Abstract
53BP1, the vertebrate ortholog of the budding yeast Rad9 and fission yeast Crb2/Rhp9 checkpoint proteins, is recruited rapidly to sites of DNA double-strand breaks (DSBs). A tandem tudor domain in human 53BP1 that recognizes methylated residues in the histone core is necessary, but not sufficient, for efficient recruitment. By analysis of deletion mutants, we identify here additional elements in 53BP1 that facilitate recognition of DNA DSBs. The first element corresponds to an independently folding oligomerization domain. Replacement of this domain with heterologous tetramerization domains preserves the ability of 53BP1 to recognize DNA DSBs. A second element is only about 15 amino acids long and appears to be a C-terminal extension of the tudor domain, rather than an independently functioning domain. Recruitment of 53BP1 to sites of DNA DSBs is facilitated by histone H2AX phosphorylation and ubiquitination. However, none of the 53BP1 domains/elements important for recruitment are known to bind phosphopeptides or ubiquitin, suggesting that histone phosphorylation and ubiquitination regulate 53BP1 recruitment to sites of DNA DSBs indirectly.
Monitoring the presence of DNA double-strand breaks (DSBs) is critical for maintaining genomic stability. In eukaryotes, the DNA DSB checkpoint pathway senses the presence of DNA DSBs and activates effectors that induce cell cycle arrest, apoptosis, or senescence. Key components of this pathway in human cells are DNA DSB sensors such as 53BP1 and the Mre11-Rad50-NBS1 complex, the signal transducing kinase ATM, and effectors downstream of ATM such as the kinase Chk2 and the transcription factor p53 (1, 16, 25).
53BP1 is one of the DNA damage response proteins that is recruited very efficiently to sites of DNA DSBs. Its recruitment can be visualized either by immunofluorescence of fixed cells or by monitoring live cells expressing 53BP1 fused to green fluorescent protein (GFP). In cells exposed to ionizing radiation (IR), the recruitment of 53BP1 to sites of DNA DSBs becomes evident by its localization to foci that are distributed throughout the nucleus; these foci are thought to correspond to sites of DNA DSBs (4, 20, 30, 33, 44). When DNA damage is induced in specific subnuclear compartments, for example, by UV laser light or by highly charged energetic particles, then 53BP1 localizes to the subnuclear compartments, where the DNA damage was induced (5, 8).
The ability to easily monitor recruitment of 53BP1 to sites of DNA DSBs has allowed significant progress to be made regarding how this protein recognizes DNA damage. Mammalian 53BP1 and its orthologs Rad9 and Crb2/Rhp9, in budding and fission yeast, respectively, recognize DNA DSBs via a tandem tudor domain that binds to methylated histones (18, 31). Human 53BP1 recognizes either methylated K79 of histone H3 or methylated K20 of histone H4 (6, 18, 32, 46), Rad9 recognizes exclusively methylated K79 of histone H3 (13, 43), and Crb2/Rhp9 recognizes exclusively methylated K20 of histone H4 (10, 31). Both K79 of histone H3 and K20 of histone H4 map to the nucleosome core, and their methylation state is apparently not regulated by DNA damage. Instead, it has been proposed that DNA DSBs induce structural changes in chromatin that make these methylated residues accessible (18, 31).
The interaction with methylated histones is critical for recognition of DNA DSBs by 53BP1, Rad9, and Crb2/Rhp9, but for all these three proteins efficient recruitment appears to require additional interactions. Rad9 and Crb2/Rhp9 interact via their BRCT domains with C-terminally phosphorylated histone H2A at DNA damage sites (10, 14, 26, 38). Recruitment of 53BP1 to sites of DNA DSBs is also facilitated by DNA damage-induced phosphorylation of the histone H2A variant H2AX (12, 40), but the BRCT domains of 53BP1 are dispensable (18, 19, 29, 40) and do not bind to phosphorylated histone H2AX (36). Instead, H2AX phosphorylation appears to regulate 53BP1 recruitment indirectly. Specifically, H2AX phosphorylation leads to recruitment of MDC1 (36), which in turn recruits the ubiquitin ligase RNF8; RNF8 then ubiquitinates histones H2A and H2AX, and this ubiquitination facilitates 53BP1 recruitment through an as-yet-unidentified mechanism (17, 22, 24).
In an effort to better understand how 53BP1 is recruited to sites of DNA DSBs, we searched for additional elements within the human protein that are critical for recognition of DNA DSBs. We identify two such elements that together with the tudor domain allow efficient recruitment of 53BP1 to sites of DNA DSBs.
MATERIALS AND METHODS
Recombinant plasmids.
Plasmids encoding a series of deletion and single-amino-acid substitution 53BP1 mutant proteins fused to the C terminus of GFP were generated from previously described mammalian expression plasmids encoding amino acids 1 to 1972 or 1220 to 1711 of human 53BP1 fused to the C terminus of GFP (18).
IR-induced focus-forming assay.
Plasmids encoding GFP-53BP1 fusion proteins were transiently transfected in U2OS osteosarcoma cells using Fugene transfection reagent (Roche Diagnostics, Basel, Switzerland). Two days later the cells were exposed to 3 Gy IR using an X-Rad 320 irradiator (Precision X-Ray, Inc., North Branford, CT) operating at 320 kV and 12.5 mA. Fifteen minutes later the cell medium was replaced with phosphate-buffered saline, and within the next 15 min the intracellular localization of the GFP-53BP1 proteins was monitored by fluorescence microscopy using a 100× water immersion lens (Zeiss, Jena, Germany). Images were acquired with an ORCA ER digital camera (Hamamatsu, Hamamatsu City, Japan) and processed using Imagevision software (Silicon Graphics Inc., Mountain View, CA).
For subnuclear irradiation, we first constructed a pIRESN2 bicistronic vector (Clontech Laboratories, Mountain View, USA) encoding a GFP-53BP1 fusion protein containing residues 1475 to 1635 of human 53BP1 and a heterologous tetramerization domain and used this vector to generate stably transfected U2OS clones. Stably transfected cells were cultured on 18-mm coverslips, which were then placed at the outlet of an X-ray microcollimator (28). The cells were exposed to IR using the X-Rad 320 irradiator operating at 30 kV and 25 mA. At 15 min after irradiation, the cells either were examined live for GFP fluorescence or were fixed and processed for immunofluorescence using antibodies specific for 53BP1 (33) and GFP (Abcam, Cambridge, United Kingdom).
Protein expression and purification and oligomerization assay.
Bacterial expression plasmids encoding various 53BP1 fragments with an N-terminal six-histidine tag were generated using a previously described bacterial expression vector (18). Proteins were expressed in Escherichia coli and purified to homogeneity with the aid of nickel chromatography resin (Talon resin; Clontech Laboratories). The proteins were then analyzed for oligomerization by size exclusion chromatography on a Sephadex 200 PC3.2/300 gel filtration column (GE Healthcare Bio-Sciences, Piscataway, NJ).
RESULTS
The oligomerization domain of human 53BP1 is required for efficient IR-induced focus formation.
A fragment of human 53BP1 containing amino acids 1220 to 1711 of the full-length protein (Fig. 1A) is recruited to IR-induced foci as efficiently as endogenous 53BP1 (18, 19, 29). In contrast, a smaller fragment, which corresponds to amino acids 1480 to 1711 and still contains an intact tudor domain, is recruited much less efficiently, with the majority of cells showing no IR-induced foci and a small minority showing weak foci (18, 19). These results suggested the presence of a region within residues 1220 to 1479 of 53BP1 that facilitates efficient recognition of DNA DSBs. To map this region we examined recruitment of GFP-53BP1 fusion proteins to IR-induced foci. The analysis was performed with live cells at 15 to 30 min after exposure to 3 Gy IR.
FIG. 1.
Mapping of a 53BP1 region required for efficient IR-induced focus formation. (A) Diagram of the human 53BP1 protein. The boundaries of the tandem tudor and BRCT domains are indicated. The oligomerization domain (OLIG) and the region C terminal to the tudor domain (RCTD) are also indicated (see below). The polypeptide corresponding to amino acids 1220 to 1711 is recruited to sites of DNA DSBs as efficiently as full-length 53BP1. (B to E) Focus-forming activities of GFP-53BP1 fusion proteins containing the indicated residues of human 53BP1. In panels C and E, all deletions were in the context of a polypeptide fragment spanning residues 1231 to 1711 of human 53BP1. The recruitment to IR-induced foci was classified as follows: wild type (+), strong foci with almost no diffuse nucleoplasmic staining; partial (/), visible foci but also clear diffuse nucleoplasmic staining; and none (−), no visible foci. (F) Sequence conservation of residues 1251 to 1271 of human 53BP1. Above the human sequence, the residues that were substituted are indicated by a / or −, depending on whether the substitution compromised or abolished focus formation. 53BP1_hs, Homo sapiens 53BP1; Crb2_sp, S. pombe Crb2; Rad9_ce, Caenorhabditis elegans Rad9; Rad9_sc, S. cerevisiae Rad9. (G) Focus-forming activities of GFP-53BP1 fusion proteins containing residues 1231 to 1711 of human 53BP1 and the indicated amino acid substitutions.
Starting with a GFP-53BP1 fusion protein containing residues 1220 to 1711 of 53BP1, we observed a very abrupt transition in focus-forming ability as 53BP1 residues were deleted: a protein containing 53BP1 residues 1231 to 1711 formed IR-induced foci very efficiently, while a protein containing residues 1241 to 1711 formed no foci in the majority of examined cells (Fig. 1B and E). A second series of deletions mutants targeted the region of 53BP1 just N terminal to the tandem tudor domain. Deletion of residues 1381 to 1475 did not affect recruitment, but larger deletions progressively compromised recruitment, such that a mutant with deletion of residues 1268 to 1475 failed to be recruited to IR-induced foci in the majority of cells (Fig. 1C and E). The gradual diminution in focus-forming activity could reflect in part steric hindrance due to close proximity of the tandem tudor domain to the region that facilitates recruitment. We therefore generated another series of deletion mutants that retained residues 1297 to 1711. In this series of mutants we observed an abrupt phenotype transition; deletion of residues 1278 to 1296 had no effect on focus formation, while deletion of residues 1268 to 1296 abolished focus formation (Fig. 1C and E).
Together, the results presented above identified a region between residues 1231 and 1277 that is important for focus formation. To examine the importance of this region in the context of full-length 53BP1, we introduced a deletion of residues 1229 to 1250 in the full-length protein and also in a 53BP1 protein lacking the C-terminal BRCT domains and examined the ability of these proteins to form foci in irradiated cells. In both cases, deletion of residues 1229 to 1250 abolished focus formation in the majority of examined cells (Fig. 1D and E).
After defining the region in human 53BP1 that facilitated efficient recognition of DNA DSBs, we noticed that part of it, corresponding to residues 1251 to 1271, is conserved in evolution (Fig. 1F). In support of the identified homology, replacement of evolutionarily conserved residues, such as Asp1256, Val1260, or Asp1261, with alanine compromised IR-induced focus formation. A double substitution of Tyr1258 and Tyr1259 with alanines, but not the single substitutions, also abolished focus formation (Fig. 1F and G).
53BP1 expressed in mammalian cell lines has been reported to homo-oligomerize in a DNA damage-independent manner, as ascertained by coimmunoprecipitation of ectopically expressed 53BP1 proteins bearing different tags. The region required for oligomerization in vivo maps to residues 1052 to 1475, which includes the region we identified above as being critical for focus formation (2). Because coimmunoprecipitation of 53BP1 proteins with different tags could indicate binding of multiple 53BP1 molecules to some third entity (for example, chromatin) and not necessarily homo-oligomerization, we examined whether 53BP1 contains a homo-oligomerization domain by using an in vitro biochemical assay.
53BP1 polypeptides corresponding to the constructs that were used to examine IR-induced focus formation in vivo (Fig. 1) were expressed in bacteria, purified to homogeneity, and monitored for homo-oligomerization by examining their elution profiles on a gel filtration column (Fig. 2). A 53BP1 polypeptide (amino acids 1231 to 1606, with amino acids 1324 to 1475 deleted) that includes the tandem tudor domain (residues 1485 to 1602) and the newly identified region that facilitates focus formation (residues 1231 to 1277) exhibited an elution profile consistent with homo-oligomerization. In contrast, a polypeptide that differs only by deletion of 10 residues from the N terminus and which did not form foci in vivo eluted from the gel filtration column as a monomer (Fig. 2).
FIG. 2.
Oligomerization of purified human 53BP1 polypeptides in vitro. His-tagged polypeptides containing the indicated residues of human 53BP1 were purified to homogeneity and assayed for oligomerization by size exclusion chromatography. The calculated molecular weight (calc. MW) of each polypeptide (in thousands) is shown to the right, and the elution of molecular weight standards is shown at the bottom. The elution profile of polypeptide 1231-1606 Δ1278-1475 is consistent with an oligomeric form that dissociates into monomers during chromatography.
Analysis of additional purified 53BP1 polypeptides showed that only those retaining amino acids 1231 to 1277 eluted as homo-oligomers, effectively identifying the region encompassing these residues as an oligomerization domain (Fig. 2). Interestingly, based on their elution profiles, the polypeptides with deletions of residues 1288 to 1475 and 1278 to 1475 did not form very stable homo-oligomers, possibly due to steric interference, as the linker between the oligomerization and tandem tudor domains became short. The ability of these two mutants to form IR-induced foci in vivo was compromised (Fig. 1C), which further strengthens the correlation between homo-oligomerization and focus formation.
Finally, we also examined the elution profile of a 53BP1 polypeptide (residues 1235 to 1297) just slightly larger than the region that facilitates focus formation. This polypeptide also eluted with a profile indicative of homo-oligomerization (Fig. 2). Thus, these results demonstrate that the novel 53BP1 region, which facilitates focus formation, is a homo-oligomerization domain.
Heterologous oligomerization domains can drive 53BP1 focus formation.
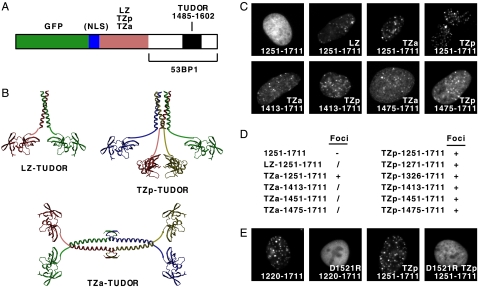
The results presented so far with the various 53BP1 mutants establish a correlation between homo-oligomerization and IR-induced focus formation. To obtain more definitive evidence for the importance of oligomerization, we examined whether heterologous oligomerization domains could rescue focus formation in mutants in which the native 53BP1 oligomerization domain had been deleted (Fig. 3A). We used three different heterologous oligomerization domains (Fig. 3B): a dimeric leucine zipper from the yeast GCN4 transcription factor (LZ), a modified GCN4 leucine zipper that assembles as a tetrameric coiled coil with all α-helices packing in a parallel orientation to each other (TZp), and a hybrid tetramerization domain that consists of the N-terminal half of the human p53 tumor suppressor oligomerization domain fused to the GCN4 leucine zipper and which is expected to have two pairs of parallel α-helices packing antiparallel to each other (TZa). All these oligomerization domains have been characterized previously and their three-dimensional structures have been determined, with the exception of TZa, whose structure, however, can be modeled on the basis of the known structures of its constituent parts (15, 27, 42).
FIG. 3.
Heterologous oligomerization domains can restore the focus-forming activity of GFP-53BP1 fusion proteins lacking the endogenous 53BP1 oligomerization domain. (A) Diagram of the GFP-53BP1 fusion proteins containing a heterologous oligomerization domain between the GFP and 53BP1 sequences and, optionally, also an NLS. LZ, leucine zipper; TZp, modified tetrameric leucine zipper with parallel α-helices; TZa, tetramerization domain generated by fusing part of the p53 tetramerization domain to a leucine zipper, resulting in two coiled coils packing antiparallel to each other. (B) Ribbon representations of the three-dimensional structures of segments of the 53BP1 fusion proteins corresponding to the heterologous oligomerization domain and the tandem tudor domain of 53BP1. The images were generated using the programs MOLSCRIPT and RASTER3D from Protein Data Bank files: 2ZTA, GCN4 leucine zipper (LZ); 1GCL, modified tetrameric GCN4 leucine zipper with parallel α-helices (TZp); 1C26 and 2ZTA, human p53 tetramerization domain and GCN4 leucine zipper (TZa); and 1XNI, tandem tudor domain of human 53BP1 (TUDOR). (C to E) Focus-forming activities of GFP-53BP1 fusion proteins containing various segments of human 53BP1 and, optionally, a heterologous oligomerization domain and/or a D1521R substitution in the 53BP1 tudor domain, as indicated. Focus-forming activities were classified as described in the legend to Fig. 1.
GFP-53BP1 fusion proteins with one of the three heterologous oligomerization domains described above inserted between the GFP and 53BP1 polypeptides were examined for recruitment to IR-induced foci in live cells. All three heterologous oligomerization domains were able to functionally substitute for the native 53BP1 oligomerization domain, with the tetramerization domains being more efficient than the leucine zipper dimerization domain (Fig. 3C and D and data not shown). Several constructs were generated to establish the minimal length of the linker between the heterologous tetramerization domains and the tudor domain that would allow IR-induced focus formation. Even the smallest linker examined, 10 residues long, allowed focus formation, as determined by examining proteins containing residues 1475 to 1711 of 53BP1 fused to the C terminus of the heterologous tetramerization domains (Fig. 3C and D). Like for full-length wild-type 53BP1, the recruitment of these fusion proteins to sites of DNA DSBs was dependent on the tandem tudor domain; replacement of D1521 with R in the tudor domain, which abrogates binding to methylated lysines (17), abrogated focus formation (Fig. 3E). We conclude that with regard to focus formation, oligomerization is the only critical function mediated by the 53BP1 residues N terminal to the tudor domain.
A short conserved region C terminal to the tudor domain is also required for 53BP1 focus formation.
All the GFP-53BP1 fusion constructs described so far contain 53BP1 fragments that extend up to residue 1711 of human 53BP1, whereas the C terminus of the tudor domain extends only up to residue 1602 (7, 18). The 109 amino acids C terminal to the tudor domain contain a nuclear localization signal (NLS), but whether they contain additional elements that are critical for IR-induced focus formation has not been examined. In the context of a GFP-53BP1 fusion protein containing the tetrameric coiled coil as an oligomerization domain and amino acids 1475 to 1711 of human 53BP1 (GFP-TZp-53BP1 1475-1711), we introduced C-terminal deletions and examined their effect on recruitment to IR-induced foci. Fusion proteins containing amino acids 1475 to 1635 and 1475 to 1631 of 53BP1 were nuclear and formed IR-induced foci, whereas a fusion protein containing amino acids 1475 to 1627 of 53BP1 was cytoplasmic (Fig. 4A). Thus, residues 1632 to 1711 are not required for focus formation, and the 53BP1 NLS maps just N terminal to residue 1631.
FIG. 4.
An element corresponding to residues 1614 to 1629 of human 53BP1 is also required for efficient IR-induced focus formation. (A and B) Focus-forming activities of GFP-53BP1 fusion proteins containing various segments of human 53BP1, the modified tetrameric GCN4 leucine zipper (TZp), and, optionally, an NLS (N), as indicated. 0 Gy, nonirradiated cells. (C) Focus-forming activities of GFP-NLS-TZp-53BP1 fusion proteins containing residues 1451 to 1631 of human 53BP1 and the indicated amino acid substitutions. (D) Focus-forming activities of GFP-full-length 53BP1 fusion proteins containing the indicated amino acid substitutions. (E) Sequence conservation of residues 1591 to 1631 of human 53BP1. Above the human sequence, the residues that were substituted are indicated by a +, /, or −, depending on whether the substitution did not affect, compromised, or abolished focus formation. The C-terminal boundary of the tudor domain at residue 1602 and the boundaries of the RCTD are also shown. 53BP1 sequences are from the following species: hs, Homo sapiens; gg, Gallus gallus; xl, Xenopus laevis; tn, Tetraodon nigroviridis; dr, Danio rerio; sp, Strongylocentrotus purpuratus. (F) Sequences of the C termini of GFP-NLS-TZp-53BP1 fusion proteins with small segments of 53BP1 replaced with residues 734 to 754 of human NBS1, residues 259 to 276 of S. cerevisiae VPS27, or residues 106 to 123 of human RAP80, as indicated. The NBS1, VPS27, and RAP80 segments are in bold letters and underlined. Asterisks indicate the free C-terminal ends of the fusion proteins. (G) Focus-forming activities of the GFP-NLS-TZp-53BP1 fusion proteins containing the human NBS1, S. cerevisiae VPS27, or human RAP80 sequences. (H) The tandem tudor domain and the RCTD function as one unit. GFP-NLS-TZp-53BP1 fusion proteins containing residues 1451 to 1631 of human 53BP1 and, optionally, amino acid substitutions targeting the tudor domain (D1521R) and/or the RCTD (L1619E) were expressed in cells and scored for IR-induced focus formation, as indicated. wt, expression of a wild-type 1451-1631 human 53BP1 fragment; wt + dm, coexpression of wild-type and double mutant (D1521R and L1619E) human 53BP1 fragments; sm + sm, coexpression of single mutant D1521R and L1619E human 53BP1 fragments.
The GFP-TZp-53BP1 fusion protein that contains residues 1475 to 1631 of human 53BP1 and forms IR-induced foci has only 29 amino acids C terminal to the tudor domain. We considered it unlikely that such a small sequence would have a function beyond nuclear localization, but to rule out this possibility we introduced a simian virus 40 NLS between the GFP moiety and the TZp tetramerization domain and reexamined IR-induced focus formation. For these experiments the N terminus of the 53BP1 fragment corresponded to residue 1451. Fusion proteins that retained residues 1451 to 1660, 1451 to 1635, or 1451 to 1631 of 53BP1 formed IR-induced foci, consistent with the results described above (Fig. 4B). Surprisingly, however, proteins that retained residues 1451 to 1627 or 1451 to 1606 of 53BP1 failed to form foci, even though they localized in the nucleus and had an intact tandem tudor domain (Fig. 4B). Deleting amino acids 1604 to 1623 or 1614 to 1623 in the context of the protein containing residues 1451 to 1631 of 53BP1 also abolished focus formation (Fig. 4B).
To map more finely the residues that are functionally important in this region, single amino acid substitutions were introduced in the GFP-TZp-53BP1 fusion protein. Targeting A1607, P1610, and L1611 did not affect focus formation; targeting A1614, A1615, S1618, and R1629 compromised focus formation; and targeting L1619 and L1622 abolished focus formation (Fig. 4C). The substitutions targeting L1619 and L1622 also abolished focus formation in the context of full-length 53BP1 (Fig. 4D). Taken together, these results define residues 1614 to 1629 as a region that facilitates focus formation. Alignment of the sequences of 53BP1 proteins from several species revealed that this region, which we refer to as the region C terminal to the tudor domain (RCTD), is conserved in higher eukaryotes (Fig. 4E). However, in lower eukaryotes we could not identify an RCTD-like sequence.
The sequence of the RCTD did not provide clues to its function. The conserved serine (S1618) in this region could in principle be phosphorylated, and this phosphorylation could mediate interaction with other proteins at sites of DNA DSBs. However, replacement of S1618 with alanine was compatible with focus formation (Fig. 4C), arguing against this possibility. We also noted weak sequence homology of this region to the C terminus of human NBS1 (the region that interacts with ATM [11]) and also to the ubiquitin interaction motifs (UIM) present in Saccharomyces cerevisiae vacuolar protein sorting protein 27 (VPS27) (37) and in the human receptor-associated protein 80 (RAP80) (the UIM in RAP80 is important for its recruitment to sites of DNA DSBs [21, 34, 39, 45]). To test whether these weak sequence homologies were functionally relevant, we replaced 53BP1 residues C terminal to the tudor domain with the homologous residues from the NBS1, VPS27, or RAP80 proteins mentioned above (Fig. 4F) and then examined for focus-forming activity in irradiated cells. As shown, none of these fusion proteins formed foci after irradiation (Fig. 4G).
Finally, we examined whether the function of the RCTD requires that it be in cis (on the same polypeptide) with the tudor domain or whether it can function in trans. We took advantage of the fact that 53BP1 forms homo-oligomers and generated mutants in which amino acid substitutions in the tudor domain and in the RCTD were present either in the same polypeptide or in different polypeptides. Further, to avoid interference with endogenous 53BP1, these substitutions were introduced in 53BP1 polypeptides fused to the parallel tetrameric zipper in place of the endogenous 53BP1 oligomerization domain (Fig. 4H). We observed IR-induced 53BP1 foci in all cells expressing a 1:1 ratio of wild-type 53BP1 and a 53BP1 mutant having substitutions in both the tudor domain and the RCTD. However, in cells expressing a 1:1 ratio of a mutant with a substitution in the tudor domain and a mutant with a substitution in the RCTD, 53BP1 foci were absent (Fig. 4H). Thus, we conclude that the tudor domain and RCTD represent one functional unit.
Validation that the ability of the GFP-53BP1 fusion proteins to form foci in irradiated cells indicates recruitment to DNA DSBs.
Throughout this project, we have considered the ability of the various GFP-53BP1 fusion proteins to form IR-induced foci to be synonymous with recruitment to sites of DNA DSBs. To verify that this is indeed the case, we developed an X-ray collimator that can limit the irradiated field to areas whose smallest dimension is in the micrometer range (28). This microcollimator consists of silicon plates with dimensions of 1 cm by 2 cm by 380 μm. On their surfaces the silicon plates have grooves that are 1 μm deep and run the entire length of the plate (Fig. 5A). Stacking of multiple such plates results in the formation of 1-μm-wide channels (Fig. 5A) that can be used to irradiate subnuclear compartments (Fig. 5B).
FIG. 5.
A minimized GFP-53BP1 fusion protein forms IR-induced foci only in irradiated subnuclear compartments. (A and B) Design of the X-ray microcollimator. Silicon wafers with 1-μm-deep grooves on one of their long surfaces are stacked against each other, such that 1-μm-wide channels are formed between the plates. (A) Cross section of one wafer (top) and of several stacked wafers (bottom). (B) Position of the silicon wafers between an X-ray source and the cells to be irradiated. For clarity, only a single wafer is shown, rather than a stack of wafers. (C and D) Formation of IR-induced foci of a GFP-TZp-53BP1 fusion protein containing residues 1475 to 1635 of human 53BP1 in irradiated subnuclear compartments. The interrupted dark blue line indicates the area exposed to IR. (C) Analysis of live cells by monitoring GFP fluorescence. (D) Analysis of fixed cells by immunofluorescence for endogenous 53BP1 and for the GFP-TZp-53BP1 fusion protein. The antibody that recognizes endogenous 53BP1 does not recognize the GFP-TZp-53BP1 fusion protein. Light blue lines mark the nuclei of the cells.
Using this microcollimator, we irradiated cells stably transfected with a plasmid expressing a fusion protein containing GFP, the parallel tetrameric zipper, and then amino acids 1475 to 1635 of human 53BP1. Within minutes after exposure to irradiation, the GFP-53BP1 fusion protein formed foci in subnuclear compartments corresponding to the areas of the nuclei that had been irradiated. This was evident both in living cells (Fig. 5C) and in fixed cells, where we could further demonstrate colocalization of the GFP-TZp-53BP1 fusion protein with endogenous 53BP1 (Fig. 5D). Thus, we conclude that the formation of IR-induced 53BP1 foci indeed reflects recruitment of 53BP1 to sites of DNA DSBs.
DISCUSSION
In this study we identified two new regions that are important for 53BP1 recruitment to IR-induced foci. The first such region is the previously identified oligomerization domain of human 53BP1. The second region is about 15 amino acids long and is located C terminal to the tudor domain.
The presence of an oligomerization domain in human 53BP1 was previously documented using a coimmunoprecipitation assay performed with extracts of cells expressing two differentially tagged 53BP1 proteins. Using this assay, the oligomerization domain was mapped within residues 1052 to 1475 (2). A subsequent study further refined the boundaries of the oligomerization domain to residues 1231 to 1270 but paradoxically also reported that this domain was not required for 53BP1 focus formation (41). Aware of these findings, we pursued our efforts to identify regions that are important for 53BP1 focus formation in an unbiased manner and made a comprehensive series of 53BP1 deletion proteins. When we concluded this analysis, the results showed that the boundaries of one of the domains needed for efficient focus formation matched precisely the boundaries of the oligomerization domain. In fact, 53BP1 proteins that are unable to oligomerize, such as proteins spanning residues 1251 to 1711 of human 53BP1, failed to form IR-induced foci, even when the endogenous 53BP1 protein was depleted (data not shown), suggesting that they cannot localize to sites of DNA DSBs, even in the absence of competition by endogenous oligomeric 53BP1. Two additional observations support our conclusion that oligomerization is critical for 53BP1 focus formation. First, the ability of purified 53BP1 polypeptides to form oligomers in vitro correlated precisely with focus-forming activity in vivo, and second, the endogenous 53BP1 oligomerization domain could be replaced with heterologous oligomerization domains.
What are the implications of oligomerization for recognition of sites of DNA DSBs by 53BP1? We speculate that changes in chromatin structure at sites of DNA DSBs expose multiple 53BP1-binding sites. As a result, a 53BP1 homo-oligomer will exhibit greater avidity than monomeric 53BP1 for chromatin at sites of DNA DSBs, akin to the example of antibodies which have two antigen-binding sites. It is also possible that oligomerization allows 53BP1 to recognize more than one methylated residue in the histone core, since the tudor domain of mammalian 53BP1 can recognize both histone H3 methylated on K79 and histone H4 methylated on K20 (6, 18, 32, 46).
Whether oligomerization is also important for recruitment of yeast 53BP1 homologs to IR-induced foci remains to be established. The oligomerization domain of human 53BP1 has weak homology to sequences present in Schizosaccharomyces pombe Crb2 and S. cerevisiae Rad9 (Fig. 1F), but it is not known if these sequences constitute oligomerization domains. Irrespective of whether they do or not, both Crb2 and Rad9 homo-oligomerize through protein-protein interactions mediated by their BRCT domains. Deleting the BRCT domains abolishes association with damaged chromatin (9, 10, 14, 26, 35), but at least for Crb2, replacing its BRCT domains with a leucine zipper does not restore IR-induced focus formation (9, 10). This indicates that the BRCT domains mediate an activity beyond oligomerization. Indeed, the BRCT domains of both Crb2 and Rad9 bind to phosphorylated histone H2A, and this binding is critical for recruitment to sites of DNA DSBs (10, 14, 26, 38). In contrast, the BRCT domains of human 53BP1 do not bind to phosphorylated H2AX (36), and deleting them does not compromise 53BP1 focus formation (18, 19, 29, 40).
The second region of human 53BP1 that facilitates IR-induced focus formation corresponds to residues 1614 to 1629 and is located just 12 amino acids after the C-terminal end of the tandem tudor domain. This region, which we refer to as RCTD, is conserved from human to sea urchin but is not discernible in yeast (Fig. 4E). We explored whether the RCTD functions as a ubiquitin-binding module, because histone H2A/H2AX ubiquitination is critical for 53BP1 focus formation (17, 22, 24). However, replacing this region with the UIM of human RAP80 or S. cerevisiae VPS27 did not rescue IR-induced focus formation, despite the fact that in RAP80 the UIM is critical for recruitment to sites of DNA damage (21, 34, 39, 45). We also explored the possibility that this region of 53BP1 may be related to the ATM-binding C terminus of NBS1, on the basis of a weak sequence homology between 53BP1 and NBS1. However, replacing residues 1615 to 1631 of human 53BP1 with the ATM-binding region of NBS1 also did not rescue focus formation. Nevertheless, we did show that the RCTD functions as one unit with the tudor domain. This could reflect a requirement of the RCTD for native folding of the tudor domain. However, structural studies of 53BP1 polypeptides containing the tandem tudor domain with or without the RCTD show that the RCTD itself is unstructured and has no effect on the three-dimensional structure of the tandem tudor domain (7, 18). Thus, the most likely possibility is that the RCTD facilitates focus formation by interacting with some component of chromatin adjacent to the tudor-binding site in the histone core, possibly DNA (3, 7, 23).
Finally, we utilized a newly developed microcollimator for X rays (28) to document that the IR-induced foci of the GFP-53BP1 fusion proteins indeed represent sites of DNA DSBs. IR-induced foci of endogenous 53BP1 were originally considered to represent sites of DNA DSBs on the basis of their colocalization with foci of phosphorylated histone H2AX and foci of NBS1 (4, 30, 33, 44). More recently, endogenous 53BP1 and GFP-53BP1 fusions proteins were shown to localize to subnuclear compartments, in which DNA damage was induced either by UV lasers or by highly charged energetic Fe particles (5, 8). By exposing micrometer-wide strips of cells to X rays, we unambiguously showed that 53BP1 foci form only in parts of cell nuclei that have been exposed to X rays. Further, a fusion protein containing residues 1475 to 1635 of human 53BP1 fused to a heterologous oligomerization domain also forms foci only in parts of the cell nuclei that have been irradiated, thus validating the analysis of the domains of 53BP1 required for efficient focus formation.
Acknowledgments
This work was supported by grants from the Swiss National Foundation (to T.D.H. and K.P.), the NIH (to T.D.H.), and the European Commission Seventh Framework Programme (GENICA).
Footnotes
Published ahead of print on 8 December 2008.
REFERENCES
- 1.Adams, M. M., and P. B. Carpenter. 2006. Tying the loose ends together in DNA double strand break repair with 53BP1. Cell Div. 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams, M. M., B. Wang, Z. Xia, J. C. Morales, X. Lu, L. A. Donehower, D. A. Bochar, S. J. Elledge, and P. B. Carpenter. 2005. 53BP1 oligomerization is independent of its methylation by PRMT1. Cell Cycle 41854-1861. [DOI] [PubMed] [Google Scholar]
- 3.Alpha-Bazin, B., A. Lorphelin, N. Nozerand, G. Charier, C. Marchetti, F. Berenguer, J. Couprie, B. Gilquin, S. Zinn-Justin, and E. Quemeneur. 2005. Boundaries and physical characterization of a new domain shared between mammalian 53BP1 and yeast Rad9 checkpoint proteins. Protein Sci. 141827-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson, L., C. Henderson, and Y. Adachi. 2001. Phosphorylation and rapid relocalization of 53BP1 to nuclear foci upon DNA damage. Mol. Cell. Biol. 211719-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bekker-Jensen, S., C. Lukas, F. Melander, J. Bartek, and J. Lukas. 2005. Dynamic assembly and sustained retention of 53BP1 at the sites of DNA damage are controlled by Mdc1/NFBD1. J. Cell Biol. 170201-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Botuyan, M. V., J. Lee, I. M. Ward, J. E. Kim, J. R. Thompson, J. Chen, and G. Mer. 2006. Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. Cell 1271361-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charier, G., J. Couprie, B. Alpha-Bazin, V. Meyer, E. Quemeneur, R. Guerois, I. Callebaut, B. Gilquin, and S. Zinn-Justin. 2004. The Tudor tandem of 53BP1: a new structural motif involved in DNA and RG-rich peptide binding. Structure 121551-1562. [DOI] [PubMed] [Google Scholar]
- 8.Costes, S. V., A. Ponomarev, J. L. Chen, D. Nguyen, F. A. Cucinotta, and M. H. Barcellos-Hoff. 2007. Image-based modeling reveals dynamic redistribution of DNA damage into nuclear sub-domains. PLoS Comput. Biol. 3e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du, L. L., B. A. Moser, and P. Russell. 2004. Homo-oligomerization is the essential function of the tandem BRCT domains in the checkpoint protein Crb2. J. Biol. Chem. 27938409-38414. [DOI] [PubMed] [Google Scholar]
- 10.Du, L. L., T. M. Nakamura, and P. Russell. 2006. Histone modification-dependent and -independent pathways for recruitment of checkpoint protein Crb2 to double-strand breaks. Genes Dev. 201583-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falck, J., J. Coates, and S. P. Jackson. 2005. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature 434605-611. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Capetillo, O., H. T. Chen, A. Celeste, I. Ward, P. J. Romanienko, J. C. Morales, K. Naka, Z. Xia, R. D. Camerini-Otero, N. Motoyama, P. B. Carpenter, W. M. Bonner, J. Chen, and A. Nussenzweig. 2002. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat. Cell Biol. 4993-997. [DOI] [PubMed] [Google Scholar]
- 13.Grenon, M., T. Costelloe, S. Jimeno, A. O'Shaughnessy, J. Fitzgerald, O. Zgheib, L. Degerth, and N. F. Lowndes. 2007. Docking onto chromatin via the Saccharomyces cerevisiae Rad9 Tudor domain. Yeast 24105-119. [DOI] [PubMed] [Google Scholar]
- 14.Hammet, A., C. Magill, J. Heierhorst, and S. P. Jackson. 2007. Rad9 BRCT domain interaction with phosphorylated H2AX regulates the G1 checkpoint in budding yeast. EMBO Rep. 8851-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harbury, P. B., T. Zhang, P. S. Kim, and T. Alber. 1993. A switch between two-, three-, and four-stranded coiled coils in GCN4 leucine zipper mutants. Science 2621401-1407. [DOI] [PubMed] [Google Scholar]
- 16.Harrison, J. C., and J. E. Haber. 2006. Surviving the breakup: the DNA damage checkpoint. Annu. Rev. Genet. 40209-235. [DOI] [PubMed] [Google Scholar]
- 17.Huen, M. S., R. Grant, I. Manke, K. Minn, X. Yu, M. B. Yaffe, and J. Chen. 2007. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell 131901-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huyen, Y., O. Zgheib, R. A. Ditullio, Jr., V. G. Gorgoulis, P. Zacharatos, T. J. Petty, E. A. Sheston, H. S. Mellert, E. S. Stavridi, and T. D. Halazonetis. 2004. Methylated lysine 79 of histone H3 targets 53BP1 to DNA double-strand breaks. Nature 432406-411. [DOI] [PubMed] [Google Scholar]
- 19.Iwabuchi, K., B. P. Basu, B. Kysela, T. Kurihara, M. Shibata, D. Guan, Y. Cao, T. Hamada, K. Imamura, P. A. Jeggo, T. Date, and A. J. Doherty. 2003. Potential role for 53BP1 in DNA end-joining repair through direct interaction with DNA. J. Biol. Chem. 27836487-36495. [DOI] [PubMed] [Google Scholar]
- 20.Jullien, D., P. Vagnarelli, W. C. Earnshaw, and Y. Adachi. 2002. Kinetochore localisation of the DNA damage response component 53BP1 during mitosis. J. Cell Sci. 11571-79. [DOI] [PubMed] [Google Scholar]
- 21.Kim, H., J. Chen, and X. Yu. 2007. Ubiquitin-binding protein RAP80 mediates BRCA1-dependent DNA damage response. Science 3161202-1205. [DOI] [PubMed] [Google Scholar]
- 22.Kolas, N. K., J. R. Chapman, S. Nakada, J. Ylanko, R. Chahwan, F. D. Sweeney, S. Panier, M. Mendez, J. Wildenhain, T. M. Thomson, L. Pelletier, S. P. Jackson, and D. Durocher. 2007. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science 3181637-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancelot, N., G. Charier, J. Couprie, I. Duband-Goulet, B. Alpha-Bazin, E. Quemeneur, E. Ma, M. C. Marsolier-Kergoat, V. Ropars, J. B. Charbonnier, S. Miron, C. T. Craescu, I. Callebaut, B. Gilquin, and S. Zinn-Justin. 2007. The checkpoint Saccharomyces cerevisiae Rad9 protein contains a tandem tudor domain that recognizes DNA. Nucleic Acids Res. 355898-5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mailand, N., S. Bekker-Jensen, H. Faustrup, F. Melander, J. Bartek, C. Lukas, and J. Lukas. 2007. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell 131887-900. [DOI] [PubMed] [Google Scholar]
- 25.Mochan, T. A., M. Venere, R. A. DiTullio, Jr., and T. D. Halazonetis. 2004. 53BP1, an activator of ATM in response to DNA damage. DNA Repair 3945-952. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura, T. M., L. L. Du, C. Redon, and P. Russell. 2004. Histone H2A phosphorylation controls Crb2 recruitment at DNA breaks, maintains checkpoint arrest, and influences DNA repair in fission yeast. Mol. Cell. Biol. 246215-6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Shea, E. K., J. D. Klemm, P. S. Kim, and T. Alber. 1991. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science 254539-544. [DOI] [PubMed] [Google Scholar]
- 28.Pataky, K., G. Villanueva, A. Liani, O. Zgheib, N. Jenkins, D. J. Halazonetis, T. D. Halazonetis, and J. Brugger. Microcollimator for micron-wide stripe irradiation of cells using 20-30 keV X-rays. Radiat. Res., in press. [DOI] [PubMed]
- 29.Pryde, F., S. Khalili, K. Robertson, J. Selfridge, A. M. Ritchie, D. W. Melton, D. Jullien, and Y. Adachi. 2005. 53BP1 exchanges slowly at the sites of DNA damage and appears to require RNA for its association with chromatin. J. Cell Sci. 1182043-2055. [DOI] [PubMed] [Google Scholar]
- 30.Rappold, I., K. Iwabuchi, T. Date, and J. Chen. 2001. Tumor suppressor p53 binding protein 1 (53BP1) is involved in DNA damage-signaling pathways. J. Cell Biol. 153613-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanders, S. L., M. Portoso, J. Mata, J. Bahler, R. C. Allshire, and T. Kouzarides. 2004. Methylation of histone H4 lysine 20 controls recruitment of Crb2 to sites of DNA damage. Cell 119603-614. [DOI] [PubMed] [Google Scholar]
- 32.Schotta, G., R. Sengupta, S. Kubicek, S. Malin, M. Kauer, E. Callen, A. Celeste, M. Pagani, S. Opravil, I. A. De La Rosa-Velazquez, A. Espejo, M. T. Bedford, A. Nussenzweig, M. Busslinger, and T. Jenuwein. 2008. A chromatin-wide transition to H4K20 monomethylation impairs genome integrity and programmed DNA rearrangements in the mouse. Genes Dev. 222048-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schultz, L. B., N. H. Chehab, A. Malikzay, and T. D. Halazonetis. 2000. p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J. Cell Biol. 1511381-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sobhian, B., G. Shao, D. R. Lilli, A. C. Culhane, L. A. Moreau, B. Xia, D. M. Livingston, and R. A. Greenberg. 2007. RAP80 targets BRCA1 to specific ubiquitin structures at DNA damage sites. Science 3161198-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soulier, J., and N. F. Lowndes. 1999. The BRCT domain of the S. cerevisiae checkpoint protein Rad9 mediates a Rad9-Rad9 interaction after DNA damage. Curr. Biol. 9551-554. [DOI] [PubMed] [Google Scholar]
- 36.Stucki, M., J. A. Clapperton, D. Mohammad, M. B. Yaffe, S. J. Smerdon, and S. P. Jackson. 2005. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell 1231213-1226. [DOI] [PubMed] [Google Scholar]
- 37.Swanson, K. A., R. S. Kang, S. D. Stamenova, L. Hicke, and I. Radhakrishnan. 2003. Solution structure of Vps27 UIM-ubiquitin complex important for endosomal sorting and receptor downregulation. EMBO J. 224597-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toh, G. W., A. M. O'Shaughnessy, S. Jimeno, I. M. Dobbie, M. Grenon, S. Maffini, A. O'Rorke, and N. F. Lowndes. 2006. Histone H2A phosphorylation and H3 methylation are required for a novel Rad9 DSB repair function following checkpoint activation. DNA Repair 5693-703. [DOI] [PubMed] [Google Scholar]
- 39.Wang, B., S. Matsuoka, B. A. Ballif, D. Zhang, A. Smogorzewska, S. P. Gygi, and S. J. Elledge. 2007. Abraxas and RAP80 form a BRCA1 protein complex required for the DNA damage response. Science 3161194-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward, I. M., K. Minn, K. G. Jorda, and J. Chen. 2003. Accumulation of checkpoint protein 53BP1 at DNA breaks involves its binding to phosphorylated histone H2AX. J. Biol. Chem. 27819579-19582. [DOI] [PubMed] [Google Scholar]
- 41.Ward, I., J. E. Kim, K. Minn, C. C. Chini, G. Mer, and J. Chen. 2006. The tandem BRCT domain of 53BP1 is not required for its repair function. J. Biol. Chem. 28138472-38477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waterman, M. J., J. L. Waterman, and T. D. Halazonetis. 1996. An engineered four-stranded coiled coil substitutes for the tetramerization domain of wild-type p53 and alleviates transdominant inhibition by tumor-derived p53 mutants. Cancer Res. 56158-163. [PubMed] [Google Scholar]
- 43.Wysocki, R., A. Javaheri, S. Allard, F. Sha, J. Cote, and S. J. Kron. 2005. Role of Dot1-dependent histone H3 methylation in G1 and S phase DNA damage checkpoint functions of Rad9. Mol. Cell. Biol. 258430-8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia, Z., J. C. Morales, W. G. Dunphy, and P. B. Carpenter. 2001. Negative cell cycle regulation and DNA damage-inducible phosphorylation of the BRCT protein 53BP1. J. Biol. Chem. 2762708-2718. [DOI] [PubMed] [Google Scholar]
- 45.Yan, J., Y. S. Kim, X. P. Yang, L. P. Li, G. Liao, F. Xia, and A. M. Jetten. 2007. The ubiquitin-interacting motif containing protein RAP80 interacts with BRCA1 and functions in DNA damage repair response. Cancer Res. 676647-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang, H., J. J. Pesavento, T. W. Starnes, D. E. Cryderman, L. L. Wallrath, N. L. Kelleher, and C. A. Mizzen. 2008. Preferential dimethylation of histone H4 lysine 20 by Suv4-20. J. Biol. Chem. 28312085-12092. [DOI] [PMC free article] [PubMed] [Google Scholar]