregulation might be stress conditions or coinfection by other microorganisms. This concept provides an intriguing explanation of the BRSV infection in cattle. Like BPIV3 and bovine herpesvirus 1, BRSV is one of the primary pathogens within the bovine respiratory disease complex. Bacterial coinfections contribute to the severity of disease. Environmental factors, e.g., housing climate and transport, are also involved in the development of the disease. Therefore, the bovine respiratory disease complex is designated a factorial disease. If either of the different factors mentioned above results in upregulation of the cellular receptor for BRSV in the respiratory epithelial cells, it would explain why BRSV infection of cattle is associated with infection of airway epithelial cells, whereas cells in the intact epithelium are rather resistant to infection. For a better understanding of the pathogenesis of the BRSV infection of cattle, it is therefore important to direct future efforts to the elucidation of the cellular receptor for BRSV.
Our results obtained with ALI cultures infected by BRSV appear to be different from those reported for HRSV. However, it should be noted that BRSV infection of cultured cells usually yields amounts of virus in the supernatant that are at best in the range of 105 to 106 PFU/ml. Such virus titers are not sufficient for infection of ALI cultures of BAEC. HRSV can be grown to tenfold-higher titers. This difference may explain why in a previous report it was found that ciliated HAEC are sensitive to HRSV. It should be noted that in that study virus was applied at an MOI of 20, which resulted in an infection rate ranging from 30 to 70% of the cells (22). Thus, it is possible that HAEC also differ in their sensitivity to infection by HRSV and HPIV3, as we have found for BRSV and BPIV3. In this case, the possibility has to be considered that children with an upregulated receptor are most endangered by HRSV.
Acknowledgments
We are grateful to Klaus Conzelmann for providing recombinant BRSV.
Financial support was provided by a grant from the Deutsche Forschungsgemeinschaft (SFB587, TPA1).
Footnotes
Published ahead of print on 3 December 2008.
REFERENCES
- 1.Bals, R., C. Beisswenger, S. Blouquit, and T. Chinet. 2004. Isolation and air-liquid interface culture of human large airway and bronchiolar epithelial cells. J. Cyst. Fibros. 249-51. [DOI] [PubMed] [Google Scholar]
- 2.Bose, S., M. Basu, and A. K. Banerjee. 2004. Role of nucleolin in human parainfluenza virus type 3 infection of human lung epithelial cells. J. Virol. 788146-8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chanock, R. M., B. R. Murphy, and P. L. Collins. 2001. Parainfluenza viruses, p. 1341-1380. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott/The Williams & Wilkins Co., Philadelphia, PA. [Google Scholar]
- 4.Ebsen, M., G. Mogilevski, O. Anhenn, V. Maiworm, D. Theegarten, J. Schwarze, and K. Morgenroth. 2002. Infection of murine precision cut lung slices (PCLS) with respiratory syncytial virus (RSV) and Chlamydophila pneumoniae using the Krumdieck technique. Pathol. Res. Pract. 198747-753. [DOI] [PubMed] [Google Scholar]
- 5.González-Reyes, L., M. B. Ruiz-Argüello, B. Garcia-Barreno, L. Calder, J. A. López, J. P. Albar, J. J. Skehel, D. C. Wiley, and J. A. Melero. 2001. Cleavage of the human respiratory syncytial virus fusion protein at two distinct sites is required for activation of membrane fusion. Proc. Natl. Acad. Sci. USA 989859-9864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray, T. E., K. Guzman, C. W. Davis, L. H. Abdullah, and P. Nettesheim. 1996. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 14104-112. [DOI] [PubMed] [Google Scholar]
- 7.Krusat, T., and H. J. Streckert. 1997. Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells. Arch. Virol. 1421247-1254. [DOI] [PubMed] [Google Scholar]
- 8.Leonard, V. H., P. L. Sinn, G. Hodge, T. Miest, P. Devaux, N. Oezguen, W. Braun, P. B. McCray, Jr., M. B. McChesney, and R. Cattaneo. 2008. Measles virus blind to its epithelial cell receptor remains virulent in rhesus monkeys but cannot cross the airway epithelium and is not shed. J. Clin. Investig. 1182448-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moscona, A., and R. W. Peluso. 1992. Fusion properties of cells infected with human parainfluenza virus type 3: receptor requirements for viral spread and virus-mediated membrane fusion. J. Virol. 666280-6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheid, A., L. A. Caliguiri, R. W. Compans, and P. W. Choppin. 1972. Isolation of paramyxovirus glycoproteins: association of both hemagglutinating and neuraminidase activities with the larger SV5 glycoprotein. Virology 50640-652. [DOI] [PubMed] [Google Scholar]
- 11.Schlender, J., G. Zimmer, G. Herrler, and K. K. Conzelmann. 2003. Respiratory syncytial virus (RSV) fusion protein subunit F2, not attachment protein G, determines the specificity of RSV infection. J. Virol. 774609-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stott, E. J., and G. Taylor. 1985. Respiratory syncytial virus: brief review. Arch. Virol. 841-52. [DOI] [PubMed] [Google Scholar]
- 13.Techaarpornkul, S., P. L. Collins, and M. E. Peeples. 2002. Respiratory syncytial virus with the fusion protein as its only viral glycoprotein is less dependent on cellular glycosaminoglycans for attachment than complete virus. Virology 294296-304. [DOI] [PubMed] [Google Scholar]
- 14.Thomas, L. H., R. N. Gourlay, E. J. Stott, C. J. Howard, and J. C. Bridger. 1982. A search for new microorganisms in calf pneumonia by the inoculation of gnotobiotic calves. Res. Vet. Sci. 33170-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valarcher, J. F., J. Furze, S. Wyld, R. Cook, K.-K. Conzelmann, and G. Taylor. 2003. Role of type I interferons in the attenuation and immunogenicity of recombinant bovine respiratory syncytial viruses (BRSV) lacking NS proteins. J. Virol. 778426-8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valarcher, J. F., J. Furze, S. G. Wyld, R. Cook, G. Zimmer, G. Herrler, and G. Taylor. 2006. Bovine respiratory syncytial virus lacking the virokinin or with a mutation in furin cleavage site RA(R/K)R109 induces less pulmonary inflammation without impeding the induction of protective immunity in calves. J. Gen. Virol. 871659-1667. [DOI] [PubMed] [Google Scholar]
- 17.Valarcher, J. F., and G. Taylor. 2007. Bovine respiratory syncytial virus infection. Vet. Res. 38153-180. [DOI] [PubMed] [Google Scholar]
- 18.Vietmeier, J., F. Niedorf, W. Bäumer, C. Martin, E. Deegen, B. Ohnesorge, and M. Kietzmann. 2007. Reactivity of equine airways: a study on precision-cut lung slices. Vet. Res. Commun. 31611-619. [DOI] [PubMed] [Google Scholar]
- 19.Walters, R. W., T. Grunst, J. M. Bergelson, R. W. Finberg, M. J. Welsh, and J. Zabner. 1999. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J. Biol. Chem. 27410219-10226. [DOI] [PubMed] [Google Scholar]
- 20.Wohlsen, A., C. Martin, E. Vollmer, D. Branscheid, H. Magnussen, W. M. Becker, U. Lepp, and S. Uhlig. 2003. The early allergic response in small airways of human precision-cut lung slices. Eur. Respir. J. 211024-1032. [DOI] [PubMed] [Google Scholar]
- 21.Zhang, L., A. Bukreyev, C. I. Thompson, B. Watson, M. E. Peeples, P. L. Collins, and R. J. Pickels. 2005. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J. Virol. 791113-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang, L., M. E. Peeples, R. C. Boucher, P. L. Collins, and R. J. Pickles. 2002. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J. Virol. 765654-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmer, G., L. Budz, and G. Herrler. 2001. Proteolytic activation of respiratory syncytial virus fusion protein: cleavage at two furin consensus sequences. J. Biol. Chem. 27631642-31650. [DOI] [PubMed] [Google Scholar]
- 24.Zimmer, G., M. Rohn, G. P. McGregor, M. Schemann, K. K. Conzelmann, and G. Herrler. 2003. Virokinin, a bioactive peptide of the tachykinin family, is released from the fusion protein of bovine respiratory syncytial virus. J. Biol. Chem. 27846854-46861. [DOI] [PubMed] [Google Scholar]