Abstract
Insulin resistance associated with Type 2 diabetes contributes to impaired vasorelaxation. Previously, we showed the phosphorylation of myosin-bound phosphatase substrate MYPT1, a marker of the vascular smooth muscle cell (VSMC) contraction, was negatively regulated by Akt (protein kinase B) phosphorylation in response to insulin stimulation. In this study we examined the role of Akt phosphorylation on impaired insulin-induced vasodilation in the Goto-Kakizaki (GK) rat model of Type 2 diabetes. GK VSMCs had impaired basal and insulin-induced Akt phosphorylation as well as increases in basal MYPT1 phosphorylation, inducible nitric oxide synthase (iNOS) expression, and nitrite/nitrate production compared with Wistar-Kyoto controls. Both iNOS expression and the inhibition of angiotensin (ANG) II-induced MYPT1 phosphorylation were resistant to the effects of insulin in diabetic GK VSMC. We also measured the isometric tension of intact and denuded GK aorta using a myograph and observed significantly impaired insulin-induced vasodilation. Adenovirus-mediated overexpression of constitutively active Akt in GK VSMC led to significantly improved insulin sensitivity in terms of counteracting ANG II-induced contractile signaling via MYPT1, myosin light chain dephosphorylation, and reduced iNOS expression, S-nitrosylation and survivin expression. We demonstrated for the first time the presence of Akt-independent iNOS expression in the GK diabetic model and that the defective insulin-induced vasodilation observed in the diabetic vasculature can be restored by the overexpression of active Akt, which advocates a novel therapeutic strategy for treating diabetes.
Keywords: diabetes, insulin, inducible nitric oxide synthase, Akt, vasodilation
vascular dysfunction characterized by increased contractility of vascular smooth muscle cells (VSMCs), abnormal vascular tone (60), and defective vasorelaxation (52) are the common abnormalities observed in atherosclerosis, diabetes, and hypertension (77). Insulin resistance often coexists in these diseases and is a well-known factor in the development of Type 2 diabetes (65). One important mechanism responsible for the defective vasorelaxation in diabetes has been impaired insulin-mediated relaxation of vasculature due to insulin resistance (38, 52).
Smooth muscle contraction and relaxation are tightly coupled to the phosphorylation and dephosphorylation, respectively, of the regulatory myosin light chain (MLC20) (32). MLC phosphorylation state is determined by the relative activities of myosin light chain kinase (MLCK) and myosin-bound phosphatase (MBP) (39). MLCK phosphorylates MLC leading to contraction (39), and MBP dephosphorylates MLC, leading to relaxation (3). Contractile agents such as angiotensin II (ANG II) activate the small GTPase, RhoA, and Rho-associated kinase α (ROKα), which then cause the phosphorylation of MYP substrate (MYPT1) at threonine-695 and the inactivation of MBP in a calcium-independent manner (27, 44, 48). The MBP inactivation, via phosphorylation of MYPT1, results in the phosphorylation of MLC20 at serine-19 and threonine-20 leading to calcium-independent cell contraction (32–34). Insulin receptor substrate-1 (IRS-1) tyrosine phosphorylation, in response to insulin, activates phosphatidylinositide 3-kinase (PI3-K)/Akt (protein kinase B) and the expression of inducible nitric oxide (NO) synthase (iNOS) (8, 38, 48, 73). The vasodilatory effects of insulin are mediated by NO (78) produced by iNOS (8, 38, 48, 73), which then activates cGK1α and results in the dephosphorylation of threonine-696 on a MYPT1 and inactivation of RhoA and ROKα (10, 11, 27, 48, 73). Akt activates endothelial NOS (eNOS) by serine-1177/1179 phosphorylation that facilitates association of the enzyme with calmodulin reducing its inhibitory interaction with caveolin-1 (53), causing NO-dependent endothelial vasodilation. Previously, we showed that insulin-induced Akt phosphorylation is essential for the dephosphorylation of ANG II-induced MYPT1 phosphorylation, resulting in VSMC relaxation via iNOS expression (48) (see depicted hypothesized signaling pathway in Fig. 7).
Studies involving the role of insulin in glucose metabolism have suggested that maintaining precise physiological levels of Akt/PKB may be critical to avoid insulin resistance. This is evidenced by data linking impaired Akt expression and activity with Type 2 diabetes (46, 47) and the increased activity observed in the renal cortex of db/db mice (26). In addition, Akt2 null mice exhibit both fasting hyperglycemia and glucose intolerance (30). Akt2/PKBβ−/− adipocytes have a reduction in insulin-induced hexose uptake and lower glucose transporter 4 (GLUT4) translocation (7). Collectively, these studies demonstrated that the absence of Akt2/PKBβ could mimic the insulin-resistant state. Given our data as well as others demonstrating that insulin causes vasodilation on VSMC via the PI3K/Akt pathway (8, 38, 48, 73), it is interesting to examine whether any abnormalities in Akt signaling might cause insulin resistance in insulin-induced vasodilation. Indeed, no studies have been conducted examining the role of Akt on insulin-induced vasorelaxation in the diabetic aorta.
We hypothesized that abnormalities in Akt activation may cause the insulin-induced vasodilation defects observed in diabetes. In this study, we used nonobese insulin-resistant Goto-Kakizaki (GK) rats, a highly inbred strain of Wistar-Kyoto (WKY) rats that spontaneously develop Type 2 diabetes (31), to dissect the pathogenesis of insulin resistance. We explored the correlation between insulin resistance, defective Akt activation, insulin-resistant iNOS expression, and impaired insulin-induced vasodilation. Using a myograph to measure isometric tension, we demonstrated impaired insulin-induced vasodilation and furthermore attempted to restore the insulin sensitivity in insulin-induced vasodilation in diabetic GK VSMC by overexpressing constitutively active Akt. This is the first study that demonstrates the role Akt phosphorylation has in insulin-resistant vasodilation using a rat model of Type 2 diabetes.
MATERIALS AND METHODS
Human insulin (recombinant DNA origin) was from Novo Nordisk Pharmaceuticals (Princeton, NJ). Synthetic human ANG II, sodium orthovanadate, bovine serum albumin, and antibodies against β-actin and Flag M2 were purchased from Sigma-Aldrich (St. Louis, MO). Anti-iNOS antibody was from Transduction Laboratories (Lexington, KY). Primocin (anti-mycoplasmic), a transfection reagent specific for smooth muscle, was purchased from Amaxa biosystems (Cologne, Germany). siCONTROL nontargeting small interfering RNA (siRNA) and custom siRNA were purchased from Dharmacon (Lafayette, CO). Enhanced chemiluminescence (ECL), anti-rabbit IgG, anti-mouse IgG (horseradish peroxide linked) were from Amersham Biosciences (Buckinghamshire, UK). Specific antibody targeting MYPT1 and phosphorylated MYPT1 on threonine-696 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody specific for survivin is purchased from Novus Biologicals (Littleton, CO). Antibody specific for Akt, phospho-Akt (Ser-473), phospho-myosin light chain 20 (Thr-18/Ser-19) were purchased from Cell Signaling Technology (Beverly, MA). Western blot reagents were from Bio-Rad Laboratories (Hercules, CA). S-Nitrosylated protein detection kit was purchased from Cayman Chemicals (Ann Arbor, MI).
Culture of VSMCs and treatment with insulin.
VSMCs in primary culture were obtained by enzymatic digestion of the aortic media of male WKY and GK rats with body weights of 200–220 g, as described in our recent publications (9, 49). Procedures involving animals and their care were conducted in conformity with institutional guidelines in compliance with international laws and policies (National Institutes of Health Guide for the Care and Use of Laboratory Animals, 1996, 7th ed.). Unless otherwise indicated, primary cultures of VSMCs were maintained in α-minimal essential medium (MEM) containing 10% FBS and 1% antibiotic-antimycotic and antimycoplasmic mixture. VSMCs isolated from diabetic GK rats were maintained in medium containing 20 mmol/l glucose to mimic a hyperglycemic condition. Subcultures of VSMCs at passage 5 were used in all experiments. All experiments on Akt, MYPT1, and MLC20 phosphorylation were performed on highly confluent cells at identical passages. Before each experiment, cells were serum starved for 24 h in serum-free α-MEM containing 1% antibiotics and 5.5 mmol/l glucose for WKY VSMC and 20 mmol/l glucose for GK VSMC. The next day cells were exposed to insulin (0–100 nM) for 10 min, ANG II (100 nM) for 15 min, or ANG II (5 min) followed by insulin.
Animal and tissue preparation.
A colony of type II GK rats was established at this institute, originally supplied by Dr. Robert V. Farese (BVA Hospital, Tampa, FL) as detailed earlier (9, 49). WKY rats were purchased from Taconic Farms (Germantown, NY). Both WKY and GK rats were euthanized at 7–8 wk of age with 95% CO2 inhalation, which was approved by Institutional Laboratory Animal Care and Use Committee of Winthrop University Hospital. The thoracic aorta of rats were rapidly and carefully excised and placed in ice-cold physiological saline solution (PSS). The fat and connective tissues were removed, and the aorta was cut into 3-mm long rings using multisectioning tool. Four rings with intact or denuded endothelium were mounted simultaneously in a Multi-Chamber myograph (model DMT 610M, AD Instruments) filled with PSS (7 ml) of the following composition (in mmol/l): 119.0 NaCl, 24 NaHCO3, 4.7 KCl, 1.6 CaCl2·2H2O, 1.17 MgSO4·7H2O, 5.5 glucose, 0.026 EDTA, and 1.18 KH2PO4. In the case of GK tissue, PSS contained 20 mmol/l of glucose to mimic hyperglycemic condition. The solution in the baths was constantly aerated with 95% O2-5% CO2 and kept at 37°C (pH 7.4).
Isometric force measurement.
Contractile force, measured with isometric transducers built into the Multi-Chamber Myograph (DMT610M, AD Instruments), was stored with a data acquisition system (PowerLab 8SP, AD Instruments) and analyzed by computer with Chart 5 (AD Instruments). As described in our previous study (49), after 45 min of equilibration with a resting tension of 2 g, aorta rings either intact or denuded, were primed by exposure to an activation solution that substitutes equal molar concentration of NaCl with KCl, until the contractile response reaches a plateau. The ring segments were then incubated with 1 μmol/l phenylephrine (PE) to get the maximal contractile response. The PE responses from each bath were used as 100% to compare each agonist-induced contractile response.
Overexpression of Akt with adenovirus (Ad-myr-AKT-Flag) treatment in VSMC-adenovirus constructed with myr-AKT-Flag were made at the Gene Transfer Vector Core (University of Iowa) as described earlier (6). Constitutively active forms of Akt have been obtained by fusion of NH2-terminal c-Src myristilation residues to Akt. VSMCs were grown to 80% confluency, and cells were washed with serum-free media and then treated with Ad-β-gal or Ad-myr-AKT-Flag for 4 h with agitation once per every hour. After 4 h, serum was added to the cells and incubated overnight. The next day cells were washed with fresh media and kept for another 24 h. Cells were serum starved for 24 h before the experiments with insulin or ANG II.
Transfection of VSMC with siAKTc.
The sequence of siRNA targeting Akt (siAKTc) targets the homologue site of rat Akt1 (1040–1058) and rat Akt2 (1043–1061) and has been shown to abolish Akt 1 and Akt 2 expression (42, 48). This site is common in rats and humans. siCONTROL, which is nontargeting siRNA no. 1 from Dharmacon, was used to show the nonspecific effect of siRNA transfection. VSMCs were transfected with Amaxa Nucleofector by electroporation with siCON or siAKTc following the manufacturer's instructions. Forty eight hours after the transfection, cells were serum starved for 24 h and experiments were done as described above.
Western blot analysis.
Cells were lysed in a buffer containing 20 mM Tris·HCl (pH 8.0), 1 mM DTT, 100 mM NaCl, 0.5% SDS, 0.75% deoxycholate, 100 mM NaCl, 100 mM NaF, 50 mM sodium pyrophosphate, 2 mM sodium orthovanadate, 2 μM microcystin, 50 mM β-glycerophosphate, 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (AEBSF), 10 μg/ml leupeptin, and 10 μg/ml aprotinin with phosphatase inhibitors. Lysates were spun down for 30 min at 14,000 g. Equal amounts of proteins were heated with sample buffer containing 2% SDS, 0.2 M Tris·HCl (pH 7.5), 20 mM EDTA, 10% glycerol, and bromophenol blue for 5 min at 95°C and then loaded on SDS-PAGE. The separated proteins were transferred to nitrocellulose membrane and probed with specific antibodies followed by incubation with horseradish peroxidase-conjugated secondary antibodies and detected by ECL. The extent of each protein was quantitated by dividing the intensity of β-actin or total specific protein. In some cases, the intensity of each protein phosphorylation was normalized to the total protein amount of target protein.
Quantitative real-time PCR.
To amplify iNOS and GAPDH cDNA, sense and antisense oligonucleotide primers were designed based on the published cDNA sequences (66). Oligonucleotides were obtained from Sigma-Genosys (St. Louis, MO). Sequences for the real-time PCR are as follows: iNOS sense 5′-AGACGCACAGGCAGAGGT-3′ and iNOS antisense 5′-AGGCACACGCAATGATGG-3′; GAPDH sense GGAGAAACCTGCCAAGTATGA-3′, and GAPDH antisense CCCTGTTGCTGTAGCCATATT-3′. Total RNA was isolated using an RNA isolation kit (QIAGEN, Valencia, CA) from WKY and GK VSMC. cDNA was synthesized with the first-strand cDNA synthesis Kit for RT-PCR (Roche Applied Science, Indianapolis, IN) for the first-strand synthesis of single-stranded cDNA from RNA for use as a PCR template using 2–3 μg total RNA in a 20-μl reaction volume. For real-time PCR, the cDNA was amplified using LightCycle 480 SYBR Green I Master for PCR and the LightCycle 480 System (Roche Applied Science, Indianapolis, IN). The double-strand DNA-specific dye SYBR Green I incorporated in the PCR reaction buffer LightCycle 480 to allow for quantitative detection of the PCR product in a 25-μl reaction volume. The temperature profile of the reaction was 95°C for 10 min, 40 cycles of denaturation at 95°C for 30 s, annealing at 62°C for 45 s, and extension at 72°C for 60 s. An internal housekeeping gene control GAPDH was used to normalize differences in RNA isolation, RNA degradation, and the efficiencies of the RT. The size of the PCR product was verified on a 1.5% agarose gel, followed by melting-curve analysis thereafter.
Nitrite measurement.
Similar number of confluent WKY and GK VSMC cells were serum starved and changed to a phenol red free α-MEM and left for 24 h before the media were collected to determine the production of NOx. Total nitrite and nitrate production was measured with a Greiss Reagents kit purchased from Calbiochem (San Diego, CA) after the conversion of nitrate to nitrite with nitrate reductase as instructed by company.
Biotin-switch assay for detection of S-nitrosylated Akt after immunoprecipitation with anti-Akt.
VSMCs were infected with either Ad-β-gal or Ad-myr-Akt to overexpress constitutively active Akt. Forty eight hours after the transfection, cells were serum starved for 24 h. Other sets of quiescent VSMC cells were serum starved and treated with insulin. The Biotin switch assay was performed using an S-nitrosylated detection kit from Cayman Chemicals on the lysates that were immunoprecipitated with anti-Akt, following the manufacturer's instructions.
Statistics.
The results are presented as means ± SE of four to six independent experiments. Paired Student's t-test was used to compare the basal versus insulin-treated preparations. Unpaired t-test or ANOVA was used to compare the mean values between treatments. A P value of <0.05 was considered statistically significant.
RESULTS
Impaired Akt basal phosphorylation and augmented MYPT1 basal phosphorylation in GK VSMC.
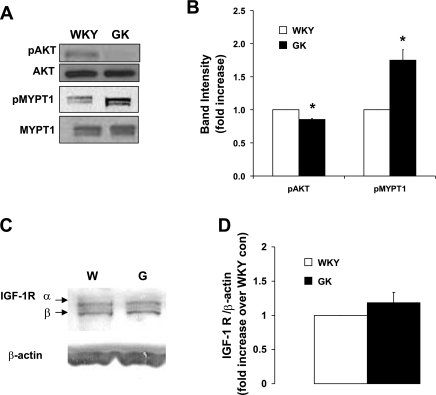
Akt phosphorylation at serine-473 is known to be important for full Akt activity (25), and recently our laboratory showed that the phosphorylation and activation of Akt by insulin plays an important role in reducing ANG II-induced contractile responses such as MYPT1 phosphorylation and MLC20 (48), resulting in the increase of insulin-induced vasodilatory MBP activity in VSMC. To better understand the defects in vascular tone regulation in diabetes, we determined the basal phosphorylation status of Akt and MYPT1. In GK VSMC, basal Akt phosphorylation was reduced to 85% of WKY (Fig. 1, A and B), whereas the total amount of Akt was similar in WKY and GK VSMC (Fig. 1A). The basal phosphorylation of MYPT1 was increased to 178% in GK VSMC (Fig. 1, A and B), demonstrating that defective Akt phosphorylation may have increased MYPT1 phosphorylation in GK VSMC.
Fig. 1.
Impaired basal Akt phosphorylation and increased myosin-bound phosphatase substrate (MYPT1) phosphorylation in Goto-Kakizaki (GK) vascular smooth muscle cells (VSMC). A: equal amounts of protein from Wistar-Kyoto (WKY) and GK cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose membrane, and the total and phosphorylated levels of Akt and MYPT1 measured by Western blot analysis. B: intensities of phosphorylated protein were quantitated by densitometric analysis and normalized to the abundance of total protein for Akt and MYPT1 (n = 6). *P < 0.05 WKY vs. GK. C: insulin-like growth factor 1 receptor (IGF-R) measured by Western blot analysis. D: quantitated densitometric analysis of IGF-1R normalized to β-actin (n = 3).
We also looked at the basal insulin receptor (IR) and insulin-like growth factor receptor (IGF-R) in GK VSMC to determine the basal insulin-signaling component. IR expression in WKY and GK were determined as similar in previously published work by Sandu et al. (74). Unlike IGF-1 recptor, IGF-IIR dose not potentiate the signaling of IGF-1 or IGF-II and is characterized as a tumor suppressor (45). Therefore, we determined whether there is any difference in the IGF-1 receptor (α and β) as shown in Fig. 1, C and D. We found that IGF-1 receptor expression was similar in WKY and GK VSMC; and thus the defective insulin-induced vasodilation is not due to the difference in IR/IGF-R expression but rather due to the changes in the insulin-signaling pathways.
Impaired insulin-induced Akt phosphorylation and Akt- independent iNOS expression in GK VSMC.
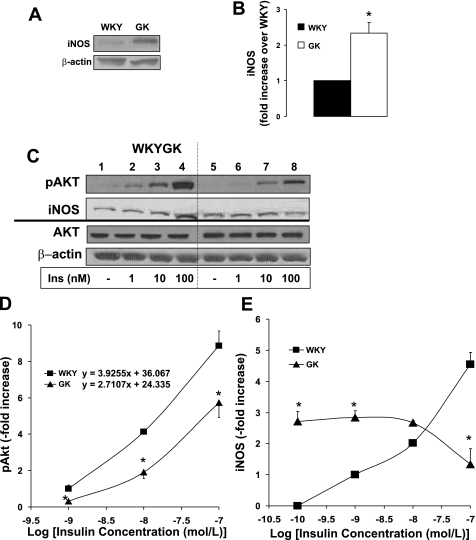
To investigate the role of Akt on insulin-dependent iNOS expression, the insulin-induced Akt phosphorylation and iNOS expression were compared in WKY control and GK diabetic VSMC. The basal iNOS expression was 2.5-fold higher than that in the WKY control (Fig. 2, A and B). Akt is phosphorylated by insulin (1, 10, and 100 nM insulin) in a dose-dependent manner in WKY and GK VSMC (Fig. 2, C and D). Insulin-induced Akt phosphorylation (10 and 100 nM) was impaired in GK VSMC to 46% and 65% compared with WKY, respectively (Fig. 2C; lanes 7 and 8; Fig. 2D). Insulin dose-response curve for pAkt showed that the WKY showed a higher slope than that of GK (Fig. 2D). Insulin (10 and 100 nM) induced iNOS to 2- and 5-fold higher over the basal in WKY VSMC (Fig. 2C; lanes 3 and 4; Fig. 2E), respectively. Whereas insulin induced iNOS expression in a dose-dependent manner in control WKY VSMC, the iNOS expression was insulin resistant and not correlated with insulin dose in GK VSMC (Fig. 2E). Insulin at 100 nM significantly decreased the iNOS expression in GK (Fig. 2C; lane 8; Fig. 2E), opposing the insulin effect in WKY control.
Fig. 2.
Impaired insulin (Ins)-induced Akt phosphorylation accompanied with abnormal inducible nitric oxide synthase (iNOS) expression in GK VSMC. Equal amounts of protein from WKY and GK cell lysate sample were resolved by SDS-PAGE and transferred to nitrocellulose membrane. A: Western blot analysis for protein expression. B: intensities of iNOS protein were quantitated by densitometric analysis and normalized to the abundance of total β-actin. Quiescent VSMCs at day 9 were stimulated with insulin (1, 10, and 100 nM) for 10 min (n = 3). C: equal amounts of protein from each cell lysate sample were resolved by SDS-PAGE, transferred to nitrocellulose membrane, and the total and phosphorylated levels of Akt and iNOS expression measured by Western blot analysis. Intensities of phosphorylated protein were quantitated by densitometric analysis and normalized to the abundance of total protein for Akt and to β-actin for iNOS. D: insulin dose-response curve versus Akt phosphorylation were plotted as fold increase. E: insulin dose-response curve versus iNOS expression were plotted. *P < 0.05 WKY vs. GK.
Impaired insulin-induced Akt phosphorylation caused an insulin-resistant reduction of ANG II-induced contraction in GK aorta and phosphorylation of MYPT1 in GK VSMC.
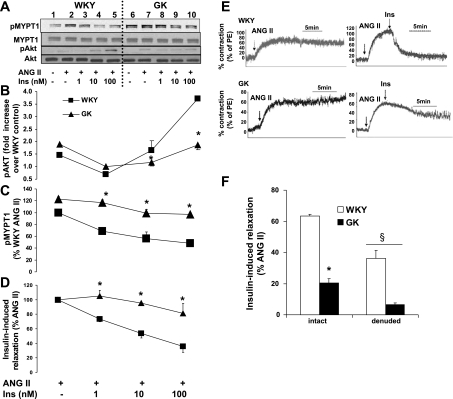
Our laboratory recently showed that insulin inhibits ANG II-induced contractile response via phosphorylation of Akt and the dephosphorylation of MYPT1 and MLC20 in VSMC (17). Thus the impaired Akt phosphorylation (Figs. 1 and 2) may have caused impaired inhibition of contractile signaling in GK diabetic VSMC. To determine the effect of defective Akt activation on insulin-induced vasodilatation and on the contractile response, such as MYPT1 in GK VSMC, the phosphorylations of Akt at serine-473 and MYPT1 were analyzed. Pretreatment of ANG II (100 nM) reduced insulin-induced Akt phosphorylation in both GK and WKY. ANG II (100 nM) completely abolished insulin (100 nM)-induced Akt phosphorylation in GK VSMC, whereas in WKY VSMC, ANG II (100 nM) only reduced insulin-induced Akt phosphorylation to 50% (Fig. 3, A and B; lane 5 vs. 10). GK VSMC showed insulin resistance when reducing the ANG II-induced MYPT1 phosphorylation. Insulin (1, 10, and 100 nM) reduced the ANG II-induced MYPT1 phosphorylation by 67%, 56%, and 48% (Fig. 3C: Fig. 3A; lanes 3–5), respectively. In contrast, the GK MYPT1 phosphorylation responses were reduced only to 95%, 80%, and 79% (Fig. 3C: Fig. 3A; lanes 8–10), respectively. Insulin-induced dephosphorylation of MYPT1 was impaired in GK VSMC, implying insulin-resistant vasorelaxation through MBP activation in diabetic GK.
Fig. 3.
Impaired insulin-induced vasorelaxation in GK aorta quiescent VSMCs at day 9 were stimulated with insulin (1, 10, and 100 nM) for 10 min following pretreatment with ANG II (100 nM; n = 5). A: equal amounts of protein from each cell lysate sample were resolved by SDS-PAGE and transferred to nitrocellulose membrane, and the total and phosphorylated levels of Akt and MYPT1 were measured by Western blot analysis. Intensities of phosphorylated protein were quantitated by densitometric analysis and normalized to the abundance of total protein for Akt and MYPT1. B: impaired insulin-induced phosphorylation of Akt. C: vasodilation effect of insulin on dephosphorylation of MYPT1. Aorta dissected from both WKY control and GK diabetic rat were mounted on the myograph for the isometric force measurement. Aorta were contracted with ANG II following treatment with insulin (1, 10, and 100 nM) to cause insulin-induced vasorelaxation in precontracted aorta by ANG II. D: impaired insulin-induced relaxation in GK rat aorta. E: representative myograph data showing impaired insulin-induced relaxation in GK diabetic rat aorta. F: effect of endothelium on insulin (10 nM)-induced vasodilation. *P < 0.05 WKY vs. GK; §P < 0.05 intact vs. denuded aorta.
We then measured the isometric tension of impaired contraction with denuded aorta using the myograph. ANG II caused the contraction in WKY and GK denuded aorta, and then insulin (1, 10, and 100 nM) relaxed the ANG II-induced contraction by 26.7%, 46.2% and 64% in WKY aorta (Fig. 3, D and E), respectively. In contrast, the insulin-induced vasodilation was only by −5%, 5%, and 19% (Fig. 3, D and E) in GK, respectively. These data show the insulin-resistant vasodilation in GK-denuded aorta.
To understand the endothelial effect on insulin-induced vasodilation, we compared the degree of insulin-induced relaxation in GK and WKY using both intact and denuded aorta. In WKY, insulin (10 nM) relaxed the ANG II-induced contraction to 63.5% in intact aorta, whereas it was only to 36% in denuded aorta (Fig. 3F). Similarly, in GK aorta, intact aorta responded better to insulin to cause relaxation on ANG II-induced contraction to 19.9% and 6.5%, respectively (Fig. 3F). Thus insulin also causes relaxation in an endothelium-dependent manner in both GK and WKY aorta.
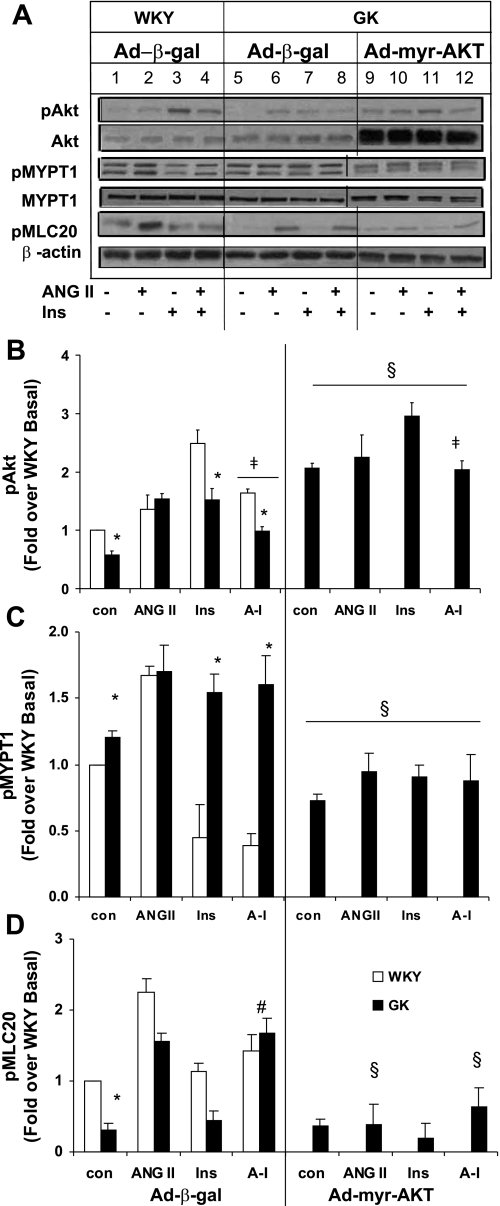
Constitutively active myristilated Ad-myr-AKT caused Akt overexpression and restored the impaired GK Akt phosphorylation. Since the impaired insulin-induced Akt phosphorylation seemed to cause the defective inhibition of ANG II-induced contraction, Ad-myr-AKT was used to overexpress constitutively active Akt in GK VSMC and determine whether the increased Akt phosphorylation restored the insulin-induced vasodilation in GK diabetic VSMC. We used a mutant Akt with a myristilated signal at the COOH-terminus as described in our earlier publication (48). Ad-myr-AKT induced the overexpression of active Akt in GK VSMC to 5.3-fold over Ad-β-gal (Fig. 4A; lanes 9–12). Basal Akt phosphorylation was increased threefold by Ad-myr-AKT in GK VSMC over Ad-β-gal control (Fig. 4A; lane 5 vs. 9; Fig. 4B). Insulin-induced Akt phosphorylation was also increased twofold compared with that in Ad-β-gal-treated GK VSMC, respectively (Fig. 4A; lane 7 vs. 11). Ad-myr-Akt restored the basal and insulin-induced Akt phosphorylation to WKY Ad-β-gal levels (Fig. 4A; lanes 1–4 vs. 9–12).
Fig. 4.
Overexpression of constitutively active Akt restores insulin-induced MYPT1 and MLC20 dephosphorylation in GK VSMC. Quiescent VSMCs infected with Ad-β-gal or Ad-myr-AKT were serum starved and pulsed with insulin (10 nM) for 10 min, ANG II (100 nM) for 15 min, or ANG II (5 min) followed by insulin (10 nM). Equal amounts of protein from each cell lysate sample were resolved by SDS-PAGE and transferred to nitrocellulose membrane. Levels of pAkt and Akt (A and B); pMYPT1 and MYPT1 (A and C), and pMLC20 compared with β-actin as an internal control (A and D) were measured by Western blot analysis. Intensities of phosphorylated protein were quantitated by densitometric analysis and normalized to the abundance of total protein for Akt and MYPT1. Densitometric analyses of four separate experiments are given below each graph. *P < 0.05 WKY vs. GK; §P < 0.05 vs. corresponding value of Ad-β-gal; ‡P < 0.05 vs. insulin; #P < 0.05 vs. ANG II.
To confirm the effect of increased Akt phosphorylation on reducing contractile responses such as MYPT1 and MLC20 phosphorylation in GK diabetic VSMC, the effects of Ad-myr-AKT on phosphorylation of MYPT1 and MLC20 were examined. The phosphorylation of MLC20 was examined as the marker of the contractile status of VSMC. Basal phosphorylation of MYPT1 was significantly reduced to 60% of the GK control (Fig. 4A; lane 5 vs. 9; Fig. 4C). Impaired insulin dephosphorylation on ANG II-induced MYPT1 phosphorylation was significantly improved to 73% of that of Ad-β-gal (Fig. 4A; lane 8 vs. 12; Fig. 4C), implying that the increased Akt phosphorylation and activity restored the insulin-induced dephosphorylation of MYPT1. Whereas basal MLC20 phosphorylation was not changed (Fig. 4A; lane 5 vs. 9; Fig. 4D), ANG II-induced MLC20 phosphorylation was completely abolished by overexpression of constitutively active Akt in GK diabetic VSMC (Fig. 4A; lane 7 vs. 11; Fig. 4D). The impaired insulin-induced dephosphorylation of ANG II-induced MLC20 phosphorylation was abolished by overexpression of constitutively active Akt (Fig. 4A; lane 8 vs. 12; Fig. 4D). These results demonstrate that the defective vasodilation can be restored by addition of active Akt in GK diabetic vasculature.
Upregulation of iNOS and opposite role of Akt on iNOS expression in GK diabetic VSMC.
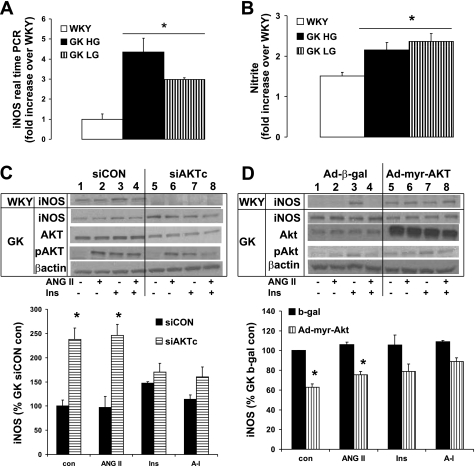
Since we found that the insulin-induced phosphorylation of Akt is essential to iNOS expression in WKY VSMC (48), we determined any abnormalities in iNOS expression in GK diabetic VSMC. First, we determined the basal expression of iNOS and then determined whether high glucose stimulated its induction. iNOS gene expression was significantly higher in GK at both high glucose (4.4-fold) and low glucose (3-fold; Fig. 5A). The production of nitrite was also twofold higher in GK VSMC (Fig. 5B). There were no significant differences between low glucose and high glucose stimulation in iNOS gene expression as well as nitrite production in GK VSMC, implying that the chronic glucose exposure may be needed to determine the effect of high glucose. There was no significant effect of high glucose on WKY VSMC compared with that of low glucose (data not shown). To determine the role of Akt on iNOS expression in GK diabetic VSMC, we used siAKTc (Fig. 5C) and constitutively active Ad-myr-Akt (Fig. 5D). siAKTc significantly increased the iNOS expression to 2.4-fold in GK diabetic VSMC (Fig. 6C), whereas in WKY, siAKTc completely abolished insulin-induced iNOS (Fig. 5C) (48). Interestingly also, the Ad-myr-Akt decreased the iNOS expression to 60% of the Ad-β-gal control (Fig. 5D). The insulin-induced iNOS expression was not significantly different by using siAKTc or Ad-myr-Akt compared with their each control, siControl, or Ad-β-gal. Thus the abnormal upregulation of iNOS and the opposite effect of Akt on iNOS expression may be linked to the abnormalities in insulin-induced vasodilation in GK VSMC.
Fig. 5.
Opposite role of Akt on iNOS expression in GK diabetic VSMC. Equal amounts of total RNA from WKY and GK VSMC post to stimulation with high glucose or low glucose for 24 h were lysed and subject to quantitative real-time PCR. A: basal iNOS gene expression were measured by real time PCR for gene expression. B: nitrite/nitrate production from the culture media of WKY and GK VSMC were measured by the Greiss methods as described in materials and methods. WKY and GK VSMC cells transfected with siAKTc (C) or Ad-myr-Akt (D) for 48 h were serum starved and pulsed with insulin (10 nM) for 10 min, ANG II (100 nM) for 15 min, or ANG II (5 min) followed by insulin (10 nM). Equal amounts of protein from each cell lysate were resolved by SDS-PAGE and transferred to nitrocellulose membrane, and the levels of iNOS, pAkt, and Akt compared with β-actin as an internal control. pAkt level was determined after total Akt amount adjusted. Densitometric analyses of four separate experiments with GK VSMC are given below each graph. *P < 0.05 siCON vs. siAKTc (C); *P < 0.05 β-gal vs. Ad-myr-Akt (D).
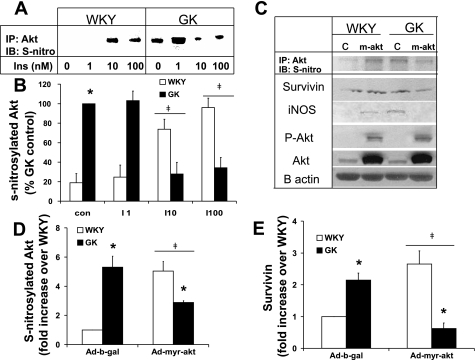
Fig. 6.
Ad-myr-Akt reduced the upregulation of anti-apopototic factors, iNOS, and survivin and decreased the S-nitrosylation in GK VSMC. Quiescent VSMC infected with Ad-β-gal or Ad-myr-AKT were serum starved for 24 h and where indicated treated with insulin, and cell lysates were prepared according to the S-nitrosylation kits′ instructions. Equal amounts of protein (500 μg) were immunoprecipitated with anti-Akt and immunoblotted with anti-S-nitrosylated protein. The levels of S-nitrosylated Akt induced by insulin (A) or overexpression of constitutively active Akt (C) are provided with the quantified results (B and D). Some lysates were resolved by SDS-PAGE and transferred to nitrocellulose membrane, and the levels of pAkt, Akt, iNOS, and survivin were measured by Western blot analysis (E). β-Actin was used as an internal control. Intensities of phosphorylated protein were quantified by densitometric analysis and normalized to the abundance of total protein for Akt or β-actin. Densitometric analyses of three separate experiments were performed for each graph. *P < 0.05 WKY vs. GK; ‡P < 0.05 vs. each Ad-β-gal.
Ad-myr-Akt reduced the upregulation of anti-apoptotic factors, iNOS, and survivin and decreased the S-nitrosylation in GK VSMC.
We speculated that the Akt-independent iNOS upregulation in the current study may have caused insulin resistance via S-nitrosylation of Akt induced by the excessive production of NO by overexpressed iNOS. Thus we measured the S-nitrosylation of Akt in WKY and GK VSMC. The basal S-nitrosylation in GK VSMC is 5.3-fold higher than that of WKY VSMC (Fig. 6A, lane 1 vs. 5; Fig. 6, B, C, and D), correlating with the basal iNOS and nitrite production. Whereas insulin increased the S-nitrosylation of Akt in a dose-dependent manner in WKY VSMC, S-nitrosylation was not insulin-dependent in GK diabetic VSMC (Fig. 6, A and B). Interestingly, constitutively active Akt increased the S-nitrosylation in WKY VSMC (Fig. 6C, lane 2; Fig. 6D), whereas overexpression of constitutively active Akt decreased the basal upregulated S-nitrosylation in GK VSMC, correlating with the iNOS expression (Fig. 6C, lane 4; Fig. 6D). As a result, the lowered basal and insulin-stimulated Akt phosphorylation is possibly due to the inactivation of Akt by S-nitrosylation and may contribute to the impaired insulin-induced vasorelaxation in GK VSMC.
Survivin (SVV), a member of the “inhibitor of apoptosis” family, functions as a key regulator of mitosis and programmed cell death (56). Recently, in vitro and in vivo studies have demonstrated an important role for SVV in the regulation of endothelial cell (EC) and SMC survival and suggest that it may be involved in the vascular injury response (12), in addition to its role in cancer biology (24). In GK diabetic VSMC, we previously demonstrated that basal apoptosis is nearly twofold higher than that of WKY (64), which correlates well with the current finding of decreased Akt activity. Thus, given the increased inflammation and apoptosis observed in GK VSMC, iNOS may be overexpressed as an anti-apoptotic factor (19). In addition, transforming growth factor (TGF)-β induces apoptosis through repressing the PI-3 kinase/Akt (85), and PI-3 kinase/Akt in turn regulates inhibition of apoptosis via the expression of SVV (59). It appears that the expression of iNOS and SVV are linked to each other (35, 68) and also share PI-3 kinase/Akt as an upstream regulator of anti-apoptosis in the vasculature (59, 85). Therefore, we decided to determine whether the level of SVV correlated with iNOS expression and apoptosis in GK VSMC. As shown in Fig. 6, C and E, we found that the basal SVV protein expression in GK VSMC is upregulated twofold over WKY and correlates well with the exaggerated proliferation found in diabetic GK VSMC (64). In addition, we found that SVV and iNOS expression were increased by upstream Akt overexpression via Ad-myr-Akt in WKY control VSMC (Fig. 6C). However, we hypothesized that the overexpression of Akt would decrease the basal iNOS and SVV due to a decrease in apoptosis and exaggerated proliferation in GK VSMC, since Akt plays as survival signal in the cell (4). We found that Ad-myr-Akt caused a decrease in both iNOS and SVV protein expression, possibly due to the inhibition of apoptosis by constitutively active Akt. Therefore, Akt-independent iNOS and SVV upregulation in GK may function as the anti-apoptotic factors when GK VSMC suffer from increased basal apoptosis (64). The restoration of Akt phosphorylation and activation by Ad-myr-Akt stabilizes GK cells by increasing the survival signal, as indicated by a decrease in iNOS and SVV (Fig. 6C).
DISCUSSION
In this study we examined defects in insulin-induced vasodilatory signaling in the Type 2 diabetic GK vasculature and found that while Akt expression was unaltered, basal and insulin-induced Akt phosphorylations were impaired (Fig. 1A and Fig. 3, C and D). Correspondingly, Akt expression and insulin stimulated activity were found to be impaired in skeletal muscle of GK rats (46), muscle biopsies from Type 2 diabetic patients (46, 47), and in insulin-resistant human adipocytes (69). Furthermore, Akt2 null mice exhibited fasting hyperglycemia and glucose intolerance (30), providing evidence for the relationship between defects in Akt and insulin resistance. Thus defects in Akt may also contribute to impaired insulin-induced vasorelaxation.
We observed increased basal MYPT1 phosphorylation (Fig. 1, A and B), which is known to be negatively regulated by Akt activation. Ad-myr-AKT restored impaired Akt phosphorylation in GK VSMC back to the level of WKY VSMC (Fig. 4, A and B), suggesting that insulin-resistant Akt phosphorylation can be restored by decreasing phosphorylation of MYPT1 and MLC20. There is, however, a slight discrepancy associated with this observation. Akt expression and activity increased in the renal cortex of db/db mice (26), a genetic model of Type 2 diabetes. Moreover, studies performed on the skeletal muscle of Type 2 diabetic patients and subjects with impaired glucose tolerance suggest insulin-induced defects are found in IRS and PI3K, while Akt activation is normal (43). Similarly, dissociation of stimulated PI3K and Akt activities were reported in adipocytes of male insulin-resistant BtB6 mice (57). Thus defects in Akt expression, phosphorylation, and activity do depend on the type of tissue and animal model being studied.
We demonstrate for the first time the presence of PI3K/Akt-independent iNOS expression in the nonobese diabetic model. In WKY VSMC, Akt is upstream of the iNOS expression, cGK1α, and MBP activity (48). iNOS is known to be expressed even after a 10-min stimulation as we and others described previously (36, 48, 74). Other studies also showed that iNOS is expressed and activated by insulin and IGF-1 after 10 min, and that pretreatment with ANG II reduced IGF-1 induced iNOS expression and activity (36). Excessive islet NO generation was reported recently in Type 2 diabetic GK rats (71), which is a similar finding to ours. We also reported that isolated aorta expressed increased iNOS in GK (49). The impaired Akt phosphorylation in vivo using GK aorta was recently reported (22). This is the first study to demonstrate iNOS might be implicated in the development of nonimmunogenic Type 2 diabetes in vascular dysfunction.
Although Akt phosphorylation was impaired in GK VSMC, the downstream event, iNOS expression, was upregulated (Fig. 2, A–C and E). Basal gene expression is upregulated in GK regardless of glucose concentration (Fig. 5A). Since the aim of the study is more to determine the abnormalities in GK diabetic VSMC than the mechanism of how high glucose caused the abnormalities, we did not investigate further the mechanism beneath the effect of high glucose in WKY and GK VSMC. In addition, nitrite/nitrate production is also increased due to the upregulation of iNOS expression (Fig. 5B). Similar to our finding, NO overproduction by iNOS was shown to be independent of PI3K/Akt activation (62, 88), and iNOS induction in obese wild-type mice skeletal muscle was associated with the impaired activation of PI-3K and Akt by insulin (62). We also detected that the increased Akt activation, by overexpression of active Akt, reduced the iNOS expression in GK diabetic VSMC (Fig. 5D). It is difficult to explain the opposite role of Akt on iNOS expression in GK diabetic VSMC. We speculated that iNOS overexpression may protect against exaggerated contraction and reflect the increased inflammation in diabetic vasculature in our recent publication (49). However, despite the host-defensive role of iNOS, excess production of NO appears to be linked to tissue damage and organ dysfunction (75). Thus, in a critical situation like the increased inflammation in diabetic vasculature, it may be possible that Akt functions as a survival signal to protect cells from apoptosis (5). Therefore, overexpression of Akt resulting in decreased expression of iNOS may be due to the reduction of the apoptosis or damage (Fig. 5D), which needs further investigation.
Strengthening our findings, diabetes and atherosclerosis accompany an increased inflammatory response due to vascular injury as evidenced by the overexpression of iNOS, reactive oxygen species (ROS), and inflammatory mediators in the diabetic population (82). Chronic low-grade inflammation has been proposed to be involved in the pathogenesis of obesity-related insulin resistance and Type 2 diabetes. The expression of pro-inflammatory cytokines, including TNF (1), IL-6 (2), and IL-1β (54), are upregulated in animal models of and patients with Type 2 diabetes. Knowing that increased inflammatory action may play an important role in the pathophysiology of cardiovascular (CV) abnormalities in hypertension, atherosclerosis, or diabetes, iNOS may be an important mediator of CV disease. Expression of iNOS is upregulated by most, if not all, inducers of insulin resistance, including pro-inflammatory cytokines (75), obesity (23, 76), free fatty acids (76), hyperglycemia (16), endotoxins (55, 79), and oxidative stress. In fact, elevated expression of iNOS was observed in skeletal muscle of mice fed a high-fat diet (62), in the hearts of Zucker diabetic rats (90), and in skeletal muscle (82), retina (14), and platelets of patients with Type 2 diabetes (81). Moreover, iNOS deficiency protects from high-fat diet-induced insulin resistance (62) in the obese Zucker rat model. These studies clearly demonstrate the important role of iNOS in insulin resistance. Thus overexpression of iNOS seen in the current study is at least partly due to enhanced inflammatory responses and insulin resistance in the GK diabetic rat. Although the mechanism of how iNOS causes or exacerbates insulin resistance remains largely unknown, it has been shown that the overproduction of NO causes S-nitroslyation of insulin-responsive molecules such as Akt, insulin receptor-β, and insulin receptor substrate-1 in ob/ob diabetic mice, resulting in insulin resistance (15, 87), and has been shown to be independent of cGMP mobilization (40). Therefore, reducing iNOS expression by knocking out the iNOS gene could improve insulin-induced glucose transport and hexose uptake (61). As seen in Fig. 6, A–D, the impaired Akt activation and insulin-induced vasodilation is partly due to S-nitrosylation of Akt in GK VSMC.
ANG II, in contrast to other contractile agents, generates ROS, a recognized player in the pathogenesis of vascular dysfunction associated with insulin resistance and non-insulin-dependent diabetes mellitus (16, 41, 77, 89). ROS such as superoxide and its reactive nitrogen derivative, peroxynitrite, are known vasoconstrictors in many vascular beds (58, 67, 84). We speculate that one possible mechanism of insulin-resistant vasorelaxation is that excessive NO together with enhanced ROS may produce peroxynitrite (ONOO−) (75, 86), an intermediate formed from the equimolar interaction of NO and superoxide known to cause impaired vasodilation by reducing NO bioavailability.
ANG II- and insulin-induced Akt activation may be two distinct and separate signaling mechanisms. ANG II is known to activate Akt both via endothelial growth factor receptor (EGFR) transactivation pathways via AT 1 receptor activation, as well as via nonreceptor tyrosine kinases, such as Src in VSMC (20, 21, 51, 80). ANG II-induced Akt activation is mediated by metabolites of arachidonic acid generated via a calmodulin-dependent kinase II (CaMKII)-stimulated Ca2+-dependent phospholipase A2 (50) and phospholipase D2 (51). Akt can be activated via a PI3-K-independent mechanism through PKA (70) and Ca2+/calmodulin-dependent kinase activation (50). ANG II-induced Akt phosphorylation is inhibited by diphenylene iodonium or overexpression of catalase, suggesting a role for NAD(P)H oxidase-derived ROS in ANG II-induced Akt activation via p38 MAP kinase pathway (80). Even though ANG II itself can phosphorylate and activate Akt similar to insulin (Fig. 3A, lane 6 vs. 7) in GK VSMC, ANG II is known to inhibit insulin signaling at multiple steps, acting through JAK-2/IRS-1/PI3-kinase, JNK, and ERK (83). ANG II increases serine phosphorylation of the insulin receptor (IR), IRS-1 and the p85 subunit of the PI3-K. At the same time, ANG II inhibits insulin-stimulated tyrosine phosphorylation of IRS-1, preventing the docking between IRS-1 and PI3-K (28, 29, 83). Thus insulin-induced Akt phosphorylation is inhibited by ANG II via inhibition of upstream of Akt, such as IR, IRS-1, and p85 subunit of the PI3-kinase (28), whereas ANG II-induced Akt phosphorylation is ROS dependent. Akt activation results in the stimulation of a variety of proteins, such as p70 ribosomal S6 kinase (RSK), glycogen synthase kinase 3β (GSK3β), Bad, and mammalian target of rapamycin (mTOR), which are specific for different functions of insulin, which include glucose uptake, glycogen synthesis, protein synthesis, and inhibition of apoptosis (25). Therefore, we believe that Akt activation by ANG II and insulin mediate distinct functions via different signaling pathways in VSMC.
As shown in our result in Fig. 3F, insulin-induced relaxation is impaired in intact aorta, implying endothelial dysfunction in GK aorta. Endothelium-dependent vascular relaxation in response to acetylcholine (ACh) and endothelium-independent vascular relaxation in response to sodium nitroprusside (SNP) were impaired in GK rats (18). Some of our studies were performed with denuded vessels to remove the effect of the endothelium in these studies. Vascular endothelial cell dysfunction lead to reduction in endothelium-derived relaxing factors such as nitric oxide via eNOS, prostacyclin, and endothelium-derived hyperpolarizing factor, or increased production of contracting factors such as endothelin-1 (ET-1) and thromboxane A2 (13). Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by the imbalance between NO and ET-1 production, suggesting that ET-1 acts as the vasoconstrictive component that is released from the endothelium to change the contractility of smooth muscle cells (63). The overall vasodilation response to insulin in intact aorta was different compared with that in the denuded aorta of GK diabetic rats, even though the presence of the endothelial dysfunction. Thus the insulin-induced relaxation may be the sum of the impaired endothelial-derived relaxing factors and increased vasoconstriction factors.
Diabetes is accompanied by alterations in MBP activation and its downstream signaling pathways (72). GK diabetic VSMC exhibited marked impairment in MBP activation by insulin, which was accompanied by failure of insulin to decrease the phosphorylation of MYPT1 and inhibit Rho kinase activity, resulting in increased MLC20 phosphorylation and VSMC contraction (8). Although GK diabetes does not affect insulin-stimulated tyrosine phosphorylation of the insulin receptor or its content, insulin-stimulated IRS-1 tyrosine phosphorylation was severely impaired. This was accompanied by marked reductions in IRS-1-associated PI3-K activity (72). Thus the impaired Akt activation (Figs. 1A and 2B), an effector of PI3-K, is well correlated with this previous finding. Even though iNOS expression is increased, as iNOS is independent from PI3K/Akt activation, downstream cGK1α is not affected by iNOS/NO signaling, given previous studies from our laboratory demonstrating lower cGK1α protein expression in GK diabetic VSMC (37). Since ROK is the upstream of MYPT1, the increased phosphorylation of MYPT1 represents the ROK activity (27, 44). As shown in Fig. 1, A and B, the increased basal and ANG-II-induced MYPT1 phosphorylation is higher than that in control VSMC, representing the increased ROK activity in GK diabetic VSMC. Thus our study compliments our previous finding that the basal and ANG II-induced ROK activity is increased in GK diabetic VSMC and denuded aorta (49, 72). Even as pMYPT1 was increased due to impaired Akt activation in GK VSMC, basal pMLC20 was lower (Fig. 3, A and D). Since the regulation of pMLC20 is also dependent on the MLCK activation, which reverses the effect of MBP, the decreased basal pMLC20 may be due to the change in the calcium-dependent contractile signaling pathway. Therefore, the multiple pathways involved with the regulation of MBP and the impact on MLC20 can be further investigated to clarify the dissociation of the increased pMYPT1 and decreased basal pMLC20 in GK VSMC. Also, further study needs to investigate any abnormalities in calcium-dependent contractile signaling in GK diabetic vasculature.
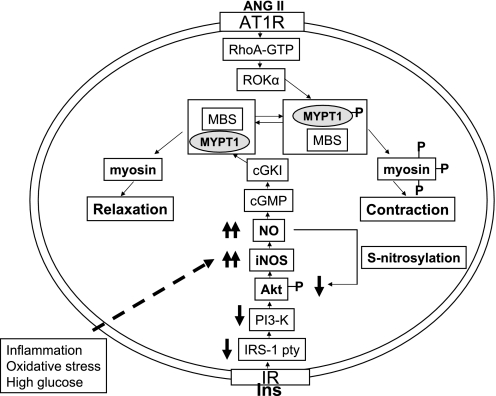
In conclusion, we demonstrate that defective Akt activation in the GK diabetic vasculature causes impaired insulin-induced vasodilation, which is rescued by the overexpression of constitutively active Akt. Upregulation of iNOS may be a protective mechanism against excessive contraction, abnormal signaling resulting from oxidative stress, and due to enhanced inflammation in the diabetic vasculature (Fig. 7), which needs further investigation to clarify. Collectively, these data advocate a novel strategy to correct defective vasodilation in diabetes.
Fig. 7.
Impaired insulin-mediated Akt activation and upregulation of iNOS/NO causes impaired insulin-mediated vasorelaxation in the GK diabetic vasculature. In GK vasculature, insulin fails to cause relaxation. Whereas insulin receptor (IR) content and tyrosine phosphorylation were similar in GK and WKY VSMC, insulin receptor substrate-1 (IRS-1) and phosphatidylinositol 3-kinase (PI3-K) activities were impaired. Therefore, phosphorylation and activation of Akt, downstream of IRS-1 and PI3-K, were impaired in GK VSMC. However, the expression of iNOS, downstream of Akt, was upregulated, due to increased inflammation, oxidative stress, and high glucose. In turn, the basal NO and nitrite production were increased, causing S-nitrosylation of Akt, possibly resulting in impaired Akt activation.
GRANTS
This Study was supported by National Heart, Lung, and Blood Institute Grant 5 R01 HL-067953-04.
Acknowledgments
We are grateful to Dr. Nikolai Kholodilov (Dept. of Neurology, Columbia University) for the generous gift of the myr-Akt construct. We thank the University of Iowa, Gene Transfer Vector Core for preparation of the adenovirus constructed with myr-AKT-Flag. We also thank Lisa Urgolites and Shichao Xia for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abu-Lebdeh H, Hodge D, Nguyen T. Predictors of macrovascular disease in patients with Type 2 diabetes mellitus. Mayo Clin Proc 76: 707–712, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Aizawa Y, Kawabe Ji Hasebe N, Takehara N, Kikuchi K. Pioglitazone enhances cytokine-induced apoptosis in vascular smooth muscle cells and reduces intimal hyperplasia. Circulation 104: 455–460, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Alessi D, MacDougall L, Sola M, Ikebe M, Cohen P. The control of protein phosphatase-1 by targetting subunits.. Eur J Biochem 210: 1023–1035, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Allen R, Krueger K, Dhume A, Agrawal D. Sustained Akt/PKB activation and transient attenuation of c-jun N-terminal kinase in the inhibition of apoptosis by IGF-1 in vascular smooth muscle cells. Apoptosis 10: 525–535, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Amaravadi R, Thompson CB. The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest 115: 2618–2624, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson RD, Haskell RE, Xia H, Roessler BJ, Davidson L. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Therapy 7: 1034–1038, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Bae SS, Cho H, Mu J, Birnbaum MJ. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J Biol Chem 278: 49530–49536, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Begum N, Duddy N, Sandu O, Reinzie J, Ragolia L. Regulation of myosin-bound protein phosphatase by insulin in vascular smooth muscle cells: evaluation of the role of Rho kinase and phosphatidylinositol-3-kinase-dependent signaling pathways. Mol Endocrinol 14: 1365–1376, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Begum N, Ragolia L, Rienzie J, McCarthy M, Duddy N. Regulation of mitogen-activated protein kinase phosphatase-1 induction by insulin in vascular smooth muscle cells. Evaluation of the role of the nitric oxide signaling pathway and potential defects in hypertension. J Biol Chem 273: 25164–25170, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Begum N, Sandu OA, Duddy N. Negative regulation of Rho signaling by insulin and its impact on actin cytoskeleton organization in vascular smooth muscle cells: role of nitric oxide and cyclic guanosine monophosphate signaling pathways. Diabetes 51: 2256–2263, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Begum N, Sandu OA, Ito M, Lohmann SM, Smolenski A. Active Rho kinase (ROK-alpha) associates with insulin receptor substrate-1 and inhibits insulin signaling in vascular smooth muscle cells. J Biol Chem 277: 6214–6222, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Blanc-Brude O, Yu J, Simosa H, Conte M, Sessa W, Altieri D. Inhibitor of apoptosis protein survivin regulates vascular injury. Nat Med 8: 987–994, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Cain A, Khalil R. Pathophysiology of essential hypertension: role of the pump, the vessel, and the kidney. Semin Nephrol 22: 3–16, 2002. [PubMed] [Google Scholar]
- 14.Carmo A, Cunha-Vaz J, Carvalho A, Lopes M. Nitric oxide synthase activity in retinas from non-insulin-dependent diabetic Goto-Kakizaki rats: correlation with blood-retinal barrier permeability. Nitric Oxide 4: 590–596, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Carvalho-Filho MA, Ueno M, Hirabara SM, Seabra AB, Carvalheira JBC, de Oliveira MG, Velloso LA, Curi R, Saad MJA. S-Nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes 54: 959–967, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol 24: 816–823, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Chen LW, Chang WJ, Wang JS, Hsu CM. Thermal injury-induced peroxynitrite production and pulmonary inducible nitric oxide synthase expression depend on JNK/AP-1 signaling. Critical Care Med 34: 142–150, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Cheng Z, Grönholm T, Louhelainen M, Finckenberg P, Merasto S, Tikkanen I, Mervaala E. Vascular and renal effects of vasopeptidase inhibition and angiotensin-converting enzyme blockade in spontaneously diabetic Goto-Kakizaki rats. J Hypertens 23: 1757–1770, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Chung H, Pae H, Choi B, Billiar T, Kim Y. Nitric oxide as a bioregulator of apoptosis. Biochem Biophys Res Commun 282: 1075–1079, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Eguchi S, Iwasaki H, Ueno H, Frank GD, Motley ED, Eguchi K, Marumo F, Hirata Y, Inagami T. Intracellular signaling of angiotensin II-induced p70 S6 kinase phosphorylation at Ser411 in vascular smooth muscle cells. Possible requirement of epidermal growth factor receptor, ras, extracellular signal-regulated kinase, and Akt. J Biol Chem 274: 36843–36851, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Eguchi S, TI. Signal transduction of angiotensin II type 1 receptor through receptor tyrosine kinase. Regul Pept 91: 13–20, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Elgebaly M, Kelly A, Harris A, Elewa H, Portik-Dobos V, Ketsawatsomkron P, Marrero M, Ergul A. Impaired insulin-mediated vasorelaxation in a nonobese model of Type 2 diabetes: role of endothelin-1. Can J Physiol Pharmacol 86: 358–364, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elizalde M, Ryden M, van Harmelen V, Eneroth P, Gyllenhammar H, Holm C, Ramel S, Olund A, Arner P, Andersson K. Expression of nitric oxide synthases in subcutaneous adipose tissue of nonobese and obese humans. J Lipid Res 41: 1244–1251, 2000. [PubMed] [Google Scholar]
- 24.Engels K, Knauer SK, Loibl S, Fetz V, Harter P, Schweitzer A, Fisseler-Eckhoff A, Kommoss F, Hanker L, Nekljudova V, Hermanns I, Kleinert H, Mann W, du Bois A, Stauber RH. NO signaling confers cytoprotectivity through the survivin network in ovarian carcinomas. Cancer Res 68: 5159–5166, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Farese RV, Sajan MP, Standaert ML. Insulin-sensitive protein kinases (atypical protein kinase C and protein kinase B/Akt): Actions and defects in obesity and Type II diabetes. Exp Biol Med 230: 593–605, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Feliers D, Duraisamy S, Faulkner J, Duch J, Lee A, Abboud H, Choudhury G, Kasinath B. Activation of renal signaling pathways in db/db mice with Type 2 diabetes. Kidney Int 60: 495–504, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem 274: 37385–37390, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Folli F, Kahn CR, Hansen H, Bouchie JL, Feener EP. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Invest 100: 2158–2169, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folli F, Saad M, Velloso L, Hansen H, Carandente O, Feener E, Kahn C. Crosstalk between insulin and angiotensin II signalling systems. Exp Clin Endocrinol Diabetes 107: 133–139, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, McNeish JD, Coleman KG. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKBβ. J Clin Invest 112: 197–208, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goto Y, Kakizaki M, Masaki N. Production of spontaneous diabetic rats by repetition of sensitive breeding. Tokoku J Exp Med 119: 85–90, 1976. [DOI] [PubMed] [Google Scholar]
- 32.Hartshorne DJ Physiology of The Gastrointestinal Tract, edited by Johnson DR. New York: Raven, 1987, p. 423–482.
- 33.Ikebe M, Hartshorne D. Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J Biol Chem 260: 10027–10031, 1985. [PubMed] [Google Scholar]
- 34.Ikebe M, Hartshorne D, Elzinga M. Identification, phosphorylation, and dephosphorylation of a second site for myosin light chain kinase on the 20,000-dalton light chain of smooth muscle myosin. J Biol Chem 261: 36–39, 1986. [PubMed] [Google Scholar]
- 35.Ikeguchi M, Ueta T, Yamane Y, Hirooka Y, Kaibara N. Inducible nitric oxide synthase and survivin messenger RNA expression in hepatocellular carcinoma. Clin Cancer Res 8: 3131–3136, 2002. [PubMed] [Google Scholar]
- 36.Isenovic ER, Meng Y, Divald A, Milivojevic N, Sowers JR. Role of phosphatidylinositol 3-kinase/Akt pathway in angiotensin II and insulin-like growth factor-1 modulation of nitric oxide synthase in vascular smooth muscle cells. Endocrine 19: 287–292, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Jacob A, Smolenski A, Lohmann SM, Begum N. MKP-1 expression and stabilization and cGK Iα prevent diabetes-associated abnormalities in VSMC migration. Am J Physiol Cell Physiol 287: C1077–C1086, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Kahn AM, Husid A, Allen JC, Seidel CL, Song T. Insulin acutely inhibits cultured vascular smooth muscle cell contraction by a nitric oxide synthase-dependent pathway. Hypertension 30: 928–933, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Kamm K, Stull J. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Ann Rev Pharmacol Toxicol 25: 593, 1985. [DOI] [PubMed] [Google Scholar]
- 40.Kapur S, Bedard S, Marcotte B, Cote C, Marette A. Expression of nitric oxide synthase in skeletal muscle: a novel role for nitric oxide as a modulator of insulin action. Diabetes 46: 1691–1700, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Katakam PVG, Snipes JA, Tulbert CD, Mayanagi K, Miller AW, Busija DW. Impaired endothelin-induced vasoconstriction in coronary arteries of Zucker obese rats is associated with uncoupling of [Ca2+]i signaling. Am J Physiol Regul Integr Comp Physiol 290: R145–R153, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Katome T, Obata T, Matsushima R, Masuyama N, Cantley LC, Gotoh Y, Kishi K, Shiota H, Ebina Y. Use of RNA interference-mediated gene silencing and adenoviral overexpression to elucidate the roles of AKT/protein kinase B isoforms in insulin actions. J Biol Chem 278: 28312–28323, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Kim Y, Nikoulina S, Ciaraldi T, Henry R, Kahn B. Normal insulin-dependent activation of Akt/potein kinase B, with diminished activation of phosphoinositide 3 kinase, in muscle in type2 diabetes. J Clin Invest 104: 733–741, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273: 245–248, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Kreiling JL, Byrd JC, MacDonald RG. Domain interactions of the mannose 6-phosphate/insulin-like growth factor II receptor. J Biol Chem 280: 21067–21077, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Krook A, Kawano Y, Song X, Efecdic S, Roth R, Wallberg-Henriksson H, Zierath J. Improved glucose tolerance restores insulin-stimulated Akt kinase activity and glucose transporter in skeletal muscle from diabetic Goto-Kakizaki rats. Diabetes 46: 2110–2114, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Krook A, Roth R, Jiang X, Zierath J, and Wallberg-Henriksson H. Insulin-stimulated Akt kinase activity is reduced in skeletal muscle from NIDDM subjects. Diabetes 47: 1281–1286, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Lee JH, Ragolia L. AKT phosphorylation is essential for insulin-induced relaxation of rat vascular smooth muscle cells. Am J Physiol Cell Physiol 291: C1355–C1365, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JH, Xia S, Ragolia L. Upregulation of AT2 receptor and iNOS impairs angiotensin II-induced contraction without endothelium influence in young normotensive diabetic rats. Am J Physiol Regul Integr Comp Physiol 295: R144–R154, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li F, Malik KU. Angiotensin II-induced Akt activation is mediated by metabolites of arachidonic acid generated by CaMKII-stimulated Ca2+-dependent phospholipase A2. Am J Physiol Heart Circ Physiol 288: H2306–H2316, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Li F, Malik KU. Angiotensin II-induced Akt activation through the epidermal growth factor receptor in vascular smooth muscle cells is mediated by phospholipid metabolites derived by activation of phospholipase D. J Pharmacol Exp Ther 312: 1043–1054, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Ludmer P, Selwyn A, Shook T, Wayne R, Mudge G, Alexander R, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med 315: 1046–1051, 1986. [DOI] [PubMed] [Google Scholar]
- 53.Luo Z, Fujio Y, Kureishi Y, Rudic R, Daumerie G, Fulton D, Sessa W, Walsh K. Acute modulation of endothelial Akt/PKB activity alters nitric oxide-dependent vasomotor activity in vivo. J Clin Invest 106: 493–499, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J Clin Invest 110: 851–860, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin E, Nathan C, Xie QW. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J Exp Med 180: 977–984, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res 14: 5000–5005, 2008. [DOI] [PubMed] [Google Scholar]
- 57.Nadler ST, Stoehr JP, Rabaglia ME, Schueler KL, Birnbaum MJ, Attie AD. Normal Akt/PKB with reduced PI3K activation in insulin-resistant mice. Am J Physiol Endocrinol Metab 281: E1249–E1254, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Oeckler RA, Kaminski PM, Wolin MS. Stretch enhances contraction of bovine coronary arteries via an NAD(P)H oxidase-mediated activation of the extracellular signal-regulated kinase mitogen-activated protein kinase cascade. Circ Res 92: 23–31, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Ohashi H, Takagi H, Oh H, Suzuma K, Suzuma I, Miyamoto N, Uemura A, Watanabe D, Murakami T, Sugaya T, Fukamizu A, Honda Y. Phosphatidylinositol 3-kinase/Akt regulates angiotensin II-induced inhibition of apoptosis in microvascular endothelial cells by governing survivin expression and suppression of caspase-3 activity. Circ Res 94: 785–793, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Okon E, Szado T, Laher I, McManus B, van Breemen C. Augmented contractile response of vascular smooth muscle in a diabetic mouse model. J Vasc Res 40: 520–530, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Ortiz PA, Garvin JL. Cardiovascular and renal control in NOS-deficient mouse models. Am J Physiol Regul Integr Comp Physiol 284: R628–R638, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med 7: 1138–1143, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, Montagnani M. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol 289: H813–H822, 2005. [DOI] [PubMed] [Google Scholar]
- 64.Ragolia L, Palaia T, Koutrouby TB, Maesaka JK. Inhibition of cell cycle progression and migration of vascular smooth muscle cells by prostaglandin D2 synthase: resistance in diabetic Goto-Kakizaki rats. Am J Physiol Cell Physiol 287: C1273–C1281, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Reaven Banting lecture 1988 GM. Role of insulin resistance in human disease. Diabetes 37: 1595–1607, 1988. [DOI] [PubMed] [Google Scholar]
- 66.Resende A, Tabellion A, Nadaud S, Lartaud I, Bagrel D, Faure S, Atkinson J, and Capdeville-Atkinson C. Incubation of rat aortic rings produces a specific reduction in agonist-evoked contraction: effect of age of donor. Life Sci 76: 9–20, 2004. [DOI] [PubMed] [Google Scholar]
- 67.Rey FE, Li XC, Carretero OA, Garvin JL, Pagano PJ. Perivascular superoxide anion contributes to impairment of endothelium-dependent relaxation: Role of gp91phox. Circulation 106: 2497–2502, 2002. [DOI] [PubMed] [Google Scholar]
- 68.Ribeiro D, Kitakawa D, Domingues M, Cabral L, Marques M, Salvadori D. Survivin and inducible nitric oxide synthase production during 4NQO-induced rat tongue carcinogenesis: a possible relationship. Exp Mol Pathol 83: 131–137, 2007. [DOI] [PubMed] [Google Scholar]
- 69.Rondinone CM, Carvalho E, Wesslau C, Smith U. Impaired glucose transport and protein kinase B activation in insulin, but not okadaic acid, in adipocytes from subjects with type II diabetic mellitus. Diabetologia 42: 819–825, 1999. [DOI] [PubMed] [Google Scholar]
- 70.Sable CL, Filippa N, Hemmings B, Van Obberghen E. cAMP stimulates protein kinase B in a Wortmannin-insensitive manner. FEBS Lett 409: 253–257, 1997. [DOI] [PubMed] [Google Scholar]
- 71.Salehi A, Abaraviciene SM, Jimenez-Feltstrom J, Östenson CG, Efendic S, Lundquist3 I. Excessive islet NO generation in Type 2 diabetic GK rats coincides with abnormal hormone secretion and is counteracted by GLP-1. PLoS ONE 3: e2165, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sandu O, Ragolia L, Begum N. Diabetes in the Goto-Kakizaki rat is accompanied by impaired insulin-mediated myosin-bound phosphatase activation and vascular smooth muscle cell relaxation. Diabetes 49: 2178–2189, 2000. [DOI] [PubMed] [Google Scholar]
- 73.Sandu OA, Ito M, Begum N. Signal transduction in smooth muscle: selected contribution: insulin utilizes NO/cGMP pathway to activate myosin phosphatase via Rho inhibition in vascular smooth muscle. J Appl Physiol 91: 1475–1482, 2001. [DOI] [PubMed] [Google Scholar]
- 74.Sandu OA, Ragolia L, Begum N. Diabetes in the Goto-Kakizaki rat is accompanied by impaired insulin-mediated myosin-bound phosphatase activation and vascular smooth muscle cell relaxation. Diabetes 49: 2178–2189, 2000. [DOI] [PubMed] [Google Scholar]
- 75.Schini-Kerth V, Vanhoutte P. Nitric oxide synthases in vascular cells. Exp Physiol 80: 885–905, 1995. [DOI] [PubMed] [Google Scholar]
- 76.Shimabukuro M, Higa M, Zhou YT, Wang MY, Newgard CB, Unger RH. Lipoapoptosis in Beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem 273: 32487–32490, 1998. [DOI] [PubMed] [Google Scholar]
- 77.Sowers JR Insulin resistance and hypertension. Am J Physiol Heart Circ Physiol 286: H1597–H1602, 2004. [DOI] [PubMed] [Google Scholar]
- 78.Steinberg H, Brechtel G, Johnson A, Fineberg N, Baron A. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 94: 1172–1179, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sugita H, Kaneki M, Tokunaga E, Sugita M, Koike C, Yasuhara S, Tompkins RG, Martyn JAJ. Inducible nitric oxide synthase plays a role in LPS-induced hyperglycemia and insulin resistance. Am J Physiol Endocrinol Metab 282: E386–E394, 2002. [DOI] [PubMed] [Google Scholar]
- 80.Taniyama Y, Ushio-Fukai M, Hitomi H, Rocic P, Kingsley MJ, Pfahnl C, Weber DS, Alexander RW, Griendling KK. Role of p38 MAPK and MAPKAPK-2 in angiotensin II-induced Akt activation in vascular smooth muscle cells. Am J Physiol Cell Physiol 287: C494–C499, 2004. [DOI] [PubMed] [Google Scholar]
- 81.Tannous M, Rabini R, Vignini A, Moretti N, Fumelli P, Zielinski B, Mazzanti L, Mutus B. Evidence for iNOS-dependent peroxynitrite production in diabetic platelets. Diabetologia 42: 539–544, 1999. [DOI] [PubMed] [Google Scholar]
- 82.Torres SH, De Sanctis JB, de Briceno LM, Hernandez N, Finol HJ. Inflammation and nitric oxide production in skeletal muscle of Type 2 diabetic patients. J Endocrinol 181: 419–427, 2004. [DOI] [PubMed] [Google Scholar]
- 83.Velloso L, Folli F, Perego L, Saad M. The multi-faceted cross-talk between the insulin and angiotensin II signaling systems. Diabetes Metab Res Rev 22: 98–107, 2006. [DOI] [PubMed] [Google Scholar]
- 84.Walia M, Samson SE, Schmidt T, Best K, Whittington M, Kwan CY, Grover AK. Peroxynitrite and nitric oxide differ in their effects on pig coronary artery smooth muscle. Am J Physiol Cell Physiol 284: C649–C657, 2003. [DOI] [PubMed] [Google Scholar]
- 85.Wang J, Yang L, Yang J, Kuropatwinski K, Wang W, Liu XQ, Hauser J, Brattain MG. Transforming growth factor β induces apoptosis through repressing the phosphoinositide 3-kinase/AKT/Survivin pathway in colon cancer cells. Cancer Res 68: 3152–3160, 2008. [DOI] [PubMed] [Google Scholar]
- 86.Wink D, Miranda K, Espey M. Effects of oxidative and nitrosative stress in cytotoxicity. Semin Perinatol 24: 20–23, 2000. [DOI] [PubMed] [Google Scholar]
- 87.Yasukawa T, Tokunaga E, Ota H, Sugita H, Martyn JAJ, Kaneki M. S-Nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem 280: 7511–7518, 2005. [DOI] [PubMed] [Google Scholar]
- 88.Yasukawa T, Tokunaga E, Ota H, Sugita H, Martyn JAJ, Kaneki M. S-Nitrosylation-dependent Inactivation of Akt/Protein Kinase B in Insulin Resistance. J Biol Chem 280: 7511–7518, 2005. [DOI] [PubMed] [Google Scholar]
- 89.Zemel MD, Reddy S, Sowers. JR. Insulin attenuation of vasoconstrictor responses to phenylephrine in Zucker lean and obese rats. Am J Hypertens 4: 537–539, 1991. [DOI] [PubMed] [Google Scholar]
- 90.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: Implications for human obesity. Proc Natl Acad Sci USA 97: 1784–1789, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]