Abstract
The vectorial transport of ions and water across epithelial cells depends to a large extent on the coordination of the apical and basolateral ion fluxes with energy supply. In this work we provide the first evidence for a regulation by the 5′-AMP-activated protein kinase (AMPK) of the calcium-activated potassium channel KCa3.1 expressed at the basolateral membrane of a large variety of epithelial cells. Inside-out patch-clamp experiments performed on human embryonic kidney (HEK) cells stably transfected with KCa3.1 first revealed a decrease in KCa3.1 activity following the internal addition of AMP at a fixed ATP concentration. This effect was dose dependent with half inhibition at 140 μM AMP in 1 mM ATP. Evidence for an interaction between the COOH-terminal region of KCa3.1 and the γ1-subunit of AMPK was next obtained by two-hybrid screening and pull-down experiments. Our two-hybrid analysis confirmed in addition that the amino acids extending from Asp380 to Ala400 in COOH-terminal were essential for the interaction AMPK-γ1/KCa3.1. Inside-out experiments on cells coexpressing KCa3.1 with the dominant negative AMPK-γ1-R299G mutant showed a reduced sensitivity of KCa3.1 to AMP, arguing for a functional link between KCa3.1 and the γ1-subunit of AMPK. More importantly, coimmunoprecipitation experiments carried out on bronchial epithelial NuLi cells provided direct evidence for the formation of a KCa3.1/AMPK-γ1 complex at endogenous AMPK and KCa3.1 expression levels. Finally, treating NuLi monolayers with the membrane permeant AMPK activator 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) caused a significant decrease of the KCa3.1-mediated short-circuit currents, an effect reversible by coincubation with the AMPK inhibitor Compound C. These observations argue for a regulation of KCa3.1 by AMPK in a functional epithelium through protein/protein interactions involving the γ1-subunit of AMPK.
Keywords: potassium channel, protein-protein interactions, cystic fibrosis
5′-amp-activated protein kinase (AMPK) is an ubiquitously expressed metabolic-sensing Ser/Thr kinase that plays a key role in the energy homeostasis of cells (26). AMPK exists as a heterotrimer constituted of a catalytic α-subunit with two regulatory subunits β and γ encoded by distinct genes (α1, α2, β1, β2, γ1, γ2, γ3). AMPK activity is largely controlled by upstream kinases such as LKB1 and calmodulin-dependent protein kinase kinase (CaMKKβ), which phosphorylate the AMPK-α subunit at Thr172 (21). It was recently reported that AMP contributes to maintain the AMPK-α subunit in an active phosphorylated state by inhibiting AMPK dephosphorylation at Thr172 (19, 20, 36). AMP can also induce an allosteric conformational change of the α-subunit leading to an increase in kinase activity (19, 20, 22, 23). Several lines of evidence point to the γ-subunit as the major site for AMP allosteric control of AMPK (9). The solved structure of the mammalian AMPK indeed confirmed the presence of two exchangeable AMP/ATP sites on opposite sides of the γ-subunit (49), in accordance with a control of the AMPK activity determined by the AMP-to-ATP ratio (19).
There is now strong evidence that AMPK plays a prominent role in coupling the transepithelial transport of ions and water in several epithelium preparations to the metabolic state of the cells. AMPK was found, for instance, to bind to the COOH-terminal tail of fibrosis transmembrane conductance regulator (CFTR) and decrease the channel open probability (17). AMPK activation was similarly reported to decrease epithelial Na channel (ENaC) currents by downregulating the number of active channels at the plasma membrane thus reducing excessive salt and water reabsorption in metabolic stress conditions (4, 7, 15). This effect was subsequently attributed to an increase by AMPK of the Nedd4-2-dependent ENaC retrieval from the plasma membrane. In addition to CFTR and ENaC, the vectorial transport of ions in airway epithelial cells also depends on the activation of K+ channels, including the Ca2+-activated K+ channel of intermediate conductance KCa3.1 also known as KCNN4, IK1, or SK4. This channel consists in a tetrameric protein with each subunit organized in six transmembrane segments. The channel Ca2+ sensitivity is conferred by the Ca2+-binding protein calmodulin (CaM), which is constitutively bound to KCa3.1 in the COOH-terminus (31). KCa3.1 activation by potentiators such as 1-ethyl-2-benzimidazolinone (EBIO) and 4-chloro-benzo[F] isoquinoline (CBIQ) was found to stimulate Cl− secretion and Na+ absorption in several epithelial cell preparations, including colonic epithelia, T84, Calu-3, and human bronchial cells (10, 30, 40, 43, 45). These observations led to conclude that KCa3.1 channels could play a prominent role in transepithelial transport by establishing a suitable driving force to maintain a sustained Cl− efflux and Na+ influx at the apical membrane of epithelial cells (3, 12, 32). KCa3.1 is also regulated by ATP, suggesting a potential link between metabolic stress and KCa3.1 activity. Recent studies have shown that internal ATP stimulates the human KCa3.1 channel activity through the phosphorylation by the nucleoside diphosphate kinase NDPK-B of a histidine residue located within the channel COOH-terminal region (42). The action of ATP required Ca2+ and resulted in an apparent greater Ca2+ sensitivity (41). PKA has also been documented to regulate the human KCa3.1 channel, but it is currently believed that KCa3.1 is not itself a target of PKA because mutations of the PKA-consensus sites in the human KCa3.1 affected neither the basal nor the ATP-activated current (13, 14). Altogether, these observations point toward a complex ATP-dependent regulation of the KCa3.1 channel activity that could involve multiple intracellular sites and/or several ATP-sensitive auxiliary proteins.
Despite the importance of KCa3.1 in modulating Cl− efflux and Na+ influx in epithelial cells, there is currently no evidence for KCa3.1 being regulated by AMPK. In this work we show a downregulation of KCa3.1 activity in response to an increase in AMP concentration, an effect we interpret as coming from an interaction between the COOH-terminal region of KCa3.1 and the AMP-binding γ1-subunit of AMPK. Therefore, our results point toward a global modification by AMPK of the ion transport properties in Cl− secreting epithelia not exclusively mediated by CFTR and ENaC but that also includes a contribution of the KCa3.1 channel.
MATERIALS AND METHODS
Cell cultures.
HEK cells were cultured in Dulbecco's high-glucose minimum essential medium (DMEM-HG) supplemented with 2.2g/l NaHCO3, 10% FBS, penicillin, and streptomycin under 5% CO2 atmosphere at 37°C. HEK-293 cells transfected with the KCa3.1 channel tagged in COOH-terminal with the Myc epitope (HIK cells) were kindly supplied by Dr. Daniel C. Devor, University of Pittsburgh, and grown in the presence of 0.1 mg/ml zeocin. Hemagglutinin (HA)-tagged KCa3.1 channels (HA-KCa3.1) were generated by inserting the HA epitope tag (YPYDVPDYA) into the channel second extracellular loop between G132 and A133 as described elsewhere (25). HA-KCa3.1 cells were obtained by transfecting HEK-293 cells with HA-KCa3.1 cDNA cloned into the pCMV-Tag5 vector (Stratagene, LaJolla, CA) using Lipofectamine 2000 (Invitrogen, Burlington, ON). Similarly, HIK+AMPK-γ1-R299G cells were obtained by transfecting HIK cells using Lipofectamine 2000 with AMPK-γ1-R299G cloned in pCMV-Tag5. Stable cell lines were generated by antibiotic selection 48 h posttransfection (0.4 mg/ml G418 for HA-KCa3.1 and 0.4 mg/ml G418 plus 0.1 mg/ml zeocin for HIK+AMPK-γ1-R299G cells). Selection was typically complete within 14 days after transfection.
For immunofluorescence, HA-KCa3.1 cells were seeded at 105 cells on poly-d-lysine hydrobromide (Sigma-Aldrich, Oakville, ON)-coated microscope cover glass (22 mm diameter, Fisher Scientific, Ottawa, ON) and grown in 35-mm plates for 4 days. An identical procedure was applied for HIK cells used in patch-clamp experiments.
Bronchial epithelial NuLi-1 (Normal Lung) cells were kindly provided by Dr. J. Zabner, University of Iowa. The NuLi-1 line was obtained by transformation of human airway epithelia of normal genotype, with a reverse transcriptase component of telomerase (hTERT) and human papillomavirus type 16 (HPV-16) E6 and E7 genes (50). The cells were cultured from passages 11 up to 17 on plastic support in presence of bronchial epithelial cell growth medium (BEGM) supplemented with hydrocortisone, bovine pituitary extract, epidermal growth factor (EGF), transferrin, bovine insulin, triiodothyronin, epinephrine, and retinoic acid (Cambrex Biosciences, Walkersville, MD). For electrophysiological experiments, NuLi cells were grown on Costar Transwell permeant filters (Costar Transwell, Toronto, ON) at the air-liquid interface in DMEM-F12 medium (Invitrogen) supplemented with UltroSerG (Biosepra SPA, Cergy-Saint-Christophe, France), which enhances ion transport (50). During the culture procedure the medium coming from the basolateral side, which appeared at the apical surface, was removed every 2–3 days, and fresh medium was added at the basolateral side. After 4 to 6 wk of culture, the formation of an air-liquid interface (luminal face exposed to air while the basolateral side was bathed in culture medium) was taken as an indication that NuLi monolayers were confluent and could form a polarized, differenciated bronchial epithelium. The mean resistances (R) and short-circuit currents (Isc) measured in Ussing chamber were under those conditions equal to 813 ± 92 Ω·cm2 and 25 ± 2 μA/cm2 (n = 23), respectively. These values are similar to that reported previously by Zabner et al. (52).
Bacteriophage two-hybrid analysis.
Two-hybrid analysis was performed using the BacterioMatch II Two-Hybrid System (Stratagene). The COOH-terminal segment of KCa3.1 extending from Leu345 to Ala400, which was used as the bait, was inserted in the frame into the pBT vector and tested against a cDNA HeLa library cloned into pTRG vector. The choice of the library was based on previous studies which showed that KCa3.1 is highly expressed in HeLa cells (37). Transformants (1–2 × 107) from HeLa cDNA library were screened, and the colonies were obtained from the initial screening plates enriched on a second selective plate. Putative positive colonies were validated using streptomycin resistance as a secondary reporter. The nucleotide sequence of the target DNA was determined (BioS&T, Lachine, Quebec, Canada) and tested against nucleotide sequence databases to identify related proteins. We verified the specificity of the interactions by one-on-one two-hybrid analysis and cotransformed the reporter strain using each purified target plasmid paired with the recombinant pBT plasmid.
Purification of recombinant GST-KCa3.1 fusion protein and affinity pull-down assays with immobilized GST fusion proteins.
A cDNA construct encoding the COOH-terminal glutathione S-transferase GST-KCa3.1 fusion protein was created by subcloning the human KCa3.1 cDNA (from Arg330 to Lys427) into the GST fusion protein vector pGEX-4T-1 (Amersham Biosciences, Piscataway, NJ). After sequencing was completed, GST and GST-KCa3.1 constructs were transformed into BL21(DE3) bacteria competent cells (Novagen, San Diego, CA), induced with isopropyl-1-thio-d-galactopyranoside (IPTG 0.2 mM) for 3 h, and lysed according to MagneGST Protein kit (Promega). The quality of the fusion proteins was assessed with Coomassie staining and tested by Western blot analysis using a rabbit anti-KCa3.1 antibody (1:200, Sigma) that was detected with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:10,000, Jackson ImmunoResearch Laboratories, West Grove, PA) as secondary antibody.
For the affinity pull-down assay, GST-KCa3.1 fusion proteins (from Arg330 to Lys427) and GST alone (50 μg each) were incubated overnight at 4°C in the presence of 1 mg of HIK cell lysates. Lysates were obtained by vortexing cells in a buffer containing 50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P40, plus 0.5% SDS complete protease inhibitor mixture (Roche Applied Science, Laval, Quebec, Canada), and centrifuged at 100,000 g for 45 min at 4°C to prepare a high-speed supernatant. Proteins from HIK cells (purified as described above) were incubated onto MagneGST-COOH-terminal KCa3.1 particles overnight at 4°C, washed twice in a phosphate buffer containing (in mM) 4.2 Na2HPO4, 2 KH2PO4, 140 NaCl, and 10 KCl, and eluted with 2× SDS sample buffer for 3 min at 85°C. AMPK-γ1 subunit pulled down by the GST-KCa3.1 fusion protein were detected by Western blot analysis. After protein transfer to nitrocellulose membrane (Hybond, Amersham Biosciences), the membrane was blocked with 5% nonfat milk in TBS containing 0.1% Tween-20 and incubated overnight at 4°C with rabbit rabbit anti AMPK-γ1 antibody (1:2,000, Invitrogen). The membrane was then washed three times with TBS containing 0.1% Tween, and the secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit IgG; Jackson ImmunoResearch Laboratories) was added at 1:20,000 dilution for 1 h at room temperature. Immunoreactive bands were visualized using ECL detection reagent (Pierce, Rockford, IL) and exposed on X-ray films.
Coimmunoprecipitation of KCa3.1 and γ1-subunit of AMPK from NuLi cells.
Lysis of NuLi cells was performed with buffer 1 (150 mM NaCl, 50 mM Tris·HCl, pH 7.5, 1% Nonidet P40, and 0.5% sodium deoxycholate) from the immunoprecipitation kit (protein A, Roche Applied Science) following the manufacturer's instructions. The lysates were homogenized and isolated by centrifugation as described previously. After quantification using the Bradford method, 1–2 mg soluble lysate was precleared with 50 μl of 50% protein A-agarose suspension. For immunoprecipitation of endogenous KCa3.1 or AMPK-γ1 proteins, precleared soluble lysates were incubated for 1–2 h with a rabbit anti-KCa3.1 antibody (1:100, Alomone Labs, Jerusalem, Israel) or with a rabbit anti-AMPK-γ1 antibody (1:100 or 1:150, Abcam, Cambridge, MA). The immunocomplexes were precipitated by incubating overnight at 4°C with 50 μl of 50% protein A-agarose suspension. After being washed twice with buffer 1 and buffer 2 (high salt, 500 mM NaCl, 50 mM Tris·HCl, pH 7.5, 0.1% Nonidet P40, 0.05% sodium deoxycholate) and once with buffer 3 (low salt, 10 mM Tris·HCl, pH 7.5, 0.1% Nonidet P40, 0.05% sodium deoxycholate), proteins bound to beads were collected by centrifugation and eluted by 30–50 μl of 2× sample buffer as described for the pull-down assays at 95°C for 5 min. The immunoprecipitated proteins were resolved on a 12% SDS-PAGE gel and revealed by Western blot analysis. Background from heavy and light immunoglobulin chains, which migrate at ∼58 and 25 kDa, respectively, was eliminated by blocking membranes with IP/Western blot ReliaBLOT reagents (Bethyl Laboratories, Montgomery, TX) overnight. The membranes were then incubated in ReliaBLOT with either rabbit anti-AMPK-γ1 antibody (1:500 or 1:1,000, Abcam) or with rabbit anti-KCa3.1 antibody (Sigma, 1:300 or 1:1,000) for 18 h. The specificity of the antibodies was verified with their respective blocking peptides. For AMPK-γ1 the blocking peptide was synthesized by BioS&T based on the sequence of the peptide used as immunogene to generate the AMPK-γ1 antibody (Abcam).
Immunofluorescence experiments.
For immunodetection, HA-KCa3.1 HEK-293 cells were fixed 15 min with paraformaldehyde 4% in PBS (in mM: 140 NaCl, 2.7 KCl, 10 Na2HPO4, 1.8 KH2PO4) at room temperature and permeabilized with 0.2% Triton-X-100 for 10 min. To block nonspecific binding sites, the cells were incubated 30 min in a PBS solution containing (in mM) 0.1 CaCl2, 1.0 MgCl2 (PBS/CM) supplemented with 1% BSA and 4% normal goat serum (NGS). The cells were then incubated for 5 min with the same solution containing 0.4% NGS and incubated for 1 h at room temperature with mouse monoclonal anti-HA antibody (1:25, Covance). After this procedure, the cells were rinsed three times with PBS/CM and incubated twice with the washing solution for 5 min. Finally, the cells were incubated with anti-mouse antibody conjugated to AlexaFluor488 (1:1,000, Invitrogen) for 45 min at room temperature. After rinsing cells were incubated with rabbit anti-AMPK-γ1 antibody (1:10, Abcam) and anti-rabbit antibody conjugated to AlexaFluor594 (Invitrogen) as described for the AlexaFluor488 protocol. The cells were rinsed three times for 5 min with PBS/CM and mounted on coverslips with MoWiol 4–88 Reagent (Calbiochem, San Diego, CA) overnight at 4°C. Negative controls were done by incubating the cells with PBS-CM containing neither KCa3.1 nor AMPK-γ1 primary antibodies. Confocal experiments were carried out at the imaging facilities in the Department of Cell Physiology at the Université de Montréal. Confocal images of labeled cells were acquired with a Leica inverted microscope DMIRBE using a ×40 oil immersion lens. Image analysis was performed with the Leica confocal software V2.61.
Patch-clamp experiments on HEK cells.
Inside-out patch-clamp experiments were performed as described previously (27). Data acquisition was performed using a Digidata 1320A acquisition system (Molecular Device, Union City, CA) at a sampling rate of 2 kHz with filtering set at 1 kHz. The percentage of current inhibition by AMP was calculated as the ratio 100 × (IATP − IAMP)/IATP, with IATP as the mean current estimated over a 5-s period 90 s after the initial addition of ATP (1 mM) to the bathing solution, and IAMP as the mean current measured over a 5-s period 2 min after the addition of AMP at a given test concentration. The mean current values were calculated using the QuB software (35). The pipette solution contained (in mM) 145 K-gluconate, 5 KCl, 2.5 MgCl2, 1 EGTA, and 10 HEPES adjusted at pH 7.4 with KOH. The bathing solution consisted of (in mM) 145 K-gluconate, 5 KCl, 2.5 MgCl2, 10 HEPES, and 1 EGTA plus CaCl2 to yield a final free Ca2+ concentration of 10 μM at pH 7.4. When using ATP-containing bath solutions, the free Ca2+ and Mg2+ concentrations were adjusted to remain constant at 10 μM and 2.5 mM, respectively. The free Ca2+ concentration for each solution was calculated using the EQCAL multiple equilibrium software (Biosoft, Cambridge, UK). To obtain a Ca2+-free bath solution, EGTA (1 mM) was added without CaCl2 (estimated free Ca2+ <10 nM). Bath solution changes were performed as described previously using a RSC-160 rapid solution changer system (BioLogic, Grenoble, France) (2). The solution exchange time was estimated to be <20 ms (27). AMP and ATP were purchased from Sigma-Aldrich. Experiments were performed at room temperature (23°C).
Ussing chamber measurements on NuLi monolayers.
NuLi epithelial cell monolayers, cultured on filters (4 cm2, Costar Transwell) at air-liquid interface for 4–6 wk, were pretreated for 1–2 h (at apical and basolateral sides) with or without 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR, 1 mM, Sigma-Aldrich), or AICAR plus Compound-C (10 μM, Merck Frosst, Whitehouse Station, NJ) in DMEM-F12. The treated monolayer was then mounted in a heated (37°C) Ussing chamber for short-circuit current measurements. KCa3.1 stimulation was induced by bath application of 1-EBIO (Tocris, Ellisville, MO), whereas TRAM-34 (Sigma-Aldrich) was used as a specific KCa3.1 inhibitor. Total Isc (Ibasal), 1-EBIO- (I1-EBIO), and Tram-34-sensitive (ITram) currents were compared either in control, AICAR, or AICAR + Compound-C conditions. Apical and basolateral sides of monolayers were perfused with warm (37°C) normal physiological solution containing (in mM) 141 NaCl, 5.4 KCl, 0.78 NaH2PO4, 0.8 MgCl2, 1.8 CaCl2, 5 glucose, and 15 HEPES at pH 7.4. Transepithelial potential difference (PD) was clamped to zero by an external voltage-clamp amplifier (VCCMC2, Physiological Instruments) with KCl agar-calomel half-cells and Ag-AgCl electrodes, and the resulting Isc was recorded continuously using the PowerLab system (ADInstrument, Toronto, Ontario, Canada) (6, 28).
RESULTS
AMP inhibits recombinant KCa3.1 channel activity in inside-out.
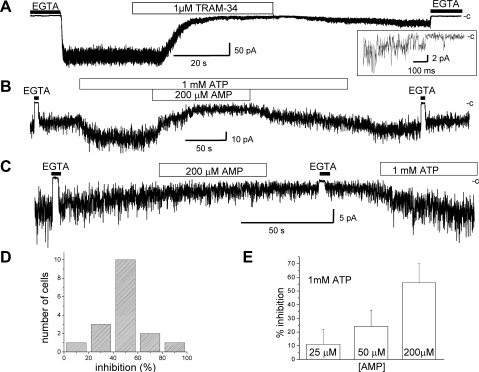
Functional KCa3.1 channel expression in HIK cells was first confirmed in inside-out patch-clamp experiments where channel activity was measured before and after addition of the KCa3.1 inhibitor Tram-34 (Fig. 1A). The internal addition of Tram-34 (1 μM) resulted in a near total inhibition of channel activity that was slowly reversible confirming that the Ca2+-sensitive currents measured under our experimental conditions correspond to KCa3.1 (24, 25, 44). A series of inside-out patch-clamp experiments was next performed to determine whether the KCa3.1 activity could be regulated by AMP. In these experiments, channel activity was measured at fixed Ca2+ (10 μM) and ATP (1 mM) concentrations. An example of inside-out patch-clamp recording illustrating the action of 200 μM AMP applied internally is presented in Fig. 1B. In most of these experiments, excision of the patch membrane resulted in a time-dependent decrease in channel activity that was reversed by the addition of 1 mM ATP to the internal medium. This effect corresponds to the well-documented stimulatory action of ATP on the KCa3.1 channel (13) and likely involves phosphorylation of KCa3.1 by the NDPK-B protein (42). The addition of 200 μM AMP in 1 mM ATP conditions caused a 46 ± 18% (n = 17) decrease in channel activity that could be partly reversed by AMP washout. Control patch-clamp experiments were also carried out to determine whether the observed inhibitory action of AMP requires the presence of ATP. As seen, patch excision in the absence of ATP resulted in a slow rundown of the channel activity typically of the order of 45% over a 1-min period (Fig. 1C). The current recording presented in Fig. 1C indicates, however, that the rundown process was not affected by the addition of 200 μM AMP in contrast to the fast AMP response illustrated in Fig. 1B. This recording also shows that channel activity could be restored by perfusing with solution containing 1 mM ATP. These observations therefore support a model whereby the KCa3.1 channel inhibition mediated by AMP is ATP dependent. Figure 1D summarizes the results obtained from 17 different cells where AMP was applied at 200 μM with ATP at 1 mM. As seen, 10 of the 17 cells studied showed a percentage of inhibition between 40% and 60%, whereas <5% of the cells studied (1/17) responded to the addition of AMP by an inhibition of <20%. The action of AMP on channel activity was dose dependent for concentrations within the physiological range expected for AMP (Fig. 1E), with a concentration for 50% inhibition estimated at 140 μM. This result is in line with the value reported from biochemical assays, where AMPK stimulation was measured under similar AMP-to-ATP ratios (23).
Fig. 1.
Inside-out experiments illustrating a regulation of the KCa3.1 activity by AMP. Inside-out patch-clamp recording performed on human epithelia kidney HEK-293 cells expressing the Myc-KCa3.1 channel. Recording obtained in 10 μM internal Ca2+ conditions at an applied membrane potential of −60 mV. Perfusion with a 0 Ca2+ solution (EGTA) is represented as a filled rectangle. The symbol c refers to the zero current level. A: internal addition of the KCa3.1-specific inhibitor Tram-34 (1 μM) caused a strong current inhibition that was slowly reversible, confirming that the Ca2+-sensitive current measured under our experimental conditions corresponds to KCa3.1. Inset, single-channel events, confirming current jumps of 2.4 pA for an unitary conductance of 40 pS as expected for KCa3.1. B: initial addition of 1 mM ATP to the internal medium caused an increase in channel activity to reach a stable current level. Perfusion with a solution containing 1 mM ATP plus 200 μM AMP led to a 46 ± 18% (n = 17) decrease in channel activity that was reversible following the washout of AMP. C: effect of AMP on channel activity in the absence of ATP. Perfusion with an internal solution containing AMP (200 μM) in the absence of ATP did not affect the time course of the channel rundown process. This result supports an effect of AMP on KCa3.1 that is ATP dependent. D: frequency histogram of the percentage of current inhibition measured at 200 μM AMP obtained from 17 different cells. E: percentage of current inhibition measured as a function of the AMP concentration for ATP at 1 mM. The AMP concentration for half inhibition was estimated at 140 μM. Each data point represents the mean ± SD of at least 3 different experiments.
Interactions between the γ1-subunit of AMPK with the COOH-terminal (CT) domain of KCa3.1.
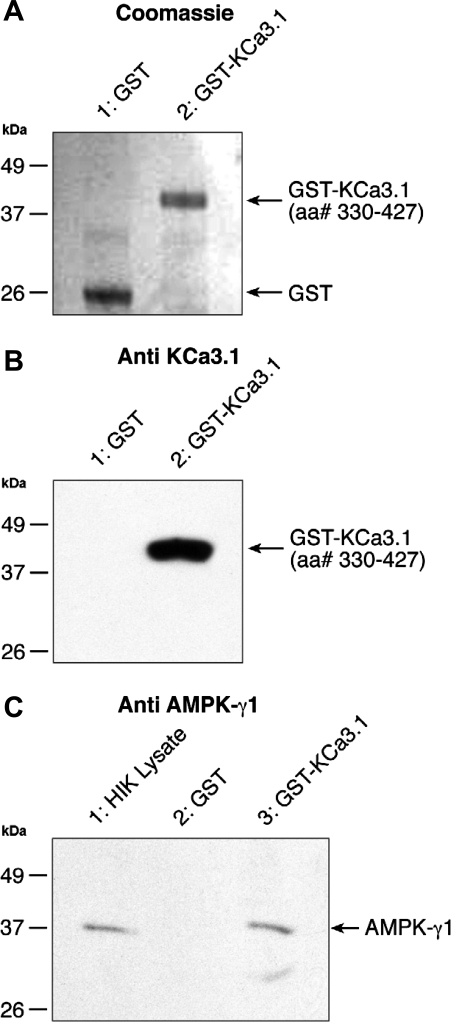
A regulation of the KCa3.1 channel activity by internal AMP suggests a potential control by AMPK. To establish whether such a regulatory mechanism involves a direct interaction KCa3.1/AMPK or the recruitment of an auxiliary protein, we screened a bacterial two-hybrid library for proteins that could act as KCa3.1 channel regulators through interactions with the channel COOH-terminal domain (Leu345-Ala400). Thirty positive clones were initially identified, of which nine remained positive following streptomycin-based screening. Out of these 9 clones, 3 were identified after sequencing as the human γ1-subunit of AMPK. The interaction between the KCa3.1/CT fragment (Leu345-Ala400) and the γ1-subunit of AMPK was further confirmed using the entire KCa3.1/CT (Arg287-Lys427) domain. Our result showed that this domain could also bind the γ1-subunit of AMPK (Table 1). No interaction between KCa3.1/CT and the γ1-subunit of AMPK could however be detected with KCa3.1/CT (Leu345-Asp380) fragment, indicating that the amino acids extending from Asp380 to Ala400 are essential to the interaction between the COOH-terminal region of KCa3.1 and the AMPK-γ1 subunit. As this 21 amino acid stretch contains a consensus sequence for a leucine zipper motif (L378ydlqqnL385ssshraL392ekqidtL399), our results suggest that the γ1-subunit of AMPK can bind to the COOH-terminal end of KCa3.1 through a leucine zipper [L - x(6)- L - x(6) - L - x(6)- L]-based mechanism. GST pull-down experiments were next performed to confirm that the COOH-terminal domain of the KCa3.1 interacts with the γ1-subunit of AMPK. The overall quality and purity of the GST/GST-KCa3.1 proteins were confirmed by Coomassie staining as seen in Fig. 2 A. Western blotting with an anti-KCa3.1 antibody revealed in addition a single band at the expected molecular mass of 40 kDa for the GST-KCa3.1 terminal fusion protein (Arg330 to Lys427) (Fig. 2B, lane 2), while no band was detected for GST alone (Fig. 2B, lane 1). Binding between the GST/KCa3.1 fusion protein containing the Arg330-Lys427 domain of KCa3.1 and the γ1-subunits of AMPK is demonstrated in the Western blots presented in Fig. 2C, lane 3. As seen, a band of 38 kDa corresponding to the expected molecular mass for the γ1-subunit of AMPK was detected in the total cell lysate (lane 1) and purified GST-KCa3.1 preparation (lane 3) using an anti AMPK-γ1 antibody. No binding was detected with GST alone (lane 2). Altogether these observations strongly argue for AMPK interacting as a complex with the COOH-terminus of KCa3.1 extending from Arg330 to Lys427.
Table 1.
Interaction between the COOH-terminal domain of KCa3.1 and the γ1-subunit of AMPK revealed by two-hybrid analysis
| Bait Plasmid (pBT) |
Prey Plasmid (pTRG) |
|
|---|---|---|
| Control | AMPK-γ1 | |
| KCa3.1/CT (Arg287-Lys427) | — | + |
| KCa3.1/CT (Leu345-Ala400)* | — | + |
| KCa3.1/CT (Leu345-Asp380) | — | — |
Positive two-hybrid responses were observed using the COOH-terminal (CT) fragments of KCa3.1 extending either from Arg287 to Lys427 or from Leu345 to Ala400. No interaction could, however, be detected using the fragment Leu345 to Asp380, indicating that the amino acids extending from Asp380 to Ala400 are essential for the interaction between KCa3.1 and the γ1-subunit of 5′-AMP protein kinase (AMPK). Detection of protein-protein interactions was based on transcriptional activation of the HIS3 reporter gene, which allows growth in the presence of 3-amino-1,2,4-triazole (3-AT), a competitive inhibitor of His3 enzyme. Positives are verified by using the aadA gene, which confers streptomycin resistance, as a secondary reporter (+).
Bait we used for our initial screening of HeLa library.
Fig. 2.
GST-KCa3.1 pull-down assay. A: Coomassie blue-stained SDS-PAGE gel of purified GST (lane 1) and GST-KCa3.1 terminal fusion protein (lane 2) confirming the purity of GST and the GST KCa3.1. B: Western blot obtained using a rabbit anti-KCa3.1 antibody raised against a synthetic peptide corresponding to amino acid residues 350–363 of rat KCa3.1 (1:1,000, Sigma-Aldrich). C: evidence for 5′-AMP-activated protein kinase γ1-subunit (AMPK-γ1) being pulled down by the GST-KCa3.1 fusion protein. Western blots obtained using a rabbit anti-AMPK-γ1 antibody (1:2,000) with horseradish peroxidase-conjugated goat anti-rabbit IgG as secondary antibody. HIK lysate (lane 1C), obtained as described in materials and methods, was added to confirm that endogenous γ1-subunit of AMPK migrates at the same MW than the AMPK-γ1 from pulldown. Western blot also demonstrates that the GST-KCa3.1 fusion protein is able to bind to the γ1-subunit of AMPK, whereas no binding is detected with GST alone.
AMPK-γ1 and KCa3.1 colocalize at the plasma membrane of HEK-293 cells expressing HA-KCa3.1.
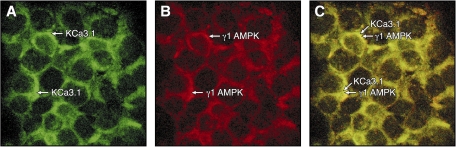
Functional protein/protein interactions with KCa3.1 requires that the auxiliary protein be localized at the plasma membrane. Immunofluorescence experiments were thus performed using HEK cells transfected with KCa3.1 containing an external HA tag at position G132. This approach was found to yield a better signal in immunofluorescence experiments compared with HEK cells transfected with KCa3.1 bearing a Myc tag. Comparing the HA-KCa3.1 and endogenous AMPK-γ1 labeling in Fig. 3 demonstrates that AMPK-γ1 is highly expressed at the plasma membrane and colocalized at the plasma membrane with HA-KCa3.1. There was no detectable labeling with the AlexaFluor488 and AlexaFluor594 probes in control experiments where cells were incubated in the absence of anti-HA/AMPK-γ1 primary antibodies (data not shown). Altogether, the colocalization of KCa3.1 and AMPK-γ1 at the plasma membrane suggests a membrane-delimited interaction between AMPK-γ1 and KCa3.1.
Fig. 3.
Membrane colocalization of HA-KCa3.1 channel and AMPK-γ1 in HEK-293 cells. A: immunostaining of HA-KCa3.1 channel performed on permeabilized HEK-293 cells expressing HA-KCa3.1 using a monoclonal anti-HA primary antibody plus an anti-rabbit antibody conjugated to AlexaFluor488 as secondary antibody. B: immunostaining of AMPK-γ1 performed on permeabilized HEK-293 cells expressing HA-KCa3.1 using an anti-AMPK-γ1 primary antibody plus an anti-rabbit antibody conjugated to AlexaFluor594 as secondary antibody. C: overlay of the AlexaFluor488 and AlexaFluor594 staining confirming the localization of AMPK-γ1 at the plasma membrane. Control experiments where no primary antibodies were added did not yield a detectable signal. Single optical sections were obtained by confocal fluorescence microscopy.
Functional AMPK-γ1 is essential for the regulation of KCa3.1 by AMP.
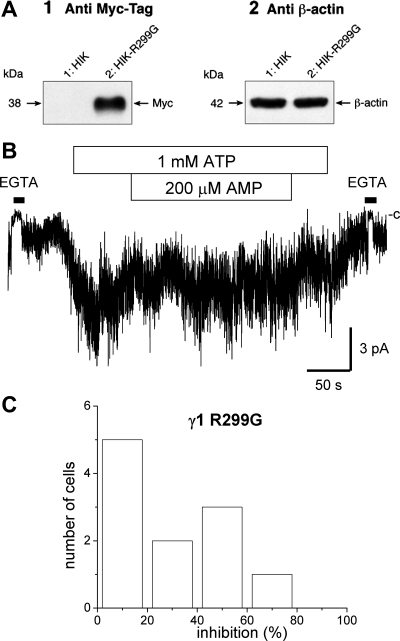
To manipulate AMPK-γ1 activity by a nonpharmacological approach, we then specifically altered the subunit activity through the generation of a dominant-negative mutant. The mutation R299G in γ1, equivalent to R531G in AMPK-γ2, has been reported to abolish the activity of the AMPKγ1β1α2 complex without any significant effect on the degree of phosphorylation at Thr172 (36). Figure 4A-1 presents an immunoblot obtained with an anti-Myc antibody using HEK cells expressing either the Myc-KCa3.1 channel alone (HIK, lane 1) or in combination with the AMPK-γ1 mutant R299G Myc tagged in COOH-terminus (HIK-R299G, lane 2). The resulting Western blot confirms the expression of the AMPK-γ1-R299G mutant, leading to a band at the expected weight of 38 kDa. In these experiments, β-actin was used as control for total protein loading with HIK cells and HIK cells expressing the negative AMPK-γ1 R299G mutant, respectively (Fig. 4A-2). Figure 4B illustrates an example of inside-out recording typical of cells cotransfected with the AMPK-γ1-R299G mutant (see Fig. 1 for comparison). As seen, the internal addition of AMP under these conditions failed to induce a reduction in KCa3.1 activity, supporting an AMP-dependent regulation of KCa3.1 mediated by AMPK-γ1. These observations are summarized in the frequency histogram presented in Fig. 4C where 5 of the 11 cells studied were found to respond to AMP by an inhibition lower than 20% of the channel activity. This result is clearly at variance with the histogram presented in Fig. 1B where only 1/17 of the cells tested responded to the addition of AMP by an inhibition of 20% or lower of the channel activity. Altogether these observations support the proposal of AMPK activation leading to a decrease in KCa3.1 channel activity.
Fig. 4.
Dominant negative approach supporting a functional link between AMPK-γ1 and KCa3.1. A1: Western blot performed with an anti-Myc-tag antibody using HIK or HIK cells expressing the Myc-tagged dominant negative AMPK-γ1 R299G mutant. The presence of a band at 38 kDa confirms the expression of the AMPK-γ1 R299G mutant in HIK cells. A2: Western blot where β-actin (Sigma, AC-15) was used as control for total protein loading with HIK cells and HIK cells expressing the AMPK-γ1 R299G mutant, respectively. B: inside-out patch-clamp recording of KCa3.1 performed using HIK cells. Recording obtained in 10 μM internal Ca2+ conditions at an applied membrane potential of −60 mV. Perfusion with a 0 Ca2+ solution (EGTA) is represented as a filled rectangle. Example of inside-out recording typical of 5/11 of HIK cells cotransfected with the dominant negative AMPK-γ1 mutant R299G. The mutation did not affect the channel activation by ATP but increased the fraction of the cells where KCa3.1 activity decreased by <20% in response to 200 μM AMP. C: frequency histogram obtained from 11 different cells showing that 5 of the 11 cells cotransfected with the AMPK-γ1-R299G mutant showed KCa3.1 currents that were inhibited by <20% following exposure to 200 μM AMP. These observations support an effect of AMP on the KCa3.1 channel activity that would be mediated by the γ1-subunit of AMPK.
Interaction between AMPK-γ1 and KCa3.1 in NuLi bronchial monolayers.
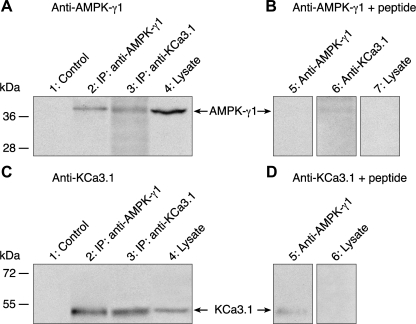
The evidence gathered so far for protein/protein interactions between KCa3.1 and AMPK-γ1 was obtained in HEK cells overexpressing tagged forms of KCa3.1. To establish if such interactions could be extrapolated to native bronchial cells, KCa3.1/AMPK-γ1 protein/protein interactions were also studied in NuLi bronchial epithelial cell monolayers. Expression of AMPK-γ1 (Fig. 5A, lane 4) and KCa3.1 (Fig. 5C, lane 4) proteins was first demonstrated by Western blot in NuLi cell extracts where bands of 38 and 51 kDa were detected using an anti-AMPK-γ1 or anti-KCa3.1 antibody, respectively. More importantly, the presence of a band at 38 kDa, corresponding to the AMPK-γ1 in Fig. 5A (lane 3) confirmed that KCa3.1 could form an immunoprecipitable complex with AMPK-γ1. Similarly, a band at 51 kDa in Fig. 5C (lane 2) provided clear evidence for KCa3.1 being capable of forming an immunoprecipitable complex with AMPK-γ1. No band was observed when membranes were blotted with the anti-AMPK-γ1 (Fig. 5B, lanes 5, 6, and 7) or anti-KCa3.1 antibodies (Fig. 5D, lanes 5 and 6) in the presence of their respective neutralizing peptides, indicating that the AMPK-γ1 and KCa3.1 signals were specific. Altogether, these results argue for KCa3.1 and AMPK-γ1 interacting in native bronchial cells.
Fig. 5.
Coimmunoprecipitation of endogenous AMPK-γ1 and KCa3.1 in NuLi cells. Immunoblots showing AMPK-γ1 (A, B) and KCa3.1 (C, D) proteins from NuLi extracts and AMPK-γ1/KCa3.1 immunoprecipitations. Membranes were blotted with anti-AMPK-γ1 (A, B) and anti-KCa3.1 (C, D) antibodies, in the presence (B, D) or absence (A, C) of neutralizing peptide. Endogenous expression of AMPK-γ1 and KCa3.1 proteins in the NuLi cell lysate are presented in lanes 4 of A and C, respectively. Control lanes (A and C, lanes 1) refer to coimmunoprecipitation experiments done in the absence of immunoprecipitating antibody. Control immunoprecipitations were also performed, AMPK-γ1 immunoprecipitation with the AMPK-γ1 antibody (A, lane 2) and KCa3.1 immmunoprecipitation with the KCa3.1 antibody (C, lane 3). Coimmunoprecipitation of endogenous AMPK-γ1 using anti-KCa3.1 antibody is illustrated in A, lane 3. Conversely, coimmunoprecipitation of endogenous KCa3.1 using anti-AMPK-γ1 antibody is shown at C, lane 2. Note that the same lysate and IP samples were used in the upper and lower parts of the membranes, blotted with AMPK-γ1 and KCa3.1 antibodies, respectively. Because of the higher AMPK-γ1 signal in cell lysates, 1/3 of the total IP was loaded for AMPK-γ1 immunoblot, whereas 2/3 of the total IP was loaded for the KCa3.1 immunoblot. No band was observed when the membranes were blotted with anti-AMPK-γ1 or anti-KCa3.1 antibodies in the presence of their respective neutralizing peptides (B and D).
Evidence of KCa3.1 currents in NuLi-polarized monolayers.
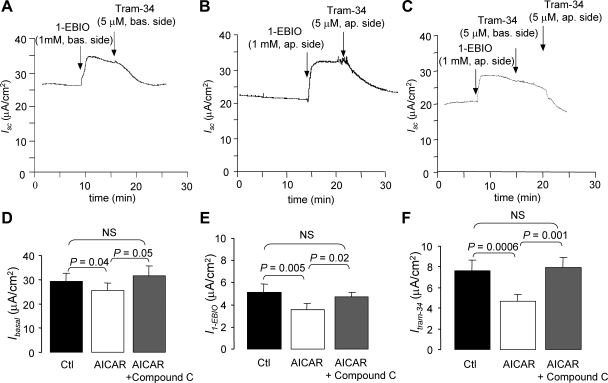
To determine whether KCa3.1 contributes to the cell ion transport properties, short-circuit measurements were also performed using NuLi cell monolayers. The mean total Isc measured in control conditions was estimated at 29 ± 3 μA/cm2 (n = 11), a value similar to that reported previously by Zabner et al. (50). The presence of functional KCa3.1 currents in NuLi monolayers was explored using the KCa3.1 potentiator 1-EBIO (1 mM) applied at the basolateral side. The current record illustrated in Fig. 6A indicates that 1-EBIO induced an additional current of 5.1 ± 0.7 μA/cm2 (n = 11) that was completely inhibited by the addition of 5 μM Tram-34, a specific KCa3.1 channel inhibitor. Since it was reported that KCa3.1 channels could be present on both apical and basolateral membranes of human bronchial 16HBEo-14 cells monolayers (3), the presence of KCa3.1 currents at the apical membrane of NuLi monolayers was also evaluated. Indeed, we observed that 1-EBIO application at the apical side also enhanced Isc currents (Fig. 6, B and C), and this apical 1-EBIO-stimulated current could be reversed by adding Tram-34 on the same side (Fig. 6, B and C). Therefore, these observations provide evidence of functional KCa3.1 channels at both apical and basolateral membranes of NuLi cells.
Fig. 6.
Impact of AMPK activity on KCa3.1currents. Short-circuit currents (Isc) measured in Ussing chamber on Nuli cell monolayers cultured for 4 to 6 wk, at air-liquid-interface. A: applying the KCa3.1 potentiator 1-EBIO (1 mM) to the basolateral side of the monolayer caused an increase in Isc, which was inhibited by a basolateral application of the KCa3.1 inhibitor Tram-34 (5 μM). This result confirms the presence of KCa3.1 at the basolateral membrane of Nuli cell monolayers. B: similarly, applying 1-EBIO (1 mM) to the apical side of the monolayer caused an increase in Isc, which was inhibited by apical application of Tram-34 (5 μM), confirming the presence of KCa3.1 at the apical membrane of Nuli cell monolayers. C: basolateral addition of Tram-34 following apical stimulation of KCa3.1 by 1-EBIO did not result in a significant Isc decrease, confirming the side specificity of the action of Tram-34 seen in B. D–F: impact of AMPK activity on KCa3.1 currents was evaluated by Isc measured in nontreated (Ctl), AICAR-treated (1 mM, 1 h, apical and basolateral side), or AICAR + Compound-C treated (10 μM, 1 h, apical and basolateral side) monolayers. AICAR is used to activate AMPK via the internal production of 5-amino-4-imidazole-carboxamide ribotide (ZMP). Basal short-circuit currents (Ibasal, D; n = 11), 1-EBIO-induced currents (I1-EBIO, E; n = 11), and 1-EBIO-induced, Tram-34 inhibited currents (ITram-34, F; n = 11) were then compared in control, AICAR, and AICAR + Compound-C-treated monolayers. The presence of Compound-C succeeded to reverse the effect of AICAR on the KCa3.1-dependent short-circuit currents. These results argue for a control of the KCa3.1 activity by AMPK in Nuli monolayers.
Impact of AMPK activity on native KCa3.1 currents.
To test the impact of AMPK activity on KCa3.1 currents, basal currents (Ibasal, Fig. 6D), 1-EBIO-stimulated Isc (I1-EBIO, Fig. 6E), and 1-EBIO-induced-Tram-34-sensitive currents (ITram-34, Fig. 6F) were compared in nontreated (Ctl) or AICAR-treated (AICAR, 1 mM, 1–2 h) NuLi monolayers. AICAR has been documented as a cell-permeable AMPK activator of which its action is mediated by the internal production of 5-amino-4-imidazole-carboxamide ribotide (ZMP) (23). We first observed that the Ibasal (29 ± 3 μA/cm2) was inhibited by AICAR treatment (P <0.05, Fig. 6D). Similarly, the I1-EBIO (5.1 ± 0.7 μA/cm2, n = 11, Fig. 6E) and ITram-34 currents (8 ± 1 μA/cm2, n = 11: Fig. 6F) were reduced to 3.5 ± 0.6 μA/cm2 (n = 11; Fig. 6E) and 4.7 ± 0.7 μA/cm2 (n = 11; Fig. 6F), respectively, in AICAR-treated monolayers (P < 0.005). Our results also show that the AMPK inhibitor Compound-C could reverse the effects observed with AICAR. As seen in Fig. 6, D–F, treating the cells with AICAR plus Compound-C (10 μM) for 1–2 h completely reversed the effect of AICAR with Ibasal (Fig. 6D), I1-EBIO (Fig. 6E), and ITram-34 (Fig. 6F) being no longer significantly different from the current values measured in control conditions.
DISCUSSION
In this work we provide the first evidence for a regulation of the KCa3.1 channel by the AMP-activated protein kinase. Inside-out patch-clamp measurements showed a decrease in the KCa3.1 activity in response to an increase in AMP concentration, a result supported by two-hybrid screening and pull-down experiments, which confirmed that the COOH-terminal region of KCa3.1 interacts with the AMP-binding γ1-subunit of AMPK. An AMPK-γ1-based control of KCa3.1 was further suggested by the observations obtained in patch-clamp experiments carried out in cells transfected with the AMPK-γ1 dominant negative R299G mutant where the fraction of cells inhibited by <20% following AMP exposure increased from 5% in control to 50% with the AMPK-γ1-R299G mutant. Finally, we present evidence through coimmunoprecipitation experiments for the formation of a KCa3.1/AMPK-γ1 complex in NuLi cells at endogenous expression levels of AMPK and KCa3.1. Short-circuit current measurements in NuLi cell monolayers confirmed in addition that AMPK activation by AICAR results in a decrease of the KCa3.1-mediated currents, an effect reversed by the AMPK inhibitor Compound-C. Altogether these observations argue for an AMPK-dependent reduction in KCa3.1 channel activity following an increase in AMP-to-ATP ratio.
AMPK as part of a regulatory complex affecting KCa3.1.
AMPK has already been documented to modulate the activity of several ion channels, including CFTR(17), the cardiac voltage-gated Na+ channel (29), ENaC (4), and more recently, the Maxi KCa1.1 channel (48). AMPK and CFTR were reported to share an apical distribution in several epithelial tissues (16), and two-hybrid experiments have demonstrated that the COOH-terminal regulatory domain of the AMPK-α1 subunit binds to the COOH-terminal tail of CFTR (18). Evidence was also provided that CFTR can be phosphorylated by AMPK in vitro leading to channel inhibition. Similarly, the α1-subunit of AMPK was found to colocalize at the plasma membrane with the Maxi KCa1.1 channel α-subunit in carotid body-type I cells and to phosphorylate KCa1.1 in vitro (48). These observations are in line with the two-hybrid, pull-down and coimmunoprecipitation results presented in this work, although our data point towards an interaction with KCa3.1 involving the γ1- rather than the α1-subunit. An interaction with AMPK-α1 or AMPK-α2 cannot, however, be entirely ruled out. Such interaction would likely be mediated by AMPK-γ, since AMPK-γ1 and AMPK-α1 or AMPK-α2 are known to participate to the formation of the AMPK complex. It is also clear from our immunofluorescence experiments that AMPK-γ1 is present a the plasma membrane, in agreement with data demonstrating that the AMPK-β subunit, which is thought to act as a scaffold protein and hold the complex together, is myristoylated (33). Such membrane localization would favor direct KCa3.1 and AMPK-γ1 membrane delimited interactions in accordance with our protein/protein interaction analysis. More importantly, our two-hybrid analysis provided evidence for the amino acids extending from Asp360 to Ala400 to be essential to the interaction between KCa3.1 and the γ1-subunit of AMPK. This stretch of 21 amino acid contains a leucine zipper motif that has already been demonstrate to be required for the correct trafficking of KCa3.1 to the plasma membrane (44). A possible role of the γ1-subunit of AMPK in KCa3.1 trafficking will require further investigation.
Data have also been presented demonstrating that the KCa3.1 COOH-terminus interacts with the nucleoside diphosphate kinase NDPK-B leading to channel activation (42). The phosphorylation site of NDPK-B has been identified as His358 and is located in proximity of the channel calmodulin-binding domain. In contrast to the stimulatory effect observed following phosphorylation by NDPK-B, our data link AMPK activation to a decrease in KCa3.1 activity. Our results therefore add to the complexity of KCa3.1 regulation through protein-protein interactions with an additional interaction involving AMPK-γ1 and the COOH-terminus of KCa3.1. The possibility of several protein kinases acting simultaneously on KCa3.1, thus modulating the effect of AMPK to various degrees, may account for the variability in the percentage of current inhibition observed from patch to patch in our inside-out experiments. In addition, because the degree of AMPK phosphorylation at Thr172 is not controlled in our patch-excised experiments, it is likely that the observed variability in the percentage of current inhibition induced by AMP also reflects cell-to-cell differences in the initial degree of phosphorylation of the AMPK complex at this site.
As an initial approach to identify a potential phosphorylation site by AMPK on KCa3.1, an analysis was undertaken of the KCa3.1 primary sequence based on the AMPK phosphorylation recognition motif φ(Xβ)XXS/TXXXφ where φ is a hydrophobic and β a basic residue (11, 47). This analysis led to the identification of Ser66 as the unique site on KCa3.1 expected to be a substrate to AMPK. As this site is located in the NH2-terminal half of the S2 transmembrane segment, it is unlikely to be phosphorylated by AMPK. We cannot rule out, however, the possibility that nonconventional sequence motifs for phosphorylation sites by AMPK may be present in KCa3.1. Within the limits of our analysis, it would be therefore unlikely for KCa3.1 to constitute per se a substrate to AMPK. AMPK could, however, be attached to KCa3.1 via the γ1-subunit as demonstrated in this work, with the α-subunit of AMPK involved in the phosphorylation of an auxiliary protein acting on KCa3.1. One possibility would be CaM, which also contains a potential site of phosphorylation by AMPK. Phosphorylation of CaM by the protein kinase CK2 has been documented to decrease the activity of the small conductance Ca2+-activated channel KCa2.2 (5). A similar AMPK-based mechanism could prevail for KCa3.1.
AMPK regulates KCa3.1 currents in functional epithelia.
NuLi cells, cultured on permeable support at air-liquid interface, have been documented to retain the normal phenotypic qualities of human airway epithelial cells and to form polarized differentiated epithelia that exhibit transepithelial resistance and ion channel physiology expected for the genotypes (50). Short-circuit current measurements carried out on polarized NuLi cell monolayers provide evidence of functional KCa3.1 channels at both the apical and basolateral membranes. The presence of KCa3.1 in NuLi cells was also confirmed through Western blotting in agreement with our short-circuit current measurements. In our Ussing chamber experiments, KCa3.1 was activated using the KCa3.1 potentiator 1-EBIO and blocked by applying the KCa3.1 inhibitor Tram-34 either to the apical or basolateral side. Because the action of 1-EBIO consists of increasing the channel apparent affinity for Ca2+ without changing the cell internal Ca2+ level (34), the current inhibition induced by Tram-34 in our experiments is likely to result from a direct block of KCa3.1 and not from a modulation of the cell internal Ca2+ concentration as documented elsewhere (38). Furthermore, we found that the application of Tram-34 in absence of 1-EBIO preactivation elicited little, if any, effect on the basal Isc current, thus arguing for an absence of unspecific effect. Finally, we recently detected an 1-EBIO-induced TRAM-34-sensitive current through apically permeabilized NuLi monolayers (in the presence of an apical to basolateral K+ gradient), demonstrating the presence of functional KCa3.1 channel at the basolateral membrane of NuLi cells (46). Altogether these observations are in line with previous evidence demonstrating an apical and basolateral distribution of KCa3.1 in wild-type and ΔF508-CFTR expressing human bronchial cell epithelia (3, 12). More importantly, KCa3.1 and AMPK-γ1 interactions could be detected in NuLi cells by coimmunoprecipitation demonstrating that KCa3.1 and AMPK-γ1 can interact under endogenous protein expression levels. These observations thus support the conclusions drawn from systems where KCa3.1 was expressed in HEK cells and argue against KCa3.1/AMPK-γ1 interactions resulting from an overexpression of the KCa3.1 channel.
Our results also demonstrate that activation of AMPK by cell treatment with AICAR caused a significant reduction of the basolateral short-circuit current associated to KCa3.1. The impact of AICAR was abolished by Compound-C. The effect of AICAR was observed in conditions where the AMP-to-ATP ratio remained unchanged because the main effect of AICAR is to cause an accumulation of AICA-ribotide or ZMP inside the cells leading to the activation of AMPK (23). Our observations thus suggest that under metabolic stress conditions, the level of ion and water transepithelial transport could be adjusted as a function of the cell energy status via an effect on KCa3.1. AMPK activation has also been reported to inhibit the cAMP-activated CFTR conductance in Calu-3 cell monolayers (17), through a decrease of the channel open probability. A contribution of CFTR to the effects of AICAR on the 1-EBIO-induced and Tram-34-sensitive short-circuit currents measured in NuLi cells is unlikely, however, because these experiments were carried out in conditions where CFTR was not activated by cAMP and thus not expected to contribute to the overall transepithelial conductance. Furthermore, a decrease in KCa3.1-mediated currents in NuLi monolayers in response to AICAR treatment is consistent with our inside-out patch-clamp results where internal AMP caused a reduction of the KCa3.1 channel activity at constant ATP and Ca2+ concentrations (23). However, because the action of AICAR requires that the molecule be transformed intracellularly into ZMP, an analog of AMP, the AICAR-based protocol used to induce KCa3.1 inhibition in short-circuit current measurements could not be extended to patch-clamp experiments.
Physiological implications.
Under normal internal metabolic conditions (ADP/ATP ≈ 0.1), the AMP-to-ATP ratio should approximate 0.01 and be too low to cause AMPK activation (20, 39). The regulation of KCa3.1 by ATP is expected therefore to depend exclusively upon NDPK-B (42) and/or additional Ser/Thr kinases (14). In response to metabolic stressors such as hypoxia or free radicals conditions, however, the ADP-to-ATP ratio can increase up to 0.5 for an AMP-to-ATP ratio of 0.25 (39). Our inside-out patch-clamp results clearly demonstrate that an AMP-to-ATP ratio of 0.2 is sufficient to cause an average 46% decrease in KCa3.1 activity at constant ATP concentration. AMPK activation has been reported to inhibit various diffusive ion-transport pathways, thereby minimizing the dissipation of ionic gradients while conserving cellular ATP by limiting ATP consumption via ATP-dependent transport processes. Because the vectoriel transport of ions in Cl− secreting epithelial cells is strongly modulated by the K+ conductance at the basolateral membrane, our results show that KCa3.1 inhibition through AMPK activation contribute to reinforce the downregulation of ion transport in these cells. The role of KCa3.1 might be, in this regard, rather crucial as one of the expected effects of metabolic stressors is to cause an increase of the intracellular Ca2+ concentration (1), thus favoring a strong KCa3.1 activation. A downregulation of KCa3.1 by AMPK transport would under these conditions preserve the intracellular ionic environment and defend the ability of the cell to generate ATP in the face of metabolic stress (4). Because AMPK acts as a metabolic sensor, AMPK activation might also determine cell survival in pathological conditions including cystic fibrosis (CF). In line with this proposal is the observation of an increased AMPK protein expression in airway cells from CF patients relative to cells from non-CF patients (15). A concomitant downregulation of KCa3.1 and ENaC by AMPK is therefore susceptible to minimize the deleterious effect of a Na+ hyperabsorption through ENaC in CF conditions. Overall our results are in agreement with a model whereby the response of Cl− secreting epithelial cells to a metabolic stress such as the one prevailing in CF conditions due to chronic infection or inflammation involves the common inhibition of both CFTR and KCa3.1 while decreasing the number of functional ENaC channels at the apical membrane (7, 15, 17).
In conclusion, AMPK is now considered as a cellular “fuel-gauge” involved in the regulation of energy metabolism. Our results supporting a regulation of KCa3.1 gating by AMPK point towards a global modification of the ion transport properties in Cl− secreting epithelia not exclusively mediated by CFTR and ENaC, but that includes a contribution of KCa3.1 as well.
GRANTS
This work was supported by grants from the Canadian Cystic Fibrosis Foundation (to R. Sauvé and E. Brochiero) and from the Institutes of Health Research (MOP 7769 to R. Sauvé).
Acknowledgments
We acknowledge the help of Julie Verner for expert cell preparation and M. Michel Lauzon for technical assistance for confocal imaging. The authors also thank Ariane Longpré-Lauzon for critical reading of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arnould T, Michiels C, Alexandre I, Remacle J. Effect of hypoxia upon intracellular calcium concentration of human endothelial cells. J Cell Physiol 152: 215–221, 1992. [DOI] [PubMed] [Google Scholar]
- 2.Banderali U, Klein H, Garneau L, Simoes M, Parent L, Sauvé R. New insights on the voltage dependence of the KCa3.1 channel block by internal TBA. J Gen Physiol: 333–348, 2004. [DOI] [PMC free article] [PubMed]
- 3.Bernard K, Bogliolo S, Soriani O, Ehrenfeld J. Modulation of calcium-dependent chloride secretion by basolateral SK4-like channels in a human bronchial cell line. J Membr Biol 196: 15–31, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4-2. J Biol Chem 281: 26159–26169, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bildl W, Strassmaier T, Thurm H, Andersen J, Eble S, Oliver D, Knipper M, Mann M, Schulte U, Adelman JP, Fakler B. Protein kinase CK2 is coassembled with small conductance Ca(2+)-activated K+ channels and regulates channel gating. Neuron 43: 847–858, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Brochiero E, Dagenais A, Prive A, Berthiaume Y, Grygorczyk R. Evidence of a functional CFTR Cl− channel in adult alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 287: L382–L392, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Carattino MD, Edinger RS, Grieser HJ, Wise R, Neumann D, Schlattner U, Johnson JP, Kleyman TR, Hallows KR. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem 280: 17608–17616, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Carling D The AMP-activated protein kinase cascade–a unifying system for energy control. Trends Biochem Sci 29: 18–24, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J 346: 659–669, 2000. [PMC free article] [PubMed] [Google Scholar]
- 10.Cowley EA, Linsdell P. Characterization of basolateral K+ channels underlying anion secretion in the human airway cell line Calu-3. J Physiol 538: 747–757, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dale S, Wilson WA, Edelman AM, Hardie DG. Similar substrate recognition motifs for mammalian AMP-activated protein kinase, higher plant HMG-CoA reductase kinase-A, yeast SNF1, and mammalian calmodulin-dependent protein kinase I. FEBS Lett 361: 191–195, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Devor DC, Bridges RJ, Pilewski JM. Pharmacological modulation of ion transport across wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol Cell Physiol 279: C461–C479, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Gerlach AC, Gangopadhyay NN, Devor DC. Kinase-dependent regulation of the intermediate conductance, calcium-dependent potassium channel, hIK1. J Biol Chem 275: 585–598, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Gerlach AC, Syme CA, Giltinan L, Adelman JP, Devors DC. ATP-dependent activation of the intermediate conductance, Ca2+-activated K+ channel, hIK1, is conferred by a COOH-terminal domain. J Biol Chem 276: 10963–10970, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Hallows KR, Fitch AC, Richardson CA, Reynolds PR, Clancy JP, Dagher PC, Witters LA, Kolls JK, Pilewski JM. Up-regulation of AMP-activated kinase by dysfunctional cystic fibrosis transmembrane conductance regulator in cystic fibrosis airway epithelial cells mitigates excessive inflammation. J Biol Chem 281: 4231–4241, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Hallows KR, Kobinger GP, Wilson JM, Witters LA, Foskett JK. Physiological modulation of CFTR activity by AMP-activated protein kinase in polarized T84 cells. Am J Physiol Cell Physiol 284: C1297–C1308, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Hallows KR, McCane JE, Kemp BE, Witters LA, Foskett JK. Regulation of channel gating by AMP-activated protein kinase modulates cystic fibrosis transmembrane conductance regulator activity in lung submucosal cells. J Biol Chem 278: 998–1004, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Hallows KR, Raghuram V, Kemp BE, Witters LA, Foskett JK. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest 105: 1711–1721, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem 67: 821–855, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays 23: 1112–1119, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 271: 27879–27887, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Henin N, Vincent MF, Gruber HE, Van den BG. Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J 9: 541–546, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Henin N, Vincent MF, Van den BG. Stimulation of rat liver AMP-activated protein kinase by AMP analogues. Biochim Biophys Acta 1290: 197–203, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Jones HM, Hamilton KL, Devor DC. Role of an S4–S5 linker lysine in the trafficking of the Ca(2+)-activated K(+) channels IK1 and SK3. J Biol Chem 280: 37257–37265, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Jones HM, Hamilton KL, Papworth GD, Syme CA, Watkins SC, Bradbury NA, Devor DC. Role of the NH2-terminus in the assembly and trafficking of the intermediate conductance Ca2+-activated K+ channel, hIK1. J Biol Chem 279: 15531–15540, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1: 15–25, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Klein H, Garneau L, Banderali U, Simoes M, Parent L, Sauvé R. Structural determinants of the closed KCa3.1 channel pore in relation to channel gating: Results from a substituted cysteine accessibility analysis. J Gen Physiol 129: 299–315, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leroy C, Dagenais A, Berthiaume Y, Brochiero E. Molecular identity and function in transepithelial transport of KATP channels in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 286: L1027–L1037, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Light PE, Wallace CH, Dyck JR. Constitutively active adenosine monophosphate-activated protein kinase regulates voltage-gated sodium channels in ventricular myocytes. Circulation 107: 1962–1965, 2003. [DOI] [PubMed] [Google Scholar]
- 30.MacVinish LJ, Hickman ME, Mufti DA, Durrington HJ, Cuthbert AW. Importance of basolateral K+ conductance in maintaining Cl- secretion in murine nasal and colonic epithelia. J Physiol 510: 237–247, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maylie J, Bond CT, Herson PS, Lee WS, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin. J Physiol 554: 255–261, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCann JD, Welsh MJ. Basolateral K+ channels in airway epithelia. II. Role in Cl− secretion and evidence for two types of K+ channel. Am J Physiol Lung Cell Mol Physiol 258: L343–L348, 1990. [DOI] [PubMed] [Google Scholar]
- 33.Mitchelhill KI, Michell BJ, House CM, Stapleton D, Dyck J, Gamble J, Ullrich C, Witters LA, Kemp BE. Posttranslational modifications of the 5′-AMP-activated protein kinase beta1 subunit. J Biol Chem 272: 24475–24479, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Pedarzani P, Mosbacher J, Rivard A, Cingolani LA, Oliver D, Stocker M, Adelman JP, Fakler B. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J Biol Chem 276: 9762–9769, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Qin F, Auerbach A, Sachs F. Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys J 70: 264–280, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J 403: 139–148, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sauvé R, Simoneau C, Monette R, Roy G. Single-channel analysis of the potassium permeability in HeLa cancer cells: evidence for a calcium-activated potassium channel of small unitary conductance. J Membrane Biol 92: 269–282, 1986. [DOI] [PubMed] [Google Scholar]
- 38.Schilling T, Eder C. TRAM-34 inhibits nonselective cation channels. Pflügers Arch 454: 559–563, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Shigemori K, Ishizaki T, Matsukawa S, Sakai A, Nakai T, Miyabo S. Adenine nucleotides via activation of ATP-sensitive K+ channels modulate hypoxic response in rat pulmonary artery. Am J Physiol Lung Cell Mol Physiol 270: L803–L809, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Singh AK, Devor DC, Gerlach AC, Gondor M, Pilewski JM, Bridges RJ. Stimulation of Cl(−) secretion by chlorzoxazone. J Pharmacol Exp Ther 292: 778–787, 2000. [PubMed] [Google Scholar]
- 41.Srivastava S, Choudhury P, Li Z, Liu G, Nadkarni V, Ko K, Coetzee WA, Skolnik EY. Phosphatidylinositol 3-phosphate indirectly activates KCa3.1 via 14 amino acids in the carboxy terminus of KCa31. Mol Biol Cell 17: 146–154, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srivastava S, Li Z, Ko K, Choudhury P, Albaqumi M, Johnson AK, Yan Y, Backer JM, Unutmaz D, Coetzee WA, Skolnik EY. Histidine phosphorylation of the potassium channel KCa3.1 by nucleoside diphosphate kinase B is required for activation of KCa31 and CD4 T cells. Mol Cell 24: 665–675, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Syme CA, Gerlach AC, Singh AK, Devor DC. Pharmacological activation of cloned intermediate- and small-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 278: C570–C581, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Syme CA, Hamilton KL, Jones HM, Gerlach AC, Giltinan L, Papworth GD, Watkins SC, Bradbury NA, Devor DC. Trafficking of the Ca2+-activated K+ channel, hIK1, is dependent upon a COOH-terminal leucine zipper. J Biol Chem 278: 8476–8486, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Szkotak AJ, Murthy M, MacVinish LJ, Duszyk M, Cuthbert AW. 4-Chloro-benzo isoquinoline (CBIQ) activates CFTR chloride channels and KCNN4 potassium channels in Calu-3 human airway epithelial cells. Br J Pharmacol 142: 531–542, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trinh NT, Prive A, Maille E, Noel J, Brochiero E. EGF and K+ channel activity control normal and cystic fibrosis bronchial epithelia repair. Am J Physiol Lung Cell Mol Physiol 295: L866–L880, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Woods A, Salt I, Scott J, Hardie DG, Carling D. The alpha1 and alpha2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett 397: 347–351, 1996. [DOI] [PubMed] [Google Scholar]
- 48.Wyatt CN, Mustard KJ, Pearson SA, Dallas ML, Atkinson L, Kumar P, Peers C, Hardie DG, Evans AM. AMP-activated protein kinase mediates carotid body excitation by hypoxia. J Biol Chem 282: 8092–8098, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao B, Heath R, Saiu P, Leiper FC, Leone P, Jing C, Walker PA, Haire L, Eccleston JF, Davis CT, Martin SR, Carling D, Gamblin SJ. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature 449: 496–500, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Zabner J, Karp P, Seiler M, Phillips SL, Mitchell CJ, Saavedra M, Welsh M, Klingelhutz AJ. Development of cystic fibrosis and noncystic fibrosis airway cell lines. Am J Physiol Lung Cell Mol Physiol 284: L844–L854, 2003. [DOI] [PubMed] [Google Scholar]