Abstract
Emerging evidence indicates that muscarinic receptors and ligands play key roles in regulating cellular proliferation and cancer progression. Both neuronal and nonneuronal acetylcholine production results in neurocrine, paracrine, and autocrine promotion of cell proliferation, apoptosis, migration, and other features critical for cancer cell survival and spread. The present review comprises a focused critical analysis of evidence supporting the role of muscarinic receptors and ligands in cancer. Criteria are proposed to validate the biological importance of muscarinic receptor expression, activation, and postreceptor signaling. Likewise, criteria are proposed to validate the role of nonneuronal acetylcholine production in cancer. Dissecting cellular mechanisms necessary for muscarinic receptor activation as well as those needed for acetylcholine production and release will identify multiple novel targets for cancer therapy.
Keywords: tumor genesis, nonneuronal, cholinergic signaling, acetylcholine, bile acids
efficient intercellular and intracellular signaling is critical for successful proliferation and survival of neoplastic cells. Depending on cancer cell type, a host of important receptors, ligands, signaling pathways, and molecules have been identified. From a clinical perspective, elucidating these signaling mechanisms is key to developing targeted, effective, and safe cancer therapeutics. In normal mammalian physiology, the critical importance of acetylcholine signaling via muscarinic receptors has long been recognized in neuronal tissue. However, the role of muscarinic signaling in neoplasia has received relatively scant attention. Recent observations, reviewed critically herein, prompted the present comprehensive review of the role of muscarinic receptors and ligands in cancer and an appraisal of the potential therapeutic implications of these experimental findings.
Acetylcholine was first discovered by Sir Henry Dale in 1914, and its function as a neurotransmitter was confirmed in 1926 by Otto Loewi (124). In both the central and peripheral nervous systems, acetylcholine plays a pivotal role in neuronal signaling (82, 83, 129). On the basis of the binding affinity of two naturally occurring substances, muscarine and nicotine, cholinergic receptors are generally classified in two broad categories, muscarinic or nicotinic. The discovery of more selective agonists and inverse agonists, along with the advent of gene cloning, led to the identification of five muscarinic receptor subtypes (designated M1R–M5R). In the present review, we focus almost exclusively on the role of muscarinic receptor signaling in cancer. Readers interested in nicotinic signaling may refer to recent excellent reviews (35, 48, 55).
It is not a novel concept that, in addition to neuronal production and release at nerve synapses, acetylcholine can be produced and released by nonneuronal cells in sufficient quantity to modulate cell function (9, 129). Nonetheless, over the past few years, a surge of information pertains to nonneuronal production and release of acetylcholine by neoplastic cells. Moreover, novel observations support the importance of muscarinic receptor expression and activation in cancer. We examined critically the purported role of muscarinic receptor signaling in the progression of neoplasia and, on the basis of the resulting analysis, propose novel criteria to validate the importance of both muscarinic signaling and nonneuronal acetylcholine production in cancer.
MUSCARINIC RECEPTORS
In 1869, Schmiedeberg and Kopp showed that increasing concentrations of extracts of the mushroom Amanita muscaria progressively slowed and arrested beating of frog hearts (17). It was discovered later that these effects were mediated by muscarinic receptors, members of a large receptor superfamily that activate guanosine 5′-phosphate (G) proteins (G protein-coupled receptors) (17). G proteins modulate the activity of adenylyl cyclase, ion channels, and phosphatidylinositol lipid turnover, thereby regulating a broad repertoire of biological responses (51, 85).
Structure and Subtypes of Muscarinic Receptors
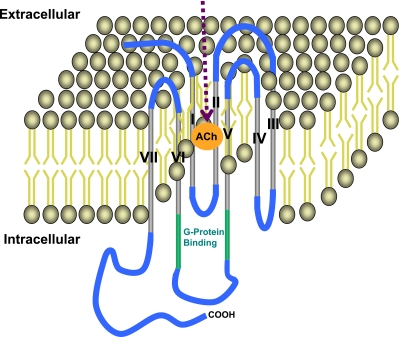
As illustrated in Fig. 1, muscarinic receptors are composed of seven transmembrane helical domains connected by three extracellular and three intracellular loops. Initially, on the basis of pharmacological selective affinity to the muscarinic agonist pirenzepine, two subtypes of muscarinic receptors, M1R and M2R, were described (61). In 1987, using molecular cloning techniques, Bonner et al. (14) described five muscarinic receptor subtypes (M1R–M5R). Within their seven transmembrane domains, these five subtypes exhibit extensive sequence homology, both with one another and with other G protein-coupled receptors (64). Receptor sequences exhibit greater variability at the extracellular amino terminus and the central portion of the third intracellular loop (127). The G protein-coupling selectivity of the individual muscarinic receptor subtypes is determined by amino acids located within the second intracellular loop and the membrane-proximal portions of the third intracellular loop (127). On activation, odd-numbered muscarinic receptors (i.e., M1R, M3R, M5R) couple to G proteins of the Gq/11 family, which activate phospholipase C-β to commence the phosphatidylinositol trisphosphate signaling cascade. Even-numbered muscarinic receptors (i.e., M2R and M4R) couple to Gi/o-type G proteins that inhibit adenylyl cyclase activity.
Fig. 1.
Illustration of general structure and key components of muscarinic receptors. The receptor is composed of 7 transmembrane domains (gray) connected by 3 intracellular and 3 extracellular loops (blue). The G protein-binding site is located at the junction of the third intracellular loop and sixth transmembrane domain (green). The acetylcholine (ACh) binding site is located in the cleft between the transmembrane domains (orange circle).
Overview of Muscarinic Receptor Distribution in Normal Tissues
Muscarinic receptors are expressed in various organ systems, and, in fact, expression of these critical signaling molecules may be ubiquitous. All five muscarinic receptor subtypes are expressed in brain (1). Likewise, all five muscarinic receptor subtypes are expressed in the eye, but M3R predominate (49). M3R and, to a lesser extent, M5R regulate cilliary muscle contraction, whereas M2R regulate pupillary constriction (13, 20, 32, 87). M1R, M2R, M3R, and M5R are expressed in the heart (126); M2R activation results in bradycardia and reduced inotropy, whereas M1R activation results in tachycardia (1, 33, 37, 67, 118). Muscarinic receptor activation produces vasodilatation in cerebral, coronary, and systemic vasculature (73, 135). While M3R activation produces relaxation of systemic vascular tone (73), M5R activation relaxes cerebral vascular tone (135). In various organs, including the gastrointestinal tract (30, 39, 52, 86), lung bronchioles (45), urinary bladder (29, 62, 125), and uterus (74), smooth muscle contraction is mediated by a mixture of M3 and M2 receptors. Whereas activation of M3R appears to be the primary regulator of smooth muscle contraction, activation of M2R potentiates this action (74).
In gastrointestinal tissue, the primary muscarinic receptor subtypes are M1R, M2R, and M3R. In salivary glands, M3R regulate flow and volume of secretion, whereas M1R regulate release of high-viscosity saliva (50, 65). M3R regulate acid secretion from gastric parietal cells (38, 113), and a mixture of M1R and M3R regulate pepsinogen secretion from chief cells (103, 119, 134). M3R also play a dominant role in regulating fluid and electrolyte secretion from enterocytes (18, 66, 120, 138, 139).
MUSCARINIC RECEPTOR LIGANDS AND SIGNALING
In humans, the only naturally occurring endogenous muscarinic receptor agonists are acetylcholine (ACh) and conjugated secondary bile acids. ACh production and release by both neuronal and nonneuronal cells is reported; these will be described separately. Evidence for the role of bile acids in muscarinic receptor signaling is also discussed in this section.
Neuronal Signaling by ACh
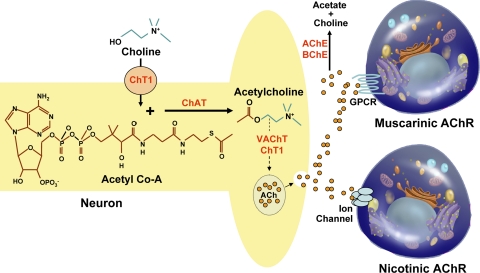
As illustrated in the overview of neuronal ACh production and release shown in Fig. 2, key components of neuronal ACh production and degradation include 1) plasma membrane choline transporters; 2) cytoplasmic choline acetyltransferase (ChAT), an enzyme that catalyzes formation of ACh from choline and acetyl coenzyme A (acetyl CoA) substrates; 3) cytoplasmic ACh transporters; and 4) cholinesterases that catalyze rapid ACh hydrolysis. Choline is actively transported into neurons by means of the choline transporter ChT-1. In a reaction catalyzed by ChAT, intracellular choline combines with acetate (derived from acetyl CoA), to form ACh and CoA. Assisted by vesicular transporters VAChT and ChT-1, newly formed ACh is stored in membrane vesicles that are transported along the axon. Following neuronal stimulation, these vesicles fuse with the plasma membrane, thereby releasing ACh into the synaptic cleft where it can interact with both muscarinic and nicotinic ACh receptors. Tissue and released acetylhydrolases [acetylcholinesterase (AChE), EC 3.1.1.7, and butyrylcholinesterase (BChE), EC 3.1.1.8] rapidly hydrolyze free ACh to form choline, acetate, and water. For ACh hydrolysis, AChE has 1.5 to 60 times greater activity than BChE, and, in humans, AChE comprises >95% of cholinesterase activity (7). Nonetheless, in rodents, inhibition of BChE results in a large increase in brain ACh (34). Hence, the enzyme primarily responsible for ACh hydrolysis may vary depending on the organ and species examined.
Fig. 2.
Illustration showing neuronal ACh production, release, degradation, and interaction with muscarinic and nicotinic cholinergic receptors. Choline acetyltransferase (ChAT) catalyzes synthesis of ACh from choline and acetyl-CoA substrates. ACh, released into the synaptic cleft following transport and fusion of vesicles with the cell membrane, binds muscarinic and nicotinic cholinergic receptors. Released ACh is rapidly hydrolyzed by tissue cholinesterases to form choline, acetate, and water. ChT1, a choline transporter that also functions as a vesicular transporter; VAChT, the major vesicular transporter; AChE, acetylcholinesterase; BChE, butyrylcholinesterase; GPCR, G protein-coupled receptor; AChR, acetylcholine receptor.
Nonneuronal Signaling by ACh
ACh released from nerve endings is rapidly hydrolyzed by cholinesterases, thereby limiting its actions to immediately neighboring cells. Hence, ACh production by nonneuronal cells can play a key role in regulating actions of cells or tissues that are not innervated by cholinergic neurons (e.g., breast and bronchial epithelial cells). To describe the production of ACh by nonneuronal tissues, independent of neuronal input, the term “nonneuronal ACh” was coined. In contrast to release of neuronal ACh at synaptic clefts, nonneuronal ACh signaling may not require expression of choline or vesicular transporters, and released ACh acts in an autocrine or paracrine mode, thereby regulating the function of ACh-releasing or neighboring cells, respectively (Fig. 3). Although expression of choline transporters is not mandatory for ACh production, in several murine nonneuronal tissues, expression of a choline transporter-like protein (CTL-1) was recently described (47, 121). However, in these studies, expression of CTL-1, which mediates choline uptake, was not necessary for ACh production. Hence, the requirement, if any, for active transport of choline into nonneuronal cells for ACh production remains to be determined.
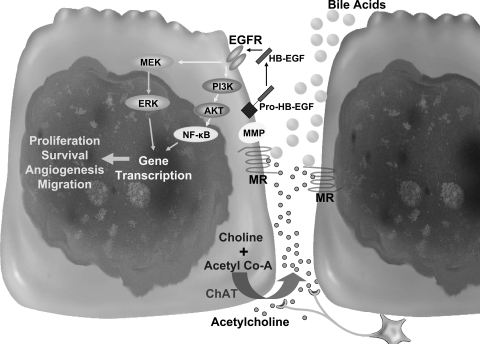
Fig. 3.
Proposed scheme for muscarinic receptor (MR) ligands and muscarinic signaling in colon cancer. ACh produced by colon cancer cells and neurons along with luminal bile acids activate muscarinic receptors, which, in turn, cleave pro-HB-EGF to its active form via matrix metalloproteinase (MMP)-7. HB-EGF activates EGF receptor (EGFR) and downstream signaling involving extracellular signal-related kinase (ERK) as well as phosphatidylinositol 3-kinase (PI3K), Akt, and nuclear factor-κB (NF-κB), resulting in gene transcription and subsequent biological actions. Nonneuronal ACh can interact with muscarinic receptors on the ACh-releasing (autocrine) and neighboring (paracrine) cells. MEK, ERK kinase.
ACh Production
ChAT (EC 2.1.3.6) is the primary enzyme that catalyzes both neuronal and nonneuronal ACh production (Figs. 2 and 3). Expression of ChAT in nonneuronal cells and tissues was reported more than 70 years ago (9). Since that initial report, ChAT expression has been identified broadly in nonneuronal cells; an incomplete list includes expression in skin keratinocytes (54, 75), lung epithelial cells (75, 115), intestinal epithelial cells (25, 75), erythrocytes (63), platelets (110), spermatozoa (11, 110), placenta and cornea epithelium (110).
Alternative (Non-ChAT) Mechanisms of ACh Production
ACh can be produced by a mechanism involving carnitine acetyltransferase (CrAT; EC 2.3.1.7), which is expressed in mammalian heart, skeletal muscle, retina, and urothelium (81, 109, 122, 123, 131). CrAT is a member of the carnitine acyltransferase family of enzymes that have substrate preferences for fatty acids and catalyze the exchange of acyl groups between carnitine and CoA (10). Recently, the structure of CrAT was explored and the importance of His343 in the active site was identified (69). While the impact of CrAT activity on the intracellular CoA pool is clear, its role in catalyzing ACh formation, using choline as substrate, is not well understood (95, 97). Using bromoacetylcarnitine, a CrAT inhibitor, Tucek (122) demonstrated 90% reduction in ACh production by homogenates of denervated rat muscle. In this system, ACh production was dependent on cellular choline concentration (122). Human urothelium also produces ACh (137), and, in this tissue, immunostaining and PCR demonstrated expression only of CrAT; ChAT was not detected (81). Hence, by using specific inhibitors, small interfering RNA (siRNA) knockdown, or other experimental approaches in a particular cell or tissue, it is important to clarify the relative contributions of ChAT and CrAT to nonneuronal ACh production.
Nonenzymatic ACh production in the presence of imidazoles has also been described both in vitro and in human cerebrospinal fluid (4, 19, 36). Burt and Silver (19) reported that imidazoles can mediate nonenzymatic time-, pH-, and concentration-dependent ACh production. It was proposed that ACh production by this mechanism involves formation of an N-acetylimidazole intermediate (19). In mammals, imidazole-containing molecules include creatinine and histamine. However, little is known regarding the tissue distribution, efficacy, kinetics, or frequency of this proposed mechanism of ACh production. As discussed below, collectively these considerations highlight the limitations of using ChAT expression alone as a surrogate marker of ACh production.
Finally, although the specificity of ChAT for choline is well characterized, the source of acetyl group donors can vary (129). For example, rat brain ChAT has the same affinity for acetyl-CoA, propionyl-CoA, and butyryl-CoA (106). Thus it is possible that, in addition to ACh, ChAT catalyzes synthesis of other molecules (e.g., propionyl- and butyrylcholine). On the basis of these additional observations, we conclude that expression of ChAT is neither necessary nor specific for de novo ACh production.
Mechanisms of Nonneuronal ACh Release
In central and peripheral nerve terminals, ACh synthesized by ChAT from acetyl-CoA and choline is translocated and stored in synaptic vesicles by vesicular ACh transporters (e.g., VAChT) (Fig. 2). After membrane depolarization, ACh-containing vesicles release ACh by exocytosis (Fig. 2). The VAChT gene is located within the ChAT gene (40). This configuration of the cholinergic gene locus (located on chromosome 10 in humans) indicates that cells expressing components of ACh production (i.e., ChAT), are also likely to express mechanisms for ACh release (i.e., VAChT).
In contrast, the mechanism underlying ACh release by normal and neoplastic nonneuronal cells is poorly understood. In cell lines derived from human small cell lung cancer (SCLC), robust expression of ChAT and VAChT, along with ACh release, is reported (115, 117). Vesamicol, a VAChT inhibitor, attenuated ACh release and proliferation of lung cancer cells (115). Nonetheless, since vesamicol did not completely inhibit ACh release from these cells, the existence of additional, nonvesicular mechanisms for ACh release is possible.
Nonneuronal expression of VAChT is also reported in rat bronchial goblet and neonatal rhesus monkey bronchial epithelial cells (80, 96, 115). However, to establish neuronlike ACh release, coexpression of ChAT and VAChT in the same cell type must be demonstrated. In contrast to demonstration of ChAT expression in a variety of nonneuronal mammalian cells, there is little evidence in nonneuronal cells for the presence of both VAChT expression and vesicular storage of ACh. These observations suggest that alternative ACh-release mechanisms are likely.
In this regard, Wessler et al. (130) demonstrated the presence of VAChT-independent release of ACh by human placenta. ACh release from placental villi was attenuated by organic cation transporter (OCT) inhibitors. OCT, members of the SLC22 and multidrug and toxin extrusion (MATE) family, mediate excretion and distribution of endogenous organic cations, xenobiotics, toxins, and environmental waste products. In human and rat bronchial epithelium and human urothelium, OCT types 1–3 are localized to the luminal membrane of ciliated epithelial cells that also express ChAT (80, 81). Moreover, OCT and VAChT have similar structural topology; 12 transmembrane domains with intracellular NH2- and COOH termini (77, 94).
Using Xenopus oocytes, transfection experiments indicate that human and murine OCT-1 and -2, but not OCT-3, are capable of transporting ACh (80). In contrast, in human placental villi, Wessler et al. (130) demonstrated that anti-sense oligonucleotides for both OCT-1 and OCT-3 reduced ACh release, whereas anti-OCT-2 oligonucleotides had no effect. These data suggest that members of the OCT family play a tissue-dependent role in nonneuronal ACh release.
Human colon cancer cells also express ChAT and produce and release ACh (25). Moreover, in surgical specimens, compared with adjacent normal tissue, colon cancer cells demonstrate increased ChAT expression (25). However, mechanisms regulating ACh release from colon cancer cells, including the role, if any, of vesicular transporters, are presently undefined. In an RT-PCR analysis of 50 cancer cell lines, colon cancer cells demonstrated expression of OCTs capable of transporting ACh, thereby raising the possibility that OCTs play a functional role in ACh release (53). Gene silencing techniques may shed additional light on the role of OCT in mediating ACh release from colon cancer cells. For example, using gene deletion, it was shown that OCT-1- and OCT-2-deficient mice have elevated levels of bronchial epithelial ACh compared with wild-type controls (78). These observations support a major role for OCT-1 and -2 in nonneuronal ACh release (70, 71, 140).
Collectively, these data indicate that various cancer cells express ChAT and membrane transporters with the potential to produce and translocate ACh, respectively. Nonetheless, whereas the mechanisms underlying neuronal production and release of ACh are well understood, much remains to be learned regarding similar mechanisms in nonneuronal tissues.
Muscarinic Receptor Signaling by Bile Acids
Deoxycholic and lithocholic acids and their glycine and taurine conjugates interact functionally with muscarinic receptors (22, 98–100). In studies using colon cancer cell lines, these bile acids stimulated cell proliferation by a mechanism involving matrix metalloproteinase (MMP)-mediated release of an EGF receptor (EGFR) ligand, and consequent post-EGFR cell signaling (Fig. 3) (24, 26).
H508 colon cancer cells, the primary in vitro model for these studies, derive from a moderately well-differentiated adenocarcinoma of the proximal colon (cecum). Cecal conjugated bile acids, determined by enzymatic assay and gas chromatography-mass spectrometry in recently deceased humans, achieved levels that stimulate colon cancer cell proliferation in vitro (10–100 μM) (60). Because of postmortem bacterial hydrolysis, it is likely that in these studies concentrations of conjugated secondary bile acids were underestimated. Cecal bile acid concentrations in persons with ileal disease, ileal resection, or colon cancer are not known. Certainly, depending on the severity and extent of injury, it is likely that ileal disease results in increased bile acid concentrations in the proximal colon, the area in which bile acids are most associated with increased cancer risk. Additional factors increase the likelihood that bile acid interaction with muscarinic receptors plays an important role in colon carcinogenesis: 1) fecal bile acids are in contact with colon epithelium for many years [the average age for developing colon cancer is >50 yr (133)]; 2) bile acids lack an ester linkage and are not hydrolyzed by tissue cholinesterases that rapidly inactivate ACh (23); 3) lipophilic lithocholic acid derivatives have access to muscarinic receptors in the lipid bilayer of colon cancer cell membranes (23); 4) neoplastic cells commonly lose cell membrane polarity, thereby leading to expression of receptors usually restricted to the basolateral membrane on the apical membrane; and 5) colon cancers have increased tight junction permeability, thereby providing access of luminal molecules to basolateral membrane receptors (112).
PROPOSED CRITERIA TO VALIDATE MUSCARINIC RECEPTOR-MEDIATED ACTIONS AND/OR NONNEURONAL PRODUCTION OF ACh IN CANCER
Muscarinic signaling is described in the four most common malignancies of men and women in the United States; breast, prostate, lung, and colon cancer. As shown in Table 1, muscarinic receptor expression is reported in tissues and cell lines derived from cancer of the brain (58), breast (41), colon (25), lung (114, 115), ovary (93), pancreas (2, 116), prostate (8, 84), skin (15, 16, 108), stomach (76), and uterus (116). Nonetheless, because muscarinic receptor expression is common if not ubiquitous in all tissues, simply demonstrating expression of muscarinic receptors is insufficient to conclude that muscarinic signaling plays an important role in cancer progression. This conclusion requires direct evidence that a specific biological action is mediated by activation of muscarinic receptor signaling.
Table 1.
Muscarinic receptor expression, activation, and signaling in cancer
| Organ/Cancer | MR Expression | Action Resulting From MR Activation | Post-MR Signaling Mediates Action | MR Agonist Mimics Action | Inhibiting ACh Production Attenuates Action | Inhibiting ACh Degradation Augments Action |
Reduced MR Expression or Activation Attenuates Action |
Reference | |
|---|---|---|---|---|---|---|---|---|---|
| In vitro | In vivo | ||||||||
| Brain (astrocytoma) | X | Proliferation | PKC-ɛ, PKC-ζ, ERK, NF-κΒ | X | ND | ND | X | ND | 6, 56–58 |
| Breast | X | Proliferation; angiogenesis | PKC-ζ, Src, ERK, PI3K | X | ND | X | X | ND | 21, 41, 42, 68 |
| Colon | X | Proliferation; survival | ERK, PI3K, Akt, NF-κB | X | X | X | X | X | 25, 27, 101, 102 |
| Lung | |||||||||
| Small cell | X | Proliferation | ERK, Akt | X | X | X | X | X | 115, 116 |
| Squamous cell | X | Proliferation | UNK | ND | ND | ND | X | X | 114 |
| Ovary | X | Reduced survival rate | UNK | ND | ND | ND | ND | ND | 93 |
| Pancreas | X | UNK | UNK | ND | ND | ND | ND | ND | 2, 31, 116 |
| Prostate | X | Proliferation | UNK | X | ND | ND | X | ND | 8, 104 |
| Skin | |||||||||
| Melanoma | X | Migration | Rho, ROK | X | ND | ND | X | ND | 15, 91, 108 |
| Merkel cell cancer | X | UNK | UNK | ND | ND | ND | ND | ND | 16 |
| Stomach | X | UNK | ERK* | ND | ND | ND | ND | ND | 76 |
| Uterine cervix | X | UNK | UNK | ND | ND | ND | ND | ND | 116 |
MR, muscarinic receptor; X, action demonstrated experimentally; ND, not demonstrated; UNK, unknown;
no proliferation despite ERK activation; PI3K, phosphatidylinositol 3-kinase; ROK, Rho kinase.
Experimentally, a functional role for muscarinic receptor expression can be demonstrated by using muscarinic receptor agonists and antagonists, by modulating muscarinic receptor ligand concentration in the cellular microenvironment, or by altering expression of muscarinic receptors. Moreover, if it is proposed that nonneuronal ACh production and release plays an important role in cancer, reducing ACh production by limiting choline availability or inhibiting ChAT activity should attenuate the proposed muscarinic action. Conversely, using AChE inhibitors, like eserine, to increase tissue concentrations of ACh, should augment the proposed action.
Data regarding muscarinic receptor signaling in cancer (Table 1) indicate that, for the most part, few investigations have taken a sufficiently rigorous experimental approach. There is heterogeneity in the literature regarding methods and criteria used to confirm the importance of muscarinic signaling. This is particularly true for studies proposing that cancers produce ACh that acts as a local trophic factor. As discussed above, demonstrating ChAT expression alone is insufficient to establish that ACh is produced by a cell or tissue. To confirm that ACh is produced nonneuronally and that it interacts in an autocrine and paracrine manner with cancer cells in vivo, at least two criteria must be met: 1) demonstration that a specific biological action is regulated by ACh and 2) measurement of ACh production in the tissue of interest. We propose that the following minimal criteria must be met to validate muscarinic receptor-mediated signaling and nonneuronal ACh production in cancer (Table 2).
Table 2.
Criteria to validate MR-mediated actions (A) and nonneuronal production of ACh (B)
A. Confirm that MR agonists regulate biological actions
|
B. Establish nonneuronal production of ACh in a tissue or cancer
|
Confirm That Muscarinic Receptor Agonists Regulate Proposed Actions
Criterion 1: demonstrate that muscarinic receptors are expressed in the tissue of interest.
Expression of muscarinic receptor subtypes (M1R–M5R) can be confirmed using immunohistochemistry, in situ hybridization, immunoblotting, and RT-PCR. Nonetheless, each of these experimental approaches has potential pitfalls. For example, immunohistochemistry and immunoblotting are dependent on the specificity of muscarinic receptor antibodies. In situ hybridization is dependent on expression of sufficient message (mRNA) for detection. The presence of muscarinic receptors can also be demonstrated using a radioligand binding approach (N-methyl-[3H]scopolamine is commonly used as a muscarinic receptor radioligand). Combining radioligand binding and immunoprecipitation using subtype-selective antibodies can detect expression of receptor subtypes with a decreased likelihood of nonspecific binding. Using any of these approaches, it is important to include appropriate positive and negative controls.
Criterion 2: muscarinic receptor agonists mimic the proposed biological action.
For these studies, ACh and carbamylcholine (carbachol), a synthetic ACh analog resistant to hydrolysis by AChE, are widely used as muscarinic receptor agonists. In a particular tissue, it is important to confirm that added ACh produces a desired effect at physiological concentrations. However, both ACh and carbachol are nonselective cholinergic agonists; they activate nicotinic as well as muscarinic receptors. To confirm that biological actions are mediated by muscarinic receptors, it is important to demonstrate that specific muscarinic receptor antagonists, at pharmacologically appropriate doses, block the actions induced by the agonist.
Criterion 3: inhibition of ACh production and/or release attenuates the proposed biological action.
ChAT is the dominant enzyme that catalyzes ACh production. In neurons and most cancer cells, ACh production can be attenuated by inhibiting ChAT expression and activity using a variety of approaches (e.g., siRNA knockdown and bromo-ACh, respectively). Bromo-ACh reacts with a sulfahydryl group in the active site of ChAT to form an inactivating thiol ester (105). Other pharmacological inhibitors are available for this purpose (122). Bromo-ACh can act as a covalent agonist for some nicotinic receptor subtypes. Thus, one should exercise caution when using bromo-ACh as a ChAT antagonist. Alternatively, ACh production can be inhibited by depleting substrates necessary for ACh production. For example, hemicholinium-3, an inhibitor of choline transport, decreases choline availability. Inhibition of ACh release with vesamicol (a VChAT inhibitor) can decrease ACh availability in the cell microenvironment. These latter approaches presume that choline or ACh transport is required for ACh production and release; limitations of such assumptions are discussed above.
Criterion 4: inhibition of ACh degradation enhances the proposed biological action.
AChE is the dominant ACh-hydrolyzing enzyme. Eserine (physostigmine) and other AChE inhibitors are commonly used to attenuate ACh degradation and, thereby, augment the proposed biological action regulated by muscarinic signaling.
Criterion 5: reduced expression or activation of muscarinic receptors attenuates the proposed biological action.
Reduced muscarinic receptor expression can be achieved by gene knockout or knockdown, using transgenic animals, siRNA, and other experimental strategies. Reduced activation of muscarinic receptors can be achieved using inverse agonists or antagonists (89). The concept of inverse agonism recognizes that muscarinic receptors are constitutively active in the absence of ligand binding (89). Demonstrating attenuation of biological actions in vivo provides the greatest translational potential for observing similar effects in humans. However, doing this requires a suitable animal model.
Establish Nonneuronal Production of ACh in a Tissue or Cancer
Criterion 1: demonstrate that the machinery needed to produce ACh exists in the tissue of interest.
ChAT is the most common enzyme that catalyzes ACh production. However, de novo ACh production may exist in tissues devoid of ChAT expression or activity, provided that an alternate enzymatic or nonenzymatic system, like CrAT or imidazoles, respectively, is identified and shown to produce ACh. To identify enzymes involved in ACh production, Tucek (122) used inhibitors of ChAT and CrAT (i.e., bromo-ACh and bromoacetylcarnitine, respectively). However, these inhibitors are not substrate specific and inhibit both ChAT and CrAT. Demonstrating expression of ChAT and CrAT mRNA and protein by RT-PCR and immunostaining/immunoblotting, respectively, is required. Moreover, in mammalian tissues the extent of nonneuronal distribution of these enzymes is unknown. Hence, once the expression of ChAT or CrAT is demonstrated, criteria 2 and 3 below must be fulfilled to establish functional significance. Imidazole-mediated nonenzymatic ACh synthesis is the least understood mechanism of ACh production. Imidazole concentrations required to mediate ACh production as well as absence of both ChAT and CrAT are necessary to confirm the importance of this nonenzymatic system.
Criterion 2: demonstrate ACh production in the tissue of interest without contamination by neuronal ACh.
In earlier studies, the primary methods for ACh detection used bioassays evaluating the ability of a test substance (e.g., tissue extract) to modulate contraction of frog rectus abdominis or a similar muscle preparation. Attenuation of these actions in the presence of an inhibitor, usually atropine, was considered sufficient evidence that ACh was present in the test material. It is evident that this approach is fraught with numerous potential pitfalls. Because other bioactive agents may contaminate tissue extracts and neuronal elements may be present, demonstration of such biological effects is non-specific. It is preferable to measure ACh directly in these tissues or cells using high performance liquid chromatography with electrochemical detection (HPLC-ED), scintigraphy, chromatography or other specific methods.
In tissue specimens, care must be taken not to confound results with ACh produced by neural tissue. If neural tissue is present in the test specimen, measured ACh deriving from neurons may result in a fallacious conclusion regarding nonneuronal production of ACh. Several approaches can be used to confirm nonneuronal ACh production. These include coimmunostaining with markers specific for neural tissue and ChAT to confirm that ChAT is expressed in nonneuronal cells. Laser capture microdissection may be particularly useful to avoid tissue contamination by neurons or other tissues (12). Using this technique, a cluster of cancer or other cells of interest can be removed from the tissue and any contaminating neural elements. This cluster of cells can then be used to amplify RNA or for other specific methods that will demonstrate expression of ChAT and other molecules needed for ACh production and release. Measuring ACh production by epithelial cell lines avoids confounding contamination by neuronal ACh.
Criterion 3: demonstrate that nonneuronal ACh produced at physiological concentrations elicits the proposed biological action.
For a given biological action, physiological concentrations of ACh may be uncertain; in most experiments this is estimated from stimulatory doses of ACh or analogs. ACh, measured by methods described above (criterion 2), should achieve concentrations that stimulate the proposed biological action. An alternative approach is to block basal activity with muscarinic receptor inhibitors. For example, Cheng et al. (25) showed that basal proliferation of H508 colon cancer cells is inhibited by adding increasing concentrations of nonselective and M3R-selective muscarinic receptor inhibitors (25). It is inferred that H508 cells produce and release a sufficient quantity of ACh to stimulate basal levels of cell proliferation. Similarly, in SCLC cells, Song et al. (115) showed that muscarinic receptor antagonists inhibit unstimulated cell proliferation.
As shown in Table 3, these criteria have been fulfilled for few human cancers.
Table 3.
Application of criteria in Table 2 to proposed nonneuronal ACh production in human cancers
| Organ | ChAT Expression | ACh Production | Physiological ACh Concentration | Reference |
|---|---|---|---|---|
| Colon | X | X | X | 25 |
| Lung | ||||
| Small cell | X | X | X | 115–117 |
| Squamous cell | X | X | ND | 114 |
| Pancreas | X | ND | ND | 116 |
| Skin (melanoma) | X | ND | ND | 108 |
| Uterine cervix | X | ND | ND | 116 |
ChAT, choline acetyltransferase; X, experimental evidence.
MUSCARINIC RECEPTOR EXPRESSION AND ACTIVATION IN CANCER
To date, in pancreatic, gastric, ovarian, cervical, and Merkel cell cancer, only the expression of muscarinic receptors has been reported (Table 1). A specific biological function following activation of these receptors has not been identified. However, in ovarian cancer, expression of muscarinic receptors is associated with reduced survival (93). In gastric cancer, ERK signaling is reported following muscarinic receptor activation. However, the failure of ERK signaling to stimulate gastric cancer cell proliferation raises questions regarding the importance of this observation (76). In human leukemia cell lines, expression of muscarinic receptors, ChAT, and CrAT, as well as ACh production, is reported (46, 72). Muscarinic receptor activation results in increased intracellular calcium and upregulation of c-fos (72). Although it is likely that these effects modulate leukemic cell function, evidence for this has yet to be reported.
As described below, more robust evidence supporting a key role for muscarinic receptor expression and activation exists for brain, breast, colon, skin, lung, and prostate cancer (Table 1).
Brain Cancer (Astrocytoma)
Muscarinic receptors (M2R, M3R, and M5R) are expressed in astrocytoma, a tumor of nonneuronal glial cells. Activation of M3R by cholinergic agonists stimulates proliferation of a primary astrocytoma cell line (58). It is proposed that proliferative actions stimulated by M3R activation are mediated by ERK and NF-κB signaling, which activate protein kinase C-ɛ and -ζ, respectively (56, 57, 59).
Breast Cancer
Breast cancer cell lines express muscarinic receptors that regulate cell proliferation and angiogenesis (41). Espanol et al. (42) concluded that M1R and M2R are involved in angiogenesis, whereas M1R, M2R, and M3R are involved in cell proliferation. Proliferation of breast cancer cells is regulated by postmuscarinic receptor activation of ERK signaling (68). In rats, Cabello et al. (21) showed that treatment with AChE inhibitors increased breast cancer risk. Studies using breast cancer cell xenografts treated with muscarinic agonists and antagonists indicated a role for nonneuronal ACh production in angiogenesis. However, compared with the use of other in vivo models to study the role of muscarinic receptors and ligands, xenograft models have limitations. For example, because breast cancer xenografts were treated with test agents before implantation, this did not exactly mimic effects on breast cancer cells in vivo. Another shortcoming of xenografts is that cell lines are maintained in vitro for years. Because of selection pressures, after passage for many generations effects on cancer cell lines may no longer be representative of the original tumor. Moreover, cells in culture lack the architectural and cellular complexity of in vivo tumors, which include inflammatory cells, vasculature, and stromal components.
Colon Cancer
Colon epithelial cells express M1R and M3R (101, 136). M3R are also expressed in colon cancer cell lines, and activation of these receptors stimulates cell proliferation that is inhibited by atropine (27, 43, 44). Using eserine to inhibit ACh degradation results in potentiation of cell proliferation, whereas reducing ACh production with a choline transport inhibitor, hemicholinium-3, attenuates proliferation (25). As shown in Fig. 3, in colon cancer cells, agonist binding to M3R results in MMP-7 activation, which cleaves pro-HB-EGF, thereby releasing HB-EGF, an EGFR ligand (26). Post-EGFR signaling, mediated by ERK activation, stimulates cell proliferation (24, 27). Downstream of EGFR, activation of an additional signaling pathway involving phosphatidylinositol 3-kinase/Akt mediates effects on cell proliferation and cell survival (25, 102). In human colon cancer cells, we recently showed that muscarinic ligands exert antiapoptotic actions that are mediated by an Akt- and NF-κB-dependent mechanism (111). Collectively, these effects of muscarinic receptor activation can stimulate colon cancer cell proliferation, survival, migration, and promote angiogenesis (Fig. 3).
Melanoma
Melanoma is a skin cancer with a high metastatic potential that arises from melanocytes. Expression of all five muscarinic receptor subtypes is reported in melanoma (108, 128). In human primary melanoma cells and cells derived from metastases, expression of M3R was demonstrated by immunohistochemistry (92). Cell migration and chemotaxis play pivotal roles in metastatic spread. In normal keratinocytes, M3R activation stimulates, whereas M4R activation inhibits, migration by means of a signaling mechanism involving Rho and Rho kinase. In health, an important role for cell migration in wound epithelialization was shown by Chernyavsky et al. (28) using M3R- and M4R-deficient mice. In melanoma cell lines, increased chemotaxis observed in response to cholinergic stimulation is attenuated by atropine (15). These findings imply that chemotaxis contributes to spread of melanoma although, to date, confirmatory in vivo studies are not reported.
Lung Cancer
In the United States, lung cancer is the most common lethal malignancy. Whereas SCLC cells are reported to express all five muscarinic receptor subtypes, squamous cell cancer (SCC) reportedly expresses only M2R, M3R, and M4R (114, 115). ACh and analogs stimulate lung cancer cell proliferation by interacting with M3R, thereby activating ERK and Akt signaling. Muscarinic receptor inverse agonists and antagonists attenuate this effect. In SCLC cells, augmented cell proliferation is observed following addition of eserine, and attenuated proliferation is observed with hemicholinium-3 (115). Although animal models of lung cancer, including SCLC, are described (88), in vivo studies to validate the role of muscarinic signaling in lung cancer were performed only using tumor xenografts. As discussed above, studying xenografts is not equivalent to using models of de novo cancer formation but technical limitations with de novo models necessitate the use of lung cancer xenografts. In SCLC xenografts, darifenacin, an M3R-selective antagonist, attenuated tumor growth (116).
Recently, Song et al. (114) reported that ACh production is upregulated in response to nicotinic stimulation. While darifenacin inhibited nicotine-induced proliferation of tumor cells in vitro, it attenuated SCC xenograft growth. Curiously, the investigators failed to demonstrate that muscarinic agonists induce tumor cell proliferation. Nevertheless, these experiments raise provocative questions regarding the potential for cross talk between nicotinic and muscarinic pathways in mediating lung cancer cell proliferation.
Prostate Cancer
Worldwide, in men, prostate cancer is the most common neoplasm. In prostate adenomas, Lepor and Kuhar (79) initially described and Ruggieri et al. (107) classified muscarinic receptors. Muscarinic receptors are expressed in both rat and human prostate cancer cell lines (8, 84). In 1997, Rayford et al. (104) reported that carbachol stimulates prostate cancer cell proliferation, an action attenuated by addition of muscarinic receptor inhibitors. On the basis of the pattern of inhibition with different muscarinic receptor antagonists, these investigators concluded that M3R mediate prostate cancer cell proliferation (104).
As discussed above, muscarinic receptor agonists commonly stimulate cancer cell proliferation. Nonetheless, there is a paucity of information regarding the role of muscarinic receptors in cell cycle regulation. In quiescent NIH 3T3 cells transfected with M3R, stimulation with carbachol results in ERK activation, expression of G1-phase cyclin-D1, and increased Rb phosphorylation, changes leading to DNA synthesis (90). Similarly, stimulation of normal human keratinocytes with carbachol upregulates cyclin-D1 (5). However, in activated NIH 3T3 and SCC-9 small cell lung cancer cells, muscarinic receptor stimulation causes transient cell cycle arrest and decreased proliferation (90, 132). These conflicting observations indicate that the results of muscarinic receptor activation on the cell cycle depend greatly on the cell model used and the cellular environment.
Summary
As reviewed above, ACh production is reported in a variety of normal and cancer cells (Table 1). Nonetheless, to date, strong evidence supports ACh production only by colon and lung (both small cell and squamous cell) cancer cells (25, 114, 116) (Table 2 and Table 3). In these cancer cells, muscarinic receptor antagonists inhibit basal cell proliferation, thereby providing further evidence that ACh is a growth factor. Although human keratinocytes and normal breast epithelial cells are reported to produce ACh, this phenomenon has not been described in cancers deriving from these cell types (54, 75, 128).
FUTURE DIRECTIONS
This review summarizes recent advances in elucidating the role of muscarinic receptors and ligands in cancer. Mechanistic observations regarding the role of muscarinic receptors derive primarily from in vitro investigation using cancer cell lines. Recent in vivo translational studies using lung cancer xenografts in nude mice (114, 116) and muscarinic receptor deficiency in mouse models of colon cancer (101), provide a useful framework to explore anti-neoplastic therapies targeted at mechanisms that underlie muscarinic receptor expression and activation, and ACh production and release.
Nonetheless, many questions persist regarding the role and mechanisms of muscarinic receptor activation in cancer. In colon cancer, although murine and human colon epithelial cells express both M3R and M1R, investigation has focused exclusively on M3 muscarinic receptors (3, 101). Although in mice M3R gene ablation reduces colon tumor number and size (101), the role of other muscarinic receptors, particularly M1R, requires investigation. It is conceivable that genetic ablation or reduced activation of both muscarinic receptor subtypes will have a greater anti-neoplastic effect than observed with just M3R deficiency.
Colon epithelial cells express and colon cancers over-express M3R. A more complete understanding of mechanisms underlying transcriptional and translational regulation of muscarinic receptor expression in cancer is needed. To investigate the role of these receptors in neoplasia, the availability of transgenic M1R- and M3R-deficient mice provides a useful tool. Future studies may benefit from transgenic models with organ-specific muscarinic receptor deficiency (for example, using Cre-lox-based techniques).
Likewise, mechanisms underlying transcriptional and translational regulation of ChAT and VAChT expression require further elucidation. Although experimental evidence indicates that these molecules play a role in ACh production and release, and autocrine and paracrine stimulation of lung and colon cancer cell proliferation, the regulation of these processes in unknown. Whereas, robust evidence supports production and release of ACh by lung and colon cancer cells, ChAT is expressed in cancer cells in other organs (e.g., pancreas and uterine cervix) (Table 1). Moreover, in some nonneuronal cells, organic cation transporters may mediate VAChT-independent ACh release. Regulation of these cellular components of nonneuronal ACh production and release require further investigation.
The overall importance of muscarinic receptor signaling in promoting neoplasia and cancer progression should be a prime focus of investigation. Clinical implications are broad. If toxicity and efficacy studies of anti-muscarinic agents in animal cancer models are favorable then human trials of these agents should be considered. Using the analogy of breast cancer, where tamoxifen treatment is targeted at tumors that express estrogen receptors, cancers with up-regulated muscarinic receptor expression may identify those most likely to benefit from anti-muscarinic receptor therapy. On the other hand, pesticides (e.g., parathione and malathione) commonly used in many parts of the world inhibit AChE activity. By reducing AChE activity, chronic exposure to these and similar agents may increase tissue concentrations of ACh, thereby stimulating muscarinic receptor activation. Hence, aside from other concerns regarding their use, exposure to these environmental hazards may increase the risk of neoplasia and promote faster progression of existent cancer. If, as indicated by the present review, muscarinic receptors and ligands are major players in cancer, then the environmental impact of these possible carcinogens is an additional concern that must be explored and addressed.
GRANTS
This work was supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs and by National Institutes of Health (NIH) Grants CA-107345 and CA-120407 (to J.-P. Raufman). N. Shah was supported by NIH Grant T32-DK-067872. S. Khurana was supported by NIH Grant K08-DK-081479.
Acknowledgments
We thank Dr. Jürgen Wess, Chief, Molecular Signaling Section, Laboratory of Bioorganic Chemistry, National Institute of Diabetes and Digestive and Kidney Diseases for critically reviewing this manuscript prior to submission.
REFERENCES
- 1.Abrams P, Andersson KE, Buccafusco JJ, Chapple C, de Groat WC, Fryer AD, Kay G, Laties A, Nathanson NM, Pasricha PJ, Wein AJ. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol 148: 565–578, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackerman MS, Roeske WR, Heck RJ, Korc M. Identification and characterization of muscarinic receptors in cultured human pancreatic carcinoma cells. Pancreas 4: 363–370, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Anini Y, Brubaker PL. Muscarinic receptors control glucagon-like peptide 1 secretion by human endocrine L cells. Endocrinology 144: 3244–3250, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Aquilonius SM, Eckernas SA. Choline acetyltransferase in human cerebrospinal fluid: non-enzymatically and enzymatically catalysed acetylcholine synthesis. J Neurochem 27: 317–318, 1976. [DOI] [PubMed] [Google Scholar]
- 5.Arredondo J, Hall LL, Ndoye A, Chernyavsky AI, Jolkovsky DL, Grando SA. Muscarinic acetylcholine receptors regulating cell cycle progression are expressed in human gingival keratinocytes. J Periodontal Res 38: 79–89, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Ashkenazi A, Ramachandran J, Capon DJ. Acetylcholine analogue stimulates DNA synthesis in brain-derived cells via specific muscarinic receptor subtypes. Nature 340: 146–150, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Ballard CG, Greig NH, Guillozet-Bongaarts AL, Enz A, Darvesh S. Cholinesterases: roles in the brain during health and disease. Curr Alzheimer Res 2: 307–318, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Batra S, Christensson PI, Hartley-Asp B. Characterization of muscarinic cholinergic receptors in membrane preparations from rat prostatic adenocarcinoma. Prostate 17: 261–268, 1990. [DOI] [PubMed] [Google Scholar]
- 9.Beyer G, Wense UT. Uber den nachweis von hormonen in einzelligen tieren (Cholin and acytylcholin in paramecium). Pflügers Arch Gesamte Physiol Menschen Tiere 237: 417–422, 1936. [Google Scholar]
- 10.Bieber LL Carnitine. Annu Rev Biochem 57: 261–283, 1988. [DOI] [PubMed] [Google Scholar]
- 11.Bishop MR, Sastry BV, Schmidt DE, Harbison RD. Occurrence of choline acetyltransferase and acetylcholine and other quaternary ammonium compounds in mammalian spermatozoa. Biochem Pharmacol 25: 1617–1622, 1976. [DOI] [PubMed] [Google Scholar]
- 12.Blatt R, Srinivasan S. Defining disease with laser precision: laser capture microdissection in gastroenterology. Gastroenterology 135: 364–369, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bognar IT, Altes U, Beinhauer C, Kessler I, Fuder H. A muscarinic receptor different from the M1, M2, M3 and M4 subtypes mediates the contraction of the rabbit iris sphincter. Naunyn Schmiedebergs Arch Pharmacol 345: 611–618, 1992. [DOI] [PubMed] [Google Scholar]
- 14.Bonner TI, Buckley NJ, Young AC, Brann MR. Identification of a family of muscarinic acetylcholine receptor genes. Science 237: 527–532, 1987. [DOI] [PubMed] [Google Scholar]
- 15.Boss A, Oppitz M, Drews U. Muscarinic cholinergic receptors in the human melanoma cell line SK-Mel 28: modulation of chemotaxis. Clin Exp Dermatol 30: 557–564, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Bowers JW, Schlauder SM, Calder KB, Morgan MB. Acetylcholine receptor expression in Merkel cell carcinoma. Am J Dermatopathol 30: 340–343, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Brown JH The Muscarinic Receptors. New York: Humana, 1990.
- 18.Browning JG, Hardcastle J, Hardcastle PT, Redfern JS. Localization of the effect of acetylcholine in regulating intestinal ion transport. J Physiol 281: 15–27, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burt AM, Silver A. Non-enzymatic imidazole-catalysed acyl transfer reaction and acetylcholine synthesis. Nat New Biol 243: 157–159, 1973. [DOI] [PubMed] [Google Scholar]
- 20.Bymaster FP, Carter PA, Yamada M, Gomeza J, Wess J, Hamilton SE, Nathanson NM, McKinzie DL, Felder CC. Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur J Neurosci 17: 1403–1410, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Cabello G, Valenzuela M, Vilaxa A, Duran V, Rudolph I, Hrepic N, Calaf G. A rat mammary tumor model induced by the organophosphorous pesticides parathion and malathion, possibly through acetylcholinesterase inhibition. Environ Health Perspect 109: 471–479, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng K, Chen Y, Zimniak P, Raufman JP, Xiao Y, Frucht H. Functional interaction of lithocholic acid conjugates with M3 muscarinic receptors on a human colon cancer cell line. Biochim Biophys Acta 1588: 48–55, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Cheng K, Khurana S, Chen Y, Kennedy RH, Zimniak P, Raufman JP. Lithocholylcholine, a bile acid/acetylcholine hybrid, is a muscarinic receptor antagonist. J Pharmacol Exp Ther 303: 29–35, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Cheng K, Raufman JP. Bile acid-induced proliferation of a human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem Pharmacol 70: 1035–1047, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Cheng K, Samimi R, Xie G, Shant J, Drachenberg C, Wade M, Davis RJ, Nomikos G, Raufman JP. Acetylcholine release by human colon cancer cells mediates autocrine stimulation of cell proliferation. Am J Physiol Gastrointest Liver Physiol 295: G591–G597, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng K, Xie G, Raufman JP. Matrix metalloproteinase-7-catalyzed release of HB-EGF mediates deoxycholyltaurine-induced proliferation of a human colon cancer cell line. Biochem Pharmacol 73: 1001–1012, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng K, Zimniak P, Raufman JP. Transactivation of the epidermal growth factor receptor mediates cholinergic agonist-induced proliferation of H508 human colon cancer cells. Cancer Res 63: 6744–6750, 2003. [PubMed] [Google Scholar]
- 28.Chernyavsky AI, Arredondo J, Wess J, Karlsson E, Grando SA. Novel signaling pathways mediating reciprocal control of keratinocyte migration and wound epithelialization through M3 and M4 muscarinic receptors. J Cell Biol 166: 261–272, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chess-Williams R Muscarinic receptors of the urinary bladder: detrusor, urothelial and prejunctional. Auton Autacoid Pharmacol 22: 133–145, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Chiba T, Bharucha AE, Thomforde GM, Kost LJ, Phillips SF. Model of rapid gastrointestinal transit in dogs: effects of muscarinic antagonists and a nitric oxide synthase inhibitor. Neurogastroenterol Motil 14: 535–541, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Chien JL, Warren JR. Differentiation of muscarinic cholinergic receptors in acinar carcinoma of rat pancreas. Cancer Res 45: 4858–4863, 1985. [PubMed] [Google Scholar]
- 32.Choppin A, Eglen RM. Pharmacological characterization of muscarinic receptors in dog isolated ciliary and urinary bladder smooth muscle. Br J Pharmacol 132: 835–842, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colecraft HM, Egamino JP, Sharma VK, Sheu SS. Signaling mechanisms underlying muscarinic receptor-mediated increase in contraction rate in cultured heart cells. J Biol Chem 273: 32158–32166, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Darvesh S, Arora RC, Martin E, Magee D, Hopkins DA, Armour JA. Cholinesterase inhibitors modify the activity of intrinsic cardiac neurons. Exp Neurol 188: 461–470, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Dasgupta P, Chellappan SP. Nicotine-mediated cell proliferation and angiogenesis: new twists to an old story. Cell Cycle 5: 2324–2328, 2006. [DOI] [PubMed] [Google Scholar]
- 36.DeKosky ST, Scheff SW, Hackney CG. Acetylcholine synthesis in human CSF: implications for study of central cholinergic metabolism. Neurochem Res 14: 191–196, 1989. [DOI] [PubMed] [Google Scholar]
- 37.Dhein S, van Koppen CJ, Brodde OE. Muscarinic receptors in the mammalian heart. Pharmacol Res 44: 161–182, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Ecknauer R, Dial E, Thompson WJ, Johnson LR, Rosenfeld GC. Isolated rat gastric parietal cells: cholinergic response and pharmacology. Life Sci 28: 609–621, 1981. [DOI] [PubMed] [Google Scholar]
- 39.Eglen RM, Harris GC. Selective inactivation of muscarinic M2 and M3 receptors in guinea-pig ileum and atria in vitro. Br J Pharmacol 109: 946–952, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erickson JD, Varoqui H, Schafer MK, Modi W, Diebler MF, Weihe E, Rand J, Eiden LE, Bonner TI, Usdin TB. Functional identification of a vesicular acetylcholine transporter and its expression from a “cholinergic” gene locus. J Biol Chem 269: 21929–21932, 1994. [PubMed] [Google Scholar]
- 41.Espanol A, Eijan AM, Mazzoni E, Davel L, Jasnis MA, Sacerdote De Lustig E, Sales ME. Nitric oxide synthase, arginase and cyclooxygenase are involved in muscarinic receptor activation in different murine mammary adenocarcinoma cell lines. Int J Mol Med 9: 651–657, 2002. [PubMed] [Google Scholar]
- 42.Espanol AJ, de la Torre E, Fiszman GL, Sales ME. Role of non-neuronal cholinergic system in breast cancer progression. Life Sci 80: 2281–2285, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Frucht H, Gazdar AF, Park JA, Oie H, Jensen RT. Characterization of functional receptors for gastrointestinal hormones on human colon cancer cells. Cancer Res 52: 1114–1122, 1992. [PubMed] [Google Scholar]
- 44.Frucht H, Jensen RT, Dexter D, Yang WL, Xiao Y. Human colon cancer cell proliferation mediated by the M3 muscarinic cholinergic receptor. Clin Cancer Res 5: 2532–2539, 1999. [PubMed] [Google Scholar]
- 45.Fryer AD, Jacoby DB. Muscarinic receptors and control of airway smooth muscle. Am J Respir Crit Care Med 158: S154–S160, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Fujii T, Tsuchiya T, Yamada S, Fujimoto K, Suzuki T, Kasahara T, Kawashima K. Localization and synthesis of acetylcholine in human leukemic T cell lines. J Neurosci Res 44: 66–72, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Fujita T, Shimada A, Okada N, Yamamoto A. Functional characterization of Na+-independent choline transport in primary cultures of neurons from mouse cerebral cortex. Neurosci Lett 393: 216–221, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Gahring LC, Rogers SW. Neuronal nicotinic acetylcholine receptor expression and function on nonneuronal cells. AAPS J 7: E885–E894, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gil DW, Krauss HA, Bogardus AM, WoldeMussie E. Muscarinic receptor subtypes in human iris-ciliary body measured by immunoprecipitation. Invest Ophthalmol Vis Sci 38: 1434–1442, 1997. [PubMed] [Google Scholar]
- 50.Gillberg PG, Sundquist S, Nilvebrant L. Comparison of the in vitro and in vivo profiles of tolterodine with those of subtype-selective muscarinic receptor antagonists. Eur J Pharmacol 349: 285–292, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Gilman AG G proteins and dual control of adenylate cyclase. Cell 36: 577–579, 1984. [DOI] [PubMed] [Google Scholar]
- 52.Gomez A, Martos F, Bellido I, Marquez E, Garcia AJ, Pavia J, Sanchez de la Cuesta F. Muscarinic receptor subtypes in human and rat colon smooth muscle. Biochem Pharmacol 43: 2413–2419, 1992. [DOI] [PubMed] [Google Scholar]
- 53.Gong S, Lu X, Xu Y, Swiderski CF, Jordan CT, Moscow JA. Identification of OCT6 as a novel organic cation transporter preferentially expressed in hematopoietic cells and leukemias. Exp Hematol 30: 1162–1169, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Grando SA, Kist DA, Qi M, Dahl MV. Human keratinocytes synthesize, secrete, and degrade acetylcholine. J Invest Dermatol 101: 32–36, 1993. [DOI] [PubMed] [Google Scholar]
- 55.Grozio A, Catassi A, Cavalieri Z, Paleari L, Cesario A, Russo P. Nicotine, lung and cancer. Anticancer Agents Med Chem 7: 461–466, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Guizzetti M, Bordi F, Dieguez-Acuna FJ, Vitalone A, Madia F, Woods JS, Costa LG. Nuclear factor kappaB activation by muscarinic receptors in astroglial cells: effect of ethanol. Neuroscience 120: 941–950, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Guizzetti M, Costa LG. Possible role of protein kinase C zeta in muscarinic receptor-induced proliferation of astrocytoma cells. Biochem Pharmacol 60: 1457–1466, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Guizzetti M, Costa P, Peters J, Costa LG. Acetylcholine as a mitogen: muscarinic receptor-mediated proliferation of rat astrocytes and human astrocytoma cells. Eur J Pharmacol 297: 265–273, 1996. [DOI] [PubMed] [Google Scholar]
- 59.Guizzetti M, Thompson BD, Kim Y, VanDeMark K, Costa LG. Role of phospholipase D signaling in ethanol-induced inhibition of carbachol-stimulated DNA synthesis of 1321N1 astrocytoma cells. J Neurochem 90: 646–653, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Hamilton JP, Xie G, Raufman JP, Hogan S, Griffin TL, Packard CA, Chatfield DA, Hagey LR, Steinbach JH, Hofmann AF. Human cecal bile acids: concentration and spectrum. Am J Physiol Gastrointest Liver Physiol 293: G256–G263, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Hammer R, Berrie CP, Birdsall NJ, Burgen AS, Hulme EC. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature 283: 90–92, 1980. [DOI] [PubMed] [Google Scholar]
- 62.Hegde SS, Eglen RM. Muscarinic receptor subtypes modulating smooth muscle contractility in the urinary bladder. Life Sci 64: 419–428, 1999. [DOI] [PubMed] [Google Scholar]
- 63.Holland WC, Greig ME. The synthesis of acetylcholine by human erythrocytes. Arch Biochem Biophys 39: 77–79, 1952. [DOI] [PubMed] [Google Scholar]
- 64.Hulme EC, Lu ZL, Saldanha JW, Bee MS. Structure and activation of muscarinic acetylcholine receptors. Biochem Soc Trans 31: 29–34, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Ikeda K, Kobayashi S, Suzuki M, Miyata K, Takeuchi M, Yamada T, Honda K. M(3) receptor antagonism by the novel antimuscarinic agent solifenacin in the urinary bladder and salivary gland. Naunyn Schmiedebergs Arch Pharmacol 366: 97–103, 2002. [DOI] [PubMed] [Google Scholar]
- 66.Inoue R, Isenberg G. Acetylcholine activates nonselective cation channels in guinea pig ileum through a G protein. Am J Physiol Cell Physiol 258: C1173–C1178, 1990. [DOI] [PubMed] [Google Scholar]
- 67.Islam MA, Nojima H, Kimura I. Muscarinic M1 receptor activation reduces maximum upstroke velocity of action potential in mouse right atria. Eur J Pharmacol 346: 227–236, 1998. [DOI] [PubMed] [Google Scholar]
- 68.Jimenez E, Montiel M. Activation of MAP kinase by muscarinic cholinergic receptors induces cell proliferation and protein synthesis in human breast cancer cells. J Cell Physiol 204: 678–686, 2005. [DOI] [PubMed] [Google Scholar]
- 69.Jogl G, Tong L. Crystal structure of carnitine acetyltransferase and implications for the catalytic mechanism and fatty acid transport. Cell 112: 113–122, 2003. [DOI] [PubMed] [Google Scholar]
- 70.Jonker JW, Wagenaar E, Mol CA, Buitelaar M, Koepsell H, Smit JW, Schinkel AH. Reduced hepatic uptake and intestinal excretion of organic cations in mice with a targeted disruption of the organic cation transporter 1 (Oct1 [Slc22a1]) gene. Mol Cell Biol 21: 5471–5477, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jonker JW, Wagenaar E, Van Eijl S, Schinkel AH. Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Mol Cell Biol 23: 7902–7908, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kawashima K, Fujii T. Extraneuronal cholinergic system in lymphocytes. Pharmacol Ther 86: 29–48, 2000. [DOI] [PubMed] [Google Scholar]
- 73.Khurana S, Chacon I, Xie G, Yamada M, Wess J, Raufman JP, Kennedy RH. Vasodilatory effects of cholinergic agonists are greatly diminished in aorta from M3R−/− mice. Eur J Pharmacol 493: 127–132, 2004. [DOI] [PubMed] [Google Scholar]
- 74.Kitazawa T, Hirama R, Masunaga K, Nakamura T, Asakawa K, Cao J, Teraoka H, Unno T, Komori S, Yamada M, Wess J, Taneike T. Muscarinic receptor subtypes involved in carbachol-induced contraction of mouse uterine smooth muscle. Naunyn Schmiedebergs Arch Pharmacol 377: 503–513, 2008. [DOI] [PubMed] [Google Scholar]
- 75.Klapproth H, Reinheimer T, Metzen J, Munch M, Bittinger F, Kirkpatrick CJ, Hohle KD, Schemann M, Racke K, Wessler I. Non-neuronal acetylcholine, a signalling molecule synthezised by surface cells of rat and man. Naunyn Schmiedebergs Arch Pharmacol 355: 515–523, 1997. [DOI] [PubMed] [Google Scholar]
- 76.Kodaira M, Kajimura M, Takeuchi K, Lin S, Hanai H, Kaneko E. Functional muscarinic m3 receptor expressed in gastric cancer cells stimulates tyrosine phosphorylation and MAP kinase. J Gastroenterol 34: 163–171, 1999. [DOI] [PubMed] [Google Scholar]
- 77.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res 24: 1227–1251, 2007. [DOI] [PubMed] [Google Scholar]
- 78.Kummer W, Wiegand S, Akinci S, Wessler I, Schinkel AH, Wess J, Koepsell H, Haberberger RV, Lips KS. Role of acetylcholine and polyspecific cation transporters in serotonin-induced bronchoconstriction in the mouse. Respir Res 7: 65, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lepor H, Kuhar MJ. Characterization and localization of the muscarinic cholinergic receptor in human prostatic tissue. J Urol 132: 397–402, 1984. [DOI] [PubMed] [Google Scholar]
- 80.Lips KS, Volk C, Schmitt BM, Pfeil U, Arndt P, Miska D, Ermert L, Kummer W, Koepsell H. Polyspecific cation transporters mediate luminal release of acetylcholine from bronchial epithelium. Am J Respir Cell Mol Biol 33: 79–88, 2005. [DOI] [PubMed] [Google Scholar]
- 81.Lips KS, Wunsch J, Zarghooni S, Bschleipfer T, Schukowski K, Weidner W, Wessler I, Schwantes U, Koepsell H, Kummer W. Acetylcholine and molecular components of its synthesis and release machinery in the urothelium. Eur Urol 51: 1042–1053, 2007. [DOI] [PubMed] [Google Scholar]
- 82.Loewi O Über humorale übertragbarkeit der Herznervenwirkung (About humoral portability of heart nervous effect). Pflügers Arch 189: 239–242, 1921. [Google Scholar]
- 83.Loewi O, Navratil E. Ueber humorale Uebertragbarkeit der Herznervenwirkung. X. Mitteilung Ueber das Schicksal des Vagusstoff. Pflügers Arch Gesamte Physiol 214: 678–688, 1926. [Google Scholar]
- 84.Luthin GR, Wang P, Zhou H, Dhanasekaran D, Ruggieri MR. Role of m1 receptor-G protein coupling in cell proliferation in the prostate. Life Sci 60: 963–968, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Malbon CC G proteins in development. Nat Rev Mol Cell Biol 6: 689–701, 2005. [DOI] [PubMed] [Google Scholar]
- 86.Matsui M, Motomura D, Fujikawa T, Jiang J, Takahashi S, Manabe T, Taketo MM. Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci 22: 10627–10632, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi S, Taketo MM. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci USA 97: 9579–9584, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meuwissen R, Berns A. Mouse models for human lung cancer. Genes Dev 19: 643–664, 2005. [DOI] [PubMed] [Google Scholar]
- 89.Nelson CP, Challiss RA. “Phenotypic” pharmacology: the influence of cellular environment on G protein-coupled receptor antagonist and inverse agonist pharmacology. Biochem Pharmacol 73: 737–751, 2007. [DOI] [PubMed] [Google Scholar]
- 90.Nicke B, Detjen K, Logsdon CD. Muscarinic cholinergic receptors activate both inhibitory and stimulatory growth mechanisms in NIH3T3 cells. J Biol Chem 274: 21701–21706, 1999. [DOI] [PubMed] [Google Scholar]
- 91.Noda S, Lammerding-Koppel M, Oettling G, Drews U. Characterization of muscarinic receptors in the human melanoma cell line SK-Mel-28 via calcium mobilization. Cancer Lett 133: 107–114, 1998. [DOI] [PubMed] [Google Scholar]
- 92.Oppitz M, Busch C, Garbe C, Drews U. Distribution of muscarinic receptor subtype M3 in melanomas and their metastases. J Cutan Pathol 35: 809–815, 2008. [DOI] [PubMed] [Google Scholar]
- 93.Oppitz M, Mobus V, Brock S, Drews U. Muscarinic receptors in cell lines from ovarian carcinoma: negative correlation with survival of patients. Gynecol Oncol 85: 159–164, 2002. [DOI] [PubMed] [Google Scholar]
- 94.Parsons SM Transport mechanisms in acetylcholine and monoamine storage. FASEB J 14: 2423–2434, 2000. [DOI] [PubMed] [Google Scholar]
- 95.Pieklik JR, Guynn RW. Equilibrium constants of the reactions of choline acetyltransferase, carnitine acetyltransferase, and acetylcholinesterase under physiological conditions. J Biol Chem 250: 4445–4450, 1975. [PubMed] [Google Scholar]
- 96.Proskocil BJ, Sekhon HS, Jia Y, Savchenko V, Blakely RD, Lindstrom J, Spindel ER. Acetylcholine is an autocrine or paracrine hormone synthesized and secreted by airway bronchial epithelial cells. Endocrinology 145: 2498–2506, 2004. [DOI] [PubMed] [Google Scholar]
- 97.Ramsay RR, Naismith JH. A snapshot of carnitine acetyltransferase. Trends Biochem Sci 28: 343–346, 2003. [DOI] [PubMed] [Google Scholar]
- 98.Raufman JP, Chen Y, Cheng K, Compadre C, Compadre L, Zimniak P. Selective interaction of bile acids with muscarinic receptors: a case of molecular mimicry. Eur J Pharmacol 457: 77–84, 2002. [DOI] [PubMed] [Google Scholar]
- 99.Raufman JP, Chen Y, Zimniak P, Cheng K. Deoxycholic acid conjugates are muscarinic cholinergic receptor antagonists. Pharmacology 65: 215–221, 2002. [DOI] [PubMed] [Google Scholar]
- 100.Raufman JP, Cheng K, Zimniak P. Activation of muscarinic receptor signaling by bile acids: physiological and medical implications. Dig Dis Sci 48: 1431–1444, 2003. [DOI] [PubMed] [Google Scholar]
- 101.Raufman JP, Samimi R, Shah N, Khurana S, Shant J, Drachenberg C, Xie G, Wess J, Cheng K. Genetic ablation of M3 muscarinic receptors attenuates murine colon epithelial cell proliferation and neoplasia. Cancer Res 68: 3573–3578, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raufman JP, Shant J, Guo CY, Roy S, Cheng K. Deoxycholyltaurine rescues human colon cancer cells from apoptosis by activating EGFR-dependent PI3K/Akt signaling. J Cell Physiol 215: 538–549, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Raufman JP, Sutliff VE, Kasbekar DK, Jensen RT, Gardner JD. Pepsinogen secretion from dispersed chief cells from guinea pig stomach. Am J Physiol Gastrointest Liver Physiol 247: G95–G104, 1984. [DOI] [PubMed] [Google Scholar]
- 104.Rayford W, Noble MJ, Austenfeld MA, Weigel J, Mebust WK, Shah GV. Muscarinic cholinergic receptors promote growth of human prostate cancer cells. Prostate 30: 160–166, 1997. [DOI] [PubMed] [Google Scholar]
- 105.Roskoski R Jr. Choline acetyltransferase: reversible inhibition by bromoacetyl coenzyme A and bromoacetylcholine. Biochemistry 13: 2295–2298, 1974. [DOI] [PubMed] [Google Scholar]
- 106.Rossier J Acetyl-coenzyme A and coenzyme A analogues. Their effects on rat brain choline acetyltransferase. Biochem J 165: 321–326, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ruggieri MR, Colton MD, Wang P, Wang J, Smyth RJ, Pontari MA, Luthin GR. Human prostate muscarinic receptor subtypes. J Pharmacol Exp Ther 274: 976–982, 1995. [PMC free article] [PubMed] [Google Scholar]
- 108.Sailer M, Oppitz M, Drews U. Induction of cellular contractions in the human melanoma cell line SK-mel 28 after muscarinic cholinergic stimulation. Anat Embryol (Berl) 201: 27–37, 2000. [DOI] [PubMed] [Google Scholar]
- 109.Sastry BV, Janson VE. Retinal cholinergic system: characterization of rat retinal acetyltransferases using specific inhibitors of choline- and carnitine-acetyltransferases. J Ocul Pharmacol Ther 10: 203–215, 1994. [DOI] [PubMed] [Google Scholar]
- 110.Sastry BV, Sadavongvivad C. Cholinergic systems in non-nervous tissues. Pharmacol Rev 30: 65–132, 1978. [PubMed] [Google Scholar]
- 111.Shant J, Cheng K, Marasa B, Wang JY, Raufman JP. Akt-dependent NF-kappaB activation is required for bile acids to rescue colon cancer cells from stress-induced apoptosis. Exp Cell Res. In Press. [DOI] [PMC free article] [PubMed]
- 112.Soler AP, Miller RD, Laughlin KV, Carp NZ, Klurfeld DM, Mullin JM. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis 20: 1425–1431, 1999. [DOI] [PubMed] [Google Scholar]
- 113.Soll AH Specific inhibition by prostaglandins E2 and I2 of histamine-stimulated [14C]aminopyrine accumulation and cyclic adenosine monophosphate generation by isolated canine parietal cells. J Clin Invest 65: 1222–1229, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Song P, Sekhon HS, Fu XW, Maier M, Jia Y, Duan J, Proskosil BJ, Gravett C, Lindstrom J, Mark GP, Saha S, Spindel ER. Activated cholinergic signaling provides a target in squamous cell lung carcinoma. Cancer Res 68: 4693–4700, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Song P, Sekhon HS, Jia Y, Keller JA, Blusztajn JK, Mark GP, Spindel ER. Acetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res 63: 214–221, 2003. [PubMed] [Google Scholar]
- 116.Song P, Sekhon HS, Lu A, Arredondo J, Sauer D, Gravett C, Mark GP, Grando SA, Spindel ER. M3 muscarinic receptor antagonists inhibit small cell lung carcinoma growth and mitogen-activated protein kinase phosphorylation induced by acetylcholine secretion. Cancer Res 67: 3936–3944, 2007. [DOI] [PubMed] [Google Scholar]
- 117.Song P, Sekhon HS, Proskocil B, Blusztajn JK, Mark GP, Spindel ER. Synthesis of acetylcholine by lung cancer. Life Sci 72: 2159–2168, 2003. [DOI] [PubMed] [Google Scholar]
- 118.Stengel PW, Gomeza J, Wess J, Cohen ML. M(2) and M(4) receptor knockout mice: muscarinic receptor function in cardiac and smooth muscle in vitro. J Pharmacol Exp Ther 292: 877–885, 2000. [PubMed] [Google Scholar]
- 119.Sutliff VE, Rattan S, Gardner JD, Jensen RT. Characterization of cholinergic receptors mediating pepsinogen secretion from chief cells. Am J Physiol Gastrointest Liver Physiol 257: G226–G234, 1989. [DOI] [PubMed] [Google Scholar]
- 120.Tapper EJ, Powell DW, Morris SM. Cholinergic-adrenergic interactions on intestinal ion transport. Am J Physiol Endocrinol Metab Gastrointest Physiol 235: E402–E409, 1978. [DOI] [PubMed] [Google Scholar]
- 121.Traiffort E, Ruat M, O'Regan S, Meunier FM. Molecular characterization of the family of choline transporter-like proteins and their splice variants. J Neurochem 92: 1116–1125, 2005. [DOI] [PubMed] [Google Scholar]
- 122.Tucek S The synthesis of acetylcholine in skeletal muscles of the rat. J Physiol 322: 53–69, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tucek S, Ricny J, Dolezal V. Acetylcoenzyme A and the control of the synthesis of acetylcholine in the brain. Acta Neurobiol Exp (Warsz) 42: 59–68, 1982. [PubMed] [Google Scholar]
- 124.Valenstein ES The War of the Soups and the Sparks: The Discovery of Neurotransmitters and the Dispute Over How Nerves Communicate. New York: Columbia Univ. Press, 2005.
- 125.Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J Pharmacol Exp Ther 273: 959–966, 1995. [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Z, Shi H, Wang H. Functional M3 muscarinic acetylcholine receptors in mammalian hearts. Br J Pharmacol 142: 395–408, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wess J Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol 10: 69–99, 1996. [DOI] [PubMed] [Google Scholar]
- 128.Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol 154: 1558–1571, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wessler I, Kirkpatrick CJ, Racke K. Non-neuronal acetylcholine, a locally acting molecule, widely distributed in biological systems: expression and function in humans. Pharmacol Ther 77: 59–79, 1998. [DOI] [PubMed] [Google Scholar]
- 130.Wessler I, Roth E, Deutsch C, Brockerhoff P, Bittinger F, Kirkpatrick CJ, Kilbinger H. Release of non-neuronal acetylcholine from the isolated human placenta is mediated by organic cation transporters. Br J Pharmacol 134: 951–956, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.White HL, Wu JC. Choline and carnitine acetyltransferases of heart. Biochemistry 12: 841–846, 1973. [DOI] [PubMed] [Google Scholar]
- 132.Williams CL, Lennon VA. Activation of muscarinic acetylcholine receptors inhibits cell cycle progression of small cell lung carcinoma. Cell Regul 2: 373–381, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D, Kirk L, Litin S, Simmang C, Gastrointestinal Consortium Panel. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology 124: 544–560, 2003. [DOI] [PubMed] [Google Scholar]
- 134.Xie G, Drachenberg C, Yamada M, Wess J, Raufman JP. Cholinergic agonist-induced pepsinogen secretion from murine gastric chief cells is mediated by M1 and M3 muscarinic receptors. Am J Physiol Gastrointest Liver Physiol 289: G521–G529, 2005. [DOI] [PubMed] [Google Scholar]
- 135.Yamada M, Lamping KG, Duttaroy A, Zhang W, Cui Y, Bymaster FP, McKinzie DL, Felder CC, Deng CX, Faraci FM, Wess J. Cholinergic dilation of cerebral blood vessels is abolished in M(5) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA 98: 14096–14101, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang WL, Frucht H. Cholinergic receptor up-regulates COX-2 expression and prostaglandin E(2) production in colon cancer cells. Carcinogenesis 21: 1789–1793, 2000. [DOI] [PubMed] [Google Scholar]
- 137.Yoshida M, Inadome A, Maeda Y, Satoji Y, Masunaga K, Sugiyama Y, Murakami S. Non-neuronal cholinergic system in human bladder urothelium. Urology 67: 425–430, 2006. [DOI] [PubMed] [Google Scholar]
- 138.Zimmerman TW, Binder HJ. Muscarinic receptors on rat isolated colonic epithelial cells. A correlation between inhibition of [3H]quinuclidinyl benzilate binding and alteration in ion transport. Gastroenterology 83: 1244–1251, 1982. [PubMed] [Google Scholar]
- 139.Zimmerman TW, Dobbins JW, Binder HJ. Mechanism of cholinergic regulation of electrolyte transport in rat colon in vitro. Am J Physiol Gastrointest Liver Physiol 242: G116–G123, 1982. [DOI] [PubMed] [Google Scholar]
- 140.Zwart R, Verhaagh S, Buitelaar M, Popp-Snijders C, Barlow DP. Impaired activity of the extraneuronal monoamine transporter system known as uptake-2 in Orct3/Slc22a3-deficient mice. Mol Cell Biol 21: 4188–4196, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]