Abstract
Activation of surface membrane receptors coupled to phospholipase C results in the generation of cytoplasmic Ca2+ signals comprised of both intracellular Ca2+ release, and enhanced entry of Ca2+ across the plasma membrane. A primary mechanism for this Ca2+ entry process is attributed to store-operated Ca2+ entry, a process that is activated by depletion of Ca2+ ions from an intracellular store by inositol 1,4,5-trisphosphate. Our understanding of the mechanisms underlying both Ca2+ release and store-operated Ca2+ entry have evolved from experimental approaches that include the use of fluorescent Ca2+ indicators and electrophysiological techniques. Pharmacological manipulation of this Ca2+ signaling process has been somewhat limited; but recent identification of key molecular players, STIM and Orai family proteins, has provided new approaches. Here we describe practical methods involving fluorescent Ca2+ indicators and electrophysiological approaches for dissecting the observed intracellular Ca2+ signal to reveal characteristics of store-operated Ca2+ entry, highlighting the advantages, and limitations, of these approaches.
I. Introduction
In many cell types, activation of hormone, neurotransmitter, or growth factor receptors coupled to phospholipase-C results in the breakdown of phosphatidylinositol 4,5-bisphoshate, resulting in production of inositol 1,4,5-trisphosphate (IP3) which stimulates a Ca2+ signaling process that is biphasic [1,2]. This biphasic response involves the release of Ca2+ ions from an intracellular organelle, the endoplasmic reticulum (ER) or a specialized component of the ER, followed by the entry of Ca2+ ions across the plasma membrane. Much is known about the first phase of intracellular Ca2+ release from an intracellular organelle, an effect mediated by IP3 acting on its own receptor, the IP3 receptor [3]. Until recently, however, the mechanisms regulating the Ca2+ entry process have been less well understood, although there is a basic and well established concept for this second phase of Ca2+ entry. That is, the degree of emptying of the Ca2+-storage organelle, generated by intracellular Ca2+ release, initiates a retrograde signaling process that regulates the rate of Ca2+ entry across the plasma membrane. This process is known as capacitative Ca2+ entry or store–operated Ca2+ entry (SOCE) [4]. The signaling processes underlying SOCE have been the subject of intense study for more than 20 years, yet only recently have the key molecular components been identified. Stim family proteins (Stim1 and 2), appear to function as Ca2+ sensors within the ER, and Orai family proteins (Orai1, 2 and 3, also known as CRACM1, 2 and 3) function as SOC channels in the plasma membrane (for recent reviews, see [5,6,7,8]).
Identification of the molecular makeup of the SOCE pathway has been facilitated by optical techniques utilizing fluorescent Ca2+ indicators [9,10]. Although the primary focus of this volume is optical techniques, for the study of Ca2+ influx mechanisms it is almost always advisable to combine the use of fluorescent indicators with electrophysiological techniques, and these will be discussed in this review as well. In developing these methodological approaches it is important to discriminate SOCE from other pathways that may influence [Ca2+]i. For example, additional mechanisms initiated by phospholipase C activation that can regulate Ca2+ entry not related to SOCE have been reported in non-excitable cells [11].
II. Fluorescence-based Measurements of SOCE
Fluorescence-based measurements of [Ca2+]i have provided a robust and widely used technique for monitoring Ca2+ signaling processes, including SOCE. These fluorescence based techniques have been productive due to the availability of a broad range of Ca2+ indicators that can be easily introduced into intact cells, and an extensive range of ‘turnkey’ equipment for measuring Ca2+ with good temporal and spatial resolution.
A. Fluorescent Ca2+ Indicator Selection
The choice of fluorescent Ca2+ indicator is the foundation for a successful study of Ca2+ signaling. It can influence the spatial and temporal information that one can collect, and the choices one has for analyzing a response. This selection will also be influenced by the available equipment for measuring fluorescence, such as excitation wavelength selection.
Single Wavelength and Ratiometric Ca2+ Indicators
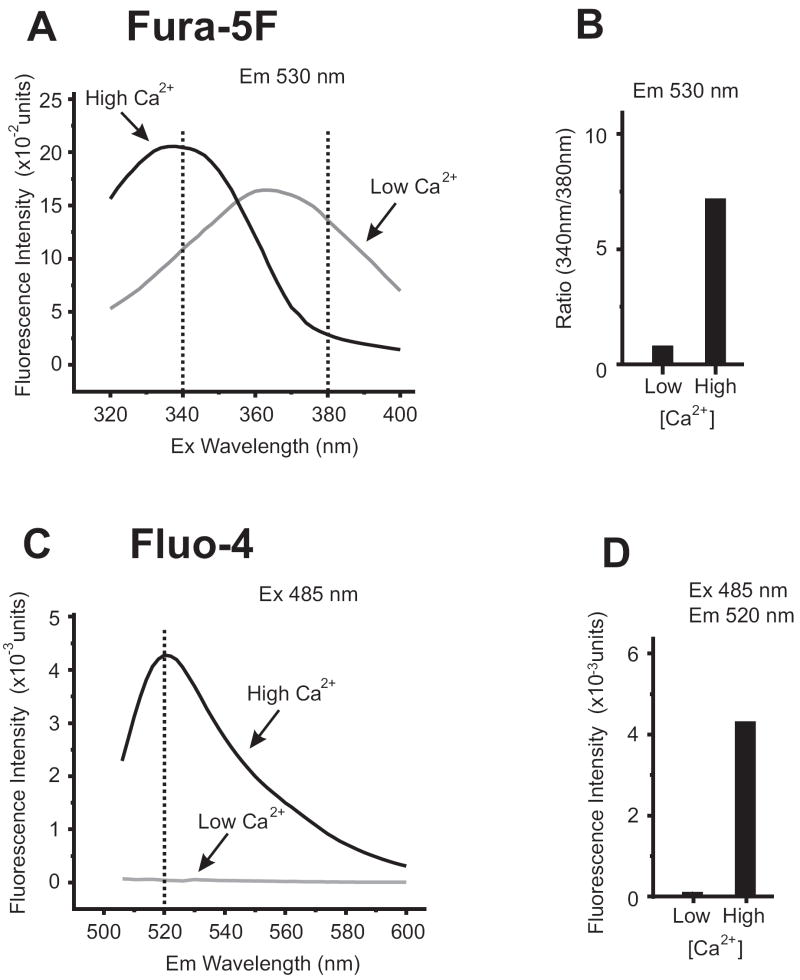
The working core of a Ca2+ indicator is centered around its ability to reversibly bind Ca2+ ions, with affinities that lie within the physiological range of cytoplasmic [Ca2+]i. With most Ca2+ indicators, such as fluo dyes, the level of [Ca2+]i can be directly monitored as a change in fluorescence intensity of the indicator where, usually, elevation of [Ca2+]i leads to higher intensity of fluorescence at a single wavelength (Fig.1) . Alternatively, there are some Ca2+ indicators that exhibit a spectral shift upon binding Ca2+ ions [12] such as the Indo and fura dyes (Fig.1). Using spectral shift indicators has the enormous advantage of providing ratiometric [Ca2+]i measurements that are independent of the concentration of the Ca2+ indicator present in the cells.
Figure. 1. Spectral Characteristic of Ratiometric and Single Wavelength Ca2+Indicators.
(A) Emission spectra of fura-5F (free-acid form) recorded at 530nm while scanning excitation wavelengths from 320-400nm. With 10 μM fura-5F dissolved in buffer containing 100 mM KCl, 20 mM HEPES, pH 7.2, switching between ‘low Ca2+‘ (buffer +200 μM BAPTA) and ‘high Ca2+’ (buffer + 1 mM CaCl2) conditions demonstrates the spectral shift characteristics of fura-5F. This [Ca2+] change can be quantified by ratioing the emission fluorescence measured at 340nm and 380nm excitation wavelengths, as shown in (B).
(C) Spectra for fluo-4 (free-acid form) was recorded with excitation at 485nm while scanning emission wavelengths from 500-600nm. Using the same buffers as in (A), switching between ‘low Ca2+‘(buffer +200 μM BAPTA) and ‘high Ca2+’ (buffer + 1 mM CaCl2) demonstrates the fluorescence intensity change in the spectra. This [Ca2+] change can be quantified by selecting a single emission wavelength at which the intensity change is maximal, as shown in (D) (measured at 520nm).
Ca2+ Binding Affinity and Ion Selectivity
Different Ca2+ indicator dyes bind Ca2+ with different affinities, and Ca2+ affinity is therefore an important consideration when choosing a dye. For example, for most experimental situations fura-5F has proven a useful, if not better, choice than fura-2 for measuring cytoplasmic [Ca2+]i. A derivative of fura-2, fura-5F is handled in exactly the same way in terms of cell loading and recording of fluorescence signals. However, fura-5F has a higher KD than fura-2 (400 nM vs. 140 nM, respectively), and given that most Ca2+ studies examine [Ca2+]i in the 100 nM – 1 μM range, the advantages of the higher KD indicator are obvious. The characteristics of fura-5F are shown in Fig.1 and tables 1 and 2, as are those of another commonly used single wavelength Ca2+ indicator, fluo-4.
Table 1.
General Characteristics of Fura-5F and Fluo4
| Ratiometric | Ba2+-entry | Mn2+-quench | KD (nM) | |
|---|---|---|---|---|
| Fura-5F | Yes | Yes | Yes | 400 |
| Fluo-4 | No | No | No | 390 |
Table 2.
Spectral and biological effects of Ca2+ and other ions on Ca2+ indicators and Ca2+ signaling processes.
| Ca2+ | Ba2+ | La3+ | Gd3+ | Mn2+ | |
|---|---|---|---|---|---|
| Fura Signal | Yes | Yes | Yes | Yes | No |
| Fura Quench | No | No | No | No | Yes |
| Fluo-4 Signal | Yes | Poor | Yes | Yes | Poor |
| Fluo-4 Quench | No | No | No | No | No |
| Block SOCE | No | No | Yes | Yes | No |
| Substrate for PMCA/SERCA | Yes | No | ? | ? | No |
| Block PMCA | No | No | Yes | Yes | ? |
While it may seem intuitive that a Ca2+ indicator should be selected based on its selectivity for Ca2+ ions over other cations, the ability of these indicators to interact with other cations can provide experimental flexibility for studying Ca2+ signaling and SOCE. This has been particularly useful with cations such as Mn2+ and Ba2+ which, in addition to their ability to interact with Ca2+ indicators, can also substitute for Ca2+ ions in a number of biological processes, or pharmacologically interfere with these same processes (Table 2). Thus, experimental approaches can be designed that involve substituting alternative cations for Ca2+ ions and these approaches may then reveal specific aspects of the underlying Ca2+ signaling process. This has been a particularly useful approach with the fura-based Ca2+ indicators and substituting Mn2+ [13] or Ba2+ [14,15] for Ca2+ ions (see below).
Introduction of Ca2+ Indicators into Cells
Since Ca2+ indicators are charged molecules and do not freely cross biological membranes, a critical factor for ensuring their successful application to biological systems was developing a way to load the indicator into the cytoplasm of intact cells. Fortunately, an innovative approach was developed whereby charged residues were chemically masked with acetoxy methyl ester groups (AM groups) [16]. The uncharged products cross the plasma membrane into the cytoplasm where non-specific esterases cleave the AM groups leaving the charged and Ca2+-sensitive form of the indicator to accumulate in that cell compartment.
One complicating problem associated with AM derivatives of Ca2+ indicators is that their accumulation may not be restricted to the cell cytoplasm. That is, the AM derivative may cross other membranes and accumulate in compartments such as the ER and mitochondria. The extent of this compartmentalization can vary depending on the type of cell being used. For example, no such problem is apparent with HEK 293 cells, whereas it presents a major obstacle in primary rat hepatocytes [17]. Fortunately, there are ways to diagnose and minimize this problem. Diagnosis can include: (i) A non-uniform and punctate spatial distribution of the Ca2+ indicator; (ii) Calibrated Ca2+ indicator values indicative of high resting cytoplasmic [Ca2+]i; (iii) Poor or lack of agonist-induced [Ca2+]i response; (iv) A combination of agonist and a sarco-endoplasmic reticulum Ca2+-ATPase (SERCA)-pump inhibitor (thapsigargin) leads to a drop in resting cytoplasmic [Ca2+] levels (See [17,18]). Minimizing compartmentalization can usually be achieved by manipulating indicator concentration, duration of incubation with AM-indicator, and the temperature of the incubation. In the event that compartmentalization cannot be resolved, one has the option of introducing free acid forms of Ca2+ indicators directly into the cytoplasm by techniques such as microinjection or electroporation [17,19].
Leakage of Ca2+ indicators out of the intact cell is an additional complication with contributing factors including the specific Ca2+ indicator choice and/or the cell type being used. In some instances, the problem appears due to the Ca2+ indicator being a substrate for organic anion transporters, an effect that can be minimized by including anion transport inhibitors such as probenicid and sulfinpyrazone in all experimental solutions [20].
Data Representation
Due to the non-linear relationship between Ca2+ indicator fluorescence changes and [Ca2+]i, calibration of the fluorescence signal [12] is advisable for instances such as looking for subtle quantitative effects on [Ca2+]i signaling, or as a means to compare experimental data collected from different instrumentation. However, in many instances a general understanding of [Ca2+]i signaling mechanisms such as SOCE can be gleaned from fluorescence intensities or fluorescence ratios. If uncalibrated ratios are used it is minimally recommended that you (i) ensure the Ca2+ indicator used has an appropriate affinity to maximize the dynamic range of the measurement and avoid the possibility of saturating the indicator, and (ii) correct data for fluorescence signals not related to Ca2+-sensitive fluorescence changes (auto-fluorescence), which minimizes cell-to-cell variation as well as variability between experiments.
In the case of ratiometric fura dyes, auto-fluorescence is readily estimated by quenching fura dyes with Mn2+ ions (treat cells with ionomycin and MnCl2, 10 μM and 20 mM, respectively, in a nominally Ca2+-free bathing solution). Single wavelength indicators like fluo-4 and Calcium Green-1 are not completely quenched by Mn2+, thus non-indicator loaded cells may provide an estimation of autofluorescence.
B. Quantitative Assessment of SOCE
Activating SOCE: SERCA Inhibitors/Ca2+-Ionophores
As described above, depletion of intracellular Ca2+-stores located within the ER plays a key role in activating SOCE and is primarily achieved through IP3-induced Ca2+-mobilization during agonist activation. The ER also serves as a critical Ca2+-buffer, with Ca2+-ATPases (SERCA pumps) that can rapidly sequester Ca2+ ions from the cell cytoplasm to prevent untoward changes in [Ca2+]i and replenish ER Ca2+-stores following agonist activation. Even in unstimulated cells, Ca2+ ions are continually cycling across the ER membrane with the actions of SERCA pumps sequestering Ca2+ balanced against a poorly defined ‘Ca2+ leak’ process out of the ER (but see [21,22]). Fortunately, there are a number of pharmacological reagents that target and inhibit the function of the SERCA Ca2+-pumps and, with the ‘Ca2+ leak’ process in effect, results in depletion of ER Ca2+-stores and full activation SOCE (Fig.2B).
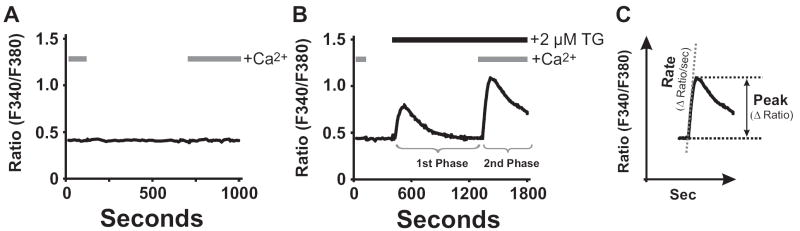
Figure. 2. ‘Ca2+re-addition’ Protocol and Biphasic Ca2+Signaling.
HEK 293 cells were loaded with fura-5F and intracellular [Ca2+]i measured as described in [57]. In (A) and (B), HEK 293 cells were subjected to the ‘Ca2+ re-addition’ protocol by switching between buffer solutions with extracellular Ca2+ present or absent. In unstimulated HEK 293 cells (A), the ‘Ca2+ re-addition’ protocol elicits no detectable change in [Ca2+]i. In (B), the ‘Ca2+ re-addition’ protocol was combined with treatment of cells with a SERCA pump inhibitor (2 μM thapsigargin) to demonstrate a biphasic Ca2+ response. In the absence of extracellular [Ca2+], the transient first phase of intracellular Ca2+ release is observed. On ‘Ca2+ re-addition’, the seconds phase of SOCE is observed. As shown in (C), the extent of SOCE activity can be quantified either as a rate of Ca2+ entry, or appeal response.
Discovery of SERCA inhibitors, namely thapsigargin, cyclopiazonic acid (CPA) and tBHQ [23] provided an important validation of the SOCE model of Ca2+ entry, especially as these reagents made it possible to deplete the IP3-sensitive Ca2+ stores and activate SOCE without formation of any inositol phosphates associated with agonist activation [24]. SERCA pump inhibitors are membrane permeant, and provide a non-invasive technique for manipulating ER Ca2+-pools and SOCE activation in intact cells. Activating SOCE with thapsigargin is, however, a difficult process to reverse. In contrast, CPA is more water soluble than thapsigargin, and appears more amenable to washing out of cells, and offers the possibility of achieving partial depletion of intracellular Ca2+ stores with a range of CPA concentrations, resulting in partial activation of SOCE [25].
An alternative to SERCA pump inhibition is to deplete ER Ca2+-pools using a Ca2+-ionophore such as ionomycin [26]. A carboxylic acid antibiotic, ionomycin is a mobile ion carrier that has proven effective in manipulating the movement of Ca2+ ions and intracellular Ca2+-pools in intact cells. Low concentrations of ionomycin (<1μM) appear to selectively release intracellular Ca2+-stores (ED50 = 50 nM) without directly increasing the permeability of the plasma membrane to extracellular Ca2+ [27,28]. At higher concentrations (1-10 μM), the ionophore increases the permeability of the plasma membrane, ER and mitochondria to Ca2+ ions.
In general, ionomycin is preferred over A23187 since, with fluorescent Ca2+ indicators excited in the UV range, A23187 can contribute significant autofluorescence to the measured signal (a non-fluorescent derivative, 4Br-A23187, is available [29].
‘Ca2+ Re-Addition’ Protocol: Dissecting out SOCE
In unstimulated cells, the concentration of Ca2+ ions in the cytoplasm (~10-7 M) reflects a steady state balance of homeostatic mechanisms involving Ca2+ pumps and channels. Cells utilize ATP-dependent Ca2+ pumps to maintain this level against a background of enormous Ca2+ gradients (extracellular milieu ~10-3 M; ER ~10-4 M). In addition in most, but not all cell types, plasma membrane permeability to Ca2+ is very low unless altered by a physiological or pathological mechanism. Figure 2A illustrates a principle method for disrupting steady state [Ca2+]i conditions by simply modifying extracellular Ca2+ conditions ([Ca2+]o). In this ‘Ca2+ re-addition’ protocol, the salt solution bathing cells is switched alternatively from one containing normal [Ca2+]o (1-2 mM) to a salt solution that is depleted or nominally Ca2+-free, and then back again to normal [Ca2+]o conditions. Depending on the water source and purity of salts used, nominally Ca2+-free buffers can contain [Ca2+] in the range of a few μM. Alternatively, one can ensure the solutions are Ca2+-free by supplementing them with a Ca2+-chelator such as EGTA or BAPTA (100-500 μM). As shown in Fig. 2A for an unstimulated HEK 293 cell loaded with fura-5F, the Ca2+ re-addition protocol does not result in any detectable change in [Ca2+]i (this is not always the case, for example see [30]). Characterizing this response in unstimulated cells is an important control to characterize the cell type being used and the full extent of SOCE activation measured in stimulated cells. In Fig.2B, the ‘Ca2+ re-addition’ protocol is now used in the same fura-5F-loaded HEK 293 cells, but this time treated with thapsigargin to empty Ca2+ stores and thus fully activate SOCE. In the absence of extracellular Ca2+, thapsigargin treatment results in a [Ca2+]i response that is transient, representing the release of Ca2+ ions from intracellular Ca2+ pools. On adding back extracellular Ca2+ to the bathing medium, the second phase of SOCE is revealed resulting in a rise in [Ca2+]i to an elevated and sustained phase. The cells will remain at this elevated [Ca2+]i level until the Ca2+-store depletion stimulus is removed, or [Ca2+]o is modified.
In summary, the basic approach described in the Ca2+ re-addition protocol is to separate the two phases of Ca2+ mobilization, Ca2+ release and SOCE. At the point of restoring [Ca2+]o, manipulations of the bathing solution, including cation substitutions, can enhance or distinguish Ca2+-entry pathways on the basis of their pharmacological properties (see below). An interesting variation of this protocol is a ‘Ca2+-overshoot protocol’. In this method, phospholipase C-coupled receptors are transiently activated in the absence of extracellular Ca2+, with Ca2+ re-addition occurring after the stimulus is removed. With this protocol, and despite the removal of the cell stimulus, intracellular Ca2+ pools remain depleted, and SOCE remains activated until extracellular Ca2+ is restored and Ca2+ pools refilled. In some cell types, this SOCE activity can be observed as a transient rise in [Ca2+]i, or‘Ca2+-overshoot’, following Ca2+ re-addition [31].
Quantifying SOCE: Peaks and Rates
Three general methods have been used to quantitatively analyze Ca2+ influx from experiments such as in Fig. 2C: initial rate of [Ca2+]i rise, peak [Ca2+]i level, and area under the [Ca2+]i curve. In theory at least, initial rates should give the cleanest indication of Ca2+ influx, although it is clear that cellular Ca2+ buffering occurs so rapidly that even this measure can be influenced by many factors. These factors can sometimes be minimized by the use of Ca2+ surrogates such as Ba2+ or Mn2+, which do not activate negative feedbacks and are poor substrates for cellular Ca2+ buffers. This is discussed in more detail below. Peak responses are probably more commonly reported, but these have the disadvantage that [Ca2+]i levels are often limited in non-linear ways by cellular feedback mechanisms and Ca2+ buffers. Least desirable is the use of integrated areas under the [Ca2+]i curve; these are generally sustained responses and thus the areas will depend upon arbitrarily selected time intervals, and will to varying degrees be even more affected by secondarily activated pumps and inactivation mechanisms.
Monitoring SOCE with Ca2+ Surrogates
The complexity of homeostatic mechanisms that regulate [Ca2+]i can lead to a number of potential artifacts when using Ca2+ ions to monitor SOCE in fluorescence-based experiments. Any mechanism that limits the extent of a [Ca2+]i rise can result in non-linear behaviors that belie appropriate quantitative analysis. These mechanisms can include activation of plasma membrane pumps to extrude Ca2+ ions and/or feedback inhibition of SOC channels. For example, a treatment that inhibits intracellular Ca2+-ATPases (such at thapsigargin) at the same time as activating SOCE may have a quantitatively different effect on elevating [Ca2+]i compared to SOCE activation by other means. These problems can sometimes by minimized by substituting for Ca2+ cations that will permeate through SOC channels but are poor substrates for other Ca2+-dependent enzymes such as Ca2+-pumps. Mn2+ or Ba2+ have proven very useful substitutes for [Ca2+]o for SOCE measurements, particularly when using the Ca2+ re-addition protocol. However, it should be noted that not all Ca2+-indicators behave in the same way to these Ca2+ surrogates (see Table 2).
Mn2+ ions appear to traverse most Ca2+-permeable channels [32] and substitute for Ca2+ in SOCE [33], despite the fact that CRAC channels are less permeable to Mn2+ than to Ca2+ [34]. In fluorescence-based [Ca2+]i measurements, fura-indicators are ideally suited to monitor Mn2+-entry as they irreversibly bind Mn2+ (due to high affinity for the cation), which quenches the fura fluorescence. Quantifying Mn2+-entry can easily be done by monitoring the Mn2+-quench process at fura-2’s isobestic wavelength (~360 nm), or by using an algebraic summation of fluorescence values obtained from excitation at two different wavelengths [35]. Thus, activation of SOCE activity can easily be quantified by measuring initial rates of Mn2+ quench in fura-loaded cells [36]. In quantifying SOCE activity, it is important to measure basal Mn2+-quench rates in unstimulated cells, since most cells exhibit a basal, constitutive permeability to Mn2+. A useful facet of the Mn2+-quench technique is that you can monitor Ca2+-release events and Mn2+-quench (SOCE) simultaneously [35,37].
There are some limitations with using the Mn2+-quench technique. One is that it is not applicable to all Ca2+-indicators, for example the single wavelength indicator Fluo-4 is poorly responsive to Mn2+ ions, and Calcium Green-1 fluorescence intensity is actually increased by Mn2+, not quenched. Another surrounds an artifact when applying Mn2+-quench to cells with significant compartmentalization of fura-indicators in the ER. While Mn2+ is not a substrate for intracellular SERCA pumps, it can easily pass through activated IP3 receptors in a retrograde manner [17]. This presents a problem when monitoring SOCE with agonist stimulation since the observed Mn2+-quench may reflect IP3 mediated movement into the ER rather than flux across the plasma membrane.
Monitoring Ba2+ entry by substituting Ba2+ for Ca2+ ions in the Ca2+ re-addition protocol has provided a useful measure of SOCE [15]. Fura-based indicators are amenable for this technique since Ba2+ can substitute for Ca2+ and render an excitation spectrum similar to that for Ca2+. Importantly, Ba2+ is a poor substrate for Ca2+-pumping ATPases and does not enter ER Ca2+-pools. Thus, monitoring Ba2+-entry during cell activation provides a measure of SOCE activity without complications due to buffering by Ca2+-pumps and Ca2+-pool refilling. As for Mn2+-quench, quantifying initial rates of Ba2+ entry is recommended, as well as taking into account basal rates of entry in the absence of cell stimulation. One potential artifact that could be encountered when using Ba2+ ions involves altering membrane potential [38], which can influence SOCE activity (see below).
III. Electrophysiological Measurement of SOC Currents
A. Biophysical Characteristics of Store-operated Currents
An understanding of SOCE and its regulation has been facilitated by the electrophysiological characterization of plasma membrane Ca2+ currents associated with SOCE. The most extensively studied and most thoroughly characterized store-operated current was first identified in mast cells as Ca2+ release-activated Ca2+ current, or Icrac [39,34]. Icrac is a small current, often below the level of reliable detection. It is largest in hematopoetic cells where it can be up to 2 pA/pF, under optimal recording conditions. But for many cell types, the measurement of store-operated entry by electrophysiological means has not always been a practical approach since the currents apparently fall near or below the limits of detection. For instance, in HEK293 cells the store-operated Ca2+ current is maximally only about 0.5 pA/pF. This limitation can be circumvented to some extent by carrying out experiments under conditions where cells are maintained in an extracellular solution that is divalent cation-free (DVF, see below). Studies on Icrac, indicate that the underlying channels have a small pore diameter [40], low permeability to Cs+, and an extremely small unitary conductance for Ca2+ and Na+ [34,41,40,42,43]. While no single channel events have been detected for Icrac, fluctuation analysis predicts a monovalent unitary conductance of less than 1 pS [40].
B. Biophysical Assessment of Store-operated Currents
Electrophysiological measurement of Icrac is usually carried out in whole-cell patch clamp mode (Fig.3A). This mode offers a number of experimental advantages, in particular the ability to define and control the conditions bathing the intracellular and extracellular milieu, and to precisely control membrane potential. With fluorescence-based [Ca2+]i measurements on SOCE, the inability to control membrane potential in intact cells is a problem that can potentially give rise to experimental artifacts (see below for discussion).
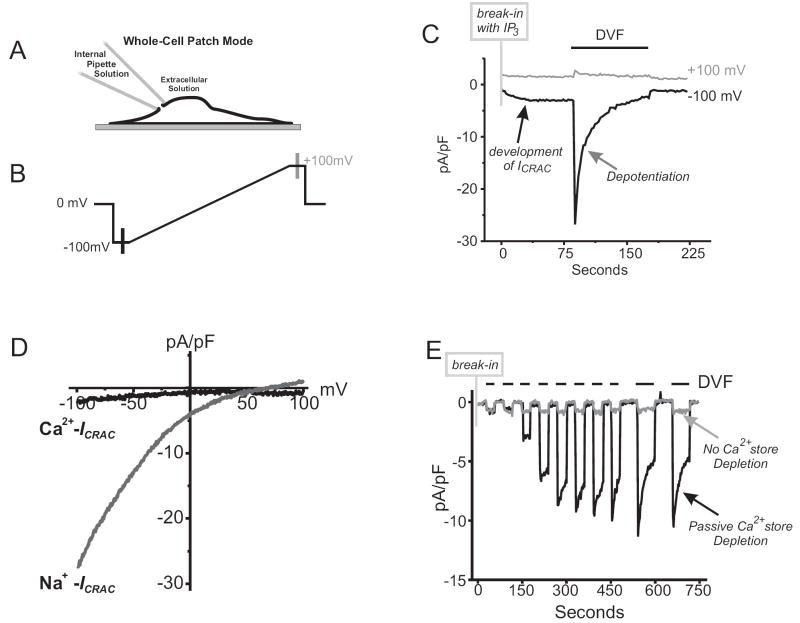
Figure 3. Biophysical Assessment of Store-Operated Currents.
(A) Diagram showing the whole-cell configuration of the patch-clamp technique. As depicted, the use of this technique allows for access to the inside of the cell through the internal pipette solution (See Table 3 for example solutions), allowing for depletion of internal Ca2+ stores passively with BAPTA alone, or in combination with activating reagents such as IP3.
(B) Schematic of a voltage ramp protocol used to assess store-operated Ca2+ currents. The protocol is repetitively applied over time, with 1 to 2 second intervals at 0 mV, to reveal currents develop during Ca2+ store depletion.
(C) An example ICRAC in an RBL cell recorded using the protocol shown in panel B, and which was repetitively applied every two seconds. Internal Ca2+ stores were actively depleted by including 20 μM IP3 and 10 mM BAPTA in the patch pipette solution. 10 mM Ca2+ was present in the external bathing solution. Following break-in with the patch pipette, a small (2 pA/pF) inward current develops at -100 mV, while no change is observed at +100 mV). Upon removal of all external divalent cations (divalent-free, DVF) in the extracellular media, Na+ ions now permeate the store-operated channel to reveal Na+-ICRAC. Over time, Na+-ICRAC depotentiates, a process which is the reciprocal of a process known as Ca2+ dependent potentiation (CDP).
(D) Current-voltage relationship of Ca2+-ICRAC and Na+-ICRAC are inwardly rectifying Ca2+- and Na+-ICRAC (data taken from recordings shown in panel C).
(E) In this whole-cell recording, DVF conditions were used to reveal ICRAC in HEK 293 cells where, normally, Ca2+- ICRAC is difficult to detect. Following break-in, the protocol entails switching the external bathing solutions between Ca2+ containing (10mM) and DVF bathing solutions. Using optimal conditions for passive depletion of Ca2+ stores (10 mM BAPTA in patch pipette solution), no ICRAC is observed with 10mM Ca2+ in the external bathing solution. By switching to DVF conditions the developing Na+-ICRAC is revealed (black line trace). In contrast, supplementing the internal pipette solution with enough Ca2+ to “clamp” free Ca2+ at 100 nM (calculated using Maxchelator software; www.stanford.edu/~cpatton/maxc) prevents the passive depletion of Ca2+ stores upon break in, and no ICRAC current develops (grey line trace) under all external bathing solution conditions.
General protocols to record Icrac using the whole-cell patch-clamp technique are reviewed in [4]. This technique involves the establishment of a tight seal between a recording pipette electrode and the surface membrane of a cell, followed by rupture of the membrane under the pipette opening to establish electrical and chemical continuity between the pipette and the cytoplasm (Fig 3A). This then allows the cell membrane potential to be clamped (usually at a depolarized potential, 0 mV) until, every 1 to 2 seconds, a voltage ramp is applied from -100 mV up to +100 mV (see Fig. 3B). With this protocol, the measured currents can reveal how Icrac develops over time (Fig. 3C) as well as its current-voltage relationship which, for Icrac, is inwardly rectifying (Fig. 3D). After completing each voltage ramp, it is routine to return to the depolarized potential (0 mV). This is an important step in the protocol as it reduces the driving force for Ca2+ ions entering the cell, and thus lessens Ca2+ dependent feedback pathways that inactivate CRAC channels.
Figure 3C illustrates a typical experiment utilizing RBL cells in the whole-cell configuration to measure Icrac activation. After break-in and establishing a whole cell patch, the cell was held at 0 mV followed by applying a voltage ramp from -100 mV to +100 mV every 2 seconds. The internal pipette solution (described in Table 3) contained IP3 (20 μM) and BAPTA (10 mM), optimal conditions to rapidly deplete ER Ca2+ stores and activate SOCE. The extracellular bathing solution (described in Table 3) contained 10 mM Ca2+. Over a period of seconds after break-in, a small inward current can be observed developing at -100 mV, while little to no change is detected at +100 mV. Plotting the current-voltage relationship of this data (Fig. 3D; please note that these figures have been leak subtracted) clearly demonstrates that Icrac, activated by Ca2+ store depletion, is strongly inwardly rectifying.
Table 3.
Basic solutions for Icrac measurements
| Extracellular Solutions (in mM) | ||
|---|---|---|
| Ca2+-Icrac | Na+-Icrac | |
| NaCl | 145 | 145 |
| KCl | 3 | 3 |
| MgCl2 | 2 | 0 |
| Glucose | 10 | 10 |
| CsCl2 | 10 | 10 |
| EGTA | 10 | 0 |
| HEPES (pH 7.4) | 0 | 0.1 |
| Internal Pipette Solutions (in mM) | ||
| Passive Depletion | No Depletion Control | |
| Cs Methansulfonate | 145 | 145 |
| MgCl2 | 8 | 8 |
| BAPTA | 10 | 10 |
| CaCl2 | 0 | 3.5 (free [Ca2+] ~100nM) |
| HEPES (pH 7.2 with CsOH) | 10 | 10 |
Note: High [Mg2+] required to block MIC currents [43].
Free [Ca2+] calculated using MaxChelator (www.stanford.edu/~cpatton/maxc)
An important characteristic of the CRAC channel is that it completely loses its Ca2+ selectivity when all extracellular divalent cations are removed, and Na+ ions are now allowed to permeate the channel. So under extracellular divalent free (DVF) conditions (see table 3), CRAC channel activity can now be measured as a function of current carried by Na+ ions. Interestingly, Na+ currents carried through CRAC channels (Na+-Icrac) run down over time by a process known as depotentiation, which is the reciprocal of a process known as Ca2+ dependent potentiation (see below).
Critically, the Na+-Icrac current densities measured under DVF conditions are large, and has provided an invaluable technique for characterizing SOC channels in cells where there is little to no detectable Ca2+-Icrac (Icrac measured with divalent cations present in extracellular solutions) (Fig. 3E, and examples in [44,45]). However, it is advisable that the Na+ currents recorded be characterized pharmacologically to ensure that they represent the activity of CRAC channels (see below).
C. Activating Store-operated Currents
The single initial signal for the activation of SOCE and Icrac is the depletion of intracellular Ca2+ stores located in the ER. As mentioned above, Icrac activation is measured using the whole-cell patch, allowing the intracellular milieu to be modified directly with the internal patch pipette solution. Thus, rather than relying of the actions of SERCA pump inhibitors like thapsigargin or ionomycin (both added to extracellular solution) to activate SOC channels, intracellular Ca2+ store depletion can be achieved by additions to the internal pipette solution.
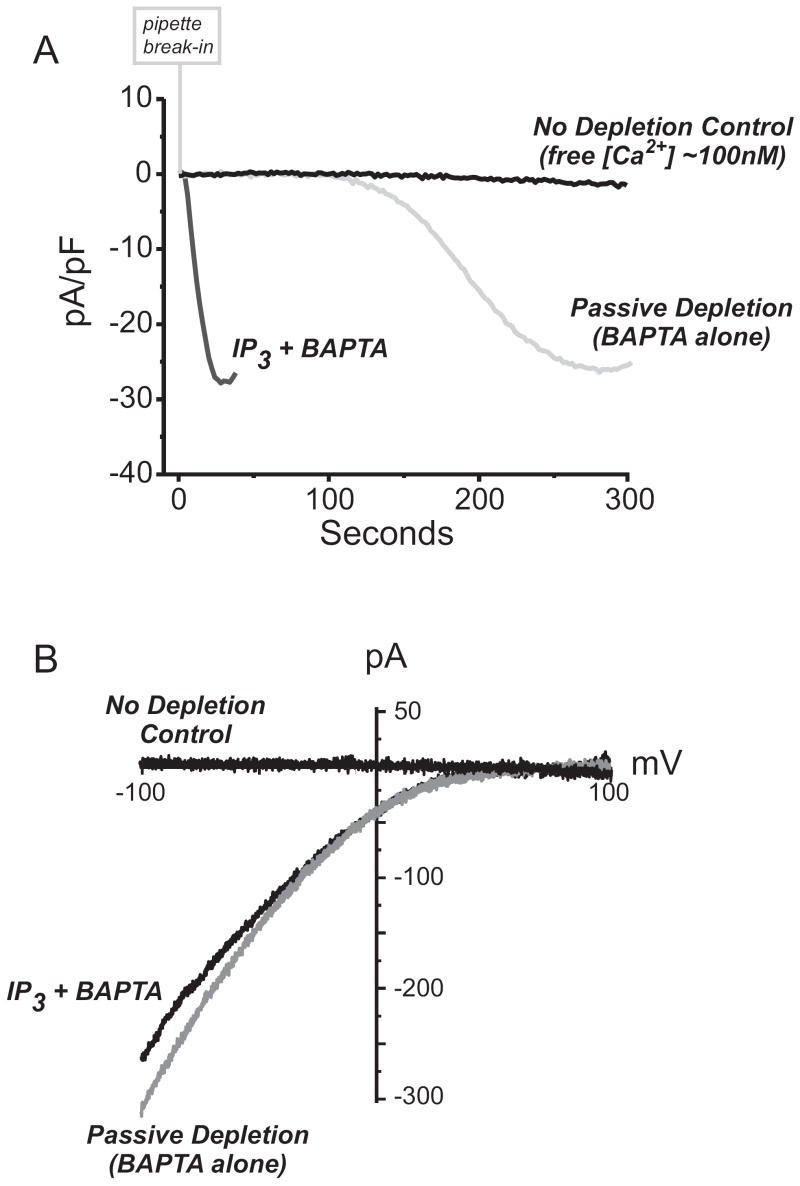
The simplest mode of Icrac activation is through ‘passive depletion’ of intracellular Ca2+ stores. By simply breaking into cells with a patch pipette solution containing a high concentration of BAPTA or EGTA and no added Ca2+ (see table 3), the intracellular Ca2+ stores are gradually lost. This technique is at least in principle equivalent to the use of SERCA inhibitors. The passive process is slow, and Icrac activation may take in the order of several minutes to fully develop (Fig.4A). Alternatively, one can use the whole-cell patch clamp technique to introduce membrane impermeant reagents to actively and rapidly deplete intracellular Ca2+ stores and activate Icrac. The most efficient way is to include metabolizable or non-metabolizable analogs of IP3 (in conjunction with EGTA or BAPTA) [46]. Under these conditions, the time course of Icrac activation is significantly faster than with passive depletion alone (Fig.4A).
Figure 4. Methods for activating ICRAC.
(A) Whole –cell currents recorded in HEK 293 cells transfected with Stim1 and Orai1. From a holding potential of 0 mV, developing store-operated currents were recorded during voltage ramps (-100 mV to +100 mV) applied every two seconds using. This data shows a time course of Ca2+-ICRAC development recorded -100 mV. With coexpression of Stim1 and Orai1, “monster” Ca2+-ICRAC currents develop, and without the need to use DVF conditions. In this experiments, we compare the time course of Ca2+-ICRAC activation when Ca2+ stores are depleted passively (20mM BAPTA alone in the internal pipette solution) or actively (20mM BAPTA plus 20 μM IP3 in the pipette). In contrast, “clamping” free [Ca2+] in the internal pipette solution to ~100 nM prevents Ca2+ store depletion, and no “monster” Ca2+-ICRAC is observed. In all recordings, the external [Ca2+] was 10 mM.
(B) Current-voltage relationships for each of the “monster” Ca2+-ICRAC currents recorded in panel A are inwardly rectifying akin to endogenous ICRAC.
Ca2+-dependent feedback regulation of Icrac
Icrac is both positively and negatively regulated by Ca2+ in a complex manner. First, Ca2+ regulates SOC channels in a positive manner through a process known as Ca2+ dependent potentiation (CDP) [47,48], and appears to result from Ca2+ ions interacting with an extracellular binding site. While this binding site has not been identified as yet, it is possible that it is the same as the Ca2+ binding site in the selectivity filter. Nonetheless, the presence of Ca2+ at this putative CDP site potentiates Icrac, and can be detected electrophysiologically when switching between external DVF solution and Ca2+ containing (10 mM) solutions. As mentioned above, the reverse of CDP can be detected when switching from a Ca2+ containing extracellular solution to DVF solutions to detect Na+Icrac, the effect being that the Na+ current depotentiates (Figure 3C and 3E).
Under conditions whereby [Ca2+]i levels are high in close proximity to the CRAC channels, CRAC channels can experience two types of Ca2+-dependent feedback regulation described as either fast or slow inactivation [34,49,50,51]. Fast inactivation of Icrac can be observed electrophysiologically by using brief hyperpolarizing steps from depolarized holding potentials (for protocol, see [34,49,51]), and is believed to result from Ca2+ entering through the channels binding directly to the channels at or near the channel mouth. This effect occurs in the millisecond time range, and differences in the rates of fast inactivation can be seen by comparing the effects of the slower Ca2+ chelator EGTA with the faster chelator, BAPTA [34,49,51] in the internal pipette solution.
In contrast, slow inactivation of Icrac occurs over a period of tens of seconds [52,50,53]. While the mechanism underlying slow inactivation is not fully understood, slow inactivation might occur due to refilling of intracellular Ca2+ stores and could occur if cytoplasmic [Ca2+]i levels were significantly elevated in spatially restricted areas and thus locally overwhelmed the Ca2+ chelator included in the internal pipette solution. Alternatively, slow inactivation might be due to a regulatory feedback mechanism on the SOCE signaling process, for example it has been suggested that PKC regulates Icrac activity [52].
In summary, feedback regulation of Icrac by Ca2+ ions is a complex multitude of processes that are yet to be fully defined. While these Ca2+ signaling pathways may play crucial roles in the physiology of many cells types, awareness of these processes is crucial for studying SOC channels electrophysiologically and for understanding how they can be controlled experimentally.
IV. Manipulating SOCE
Cytoplasmic [Ca2+]i reflects a homeostatic balance of Ca2+ pumps and Ca2+ channel activities. Any disturbance in these processes, through receptor-dependent activation of Ca2+ channel activity for example, can lead to the rapid movement of Ca2+ down concentration gradients until a new steady state is achieved in concert with Ca2+ pump and feedback mechanisms. Experimental manipulations and pharmacological agents that interfere with any of these Ca2+ homeostatic processes can have a profound effect on the cytoplasmic [Ca2+]i and plasma membrane currents. Thus, an intimate understanding of the underlying Ca2+ homeostatic processes and the various ways they can be manipulated will allow the observed fluorescence-based [Ca2+]i signals and membrane currents to be interpreted in meaningful ways that speak to the underlying Ca2+ signaling process. Some of these approaches are outlined below.
A. Pharmacological Manipulation of SOCE
Pharmacological manipulation of SERCA pumps with inhibitors such as thapsigargin was crucial for developing our understanding of SOCE (see above). Unfortunately, our ability to manipulate SOC channels directly and inhibit them with any degree of specificity has been rather limited. Amongst the arsenal of tools proposed as SOC channel inhibitors [23,4], lanthanides and 2-aminoethyldiphenyl borate (2-APB) have proven most useful in manipulating SOC entry, with lanthanides appearing to be the most selective (see below).
Due to their physical chemistry [54], lanthanides were exploited as tools to block both Ca2+ entry and efflux processes [55]. Fortunately, SOCE is much more sensitive to inhibition by lanthanides than Ca2+-efflux across the plasma membrane. The lanthanide Gd3+ is generally used in this respect and appears specific for SOCE over non-SOCE pathways when used in the appropriately low (≤5 μM) concentration range [56], and only begins to block PMCA activity above 100 μM [57].
When using this approach, care should be taken to use freshly prepared lanthanide salt solutions in buffers devoid of divalent anions. Also the presence of extracellular BAPTA or EGTA will bind lanthanides with high affinity [56] and adversely affect their concentration in solution. Another potential problem stems from the ability of lanthanides to render an excitation spectrum with fura-based Ca2+-indicators. Usually, lanthanides poorly traverse the plasma membrane of intact cells, if at all [15]. However, should bathing an unstimulated cell with a lanthanide increase the fura-signal, this could be indicative of ‘leaky’ or unhealthy cells.
2-APB has proven a somewhat selective inhibitor for SOCE [58,59,60,61,62], an effect that appears to be extracellular at the plasma membrane [59,61,63,64], and independently of IP3 receptor inhibition [65]. However, a careful evaluation of 2-APB effects on Ca2+-signaling processes is advised. Originally shown to be a membrane permeable inhibitor of IP3 receptors [66], the effects of 2-APB are proving complex and this drug may lack the desired specificity. These effects can include inhibiting a magnesium-inhibitable cation channel [59,43], or activating distinct ion channel activity depending on the concentration of 2-APB [67,68,69].
B. Membrane Potential
Contrary to voltage-activated Ca2+ channels, membrane depolarization does not play a role in SOCE activation. However, since Ca2+ entry through SOC channels is an electrogenic process driven by chemical (concentration gradient) and electrical (membrane potential) forces, plasma membrane potential can regulate SOCE such that less Ca2+ enters upon depolarization and membrane hyperpolarization promotes Ca2+ influx [70,71].
Membrane potential is well controlled when using voltage-clamp techniques to measure SOC channel activity (see below). However, when measuring SOCE with fluorescent Ca2+-indicators alone, changes in membrane potential are usually not controlled, but it is possible to carry out fluorescence measurements of [Ca2+]i under voltage clamped conditions [72]. Lack of control of membrane potential must be considered when characterizing pharmacological agents that affect SOCE [73], since modulation of the Ca2+-entry signal could result from depolarization/hyperpolarization rather than direct effects on the SOC channel itself.
Monitoring membrane potential and characterizing the effects of candidate pharmacological agents for SOCE is one way to control for this. Alternatively, these complications can be avoided by combining voltage-clamp techniques with fluorescence Ca2+ measurements. If this option is not available, one can manipulate the bathing solution to contain high [KCl] and create a “poor man’s voltage-clamp” and carry out fluorescence experiments with the plasma membrane fully depolarized [74,75,76].
C. Manipulating the Molecular Players for SOCE
As described above, the main limitation to developing approaches that specifically manipulate SOCE has been our poor understanding of the molecular players involved in this process. Fortunately, the recent identification of STIM and Orai (or CRACM) family proteins has facilitated our understanding of the target plasma membrane channel mediating SOCE, as well as the mechanism by which depletion of intracellular Ca2+ stores is communicated to these SOC channels at the plasma membrane [77].
These discoveries have opened new experimental approaches to dissect and manipulate SOCE through techniques such as RNAi [78,79,80,81,82,83], protein modification and expression [84], and the generation of animal models [45]. These approaches have enabled structure function studies on Orai family proteins to identify residues involved in pore formation and the selectivity filter [85,86,87]. These studies may very well lay the foundation to develop new pharmacological strategies to manipulate SOCE [88].
V. Concluding Remarks
The purpose of this review is to delineate useful strategies for dissecting Ca2+ signaling processes and studying SOCE using experimental approaches that employ fluorescent Ca2+ indicators and electrophysiological techniques. The ability to use these diverse experimental approaches to monitor [Ca2+]i and SOC currents in real time, with varying degrees of spatial and temporal resolution, has significantly contributed to our current understanding of the basic processes of regulated SOCE.
It remains a challenge to continually refine protocols and experimental conditions to optimally discriminate SOCE from other possible routes of Ca2+-entry across the plasma membrane. This is especially important when identifying a role for SOCE under conditions of physiological activation of phospholipase C-linked pathways and SOCE, or in considering a role for SOCE in excitable cells. The identification of the molecular players for SOCE, STIM and Orai family proteins, has also opened up exciting possibilities to derive new strategies to specifically manipulate SOCE, as well as delineate all the steps involved in this Ca2+ signaling pathway.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Putney JW, Poggioli J, Weiss SJ. Phil Trans R Soc Lond B. 1981;296:37–45. doi: 10.1098/rstb.1981.0169. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Irvine RF. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ. Ann Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- 4.Parekh AB, Putney JW. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 5.Cahalan MD, Zhang SL, Yeromin AV, Ohlsen K, Roos J, Stauderman KA. Cell Calcium. 2007;42:133–144. doi: 10.1016/j.ceca.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vig M, Kinet JP. Cell Calcium. 2007;42:157–162. doi: 10.1016/j.ceca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Cell Calcium. 2007;42:145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Lewis RS. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 9.Tsien RY. Annual Review of Neuroscience. 1989;12:227–253. doi: 10.1146/annurev.ne.12.030189.001303. [DOI] [PubMed] [Google Scholar]
- 10.Vorndran C, Minta A, Poenie M. Biophysical Journal. 1995;69:2112–2124. doi: 10.1016/S0006-3495(95)80082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shuttleworth TJ, Thompson JL. The Biochemical Journal. 1996;316:819–824. doi: 10.1042/bj3160819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grynkiewicz G, Poenie M, Tsien RY. Journal of Biological Chemistry. 1986;260:3440–3450. [PubMed] [Google Scholar]
- 13.Hallam TJ, Jacob R, Merritt JE. The Biochemical Journal. 1988;255:179–184. doi: 10.1042/bj2550179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almers W, McCleskey EW. J Physiol. 1984;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwan CY, Putney JW. Journal of Biological Chemistry. 1990;265:678–684. [PubMed] [Google Scholar]
- 16.Tsien RY. Nature. 1981;290:527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- 17.Glennon MC, Bird GStJ, Kwan C-Y, Putney JW. Journal of Biological Chemistry. 1992;267:8230–8233. [PubMed] [Google Scholar]
- 18.Glennon MC, Bird GS, Takemura H, Thastrup O, Leslie BA, Putney JW. J Biol Chem. 1992;267:25568–25575. [PubMed] [Google Scholar]
- 19.Quintana A, Hoth M. Cell Calcium. 2004;36:99–109. doi: 10.1016/j.ceca.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Di Virgilio F, Steinberg TH, Silverstein SC. Cell Calcium. 1990;11:57–62. doi: 10.1016/0143-4160(90)90059-4. [DOI] [PubMed] [Google Scholar]
- 21.Lomax RB, Camello C, Van Coppenolle F, Petersen OH, Tepikin AV. Journal of Biological Chemistry. 2002;277:26479–26485. doi: 10.1074/jbc.M201845200. [DOI] [PubMed] [Google Scholar]
- 22.Ong HL, Liu X, Sharma A, Hegde RS, Ambudkar IS. Pflugers Archiv European Journal of Physiology. 2006 doi: 10.1007/s00424-006-0163-5. [DOI] [PubMed] [Google Scholar]
- 23.Putney JW. Mol Interventions. 2001;1:84–94. [PubMed] [Google Scholar]
- 24.Takemura H, Hughes AR, Thastrup O, Putney JW. Journal of Biological Chemistry. 1989;264:12266–12271. [PubMed] [Google Scholar]
- 25.Sedova M, Klishin A, Hüser J, Blatter LA. J Physiol (Lond) 2000;523:549–559. doi: 10.1111/j.1469-7793.2000.t01-3-00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu C-M, Herman TE. Journal of Biological Chemistry. 1979;253:5892–5894. [PubMed] [Google Scholar]
- 27.Albert PR, Tashjian AH., Jr Journal of Biological Chemistry. 1984;259:15350–15363. [PubMed] [Google Scholar]
- 28.Morgan AJ, Jacob R. The Biochemical Journal. 1994;300:665–672. doi: 10.1042/bj3000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deber CM, Tom-Kun J, Mack E, Grinstein S. Anal Biochem. 1985;146:349–352. doi: 10.1016/0003-2697(85)90550-0. [DOI] [PubMed] [Google Scholar]
- 30.Bird GStJ, Takemura H, Thastrup O, Putney JW, Menniti FS. Cell Calcium. 1991;13:49–58. doi: 10.1016/0143-4160(92)90029-r. [DOI] [PubMed] [Google Scholar]
- 31.Takemura H, Putney JW. The Biochemical Journal. 1989;258:409–412. doi: 10.1042/bj2580409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson A. Journal of General Physiology. 1983;81:805–827. doi: 10.1085/jgp.81.6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hallam TJ, Rink TJ. FEBS Letters. 1985;186:175–179. doi: 10.1016/0014-5793(85)80703-1. [DOI] [PubMed] [Google Scholar]
- 34.Hoth M, Penner R. J Physiol (Lond) 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shuttleworth TJ. Cell Calcium. 1994;15:457–466. doi: 10.1016/0143-4160(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 36.Chiavaroli C, Bird GStJ, Putney JW. Journal of Biological Chemistry. 1994;269:25570–25575. [PubMed] [Google Scholar]
- 37.Loessberg PA, Zhao H, Muallem S. Journal of Biological Chemistry. 1991;266:1363–1366. [PubMed] [Google Scholar]
- 38.Franchini L, Levi G, Visentin S. Cell Calcium. 2004;35:449–459. doi: 10.1016/j.ceca.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Hoth M, Penner R. Nature. 1992;355:353–355. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 40.Prakriya M, Lewis RS. J Gen Physiol. 2006;128:373–386. doi: 10.1085/jgp.200609588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bakowski D, Parekh AB. Cell Calcium. 2002;32:379–391. doi: 10.1016/s0143416002001914. [DOI] [PubMed] [Google Scholar]
- 42.Prakriya M, Lewis RS. Cell Calcium. 2003;33:311–321. doi: 10.1016/s0143-4160(03)00045-9. [DOI] [PubMed] [Google Scholar]
- 43.Prakriya M, Lewis RS. Journal of General Physiology. 2002;119:487–507. doi: 10.1085/jgp.20028551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeHaven WI, Smyth JT, Boyles RR, Putney JW. J Biol Chem. 2007;282:17548–17556. doi: 10.1074/jbc.M611374200. [DOI] [PubMed] [Google Scholar]
- 45.Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parekh AB, Penner R. Physiological Reviews. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 47.Christian EP, Spence KT, Togo JA, Dargis PG, Patel J. J Membrane Biol. 1996;150:63–71. doi: 10.1007/s002329900030. [DOI] [PubMed] [Google Scholar]
- 48.Zweifach A, Lewis RS. Journal of General Physiology. 1996;107:597–610. doi: 10.1085/jgp.107.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zweifach A, Lewis RS. Journal of General Physiology. 1995;105:209–226. doi: 10.1085/jgp.105.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zweifach A, Lewis RS. Journal of Biological Chemistry. 1995;270:14445–14451. doi: 10.1074/jbc.270.24.14445. [DOI] [PubMed] [Google Scholar]
- 51.Fierro L, Parekh AB. J Membr Biol. 1999;168:9–17. doi: 10.1007/s002329900493. [DOI] [PubMed] [Google Scholar]
- 52.Parekh AB, Penner R. Proceedings of the National Academy of Sciences USA. 1995;92:7907–7911. doi: 10.1073/pnas.92.17.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parekh AB. Journal of Biological Chemistry. 1998;273:14925–14932. doi: 10.1074/jbc.273.24.14925. [DOI] [PubMed] [Google Scholar]
- 54.Lettvin JY, Pickard WF, MCCULLOCH WS, PITTS W. Nature. 1964;202:1338–1339. doi: 10.1038/2021338a0. [DOI] [PubMed] [Google Scholar]
- 55.Van Breemen C, Farinas B, Gerba P, McNaughton ED. Circulation Research. 1972;30:44–54. doi: 10.1161/01.res.30.1.44. [DOI] [PubMed] [Google Scholar]
- 56.Broad LM, Cannon TR, Taylor CW. J Physiol (Lond) 1999;517:121–134. doi: 10.1111/j.1469-7793.1999.0121z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bird GS, Putney JW. J Physiol. 2005;562:697–706. doi: 10.1113/jphysiol.2004.077289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma H-T, Patterson RL, van Rossum DB, Birnbaumer L, Mikoshiba K, Gill DL. Science. 2000;287:1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- 59.Braun F-J, Broad LM, Armstrong DL, Putney JW. Journal of Biological Chemistry. 2001;276:1063–1070. doi: 10.1074/jbc.M008348200. [DOI] [PubMed] [Google Scholar]
- 60.Gregory RB, Rychkov G, Barritt GJ. The Biochemical Journal. 2001;354:285–290. doi: 10.1042/0264-6021:3540285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bakowski D, Glitsch MD, Parekh AB. J Physiol (Lond) 2001;532:55–71. doi: 10.1111/j.1469-7793.2001.0055g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bootman MD, Collins TJ, Mackenzie L, Roderick HJ, Berridge MJ, Peppiatt CM. The FASEB Journal. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- 63.Prakriya M, Lewis RS. J Physiol (Lond) 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iwasaki H, Mori Y, Hara Y, Uchida K, Zhou H, Mikoshiba K. Receptors and Channels. 2001;7:429–439. [PubMed] [Google Scholar]
- 65.Broad LM, Braun F-J, Lièvremont J-P, Bird GStJ, Kurosaki T, Putney JW. Journal of Biological Chemistry. 2001;276:15945–15952. doi: 10.1074/jbc.M011571200. [DOI] [PubMed] [Google Scholar]
- 66.Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. Journal of Biochemistry. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 67.Braun FJ, Aziz O, Putney JW. Molecular Pharmacology. 2003;63:1304–1311. doi: 10.1124/mol.63.6.1304. [DOI] [PubMed] [Google Scholar]
- 68.Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. Journal of Neuroscience. 2004;24:5177–5182. doi: 10.1523/JNEUROSCI.0934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, Lee LY, Wood JD, Zhu MX. Journal of Biological Chemistry. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- 70.Merritt JE, Rink TJ. Journal of Biological Chemistry. 1987;262:17362–17369. [PubMed] [Google Scholar]
- 71.Di Virgilio F, Lew PD, Andersson T, Pozzan T. Journal of Biological Chemistry. 1987;262:4574–4579. [PubMed] [Google Scholar]
- 72.Huang Y, Putney JW. Journal of Biological Chemistry. 1998;273:19554–19559. doi: 10.1074/jbc.273.31.19554. [DOI] [PubMed] [Google Scholar]
- 73.Franzius D, Hoth M, Penner R. Pflügers Arch. 1994;428:433–438. doi: 10.1007/BF00374562. [DOI] [PubMed] [Google Scholar]
- 74.Schilling WP, Rajan L, Strobl-Jager E. Journal of Biological Chemistry. 1989;264:12838–12848. [PubMed] [Google Scholar]
- 75.Pittet D, Di VF, Pozzan T, Monod A, Lew DP. Journal of Biological Chemistry. 1990;265:14256–14263. [PubMed] [Google Scholar]
- 76.Lievremont JP, Bird GS, Putney JW. Am J Physiol Cell Physiol. 2004;287:C1709–C1716. doi: 10.1152/ajpcell.00350.2004. [DOI] [PubMed] [Google Scholar]
- 77.Putney JW., Jr Journal of Cell Science. 2007;120:1959–1965. doi: 10.1242/jcs.03462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. Journal of Cell Biology. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 82.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Proc Natl Acad Sci U S A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wedel B, Boyles RR, Putney JW, Bird GS. J Physiol. 2007;579:679–689. doi: 10.1113/jphysiol.2006.125641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liou J, Fivaz M, Inoue T, Meyer T. Proc Natl Acad Sci U S A. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 86.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smyth JT, DeHaven WI, Bird GS, Putney JW., Jr Journal of Cell Science. 2008;121:762–772. doi: 10.1242/jcs.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]