Abstract
Pannexin 1 forms a large membrane channel that, based on its biophysical properties and its expression pattern, is a prime candidate to represent an ATP release channel. Pannexin 1 channel activity is potentially deleterious for cells as indicated by its involvement in the P2X7 death complex. Here we describe a negative feedback loop controlling pannexin 1 channel activity. ATP, permeant to pannexin 1 channels, was found to inhibit its permeation pathway when applied extracellularly to oocytes expressing pannexin 1 exogenously. ATP analogues, including benzoylbenzoyl-ATP, suramin, and brilliant blue G were even more effective inhibitors of pannexin 1 currents than ATP. These compounds also attenuated the uptake of dyes by erythrocytes, which express pannexin 1. The rank order of the compounds in attenuation of pannexin 1 currents was similar to their binding affinities to the P2X7 receptor, except that receptor agonists and antagonists both were inhibitory to the channel. Mutational analysis identified R75 in pannexin 1 to be critical for ATP inhibition of pannexin 1 currents.
Keywords: ATP release, brilliant blue G, benzylbenzoyl-ATP, P2X7 receptor, erythrocyte
pannexin 1 forms one of the largest channels found in cell membranes of vertebrates. The single channel conductance is ∼500 pS, and the channel is accessible to molecules in the size range of ∼1.5 kDa (1, 35). Opening of this channel, therefore, is potentially lethal to cells because of the ensuing rundown of ionic gradients and loss of precious cellular constituents. Indeed, prolonged activation of pannexin 1 channels by ATP binding to coexpressed P2X7 receptors results in cell death (20). Pannexin 1 is widely expressed and expression levels can be high as evidenced by strong labeling of plasma membranes in various tissues, including neurons and astrocytes, with anti-pannexin 1 antibodies (2, 9, 14, 19, 25, 30, 36). Considering the potentially lethal effects of channel opening, there must be effective mechanisms keeping pannexin 1 channels in check. Here we show that extracellular ATP is an inhibitor of pannexin 1-mediated currents. ATP apparently binds to extracellular moieties of the channel protein, and the pharmacological properties of pannexin 1 channel inhibition resemble those of the P2X7 receptor.
Pannexins were discovered by virtue of their sequence homology to the invertebrate gap junction proteins, the innexins (25). However, in contrast to innexins, pannexins do not appear to form intercellular gap junction channels in vivo (4, 9, 14, 27). Instead, pannexin 1 operates probably exclusively as an unapposed membrane channel. Channel activities attributable to pannexin 2 or pannexin 3 have not been demonstrated (6). Because of its inherent properties, including high ATP permeability, pannexin 1 is a prime candidate for the long-sought ATP release channel. When coexpressed with purinergic receptors the pannexin 1 channel can be opened by extracellular ATP (20, 21). Given the high ATP permeability, this should result in a positive feedback loop keeping the channels permanently open. However, the opposite is observed. Exposure to ATP of cells coexpressing pannexin 1 with either P2Y or P2X7 receptors results only in a transient activation of pannexin 1 channels; they inactivate in the presence of ATP (20, 21). Thus an inhibitory mechanism of pannexin 1 channel activity must exist.
MATERIALS AND METHODS
Preparation of oocytes.
Preparation of oocytes and electrophysiological recording were performed as described (10). Mouse pannexin 1 was kindly provided by Dr. Rolf Dermietzel (University of Bochum), and Cx46 (connexin 46) was obtained from Dr. D. L. Paul (Harvard University). The plasmid containing mouse pannexin 1 in pCS2 was linearized with NotI and the plasmid containing Cx46 (rSP64T) was linearized with EcoR1. In vitro transcription was performed with SP6 polymerase, using the Message Machine kit (Ambion, Austin, TX). mRNAs were quantified by absorbance (260 nm), and the proportion of full-length transcripts was checked by agarose gel electrophoresis. In vitro transcribed mRNAs (∼40 nl) were injected into Xenopus oocytes. Cells were kept in regular frog Ringer solution OR2 (in mM: 82.5 NaCl, 2.5 KCl, 1 Na2HPO4, 1 MgCl2, 1 CaCl2, and 5 HEPES) with 10 mg/ml streptomycin.
Electrophysiology.
Whole cell membrane current of single oocytes was measured using a two-electrode voltage clamp and recorded with a chart recorder. Both voltage-measuring and current-passing microelectrodes were pulled with a vertical puller (Kopf) and filled with 3 M KCl. The recording chamber was perfused continuously with solution. Membrane conductance was determined using voltage pulses. Oocytes expressing Cx46 were held at −10 mV, and depolarizing pulses of 5 s duration and of 10 mV amplitude were applied. Oocytes expressing pannexin 1 were held at −50 mV, and pulses to +50 mV were applied to transiently open the channels.
Single-channel patch clamp.
Single pannexin 1 channels were studied by the patch-clamp technique (13) using a WPC 100 amplifier (E. S. F. Electronic, Goettingen, Germany). The vitelline membrane of the oocyte was manually removed and the oocyte was washed once before transfer into a new dish containing NaCl solution (in mM: 140 NaCl, 10 KCl, and 5 TES; pH 7.5). Electrode pipettes made from glass capillary tubing (1.5–0.86 mm, no. GC150F-15, Warner Instrument) were pulled using a Flaming-Brown Micropipette Puller (model P-97, Sutter Instrument) and polished with a microforge (Narishige Scientific Instruments) to 0.5–1 μm with resistances of 10–20 MΩ in NaCl solution. Both the standard pipette and bath solution were NaCl solution. After an outside-out patch was excised from the membrane and the pannexin 1 channel was identified, the patch was transferred into a microperfusion chamber, which was continuously perfused with solution. The perfusion system was driven by gravity at a flow rate of 100 μl/s.
Dye uptake.
Xenopus erythrocytes were washed three times in Ringer solution by low-speed centrifugation. Erythrocytes were suspended at 0.1% hematocrit and aliquots of 75 μl were plated onto poly-d-lysine-coated 96-well plates (BioCoat, Becton Dickinson). OR2 alone (25 μl) or with four times concentration of drugs were added and preincubated for 10 min (final volume 100 μl). Solution (85 μl) was removed from the well and dye uptake was initiated by adding 100 μl KGlu (in mM: 140 potassium gluconate, 10 potassium chloride, and 5 TES; pH 7.5) solution with 5 μM YoPro-1 with or without drugs. Addition of 100 μl OR2 with YoPro-1 instead of KGlu served as negative control. Images were acquired with a Canon Powershot S3 IS digital camera with an exposure time of 6 s and an aperture setting of 3.2 attached to the phototube of an inverted fluorescence microscope (model DMIL, Leica).
ATP-release assay.
ATP flux was determined by luminometry. Oocytes, 2 days after injection of pannexin 1 messenger RNA, were pretreated in OR2 solution with and without brilliant blue-G (BBG) for 10 min and stimulated by incubation in OR2 solution (negative control), KGlu solutions (positive control), and KGlu solution with BBG, respectively, for 10 min. The supernatant was collected and assayed with luciferase-luciferin (Promega, Madison).
Site-directed mutagenesis.
The alanine mutants were engineered with QuickChange II site-directed mutagenesis kit (Stratagene) according to the manufacturer's specifications.
RESULTS
Inhibition of pannexin 1 currents by ATP and analogues.
When held at negative potentials, pannexin 1 channels are closed, and application of ATP is inconsequential unless purinergic receptors are present and activated (20, 21). Pannexin 1 channels open at positive membrane potentials (1, 6). To test ATP effects on open pannexin 1 channels, we used Xenopus oocytes expressing mouse pannexin 1 exogenously. From a holding potential of −50 mV we applied voltage steps to +50 mV to induce pannexin 1-mediated currents. Application of ATP attenuated these currents reversibly (Fig. 1). The dose dependency of this inhibition (Fig. 1C) indicated that significant inhibition was prominent at ATP concentrations slightly higher than those required for activation of purinergic receptors, including P2X7 and P2Y2. Thus an ATP-induced ATP release mechanism attributed to pannexin 1 channels (21) is at least transiently possible despite the inhibitory effect of ATP on the release channel. The effect of ATP on pannexin 1 channels appeared to be specific as channels of similar pore size formed by Cx 46 remained unaffected by ATP (Fig. 1D).
Fig. 1.
ATP and benzoyl-benzoyl-ATP (BzATP) inhibition of pannexin 1 currents but not of currents carried by Cx46. A: −50-mv to +50-mv voltage pulses induced large membrane currents in oocytes expressing mouse pannexin 1 channels, which were attenuated by 200 μM ATP reversibly. B: currents were inhibited by 20 μM BzATP to the same extent. C: inhibition of pannexin 1 currents by ATP and BzATP was concentration dependent. Plotted values are means ± SE (n = 4). D: 200 μM ATP and 50 μM BzATP had no inhibitory effect on currents carried by Cx46, which were induced by −10-mV to 0-mV voltage steps.
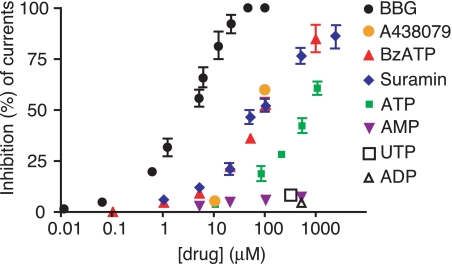
Purinergic receptors interact with a variety of ATP analogues and ATP metabolites, some activating, others inhibiting the receptors (7). The affinity of these agents to different purinergic receptors varies and has given rise to a classification of purinergic receptors, which subsequently was confirmed by cloning of the various P2Y and P2X subtypes. To test the specificity of ATP on pannexin 1 currents, we used purinergic receptor agonists and antagonists. Figure 2 shows that the ATP metabolites ADP and AMP were ineffective in inhibiting currents through pannexin 1 channels. The P2X7 “specific” agonist benzoylbenzoyl-ATP (BzATP) was more powerful than ATP at equal concentrations as a pannexin 1 channel inhibitor. The strongest inhibition was observed with BBG, which attenuated pannexin 1 channel currents with an IC50 of ∼3 μM (Fig. 2). The competitive P2X7 antagonist A438079 (11) inhibited pannexin 1 channels to a similar degree as BzATP (Fig. 2). The activity spectrum with which the various substances affected pannexin 1 currents in general closely resembles that of their interaction with the P2X7 receptor (17, 24), except that receptor agonists and antagonists both inhibited pannexin 1 currents.
Fig. 2.
Dose-response curves for inhibition of pannexin 1 currents by ATP and various analogues. Plotted values are means ± SE (n = 3–5). ATP could not be tested at higher concentrations because of activation of an endogenous conductance seen even in uninjected oocytes that was insensitive to probenecid, a pannexin 1 inhibitor (32), and thus not related to pannexin 1 (see supplemental Fig. 4).
Typically, the larger ligands yielded stronger channel inhibition. For example, the rank order of inhibition follows the size for BBG>BzATP>ATP. However, not all compounds obeyed this relationship. Notably, suramin was considerably less effective in inhibiting currents than BBG despite its larger size. BBG, on the other hand has much higher affinity for P2X7 receptors than suramin. A combination of effects, therefore, has to be considered. Consequently, the inhibition of pannexin channels may be on the basis of an affinity-dominated steric block of the channel.
Discrimination of gating and steric effects on channels typically can easily be achieved by single channel analysis. However, pannexin 1 channels are not conducive for this type of analysis because of their unusual properties. Already in the absence of interfering agents, pannexin 1 channels exhibit multiple subconductance levels. Supplemental Fig. 1 shows a record from an excised outside-out membrane patch containing more than one pannexin 1 channel. Channel activity can be ascribed to pannexin 1 because of the attenuation by carbenoxolone, a known pannexin 1 inhibitor (5), which has no endogenous target channel in Xenopus oocytes. Application of BzATP or BBG had similar qualitative effects on channel activity as carbenoxolone. The complex channel currents were reduced to discernible unitary events (supplemental Fig. 1b) with conductances that are only a fraction of the full conductance of ∼500 pS of pannexin 1 channels. The drugs, therefore, either attenuated the channel conductance by steric block or they forced the channel into any one of the lower subconductance states.
Inhibition of ATP release and dye uptake by ATP or analogues.
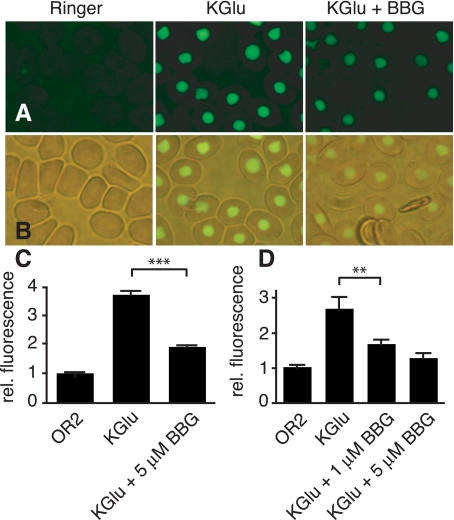
The effect of ATP analogues on pannexin 1 channels is not limited to inhibition of ionic currents. BBG, for example, also inhibited ATP release from oocytes expressing pannexin 1 exogenously (Fig. 3). Oocytes, when depolarized by potassium, release ATP by an endogenous mechanism that is sensitive to Brefeldin and consequently thought to be vesicular (22). Pannexin 1 expressing oocytes when exposed to high potassium solution released ATP at a significantly higher level than uninjected oocytes (1, 32). This release was attenuated by BBG in a dose-dependent fashion.
Fig. 3.
Inhibition by brilliant blue-G (BBG) of ATP release from oocytes expressing pannexin 1. ATP release was induced by high potassium solution, and ATP in the medium was measured by luminometry using a luciferase assay. ATP release was attenuated by BBG in a dose-dependent way. To control for effects of the solutions themselves on the luciferase assay, the various solutions were tested in the presence of 10 nM ATP. Plotted values are means ± SE (n = 5). *P < 0.05.
To test whether the inhibition of pannexin 1 channels by ATP and its analogues may also apply in vivo, we examined erythrocytes. These cells release ATP under shear stress and in response to a low oxygen environment, and the release appears to be mediated by pannexin 1 channels (3, 19, 33). Under conditions of ATP release, a concomitant uptake of extracellular dyes like carboxyfluorescein can be observed, which is a convenient surrogate measure for ATP release.
We used the nucleated frog erythrocytes in combination with YoPro, a dye that fluoresces highly only when bound to nucleic acid. Although the presence of pannexin 1 has not been directly demonstrated in frog erythrocytes, it is probable that they do. Human erythrocytes express this protein at high levels, and evidence indicates that pannexin 1 mediates ATP release and dye uptake (19). Dye uptake in frog erythrocytes is similarly blocked by pannexin 1 inhibitors (32), consistent with a pannexin 1 involvement in this process. Figure 4 shows that the uptake of YoPro by frog erythrocytes was attenuated by BBG. Curiously, when applied at their respective IC50 concentrations for current inhibition the rank order for inhibition was altered, BzATP was most effective in inhibiting dye uptake, followed by ATP and BBG, which was least effective (Fig. 4 and supplemental Fig. 2).
Fig. 4.
Uptake of YoPro by Xenopus erythrocytes. A: fluorescence micrographs were taken 15 min after application of YoPro (5 μM) in OR2 (left), KGlu (middle), and KGlu with 5 μM BBG (right). B: combined brightfield and fluorescence micrographs show the nuclear localization of the dye. C: quantitative analysis of dye uptake in A using the NIH Image analysis program using the profile plot function. Data were normalized to fluorescence in unstimulated erythrocytes. Plotted values are means ± SE (n = 50 erythrocytes). Two additional experiments yielded similar results. D: quantitative data obtained by plate reader analysis. Plotted values are means ± SE; n = 9 (3 wells × 3 experiments). ***P < 0.0001, **P < 0.01.
Mutational analysis of putative ATP binding site.
The inhibition of pannexin 1-mediated currents by ATP and analogues is probably not by simple partitioning of these molecules in the channel and thereby interfering with the flow of smaller ions. The concentrations of the reagents required for observing current inhibition were too low for such an effect. Furthermore, the deviation from a strict size dependence is not consistent with such a mechanism. Instead, the binding of ATP to the pannexin 1 protein appears to be a plausible mechanism.
The effective concentrations for channel inhibition by ATP applied from the extracellular side are considerably lower than the ATP concentration prevailing in the cytoplasm. Thus putative binding sites can be expected to be on the external portion of the pannexin 1 protein. Further support for an extracellular site comes from the observation that extracellular application of ATP (not shown) or BBG to the closed channel affected the channel upon reopening (supplemental Fig. 3).
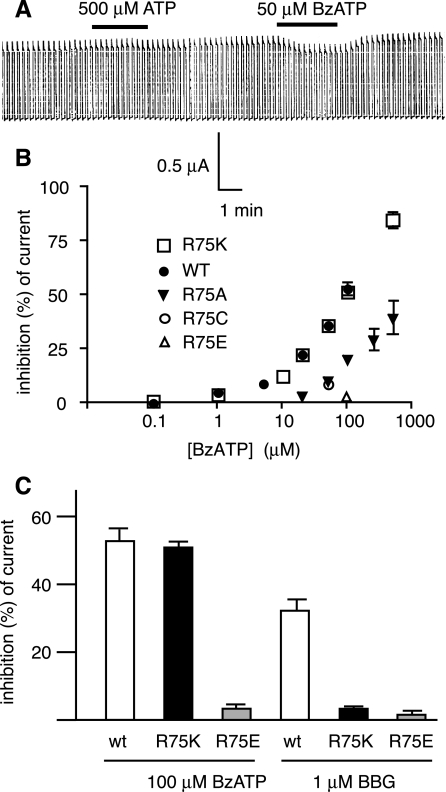
None of the canonical ATP binding sites has been found in the pannexin 1 sequence. Whereas the same applies to P2X7 receptors, the contribution of positively charged amino acids to ATP binding has been established (12, 18). Because the inhibition of pannexin 1 channels exhibited a similar pharmacology to the P2X7 receptor, we tested whether mutations of the positively charged amino acids in the putative extracellular loops altered the effect of ATP on pannexin 1 channels. Figure 5 shows that conversion of arginine in position 75 to alanine attenuated the BzATP inhibition of channel currents. Mutation of any other of the remaining five arginines or lysines was inconsequential for both channel activity and its inhibition by ATP or analogues in three mutants, whereas the remaining two mutants did not yield functional channels (data not shown). We therefore focused on position 75 and made additional mutations. Replacement of the arginine with lysine was of no consequence for the inhibitory effect of BzATP on pannexin 1 currents. Replacement of R75 with glutamate completely abolished the effect of BzATP on pannexin 1 currents. Cysteine replacement decreased the sensitivity for BzATP to a similar extent as the alanine replacement. The inhibition of pannexin 1 channels by BBG was also altered in the R75 mutants. However, the effect depended on the amino acid replacing R75. For example, BzATP and BBG inhibition of channel activity was almost nonexistent in the R75E mutant. In contrast, the R75K mutation was without consequence for the effect of BzATP while it highly attenuated the inhibitory effect of BBG (Fig. 5C).
Fig. 5.
Effect ATP and BzATP on pannexin 1 R75 mutants. A: large membrane currents in R75A mutant expressing oocytes were not affected by 500 μM ATP and decreased slightly in 50 μM BzATP. B: BzATP dose-response curve of wild-type pannexin 1 and mutants R75A, R75K, R75C, and R75E. Plotted values are means ± SE (n = 3–5). C: effects of BzATP and BBG on mutants R75K and R75E. BzATP and BBG were applied at 100 and 1 μM, respectively.
DISCUSSION
Accumulating evidence indicates that pannexin 1 forms ATP release channels. The biophysical properties of pannexin 1 channels are consistent for such a role, as is the expression pattern of pannexin 1 at the cellular and subcellular level. The channel is highly permeable to ATP (1), is mechanosensitive, and can be activated at the resting membrane potential by ATP by means of P2Y purinergic receptors (21) or P2X7 receptors (20, 26). Pannexin 1 is expressed in ATP-releasing cells, including erythrocytes, endothelial cells, astrocytes, and bronchial epithelial cells (9, 14, 15, 19, 29, 31). In the last cells it is localized to the luminal membrane, the site of ATP release.
If pannexin 1 forms ATP-release channels, it seems counterintuitive that ATP would inhibit channel activity. However, negative regulation of a channel by its permeant has previously been reported. For example, as part of the desensitization at synapses, calcium ion entry inactivates the permeation pore Cav2.1 (23). However, the action of calcium is not direct but involves a Ca2+-sensor protein. A direct action of the permeant ion on its conduction pathway has been demonstrated for Cl− on ClC channels, but its effect is a facilitation of ion permeation (8).
Pannexin 1 forms a potentially deadly channel. Its large pore size combined with low selectivity will result in fast rundown of electrochemical gradients and loss of essential cellular constituents. Indeed, prolonged or repeated activation of pannexin 1 channels can result in cell death (20). Pannexin 1 channels can be opened at the resting membrane potential by extracellular ATP via P2Y or P2X7 receptors. This represents a positive feedback loop that probably underlies ATP-induced ATP release in various tissues. Yet, cell death typically is not encountered when pannexin 1 channels are activated through purinergic receptors, with the exception of prolonged activation of P2X7 receptors. In fact, activation through P2Y2 results in a transient response; the current attenuates in the presence of ATP (21). This could be due to receptor desensitization or channel inhibition. The present results indicate that channel inhibition is at least a contributing factor, providing a negative feedback loop opposing the positive one involving purinergic receptor-mediated pannexin 1 channel activation.
The effect of ATP on pannexin 1 channels appears to be direct; no intermediates probably are involved. Aside from the effect of ATP on channels in excised membrane patches, this is further indicated by the attenuation of the effect of ATP and its analogues by mutations in pannexin 1. Although no overt canonical binding site for ATP is present in the pannexin 1 sequence, replacement of an arginine in position 75 with alanine drastically attenuated the inhibitory action of ATP and analogues on pannexin 1 currents. Of the other five basic amino acids in the extracellular portions and conserved in pannexin 1 of different species, alanine substitutions resulted in loss of function in two of them (K248A and K265A), whereas alanine substitutions of the remaining basic amino acids had no consequence for ATP effects on channel currents. Typically, ATP binding sites involve more than one basic amino acid. It is, therefore, important to evaluate the basic amino acids in positions 248 and 265 in more detail.
As in any mutagenesis approach, it has to be considered that the mutations have remote effects; i.e., that they do not necessarily identify the binding amino acid but induce conformational changes with long-range effects on the actual binding site. The observations that the mutational effects were replacement specific and that ATP and BBG effects were differentially modified do not support remote effects but suggest that R75 indeed is involved in ATP binding by pannexin 1.
The pharmacology of pannexin 1 inhibition is very similar to that of the P2X7 receptor except that receptor agonists and antagonists work similarly on the pannexin 1 channels. Even the competitive P2X7-specific antagonist A438079 effectively inhibited pannexin 1 channel currents. Thus it can be expected that the binding site in pannexin 1 is probably similar to that of the as-yet-unidentified ATP binding site of the P2X7 receptor. The involvement of basic amino acids in the ATP binding site in P2X7 has also been established (12, 18). The R75 mutation even affected the inhibition of pannexin 1 channel currents by BBG, a noncompetitive inhibitor of P2X7 receptors (17). The various mutants, however, influenced the inhibitory potency of BBG differently from that of BzATP. Thus the BBG and ATP binding sites, although distinct, may partially overlap.
The mechanism of action of ATP in inhibiting pannexin 1 channel activity appears to have a steric component. This is indicated by the considerably higher inhibitory effect of ATP or analogues on ATP release and dye uptake than on ion flux. Mismatch of effects on membrane currents and flux of larger molecules is typical for large bore channels, including connexin and pannexin channels. For example, a voltage gate in Cx46 channels drives the channel into a subconductance state with increased open probability thereby not affecting macroscopic conductance while inhibiting the flux of dyes and of second messengers (28). Inhibition of pannexin 1 channels by mimetic peptides or by appropriately sized polyethylene glycol, probably by steric block, reduces membrane currents only marginally while blocking dye flux almost completely (35).
The potency of the various drugs to inhibit pannexin currents, however, is only poorly correlated with molecular size of the drugs. For example suramin, the largest molecule used in this study, was less effective in inhibiting pannexin 1 currents than the smaller BBG. However, if the affinity of the drugs to the binding site in pannexin 1 is similar to that in the P2X7 receptor, suramin would have the lowest affinity to pannexin 1. Thus it is plausible that a combination of affinity and molecular size determine the potency of the drugs to inhibit pannexin 1 activity.
The multimeric nature of the channels (4), with six pannexin 1 molecules forming a channel, is yet another factor potentially skewing the molecular size-inhibition relationship. For example, if a channel can only accommodate a single large molecule, the inhibitory effect of the molecule can be significantly less pronounced than that exerted by a smaller compound that can bind to several or all subunits of the channel.
Regardless of the mechanism, the present findings indicate that caution is warranted when data involving these drugs are interpreted. The effects may be attributable to either P2X7 receptors, pannexin 1 channels, or both. The issue is furthermore complicated by the association of pannexin 1 with the P2X7 receptor (16, 20, 26). For example, ATP release and its surrogate measure, dye uptake, are inhibited by BBG, as is the propagation of calcium waves (34). In the past this would have been a clear indication that the P2X7 receptor itself mediates ATP release and calcium wave propagation. Although that conclusion is no longer tenable, an involvement of the P2X7 receptor in calcium wave propagation as activator of pannexin 1 channels cannot be excluded.
GRANTS
This work was supported by National Institutes of Health Grant GM-48610.
Supplementary Material
Acknowledgments
We thank Drs. W. Silverman and K. Muller for valuable discussions and reading of an early version of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572: 65–68, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics 83: 706–716, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 26: 40–47, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem 282: 31733–31743, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem 92: 1033–1043, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci USA 100: 13644–13649, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnstock G Current state of purinoceptor research. Pharm Acta Helv 69: 231–242, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Chen TY Coupling gating with ion permeation in ClC channels. Sci STKE 2003: pe23, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Dahl G, Locovei S. Pannexin: To Gap or not to Gap, is that a question? IUBMB Life 58: 409–419, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Dahl G, Pfahnl A. Mutagenesis to study channel structure. Methods Mol Biol 154: 251–268, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly-Roberts DL, Jarvis MF. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol 151: 571–579, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ennion S, Hagan S, Evans RJ. The role of positively charged amino acids in ATP recognition by human P2X1 receptors. J Biol Chem 275: 35656, 2000. [PubMed] [Google Scholar]
- 13.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Grinspan JB, Abrams CK, Scherer SS. Pannexin1 is expressed by neurons and glia but does not form functional gap junctions. Glia 55: 46–56, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA 104: 6436–6441, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. P2X7 receptor-pannexin 1 complex: pharmacology and signaling. Am J Physiol Cell Physiol 295: C752–C760, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol Pharmacol 58: 82–88, 2000. [PubMed] [Google Scholar]
- 18.Jiang LH, Rassendren F, Surprenant A, North RA. Identification of amino acid residues contributing to the ATP-binding site of a purinergic P2X receptor. J Biol Chem 275: 34190–34196, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA 103: 7655–7659, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex. FEBS Lett 581: 483–488, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett 580: 239–244, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Maroto R, Hamill OP. Brefeldin A block of integrin-dependent mechanosensitive ATP release from Xenopus oocytes reveals a novel mechanism of mechanotransduction. J Biol Chem 276: 23867–23872, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Mochida S, Few AP, Scheuer T, Catterall WA. Regulation of presynaptic Ca(V)2.1 channels by Ca2+ sensor proteins mediates short-term synaptic plasticity. Neuron 57: 210–216, 2008. [DOI] [PubMed] [Google Scholar]
- 24.North RA, Barnard EA. Nucleotide receptors. Curr Opin Neurobiol 7: 346–357, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol 10: R473–R474, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X(7) receptor. EMBO J, 2006. [DOI] [PMC free article] [PubMed]
- 27.Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, Laird DW. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci 120: 3772–3783, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Qu Y, Dahl G. Function of the voltage gate of gap junction channels: selective exclusion of molecules. Proc Natl Acad Sci USA 99: 697–702, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ransford GA, Dahl G, Fregien N, Conner GE, Salathe M. Pannexin 1 expression in airway epithelia. Am J Respir Crit Care Med 175: A887, 2007. [Google Scholar]
- 30.Ray A, Zoidl G, Weickert S, Wahle P, Dermietzel R. Site-specific and developmental expression of pannexin1 in the mouse nervous system. Eur J Neurosci 21: 3277–3290, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Scemes E, Suadicani SO, Dahl G, Spray DC. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol 3: 199–208, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol 295: C761–C767, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart Circ Physiol 275: H1726–H1732, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci 26: 1378–1385, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Ma M, Locovei S, Keane RW, Dahl G. Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol 293: C1112–C1119, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Zoidl G, Petrasch-Parwez E, Ray A, Meier C, Bunse S, Habbes HW, Dahl G, Dermietzel R. Localization of the Pannexin1 protein at postsynaptic sites in the cerebral cortex and hippocampus. Neuroscience 146: 9–16, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.