Abstract
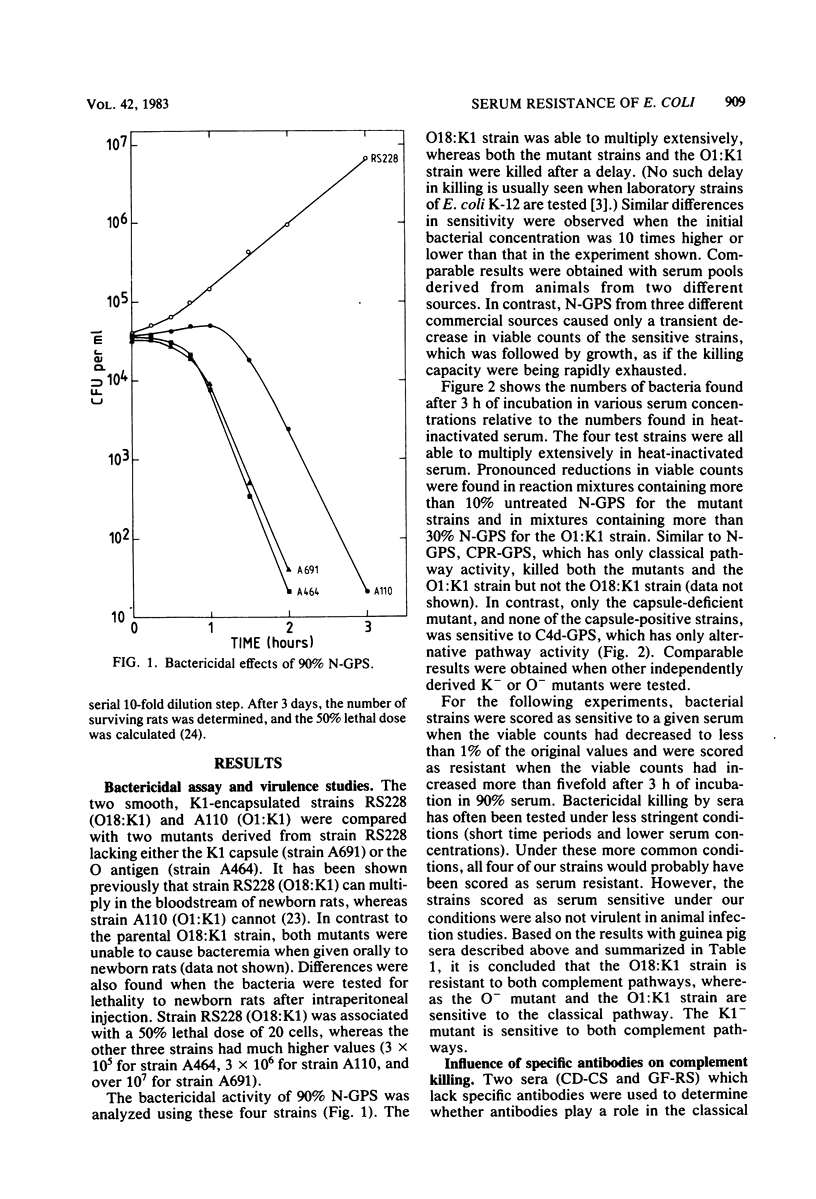
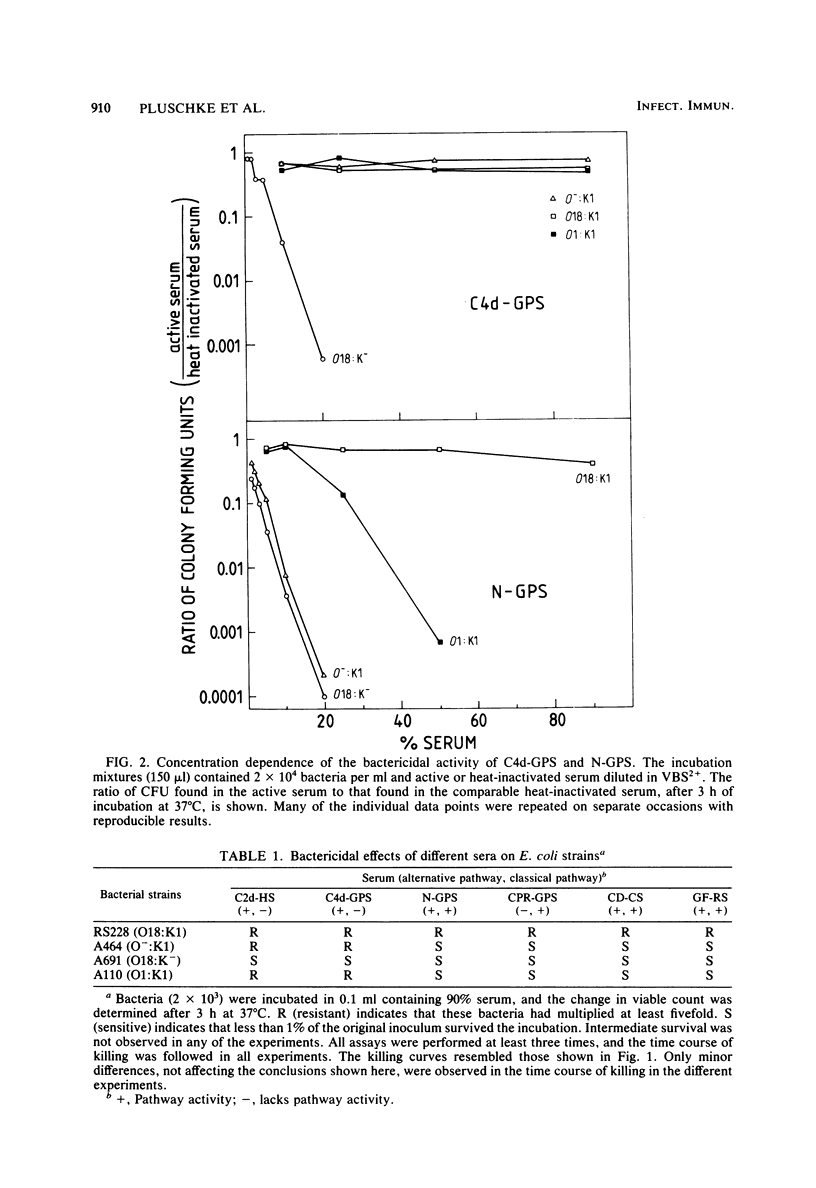
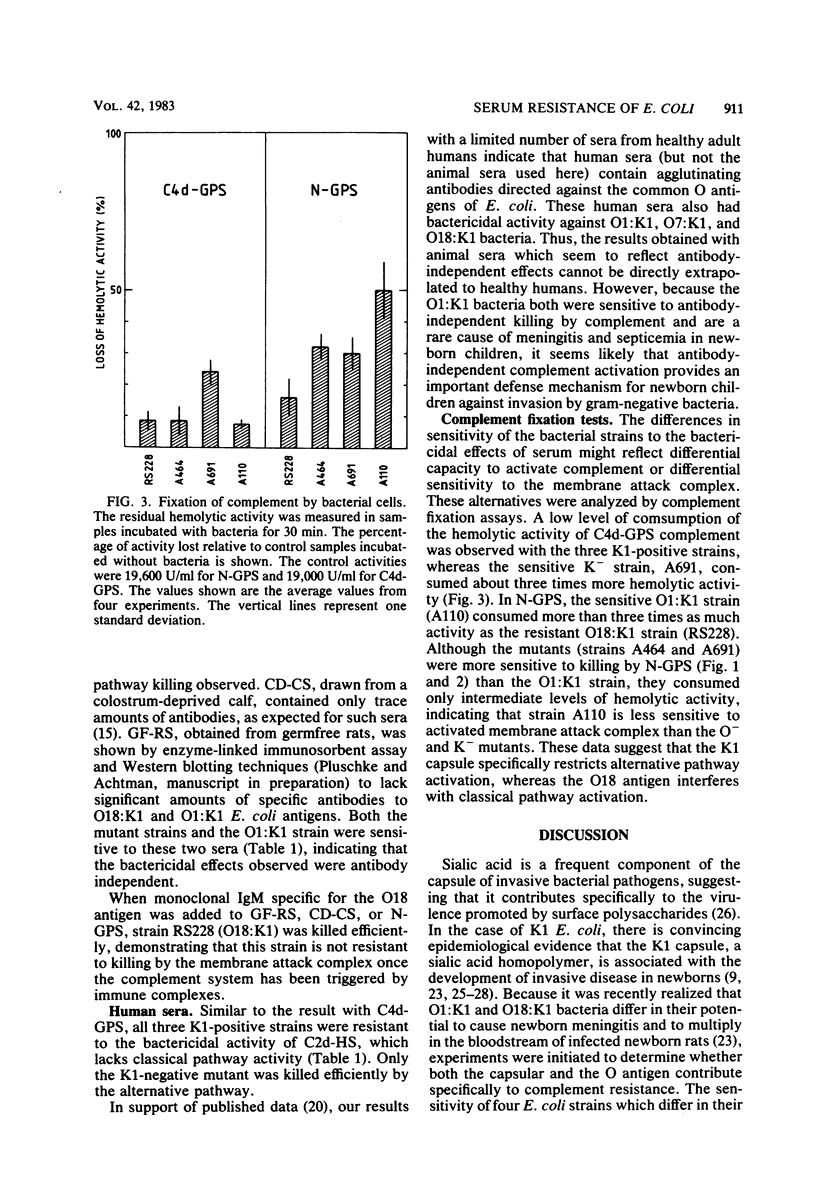
Epidemiological data show that O18:K1 Escherichia coli is a common cause of neonatal bacteremia and meningitis. These bacteria were capable of multiplying in the bloodstream of newborn rats and were resistant to the bactericidal effects of complement in the absence of specific antibodies. The roles played by the O antigen and the K antigen in complement resistance were analyzed by comparing the bactericidal effects of normal sera and of sera deficient in various complement components or in immunoglobulins. These sera were tested on O18:K1 bacteria and on mutants lacking either the lipopolysaccharide O antigen or the K1 capsular polysaccharide. In addition, O1:K1 cells, which can cause pyelonephritis but which are rare in newborn meningitis and which do not multiply in the bloodstream of newborn rats, were also examined. Different mechanisms of protection against the alternative and classical pathways were recognized: K1-positive cells were resistant to the bactericidal activity of sera deficient in classical complement pathway components, whereas K1-negative cells were sensitive to these sera. Based on these results and on those from complement fixation assays, the K1 sialic acid polysaccharide impedes the activation of, and thus protects the bacteria against, the alternative complement pathway. Not only the K1-negative mutant cells but also O1:K1 bacteria and mutants lacking the O18 oligosaccharide repeating units of the lipopolysaccharide were sensitive to the classical complement pathway. These bactericidal effects were observed even in the absence of specific antibodies. It is proposed that both the K1 capsule and the O18 oligosaccharide restrict antibody-independent classical pathway activation by shielding deeper structures on the cell membrane that are capable of activating this pathway.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz S. J., Isliker H. Antibody-independent interactions between Escherichia coli J5 and human complement components. J Immunol. 1981 Nov;127(5):1748–1754. [PubMed] [Google Scholar]
- Binns M. M., Mayden J., Levine R. P. Further characterization of complement resistance conferred on Escherichia coli by the plasmid genes traT of R100 and iss of ColV,I-K94. Infect Immun. 1982 Feb;35(2):654–659. doi: 10.1128/iai.35.2.654-659.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolussi R., Ferrieri P., Björkstén B., Quie P. G. Capsular K1 polysaccharide of Escherichia coli: relationship to virulence in newborn rats and resistance to phagocytosis. Infect Immun. 1979 Jul;25(1):293–298. doi: 10.1128/iai.25.1.293-298.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clas F., Loos M. Requirement for an additional serum factor essential for the antibody-independent activation of the classical complement sequence by Gram-negative bacteria. Infect Immun. 1982 Sep;37(3):935–939. doi: 10.1128/iai.37.3.935-939.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F., Ruddy S. Properdin factor D: characterization of its active site and isolation of the precursor form. J Exp Med. 1974 Feb 1;139(2):355–366. doi: 10.1084/jem.139.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glode M. P., Sutton A., Moxon E. R., Robbins J. B. Pathogenesis of neonatal Escherichia coli meningitis: induction of bacteremia and meningitis in infant rats fed E. coli K1. Infect Immun. 1977 Apr;16(1):75–80. doi: 10.1128/iai.16.1.75-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Cole R. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J Exp Med. 1982 Mar 1;155(3):797–808. doi: 10.1084/jem.155.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. II. C8 and C9 release C5b67 from the surface of Salmonella minnesota S218 because the terminal complex does not insert into the bacterial outer membrane. J Exp Med. 1982 Mar 1;155(3):809–819. doi: 10.1084/jem.155.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M., Ihara I., Suzuki A., Harada Y. Properties of a new complement-dependent bactericidal factor specific for Ra chemotype salmonella in sera of conventional and germ-free mice. J Immunol. 1982 Nov;129(5):2198–2201. [PubMed] [Google Scholar]
- Kazatchkine M. D., Fearon D. T., Austen K. F. Human alternative complement pathway: membrane-associated sialic acid regulates the competition between B and beta1 H for cell-bound C3b. J Immunol. 1979 Jan;122(1):75–81. [PubMed] [Google Scholar]
- Kraehenbuhl J. P., Bron C., Sordat B. Transfer of humoral secretory and cellular immunity from mother to offspring. Curr Top Pathol. 1979;66:105–157. doi: 10.1007/978-3-642-67205-7_4. [DOI] [PubMed] [Google Scholar]
- Leist-Welsh P., Bjornson A. B. Immunoglobulin-independent utilization of the classical complement pathway in opsonophagocytosis of Escherichia coli by human peripheral leukocytes. J Immunol. 1982 Jun;128(6):2643–2651. [PubMed] [Google Scholar]
- Loos M., Wellek B., Thesen R., Opferkuch W. Antibody-independent interaction of the first component of complement with Gram-negative bacteria. Infect Immun. 1978 Oct;22(1):5–9. doi: 10.1128/iai.22.1.5-9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N., Yasuda T., Okada H. Restriction of alternative complement pathway activation by sialosylglycolipids. Nature. 1982 Sep 16;299(5880):261–263. doi: 10.1038/299261a0. [DOI] [PubMed] [Google Scholar]
- Olling S., Hanson L. A., Holmgren J., Jodal U., Lincoln K., Lindberg U. The bactericidal effect of normal human serum on E. coli strains from normals and from patients with urinary tract infections. Infection. 1973;1(1):24–28. doi: 10.1007/BF01638251. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Complement C3 convertase: cell surface restriction of beta1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2416–2420. doi: 10.1073/pnas.75.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Mercer A., Kusećek B., Pohl A., Achtman M. Induction of bacteremia in newborn rats by Escherichia coli K1 is correlated with only certain O (lipopolysaccharide) antigen types. Infect Immun. 1983 Feb;39(2):599–608. doi: 10.1128/iai.39.2.599-608.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B., McCracken G. H., Jr, Gotschlich E. C., Orskov F., Orskov I., Hanson L. A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- Siegel J. D., McCracken G. H., Jr Sepsis neonatorum. N Engl J Med. 1981 Mar 12;304(11):642–647. doi: 10.1056/NEJM198103123041105. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Huggins M. B. The association of the O18, K1 and H7 antigens and the Co1V plasmid of a strain of Escherichia coli with its virulence and immunogenicity. J Gen Microbiol. 1980 Dec;121(2):387–400. doi: 10.1099/00221287-121-2-387. [DOI] [PubMed] [Google Scholar]
- Stevens P., Huang S. N., Welch W. D., Young L. S. Restricted complement activation by Escherichia coli with the K-1 capsular serotype: a possible role in pathogenicity. J Immunol. 1978 Dec;121(6):2174–2180. [PubMed] [Google Scholar]
- Wright S. D., Levine R. P. How complement kills E. coli. I. Location of the lethal lesion. J Immunol. 1981 Sep;127(3):1146–1151. [PubMed] [Google Scholar]


