Abstract
Colonic epithelial stem cells are believed to be located at the crypt base where they have previously been shown to express musashi-1. The colonic stem cell niche, which includes extracellular matrix and myofibroblasts (together with other cell types), is likely to be important in maintaining the function of the progenitor cells. The aims of our studies were to characterize stem cells in isolated and disaggregated human colonic crypt epithelial cells and investigate their interactions with monolayers of primary human colonic myofibroblasts. In unfractionated preparations of disaggregated colonic crypts, musashi-1 positive cells preferentially adhered to colonic myofibroblasts, despite the presence of excess blocking anti-β1-integrin antibody. These adherent epithelial cells remained viable for a number of days and developed slender processes. Cells with side population characteristics (as demonstrated by ability to expel the dye Hoechst 33342) were consistently seen in the isolated colonic crypt epithelial cells. These side population cells expressed musashi-1, β1-integrin, BerEP4, and CD133. Sorted side population crypt epithelial cells also rapidly adhered to primary colonic myofibroblasts. In conclusion, in preparation of isolated and disaggregated human colonic crypts, cells with stem cell characteristics preferentially adhere to primary human colonic myofibroblasts in a β1-integrin-independent fashion.
Keywords: colonic progenitor cells, side population cells
the stem cells are believed to be located at specific positions along the crypt-villus axis of the small and large intestine. To date, small intestinal stem cells have been studied the most and are believed to give rise to four types of terminally differentiated cells, enterocytes (columnar cells), goblet cells, Paneth cells and enteroendocrine cells (5, 7, 9, 44). All four of the differentiated epithelial cell types can also be present in the chronically inflamed colon, but Paneth cells are usually absent in the normal colonic mucosa (14, 38). Recent studies using transgenic and knockout mice have demonstrated the importance of distinct signaling pathways in intestinal stem cell function. These pathways include Wnt, Notch, bone morphogenetic protein, and phosphatidylinositol(3,4,5) kinase (5, 12, 13, 44). The stem cells also express Hes1, a basic helix-loop-helix transcriptional repressor of the secretory cell lineage (23, 45). These signaling pathways are likely to involve interactions with the microenvironment surrounding the stem cells (stem cell niche).
The stem cell niche is believed to be important in maintaining the functions of progenitor cells in a number of different organs (18). This is also likely to be the case in the intestine (35, 47), where non-stem cell constituents of the niche include extracellular matrix, myofibroblasts, fibroblasts, smooth muscle cells, endothelial cells, neural cells, lymphocytes, and macrophages. Epithelial-mesenchymal interactions enable specification of epithelial stem cells during development (6), but the requirement and nature of these communications in adult stem cells remain to be characterized. Intestinal myofibroblasts demonstrate characteristics of both fibroblasts and smooth muscle cells and are present under the basement membrane, immediately subjacent to the overlying epithelial cells (40). In addition to communications via secreted products, direct contact between epithelial cells and myofibroblasts may occur via processes that project through pores in the basement membrane (31). Following the loss of the overlying epithelial cells, myofibroblasts have also been shown to migrate toward the luminal surface via the basement membrane pores (29). While lying over the basement membrane, the myofibroblasts may facilitate epithelial restitution and enhance barrier function via secreted transforming growth factor-β (4, 33). Intestinal myofibroblasts are also capable of secreting a wide range of other peptide factors (1, 2, 22, 40), cyclooxygenase products (4, 16, 29), extracellular matrix proteins (1, 29), matrix metalloproteinases, and tissue inhibitors of matrix metalloproteinases (34). Moreover, myofibroblasts in the pericryptal region have been proposed as an important source of Wnt and other signals (44, 46). The above biological properties and the proximity of some of these cells to the stem cells suggest that intestinal myofibroblasts may play a major role in regulating the function of the progenitor cells.
In this study, we have investigated interactions between monolayers of isolated primary human colonic myofibroblasts and stem cell-containing epithelial cell preparations obtained from isolated human colonic crypts.
MATERIALS AND METHODS
Tissue.
Histologically normal colonic mucosal samples were obtained (>5 cm from tumor) from fresh intestinal specimens resected for cancer. Mucosal samples, which were surplus to clinical requirements, were only used following informed consent from patients. The use of such mucosal samples for research was approved by the Nottingham Research Ethics Committee.
Isolation of colonic crypts and their disaggregation.
The tissue samples were washed thoroughly with calcium- and magnesium-free Hanks’ balanced salt solution (HBSS) to remove any adherent fecal material. Mucosal strips were then dissected from the submucosa in approximately 0.5 to 1 cm2 pieces, and epithelial cells were isolated as previously described (30), with minor modifications. In brief, the mucosal strips were treated with 1 mmol/l EDTA plus 0.05% mmol/l dithiothreitol (DTT) in a shaking water bath at 37°C. After 30 min of incubation in EDTA, the mucosal tissue samples were shaken to release epithelial cells, which were collected by washing with calcium- and magnesium-free HBSS. This process was repeated on two further occasions. Cells in crypt-containing fractions were disaggregated as previously described by Whitehead et al. (48), by incubation at room temperature in 0.25% pancreatin (Sigma) for 90 min with occasional shaking. The disaggregated crypt epithelial cells were subsequently washed once in cold (4°C) calcium- and magnesium-free HBSS and treated with 0.05 mmol/l DTT solution to dissolve the mucus. A small aliquot of cells was used to assess the cell count and viability. If the proportion of cells in aggregates was >20%, they were gently passed through a 21-gauge needle and recounted. The disaggregated epithelial cells were used for cytospin preparations, for investigation of adhesion to myofibroblasts, and flow cytometry.
Myofibroblast isolation and culture.
Myofibroblasts were isolated from tissue samples as previously reported by us (29). Briefly, colonic mucosal samples denuded of epithelial cells (as described above) were washed in HBSS and placed in culture dishes (Corning Costar) containing RPMI 1640 medium (GIBCO) supplemented with 10% fetal calf serum (FCS), penicillin, streptomycin, gentamicin, and glutamine and incubated at 37°C. The myofibroblasts migrate from the mucosa in 2 to 3 wk and adhere to the culture dishes. Once the adherent colonies of myofibroblasts had reached a critical density, the mucosal strips were removed from the culture dish and the RPMI growth medium was changed to Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FCS, penicillin, streptomycin, gentamicin, 1% nonessential amino acids (GIBCO), and 200 mM glutamine (Sigma). Colonies of primary myofibroblasts were then cultured to confluency before passage. Phenotype of the myofibroblasts was confirmed by expression of α-smooth muscle actin and vimentin by immunohistochemistry (29).
Interaction between colonic epithelial cells and myofibroblasts.
For experiments to study the interactions between epithelial cells and myofibroblasts, the latter were grown to confluency on glass 13-mm coverslips placed in 24-welled plates. Disaggregated colonic crypt epithelial cells (in prewarmed DMEM containing 10% FCS, penicillin, streptomycin, and gentamicin, 200 mM glutamine, and 1× nonessential amino acids) were seeded (0.25 × 106 viable cells per well) onto confluent monolayers of myofibroblasts established on coverslips. After 30-min coculture, the wells were washed gently to remove the nonadherent cells and the coverslips were fixed in acetone or 4% paraformaldehyde (PFA)/sucrose and stored at −20°C, before immunohistochemistry. Some coverslips were fixed in 2.5% glutaraldehyde (in 0.1 M cacodylate buffer, pH 7.4) for scanning electron microscopy.
In some of the experiments, the isolated and disaggregated colonic epithelial cells were incubated (for 30 min at 37°C) with excess anti-β1-integrin antibody (4B4 clone, Beckmann Coulter) or control antibody (IgG1 antibody, Beckmann Coulter) before application to monolayers of myofibroblasts for 30 min, followed by washing and fixation as described above.
Immunohistochemistry and immunocytochemistry.
Immunohistochemical studies were undertaken using sections from paraffin-embedded samples of normal colonic mucosa. Sections were dewaxed in xylene and rehydrated through graded alcohols. Peroxidase activity was quenched using 3% H2O2 in methanol for 20 min followed by antigen retrieval in boiling 10 mM citrate buffer. For immunocytochemistry, cytospin preparations and coverslips were allowed to thaw initially and then hydrated at room temperature. Endogenous peroxidase activity was quenched in 0.3% H2O2 in methanol for 30 min before blocking with 3% horse serum (GIBCO) and 0.1% Triton X (Fluka) in Tris-buffered saline buffer for 40 min.
Immunoperoxidase staining was performed using a Vectastain Elite Universal ABC kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's instructions. In brief, the specimens (tissue sections or cytospins) were either incubated with anti-cytokeratin (Sigma-Aldrich, St. Louis, MO), anti-BerEP4 (Dako), anti-musashi-1 (Chemicon), or anti-DCAMKL-1 (doublecortin and CaM kinase-like-1) antibody, followed by biotinylated secondary antibody and avidin-biotin complex. Peroxidase activity was developed with either VECTOR VIP peroxidase substrate (H2O2) or DAB solution as per the manufacturer's protocol.
RT-PCR.
Total RNA was extracted with the RNeasy Plus mini kit (Qiagen) using the manufacturer's recommended protocol for eukaryotic cellular RNA. Complementary DNA was made with Quantitect Reverse Transcription kit (Qiagen) as per the manufacturer's protocol. The following primer pairs were used for PCR: 5′-GGCTTC-GTCACTTTCATGGACCAGGCG-3′ and 5′-GGGAACTGGTAGGTGTAGC-3′ to amplify musashi-1 cDNA, 5′-TGATTTTGGATGCTCTGAAGAA-3′ and 5′-GGTACT-TCCCCAGCACACTT-3′ to amplify Hes-1 cDNA, 5′-TGAAGCTGGGTGAC-TTTGG-3′ and 5′-GTCCACCTTGAGGCCGTAT-3′ to amplify DCAMKL-1 cDNA, 5′-TTGCGAAGCCTTCAATCC-3′ and 5′-CTGGACGGGGATTTCTGTTA-3′ to amplify Lgr5 cDNA, and 5′-TGTAAAACGTGTATTGTTCGTTACC-3′ and 5′-CAATATCTT-GGAGAGTTTTATCTGACC-3′ to amplify Bmi-1 cDNA. GAPDH primers, 5′-CCACCCATGGCAAATTCCATGGCA-3′ and 5′-TCTAGACGGCAGGTCAGGTCC-ACC-3′, were used as a positive control for the reactions. PCR products were analyzed using 1% agarose gels.
Flow cytometric analysis of epithelial cells.
For analysis of β1-integrin expression, disaggregated colonic crypt epithelial cells (unfractionated or myofibroblast-adherent) were incubated for 30 min (on ice) with phycoerythrin-conjugated anti-β1-integrin antibody (Beckman Coulter, Miami, FL) or control antibody of the same isotype. Cells were analyzed with an EPICS flow cytometer (Beckman Coulter), and the WinMDI (multiple document interface for flow cytometry) V2.8 software was used for data analysis.
Side population studies were performed using the Hoechst 33342 staining method outlined by Goodell et al. (20). Isolated colonic epithelial cells were suspended in DMEM at concentration of 106 cells/ml containing 2% FCS and 10 mM HEPES, and 2.5 μg/ml Hoechst 33342 was added, with or without prior addition of either 50 μM verapamil HCl (Sigma) or 10 μM of the specific ATP binding cassette (ABC) G2 blocker, fumitremorgin C (Axxora). After incubation in a shaking water bath (at 37°C) for 30 min, the cells were placed on ice to terminate dye efflux followed by a wash step in 2 ml cold HBSS containing 2% FCS.
The Hoechst dye was excited with a krypton ultraviolet laser at 337–356 nm, and its fluorescence was measured with a 465/30 BP filter (Hoechst Blue) and a 675 BP optical filter (Hoechst Red) using a Beckman Coulter Altra flow cytometer. Hoechst low side population cells were sorted and subsequently incubated with confluent monolayers of human colonic myofibroblasts (on glass coverslips) for 30 min, before gentle washing and fixation as described above. The cocultures were subsequently used for immunocytochemistry.
For phenotypic studies, the cells were labeled (for 30 min at 4°C, after staining with Hoechst) with the following antibodies: anti-BerEP4-FITC (Dako), anti-β1-integrin-PE (Beckmann Coulter), anti-CD133-PE (Miltenyi Biotec), or anti-CD34-ECD (Beckman Coulter). The cells were subsequently resuspended in cold HBSS containing 2% FCS and 10 mM HEPES and maintained at 4°C until analysis by flow cytometry. Before analysis, 2 μg/ml propidium iodide was added to exclude the dead cells.
The antibody-labeled side population cells were sorted into formaldehyde fixative and subsequently studied by flow cytometry using the blue laser for the surface markers. For each side population sample, a representative sample from the non-side population region was also collected and labeled with the above antibodies to aid a direct comparison in the levels of expression of the relevant markers. Side population and non-side population cells were also collected in media for cytospin preparations followed by fixation in 4% PFA/sucrose.
Statistical analysis.
Data are expressed as means ± SE and were analyzed by paired t-test.
RESULTS
Musashi-1 immunohistochemistry and characterization of isolated and disaggregated colonic crypt epithelial cells.
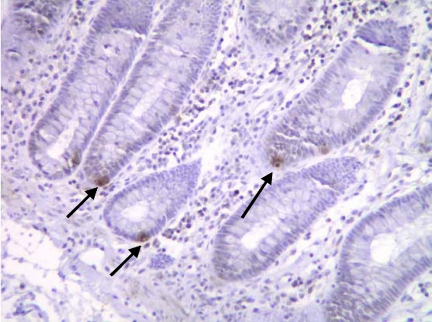
Immunohistochemical studies using a specific antibody showed individual musashi-1 positive cells at the crypt base in sections of normal colonic mucosa, in a position where putative intestinal stem cells are believed to be located (Fig. 1).
Fig. 1.
Musashi 1-expressing cells in a section of normal human colon (sigmoid). Immunoreactive cells are seen at the crypt base (arrowed).
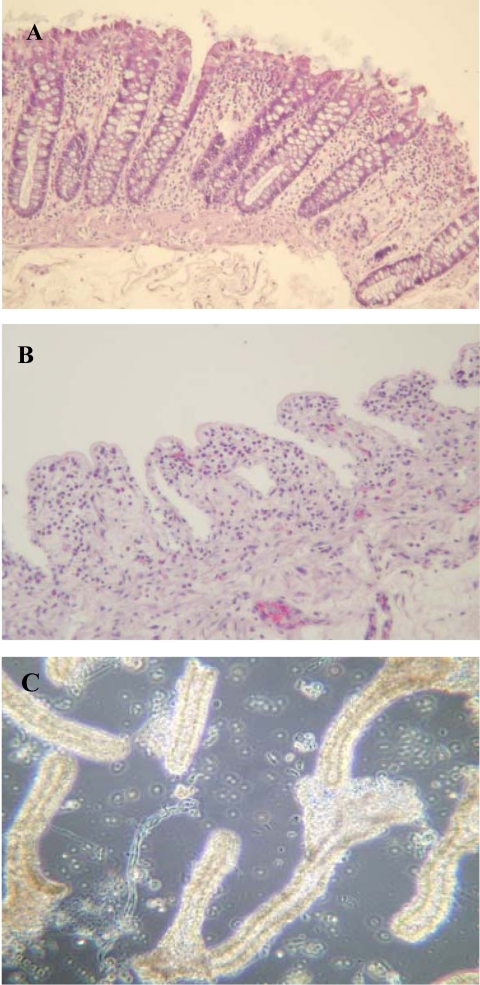
Treatment of mucosal samples (Fig. 2A) with EDTA released crypts (Fig. 2C), leaving behind empty crypt spaces (Fig. 2B). Following treatment with 0.25% pancreatin, the isolated colonic crypts disaggregated into predominantly single cells, which were used for further characterization. The average cell viability (assessed using Trypan blue) of the isolated and disaggregated crypt epithelial cells was 71.8 ± 1.8% (mucosal samples from colonic resection specimens from 64 individuals were used). In cytospin preparations, 90.5 ± 3.89% of these cells were immunoreactive for cytokeratin (Fig. 3A). Studies using anti-musashi-1 antibody showed only occasional (<1%) immunoreactive cells in these cytospin preparations (Fig. 3B). Studies by RT-PCR confirmed the presence of transcripts for musashi-1, Hes-1, DCAMKL-1, Lgr5, and Bmi-1 in our isolated and disaggregated colonic crypt epithelial cells (not shown).
Fig. 2.
Isolation of colonic crypts. Hematoxylin and eosin-stained section of normal colonic mucosa before (A; with intact epithelial crypts) and after (B) treatment with EDTA. Released colonic crypts are shown (by phase contrast microscopy; C).
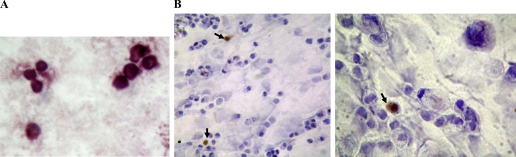
Fig. 3.
Cytokeratin (A) and musashi-1 (B) immunoreactivity in isolated and disaggregated colonic crypt epithelial cells. Colonic crypt epithelial cells were isolated from mucosal samples using EDTA and disaggregated with pancreatin. Cytospin preparations were subsequently used for immunocytochemistry. Musashi-1-immunoreactive cells (B) are arrowed.
Interactions between isolated, unfractionated colonic crypt epithelial cells, and monolayers of primary colonic myofibroblasts.
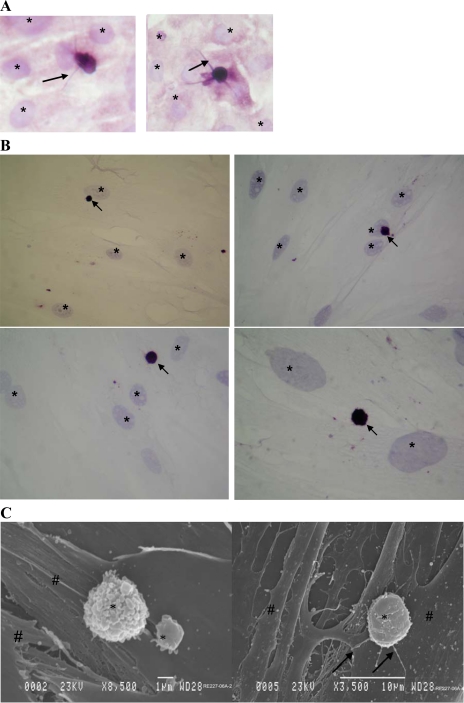
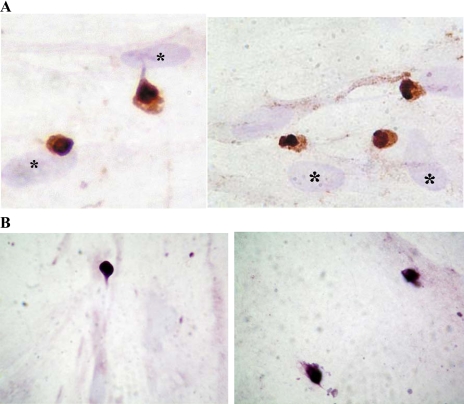
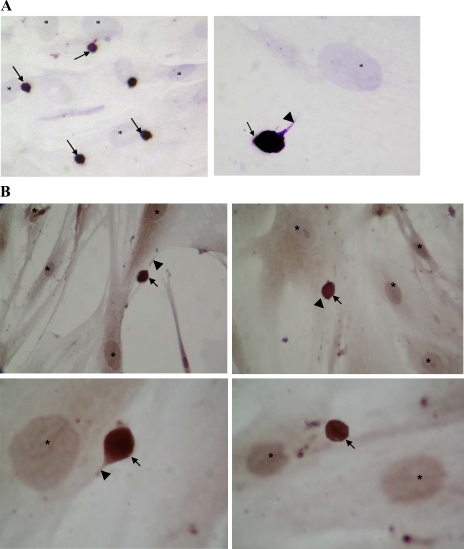
A small (<2%) proportion of the isolated and disaggregated colonic crypt epithelial cells rapidly (within 10 min) adhered to colonic myofibroblasts during culture (at 37°C). Immunocytochemical studies of epithelial-myofibroblast cocultures showed that the adherent epithelial cells were much smaller that the myofibroblasts (Fig. 4, A and B). Moreover, slender processes were seen emanating from a number of the epithelial cells (Fig. 4A). This finding was confirmed by scanning electron microscopy (Fig. 4C). Immunohistochemical studies also showed that many (58.9 ± 11.9%) of the colonic crypt epithelial cells that remained adherent to myofibroblasts were musashi-1 immunoreactive (Fig. 5A). These epithelial cells also showed DCAMKL-1 immunoreactivity (Fig. 5B). These epithelial cells maintained their morphology and adherence to the myofibroblasts for up to 5 days.
Fig. 4.
Isolated and disaggregated colonic crypt epithelial cells adherent to primary colonic myofibroblasts. The isolated crypt epithelial cells were applied to monolayers of primary colonic myofibroblasts, and following culture (at 37°C) for 10 min, the monolayers were washed and the cocultures were either fixed immediately (A) or after 24-h culture (B and C). Immunohistochemical studies were undertaken using anti-cytokeratin (A) or anti-BerEP4 (B) antibody. In A, slender processes of adherent cytokeratin immunostained epithelial cells are arrowed. In B, BerEP4 immunoreactive adherent epithelial cells are arrowed. Hematoxylin-stained nuclei of myofibroblasts (which are bigger than the adherent epithelial cells) are marked (*) in all of the figures. C: scanning electron micrographs of isolated colonic crypt epithelial cells (*) adherent to primary colonic myofibroblasts (#). Slender processes arising from the adherent epithelial cells are arrowed.
Fig. 5.
Expression of musashi-1 (A) and doublecortin and CaM kinase-like-1 (DCAMKL-1) (B) by colonic crypt epithelial cells adherent to colonic myofibroblasts. Isolated and disaggregated colonic crypt epithelial cells were applied to monolayers of primary colonic myofibroblasts and cultured for 30 min, followed by removal of nonadherent cells by washing. Immunocytochemical studies were undertaken on the cocultures using anti-musashi-1 (A) or anti-DCAMKL-1 (B) antibody. In A, musashi-1-expressing cells are stained brown and are seen close to the larger hematoxylin-stained nuclei of myofibroblasts (*).
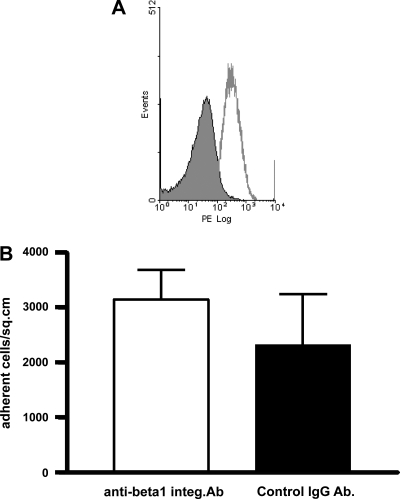
Flow cytometric analysis of the isolated and disaggregated crypt epithelial cells showed surface expression of β1-integrin [median fluorescence intensity (MFI): 18.7 ± 4.3; 77.9 ± 3.7% cells positive, n = 5; Fig. 6A]. The majority of the myofibroblast-adherent (studied after detachment) and nonadherent epithelial cells also expressed β1-integrin (MFI: 10.6 ± 1.7 and 13.2 ± 4.6, respectively). Prior incubation of the isolated crypt epithelial cells with blocking anti-β1-integrin antibody did not affect their adherence to myofibroblast monolayers (Fig. 6B). As expected, the blocking anti-β1-integrin antibody did inhibit adhesion of the isolated colonic crypt epithelial cells to collagen I (not shown).
Fig. 6.
Integrin-β1 expression by isolated and disaggregated colonic crypt epithelial cells (A) and effect of blocking integrin-β1 on the adherence of isolated colonic crypt epithelial cells to myofibroblasts (B). A: epithelial cells were labeled with phycoerythrin (PE)-conjugated anti-β1-integrin antibody (line without filling) or appropriate isotype control antibody (line with filling). The figure is representative of 5 experiments using cells isolated from colonic mucosal samples of different subjects. B: isolated and disaggregated colonic crypt epithelial cells were incubated (for 30 min) with anti-β1-integrin antibody or control antibody before coculture with monolayers of primary colonic myofibroblasts for 30 min. After washing, adherent cells were counted and are expressed per square centimeter. The figure represents data from 3 experiments.
Investigation of cells with side population characteristics in isolated colonic crypt epithelial cells.
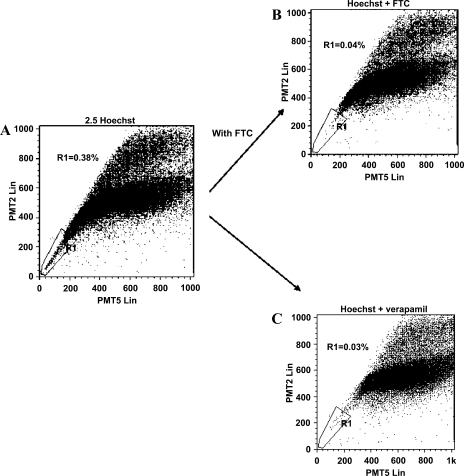
Flow cytometric analysis of Hoechst 33342-labeled isolated and disaggregated colonic crypt epithelial cells for blue and red fluorescence demonstrated a small population of cells with low fluorescence, implying active extrusion of the dye (Fig. 7A). The side population characteristics of the majority (81.7 ± 2.7%; cells isolated from colonic resection specimens from 27 individuals) of these cells with low fluorescence was confirmed by “normal fluorescence” (Fig. 7, B and C) following treatment with inhibitors of ABC transporters verapamil and fumitremorgin C (under these conditions, the cells are not able to expel the dye). Using inhibitors of ABC transporters for characterization, 0.7 ± 0.3% of the total disaggregated colonic crypt epithelial cells (isolated from colonic resection specimens from 27 individuals) demonstrated side population characteristics.
Fig. 7.
Cells with side population characteristics in an isolated colonic crypt epithelial cell preparation. Following isolation and disaggregation, the colonic crypt epithelial cells were stained with Hoechst 33342 for 30 min, in the absence (A) or presence of fumitremorgin C (B) or verapamil (C). Subsequent analysis was undertaken by flow cytometry using ultraviolet laser. A small population of cells with side population characteristics (as demonstrated by low staining) is shown in region R1 (A), which is largely lost in the presence of inhibitors of the ATP binding cassette (ABC) transporters fumitremorgin C (B) and verapamil (C). The figure is representative of experiments using cells isolated from colonic mucosal samples of 27 subjects.
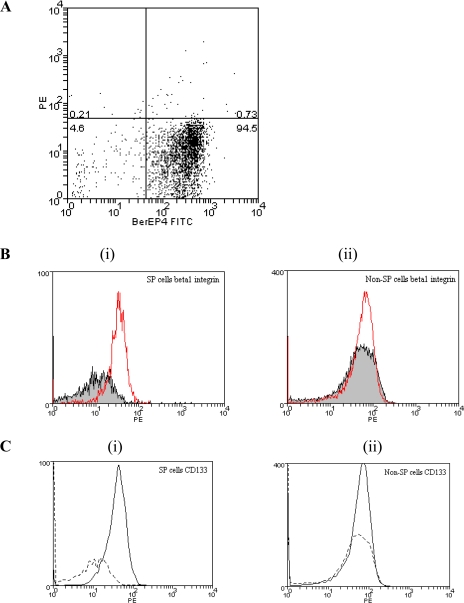
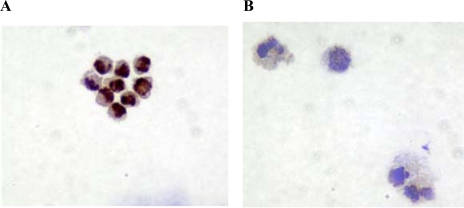
Further characterization of the side population cells was undertaken after separating (sorting) them in a flow cytometer. Subsequent analyses showed that the majority (82.3 ± 8.2%) of the side population cells were labeled by an antibody to the epithelial cell-specific marker BerEP4 (Fig. 8A). Comparison between BerEP4 positive side population and non-side population cells showed that a greater proportion of the former cells expressed β1-integrin (32.7 ± 4.7% vs. 5.3 ± 2.6 %, P < 0.05; Fig. 8B). The BerEP4-positive side population cells also expressed CD133 (Fig. 8C). However, none of these cells were labeled with an antibody to CD34 (not shown), a hematopoetic stem cell marker.
Fig. 8.
Characterization of side population cells by flow cytometry. A: BerEP4 status of sorted colonic crypt epithelial cells with side population characteristics. Side population cells (see Fig. 7) from preparations of isolated and disaggregated colonic crypt epithelial cells were labeled with FITC-conjugated anti-BerEP4 antibody. The figure is representative of 3 experiments. B: β1-integrin expression by side population cells (Bi), but not non-side population cells (Bii). Cells with side population characteristics were sorted from preparations of isolated and disaggregated colonic crypt epithelial cells. Side population and non-side population cells were labeled with FITC-conjugated anti-BerEP4 and PE-conjugated anti-β1-integrin (and their respective isotype control) antibodies. The histograms show median fluorescence intensity of BerEP4-positive (gated) side population (Bi) and non-side population (Bii) cells labeled with PE-conjugated anti-β1-integrin antibody (line without filling) or isotype control antibody (line with filling). The figure is representative of experiments undertaken using cells isolated from mucosal samples of 3 subjects. C: CD133 expression by side population (Ci) but not non-side population (Cii) cells. Cells with side population characteristics were sorted from preparations of isolated and disaggregated colonic crypt epithelial cells. Side population and non-side population cells were labeled with FITC-conjugated anti-BerEP4 and PE-conjugated anti-CD133 (and their respective isotype control) antibodies. The histograms show median fluorescence intensity of BerEP4 positive (gated) side population (Ci) and non-side population (Cii) cells labeled with PE-conjugated anti-CD133 antibody (solid black line) or isotype control antibody (dashed line). The figure is representative of experiments undertaken using cells isolated from mucosal samples of 3 subjects.
Musashi-1 expression by the sorted side population cells (Fig. 9) was demonstrated by immunocytochemistry in cytospin preparations (66.1 ± 7.0% of side population cells were musashi-1 immunoreactive compared with 9.9 ± 4.8% of non-side population cells). The sorted side population cells (which are small in size) were also able to adhere to monolayers of colonic myofibroblasts (Fig. 10, A and B).
Fig. 9.
Musashi-1 expression by side population cells (A) but not non-side population cells (B). Cytospins of sorted side population (A) and non-side population (B) fractions of isolated and disaggregated colonic crypt epithelial cells were used for immunocytochemistry using anti-musashi-1 antibody. In contrast to non-side population cells, the side population cells are strongly immunoreactive.
Fig. 10.
Colonic crypt epithelial side population cells can adhere to colonic myofibroblasts. Sorted side population cells were cultured on top of colonic myofibroblasts for 30 min. After being washed, cocultures were fixed and used for immunocytochemistry using anti-BerEP4 (A) or anti-musashi-1 (B) antibody. Strongly BerEP4 (A) and musashi-1 (B) immunoreactive side population cells (arrowed) are seen, a number with prominent processes (arrowhead). These side population cells are smaller than the nuclei of myofibroblasts (*).
DISCUSSION
There has recently been significant interest in the identification of markers of epithelial stem cells in the small intestine (3, 26, 32, 39, 43). While there is ongoing debate regarding the position (at the crypt base and/or above Paneth cells) of stem cells in the small intestine, musashi-1 is expressed by cells in both locations (26, 39). Although less well studied, stem cells in the colon are believed to be located at the crypt base where they also express musashi-1 (26, 39) (as also demonstrated in the present study). We found that our isolated and disaggregated crypt cells also expressed transcripts for Lgr5, DCAMKL-1 and Bmi-1, which are the other recently described markers of intestinal stem cells (3, 32, 43).
Specific markers for stem cells may enable their identification following isolation, and cell surface markers may also facilitate their purification. However, the above markers are expressed intracellularly and therefore can only be used to identify isolated stem cells. Cells with stem cell properties have also been characterized from mixed populations of cells isolated from different tissue sources, based on the ability of the progenitor cells to efflux the DNA-binding dye Hoechst 33342 (20, 21, 27, 36, 49). The so-called side population, which is Hoechst low in the absence of inhibitors of ABC transporters, can be identified and sorted by flow cytometry, and this opens up the potential to further analyze and purify these putative stem cells. The ability of stem cells to preferentially adhere to extracellular matrix, due to high surface integrin expression, may also be exploited for their separation (24). Colonic epithelial stem cells are normally adherent to the basement membrane, components of which are synthesized by myofibroblasts that lie immediately below the epithelium (1, 29). We therefore undertook studies to determine whether colonic stem cells may preferentially adhere to either the myofibroblasts or their secreted extracellular matrix.
As would be expected from the immunohistochemical studies in tissue sections, our preparations of disaggregated colonic crypt epithelial cells contained only occasional musashi-1-immunoreactive cells. However, of the small fraction of the disaggregated crypt epithelial cells that adhered rapidly to monolayers of primary human colonic myofibroblasts, a large proportion was immunoreactive for musashi-1 and also DCAMKL-1. This suggests that musashi-1- and DCAMKL-1-expressing crypt epithelial cells preferentially adhere to intestinal myofibroblasts and this property can enable enrichment of these cells. It is also of interest that the adherent epithelial cells were consistently seen to be much smaller in size than the myofibroblasts. We are not aware of previous reports describing such a finding for putative colonic epithelial stem cells; however, human ocular side population cells with clonogenic potential have been reported to contain a high proportion of cells with diameters smaller than 10 μm (11).
We also confirmed a previous report regarding the expression of β1-integrin on the surface of human intestinal crypt epithelial cells (19). While a majority of the isolated and disaggregated crypt epithelial cells expressed β1-integrin, we were surprised to find that a lower proportion of side population cells expressed this adhesion molecule. One possible reason for this finding is that further manipulation of the isolated and disaggregated crypt epithelial cells may have led to the loss of this integrin. Further studies are required to investigate this possibility.
Our previous studies have shown that primary human colonic myofibroblasts express the extracellular matrix proteins collagen type IV, laminin-β1 and -γ1, and fibronectin (29). Recently, we have also confirmed studies by Andoh et al. (1) that these cells express collagen I (not shown). Further studies were therefore undertaken to determine whether adhesion of the disaggregated crypt epithelial cells (to myofibroblasts) was mediated via β1-integrin. However, blocking anti-β1-integrin antibody did not affect adherence of the disaggregated crypt epithelial cells to monolayers of colonic myofibroblasts.
Although the mechanism(s) by which isolated musashi-1-expressing intestinal crypt epithelial cells adhere to intestinal myofibroblasts in vitro remain to be determined, recent studies suggest that β1-integrin may not play a major role in the adhesion of intestinal crypt epithelial cells to extracellular matrix. Thus, conditional deletion of β1-integrins in the murine intestinal epithelium did not result in decreased cell adhesion and proliferation but instead caused a marked increase in epithelial proliferation and dysplasia (25). Using conditional deletion of β1-integrins in murine skin and mammary epithelia, previous studies had demonstrated the importance of β1-integrin-mediated epithelial cell adhesion in these tissues in vivo (8, 17, 42). Thus, there are significant differences between epithelial cells of the intestine and those of the skin and mammary glands in the mechanisms by which adhesion to extracellular matrix occurs in vivo.
To further characterize cells with properties of stem cells in our isolated and disaggregated colonic crypt epithelial cells, their ability to actively expel the dye Hoechst 33342 was investigated. We found that a mean 0.7% of the isolated and disaggregated human colonic crypt epithelial cells demonstrated side population characteristics. A small proportion (1–2% of the total viable cells) of isolated murine jejunal epithelial cells have recently also been reported to show side population characteristics (15). In our studies, the majority of the side population cells expressed the epithelial cell-specific marker BerEP4, musashi-1, and a significant proportion also expressed β1-integrin and CD133. Recently, colon cancer stem cells have also been shown to express CD133 (37). Thus, we believe that the musashi-1-positive isolated and disaggregated human colonic crypt epithelial cells with side population characteristics are stem cells. Our studies also suggest that these side population cells have the ability to rapidly adhere to primary colonic myofibroblasts and they may subsequently remain viable for a number of days. The adherent side population cells were much smaller than the myofibroblasts, as was the case for epithelial cells in unfractionated disaggregated colonic crypt cells that also adhered to the myofibroblasts. This similarity in size, together with expression of musashi-1, suggests that the majority of the disaggregated colonic crypt epithelial cells with the capacity to rapidly adhere to myofibroblasts are stem cells.
The presence of stem cells in isolated and disaggregated human colonic crypt epithelial cells is supported by studies by Whitehead and colleagues (19, 48), who used conditioned medium from a colon carcinoma cell line to demonstrate clonogenic growth from single-cell suspension. It is anticipated that future in vitro studies using isolated adult intestinal stem cells will facilitate investigation of the contribution of not only myofibroblasts and related cells (10, 28) but also other cellular components (41) of the stem cell niche in regulating the progenitor cell function.
GRANTS
This study was supported by the Medical Research Council.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A, Shimizu N, Fujiyama Y. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 129: 969–984, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj-Elliott M, Breese E, Poulsom R, Fairclough PD, MacDonald TT. Keratinocyte growth factor in inflammatory bowel disease. Increased mRNA transcripts in ulcerative colitis compared with Crohn's disease in biopsies and isolated mucosal myofibroblasts. Am J Pathol 151: 1469–1476, 1997. [PMC free article] [PubMed] [Google Scholar]
- 3.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Beltinger J, McKaig BC, Makh S, Stack WA, Hawkey CJ, Mahida YR. Human colonic subepithelial myofibroblasts modulate transepithelial resistance and secretory response. Am J Physiol Cell Physiol 277: C271–C279, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Bjerknes M, Cheng H. Gastrointestinal stem cells. II. Intestinal stem cells. Am J Physiol Gastrointest Liver Physiol 289: G381–G387, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell 128: 445–458, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Booth C, Potten CS. Gut instincts: thoughts on intestinal epithelial stem cells. J Clin Invest 105: 1493–1499, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, Svensson M, Herken R, Sasaki T, Timpl R, Werner S, Fassler R. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J 19: 3990–4003, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brittan M, Wright NA. Stem cell in gastrointestinal structure and neoplastic development. Gut 53: 899–910, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, Stenson WF, Stappenbeck TS. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest 117: 258–269, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budak MT, Alpdogan OS, Zhou M, Lavker RM, Akinci MA, Wolosin JM. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J Cell Sci 118: 1715–1724, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clevers H Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet 7: 349–359, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Cunliffe RN, Rose FR, Keyte J, Abberley L, Chan WC, Mahida YR. Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut 48: 176–185, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dekaney CM, Rodriguez JM, Graul MC, Henning SJ. Isolation and characterization of a putative intestinal stem cell fraction from mouse jejunum. Gastroenterology 129: 1567–1580, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Di Mari JF, Saada JI, Mifflin RC, Valentich JD, Powell DW. HETEs enhance IL-1-mediated COX-2 expression via augmentation of message stability in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol 293: G719–G728, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Faraldo MM, Deugnier MA, Thiery JP, Glukhova MA. Growth defects induced by perturbation of beta1-integrin function in the mammary gland epithelium result from a lack of MAPK activation via the Shc and Akt pathways. EMBO Rep 2: 431–437, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell 116: 769–778, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto K, Beauchamp RD, Whitehead RH. Identification and isolation of candidate human colonic clonogenic cells based on cell surface integrin expression. Gastroenterology 123: 1941–1948, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 183: 1797–1806, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401: 390–394, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Hata K, Andoh A, Shimada M, Fujino S, Bamba S, Araki Y, Okuno T, Fujiyama Y, Bamba T. IL-17 stimulates inflammatory responses via NF-κB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol 282: G1035–G1044, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet 24: 36–44, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73: 713–724, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Jones RG, Li X, Gray PD, Kuang J, Clayton F, Samowitz WS, Madison BB, Gumucio DL, Kuwada SK. Conditional deletion of beta1 integrins in the intestinal epithelium causes a loss of Hedgehog expression, intestinal hyperplasia, and early postnatal lethality. J Cell Biol 175: 505–514, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayahara T, Sawada M, Takaishi S, Fukui H, Seno H, Fukuzawa H, Suzuki K, Hiai H, Kageyama R, Okano H, Chiba T. Candidate markers for stem and early progenitor cells, Musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett 535: 131–135, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Kim M, Morshead CM. Distinct populations of forebrain neural stem and progenitor cells can be isolated using side-population analysis. J Neurosci 23: 10703–10709, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosinski C, Li VS, Chan AS, Zhang J, Ho C, Tsui WY, Chan TL, Mifflin RC, Powell DW, Yuen ST, Leung SY, Chen X. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci USA 104: 15418–15423, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahida YR, Beltinger J, Makh S, Goke M, Gray T, Podolsky DK, Hawkey CJ. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol Gastrointest Liver Physiol 273: G1341–G1348, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Mahida YR, Wu KC, Jewell DP. Respiratory burst activity of intestinal macrophages in normal and inflammatory bowel disease. Gut 30: 1362–1370, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathan M, Hermos JA, Trier JS. Structural features of the epithelio-mesenchymal interface of rat duodenal mucosa during development. J Cell Biol 52: 577–588, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells 26: 630–637, 2008. [DOI] [PubMed] [Google Scholar]
- 33.McKaig BC, Makh SS, Hawkey CJ, Podolsky DK, Mahida YR. Normal human colonic subepithelial myofibroblasts enhance epithelial migration (restitution) via TGF-β3. Am J Physiol Gastrointest Liver Physiol 276: G1087–G1093, 1999. [DOI] [PubMed] [Google Scholar]
- 34.McKaig BC, McWilliams D, Watson SA, Mahida YR. Expression and regulation of tissue inhibitor of metalloproteinase-1 and matrix metalloproteinases by intestinal myofibroblasts in inflammatory bowel disease. Am J Pathol 162: 1355–1360, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mills JC, Gordon JI. The intestinal stem cell niche: there grows the neighborhood. Proc Natl Acad Sci USA 98: 12334–12336, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montanaro F, Liadaki K, Volinski J, Flint A, Kunkel LM. Skeletal muscle engraftment potential of adult mouse skin side population cells. Proc Natl Acad Sci USA 100: 9336–9341, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445: 106–110, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Paterson JC, Watson SH. Paneth cell metaplasia in ulcerative colitis. Am J Pathol 38: 243–249, 1961. [PMC free article] [PubMed] [Google Scholar]
- 39.Potten CS, Booth C, Tudor GL, Booth D, Brady G, Hurley P, Ashton G, Clarke R, Sakakibara S, Okano H. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation 71: 28–41, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts II. Intestinal subepithelial myofibroblasts. Am J Physiol Cell Physiol 277: C183–C201, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA 102: 99–104, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol 150: 1149–1160, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40: 915–920, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology 134: 849–864, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki K, Fukui H, Kayahara T, Sawada M, Seno H, Hiai H, Kageyama R, Okano H, Chiba T. Hes1-deficient mice show precocious differentiation of Paneth cells in the small intestine. Biochem Biophys Res Commun 328: 348–352, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111: 241–250, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Walker MR, Stappenbeck TS. Deciphering the ‘black box’ of the intestinal stem cell niche: taking direction from other systems. Curr Opin Gastroenterol 24: 115–120, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Whitehead RH, Demmler K, Rockman SP, Watson NK. Clonogenic growth of epithelial cells from normal colonic mucosa from both mice and humans. Gastroenterology 117: 858–865, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Yamahara K, Fukushima S, Coppen SR, Felkin LE, Varela-Carver A, Barton PJ, Yacoub MH, Suzuki K. Heterogeneic nature of adult cardiac side population cells. Biochem Biophys Res Commun 371: 615–620, 2008. [DOI] [PubMed] [Google Scholar]