Abstract
Human acid-sensing ion channel 1b (hASIC1b) is a H+-gated amiloride-sensitive cation channel. We have previously shown that glioma cells exhibit an amiloride-sensitive cation conductance. Amiloride and the ASIC1 blocker psalmotoxin-1 decrease the migration and proliferation of glioma cells. PKC also abolishes the amiloride-sensitive conductance of glioma cells and inhibits hASIC1b open probability in planar lipid bilayers. In addition, hASIC1b's COOH terminus has been shown to interact with protein interacting with C kinase (PICK)1, which targets PKC to the plasma membrane. Therefore, we tested the hypothesis that PKC regulation of hASIC1b at specific PKC consensus sites inhibits hASIC1b function. We mutated three consensus PKC phosphorylation sites (T26, S40, and S499) in hASIC1b to alanine, to prevent phosphorylation, and to glutamic acid or aspartic acid, to mimic phosphorylation. Our data suggest that S40 and S499 are critical sites mediating the modulation of hASIC1b by PKC. We expressed mutant hASIC1b constructs in Xenopus oocytes and measured acid-activated currents by two-electrode voltage clamp. T26A and T26E did not exhibit acid-activated currents. S40A was indistinguishable from wild type (WT), whereas S40E, S499A, and S499D currents were decreased. The PKC activators PMA and phorbol 12,13-dibutyrate inhibited WT hASIC1b and S499A, and PMA had no effect on S40A or on WT hASIC1b in oocytes pretreated with the PKC inhibitor chelerythrine. Chelerythrine inhibited WT hASIC1b and S40A but had no effect on S499A or S40A/S499A. PKC activators or the inhibitor did not affect the surface expression of WT hASIC1b. These data show that the two PKC consensus sites S40 and S499 differentially regulate hASIC1b and mediate the effects of PKC activation or PKC inhibition on hASIC1b. This will result in a deeper understanding of PKC regulation of this channel in glioma cells, information that may help in designing potentially beneficial therapies in their treatment.
Keywords: two-electrode voltage clamp; Xenopus laevis oocytes; phorbol 12-myristate 13-acetate; chelerythrine; mutagenesis; phorbol 12,13-dibutyrate; protein kinase C
acid-sensing ion channels (ASICs) are activated by extracellular protons and belong to the epithelial Na+ channel (ENaC)/degenerin (Deg) family of ion channels. Four ASIC genes (ASIC1–ASIC4) have been cloned, and ASIC1–ASIC3 have splice variants (19, 28, 29, 31, 42). ASICs are permeable to Na+ but also to other monovalent and divalent cations like Ca2+ (in the case of ASIC1), Li+, K+, and H+ (41, 42). ASIC subunits are expressed in peripheral nervous system neurons, where they have been implicated in nociception during tissue acidosis and in mechanosensation (42). In the central nervous system (CNS), ASIC1 has been mostly studied in mouse and rat brains, where it is abundantly expressed, and ASIC1a, ASIC1b, and ASIC2 have been found to be expressed in the human brain (17). Mouse ASIC1a modulates synaptic plasticity, contributing to learning, memory, and fear conditioning (42). ASICs are also expressed in non-neuronal tissue and cells such as the retina, osteoblasts, ear, taste buds, lung, testis, astrocytes, and intestine, where they may sense extracellular acid changes (38, 42).
The recent crystallization of chicken ASIC1 at low pH has confirmed the protein structure and has also revealed that ASIC1 forms a homotrimer (26). The protein consists of short intracellular NH2 and COOH termini, two transmembrane-spanning α-helixes, and a large extracellular loop (28, 29, 40, 42). Different ASIC subunits are capable of forming heteromultimers with other ASIC subunits and, in some cases, with other ENaC/Deg subunits (2, 4, 5, 15, 32). The extracellular domain senses protons and interacts with modulators, including proteases, Zn2+, Ca2+, and redox reagents (42). The cytoplasmic NH2 and COOH termini contain phosphorylation sites and interact with other proteins such as protein interacting with C kinase 1 (PICK1) (14, 25, 42), postsynaptic density protein 95, abnormal cell lineage 7b (24), and annexin (13), resulting in changes in the current density or cellular localization of ASIC. For example, PICK1 increases the ASIC2a current amplitude by potentiating the phosphorylation of ASIC2a at T39 [equivalent to site S40 on human (h)ASIC1b] (3). Also, PKC phosphorylation of the COOH terminus of ASIC3 (at S523) increases the binding of ASIC3 to Na+/H+ exchanger regulatory factor 1, which, in turn, increases the current of ASIC3 expressed in Xenopus oocytes (12). Furthermore, PKA phosphorylates hASIC1b at S479, and this phosphorylation interferes with PICK1 binding (30). Berdiev et al. (6) showed that PKC holoenzyme phosphorylates hASIC1b in vitro, and the addition of PKC to the intracellular side of hASIC1b in lipid bilayers decreases its open probability. Generally, the direction of the effect of PKC on ion channels is cell type specific and channel subtype specific, with PKC activation resulting in either an increase or a decrease of the current (11). For example, the activation of PKC increased transepithelial Na+ transport measured as amiloride-sensitive short-circuit current (Isc) across the skins of two different species of frogs and inhibited Isc across the bladders of the same animals (10).
We focused on characterizing the consensus PKC phosphorylation sites of hASIC1b and on describing the effect of PKC activation or inhibition on hASIC1b function because of evidence suggesting that this ion channel and PKC are involved in the regulation of an amiloride-sensitive cation conductance in high-grade glioma cells. We have previously shown that this conductance is expressed only in high-grade glioma, and it is absent in normal astrocytes and in low-grade glioma (6–9, 38). These cells express only the hASIC1b splice variant (38), and previous evidence has suggested that hASIC1b is a component of the amiloride-sensitive channel complex (7, 38, 39). The physiological importance for glioma cells to express this amiloride-sensitive conductance becomes clear because inhibition of this conductance affects glioma cell migration and proliferation. Amiloride inhibits glioma cell migration and proliferation (23, 34, 38), whereas psalmotoxin 1, an ASIC1 blocker, inhibits the glioma conductance (8) and the regulatory volume increase of glioma cells after a hyperosmotic challenge (33). Berdiev et al. (6) also showed that the addition of PKC abolished the inward current of a glioma cell and that PKC inhibition is one of the necessary steps required to observe a similar amiloride-sensitive current in normal astrocytes. Therefore, we hypothesized that differential regulation of hASIC1b by PKC may turn this conductance on in glioma cells and off in normal astrocytes. Additionally, the hASIC1b splice variant is the most similar to rat and mouse ASIC1a (Fig. 1), both of which are expressed and play a role in the CNS. Thus, it is important to determine specifically if PKC can regulate hASIC1b function.
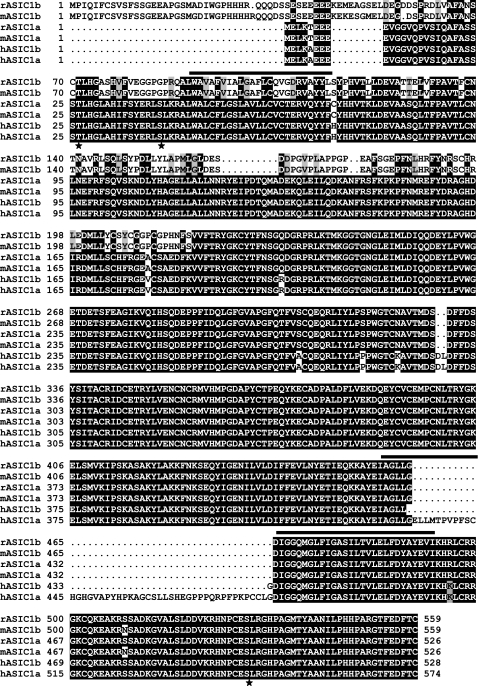
Fig. 1.
Alignment of acid-sensing ion channel 1 (ASIC1) isoforms from different species. Sequence alignment of ASIC1 isoforms from the human (hASIC1a, NM_020039; and hASIC1b, NM_001095), mouse (mASIC1a, NM_009597; and mASIC1b, AB208022), and rat (rASIC1a, NM_024154; and rASIC1b, AJ309926) was done with Clusta IW, and shading was done with the Boxshade program (http://www.ch.embnet.org/). Identical amino acids are shown as white letters on a black background, and conserved amino acids are shown as black letters on a shaded background. The positions of the transmembrane domains were obtained from Jasti et al. (26) and are shown by the black horizontal bars. The amino acid sequence of hASIC1b was analyzed with Genetics Computer Group (University of Alabama at Birmingham) and Scansite (MIT) software for consensus PKC phosphorylation sites. The three consensus PKC phosphorylation sites on hASIC1b are shown (★), and they are T26, S40 (on the cytoplasmic NH2 terminus), and S499 (on the cytoplasmic COOH terminus).
The presence of a PDZ-binding domain in the COOH terminus of hASIC1b and the fact that the hASIC1b COOH terminus interacts with PICK1 (25) also suggest that phosphorylation by PKC might regulate hASIC1b. Moreover, queries with databases that search for motifs in the amino acid sequence of a protein have shown that there are three consensus PKC phosphorylation sites in the cytoplasmic tails of hASIC1b. The aim of this study was to determine if these sites are important for the effect of PKC on hASIC1b currents. To study the effect that specific phosphorylation sites have on hASIC1b function, we heterologously expressed hASIC1b and several phosphorylation-null or mimic mutants of hASIC1b in Xenopus oocytes and measured acid-activated currents by two-electrode voltage clamp (TEV). Xenopus oocytes provide a good model system for studying the regulation of proteins by PKC because they do not exhibit endogenous acid-activated currents (37) and express most of the PKC isoforms (PKC-α, -βI, -βII, -γ, -δ, -ζ, and -ɛ) (27, 44). We found that mutations in hASIC1b consensus PKC phosphorylation sites decrease hASIC1b functional expression in Xenopus oocytes and that these sites determine if PKC activation or inhibition has an effect on hASIC1b.
MATERIALS AND METHODS
Materials.
The PKC activator PMA (Calbiochem, San Diego, CA) was stored as a 1 mM stock in DMSO (Sigma, St. Louis, MO) at −80°C. Phorbol 12,13-dibutyrate (PdBu; Calbiochem) was stored as a 10 mM stock in DMSO. The inactive phorbol ester 4-α-PMA (Promega, Madison, WI) was kept as a 5 mM stock in DMSO at −20°C. Chelerythrine HCl (Calbiochem or Sigma) was stored as a 2 mM stock in DMSO at −20°C. The PKC inhibitory peptide 19–31 was dissolved in 5% acetic acid at 1 mg/ml and stored at −20°C.
Generation of hASIC1b phosphorylation mutants.
The full-length sequence of human ASIC1b (GenBank Accession No. NM001095) was entered into the Genetics Computer Group sequence analysis suite (GCG) at the University of Alabama at Birmingham (UAB) and into Scansite software (http://scansite.mit.edu) to determine the PKC consensus phosphorylation sites. There are three PKC consensus phosphorylation sites located in the intracellular aspect of hASIC1b: T26, S40, and S499. These sites were mutated to alanine, to prevent their phosphorylation, or to glutamic acid or aspartic acid, to mimic phosphorylation. Site-directed mutagenesis was performed using the Excite kit for T26A, S40A, and S499A mutants or the Quickchange II XL kit (both kits from Stratagene, La Jolla, CA) for T26E, S40E, S499D, S40A/S499A, S40E/S499A, and S40E/S499D mutants. Each human ASIC1b construct was subjected to PCR with sense and antisense primers with the necessary base changes to result in the desired amino acid mutations. The PCR product was digested overnight at 37°C with DpnI to remove nonmutated DNA, and it was transformed into XL-10 Gold Escerichia coli following the manufacturer's instructions. Transformed E. coli were plated on Luria Bertani (LB) plates with 50 μg/ml ampicillin and grown for 16 h at 37°C. DNA was isolated from 5 ml LB + ampicillin cultures of each colony using a miniprep kit (Qiagen, Valencia, CA), and the presence of the mutations was confirmed by sequencing (Heflin Genetics Center, UAB). Colonies were then grown into 250 ml LB + ampicillin cultures, and DNA was isolated with a maxiprep kit (Qiagen). Full-length hASIC1b was sequenced again after this step.
Generation of hemagglutinin-tagged hASIC1b.
To insert the hemagglutinin (HA) tag (YPYDVPDYA) of the influenza virus between F147 and K148 residues of the extracellular loop of hASIC1b, we used a similar method to Geiser et al. (18). Oligonucleotide primers were obtained PAGE purified from Integrated DNA Technologies (Coralville, IA). The forward primer was 63 bp long with the 5′-end formed by 36 nucleotides corresponding to the hASIC1b DNA sequence upstream of the insertion of the HA tag followed by the 27 nucleotides encoding the HA tag. The reverse primer was 131 bp long with the 5′-end consisting of 104 bases identical to the hASIC1b DNA sequence downstream of the HA tag position. PCR fragments were obtained by PCR of 3 μg of each of the forward and reverse primers with Taq polymerase. Primers were denatured at 94°C for 10 min, annealed by decreasing the temperature from 94 to 60°C for 34 min at a rate of 1°C/min, and extended at 68°C for 10 min. The PCR product was gel purified with a Qiaquick Gel Extraction Kit (Qiagen); 300 ng of this gel-purified PCR fragment and 100 ng of recipient plasmid DNA (hASIC1b) were used in a mutagenesis reaction using the Quickchange II XL Site-Directed Mutagenesis kit (Stratagene) as described in the kit's instructions. After the transformation of XL-10 Gold E. Coli and the isolation of plasmid DNA by miniprep, the presence of the HA tag in the correct position on hASIC1b was confirmed by DNA sequencing.
cRNA preparation.
Wild-type (WT) hASIC1b and phosphorylation mutants or HA-tagged hASIC1b were linearized with XbaI. After proteinase K digestion, Na-acetate precipitation, and phenol-chloroform followed by chloroform extraction, purified DNA in water was used for cRNA transcription using the MegaScript High Yield In Vitro Transcription kit (T7) and Cap Analog or ARCA (Ambion, Austin, TX). Quality and size of the synthesized cRNA were checked by denaturing agarose-formaldehyde gel electrophoresis. The cRNA concentration was determined spectroscopically by measuring optical density at 260 nm.
Isolation of Xenopus laevis oocytes.
Oocytes were isolated surgically from anesthetized female Xenopus laevis frogs (Xenopus I, Dexter, MI) and placed in Ca2+-free ND96 buffer (96 mM NaCl, 1mM MgCl2, 2 mM CaCl2, 2 mM KCl, and 5 mM HEPES; pH 7.4). Oocytes were first dissociated manually with forceps into small groups. They were then digested with 2 mg/ml collagenase A (Roche Applied Science, Indianapolis, IN) in Ca2+-free ND96 pH 7.4 buffer for 75–100 min, after which they were washed with Ca2+-free ND96 followed by three washes in ND96 with 2mM CaCl2. Stage V-VI oocytes were incubated (before and after injection) at 18°C in ND96 buffer with 10 mM sodium pyruvate (Sigma) and 10 mg/ml gentamycin (Lonza, Basel, Switzerland). These experimental procedures are in accordance with and were approved by the Institutional Animal Care and Use Committee of UAB.
Expression of hASIC1b and phosphorylation mutants in Xenopus oocytes.
Oocytes were injected 18–24 h after collagenase digestion with 11.5 ng of a 0.5 μg/μl cRNA dilution. We used a nanoliter injector (World Precision Instruments, Sarasota, FL) and 10-μl microdispensers (Drummond Scientific, Broomall, PA) pulled with a vertical Kopf Model 700D micropipette puller (David Kopf Instruments, Tujunga, CA). cRNAs used to express each WT or phosphorylation mutant construct in oocytes were in vitro transcribed three to four different times and were not all from one batch of cRNA, with the exception of S40A/S499E and S40E/S499D cRNAs. Also, acid-activated currents were measured in oocytes injected with cRNAs for the same construct, in vitro transcribed in different days, to ensure that the results were not affected by cRNAs of different batches. Experiments were performed at room temperature 1–4 days postinjection.
TEV experiments.
TEV experiments were performed using a Digidata 1200 Series interface and a Dagan TEV 200 or Geneclamp 500B amplifier with a steady-state restore switch modification (Axon Instruments, Sunnyvale, CA). The oocyte was placed in a recording chamber with bath volume of 500 μl and impaled with two electrodes. Micropipettes were made from VWR calibrated (100 μl) borosilicate glass capillaries (World Precision Instruments) pulled with a Kopf model 700D vertical pipette puller (Kopf Instruments) and had resistances of 0.5–2.0 MΩ when filled with 3 M KCl and inserted into the bath solution. A SF-77B Perfusion Fast-Step (Warner Instruments, Hamden, CT) set at 3 steps/position was used for rapid exchange of solution bathing the oocyte from ND96 pH 7.4 to ND96 pH 4.0 (with 5 mM MES substituted for 5 mM HEPES) to activate the heterologously expressed hASIC1b channel. The protocol used exposed the oocyte to pH 7.4 solution for 13 s, to pH 4.0 for 13 s, and again to pH 7.4 for 13 s to allow for complete recovery of the acid-activated channel. Before acid-activated currents were recorded, the clamp gain and stability were set for each oocyte using capacitive transients of a step protocol from −40 to −50 mV. Oocytes were clamped at −60 mV for all the experiments. The flow of solutions out of the perfusion fast step was controlled with a VC-6 Six Valve Controller (Warner Instruments), and the solution flow rate was 1–2 ml/min.
For pH activation curves, the solution flowing out of one barrel of the perfusion system was ND96 pH 7.4 while the solution flowing out of the second barrel was changed to ND96 pH 7.0, 6.5, 6.0, 5.5, 5.0, and 4.0 sequentially. These solutions were buffered with 5 mM HEPES (for pH 7.4 to 6.0) or 5 mM MES (for pH 5.5 to 4.0). Acid-activated currents at each pH were normalized to the peak pH 4.0 current (IpH4.0) of the oocyte. Pooled normalized values were fitted to the sigmoidal dose-response (variable slope) equation in GraphPad Prism 3 to obtain pH50 values and Hill coefficients.
For PKC activator experiments, five sweeps of acid-activated currents (activated with ND96 pH 4.0 solution) were recorded, the flow of solutions was stopped, and the oocyte was unclamped before and during the addition of PMA or PdBu to the bath solution to a final 1 μM concentration for 5 min (21). After the PMA incubation period, the oocyte was clamped at −60 mV, and another five acid-activated peaks were recorded. As controls, we used 5 min of no treatment, DMSO (1:1,000, the equivalent of DMSO in the PMA experiment), or 4-α-PMA (an inactive PMA analog) at a final concentration of 1 μM. The peak acid-induced current recorded after the addition of a treatment (no treatment, DMSO, 4-α-PMA, PMA, or PdBu) was normalized to the current from the last sweep before treatment. In some experiments, oocytes were preincubated in ND96 pH 7.4 with 1 μM chelerythrine for 1 h before recording. PKC inhibitory peptide 19–31 was injected in the oocytes 1 h before recording at a final concentration of 6 μM.
Data were acquired with pCLAMP 8.0 software at 200 Hz and analyzed with Clampfit 9.0. After acquisition, data were filtered with an eight-pole Bessel filter with a 10 Hz −3-dB cutoff, and baselines were subtracted.
Cell culture and transfection.
Chinese hamster ovary (CHO)-K1 cells were maintained in continuous culture in 50:50 DMEM-F-12 medium (Invitrogen) supplemented with 10% FBS (Hyclone). For electrophysiological recordings, cells were split into six-well tissue culture dishes and transiently transfected using 4 μg pBi-enhanced green fluorescent protein (eGFP)-hASIC1b plasmid DNA and 10 μl Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's protocols. After transfection, cells were replated using PBS-EDTA onto flame-sterilized glass coverslips and patched within 24–48 h.
Patch clamp.
Micropipettes were prepared using a Narashigi PP-83 two-stage micropipette puller and were filled with 120 mM KCl, 5 mM NaCl, 10 mM HEPES, 0.4 mM CaCl2, 2 mM MgCl2, 1 mM EGTA, and 2 mM MgATP (pH 7.4). Pipette electrical resistance was 3–5 MΩ. Outside-out patches were formed by abutting the pipette tip to the cell surface, applying suction to form a GΩ seal, rupturing the membrane with increased suction, and slowly removing the pipette from the cell surface to form an outside-out patch. The patch/pipette tip was moved just in front of the barrels of a VC-77MCS perfusion system (Warner Instruments). Signals were recorded on a personal computer running pCLAMP 9 using an Axopatch 200B patch-clamp amplifier and a DigiData 1320 digitizer. The signal was sampled at 5 kHz and low pass filtered at 5 kHz with the 200B's four-pole Bessel filter. Currents were recorded by holding the membrane voltage at −60 mV and perfusing with Krebs buffer (130 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM d-glucose, and 10 mM HEPES; pH 7.4). Acid-induced currents were evoked by pH 6.0 Krebs buffer in which 5 mM HEPES was replaced with 5 mM MES and the pH was adjusted with HCl.
For PMA experiments, 100 nM PMA was added to the pH 7.4 and 6.0 solutions, with an equivalent amount of DMSO [1/10,000 (vol/vol)] being added to the pH 7.4 and 6.0 control solutions. An ∼30-s protocol was used: 5 s at pH 7.4, 10 s at pH 6.0, and 15 s at pH 7.4. Little to no desensitization of the current was observed with this protocol for this activation pH. Cells were patched on a Nikon TE200 with an epifluorescent attachment, allowing for the visualization of GFP in transfected cells. Outside-out patches showing <100 pA of acid-induced current were excluded to allow for an acceptable dynamic range. In these conditions, no acid-induced currents were observed in nonfluorescent cells.
Whole oocyte membrane preparation.
To assess the whole oocyte expression of WT hASIC1b and each phosphorylation mutant, we used a modified procedure of Turk et al. (36). Ten oocytes per group were rinsed in sterile ND96 pH 7.4 solution and homogenized in lysis buffer [20 μl/oocyte; 100 mM NaCl, 20 mM Tris·HCl (pH 7.6), 1% Triton X-100, and 1 Complete protease inhibitor tablet (Roche Applied Science) per 50 ml] in a 1.5-ml tube with an Eppendorf micropestle. Homogenized samples were centrifuged for 10 min at 12,000 rpm, and the middle liquid layer was retained for gel electrophoresis. Protein concentrations were determined with a BCA assay kit (Pierce, Rockford, IL).
Electrophoresis and immunoblot analysis.
Equivalent amounts of protein (30 μg) from the oocyte total membrane preparations were boiled in 1× Laemmli sample buffer [62.5 mM Tris·HCl (pH 6.8), 25% glycerol, 2% SDS, 5% β-mercaptoethanol, and 0.01% bromophenol blue] at 95°C for 5 min and subjected to SDS-PAGE with an 8% separating gel and a 4% stacking gel. Proteins were electrotransferred to methanol-treated polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA) for 1.5 h at 100 V at 4°C in a Tris-glycine buffer [25 mM Tris, 192 mM glycine, and 20% (vol/vol) methanol]. Membranes were blocked in 5% nonfat dry milk in Tris-buffered saline with Tween (TBST) buffer [100 mM Tris (pH 7.5), 150 mM NaCl, and 0.1% Tween] for 1 h at room temperature. Membranes were incubated overnight at 4°C with rabbit polyclonal antibody to ASIC1 (Alomone Labs, Jerusalem, Israel) at 1:200 or with mouse anti-actin (Chemicon, Billerica, MA) at 1:5,000 in 2% milk in TBST. Primary antibody incubation was followed by three washes of 15 min each with TBST. A goat anti-rabbit IgG horseradish peroxidase (HRP)-conjugated antibody (Pierce) diluted 1:10,000 or a goat anti-mouse IgG HRP-conjugated antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted 1:20,000 in 2% milk in TBST was used to detect the primary antibodies for 1 h at room temperature. After three 15-min washes with TBST, membranes were treated with SuperSignal West Pico Substrate (Pierce) and exposed to Kodak Biomax XAR film (Perkin-Elmer, Waltham, MA).
Single oocyte chemiluminescence.
To quantify the expression of hASIC1b at the plasma membrane of oocytes in control or PKC-modulated conditions, we used the method of single oocyte chemiluminescence (SOC) as described by Zerangue et al. (45). Oocytes expressing HA-tagged WT hASIC1b were treated with PMA (1 μM for 5 min), PdBu (1 μM for 5 min), chelerythrine (1 μM for 1 h) or both chelerythrine (1 μM for 1 h) and PMA (1 μM for 5 min), as was done for recording peak pH 4.0-activated currents. They were then fixed in 4% paraformaldehyde in ND96 pH 7.4 solution for 15 min at 4°C. All subsequent steps were performed at 4°C. Oocytes were washed three times for 5 min each time in ND96 pH 7.4 solution and incubated in blocking solution (1% BSA in ND96 pH 7.4 solution) overnight. They were then incubated in rat anti-HA antibody (clone 3F10, Roche) at 1 μg/ml for 2 h. They were washed twice in blocking solution for 30 min each time and incubated in goat anti-rat HRP-conjugated secondary antibody (Pierce) at 1:400 for 1 h. The secondary antibody was washed twice for 30 min each time with blocking solution, and oocytes were finally washed with ND96 pH 7.4 solution for 10 min. Each oocyte was then placed in a 1.5-ml Eppendorf tube, and all the solution was removed. ELISA Supersignal West Femto Chemiluminescence substrate (50 μl, Pierce) was added, and luminescence was measured with a Turner TD20/20 luminometer (Turner BioSystems, Sunnyvale, CA).
Data analysis and statistics.
Data are presented as means (SD). Statistical significance was determined with two-tailed paired or unpaired Student's t-tests (in Excel) for two-group comparisons or one-way ANOVA followed by Tukey's post hoc test for comparisons of three or more groups (GraphPad Prism 3). A statistically significant difference was accepted if P < 0.05; 95% confidence intervals (CIs) were also determined for each mean (GraphPad Prism 3).
RESULTS
Mutations in hASIC1b consensus PKC phosphorylation sites and hASIC1b expression.
We analyzed the amino acid sequence of hASIC1b for PKC phosphorylation motifs with GCG (UAB) and Scansite (MIT) software. This analysis resulted in several consensus PKC phosphorylation sites; however, we decided to concentrate on the three amino acids that are located on the intracellular NH2 and COOH termini of hASIC1b: T26, S40, and S499 (Fig. 1) because the phosphorylation of membrane proteins is an intracellular event (43). We used site-directed mutagenesis to generate phosphorylation mutants at each of these three sites. Mutations to alanine prevent phosphorylation, and mutations to glutamic acid or aspartic acid can often mimic a phosphorylated amino acid.
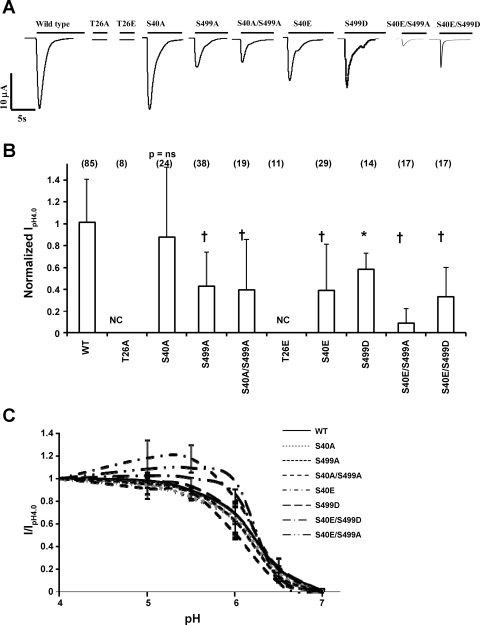
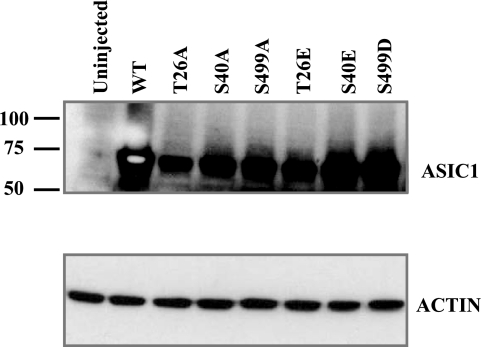
We first assessed the effect of these phosphorylation site mutations on the functional expression of each construct in Xenopus oocytes. Oocytes were injected with WT hASIC1b or mutant cRNA for each phosphorylation site. Electrophysiological recordings were obtained 1–4 days postinjection. Oocytes were voltage clamped at −60 mV, and acid-activated currents were recorded by rapidly switching the solution bathing the oocyte from ND96 pH 7.4 to ND96 pH 4.0 solution. Representative traces of these recordings are shown in Fig. 2A. Uninjected oocytes did not exhibit any acid-activated currents (not shown; also, there is no acid-activated current in oocytes injected with two of the hASIC1b mutants in Fig. 2). Peak IpH4.0 of each mutant was normalized to the average peak IpH4.0 of WT hASIC1b constructs recorded from the same batch of oocytes on the same day postinjection [1.01 (SD 0.39), n = 85, 95% CI (0.925, 1.10); Fig. 2B]. Mutation of S40 to alanine had no effect on the functional expression of hASIC1b, as S40A exhibited average peak pH 4.0-activated currents that were not statistically different than WT hASIC1b [0.88 (SD 0.63), n = 24, P = not significant (NS) by one-way ANOVA, 95% CI (0.609, 1.15), P < 0.05 vs. S499D]. Mutation of S40 to glutamic acid, a phosphorylation mimic, resulted in a decrease in IpH4.0 of hASIC1b [0.39 (SD 0.42), n = 28, P < 0.001, 95% CI (0.231, 0.552)]. At site S499, mutation to either alanine or aspartic acid also resulted in a decrease in IpH4.0 [0.43 (SD 0.31), n = 38, P < 0.001, 95% CI (0.327, 0.532), for S499A and 0.58 (SD 0.15), n = 14, P < 0.01, 95% CI (0.498, 0.670), for S499D]. The double mutant construct S40A/S499A exhibited reduced functional expression as well [0.39 (SD 0.46), n = 19, P < 0.001, 95% CI (0.170, 0.161)]. The S40E/S499A mutation had an even greater effect [0.0877 (SD 0.137), n = 17, P < 0.001, 95% CI (0.0172, 0.158), P < 0.05 vs. S499D]. The S40E/S499D mutation also expressed reduced IpH4.0 compared with WT [0.330 (SD 0.271), n = 17, P < 0.001, 95% CI (0.191, 0.470)]. We were not able to measure any acid-activated currents in oocytes expressing T26A (n = 8) or T26E (n = 11) hASIC1b constructs. The translation of these constructs in oocytes was confirmed by immunoblot analysis of total membranes of uninjected oocytes or oocytes injected with each of the ASIC1b constructs with an ASIC1 antibody (Fig. 3).
Fig. 2.
Mutations in PKC consensus phosphorylation sites on hASIC1b affect its function in Xenopus oocytes. cRNA (11.5 ng) for wild type (WT) hASIC1b or hASIC1b PKC phosphorylation mutants was expressed in Xenopus oocytes. Acid-activated currents at pH 4.0 were measured by two-electrode voltage clamp (TEV) at 1–4 days postinjection as described in materials and methods. A: representative traces of acid-activated currents of WT hASIC1b and each hASIC1b mutant. B: peak pH 4.0 current (IpH4.0) for each phosphorylation mutant was normalized to the average peak IpH4.0 recorded on the same day in oocytes of the same batch expressing WT hASIC1b. Shown is the summary of 3–6 experiments; values are means + SD. The number of individual oocytes recorded is shown in parentheses on top of each bar. P values were determined with one-way ANOVA with Tukey's post-hoc test. *P < 0.01 and †P < 0.001 vs. WT. NC, no acid-activated current. C: pH activation curves for WT hASIC1b and hASIC1b phosphorylation mutants. Peak acid-activated currents were recorded by switching the extracellular solution to pH 7.0, 6.5, 6.0, 5.5, 5.0, and 4.0 sequentially, as described in materials and methods, and were normalized to peak IpH4.0. Normalized values (I/IpH4.0) were fitted to the Hill equation to obtain pH50 values and Hill coefficients. n = 13 oocytes for WT, 10 oocytes for S40A, 11 oocytes for S499A, 8 oocytes for S40A/S499A, 8 oocytes for S40E, 8 oocytes for S499D, 5 oocytes for S40E/S499A, and 5 oocytes for S40E/S499D. T26A (n = 8) and T26E (n = 8) constructs did not exhibit any acid-activated current at any pH (data not shown).
Fig. 3.
hASIC1b expression in Xenopus oocytes. Oocytes were injected with 11.5 ng RNA for WT hASIC1b or each hASIC1b mutant, and total membranes were isolated as described in materials and methods. Protein (30 μg) was resolved by SDS-PAGE, transferred to a polyvinylidene difluoride (PVDF) membrane, and immunoblotted with an antibody to ASIC1 (1:200) or to actin (1:5,000) as a loading control. The blot shown is representative of 2–4 experiments comparing WT hASIC1b expression with that of each hASIC1b phosphorylation mutant.
Because ASIC1 is a ligand-gated channel, activated by H+, we determined if the mutations in the consensus PKC phosphorylation sites have any effect on the affinity of the channel for protons. Oocytes were injected with cRNA for each hASIC1b mutant, and acid-activated currents were recorded at different activation pHs ranging from pH 7.0 to 4.0 (Fig. 2C). The oocyte was sequentially exposed to solutions of higher pH and then to lower pH. To determine if hASIC1b current exhibited rundown, i.e., a diminution of the peak current with repeated acid challenges, two consecutive activation curves were taken, and no significant rundown of the current was observed (not shown). Peak acid-activated currents at each pH were normalized to peak IpH4.0 (Fig. 2C). pH50 values of activation and Hill coefficients were determined for each individual oocyte by fitting normalized current (I)/IpH4.0 at each test pH to the sigmoidal dose-response (variable rate) equation in Prism 3. The pH activation curve shown is the average of all the oocytes. Means and SDs of pH50 values and Hill coefficients are shown in Table 1. pH50 values of hASIC1b phosphorylation mutants were not significantly different than the pH50 value of WT hASIC1b. However, there was a statistically significant difference in Hill coefficients (Table 1).
Table 1.
pH50 values for half-maximal activation and Hill coefficients for WT hASIC1b and hASIC1b phosphorylation mutants
| pH50 | Hill Coefficient | n | |
|---|---|---|---|
| WT | 6.17 (SD 0.186) | 1.78 (SD 0.405) | 13 |
| S40A | 6.49 (SD 1.17) | 1.83 (SD 0.704) | 10 |
| S499A | 6.11 (SD 0.141) | 2.03 (SD 0.736) | 11 |
| S40A/S499A | 6.11 (SD 0.0542) | 2.77 (SD 0.595)a | 8 |
| S40E | 6.18 (SD 0.0229) | 3.21 (SD 0.717)b,c | 8 |
| S499D | 6.17 (SD 0.0732) | 2.39 (SD 0.633) | 8 |
| S40E/S499A | 6.26 (SD 0.0247) | 4.30 (SD 0.435)c,d,e | 5 |
| S40E/S499D | 6.24 (SD 0.0917) | 3.37 (SD 0.498)b,c | 5 |
Data are means (SD); n is the number of individual oocytes. pH50 values and Hill coefficients were obtained by fitting normalized pH response values to the Hill equation on GraphPad Prism 3 for each individual oocyte. pH50 values were not statistically different (P = 0.71 by one-way ANOVA). Hill coefficients were statistically analyzed with one-way ANOVA followed by a Tukey's post hoc test:
P < 0.05 vs. wild type (WT) and S40A;
P < 0.01 vs. S499A;
P < 0.001 vs. WT and S40A;
P < 0.001 vs. S499A and S499D;
P < 0.01 vs. S40A/S499A.
The effect of the PKC activator PMA on acid-activated currents of hASIC1b is mediated by site S40.
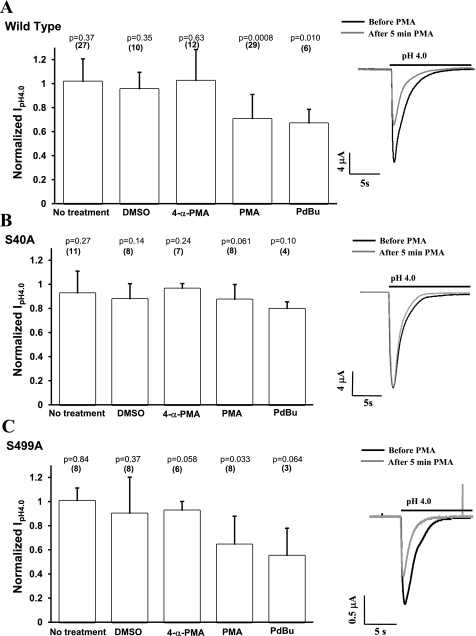
We examined the effect of PKC activation with the phorbol esters PMA or PdBu on hASIC1b currents. PMA and PdBu are cell-permeable diacylglycerol (DAG) analogs that activate classical and novel PKC isoforms. Oocytes were injected with WT hASIC1b, S40A hASIC1b, or S499A hASIC1b to examine the importance of the amino acids that are consensus PKC phosphorylation sites. Acid-activated currents were recorded in the same oocyte before and after 1 μM PMA or 1 μM PdBu addition to the bath solution for 5 min. Incubation of the oocyte with PMA decreased IpH4.0 of WT hASIC1b [0.70 (SD 0.20), n = 29, P = 0.0008 by two-tailed paired t-test of IpH4.0 before and after treatment]. PdBu also inhibited WT hASIC1b current [0.67 (SD 0.12), n = 6, P = 0.010 by two-tailed paired t-test]. Incubation of WT hASIC1b-injected oocytes in the bath solution (ND96 pH 7.4 solution) for 5 min without treatment (n = 27), with DMSO (1:1,000, n = 10), or with the inactive PMA analog 4-α-PMA (1 μM, n = 12) had no effect on acid-activated currents (Fig. 4A). The 95% CIs for the means of the ratio of IpH4.0 after treatment to IpH4.0 before treatment for the WT hASIC1b construct were (0.940, 1.09) for 5 min of no treatment, (0.852, 1.05) for DMSO, (0.856, 1.19) for 4-α-PMA, (0.626, 0.783) for PMA, and (0.544, 0.792) for PdBu. A statistically significant effect of PMA or PdBu on hASIC1b currents was not observed in oocytes expressing S40A hASIC1b [0.87 (SD 0.13), n = 8, P = 0.061 by two-tailed paired t-test for PMA treatment and 0.80 (SD 0.059), n = 4, P = 0.10 for PdBu treatment; Fig. 4B]. The 95% CIs for the ratios of IpH4.0 after treatment to IpH4.0 before treatment for the S40A construct were (0.799, 1.05) for 5 min of no treatment, (0.768, 0.984) for DMSO, (0.922, 1.00) for 4-α-PMA, (0.766, 0.978) for PMA, and (0.7, 0.89) for PdBu. The S499A hASIC1b construct responded to the activation of endogenous oocyte PKC with PMA or PdBu in a similar manner to WT hASIC1b, decreasing by a similar amount [0.64 (SD 0.24), n = 8, P = 0.033 by two-tailed paired t-test for the PMA effect and 0.55 (SD 0.23), n = 3, P = 0.064 by two-tailed paired t-test for the PdBu effect; Fig. 4C]. The 95% CIs for the mean ratios of IpH4.0 after treatment to IpH4.0 before treatment for the S499A channel were (0.914, 1.09) for 5 min of no treatment, (0.645, 1.15) for DMSO, (0.844, 1.01) for 4-α-PMA, and (0.461, 0.824) for PMA.
Fig. 4.
PKC activators PMA and phorbol 12,13-dibutyrate (PdBu) reduce acid-activated currents of WT hASIC1b and S499A hASIC1b but not that of S40A hASIC1b. Oocytes were injected with RNA for WT hASIC1b (A), S40A (B), or S499A (C). Acid-activated currents were measured by TEV before and after the addition of 1 μM PMA or 1 μM PdBu to the bath for 5 min to activate endogenous oocyte PKC. The negative controls used were either no treatment, vehicle (DMSO, 1:1,000), or an inactive PMA analog (4-α-PMA, 1 μM) added to the bath for 5 min between current measurements. Bar graph values are fractions of peak IpH4.0 recorded after treatment over peak IpH4.0 recorded before the treatment. Representative acid-activated current traces before (solid line) and after (shaded line) 1 μM PMA was added to the bath are also shown to the right of each graph. The numbers in parentheses on top of each bar indicate the number of oocytes. P values were determined by a two-tailed paired Student's t-test comparing the sets of peak IpH4.0 values before and after each corresponding treatment. Data are means + SD.
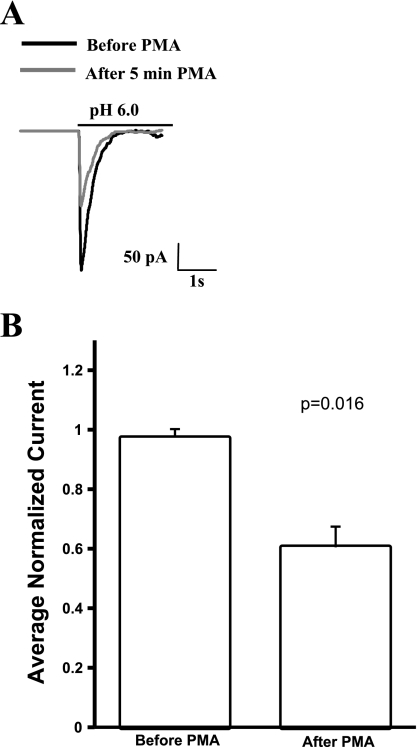
Because PKC can have cell type-specific effects (11), we repeated the experiment of activating PKC with PMA in CHO cells transfected with WT hASIC1b-eGFP bicistronic vector. Acid-activated currents were recorded before and after the superfusion of 100 nM PMA in the chamber. To normalize for differences in patch size and current density, peak acid-activated currents were normalized to the first peak current for the patch. Three peaks before PMA and three peaks 5–7 min after PMA were averaged to assay the effect of PMA on acid-induced currents. Treatment with 100 nM PMA reduced the peak acid-activated current of WT hASIC1b in outside-out patches to 0.605 (SD 0.0697), a decrease that was statistically significant compared with normalized current values before PMA (Fig. 5). This is consistent with our findings in the TEV system with Xenopus oocytes.
Fig. 5.
PMA inhibits peak acid-induced currents of hASIC1b in transfected Chinese hamster ovary (CHO)-K1 cells. A: in outside-out patches of CHO-K1 cells transfected with a bicistronic plasmid encoding WT hASIC1b and enhanced green fluorescent protein, acid-induced currents were observed. Treatment with 100 nM PMA significantly reduced the current. B: for analysis, currents were normalized to address expression variability and patch size. Data are shown as average normalized currents for 3 pulses before PMA application and 3 pulses 5 min post-PMA application; n = 3 cells. Data were analyzed with a paired Student's t-test.
We also determined the changes in channel gating kinetics upon PKC activation with PMA of hASIC1b constructs expressed in Xenopus oocytes. We determined the peak half-width [defined as the time (in ms) between the two points that are 50% of the peak current amplitude from the baseline], half-activation time [or rise time (in ms), defined as the time from 0% to 50% of the peak amplitude], and half-inactivation time [or decay time (in ms), defined as the time from 100% to 50% of the peak amplitude]. A summary of these parameters for each construct (WT, S40A, and S499A) is shown in Table 2. The half-width and inactivation time of WT hASIC1b decreased after PMA treatment. PMA had no effect on the half-width, activation time, and inactivation time of S40A acid-induced currents. The half-width of the S499A current was also decreased upon the application of PMA.
Table 2.
Properties of pH 4.0-induced currents of human acid-sensing ion channel 1b before and after PMA
|
No Treatment |
DMSO
|
4-γ-PMA
|
PMA
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |||||||||
| Half-width, ms | ||||||||||||||||
| WT | 711 (SD 326) | 681 (SD 289) | 2013 (SD 903) | 1793 (SD 970) | 1804 (SD 1124) | 1761 (SD 1114) | 802 (SD 269) | 724 (SD 264) | ||||||||
| n | 22 | 10 | 10 | 22 | ||||||||||||
| P value | 0.449 | 0.0879 | 0.566 | 0.00741* | ||||||||||||
| S40A | 795 (SD 337) | 719 (SD 232) | 870 (SD 153) | 906 (SD 209) | 1039 (SD 349) | 965 (SD 296) | 1222 (SD 422) | 1149 (SD 369) | ||||||||
| n | 9 | 7 | 5 | 10 | ||||||||||||
| P value | 0.133 | 0.632 | 0.234 | 0.33 | ||||||||||||
| S499A | 673 (SD 157) | 677 (SD 195) | 750 (SD 294) | 689 (SD 222) | 1137 (SD 367) | 932 (SD 344) | 973 (SD 227) | 766 (SD 102) | ||||||||
| n | 8 | 7 | 8 | 7 | ||||||||||||
| P value | 0.934 | 0.182 | 0.0497 | 0.00968* | ||||||||||||
| τ1/2 activation, ms | ||||||||||||||||
| WT | 185 (SD 87) | 188 (SD 66) | 163 (SD 46) | 155 (SD 40) | 114 (SD 29) | 132 (SD 58) | 120 (SD 34) | 143 (SD 71) | ||||||||
| n | 21 | 8 | 6 | 18 | ||||||||||||
| P value | 0.435 | 0.649 | 0.449 | 0.19 | ||||||||||||
| S40A | 149 (SD 101) | 163 (SD 66) | 146 (SD 43) | 155 (SD 40) | 268 (SD 162) | 182 (SD 94) | 132 (SD 38) | 135 (SD 59) | ||||||||
| n | 9 | 5 | 6 | 7 | ||||||||||||
| P value | 0.76 | 0.727 | 0.148 | 0.906 | ||||||||||||
| S499A | 108 (SD 45) | 128 (SD 67) | 185 (SD 108) | 153 (SD 78) | 113 (SD 24) | 119 (SD 27) | 137 (SD 55) | 115 (SD 30) | ||||||||
| n | 6 | 5 | 7 | 9 | ||||||||||||
| P value | 0.217 | 0.55 | 0.624 | 0.222 | ||||||||||||
| τ1/2 inactivation, ms | ||||||||||||||||
| WT | 387 (SD 167) | 364 (SD 129) | 1426 (SD 671) | 1264 (SD 636) | 1078 (SD 639) | 1050 (SD 649) | 556 (SD 212) | 469 (SD 175) | ||||||||
| n | 20 | 10 | 9 | 22 | ||||||||||||
| P value | 0.392 | 0.167 | 0.694 | 0.00191* | ||||||||||||
| S40A | 500 (SD 200) | 474 (SD 160) | 625 (SD 112) | 668 (SD 196) | 931 (SD 495) | 974 (SD 710) | 879 (SD 335) | 826 (SD 293 | ||||||||
| n | 8 | 7 | 6 | 10 | ||||||||||||
| P value | 0.242 | 0.551 | 0.703 | 0.515 | ||||||||||||
| S499A | 496 (SD 143) | 486 (SD 195) | 507 (SD 188) | 485 (SD 158) | 813 (SD 273) | 739 (SD 340) | 641 (SD 217) | 537 (SD 83) | ||||||||
| n | 8 | 7 | 8 | 8 | ||||||||||||
| P value | 0.839 | 0.377 | 0.52 | 0.064 | ||||||||||||
Values are means (SD); n is the number of individual oocytes. Half-width, time between the two points that are 50% of the peak current amplitude from the baseline; τ1/2 activation, time from 0% to 50% of the peak amplitude during activation; τ1/2 inactivation, time from 100% to 50% of the peak amplitude during inactivation.
Statistically significant P values obtained with two-tailed paired Student's t-tests between the before and after pairs for each construct for each condition.
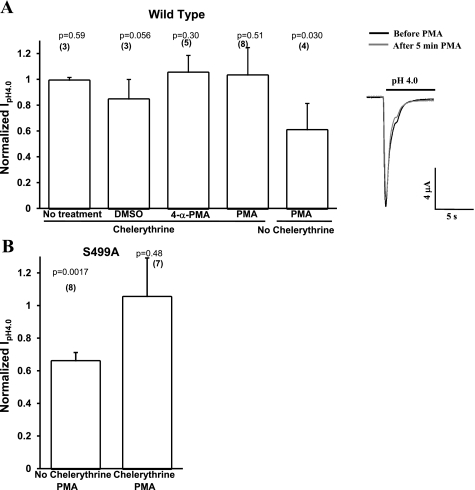
We were able to prevent the inhibitory effect of PMA on acid-induced currents of WT hASIC1b or S499A hASIC1b expressed in oocytes by pretreating the oocytes with 1 μM chelerythrine (Fig. 6). Chelerythrine is a potent and specific PKC antagonist that inhibits the catalytic domain of PKC and does not interfere with DAG and, therefore, also with PMA binding. These data suggest that the effect of PMA on WT or S499A hASIC1b is mediated by PKC and is not an artifact of nonspecific PMA effects. This is also supported by the lack of effect of the inactive PMA analog 4-α-PMA (Fig. 4, A–C). PKC activation has an inhibitory effect on the functional expression of hASIC1b, and this effect is mediated by site S40 of hASIC1b.
Fig. 6.
The PKC inhibitor chelerythrine abolishes the effect of PMA on WT and S499A hASIC1b. A: oocytes expressing WT hASIC1b were pretreated with the PKC inhibitor chelerythrine (1 μM) for 1 h, and peak IpH4.0 was measured before and after 5 min of 1 μM PMA, 1 μM 4-α-PMA, DMSO (1:1,000), or no treatment. Shown are representative pH 4.0-activated currents recorded by TEV before (solid line) and after the addition of 1 μM PMA to the bath for 5 min (shaded line) in oocytes expressing WT hASIC1b pretreated with 1 μM chelerythrine for 1 h. B: oocytes expressing S499A hASIC1b were pretreated with 1 μM chelerythrine overnight. Peak pH 4.0-activated currents were measured before and after the addition of 1 μM PMA to the bath. Peak IpH4.0 recorded after 5 min of treatment was normalized to peak IpH4.0 recorded before treatment on the same oocyte. The effect of PMA on control untreated oocytes is also shown. Bar graphs represent mean normalized values + SD. P values shown on top of each bar were determined with two-tailed paired Student's t-test on sets of before and after peak IpH4.0 values for each treatment. The number of oocytes is shown in parentheses.
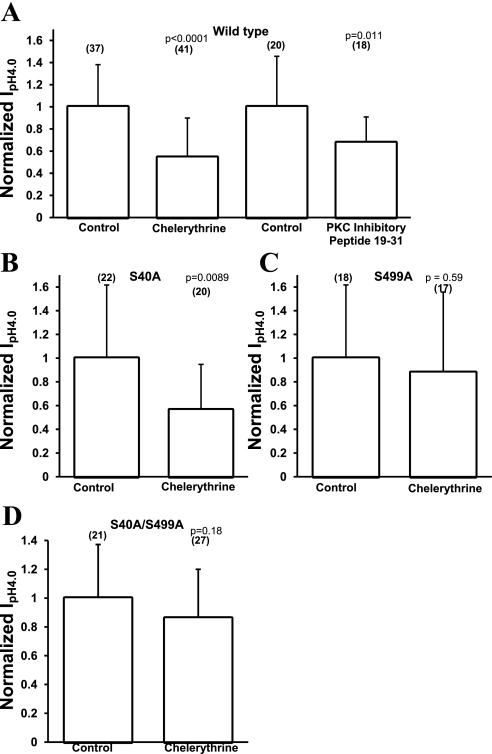
The PKC inhibitor chelerythrine decreases acid-activated current of WT hASIC1b and S40A but not of S499A hASIC1b.
Because the activation of PKC with PMA decreased the acid-activated current of hASIC1b, and the presence of site S40 was necessary to observe this effect, we inhibited PKC with chelerythrine and measured acid-activated currents in control oocytes and chelerythrine-treated oocytes injected with WT hASIC1b, S40A hASIC1b, S499A hASIC1b, or S40A/S499A hASIC1b. Chelerythrine was applied for 1 h at 1 μM in ND96 pH 7.4 solution. Control oocytes were incubated in ND96 pH 7.4 solution with no drugs for 1 h. Peak IpH4.0 were normalized to the average peak IpH4.0 of control untreated oocytes expressing the appropriate hASIC1b construct being studied. PKC inhibition with chelerythrine decreased acid-activated currents of WT hASIC1b [0.54 (SD 0.36), n = 41, P < 0.0001 by two-tailed unpaired t-test; Fig. 7A] and of S40A hASIC1b [0.56 (SD 0.38), n = 20, P = 0.0089; Fig. 7B]. The 95% CIs for normalized WT hASIC1b IpH4.0 were (0.873, 1.13) for the control, (0.431, 0.656) for chelerythrine-treated WT hASIC1b, and (0.726, 1.27) and (0.394, 0.734) for control and chelerythrine-treated S40A, respectively. Another PKC inhibitor, PKC inhibitory peptide 19–31, when injected in a 50-nl volume for a final concentration of ∼6 μM in the oocyte, also inhibited acid-activated currents of WT hASIC1b-expressing oocytes [0.68 (SD 0.23), n = 18, P = 0.011 by two-tailed unpaired t-test vs. control, 95% CI of PKC inhibitory peptide 19–31 (0.561, 0.792) and 95% CI of control (0.786, 1.21); Fig. 7A]. Control oocytes were injected with 50 nl vehicle (5% acetic acid) only.
Fig. 7.
Effect of PKC inhibitors on acid-activated currents of WT, S40A, S499A, or S40A/S499A hASIC1b. Oocytes were injected with 11.5 ng RNA for WT hASIC1b, S40A, S499A, or S40A/S499A. Two to three days postinjection, they were pretreated with 1 μM chelerythrine for 1 h (A–D) or injected with PKC inhibitory peptide 19–31 (PKC IP 19–31) at a final concentration of 6 μM for 1 h (A). pH 4.0-activated hASIC1b currents were recorded by TEV. Bar graph values are average peak IpH4.0 from chelerythrine-treated oocytes or oocytes injected with PKC IP 19–31 normalized to average peak IpH4.0 from untreated oocytes or oocytes injected with vehicle for PKC IP 19–31 (5% acetic acid). Data are summaries of 3–6 experiments, and values are means of individual oocytes + SD. The number of oocytes is shown in parentheses. P values were determined using a two-tailed unpaired Student's t-test vs. control.
Chelerythrine-treated oocytes expressing S499A hASIC1b exhibited currents that were not different from control oocytes [0.88 (SD 0.68), n = 17, P = 0.59; Fig. 7C], with 95% CIs of (0.693, 1.31) and (0.531, 1.23) for control S499A and chelerythrine-treated S499A, respectively. Chelerythrine did not have a statistically significant effect on the S40A/S499A double mutant [0.86 (SD 0.34), n = 27, P = 0.18 by two-tailed t-test vs. control, 95% CI of the chelerythrine-treated group (0.727, 0.995) and 95% CI of the control group (0.83, 1.17); Fig. 7D]. These data suggest that site S499 mediates the inhibitory effect of PKC inhibition on the functional expression of hASIC1b. Some basal level of PKC activity seems to be necessary for the amplitude of acid-activated currents of WT hASIC1b. This could be due to a basal phosphorylation at site S499, because when this site is mutated to alanine, there is no inhibition in the amplitude of hASIC1b acid-activated currents after chelerythrine treatment.
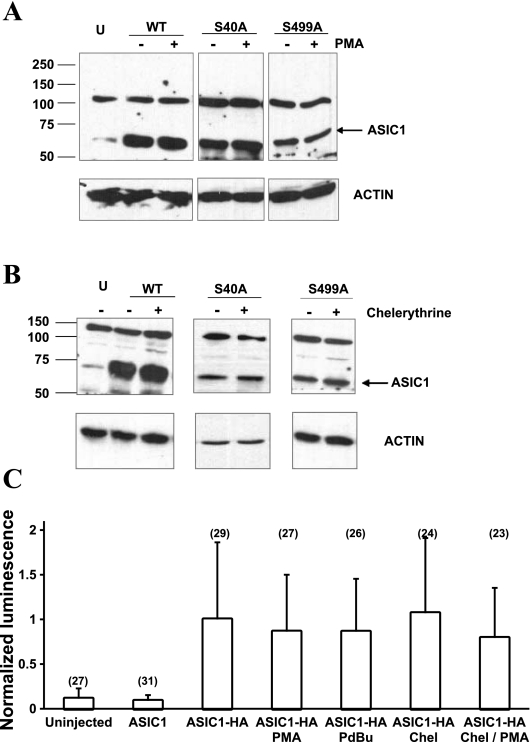
Activation or inhibition of PKC has no effect on the total protein expression or on the surface expression of WT hASIC1b.
Because PKC activation or inhibition could have an effect on the total expression of hASIC1b or on hASIC1b expression at the plasma membrane, we detected total membrane levels and plasma membrane levels of hASIC1b. The total expression of hASIC1b was assessed by immunoblots with an ASIC1 antibody to detect whole oocyte WT hASIC1b, S40A, and S499A protein expression levels from membrane preparations of control oocytes and in oocytes treated with 1 μM PMA for 5 min, as in the TEV experiments (Fig. 8A). No change in total protein expression of hASIC1b was observed due to PKC activation with PMA. Protein expression analysis with Western blot analysis of whole lysates of oocytes expressing WT hASIC1b, S40A, or S499A under control conditions or PKC inhibition with chelerythrine showed that treatment of oocytes with 1 μM chelerythrine for 1 h did not affect the total level of hASIC1b protein expression (Fig. 8B).
Fig. 8.
PMA and chelerythrine have no effect on the total expression or surface expression of hASIC1b. A and B: immunoblots showing hASIC1b expression with and without PMA (A) or chelerythrine (B) treatment in total membranes prepared as described in materials and methods 3 days postinjection of oocytes with RNA for WT, S40A, or S499A hASIC1b. U, uninjected oocytes. Equivalent amounts of protein (30 μg) were loaded on each lane and separated by SDS-PAGE. Proteins were transferred to PVDF membranes, which were blotted for hASIC1 (1:200) and actin (1:5,000) as a loading control. As shown by the actin immunoblot, these experiments were repeated 2–4 times. C: mean normalized luminescence of oocytes expressing hemagglutinin (HA)-tagged WT hASIC1b. There was no statistically significant difference between WT hASIC1b surface expression in control or treated oocytes (P = 0.628 by one-way ANOVA). Shown is a summary of 3 experiments. The number in parentheses on top of each bar indicates the number of individual oocytes measured.
Next, we assessed the surface expression of WT hASIC1b with SOC in control oocytes or in oocytes treated with 1 μM PMA or 1 μM PdBu for 5 min. The luminescence of each oocyte was normalized to the average luminescence of control oocytes injected with WT HA-tagged hASIC1b [1.00 (SD 0.863), n = 29, 95% CI (0.683, 1.32); Fig. 8C]. The WT HA-hASIC1b construct exhibited whole cell IpH4.0 that were significantly lower than those obtained in oocytes expressing untagged WT hASIC1b; however, it responded to PMA treatment in the same way as untagged WT hASIC1b (not shown). The luminescence from uninjected oocytes or oocytes injected with untagged WT hASIC1b was minimal (Fig. 8C). PKC activators PMA and PdBu did not have a significant effect on the surface expression of HA-tagged WT hASIC1b [normalized luminescence value was 0.863 (SD 0.637), n = 27, 95% CI (0.611, 1.12) for PMA and 0.862 (SD 0.592), n = 26, 95% CI (0.623, 1.10) for PdBu]. The PKC inhibitor chelerythrine also had no effect on the surface expression of HA-tagged WT hASIC1b [1.07 (SD 0.841), n = 24, 95% CI (0.738, 1.40)]. In some TEV experiments, we pretreated oocytes with chelerythrine to inhibit endogenous oocyte PKC and then tested the effect of PMA on IpH4.0. The luminescence experiment showed that there was no effect of chelerythrine and PMA on the surface expression of WT hASIC1b [0.793 (SD 0.561), n = 23, 95% CI (0.550, 1.04); Fig. 8C]. Since there was an effect of these drugs on WT hASIC1b current but no effect on its surface expression, we did not test the effect of these treatments on the surface expression of mutant hASIC1b channels. We conclude that the decrease in IpH4.0 of WT hASIC1b upon the activation or inhibition of PKC is not due to a change in the total protein expression or expression of the channel at the plasma membrane.
DISCUSSION
In this study, we tested the hypothesis that PKC consensus phosphorylation sites on hASIC1b and PKC affect the functional expression of hASIC1b. We used molecular, biological, and electrophysiological techniques to better understand how hASIC1b is regulated by PKC. There are three intracellular PKC consensus phosphorylation sites on hASIC1b, and, using site-directed mutagenesis, they were mutated to alanine, to prevent phosphorylation, and to glutamic acid and aspartic acid, to mimic phosphorylation. The cRNAs encoding each phosphorylation mutant were expressed in Xenopus oocytes, and acid-activated currents at pH 4.0 were measured by TEV. We found that mutating site T26 either to alanine or to glutamic acid resulted in nonfunctional channels, because we are not able to measure any acid-activated currents in oocytes expressing either T26A or T26E. A similar observation has been reported for the similar residue T92 in α-ENaC (20). Mutation of T92 to alanine results in a significant reduction of α-ENaC Na+ current (INa) compared with INa of WT α-ENaC expressed in oocytes, without affecting its surface expression (20). Two conserved residues involved in the gating of ENaC (GH) are located at positions 94–95 in α-ENaC and 28–29 in hASIC1b (Fig. 1), suggesting that nonconservative mutations of T26 might negatively affect the gating of the channel because of the proximity of this amino acid to the GH region (20). We did not pursue the study of T26A and T26E mutants further because they do not express acid-activated currents.
Site S40 seems to mediate the inhibitory effect of PKC on hASIC1b. When S40 is mutated to alanine, an amino acid with a nonphosphorylatable CH3 side chain, the peak IpH4.0 is similar to that of WT; however, the S40E mutant, which mimics phosphorylation because the COO− group of glutamic acid mimics the negative charge of an added phosphate (PO43−), expresses reduced IpH4.0 compared with WT. This result suggests that phosphorylation at S40 inhibits the functional expression of hASIC1b. In agreement with this idea, PKC activators (PMA or PdBu) inhibit the current of WT and S499A hASIC1b, but they have no statistically significant effect on S40A hASIC1b, suggesting that the S40 site mediates the inhibition of hASIC1b currents upon PKC activation. There is evidence for the direct modulation of hASIC1b kinetics as well as for the trafficking of related channels in response to PKC. Previous experiments in planar lipid bilayers have shown that PKC addition to the intracellular side of hASIC1b decreased its open probability and had no effect on the channel conductance (6). We can also speculate from data on the channel gating kinetics before and after the addition of PMA (Table 2) that the observed reduction in the magnitude of whole cell pH 4.0-activated currents could be due to changes in the gating of the channel. PMA does not affect the half-width, activation time, or inactivation time of S40A hASIC1b pH 4.0-activated current, but it decreases the half-width of WT and S499A and the inactivation time of WT hASIC1b. Another possibility is that the PKC activators cause membrane retrieval of the channel, therefore decreasing its functional expression. This has been reported for other membrane proteins expressed in oocytes like αβγ-rENaC (1), type II Na+-Pi cotransporters (16), glutamate transporter EAAC1 (35), and anion exchanger SLC26A6 (22). The effects of PMA on membrane retrieval could be specific to the membrane protein expressed in oocytes or general endocytosis of the plasma membrane (1). Because the mutation in the PKC consensus site S40 is able to prevent the inhibitory effect of PMA or PdBu on hASIC1b, this is probably a specific effect on hASIC1b rather than general membrane endocytosis. Moreover, measurements of the amount of WT hASIC1b channel expressed at the plasma membrane by SOC showed that there is no effect of PMA or PdBu on the surface expression of hASIC1b (Fig. 8C).
While the S40 site seems to mediate the effect of PMA on hASIC1b, the S499 site mediates the effect of the PKC inhibitor chelerythrine on hASIC1b. Pretreatment of oocytes with this PKC inhibitor decreased the peak IpH4.0 of WT (Fig. 7), without decreasing its surface expression (Fig. 8C). This suggests that PKC activity could maintain a basal level of phosphorylation of the channel. Chelerythrine has the same effect on S40A hASIC1b, but no effect was observed on the S499A hASIC1b peak IpH4.0. If there is basal phosphorylation of the S499 site and if this phosphorylation is necessary for either trafficking of the channel to the plasma membrane or for a certain level of channel conductance, then mutation of this site to alanine would decrease the amplitude of the current observed by TEV. In agreement with this hypothesis, S499A and S40A/S499A mutants exhibited reduced peak IpH4.0 compared with the WT. One would then expect the phosphorylation mimic S499D to have a peak IpH4.0 similar to the WT channel. However, S499D hASIC1b also exhibited slightly but statistically significant reduced current compared with WT. A discrepancy between the phosphorylation of an amino acid and its substitution with a phosphorylation mimic has been reported previously for rat ASIC2a. PKC phosphorylation at site T39 on rat ASIC2a stimulated the peak current, but the phosphorylation mimic mutation T39D did not significantly increase it as expected (3).
If there is basal phosphorylation at S499, then in control untreated oocytes, basal PKC activity would allow for the phosphorylation at S499 in WT and S40A hASIC1b. In chelerythrine-treated oocytes, PKC is inhibited and unable to phosphorylate the S499 site located on WT or S40A hASIC1b. The observed decrease in peak IpH4.0 of WT and S40A upon treatment with a PKC inhibitor is consistent with this idea. Additionally, PKC inhibition with chelerythrine does not affect S499A hASIC1b. These data are somewhat at odds with those of Leonard et al. (30), who found little phosphorylation by PKC in a hASIC1b COOH-terminus peptide containing amino acids 459–528, but in this study, the focus was on phosphorylation by PKA, and there was no positive control showing in vitro phosphorylation by PKC.
Although further experiments determining the phosphorylation status of hASIC1b at each site are needed to support these interpretations, this work shows that S40 and S499 sites mediate the effect of PKC activation and PKC inhibition on hASIC1b and that the effects of chelerythrine and PMA on hASIC1b current are not due to a decreased surface expression of the channel. This modulation of hASIC1b by PKC may be relevant for physiological and pathophysiological situations involving ASIC1 function.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-37206.
Acknowledgments
The authors thank Dr. Jian Ping Wu, Dr. Xiaofen Liu, and the University of Alabama at Birmingham Animal Resources Program for technical assistance with the frog surgeries. The human ASIC1b construct was a kind gift of Dr. David Corey. The authors also thank the American Physiological Society workshop on Scientific Writing and the 2008 small group (Dr. Kim Barrett, Dr. Mark Hernandez, Alencia Woodard-Grice, and Dr. Zan Pan). Also, we thank Dr. Mark Bevensee, Dr. Wanda Vila-Carriles, Dr. Bakhram Berdiev, and Melissa McCarthy for helpful discussions and Theresa Henson, Jan Philips, Rebecca Todd, and Faith Foster for assistance with the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Awayda MS Specific and nonspecific effects of protein kinase C on the epithelial Na+ channel. J Gen Physiol 115: 559–570, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babinski K, Catarsi S, Biagini G, Seguela P. Mammalian ASIC2a and ASIC3 subunits co-assemble into heteromeric proton-gated channels sensitive to Gd3+. J Biol Chem 275: 28519–28525, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Baron A, Deval E, Salinas M, Lingueglia E, Voilley N, Lazdunski M. Protein kinase C stimulates the acid-sensing ion channel ASIC2a via the PDZ domain-containing protein PICK1. J Biol Chem 277: 50463–50468, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bassilana F, Champigny G, Waldmann R, de Weille JR, Heurteaux C, Lazdunski M. The acid-sensitive ionic channel subunit ASIC and the mammalian degenerin MDEG form a heteromultimeric H+-gated Na+ channel with novel properties. J Biol Chem 272: 28819–28822, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA 99: 2338–2343, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berdiev BK, Xia J, Jovov B, Markert JM, Mapstone TB, Gillespie GY, Fuller CM, Bubien JK, Benos DJ. Protein kinase C isoform antagonism controls BNaC2 (ASIC1) function. J Biol Chem 277: 45734–45740, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Berdiev BK, Xia J, McLean LA, Markert JM, Gillespie GY, Mapstone TB, Naren AP, Jovov B, Bubien JK, Ji HL, Fuller CM, Kirk KL, Benos DJ. Acid-sensing ion channels in malignant gliomas. J Biol Chem 278: 15023–15034, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Bubien JK, Ji HL, Gillespie GY, Fuller CM, Markert JM, Mapstone TB, Benos DJ. Cation selectivity and inhibition of malignant glioma Na+ channels by Psalmotoxin 1. Am J Physiol Cell Physiol 287: C1282–C1291, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Bubien JK, Keeton DA, Fuller CM, Gillespie GY, Reddy AT, Mapstone TB, Benos DJ. Malignant human gliomas express an amiloride-sensitive Na+ conductance. Am J Physiol Cell Physiol 276: C1405–C1410, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Chalfant ML, Civan JM, Peterson-Yantorno K, DiBona DR, O'Brien TG, Civan MM. Regulation of epithelial Na+ permeability by protein kinase C is tissue specific. J Membr Biol 152: 207–215, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Conn PJ, Sweatt JD. Protein kinase C in the nervous system. In: Protein Kinase C, edited by Kuo JF. New York, NY: Oxford Univ. Press, 1994.
- 12.Deval E, Friend V, Thirant C, Salinas M, Jodar M, Lazdunski M, Lingueglia E. Regulation of sensory neuron-specific acid-sensing ion channel 3 by the adaptor protein Na+/H+ exchanger regulatory factor-1. J Biol Chem 281: 1796–1807, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Donier E, Rugiero F, Okuse K, Wood JN. Annexin II light chain p11 promotes functional expression of acid-sensing ion channel ASIC1a. J Biol Chem 280: 38666–38672, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Duggan A, Garcia-Anoveros J, Corey DP. The PDZ domain protein PICK1 and the sodium channel BNaC1 interact and localize at mechanosensory terminals of dorsal root ganglion neurons and dendrites of central neurons. J Biol Chem 277: 5203–5208, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, Moinier D, Menez A, Lazdunski M. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem 275: 25116–25121, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Forster IC, Traebert M, Jankowski M, Stange G, Biber J, Murer H. Protein kinase C activators induce membrane retrieval of type II Na+-phosphate cotransporters expressed in Xenopus oocytes. J Physiol 517: 327–340, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Anoveros J, Derfler B, Neville-Golden J, Hyman BT, Corey DP. BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc Natl Acad Sci USA 94: 1459–1464, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geiser M, Cebe R, Drewello D, Schmitz R. Integration of PCR fragments at any specific site within cloning vectors without the use of restriction enzymes and DNA ligase. Biotechniques 31: 88–90, 92, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Grunder S, Geissler HS, Bassler EL, Ruppersberg JP. A new member of acid-sensing ion channels from pituitary gland. Neuroreport 11: 1607–1611, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Grunder S, Jaeger NF, Gautschi I, Schild L, Rossier BC. Identification of a highly conserved sequence at the N-terminus of the epithelial Na+ channel alpha subunit involved in gating. Pflugers Arch 438: 709–715, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Hahnenkamp K, Durieux ME, Hahnenkamp A, Schauerte SK, Hoenemann CW, Vegh V, Theilmeier G, Hollmann MW. Local anaesthetics inhibit signalling of human NMDA receptors recombinantly expressed in Xenopus laevis oocytes: role of protein kinase C. Br J Anaesth 96: 77–87, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Hassan HA, Mentone S, Karniski LP, Rajendran VM, Aronson PS. Regulation of anion exchanger Slc26a6 by protein kinase C. Am J Physiol Cell Physiol 292: C1485–C1492, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Hegde M, Roscoe J, Cala P, Gorin F. Amiloride kills malignant glioma cells independent of its inhibition of the sodium-hydrogen exchanger. J Pharmacol Exp Ther 310: 67–74, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Hruska-Hageman AM, Benson CJ, Leonard AS, Price MP, Welsh MJ. PSD-95 and Lin-7b interact with acid-sensing ion channel-3 and have opposite effects on H+-gated current. J Biol Chem 279: 46962–46968, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Hruska-Hageman AM, Wemmie JA, Price MP, Welsh MJ. Interaction of the synaptic protein PICK1 (protein interacting with C kinase 1) with the non-voltage gated sodium channels BNC1 (brain Na+ channel 1) and ASIC (acid-sensing ion channel). Biochem J 361: 443–450, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449: 316–323, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Johnson J, Capco DG. Progesterone acts through protein kinase C to remodel the cytoplasm as the amphibian oocyte becomes the fertilization-competent egg. Mech Dev 67: 215–226, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Krishtal O The ASICs: signaling molecules? Modulators? Trends Neurosci 26: 477–483, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Leonard AS, Yermolaieva O, Hruska-Hageman A, Askwith CC, Price MP, Wemmie JA, Welsh MJ. cAMP-dependent protein kinase phosphorylation of the acid-sensing ion channel-1 regulates its binding to the protein interacting with C-kinase-1. Proc Natl Acad Sci USA 100: 2029–2034, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lingueglia E Acid-sensing ion channels in sensory perception. J Biol Chem 282: 17325–17329, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Meltzer RH, Kapoor N, Qadri YJ, Anderson SJ, Fuller CM, Benos DJ. Heteromeric assembly of acid-sensitive ion channel and epithelial sodium channel subunits. J Biol Chem 282: 25548–25559, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Ross SB, Fuller CM, Bubien JK, Benos DJ. Amiloride-sensitive Na+ channels contribute to regulatory volume increases in human glioma cells. Am J Physiol Cell Physiol 293: C1181–C1185, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Szolgay-Daniel E, Carlsson J, Zierold K, Holtermann G, Dufau E, Acker H. Effects of amiloride treatment on U-118 MG and U-251 MG human glioma and HT-29 human colon carcinoma cells. Cancer Res 51: 1039–1044, 1991. [PubMed] [Google Scholar]
- 35.Trotti D, Peng JB, Dunlop J, Hediger MA. Inhibition of the glutamate transporter EAAC1 expressed in Xenopus oocytes by phorbol esters. Brain Res 914: 196–203, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Turk E, Kerner CJ, Lostao MP, Wright EM. Membrane topology of the human Na+/glucose cotransporter SGLT1. J Biol Chem 271: 1925–1934, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Ugawa S, Ishida Y, Ueda T, Inoue K, Nagao M, Shimada S. Nafamostat mesilate reversibly blocks acid-sensing ion channel currents. Biochem Biophys Res Commun 363: 203–208, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Vila-Carriles WH, Kovacs GG, Jovov B, Zhou ZH, Pahwa AK, Colby G, Esimai O, Gillespie GY, Mapstone TB, Markert JM, Fuller CM, Bubien JK, Benos DJ. Surface expression of ASIC2 inhibits the amiloride-sensitive current and migration of glioma cells. J Biol Chem 281: 19220–19232, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Vila-Carriles WH, Zhou ZH, Bubien JK, Fuller CM, Benos DJ. Participation of the chaperone Hsc70 in the trafficking and functional expression of ASIC2 in glioma cells. J Biol Chem 282: 34381–34391, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Waldmann R, Champigny G, Lingueglia E, De Weille JR, Heurteaux C, Lazdunski M. H+-gated cation channels. Ann NY Acad Sci 868: 67–76, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Waldmann R, Lazdunski M. H+-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol 8: 418–424, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci 29: 578–586, 2006. [DOI] [PubMed] [Google Scholar]
- 43.West JW, Numann R, Murphy BJ, Scheuer T, Catterall WA. Phosphorylation of a conserved protein kinase C site is required for modulation of Na+ currents in transfected Chinese hamster ovary cells. Biophys J 62: 31–33, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao GQ, Qu Y, Sun ZQ, Mochly-Rosen D, Boutjdir M. Evidence for functional role of epsilonPKC isozyme in the regulation of cardiac Na+ channels. Am J Physiol Cell Physiol 281: C1477–C1486, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron 22: 537–548, 1999. [DOI] [PubMed] [Google Scholar]