Abstract
Objective
To investigate the association of longitudinal change in serum gamma-glutamyltransferase (GGT) with mortality from cardiovascular disease (CVD).
Methods and Results
A population-based cohort of 76,113 Austrian men and women with 455,331 serial GGT measurements was prospectively followed-up for a median of 10.2 years after assessment of longitudinal GGT-change during an average period of 6.9 years. Cox proportional hazards models with time-varying covariates were used to evaluate GGT-change as an independent predictor for CVD death. Independently of baseline GGT and other classical CVD risk factors, a pronounced increase in GGT (7-year-change >9.2 U/L) was significantly associated with increased total CVD mortality in men (p=0.005); the adjusted hazard ratio (95% confidence interval) in comparison to stable GGT (7-year-change -0.7-1.3 U/L) was 1.40 (1.09-1.81). Similarly, CVD risk was elevated for increasing GGT in women, although effects were less pronounced and statistically significant only in a sub-analysis regarding coronary heart disease. Age of participants significantly modified the relation between GGT-change and CVD mortality, with markedly stronger associations to be observable for younger individuals.
Conclusion
Our study is the first to demonstrate that a longitudinal increase in GGT, independently of baseline GGT and even within its normal range, significantly increases risk of fatal CVD.
Keywords: cardiovascular disease mortality, gamma-glutamyltransferase, longitudinal change, risk factor, epidemiology, Strasak: GGT-change and CVD mortality
Gamma-glutamyltransferase (GGT), present on the external surface of most cells and in serum, is the enzyme responsible for the extracellular catabolism of glutathione (GSH), the main thiol antioxidant in mammalian cells.1-3 Despite its well-established clinical use as an indicator of hepato-biliary diseases and marker of excessive alcohol intake,1,4,5 in recent years, several epidemiologic studies have sparked further interest in elevated GGT as an independent predictor for morbidity and mortality from causes other than liver disease.6
Specifically, it was reported that GGT is related to incidence of and mortality from cardiovascular disease (CVD),7-14 and correlated with most cardiovascular risk factors, including diabetes, hypertension, dyslipidemia and the metabolic syndrome.15-18 It has been speculated that GGT may be a sensitive and reliable marker of oxidative stress,7 which has been shown to be a key mechanism in the pathogenesis of many metabolic disorders, CVD, and other degenerative and lifestyle-related diseases.19-22
Despite a growing body of epidemiologic evidence on the predictive significance of baseline GGT for prevalent health conditions and/or mortality, few data have been published on longitudinal changes in GGT. Although GGT displays a considerable intra-individual stability and strong “tracking” pattern,23 evidence from large-scale population studies indicates also a perceptible increase in GGT during the past few decades, with a strong secular trend.19,24 It has been reported that an increase in GGT over time is positively correlated with an increase in body-mass-index in both men and women, although other CVD risk factors, including systolic blood pressure, total and HDL-cholesterol and triglycerides were associated differently in the genders.24 A recent study reported a three-year increase of GGT (>5 U/L), even within its normal range, to be significantly related to insulin resistance and risk of incident type 2 diabetes, independently of baseline GGT, which is itself a diabetes risk factor.25
In the present study, we prospectively investigated the association of longitudinal change in serum GGT during an average period of 7 years with subsequent all-cause mortality, mortality from total CVD, coronary heart disease (CHD), congestive heart failure (CHF) and stroke in a population-based cohort of 76,113 Austrian men and women. Although previous studies have investigated the association of baseline GGT with morbidity and mortality from CVD, to our knowledge, the present study comprises the first investigation to assess the predictive value of GGT-change over time for mortality from overall CVD and all major subforms.
Methods
Study Population
The Vorarlberg Health Monitoring and Promotion Program [VHM&PP]8,23,26 started in 1985 and conducted by the Agency for Social and Preventive Medicine in Vorarlberg, the westernmost province of Austria, is one of the world’s largest ongoing population-based risk factor surveillance programs. All adults of the region are invited to participate by a combination of different measures including written invitations, television, radio and newspaper reports. Active follow-up of study participants is performed through a recall-system of written biennial re-invitation letters. Socio-demographic data are recorded, and a voluntary physical examination is conducted regularly in a standardized manner by trained local physicians and internists. During the exam, a fasting blood sample is taken. Costs are covered by the participant’s (compulsory) health insurance. A more detailed description of the program methodology has been reported elsewhere.23
Between 1985 and 2005, a total of 184,774 male and female Vorarlberg residents (aged >18 years) were enrolled in the VHM&PP. Participants had between 1 and 19 routine health examinations. The current investigation was restricted to individuals with at least two examinations over a 5- to 9-year time interval, during which longitudinal GGT-change from the first (baseline) visit was assessed. To minimize possible effects of reverse causality due to pre-existing disease, we further excluded participants dying within the first year after GGT-change assessment. Consequently, to avoid attrition/censoring bias in our analyses, we also excluded participants with follow-up periods <1 year, not experiencing a fatal event, yielding a total of 32,365 men and 43,748 women with complete and valid data on longitudinal GGT-change eligible for analyses. Baseline characteristics of the present study cohort were very similar in comparison to the total VHM&PP cohort (Supplemental Table 1); however, since participants suffering from early deaths were not routinely eligible for the present investigation, mortality rates were slightly lower in both men and women.
All participants signed informed consents to have personal data stored and processed. For this study, institutional review board approval was obtained by the Ethics Committee of the province of Vorarlberg.
Data Collection
Measurements of height, weight, systolic and diastolic blood pressure, total cholesterol, triglycerides, blood glucose, GGT and smoking status (current, former, never) are routinely obtained for each study participant. Individuals who reported smoking of at least one cigarette per day during the year before examination were classified as current smokers. Occupational status (blue collar, white collar or self-employed) was determined by the insurance number of participants and used as a surrogate measure of socioeconomic status. Participants who were retired at baseline were classified according to their former occupation, and housewives were classified according to their husband’s job.
Laboratory Measurements
Two central laboratories undergoing regular internal and external quality procedures enzymatically determined GGT concentrations on fasting blood samples. Within 60 to 240 minutes after venous blood sample collection from a cubital vein, serum was obtained by centrifugation for 15 minutes at 4000 rotations per minute. Subsequently, GGT concentrations were measured at 37°C and were given as units per liter (U/L). In order to check calibration, 3 daily control samples were included. If average values of the control samples of each run were not within 3% of the true value, the run was repeated. Day-by-day variation had to be within 5%.
End Points
By the end of 2005, a total of 4,551 deaths were recorded in our database of which 1,955 (43.0%) were cardiovascular- or cerebrovascular-related deaths. Date and cause of death information was provided by the local health authority and was linked in the database with the use of a validated procedure. All deaths were identified from death certificates that were confirmed by authorized physicians only. In cases of unclear causes of death, autopsies were performed. For analyses, deaths from total CVD (ICD-9 401-443 [except 415-417]; ICD-10 I10-I79 [except I26-I28])8,27 were grouped into the following CVD subcategories: CHD (ICD-9 410-414; ICD-10 I20-I25 [except I25.5]), CHF (ICD-9 425, 428, 429.0, 429.1, 429.3; ICD-10 I25.5, I42, I43, I50, I51.5, I51.7) and stroke (ICD-9 430-438; ICD-10 I60-I69).
Statistical Analyses
A two-stage regression approach, including (1) linear regression models to calculate least-square estimates of slopes for assessing longitudinal within-subject change in serum GGT per year28-30 and (2) extended Cox proportional hazards models with time-varying covariates31 to calculate hazard ratios with their 95% confidence intervals for the association of GGT-change with all-cause and CVD mortality, was used. Since the number of examinations and the time between examinations differed among study participants, we chose a 5- to 9-year time period (mean 6.9 years) to assess longitudinal GGT-change in order to balance the length of the assessment period against the length of the follow-up period after GGT-change assessment. Specifically, the time period for GGT-change assessment ended at the study visit that occurred closest to 7 years after baseline. All measurements obtained during the period of GGT-change assessment were used to construct linear regression models for each participant, employing classical least-squares estimation of the slope of the trend in GGT (measured by the size of the regression coefficients) against time as an indicator of their individual trend for GGT-change, with each data point weighted equally. 28-30,32 Consequently, subjects were stratified into seven, equally-sized, gender-specific categories according to the distribution of slopes of GGT-change with 3 categories for each, increasing and decreasing GGT (mild, moderate, pronounced) and stable GGT.33 The assumption of linearity in longitudinal GGT-change in the individual linear regression models was checked by visual inspection of residual plots in a randomly selected sub-sample of 10% of study participants with at least 3 GGT measurements and was found to be approximately fulfilled. Additionally, since the variability between person’s slopes was considerably greater than the residual or within person’s variability, the estimates of the individual slopes have been shown to be approximately the same regardless of the method of estimation used.32,34
Follow-up started after assessment of longitudinal GGT-change and ended at incidence of cardiovascular death or at censoring. Censoring events were non-cardiovascular death, loss to follow-up and end of study. We first estimated hazard ratios with 95% confidence intervals for the association of baseline GGT with all-cause and CVD mortality by calculating Cox proportional hazards models, adjusted for baseline values of age, body-mass-index, smoking status, occupational status, triglycerides, total cholesterol, systolic and diastolic blood pressure and blood glucose. Due to skewed distributions, GGT and triglycerides were transformed using logarithm based 10. To incorporate repeated measurements and to further estimate hazard ratios for GGT as a time-varying variable, we recalculated all hazard ratios, using extended Cox proportional hazards models simultaneously adjusting for the above covariates as time-varying variables.31 To estimate hazard ratios with 95% confidence intervals for the association of GGT-change (in seven categories) with all-cause and CVD mortality, we fitted the same extended Cox models with time-varying covariates and additional adjustment for baseline GGT (logarithmically transformed) and the period of GGT-change assessment. The proportional hazards assumption was checked using Schoenfeld residuals31 and visual inspection of the hazards plots. To assess dose-response relationships of GGT-change and risk of CVD and total mortality, log-linear trends across categories of increasing and decreasing GGT, in respect to stable GGT as the reference category, were tested using the median GGT-change for each category as an ordinal variable in our Cox models.
In sensitivity analyses, we evaluated whether the GGT-change-CVD association was confounded by severe pre-existing illness excluding the first two years of follow-up. To investigate possible effect modification by age, we included multiplicative interaction terms in our Cox models and used stratified analyses. We further recalculated all results, excluding (1) participants with elevated GGT at baseline (>60 U/L in men and >36 U/L in women) and/or participants increasing into the abnormal range of GGT. To investigate possible effects of within-subject variability in GGT-change over time, we included the root mean square error, obtained from linear regression analyses, as an additional covariate in our regression runs. Two sided p-values <0.05 were considered statistically significant. All statistical analyses were conducted using STATA 9.0 and SPSS 15.0 statistical software.
Results
Characteristics of study population and categories of GGT-change
Demographic and clinical characteristics of the study population are shown in Table 1. The average period of longitudinal GGT-change assessment was 6.9±0.9 years and median follow-up after GGT-change assessment corresponded to 9.8 years in men and 10.6 years in women, with a total of 716,098 person-years at risk. Most participants (96.7%) were followed-up for at least two years after GGT-change assessment and 72.5% had follow-up times of 7 or even more years. Mean age at study entry was 42.1 years in men and 42.0 years in women. During follow up, 1,955 (2.6%) CVD deaths were recorded in our database. On average, six GGT measurements were obtained for each participant, with median baseline GGT concentrations of 28.6 U/L in men and 17.9 U/L in women. Stratification of subjects into seven, equally-sized, gender-specific categories according to the distribution of slopes of GGT-change yielded the following cut-off values: “stable” (7-year-change -0.68 to 1.26 U/L in men; -0.55 to 0.79 U/L in women), “mild increase” (7-year-change 1.27 to 3.76 U/L in men; 0.80 to 2.27 U/L in women), “moderate increase” (7-year-change 3.77 to 9.16 U/L in men; 2.28 to 5.15 U/L in women), “pronounced increase” (7-year-change >9.17 U/L in men; >5.15 U/L in women), “mild decrease” (7-year-change -3.02 to -0.69 U/L in men; -1.97 to -0.56 U/L in women), “moderate decrease” (7-year-change -7.98 to -3.03 U/L in men; -4.49 to -1.98 U/L in women) and “pronounced decrease” (7-year-change <(-7.99) U/L in men; <(-4.50) U/L in women).
Table 1.
Characteristics of study population, VHM&PP 1985-2005
| Characteristic | Males | Females |
|---|---|---|
| Eligible Participants with complete and valid data for longitudinal GGT-Change assessment - no.* |
32,365 | 43,748 |
| Routine health examination with longitudinal GGT measurements - no. | 187,635 | 267,696 |
| Period of longitudinal GGT-Change assessment, mean±SD (range), y | 6.9±0.9 (5.0-9.0) | 6.9±0.9 (5.0-9.0) |
| Follow-up after longitudinal GGT-Change assessment, mean±SD (median), y | 9.1±3.8 (9.8) | 9.7±3.7 (10.6) |
| Person years at risk after longitudinal GGT-Change assessment | 293,259 | 422,830 |
| Age, mean±SD (range), y† | 42.1±13.6 (18-89) | 42.0±14.4 (19-90) |
| Body-mass-index, mean±SD (median), kg/m2† | 25.3±3.4 (25.0) | 24.1±4.3 (23.3) |
| Glucose, mean±SD (median), mg/dL† | 86.4±22.0 (84.0) | 84.3±18.4 (83.0) |
| Triglycerides, mean±SD (median), mg/dL† | 152.4±105.4 (123.0) | 111.4±64.9 (95.0) |
| Total Cholesterol, mean±SD (median), mg/dL† | 220.9±46.8 (218.0) | 216.1±46.5 (211.0) |
| Gamma-glutamyltransferase, mean±SD (median), U/L† | 42.2±55.7 (28.6) | 23.7±29.2 (17.9) |
| Systolic Blood Pressure, mean±SD (median), mm Hg† | 131.9±18.0 (130.0) | 127.6±20.8 (125.0) |
| Diastolic Blood Pressure, mean±SD (median), mm Hg† | 81.8±10.7 (80.0) | 79.3±11.0 (80.0) |
| Occupational status† | ||
| Blue collar - (%) | 31.7 | 36.4 |
| White collar - (%) | 58.3 | 56.5 |
| Self-employed - (%) | 10.0 | 7.1 |
| Current or Former Cigarette Smoking - (%)† | 31.5 | 23.4 |
| Total Mortality - no (%) | 2,304 (7.1) | 2,247 (5.1) |
| Cardiovascular/cerebrovascular deaths - no. (%) | 986 (3.0) | 969 (2.2) |
| Other death cause - no. (%) | 1,318 (4.1) | 1,278 (2.9) |
Participants diagnosed with malignancies prior to enrolment, during assessment of longitudinal GGT-change, or within 1 year after longitudinal GGT-change assessment were excluded.
Values referring to baseline (i.e. measurement at first visit)
Association of baseline and time-varying GGT with all-cause and CVD mortality
The association of GGT, modelled as baseline and time-varying covariate, with all-cause mortality, total CVD mortality and mortality from CHD, CHF and stroke is shown in Table 2. In Cox proportional hazards models adjusted for baseline values of age, body-mass-index, smoking status, occupational status, triglycerides, total cholesterol, systolic and diastolic blood pressure and blood glucose, baseline GGT was independently associated with all-cause mortality in both men and women; the adjusted hazard ratios (95%CI) per baseline GGT log-unit increase were 2.18 (1.89-2.53) and 1.53 (1.31-1.80), respectively. In men, baseline GGT was further significantly associated with total CVD mortality, mortality from CHD, CHF and stroke (all p<0.01, Table 2).
Table 2.
Estimated adjusted hazard ratios with 95% confidence intervals for the association of gamma-glutamyltransferase, modelled as baseline and time-varying covariate, with all-cause mortality and mortality from cardiovascular disease in 76,113 Austrian adults, VHM&PP 1985-2005
| All-cause mortality | Total mortality from Cardiovascular Disease |
Mortality from Coronary Heart Disease |
Mortality from Congestive Heart Failure |
Stroke mortality | |
|---|---|---|---|---|---|
| Males (n=32,365) | |||||
| Fatal Events - no (%) | 2,304 (7.1) | 986 (3.0) | 541(1.7) | 91 (0.3) | 228 (0.7) |
| HR (95%CI, p-value) per baseline GGT log-unit increase* | 2.18 (1.89-2.53, p<0.0001) |
1.87 (1.48-2.36, p<0.0001) |
1.80 (1.32-2.46, p<0.0001) |
3.04 (1.45-6.23, p=0.003) |
2.25 (1.40-3.61, p=0.001) |
| HR (95%CI, p-value) per GGT log-unit increase with GGT as time-varying covariate† |
2.53 (2.22-2.89, p<0.0001) |
2.13 (1.73-2.62, p<0.0001) |
2.22 (1.68-2.93, p<0.0001) |
1.74 (0.89-3.41, p=0.11) |
2.29 (1.51-3.48, p<0.0001) |
| Females (n=43,748) | |||||
| Fatal Events - no (%) | 2,247 (5.1) | 969 (2.2) | 442 (1.0) | 97 (0.2) | 266 (0.6) |
| HR (95%CI, p-value) per baseline GGT log-unit increase* | 1.53 (1.31-1.80, p<0.0001) |
1.21 (0.94-1.56, p=0.13) |
1.40 (0.97-2.02, p=0.07) |
0.89 (0.38-2.07, p=0.79) |
1.29 (0.80-2.08, p=0.30) |
| HR (95%CI, p-value) per GGT log-unit increase with GGT as time-varying covariate† |
1.92 (1.65-2.24, p<0.0001) |
1.72 (1.35-2.18, p<0.0001) |
1.45 (1.01-2.09, p=0.046) |
3.18 (1.62-6.22, p=0.001) |
2.12 (1.37-3.28, p=0.001) |
Estimated from Cox proportional hazards models adjusted for baseline values of age, body-mass-index, smoking status, occupational status, triglycerides, total cholesterol, systolic and diastolic blood pressure and blood glucose. GGT and triglycerides were log-transformed.
Estimated from body-mass-index, smoking status, occupational status, triglycerides, total cholesterol, systolic and diastolic blood pressure extended Cox proportional hazards models for age, and blood glucose as time-varying covariates. GGT and triglycerides were log-transformed.
When GGT was modelled as time-varying covariate, with additional adjustment for all above confounding factors as time-varying variables, associations with all-cause mortality markedly increased in both men and women. Similarly, time-varying GGT became an independent predictor for total CVD mortality, mortality from CHD, CHF and stroke also in women (all p<0.05), while the association of GGT with mortality from CHF turned out to be non-significant in men. (p=0.11, Table 2).
Association of longitudinal GGT-change with all-cause and CVD mortality
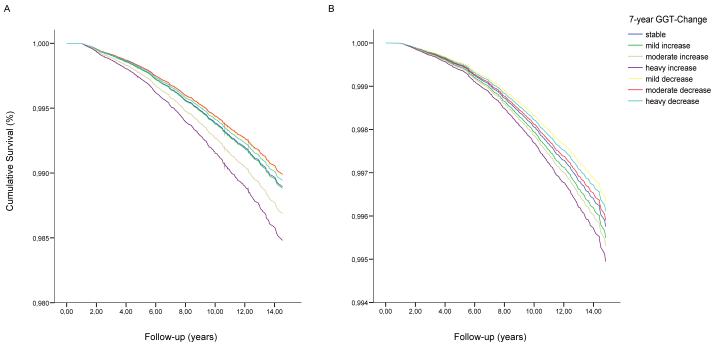
The association of longitudinal GGT-change with all-cause and CVD mortality in men and women is shown in Tables 3 and 4, respectively. In extended Cox proportional hazards models, adjusted for baseline log-GGT, period of GGT-change assessment and age, body-mass-index, smoking status, occupational status, log-triglycerides, total cholesterol, systolic and diastolic blood pressure and blood glucose as time-varying covariates, a pronounced 7-year increase in GGT was independently related to increased risk of all-cause mortality in both sexes (p for trend both <0.01); the hazard ratios (95%CI) in comparison to stable GGT were 1.36 (1.16-1.61) and 1.26 (1.06-1.49), respectively.
Table 3.
All-cause mortality and mortality from cardiovascular disease according to categories of longitudinal GGT-change in 32,365 men, VHM&PP 1985-2005
| Longitudinal GGT-change |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Decrease |
Stable | Increase |
|||||||
| Pronounced n=4625 |
Moderate n=4625 |
Mild n=4625 |
n=4625 | Mild n=4625 |
Moderate n=4625 |
Pronounced n=4615 |
|||
| Longitudinal 7-year GGT-change (U/L) - Range |
<(-7.99) | -7.98-(-3.03) | -3.02-(-0.69) | -0.68-1.26 | 1.27-3.76 | 3.77-9.16 | >9.17 | ||
| Longitudinal 7-year GGT-change - mean±SD (median) (U/L) |
-30.1±52.7 (-15.8) | -5.0±1.4 (-4.8) | -1.8±0.7 (-1.7) | 0.3±0.6 (0.2) | 2.5±0.7 (2.4) | 6.0±1.5 (5.8) | 31.8±52.9 (17.9) | p for trend GGT decrease* |
p for trend GGT increase* |
| Baseline GGT - mean±SD (median) (U/L) |
100.2±115.8 (69.0) |
37.8±20.2 (34.0) | 28.9±16.6 (25.1) | 25.6±15.7 (21.5) | 25.1±16.2 (21.5) | 29.5±20.3 (25.1) | 48.3±49.0 (34.0) | ||
| ALL-CAUSE MORTALITY | |||||||||
| Fatal events - no.(%) | 490 (10.6) | 385 (8.3) | 283 (6.1) | 266 (5.8) | 243 (5.3) | 242 (5.2) | 395 (8.6) | ||
| HR (95% CI)† | 0.93 (0.78-1.11) | 1.01 (0.86-1.18) | 0.89 (0.75-1.06) | 1.00 (Ref) | 1.05 (0.88-1.25) | 1.07 (0.90-1.28) | 1.36 (1.16-1.61) | 0.54 | <0.0001 |
| TOTAL CVD MORTALITY | |||||||||
| Fatal events - no.(%) | 221 (4.8) | 159 (3.4) | 139 (3.0) | 117 (2.5) | 102 (2.2) | 113 (2.4) | 135 (2.9) | ||
| HR (95% CI)† | 1.01 (0.78-1.32) | 0.97 (0.76-1.25) | 0.92 (0.71-1.18) | 1.00 (Ref) | 1.04 (0.79-1.37) | 1.16 (0.88-1.51) | 1.40 (1.09-1.81) | 0.85 | 0.005 |
| CORONARY HEART DISEASE | |||||||||
| Fatal events - no.(%) | 125 (2.7) | 87 (1.9) | 80 (1.7) | 60 (1.3) | 58 (1.3) | 65 (1.4) | 66 (1.4) | ||
| HR (95% CI)† | 0.94 (0.66-1.34) | 0.92 (0.66-1.29) | 0.92 (0.65-1.30) | 1.00 (Ref) | 1.09 (0.76-1.56) | 1.23 (0.87-1.76) | 1.13 (0.79-1.61) | 0.70 | 0.36 |
| CONGESTIVE HEART FAILURE | |||||||||
| Fatal events - no.(%) | 21 (0.5) | 13 (0.3) | 13 (0.3) | 11 (0.2) | 7 (0.2) | 10 (0.2) | 16 (0.3) | ||
| HR (95% CI)† | 0.97 (0.42-2.25) | 0.77 (0.34-1.76) | 1.07 (0.48-2.36) | 1.00 (Ref) | 0.62 (0.23-1.68) | 0.81 (0.31-2.11) | 1.57 (0.70-3.50) | 0.69 | 0.35 |
| STROKE | |||||||||
| Fatal events- no.(%) | 54 (1.2) | 38 (0.8) | 29 (0.6) | 26 (0.6) | 22 (0.5) | 24 (0.5) | 35 (0.8) | ||
| HR (95% CI)† | 1.29 (0.74-2.26) | 1.19 (0.70-2.00) | 0.86 (0.48-1.52) | 1.00 (Ref) | 0.98 (0.53-1.80) | 1.32 (0.74-2.34) | 1.89 (1.11-3.22) | 0.67 | 0.002 |
Log-linear trends across categories of increasing or decreasing GGT, in respect to stable GGT, were tested using the median GGT-change for each category as an ordinal variable in an extended Cox proportional hazards model adjusted for age, body-mass-index, smoking status, occupational status, triglycerides, total cholesterol, systolic and diastolic blood pressure and blood glucose as time-varying covariates. All models are additionally adjusted for baseline GGT and the period of GGT-change assessment. GGT and triglycerides were log-transformed.
Estimated extended Cox proportional hazards models adjusted for age, body-mass-index, smoking status, occupational status, triglycerides, from total cholesterol, systolic and diastolic blood pressure and blood glucose as time-varying covariates. All models are additionally adjusted for baseline GGT and period of GGT-change assessment. GGT and triglycerides were log-transformed.
Table 4.
All-cause mortality and mortality from cardiovascular disease according to categories of longitudinal GGT-change in 43,748 women, VHM&PP 1985-2005
| Longitudinal GGT-change |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Decrease |
Stable | Increase |
|||||||
| Pronounced n=6251 |
Moderate n=6253 |
Mild n=6250 |
n=6252 | Mild n=6251 |
Moderate n=6253 |
Pronounced n=6238 |
|||
| Longitudinal 7-year GGT-change (U/L) - Range |
<(-4.50) | -4.49-(-1.98) | -1.97-(-0.56) | -0.55-0.79 | 0.80-2.27 | 2.28-5.15 | >5.15 | ||
| Longitudinal 7-year GGT-change - mean±SD (median) (U/L) |
-15.4±27.0 (-8.2) | -3.1±0.7 (-3.0) | -1.2±0.4 (-1.2) | 0.1±0.3 (0.0) | 1.5±0.4 (1.4) | 3.5±0.9 (3.4) | 19.3±32.1 (9.9) | p for trend GGT decrease* |
p for trend GGT increase* |
| Baseline GGT - mean±SD (median) (U/L) |
49.4±55.9 (34.0) | 21.4±10.7 (19.7) | 18.1±8.6 (16.1) | 16.6±7.4 (14.3) | 16.1±8.3 (14.3) | 16.9±10.7 (14.3) | 26.6±26.6 (19.7) | ||
| ALL-CAUSE MORTALITY | |||||||||
| Fatal events - no.(%) | 512 (8.2) | 331 (5.3) | 246 (3.9) | 240 (3.8) | 239 (3.8) | 258 (4.1) | 421 (6.7) | ||
| HR (95% CI)† | 0.99 (0.83-1.18) | 1.03 (0.87-1.22) | 0.96 (0.80-1.15) | 1.00 (Ref) | 1.03 (0.86-1.24) | 1.14 (0.95-1.37) | 1.26 (1.06-1.49) | 0.72 | 0.003 |
| TOTAL CVD MORTALITY | |||||||||
| Fatal events - no.(%) | 214 (3.4) | 153 (2.4) | 113 (1.8) | 117 (1.9) | 114 (1.8) | 106 (1.7) | 152 (2.4) | ||
| HR (95% CI)† | 0.91 (0.70-1.19) | 0.97 (0.75-1.26) | 0.85 (0.64-1.13) | 1.00 (Ref) | 1.02 (0.78-1.34) | 1.13 (0.86-1.48) | 1.18 (0.91-1.51) | 0.91 | 0.23 |
| CORONARY HEART DISEASE | |||||||||
| Fatal events - no.(%) | 103 (1.6) | 74 (1.2) | 44 (0.7) | 50 (0.8) | 53 (0.8) | 53 (0.8) | 65 (1.0) | ||
| HR (95% CI)† | 1.15 (0.77-1.73) | 1.24 (0.84-1.84) | 0.81 (0.51-1.27) | 1.00 (Ref) | 1.24 (0.81-1.88) | 1.32 (0.87-2.00) | 1.42 (0.96-2.10) | 0.33 | 0.06 |
| CONGESTIVE HEART FAILURE | |||||||||
| Fatal events - no.(%) | 19 (0.3) | 15 (0.2) | 10 (0.2) | 15 (0.2) | 10 (0.2) | 11 (0.2) | 17 (0.3) | ||
| HR (95% CI)† | 0.77 (0.34-1.74) | 0.62 (0.27-1.45) | 1.01 (0.46-2.21) | 1.00 (Ref) | 0.78 (0.33-1.85) | 1.12 (0.50-2.49) | 1.06 (0.46-2.21) | 0.87 | 0.99 |
| STROKE | |||||||||
| Fatal events- no.(%) | 59 (0.9) | 36 (0.6) | 40 (0.6) | 30 (0.5) | 33 (0.5) | 26 (0.4) | 42 (0.7) | ||
| HR (95% CI)† | 0.69 (0.41-1.11) | 0.71 (0.43-1.16) | 0.92 (0.57-1.49) | 1.00 (Ref) | 0.79 (0.47-1.31) | 0.92 (0.56-1.51) | 0.91 (0.57-1.46) | 0.14 | 0.70 |
Log-linear trends across categories of increasing or decreasing GGT, in respect to stable GGT, were tested using the median GGT-change for each category as an ordinal variable in an extended Cox proportional hazards model adjusted for age, body-mass-index, smoking status, occupational status, triglycerides, total cholesterol, systolic and diastolic blood pressure and blood glucose as time-varying covariates. All models are additionally adjusted for baseline GGT and the period of GGT-change assessment. GGT and triglycerides were log-transformed.
Estimated extended Cox proportional hazards models adjusted for age, body-mass-index, smoking status, occupational status, triglycerides, total cholesterol, systolic and diastolic blood pressure and bloodfrom glucose as time-varying covariates. All models are additionally adjusted for baseline GGT and period of GGT-change assessment. GGT and triglycerides were log-transformed.
In men, a pronounced increase in GGT was further independently associated with increased risk of total CVD mortality; the hazard ratio (95%CI) in comparison to stable GGT was 1.40 (1.09-1.81, p for trend 0.005). Although total CVD risk similarly was elevated for all categories of increasing GGT also in women, effects were less pronounced in comparison to men and not statistically significant. For a pronounced decrease in GGT concentrations over time, we observed a marked increase in CVD death for both genders; however, after full adjustment for confounding factors as time-varying covariates in our multivariate regression runs, these associations proved to be non-significant in both men and women (Tables 3 and 4). In CVD subcategories, a pronounced increase in GGT was significantly associated with stroke mortality in men (hazard ratio in comparison to stable GGT 1.89 (1.11-3.22), p for trend 0.002, Table 3), while we found no such association in women. In women, however, pronounced increasing GGT slightly increased risk for fatal CHD (hazard ratio in comparison to stable GGT 1.42 (0.96-2.10), p for trend 0.06), while this association was not observable for men.
Exclusion of the first two years of follow-up in all above analyses did not change our findings. Including the root mean square error of the slope of GGT-change for participants with at least 3 GGT measurements as an additional covariate in our regression models did not indicate an independent effect of variability of GGT on risk of total or CVD mortality in neither, men nor women. When recalculating all results, excluding participants with elevated GGT at baseline (>60 U/L in men and >36 U/L in women) and/or participants increasing into the abnormal range of GGT, the above reported associations of increasing GGT with CVD mortality remained stable in magnitude of effect in both men and women (Figure 1, Panels A and B; Supplemental Tables 2 and 3). Additionally, for the association of GGT-change with CHD mortality in women, the hazard ratio for a pronounced GGT-increase in comparison to stable GGT markedly increased to 1.80 (1.15-2.81), with a test for trend across categories of increasing GGT becoming highly significant (p=0.008, Supplementary Table 3).
Figure 1. Panels a and b.
Adjusted cumulative survival from all cardiovascular events according to categories of 7-year-increase of serum gamma-glutamyltransferase (GGT) among A) 26,105 male (fatal CVD events n=700) and B) 36,822 female (fatal CVD events n=687) Austrian adults in the VHM&PP estimated at the average values of covariates. Participants with baseline GGT concentrations >60 U/L in men and >36 U/L in women and/or participants increasing into the abnormal range of GGT (>60 U/L in men and >36 U/L in women) were excluded. Survival curves were estimated from extended Cox proportional hazards models adjusted for baseline log-GGT, period of GGT-change assessment and age, body-mass-index, smoking status, occupational status, log-triglycerides, total cholesterol, systolic and diastolic blood pressure and blood glucose as time-varying covariates.
Adjusted cumulative overall mortality form cardiovascular disease (n=1955) according to categories of 7-year GGT-Change among A) 33265 male and B) 43748 female Austrian adults in the VHM&PP estimated at the average values of covariates. Survival curves were calculated from Cox proportional hazards models adjusted for age, body-mass index, smoking status, occupational status, triglycerides, total cholesterol, systolic and diastolic blood pressure, blood glucose, baseline GGT and period of GGT-Change assessment.
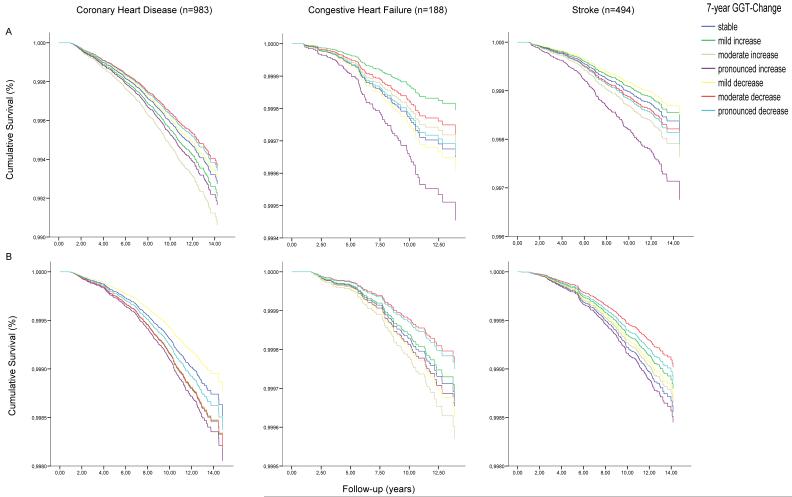
Age of participants significantly modified the association between GGT-change and CVD mortality for both men and women (p for multiplicative interaction age*GGT-change, both <0.001). In stratified analyses, the association of GGT-change with total CVD mortality markedly increased in male participants aged ≤60 years at baseline (hazard ratio for pronounced increasing GGT vs. stable GGT 1.72 (1.10-2.69, p for trend 0.009), Figure 2, Panel A) and was attenuated to a non-significant level in men aged >60 years at baseline (hazard ratio for pronounced increasing GGT vs. stable GGT 1.29 (0.93-1.79, p for trend 0.065; Supplemental Table 4). A similar age-interaction was observable for women, although effects were still less pronounced and did not reach statistical significance (Figure 2, Panel B, Supplemental Table 5). Likewise, also the predictive value of GGT, modelled as baseline variable only or as time-varying covariate, was attenuated in participants aged >60 years at enrolment (Supplemental Tables 4 and 5) and entirely disappeared when using a cut-off value of 75 years (data not shown).
Figure 2. Panels a and b.
Adjusted cumulative survival from all cardiovascular events according to categories of 7-year-increase of serum gamma-glutamyltransferase (GGT) among A) 28,681 male (fatal CVD events n=358) and B) 37,987 female (fatal CVD events n=188) Austrian adults in the VHM&PP aged <60 years at enrolment. Survival curves were estimated at the average values of covariates from extended Cox proportional hazards models adjusted for baseline log-GGT, period of GGT-change assessment and age, body-mass-index, smoking status, occupational status, log-triglycerides, total cholesterol, systolic and diastolic blood pressure and blood glucose as time-varying covariates.
Adjusted cumulative mortality form coronary heart disease, congestive heart failure and stroke according to categories of 7-year GGT-Change among A) 33265 male and B) 43748 female Austrian adults in the VHM&PP estimated at the average values of covariates. Survival curves were calculated from Cox proportional hazards models adjusted for age, body-mass index, smoking status, occupational status, triglycerides, total cholesterol, systolic and diastolic blood pressure, blood glucose, baseline GGT and period of GGT-Change assessment.
Discussion
The present study, involving more than 76,000 apparently healthy Austrian men and women across a wide age range, comprises the first epidemiologic investigation of the association of GGT-change over time with risk of subsequent CVD death. As suggested by previous investigations,7-14 our data confirm an independent effect of baseline GGT on CVD, especially in younger men. However, after adjustment for baseline GGT and other classical CVD risk factors as time-varying covariates, we still found male individuals, even with baseline GGT concentrations within the normal range (<60 U/L), exhibiting a longitudinal 7-year increase in GGT >9.17 U/L, to have a 36% greater risk of fatal cardiovascular events, in comparison to male individuals with stable GGT concentrations. This risk substantially increased to 72% for men ≤60 years at baseline, strongly suggesting that not only GGT per se, but also a longitudinal increase in GGT, even within its normal range, may be related to adverse cardiovascular/cerebrovascular outcome in men. Although similar associations of longitudinally increasing GGT with CVD mortality were observable in women, these effects were less pronounced in comparison to men. In line with this, sex-specific differences in trends of CVD have previously been well established, although the precise underlying mechanisms remain unclear.35,36 Mortality rates from heart disease differ across the life course of men and women, both in timing of incidence and in clinical presentation, attributed by some investigators to alterations in lipoprotein and hemostatic systems occurring in the postmenopausal period in women.37 Additionally, women in our cohort had substantially lower absolute levels of GGT, resulting in lower differences among categories of GGT-change and experienced fatal CVD events lagged by almost a decade after men, with 10-year follow-up analyses possibly not capturing this fully.
In the only epidemiologic investigation related to the topic, André and colleagues25 recently demonstrated that a 3-year increase in GGT (>5 U/L), in comparison to decreasing GGT during the same time period, was associated with a significant increase of risk for incident type 2 diabetes in both sexes, after adjusting for age and baseline GGT. After further adjustment for several confounding factors, including body-mass-index, smoking habits and fasting insulin, this association was attenuated to borderline significance in women, while even more pronounced in men.
It has been shown that a change in GGT over time is correlated with a longitudinal change of other CVD risk factors. However, these associations proved to exhibit a strong sex-specific pattern.24 In males, a 7-year increase in GGT was reported to positively correlate with an increase in body-mass-index, total serum cholesterol, HDL cholesterol and number of cigarettes per day. Conversely, in women, longitudinal change of all the above reported CVD risk factors, with the exception of body-mass-index, showed negative correlations with increasing GGT. Increase in frequency of inebriation resulted in an increase in GGT only in males. In women, increasing GGT was positively associated with use of oral contraceptives and occurrence of menopause.24
Given the epidemiological nature of our observations, the mechanisms, causing a longitudinal change in GGT over the 7-year assessment period, cannot be directly addressed in the present study. Despite evidence for a sensible secular trend in GGT during the past few decades,19,24,38 several cross-sectional studies have reported a positive association between age and serum GGT. However, this age-effect was estimated to be of approximately 3 U/L during a 7-year time period19 and thus, cannot sufficiently explain a pronounced variation in the extreme categories of GGT-change in our cohort. Considering mild to moderate variation in GGT during the 7-year assessment period, it cannot be ruled out, however, that intra-individual variability or methodological artefacts, including measurement error, year-to-year laboratory variation or “regression towards the mean” may have resulted in minor classification bias.
Our study had several strengths and potential limitations that should be considered. Major strengths are the prospective design, the large sample size, length of follow-up, and the standardized protocol performed by experienced physicians. Even though information on all major CVD risk factors was collected, our study was unable to account for additional factors that might have residually confounded the relationship between GGT-change and CVD death, including lipid subfractions or apolipoproteins, C-reactive protein, homocysteine, alcohol consumption, physical activity, diet, and genetic and psychosocial factors. A further limitation of this study is the inability to examine the effect of medication use (e.g. statins, antihypertensive drugs) on the relationship of GGT-change with CVD. In regard to statins, however, there is little, if any effect, as 75% of the study participants were examined before the implementation of statin therapy in Austria in 1995.
Alcohol consumption has not been documented routinely in the VHM&PP and we therefore, were unable to examine for confounding or effect modification by alcohol use. Numerous epidemiological studies have investigated the relationship of alcohol consumption to heart disease and stroke, and there is evidence supporting a moderate beneficial effect of alcohol consumption on CVD risk.39 However, because of its multiple pathways of both positive and negative influences, there is still no conclusive evidence about its biological mechanism. It has been shown that alcohol affects lipid metabolism, as well as hemostatic and oxidative factors.40,41 Mainly because of caloric intake, alcohol consumption has been shown to be associated with obesity and elevated blood pressure.42 Although our findings of longitudinally increasing GGT showing more prominent effects in males suggests a role of alcohol, GGT was recently reported to increase risk for CVD in never-drinkers as well, as shown in a study of non-drinking Japanese women.10 Moreover, the observed monotonic increasing trend of all-cause and CVD mortality with increasing GGT, among both men and women is unlikely to simply reflect the effects of alcohol consumption since previous studies have rather shown U-or J-shaped relationships of alcohol drinking to cardiovascular and all-cause mortality.39,43,44 Additionally, in a randomly selected sub-sample of 731 participants from the VHM&PP that provided self-reported alcohol data, only a weak, though statistically significant age- and sex-adjusted correlation of GGT with the average number of alcoholic units per week was observed (r=0.12).45
In summary, the present study prospectively investigated the association of longitudinal change in serum GGT with risk of subsequent all-cause and CVD mortality in more than 76,000 Austrian men and women. Our findings, for the first time demonstrate that a longitudinal increase in GGT, independently of baseline GGT and even within its normal range, significantly increases risk of fatal CVD and all-cause mortality, particularly in individuals at younger ages. Although our findings need to be confirmed in other populations, longitudinal monitoring of GGT-change may be beneficial in primary prevention of CVD, especially since GGT is a low cost and widely used laboratory measurement.
Supplementary Material
Acknowledgements
We would like to thank all the participants and physicians of the VHM&PP. We are grateful to the Government of the State of Vorarlberg, Austria for funding the program.
Sources of funding
This work was supported by Austrian National Bank Grant OENB-12737 (to H.U.). Dr. Brant was supported by funds from the intramural research program of the National Institute on Aging.
Footnotes
Conflicts of interest None
Condensed abstract We prospectively investigated the association of longitudinal GGT-change with CVD mortality in 76,113 men and women. We found increasing GGT, even within its normal range, to significantly increase risk of fatal CVD, independently of baseline GGT and other classical CVD risk factors.
References
- 1.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- 2.Emdin M, Passino C, Michelassi C, Titta F, L’abbate A, Donato L, Pompella A, Paolicchi A. Prognostic value of serum gamma-glutamyl transferase activity after myocardial infarction. Eur Heart J. 2001;22:1802–7. doi: 10.1053/euhj.2001.2807. [DOI] [PubMed] [Google Scholar]
- 3.Meister A. Metabolism and transport of glutathione and other gamma-glutamyl compounds. In: Larsson A, Orrenius S, Holmgren A, Mannervik B, editors. Functions of glutathione: biochemical, toxicological and clinical aspects. Raven Press; New York: 1983. pp. 1–22. [Google Scholar]
- 4.Rollason JG, Pincherle G, Robinson D. Serum gammaglutamyltranspeptidase in relation to alcohol consumption. Clin Chim Acta. 1972;39:75–80. doi: 10.1016/0009-8981(72)90301-4. [DOI] [PubMed] [Google Scholar]
- 5.Skinner HA, Holt S, Schuller R, Roy J, Israel Y. Identification of alcohol abuse using laboratory tests and a history of trauma. Ann Intern Med. 1984;101:847–51. doi: 10.7326/0003-4819-101-6-847. [DOI] [PubMed] [Google Scholar]
- 6.Kazemi-Shirazi L, Endler G, Winkler S, Schickbauer T, Wagner O, Marsik C. Gamma glutamyltransferase and long-term survival: is it just the liver? Clin Chem. 2007;53:940–6. doi: 10.1373/clinchem.2006.081620. [DOI] [PubMed] [Google Scholar]
- 7.Pompella A, Emdin M, Passino C, Paolicchi A. The significance of serum gamma-glutamyltransferase in cardiovascular diseases. Clin Chem Lab Med. 2004;42:1085–91. doi: 10.1515/CCLM.2004.224. [DOI] [PubMed] [Google Scholar]
- 8.Ruttmann E, Brant LJ, Concin H, Diem G, Rapp K, Ulmer H, the Vorarlberg Health Monitoring and Promotion Program Study Group γ-Glutamyltransferase as a risk factor for cardiovascular disease mortality. An epidemiological investigation in a cohort of 163944 Austrian adults. Circulation. 2005;112:2130–7. doi: 10.1161/CIRCULATIONAHA.105.552547. [DOI] [PubMed] [Google Scholar]
- 9.Meisinger C, Döring A, Schneider A, Löwel H, KORA Study Group Serum gamma-glutamyltransferase is a predictor of incident coronary events in apparently healthy men from the general population. Atherosclerosis. 2006;189:297–302. doi: 10.1016/j.atherosclerosis.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Hozawa A, Okamura T, Kadowaki T, Murakami Y, Nakamura K, Hayakawa T, Kita Y, Nakamura Y, Okayama A, Ueshima H, NIPPON DATA90 Research Group Gamma-Glutamyltransferase predicts cardiovascular death among Japanese women. Atherosclerosis. 2007;194:498–504. doi: 10.1016/j.atherosclerosis.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 11.Wannamethee G, Ebrahim S, Shaper AG. Gamma-glutamyltransferase: determinants and association with mortality from ischemic heart diseases and all cause. Am J Epidemiol. 1995;142:699–708. doi: 10.1093/oxfordjournals.aje.a117699. [DOI] [PubMed] [Google Scholar]
- 12.Jousilahti P, Rastenyte D, Tuomilehto J. Serum gamma-glutamyl transferase, self reported alcohol drinking, and the risk of stroke. Stroke. 2000;31:1851–5. doi: 10.1161/01.str.31.8.1851. [DOI] [PubMed] [Google Scholar]
- 13.Bots ML, Salonen JT, Elwood PC, Nikitin Y, de Concalves A Freire, Inzitari D, Sivenius J, Trichopoulou A, Tuomilehto J, Koudstaal PJ, Grobbee DE. Gamma-glutamyltransferase and risk of stroke: the EUROSTROKE project. J Epidemiol Community Health. 2002;56(suppl1):25–9. doi: 10.1136/jech.56.suppl_1.i25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee DH, Silventoinen K, Hu G, Jacobs DR, Jr, Jousilahti P, Sundvall J, Tuomilehto J. Serum gamma-glutamyltransferase predicts non-fatal myocardial infarction and fatal coronary heart disease among 28838 middle-aged men and women. Eur Heart J. 2006;27:2170–6. doi: 10.1093/eurheartj/ehl086. [DOI] [PubMed] [Google Scholar]
- 15.Lee DS, Evans JC, Robins SJ, Wilson PW, Albano I, Fox CS, Wang TJ, Benjamin EJ, D’Agostino RB, Vasan RS. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2007;27:127–33. doi: 10.1161/01.ATV.0000251993.20372.40. [DOI] [PubMed] [Google Scholar]
- 16.Rantala AO, Lilja M, Kauma H, Savolainen MJ, Reunanen A, Kesaniemi YA. Gamma-glutamyl transpeptidase and the metabolic syndrome. J Intern Med. 2000;248:230–8. doi: 10.1046/j.1365-2796.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee DH, Ha MH, Kim JR, Gross M, Jacobs DR., Jr Gamma-glutamyltransferase, alcohol, and blood pressure. A four year follow-up study. Ann Epidemiol. 2002;12:90–6. doi: 10.1016/s1047-2797(01)00252-6. [DOI] [PubMed] [Google Scholar]
- 18.Lee DH, Ha MH, Kim JH, Christiani DC, Gross MD, Steffes M, Blomhoff R, Jacobs DR., Jr Gamma-glutamyltransferase and diabetes - a 4 year follow-up study. Diabetologia. 2003;46:359–64. doi: 10.1007/s00125-003-1036-5. [DOI] [PubMed] [Google Scholar]
- 19.Lee DH, Ha MH, Kam S, Chun B, Lee J, Song K, Boo Y, Steffen L, Jacobs DR., Jr A strong secular trend in serum gamma-glutamyltransferase from 1996 to 2003 among South Korean men. Am J Epidemiol. 2006;163:57–65. doi: 10.1093/aje/kwj006. [DOI] [PubMed] [Google Scholar]
- 20.Lee DH, Blomhoff R, Jacobs DR. Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res. 2004;38:535–9. doi: 10.1080/10715760410001694026. [DOI] [PubMed] [Google Scholar]
- 21.Ross JS, Stagliano NE, Donovan MJ, Breitbart RE, Ginsburg GS. Atherosclerosis and cancer: common molecular pathways of disease development and progression. Ann N Y Acad Sci. 2001;947:271–92. [PubMed] [Google Scholar]
- 22.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–85. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 23.Ulmer H, Kelleher C, Diem G, Concin H. Long-term tracking of cardiovascular risk factors among men and women in a large population-based health system: the Vorarlberg Health Monitoring & Promotion Programme. Eur Heart J. 2003;24:1004–13. doi: 10.1016/s0195-668x(03)00170-2. [DOI] [PubMed] [Google Scholar]
- 24.Nilssen O, Førde OH. Seven-year longitudinal population study of change in gamma-glutamyltransferase: The Tromsø Study. Am J Epidemiol. 1994;139:787–92. doi: 10.1093/oxfordjournals.aje.a117075. [DOI] [PubMed] [Google Scholar]
- 25.André P, Balkau B, Born C, Charles MA, Eschwège E, D.E.S.I.R. study group Three-year increase of gamma-glutamyltransferase level and development of type 2 diabetes in middle-aged men and women: the D.E.S.I.R. cohort. Diabetologia. 2006;49:2599–603. doi: 10.1007/s00125-006-0418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strasak A, Ruttmann E, Brant L, Kelleher C, Klenk J, Concin H, Diem G, Pfeiffer KP, Ulmer H, the VHM&PP Study Group Serum uric acid and risk of cardiovascular mortality: A prospective long-term study in 83683 Austrian men. Clin Chem. 2008;54:273–84. doi: 10.1373/clinchem.2007.094425. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization International Classification of Diseases (ICD) http://www.who.int/classifications/icd/en
- 28.Lissner L, Odell PM, D’Agostino RB, Stokes J, 3rd, Kreger BE, Belanger AJ, Brownell KD. Variability of body weight and health outcomes in the Framingham population. N Engl J Med. 1991;324:1839–44. doi: 10.1056/NEJM199106273242602. [DOI] [PubMed] [Google Scholar]
- 29.Rapp K, Klenk J, Ulmer H, Concin H, Diem G, Oberaigner W, Schroeder J. Weight change and cancer risk in a cohort of more than 65 000 adults in Austria. Ann Oncol. 2008;19:641–8. doi: 10.1093/annonc/mdm549. [DOI] [PubMed] [Google Scholar]
- 30.Golla A, Strauch K, Dietter J, Baur MP. Quantitative trait linkage analysis of longitudinal change in body weight. BMC Genet. 2003;4(Suppl 1):S7. doi: 10.1186/1471-2156-4-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. Springer; New York: 2000. [Google Scholar]
- 32.Feldman HA. Families of lines: random effects in linear regression analysis. J Appl Physiol. 1988;64:1721–32. doi: 10.1152/jappl.1988.64.4.1721. [DOI] [PubMed] [Google Scholar]
- 33.Drøyvold WB, Nilsen TI Lund, Lydersen S, Midthjell K, Nilsson PM, Nilsson JA, Holmen J, the Nord-Trøndelag Health Study Weight change and mortality: the Nord-Trøndelag Health Study. J Intern Med. 2005;257:338–45. doi: 10.1111/j.1365-2796.2005.01458.x. [DOI] [PubMed] [Google Scholar]
- 34.Verbeke G, Lesaffre E. A linear mixed-effects model with heterogeneity in the random-effects population. J Am Stat Assoc. 1996;91:217–21. [Google Scholar]
- 35.Wingard DL, Suarez L, Barrett-Connor E. The sex differential in mortality from all causes and ischemic heart disease. Am J Epidemiol. 1983;117:165–72. doi: 10.1093/oxfordjournals.aje.a113527. [DOI] [PubMed] [Google Scholar]
- 36.Barrett-Connor E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation. 1997;95:252–64. doi: 10.1161/01.cir.95.1.252. [DOI] [PubMed] [Google Scholar]
- 37.Meade TW, Dyer S, Howarth DJ, Imeson JD, Stirling Y. Antithrombin- III and procoagulant activity—sex differences and effects of the menopause. Br J Haematol. 1990;74:77–81. doi: 10.1111/j.1365-2141.1990.tb02541.x. [DOI] [PubMed] [Google Scholar]
- 38.Ulmer H, Kelleher CC, Fitz-Simon N, Diem G, Concin H. Secular trends in cardiovascular risk factors: an age-period cohort analysis of 698,954 health examinations in 181,350 Austrian men and women. J Intern Med. 2007;261:566–76. doi: 10.1111/j.1365-2796.2007.01779.x. [DOI] [PubMed] [Google Scholar]
- 39.Hill DA. In vino veritas: alcohol and heart disease. Am J Med Sci. 2005;329:124–35. doi: 10.1097/00000441-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 40.De Oliveira E Silva ER, Foster D, Harper M McGee, Seidman CE, Smith JD, Breslow JL, Brinton EA. Alcohol consumption raises HDL cholesterol levels by increasing the transport rate of apolipoproteins A-I and A-II. Circulation. 2000;102:2347–52. doi: 10.1161/01.cir.102.19.2347. [DOI] [PubMed] [Google Scholar]
- 41.Mukamal KJ, Jadhav PP, D’Agostino RB, Massaro JM, Mittleman MA, Lipinska I, Sutherland PA, Matheney T, Levy D, Wilson PW, Ellison RC, Silbershatz H, Muller JE, Tofler GH. Alcohol consumption and hemostatic factors: analysis of the Framingham Offspring cohort. Circulation. 2001;104:1367–73. doi: 10.1161/hc3701.096067. [DOI] [PubMed] [Google Scholar]
- 42.Langer RD, Criqui MH, Reed DM. Lipoproteins and blood pressure as biological pathways for effect of moderate alcohol consumption on coronary heart disease. Circulation. 1992;85:910–5. doi: 10.1161/01.cir.85.3.910. [DOI] [PubMed] [Google Scholar]
- 43.Leppala JM, Paunio M, Virtamo J, Fogelholm R, Albanes D, Taylor PR, Heinonen OP. Alcohol consumption and stroke incidence in male smokers. Circulation. 1999;100:1209–14. doi: 10.1161/01.cir.100.11.1209. [DOI] [PubMed] [Google Scholar]
- 44.Gronbaek M, Johansen D, Becker U, Hein HO, Schnohr P, Jensen G, Vestbo J, Sorensen TI. Changes in alcohol intake and mortality: a longitudinal population-based study. Epidemiology. 2004;15:222–8. doi: 10.1097/01.ede.0000112219.01955.56. [DOI] [PubMed] [Google Scholar]
- 45.Ulmer H, Diem G, Bischof HP, Ruttmann E, Concin H. Recent trends and sociodemographic distribution of cardiovascular risk factors: results from two population surveys in the Austrian WHO CINDI demonstration area. Wien Klin Wochenschr. 2001;113:573–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.