Abstract
PDGF B chain or PDGF receptor (PDGFR)-β-deficient (−/−) mice lack mesangial cells. To study responses of α- and β-receptor activation to PDGF ligands, metanephric mesenchymal cells (MMCs) were established from embryonic day E11.5 wild-type (+/+) and −/− mouse embryos. PDGF BB stimulated cell migration in +/+ cells, whereas PDGF AA did not. Conversely, PDGF AA was chemotactic for −/− MMCs. The mechanism by which PDGFR-β inhibited AA-induced migration was investigated. PDGF BB, but not PDGF AA, increased intracellular Ca2+ and the production of reactive oxygen species (ROS) in +/+ cells. Transfection of −/− MMCs with the wild-type β-receptor restored cell migration and ROS generation in response to PDGF BB and inhibited AA-induced migration. Inhibition of Ca2+ signaling facilitated PDGF AA-induced chemotaxis in the wild-type cells. The antioxidant N-acetyl-l-cysteine (NAC) or the NADPH oxidase inhibitor diphenyleneiodonium (DPI) abolished the BB-induced increase in intracellular Ca2+ concentration, suggesting that ROS act as upstream mediators of Ca2+ in suppressing PDGF AA-induced migration. These data indicate that ROS and Ca2+ generated by active PDGFR-β play an essential role in suppressing PDGF AA-induced migration in +/+ MMCs. During kidney development, PDGFR β-mediated ROS generation and Ca2+ influx suppress PDGF AA-induced chemotaxis in metanephric mesenchyme.
Keywords: reactive oxygen species, calcium
proliferation and migration are key biological processes involved in organ development, including development of the metanephric kidney. Two structurally similar transmembrane platelet-derived growth factor receptors (PDGFRs) with intrinsic tyrosine kinase activity have been identified, PDGFR-α and PDGFR-β (11, 43, 63). Biologically active platelet-derived growth factor (PDGF) is a dimer (AA, AB, BB, CC or DD). The α-receptor has greater affinity for PDGF AA than BB. PDGFR-β binds PDGF BB and DD with high affinity (5, 11, 26, 39, 52). Activation of PDGFR-β mediates proliferation, migration, survival, and differentiation in a variety of cell types (1, 18). The role of PDGF B chain and PDGFR-β in development of the renal glomerular microvasculature has been conclusively demonstrated in two studies utilizing mice carrying mutations in either PDGFR-β or PDGF B chain ligand (38, 54).
Homozygous PDGFR-β-deficient mice (−/−) do not survive past birth. They are afflicted with anemia, thrombocytopenia, and form rudimentary glomeruli that lack mesangial cells (54). The phenotype is fully penetrant and observed in mature glomeruli of 16–18 days postconception (dpc) embryos. Mice carrying a null mutation in the gene encoding PDGF B chain exhibit a similar glomerular phenotype (38). In these studies, PDGFR-α was found to be intact and functional, yet not compensatory for the loss of PDGFR-β. Mice deficient in PDGFR-α (e.g., the Patch mutant) are characterized by maldevelopment of mesenchymal structures and have a deficiency in renal fibroblasts (6, 51), but there are no reports of mesangial cell abnormalities. Therefore, PDGFR-β has a principle role in mesangial cell development.
Binding of a PDGF dimer to the extracellular domain of the receptor induces homo- or heterodimerization (depending on the ligand) and subsequent transphosphorylation. Autophosphorylation of the receptors provides docking sites or activates intracellular effector proteins including phosphatidylinositol 3-kinase (PI3-K), phospholipase C-γ1 (PLCγ1), and Ras. Downstream effects include activation of the mitogen activated protein kinase/extracellular regulated kinase kinase (MEK), and mitogen activated protein kinase (MAPK) pathways (1, 8, 27, 41). It is known that PDGFR-β-mediated chemotaxis depends on PI3-K (64). Cells expressing a PDGFR-β mutant incapable of activating PI3-K do not demonstrate chemotaxis to PDGF (59, 62). PDGF B chain and PDGFR-β are essential for mesangial cell development. Signaling through PDGFR-α does not compensate for the loss of PDGFR-β signaling. Therefore, the biological effects of PDGF AA and PDGF BB in wild-type (+/+) and PDGFR-β-deficient (−/−) MMCs were examined.
In this study, we established wild-type and −/− MMCs. We hypothesized that defective α-receptor signaling and aberrant cell migration may explain the inability of the α-receptor to compensate for the β-receptor in mesangial cell development. To our surprise, PDGF AA and α-receptors signal normally and induce MMC migration in the PDGFR-β −/− cells. In wild-type metanephric mesenchymal cells, PDGFR-β negatively regulates PDGF AA-induced cell migration through reactive oxygen species (ROS)- and Ca2+-mediated signals. ROS are generated via PDGFR-β and stimulate extracellular Ca2+ influx, suppressing PDGF AA-induced migration in wild-type cells.
MATERIALS AND METHODS
Materials.
Tissue culture materials were purchased from GIBCO BRL (Rockville, MD). Recombinant PDGF AA and PDGF BB were obtained from R&D Systems (Minneapolis, MN). BAPTA, A23187, fura 2, and 2′,7′-dichlorofluorescein diacetate (DCFDA) were purchased from Molecular Probes (Eugene, OR). Primary antibody to PDGFR-β (958) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). PDGFR-α antibody (C-20) was from Cell Signaling (Boston, MA). Antibodies against vimentin, pan cytokeratin, E-cadherin, and α-smooth muscle actin were purchased from Sigma (St. Louis, MO). Secondary FITC-conjugated antibodies were obtained from Chemicon (Temecula, CA). Lipofectamine Plus was purchased from GIBCO BRL. Protein measurement and polyacrylamide gel reagents were purchased from Bio-Rad (Hercules, CA). Agarose gel products, SeaKem GTG agarose, and NuSieve GTG low-melting-temperature agarose were obtained from FMC BioProducts (Rockland, ME). All other reagents were high-quality analytic grade.
Establishment and characterization of metanephric mesenchymal cells in culture.
Primary cultures of mouse metanephric mesenchymal cells (MMCs) were prepared as recently described for rat MMCs (3). All animal protocols were reviewed by the Alexion Institutional Animal Care and Use Committee. Heterozygous PDGFR-β-deficient mice were mated. At postcoital day 11.5, pregnant mice were anesthetized by intramuscular injection with a mixture of ketamine (30%) and Rompun (20%) in 0.9% NaCl, and embryos were collected. The age of the embryo was counted from the day of the vaginal plug (day 0). Embryos were dissected in 1× phosphate-buffered saline using an Olympus SZH stereo zoom model microscope. Metanephric blastemas were collected from each embryo individually by removing the embryonic kidney and separating the metanephric mesenchyme from the ureteric bud. Tissue from each embryo was also collected for genotyping. Once metanephric mesenchyme was collected, cells were propagated in Dulbecco's modified Eagle's medium (GIBCO BRL) including 10% fetal calf serum and grown at 37°C, in a 5% CO2 atmosphere. Cells from littermates of the same genotype were pooled. Cells were grown for 3 days in growth media and then transformed with a retroviral vector expressing the human papilloma virus E6/E7 oncoproteins (MFGS). Genotypic analysis of the 11.5-dpc embryo showed the presence of null, heterozygous, and +/+ alleles. Once genotypes of the embryos were determined, collected embryonic kidneys of the same genotype were pooled to propagate +/+ and PDGFR-β-deficient cell lines. The genotype of the cultured cells was confirmed by PCR using the same primer sets. The results demonstrate isolated pure cultures of +/+, −/−, and heterozygous cells (60).
At this stage of development, the +/+ MMCs express both PDGF receptors α and β. The +/+ and −/− established cell lines grow at similar rates in culture. To demonstrate mesenchymal characteristics of the cultured cells, immunohistochemistry using mesenchymal and epithelial cell markers was performed. Both +/+ and −/− MMCs stained for vimentin, a mesenchymal intracellular filament protein. Each cell line was also positive for α-smooth muscle actin. No cell surface staining for the epithelial cell markers E-cadherin or cytokeratin was observed in either cell line. Dolicus biflorus, a cell surface glycoprotein expressed in the developing ureter but not in mesenchyme, was also negative in each cell line, but positive in the ureteric bud cells that we also isolated (50). These results indicate that the two cell lines, (+/+) and (−/−), derived from the embryonic metanephric blastemas at 11.5 dpc had mesenchymal characteristics and were free of epithelial ureteric bud cell contamination (60).
Cell transfections.
Human PDGFR-β was transfected into −/− MMCs by Lipofectamine Plus. Transfection experiments were carried out in antibiotic-free media and were performed using conditions specified by the manufacturer. Cells were grown in 100-mm2 cell culture dishes until ∼70% confluent. A total of 1 μg/ml of DNA was used, and stable cell lines were established.
DNA synthesis.
MMCs were plated at 7.5 × 104 cells/24-well dish, grown to confluency, and serum-deprived for 48 h. Cells were stimulated with PDGF isoforms. One microcurie of [3H]thymidine was added to each well. DNA synthesis was measured as incorporation of [3H]thymidine into trichloroacetic acid (TCA)-insoluble material (23).
Chemotaxis assays.
Cellular chemotaxis in response to PDGF was determined using blind well (modified Boyden) chamber assays. Polycarbonate filters (14 μm, Osmonics, Minnetonka, MN) were coated with rat tail collagen (4 μg/filter, Becton-Dickinson, Becton, MA). Confluent MMCs were serum-deprived overnight, and the monolayer of cells was briefly trypsinized and resuspended in serum-free media. The cell suspension (2 × 105 cells/ml) was added to the top chamber, while PDGF was added to the bottom chamber of the apparatus. After 4 h at 37°C, the filters were inverted on glass slides and fixed with methanol, stained with Giemsa (Fischer Scientific), dried, and mounted in Permount. Cells were counted in 10 high-power fields (magnification, ×450) in the center of each filter. The data are presented as number of cells per high-power field (24). Cells in suspension were treated with 5 mM EGTA, 20 μM BAPTA, or 20 mM N-acetyl-l-cysteine (NAC) for 2, 15, or 20 min and then added to the top chamber. Alternatively, cells were treated with 1 μM A32187 or 200 μM H2O22 for 1 or 20 min and washed with serum-free media before being added to the top chamber.
Immunohistochemistry.
Both +/+ and PDGFR β-deficient cells were grown to near-confluency on coverslips and fixed with methanol. Direct and indirect immunofluorescence staining was used to examine the expression of vimentin, α-smooth muscle actin, cytokeratin, E-cadherin. and D. biflorus (rhodamine-conjugated lectin specific for ureteric bud) as previously described (3). The primary antibodies were omitted or replaced by normal mouse or rabbit IgG (5 μg/ml) as controls. Sections were viewed and photographed with an Olympus AX70 research microscope.
PCR.
Genomic DNA was isolated from each embryo or from cultured cells lines. PCR for PDGFR-β was performed using two forward primers, P1: 5′-ACA ATT CCG TGC CGA GTG ACA-3′, P2: 5′-AAA AGT ACC AGT GAA ACC TCG CTG-3′; and one reverse primer, P3: 5′-ATC AGC CTC GAC TGT GCC TTC TAG-3′. Cycling temperatures were as follows: denaturing, 93°C, 30 s; annealing, 58°C, 30 s; and extending, 65°C, 45 s; for 35 cycles. PCR products were separated in 1% SeaKem GTG agarose (FMC BioProducts) with 2.7% NuSieve GTG low-melting-temperature agarose TEA gel (60).
RT-PCR.
Monolayers of +/+ or −/− cells were washed, and total RNA was isolated with a TRIzol Plus RNA Purification Kit (Invitrogen). Primers for mouse PDGFR-α (determined by P3) were 5′-ggg gac aga ctg tga ggt gt-3′ and 5′-gtt ggt gct gtt ggt gat tg-3′.
Western blotting.
Equal amounts of protein from cell lysates were separated on a 12.5% SDS-PAGE and transferred by electrophoresis to a polyvinyl membrane. The membrane was blocked with 5% nonfat milk prepared in TBST buffer, washed with TBST and incubated with PDGFR-β primary antibody (1:750 dilution, Santa Cruz Biotechnology). The membrane was then washed and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG. The blot was developed with ECL reagent according to the manufacturer's directions.
Immune complex kinase assay.
Serum-deprived confluent MMCs were treated with PDGF and lysed in RIPA with sodium orthovanadate (21). One hundred micrograms of protein were immunoprecipitated with 1 μg of the indicated antibody, and the immune complex kinase assay was conducted as described (10).
Laser-scanning confocal microscopy.
Cells were seeded on slides, grown to < 30% confluency, washed with PBS, and fixed with 4% paraformaldehyde for 15 min. Membranes were permeabilized with 0.2% Triton X in PBS for 5 min, washed, and blocked with 5% BSA in PBS at 4°C overnight. Anti-PDGFR-β and monoclonal anti-PDGFR-α (16A1) at 1:250 dilution were applied for 30 min, followed by 1:250 secondary antibody, FITC- and Alexa Fluor 680-conjugated anti-rabbit and -mouse, respectively. Images were obtained using an inverted microscope with a ×60 oil objective (Olympus).
Flow cytometry.
Serum-deprived monolayers were washed three times with PBS, detached from plates using nonenzymatic cell dissociation solution (Sigma), centrifuged, and fixed with 1% paraformaldehyde for 10 min. Cells were permeabilized with cold methanol for 10 min, rinsed, and blocked with 0.5% BSA in PBS. Cells were incubated with 1:400 primary antibody (rabbit IgG isotype control, anti-PDGFR-β, or C-20 anti-PDGFR-α) for 30 min, then 1:100 FITC-conjugated anti-rabbit (Jackson) for 15 min. Cells were sorted (FACS Calibur, Becton-Dickinson Immunocytometry Systems, San Jose, CA) with an excitation wavelength of 488 nm, 530/30-nm band-pass filter. Data were obtained by CellQuest (BDIS).
Ca2+ measurements.
Cytosolic Ca2+ concentration ([Ca2+]i) was determined by spectrofluorometric measurements in cell suspensions as previously described (23). Briefly, cells were trypsinized and resuspended in growth media. Cells were washed with PBS and loaded with 1.2 μM fura 2-AM at 37°C for 30 min with gentle agitation. Cells were brought to room temperature and washed two times to remove extracellular dye and resuspended at 1 × 106 cells/ml. The cell suspension was placed in a fluorometer, and 10 ng/ml of PDGF BB or 100 ng/ml of PDGF AA was added. An excitation ratio of 340/380 was measured with a PTI Delta Scan spectrofluorometer (Photon Technology International, South Brunswick, NJ) using 340- and 380-nm wavelengths for excitation and 510 nm for emission. The following calculation was used: [Ca2+]i (nM) = Kd(R − Rmin)/(Rmax − R), where R is the fluorescent ratio of 340/380; Rmin and Rmax are minimal and maximal fluorescent ratios, respectively; and Kd is the dissociation constant (taken as 224 nM) of fura 2 for Ca2+.
Measurement of superoxide anion production in intact MMCs.
Measurement of the superoxide anion (O2−) released into the media of +/+ and PDGFR-β-deficient MMCs was performed by detection of ferricytochrome c reduction, as described by Johnston et al. (30). Medium from growth-arrested MMCs grown in six-well plates (2 × 106 cells/well) was aspirated and replaced with 1 ml of Hanks' balanced salt solution without phenol red containing 80 μM cytochrome c with or without 100 ng/ml PDGF AA or 10 ng/ml PDGF BB. At the end of the incubation, the medium was removed at the times indicated and centrifuged for 2 min at 10,000 g at 4°C to stop the reaction. The optical density was measured by spectrophotometry at 550 nm and converted to nanomoles of cytochrome c reduced using the extinction coefficient ΔE550 = 21.0 × 103 M/cm. The reduction of cytochrome c that was inhibited by pretreatment with superoxide dismutase (SOD; 50 μg/ml) reflected O2− release.
Detection of intracellular H2O2.
The H2O2-sensitive fluorescent probe DCFDA was used to assess the generation of H2O2. This compound is converted by intracellular esterases to 2′,7′-dichlorofluorescein, which is then oxidized by H2O2 to the highly fluorescent 2′,7′-dichlorofluorescein (DCF). Both +/+ and PDGFR-β-deficient MMCs were grown to near confluence in cover glass chambers and serum-deprived for 48 h. Cells were incubated with 10 μM DCFDA for 30 min at 37°C. The supernatant was removed and replaced with fresh media before treatment of MMCs with 100 ng/ml of PDGF AA or 10 ng/ml PDGF BB. Differential interference contrast images were obtained simultaneously using an Olympus inverted microscope with a ×40 Aplanfluo objective and an Olympus fluoview confocal laser-scanning attachment (22). The DCF fluorescence was measured with an excitation wavelength of 488-nm light, and its emission was detected using a 510- to 550-nm band-pass filter. Cells were treated with 10 mM diphenylene iodonium as described (21).
Statistical analysis.
Results are expressed as means ± SE. Statistical significance was assessed by Student's unpaired t-test. Significance was determined as P < 0.05.
RESULTS
Effect of PDGF receptor activation on migration and DNA synthesis in +/+ and PDGFR-β-deficient MMCs.
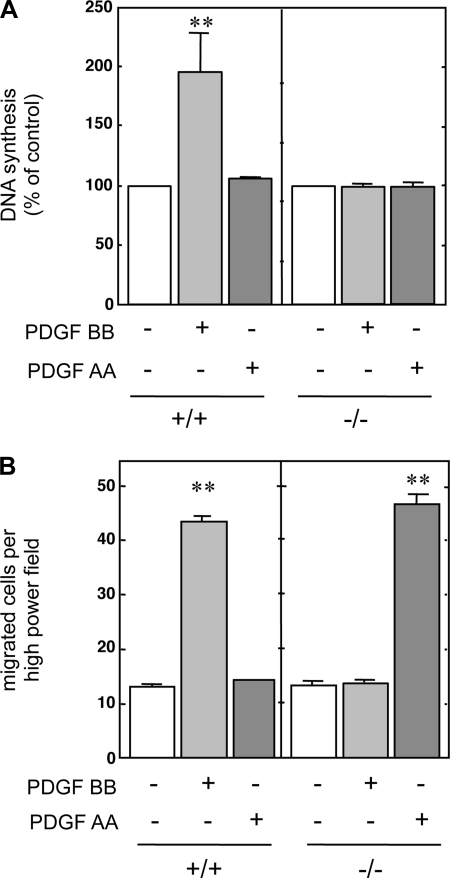
Metanephretic blastemas were isolated from 11.5-dpc mouse embryos. MMCs were established from +/+ and −/− embryos as described elsewhere (60). The ability of the PDGF isoforms AA and BB to induce DNA synthesis or cellular migration was examined in +/+ and −/− cells. PDGF BB induced DNA synthesis only in +/+ cells. As expected, PDGF BB had no mitogenic effect in −/− MMCs even at concentrations of 100 ng/ml (Fig. 1A). PDGF AA, even at doses up to 100 ng/ml, had no effect on DNA synthesis in either +/+ or −/− MMCs. These data indicate that PDGFR-β, but not PDGFR-α, is necessary for PDGF-induced mitogenesis in +/+ cells. PDGF BB induced migration of +/+ MMCs fourfold above basal levels, whereas PDGF AA did not have an effect (Fig. 1B). Surprisingly, PDGF AA stimulated migration in the −/− cells, whereas PDGF BB had no effect (Fig. 1B).
Fig. 1.
Effect of platelet-derived growth factor (PDGF) on DNA synthesis and migration of wild-type (+/+) and PDGF receptor-β (PDGFR-β)-deficient (−/−) cells. Serum-deprived quiescent mouse metanephric mesenchymal cells (MMCs) were used in DNA synthesis or cell migration assays in the presence of 100 ng/ml of PDGF AA or 10 ng/ml PDGF BB, as described in materials and methods. A: DNA synthesis measured by [3H]thymidine incorporation into TCA-precipitable material. B: cell migration was quantified as the number of cells that migrated through a polycarbonate filter under high magnification (×450). Values are means ± SE of 3 independent experiments. **P < 0.01 vs. control.
Introduction of wild-type PDGFR-β into PDGFR-β-deficient cells inhibits PDGF AA-induced migration.
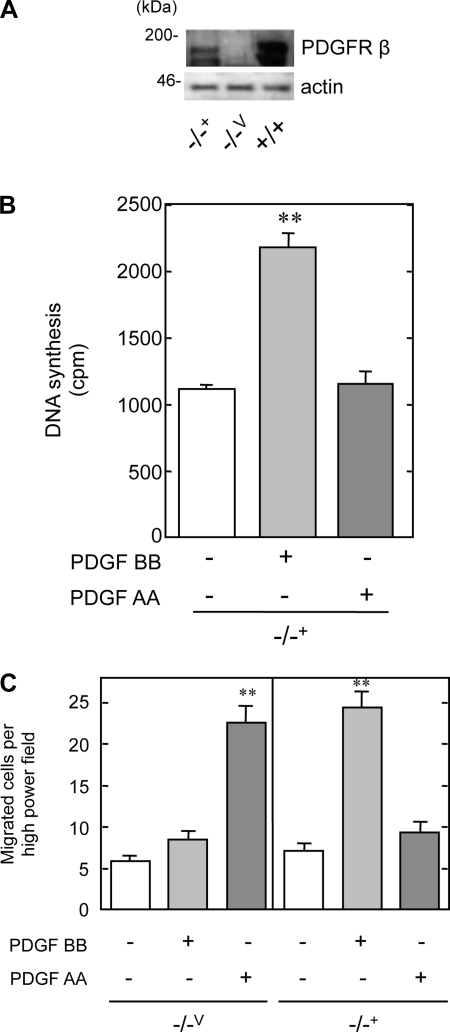
We next determined whether the differential migratory response between the +/+ and −/− MMCs to PDGF AA was due to PDGFR-β itself rather than to another alteration in the genetic program at early stages of development. Wild-type human PDGFR-β was transfected into the −/− MMCs and stable cell lines (−/−+) were established. PDGFR-β was expressed in the −/−+ cells, but not in cells transfected with an empty vector (−/−V) (Fig. 2A).
Fig. 2.
PDGFR-β suppresses PDGF AA-induced chemotaxis in +/+ MMCs. A: Western blot showing expression of PDGFR-β. PDGFR-β-deficient cells were transfected with vector (−/−V) or human PDGFR-β (−/−+), and immunoblotting was performed for the receptor. B: quiescent −/−+ MMCs were treated with PDGF AA (100 ng/ml) or BB (10 ng/ml), and DNA synthesis was measured as described in materials and methods. Values are means ± SE. **P < 0.01 vs. control. C: chemotaxis in response to PDGF AA or PDGF BB was assessed by the modified Boyden chamber technique. **P < 0.01 vs. control.
DNA synthesis and chemotaxis assays were performed on −/−Vand −/−+ MMCs to examine whether the transfected PDGFR-β could rescue the phenotypes we observed in the −/− MMCs. As expected, the −/−V cells demonstrated a lack of mitogenic response to either PDGF AA or BB (data not shown), similar to that in −/− MMCs. In contrast, PDGF BB-induced DNA synthesis was restored to normal in the −/−+ cells (Fig. 2B). These data demonstrate that expression of wild-type PDGFR-β in the −/− MMCs rescued the mitogenic effect of PDGF BB. PDGF AA induced migration fourfold over basal levels in −/−V MMCs, whereas PDGF BB had no effect, identical to the −/− MMCs (Fig. 2C). PDGF BB induced cell migration in −/−+, whereas PDGF AA did not have an effect (Fig. 3C, right). In total, introduction of human wild-type PDGFR-β into −/− MMCs restored the +/+ phenotype.
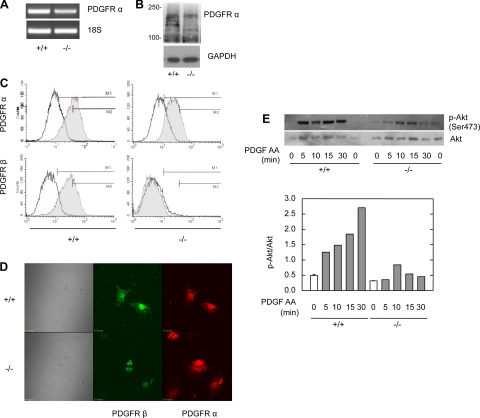
Fig. 3.
PDGFR-α is not abundantly expressed in the −/− MMCs. A: PDGFR-α mRNA in +/+ and −/− MMCs. RT-PCR was performed for PDGFR-α in the cells. 18S mRNA was used as a control. B: immunoblot for PDGFR-α in quiescent +/+ and −/− MMCs. C: flow cytometry for PDGFR-α and -β in +/+ and −/− MMCs. PDGFR expression (gray histogram) was compared with isotype control (unshaded) in nonstimulated, serum-deprived cells. D: PDGFR expression in +/+ and −/− cells by laser-scanning confocal microscopy. E: equal amounts of total protein from the treated cell lysates were loaded and separated by SDS-PAGE. Akt phosphorylation was analyzed by Western blotting using a phospho-specific (Ser 473) anti-Akt antibody. Total Akt amounts are also shown.
PDGFR-α is not upregulated in −/− MMCs.
PDGF AA is not a ligand for PDGFR-β, but it does activate PDGFR-α. The coexpression of the PDGFRs α and β may account for the lack of PDGF AA-induced migration in the +/+ cells. Because a greater level of PDGFR-α expression in the −/− MMCs could explain PDGF AA-induced migration, the extent of PDGFR-α-mediated signaling and the degree of its expression were examined.
The demonstration that PDGFR-α was not upregulated in the −/− cells was performed by multiple techniques. RT-PCR was performed on +/+ and −/− cells to assess the quantity of mRNA transcript for the PDGFR-α (Fig. 3A). There was a negligible difference between the cell lines. Immunoblots for PDGFR-α and -β were performed on +/+ and −/− lysates (Fig. 3B). There was no difference in the expression of PDGFR-α between +/+ and −/− cells. The −/− cells lacked PDGFR-β as detected by flow cytometry and did not appear to differ in the quantity of PDGFR-α expression from the +/+ cells (Fig. 3C). Laser-scanning confocal microscopy was employed to assess the expression of PDGFR-α among populations of +/+ and −/− cells (Fig. 3D). PDGFR-β was again absent in the −/− cells, and there was no discernable difference in PDGFR-α between the two cell lines. Furthermore, the −/− cells were homogenous in the degree of PDGFR-α expression.
Effect of PDGF isoforms on PI3-K-dependent signaling pathways in MMCs.
PI3-K has previously been shown to associate with tyrosine-phosphorylated PDGFRs and is a major component in PDGF-induced migration. Using the pharmacological PI3-K inhibitors wortmannin and LY294002, we have previously shown that PI3-K is the major pathway involved in PDGF BB-induced migration in rat MMCs (49). Both wortmannin and LY294002 attenuated PDGF BB- and AA-induced migration in +/+ and −/− MMCs, respectively (data not shown). One of the downstream targets of PI3-K is the serine threonine kinase Akt (15). Because Akt has been implicated in cell migration (44), we explored the activation of Akt pathway in MMCs. Cells were stimulated with PDGF AA, and lysate-derived protein was analyzed by immunoblotting using a specific antibody against the phosphorylated form of Akt. PDGF AA treatment led to higher phosphorylation of Akt in +/+ than in −/− MMCs over a similar time period (Fig. 3E).
In total, these data indicate that the PDGFR-α is neither overexpressed nor overactive in the −/− cell line. Therefore, the differential biological activities in PDGFR-β −/− cells observed in Fig. 2 were not due to alteration of any genetic program during development of these −/− cells; rather, they were due to an absence of the β-receptor. Thus we conclude that PDGF BB-induced proliferation and migration are mediated through PDGFR-β and that the presence of PDGFR-β inhibited PDGF AA-induced migration.
Involvement of Ca2+ in PDGF isoform-induced migration of MMCs.
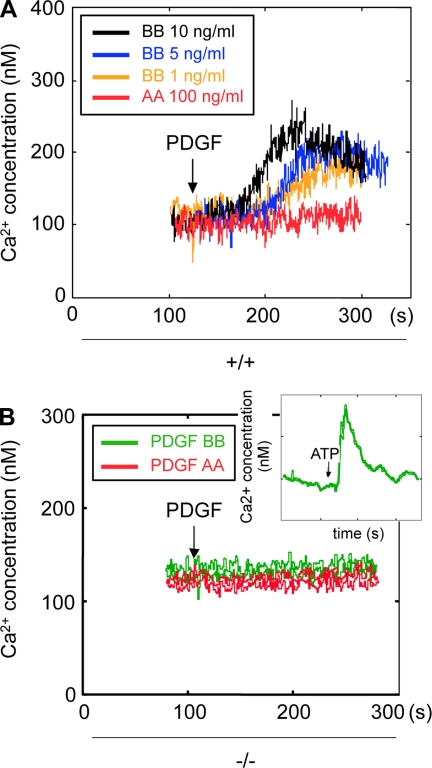
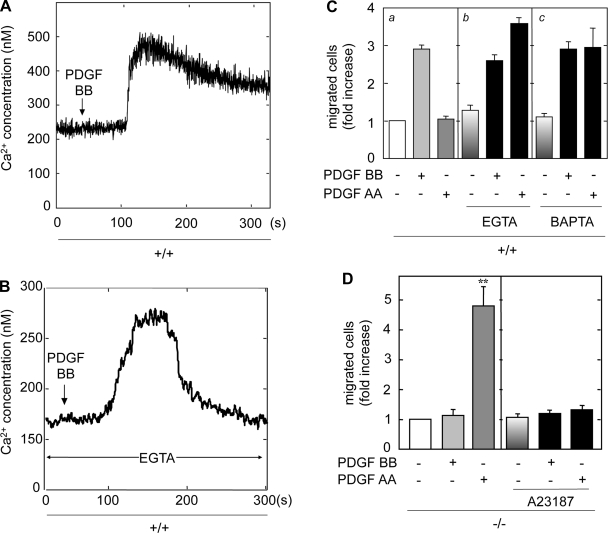
Between the PDGF receptors, activation of only the β-isoform leads to DNA synthesis (Figs. 1A and 2B). There is clearly differential regulation of migration by PDGFR-α and -β in the MMCs (Figs. 1B and 2C). These data do not explain these differential effects in PDGF-induced cell migration in +/+ and −/− MMCs. Therefore, we explored the effect of PDGF isoforms on Ca2+ flux in MMCs. Relative free cytosolic Ca2+ in the cells was measured by the ratio of fluorescence in fura 2-loaded cells (Fig. 4A). In +/+ MMCs, PDGF BB induced a rapid initial increase in intracellular Ca2+ in a dose-dependent manner; this Ca2+ was likely from internal stores. This was followed by a sustained increase in intracellular Ca2+, likely due to influx from the extracellular compartment. In contrast, PDGF AA did not have any effect on intracellular Ca2+ in +/+ cells. Neither PDGF AA nor PDGF BB induced an increase in intracellular Ca2+ in the −/− MMCs (Fig. 4B). To determine whether −/− cells are capable of generating an intracellular Ca2+ flux, cells were stimulated with ATP, which did induce a rapid increase in intracellular levels of Ca2+ (Fig. 4B, inset). These results indicate that PDGFR-β is required for a PDGF BB-induced increase in intracellular Ca2+ and that neither PDGF AA nor PDGF BB could induce a response through the PDGFR-α in the absence of PDGFR-β.
Fig. 4.
PDGF BB, but not PDGF AA, induces intracellular Ca2+ flux in +/+ cells. A: serum-deprived quiescent +/+ MMCs were loaded with fura 2 as described in materials and methods. After baselines were established, +/+ MMCs were treated with 1–10 ng/ml PDGF BB or 100 ng/ml PDGF AA and intracellular Ca2+ concentration ([Ca2+]i ) was measured. B: PDGFR β-deficient MMCs were stimulated with PDGF AA (100 ng/ml) or PDGF BB (10 ng/ml) and [Ca2+]i was measured as described.
EGTA and BAPTA are Ca2+ chelators and abolish sustained increases in cytosolic Ca2+ from the extracellular compartment. To determine the effects of EGTA on PDGF-induced intracellular Ca2+ flux, +/+ MMCs were treated with EGTA before stimulation with PDGF BB, and cytosolic Ca2+ was measured (Fig. 5, A and B). Chelation of extracellular Ca2+ with EGTA slightly reduced the level of initial Ca2+ flux and completely abolished the sustained plateau of intracellular Ca2+ influx from extracellular stores.
Fig. 5.
Extracellular Ca2+ chelator pretreatment unmasks PDGF AA-induced migration in +/+ cells. A: serum-deprived quiescent +/+ MMCs were loaded with fura 2 as described. B: quiescent +/+ MMCs were treated with 5 mM EGTA before stimulation with PDGF BB and intracellular Ca2+ flux was measured. Chelating extracellular Ca2+ with EGTA slightly reduced the level of the initial Ca2+ flux; however, EGTA completely abolished the sustained plateau of Ca2+ levels that enter the cells from extracellular stores. C: serum-deprived quiescent +/+ MMCs were used in cell migration assays in the presence of 100 ng/ml of PDGF AA or 10 ng/ml PDGF BB (a). Serum-deprived quiescent +/+ MMCs were pretreated with 5 mM EGTA (b) or 2 mM BAPTA (c) and were used in cell migration assays in the presence of 100 ng/ml of PDGF AA or 10 ng/ml PDGF BB. D: serum-deprived quiescent −/− MMCs were pretreated with 1 μM A23187 and were used in cell migration assays in the presence of 100 ng/ml of PDGF AA or 10 ng/ml PDGF BB, as described in materials and methods. Values are means ± SE of 3 independent experiments. **P < 0.01 vs. untreated control.
To address the role of Ca2+ in the migration of MMCs, EGTA and BAPTA were used in combination with the PDGF isoforms in cell chemotaxis assays (Fig. 5C). PDGF BB induced migration of +/+ MMCs, whereas PDGF AA had no effect (Fig. 5Ca). Treatment of these cells with the extracellular Ca2+ chelators did not affect PDGF BB-induced migration (Fig. 5C, b and c), indicating that the initial rapid peak of cytosolic Ca2+ from internal stores may be sufficient for PDGF BB-induced migration. However, in the presence of the extracellular chelators, PDGF AA induced migration in +/+ MMCs to similar levels as PDGF BB in unpretreated cells. These data indicate that activation of cell migration in response to PDGF AA does not require sustained cellular Ca2+ signaling but is rather inhibited by it. EGTA and BAPTA themselves had no apparent effect on cell viability or basal migration.
To further test the hypothesis that an increase in intracellular Ca2+ inhibits PDGF AA-induced migration in MMCs, −/− cells were treated with the Ca2+ ionophore A23187. This was used to enhance the influx of extracellular Ca2+ into the cytosol independently of sarco/endoplasmic reticulum Ca2+ ATPase (SERCA). Treatment of cells with AT23187 completely inhibited PDGF AA-induced cell migration (Fig. 5D), whereas basal migration was unaffected, indicating that an increase in extracellular Ca2+ flux inhibits PDGF AA-induced migration in −/− MMCs.
PDGF-induced ROS regulate Ca2+ production and migration in MMCs.
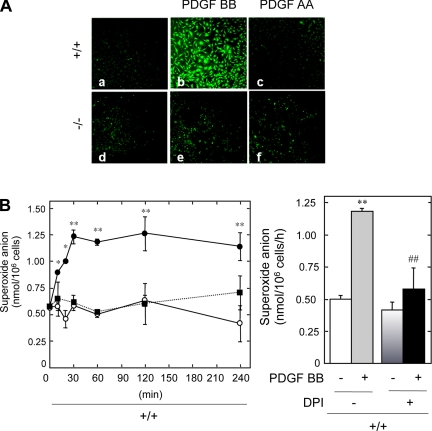
Second messengers such as ROS including O2− and H2O2 have been implicated in PDGF-induced biological activity in various cells. A role for H2O2 in PDGF-induced mitogenesis has been proposed (20, 56, 60). PDGF-induced H2O2 production was measured in +/+ and −/− MMCs with a fluorescence-based assay using a peroxide-sensitive fluorophore, DCFDA, and laser-scanning confocal microscopy. PDGF BB significantly increased the DCFDA fluorescence in +/+ MMCs, indicating the production of H2O2 in these cells (Fig. 6A, compare b with a). PDGF AA did not increase H2O2 production in either +/+ (Fig. 6Ac) or −/− MMCs (Fig. 6Af). One mechanism by which H2O2 can be produced is the spontaneous dismutation of O2−. We therefore assessed O2− generation in MMCs by measuring the SOD-inhibitable reduction of ferricytochrome c. Treatment of MMCs with PDGF BB resulted in a rapid and time-dependent increase in O2− generation only in +/+ MMCs (Fig. 6B, left). This generation of O2− was maximal at 30 min and sustained for up to 4 h. PDGF AA did not have a significant effect on O2− generation. These data indicate that PDGF BB increases ROS through PDGFR-β in +/+ MMCs, while in the absence of PDGFR-β neither O2− nor H2O2 is produced in response to PDGF. We have previously demonstrated that the generation of ROS may be via an NAD(P)H oxidase (60); therefore, PDGF BB-stimulated +/+ cells were pretreated with the flavoprotein inhibitor diphenylene iodonium (DPI) (Fig. 6B, right). DPI treatment suppressed PDGF BB-induced O2− generation without affecting basal levels. These data demonstrate that PDGF BB elicits ROS generation via a DPI-inhibitable source, such as an NAD(P)H oxidase.
Fig. 6.
PDGF BB is a potent stimulator of reactive oxygen species (ROS) generation in PDGFR β-expressing MMCs. A: representative photomicrographs of DCF fluorescence in +/+ MMCs under basal conditions (a), 120 min after addition of 10 ng/ml PDGF BB (b) or 100 ng/ml PDGF AA (c), and representative photomicrographs of DCF fluorescence in −/− MMCs under basal conditions (d), 120 min after addition of 10 ng/ml of PDGF BB (e) or 100 ng/ml of PDGF AA (f). B: quiescent +/+ MMCs were stimulated with PDGF BB (•), PDGF AA (▪, dashed line), or left untreated (○) for increasing time intervals at 37°C in Hanks' balanced salt solution containing 80 μM ferricytochrome c (left). Superoxide-specific reduction of ferricytochrome c was calculated from the difference in absorbance between cells incubated with or without SOD (50 μg/ml) by use of an extinction coefficient of 21.0 × 103 M/cm and expressed as nanomoles superoxide/106 cells. Reduction of ferricytochrome c was calculated as described in materials and methods. Values are means ± SE of 3 separate experiments. Pretreatment with the NADPH oxidase inhibitor diphenyleneiodonium (DPI; 10 μM) suppressed PDGF BB-induced superoxide generation in +/+ cells (right). Values are means ± SE of 3 independent experiments. **P < 0.01 with respect to control. ##P < 0.01 with respect to the PDGF-treated group.
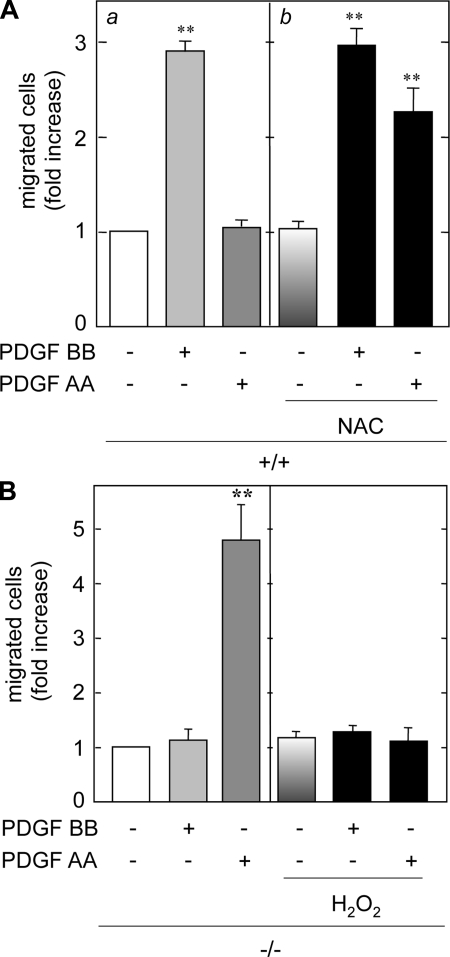
Induction of ROS by PDGFR-β activation may be responsible for suppressing PDGF AA-induced migration in +/+ MMCs. To address this, the MMCs were treated with the antioxidant NAC and PDGF-induced migration was assessed. NAC is an antioxidant thiol compound that works by increasing glutathione stores. Treatment of +/+ MMCs with NAC resulted in a significant increase in PDGF AA-induced migration (Fig. 7A). Given that an antioxidant unmasked the inhibitory effect of PDGF BB on AA-induced migration in +/+ cells, the sensitivity of PDGF AA-induced migration to an oxidant was examined in −/− cells. Pretreatment with H2O2 completely blocked PDGF AA-induced migration (Fig. 7B). These results indicate that ROS negatively regulate PDGF AA-induced migration in +/+ cells. PDGF BB-induced increases in cytosolic Ca2+ or ROS generation are not key mediators in PDGF BB-induced migration (Figs. 5C and 7A). Nonetheless, these second messengers may play a significant role in suppressing PDGF AA-induced migration in MMCs when PDGFR-β is expressed.
Fig. 7.
PDGF-induced chemotaxis is redox dependent. A: quiescent +/+ MMCs were pretreated with 20 mM N-acetyl-l-cysteine (NAC; b), stimulated with PDGF AA or BB, and migration was assessed. B: quiescent −/− MMCs were pretreated with H2O2, stimulated with PDGF AA or BB, and migration assessed as described in materials and methods.
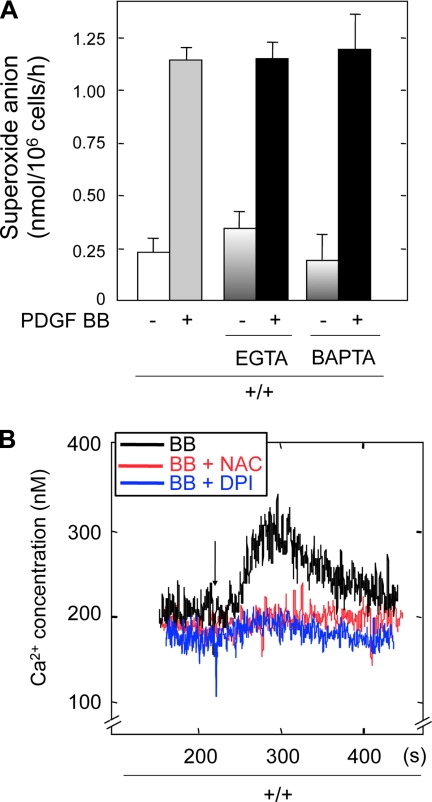
To investigate cross talk between Ca2+ flux and redox pathways induced by PDGF BB, we examined the effect of Ca2+ chelators EGTA or BAPTA on PDGF BB-induced O2− production. There was no effect of either chelator on PDGF BB-induced O2− generation (Fig. 8A). These data indicate that the increase in Ca2+ flux does not regulate the redox pathway induced by PDGF BB. However, this observation does not rule out the possibility of redox regulation of Ca2+ by PDGF BB. Therefore, the effects of NAC and DPI on PDGF BB-induced intracellular Ca2+ fluxes were examined. Pretreatment of +/+ MMCs with either compound significantly inhibited PDGF BB-induced cytosolic Ca2+ (Fig. 8B). These data indicate that ROS are upstream regulators of the PDGF BB-induced increase in intracellular Ca2+.
Fig. 8.
ROS act as upstream mediators of Ca2+ flux. A: quiescent +/+ MMCs were treated with EGTA (5 mM) or BAPTA (2 mM) for 2 min before being stimulated with PDGF BB (10 ng/ml) or PDGF AA (100 ng/ml) for 120 min at 37°C in Hanks' balanced salt solution containing 80 μM ferricytochrome c. Superoxide-specific reduction of ferricytochrome c was calculated as in Fig. 7. Values are means + SE of 3 separate experiments. B: serum-deprived +/+ MMCs were loaded with fura 2 and incubated with NAC or DPI for 20 min. After baselines were established, +/+ MMCs were treated with PDGF BB, and [Ca2+]i was measured.
DISCUSSION
Activation of PDGFR-β is typically promitogenic and chemotactic in a number of cell types, whereas PDGFR-α-mediated migration is particular to specific cells (64). PDGF AA can inhibit cell migration in certain cell types (34, 45). In vascular smooth muscle cells and 3T3 fibroblasts, PDGFR-β mediates the mobilization of intracellular Ca2+ more efficiently than PDGFR-α (12, 13). It is possible that these differences reflect cell type-specific or context-dependent signaling mechanisms.
Signaling through PDGFR-α does not compensate for the loss of PDGFR-β signaling in regulating the development of MMCs to mature mesangial cells. We show that PDGF BB is mitogenic and chemotactic through PDGFR-β in +/+ MMCs isolated from 11.5-dpc embryos. PDGF AA is chemotactic only in −/− cells. In the absence of PDGFR-β, signaling through PDGFR-α may cause aberrant migration of MMCs during nephrogenesis and therefore may help explain the lack of mature mesangial cells in the glomerular tuft of PDGFR-β-deficient animals (54). We demonstrate that the lack of PDGF AA-induced migration in +/+ MMCs is specifically due to the presence of PDGFR-β and not due to differential activation of the Akt pathway, which is known to regulate PDGF-induced migration. We present evidence that PDGFR-β-induced generation of ROS and Ca2+ flux negatively regulate AA-induced migration and thus provide a mechanism by which specific signals generated by PDGFR-β suppress PDGF AA-induced migration of MMCs during kidney development.
Many studies have confirmed similarities as well as differences differences in the signaling events mediated by PDGFR-α and PDGFR-β. Both receptors are protein tyrosine kinases that activate similar sets of signaling molecules. However, in vivo studies of targeted deletions of the respective receptors reveal markedly different phenotypes. Mice lacking PDGFR-α die between embryonic days E8 and E16, with phenotypic abnormalities including cleft facies, skeletal defects, abnormal somite patterning, and hemorrhage (55). Mice lacking PDGFR-β die between E16 and birth, exhibiting cardiovascular, hematological, and renal defects including abnormal capillary formation within the glomerular tuft of the developing kidney and a total lack of mesangial cells (54). To test the specificity of PDGFR signaling in vivo, Klinghoffer et al. (33) created complementary lines of knockin mice expressing mutant PDGFR-β with the intracellular signaling domains of PDGFR-α and mice expressing mutant PDGFR-α with the intracellular signaling domains of PDGFR-β. Both lines demonstrated substantial rescue of normal development, in particular the ability of the PDGFR-β intracellular domain to compensate for PDGFR-α. However, substitution of the PDGFR-β signaling domain with that of PDGFR-α resulted in varying degrees of vascular disease, including glomeruli devoid of mesangial cells. These data conclusively demonstrated the importance of PDGF B-dependent β-receptor signaling in the development of mesangial cells.
Since both PDGFR-β and PDGF B-chain −/− mice die perinatally and exhibit a total lack of mesangial cells within the glomerular tuft, it is difficult to study the mechanisms of this pathway in the development of mature mesangial cells. Studies in the PDGF B-chain deficient mouse demonstrate PDGFR-β expression in the developing S-shaped nephron segment and in the early cup-shaped glomeruli. In late-stage glomerular development in the PDGF B-null kidney, PDGFR-β-positive cells are found only in the juxtaglomerular region. Lindahl et al. (40) have generated chimeric mice from +/+ blastocysts injected with PDGFR-β-deficient embryonic stem cells to provide insight into the ontogeny of mesangial cells. They noted that the glomerular mesangium invariably expressed PDGFR-β, implying a direct effect of PDGF-B on the development of mesangial cell lineage. The glomerular pathology of the −/− mice indicates that mesangial cells or their progenitors are critical targets for PDGF B-mediated signals. The absence of mesangial cells in the mutant glomeruli may also be a result of indirect effects of PDGF B on PDGFR-β signaling. The cells that populate the glomerulus may use PDGF B and PDGFR-β signaling in the maintenance of cell survival during the transition from proliferating to terminally differentiating cells. Additionally, PDGF B/PDGFR-β may serve to suppress PDGF-A/PDGFR-α signaling while other factors promote the differentiation program. Although many signaling pathways of PDGFR-α or -β may be redundant, certain signals are specific to each.
We report that PDGF BB induces ROS production and Ca2+ flux in +/+ MMCs, whereas PDGF AA does not. Neither PDGF AA nor BB increases ROS production or Ca2+ flux in −/− MMCs. Increased cytosolic Ca2+ can activate downstream signals involved in localized structural changes and directed cell migration (53). Many reports have suggested that Ca2+ positively regulates the pathways involved in cell migration using the Ca2+/calmodulin signaling pathway. Previous reports have also suggested that the motility of endothelial cells can be sensitive or insensitive to extracellular Ca2+, depending on the immobilized extracellular matrix and integrins involved (37). These studies focused on haptotaxis, whereas we now demonstrate sensitivity to Ca2+ in a chemotactic system. Other studies in various cell types have implicated Ca2+ as a mediator of migration, but only a few studies have shown that Ca2+ influx inhibits migration. Hodgson et al. (28) have reported that Ca2+ downregulates α2β1-integrin-mediated cell motility in A2058 human melanoma cells. Horgan et al. (29) have shown G protein-mediated inhibition of neuronal migration requires Ca2+ influx. In the present study, we provide evidence that signals mediated by PDGFR-β, such as an increase in Ca2+ influx, block PDGF AA-induced migration of +/+ MMCs. In developing metanephroi, the absence of PDGFR-β-mediated Ca2+ influx in −/− MMCs allows PDGF AA-induced migration of these cells. This may result in aberrant localization of mesangial cell precursors in the −/− animals.
In nonphagocytic cells, there is considerable evidence that ROS act as classic second-messenger molecules in response to stimulation with a variety of growth factors and cytokines (17, 19). Cytosolic Ca2+ levels can be increased in response to ROS in various cell types through the mobilization of intracellular Ca2+ stores and/or through the influx of extracellular Ca2+ (14, 16, 17, 25, 35, 46–48). This suggests a physiological role of the redox state in the regulation of Ca2+ signaling (31, 32). The exact source of Ca2+ release in response to oxidants remains controversial, and molecular targets of oxidants have not yet been clearly defined (57). On the other hand, oxygen radicals may not be involved in Ca2+ flux (61), but rather the Ca2+ mobilization may be responsible for ROS production (58). We show that ROS are upstream mediators of Ca2+ flux in MMCs and that ROS-dependent Ca2+ flux negatively regulates PDGF AA-induced migration.
Activation of PDGFR-β stimulates production of O2− and H2O2 (4, 42, 56). The differential effect of the PDGF isoforms on ROS generation observed in MMCs correlates well with the data reported by Marumo et al. (42), who showed that PDGF BB, rather than PDGF AA, stimulated ROS generation in aortic smooth muscle cells. PDGF BB has been reported to elicit ROS generation in various cell types (2, 7, 36). For instance, Bae et al. (4) demonstrated that PI3-K and activation of Rac1 were required for PDGFR-β-induced H2O2 production in HepG2 cells. Cells from mice deficient for the mammalian 2-Cys peroxiredoxin type II (Prx II) demonstrate increased H2O2 production and increased PDGFR-β activity (9). In cultured mouse embryonic fibroblasts, overexpression of Prx II suppressed protein tyrosine phosphorylation (including of PLC-γ1) and inhibited PDGF-induced inositol-1,4,5-triphosphate production and chemotaxis. The authors concluded that Prx II is a principle inhibitory regulator of PDGFR-β signaling, as endogenous H2O2 amplifies PDGFR phosphorylation in a site-specific manner, and Prx II inhibits this phosphorylation and subsequent receptor activity (9). Our data indicate that PDGFR-β blunts activity of PDGFR-α via ROS. In vivo, there may be PDGF BB-induced redox-sensitive mediators that suppress PDGFR-α activation, such as the peroxiredoxins.
PDGFR-α may have a role in the development of interstitial cells, including adventitial fibroblasts (51). Mouse blastemas lacking this receptor (i.e., Patch mutation) have a severe phenotype, characterized by a diffuse paucity of fibroblasts (51) and deficiencies in renal interstitial mesenchyme, the precursors of vascular smooth muscle cells and mesangial cells (6). At later stages of nephrogenesis, PDGFR-β is expressed in MC precursors in the cleft of the comma-shaped and S-shaped bodies and in more mature glomeruli in a mesangial distribution (3). Because PDGFR-α-deficient mice have a deficit in renal interstitial mesenchyme (6, 39), it is our belief that PDGFR-α may serve as the means by which loose mesenchyme differentiates into interstitial mesenchymal cells before the S-shaped body phase, when PDGFR-β-expressing cells hone to the developing glomerular cleft.
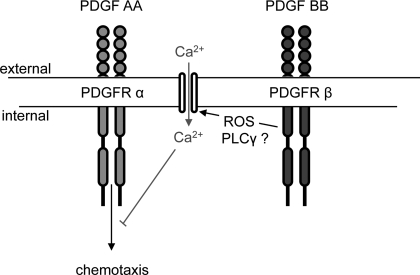
In this study, PDGF BB induced migration of +/+ MMCs, whereas PDGF AA had minimal effects. In the absence of PDGFR-β, PDGF AA induced migration in MMCs and the response was suppressed by H2O2 or a Ca2+ ionophore. By chelating extracellular Ca2+ or scavenging ROS in +/+ MMCs, PDGF AA seems to be capable of inducing migration. These results suggest that in the absence of PDGFR-β, PDGFR-α is capable of inducing migration, which does not normally occur in the presence of PDGFR-β, and that ROS and Ca2+ influx are involved in negatively regulating PDGFR-α-mediated migration (Fig. 9). These data may help explain why PDGFR-α is unable to compensate for the lack of PDGFR-β in the development of mesangial cells during embryogenesis. The factors involved in the regulation of mesangial cell development are multifactorial and likely depend on the temporospatial distribution of the PDGFRs and ligands, the binding affinities of the ligands to their respective receptors, and the specificity of the signals. Further studies are needed to correlate the kinetics of ROS and Ca2+ signaling induced by PDGFR-β to particular cytoplasmic and nuclear events. A comprehensive examination of molecules activated by PDGFR-β may lead to an understanding of the mechanism by which PDGFR-β activation results in mesangial cell development and maturation.
Fig. 9.
Proposed model of PDGFR β-mediated suppression of PDGF AA-induced migration in MMCs.
GRANTS
These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-33665 (H. E. Abboud), DK-50190, and DK-55815 (G. G. Choudhury). Y. Gorin is supported through a Scientist Development Grant from the National American Heart Association, a Juvenile Diabetes Research Foundation Regular Research Grant, a National Kidney Foundation Young Investigator Award, and a National Kidney Foundation of South and Central Texas, Research Program Award. G. G. Choudbury is supported by a Department of Veterans Affairs Merit Review grant, Regular Research Grant from the Juvenile Diabetes Research Foundation, and by a Department of Veterans Affairs Research Career Scientist Award. B. Wagner is the recipient of the National Institutes of Health Loan Repayment Program, National Kidney Foundation Research Fellowship, National Kidney Foundation Jack C. Kent Third Year Research Fellowship, and an American Heart Association Fellow-to-Faculty Transition Award.
Acknowledgments
We thank Dr. Philippe Soriano at the Program in Developmental Biology and Division of Basic Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, for providing the PDGFR β-deficient mice. Images were generated in the Core Optical Imaging Facility which is supported by UTHSCSA, National Institutes of Health (NIH)-NCI P30 CA54174 (San Antonio Cancer Institute), NIH-NIA P30 AG013319 (Nathan Shock Center), and (NIH-NIA P01AG19316). The flow cytometry part of this work was performed at the Institutional/SACI Flow Cytometry Core Laboratory. We also are indebted to Bridget Fagg, Andrea Barrentine-Fourcaudot, and Yohann Wittrant for experiments used in this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abboud HE Role of platelet-derived growth factor in renal injury. Annu Rev Physiol 57: 297–309, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Adachi T, Togashi H, Suzuki A, Kasai S, Ito J, Sugahara K, Kawata S. NAD(P)H oxidase plays a crucial role in PDGF-induced proliferation of hepatic stellate cells. Hepatology 41: 1272–1281, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Arar M, Xu YC, Elshihabi I, Barnes JL, Choudhury GG, Abboud HE. Platelet-derived growth factor receptor beta regulates migration and DNA synthesis in metanephric mesenchymal cells. J Biol Chem 275: 9527–9533, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bae YS, Sung JY, Kim OS, Kim YJ, Hur KC, Kazlauskas A, Rhee SG. Platelet-derived growth factor-induced H2O2 production requires the activation of phosphatidylinositol 3-kinase. J Biol Chem 275: 10527–10531, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bergsten E, Uutela M, Li X, Pietras K, Ostman A, Heldin CH, Alitalo K, Eriksson U. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat Cell Biol 3: 512–516, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Betsholtz C, Lindblom P, Bjarnegard M, Enge M, Gerhardt H, Lindahl P. Role of platelet-derived growth factor in mesangium development and vasculopathies: lessons from platelet-derived growth factor and platelet-derived growth factor receptor mutations in mice. Curr Opin Nephrol Hypertens 13: 45–52, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Boureux A, Furstoss O, Simon V, Roche S. Abl tyrosine kinase regulates a Rac/JNK and a Rac/Nox pathway for DNA synthesis and Myc expression induced by growth factors. J Cell Sci 118: 3717–3726, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Cantley LC, Auger KR, Carpenter C, Duckworth B, Graziani A, Kapeller R, Soltoff S. Oncogenes and signal transduction. Cell 64: 281–302, 1991. [DOI] [PubMed] [Google Scholar]
- 9.Choi MH, Lee IK, Kim GW, Kim BU, Han YH, Yu DY, Park HS, Kim KY, Lee JS, Choi C, Bae YS, Lee BI, Rhee SG, Kang SW. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature 435: 347–353, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Choudhury GG, Grandaliano G, Jin DC, Katz MS, Abboud HE. Activation of PLC and PI 3 kinase by PDGF receptor alpha is not sufficient for mitogenesis and migration in mesangial cells. Kidney Int 57: 908–917, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Claesson-Welsh L, Eriksson A, Westermark B, Heldin CH. cDNA cloning and expression of the human A-type platelet-derived growth factor (PDGF) receptor establishes structural similarity to the B-type PDGF receptor. Proc Natl Acad Sci USA 86: 4917–4921, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diliberto PA, Gordon G, Herman B. Regional and mechanistic differences in platelet-derived growth factor-isoform-induced alterations in cytosolic free calcium in porcine vascular smooth muscle cells. J Biol Chem 266: 12612–12617, 1991. [PubMed] [Google Scholar]
- 13.Diliberto PA, Gordon GW, Yu CL, Earp HS, Herman B. Platelet-derived growth factor (PDGF) alpha receptor activation modulates the calcium mobilizing activity of the PDGF beta receptor in Balb/c3T3 fibroblasts. J Biol Chem 267: 11888–11897, 1992. [PubMed] [Google Scholar]
- 14.Doan TN, Gentry DL, Taylor AA, Elliott SJ. Hydrogen peroxide activates agonist-sensitive Ca2+-flux pathways in canine venous endothelial cells. Biochem J 297: 209–215, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downward J Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol 10: 262–267, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Dreher D, Junod AF. Role of oxygen free radicals in cancer development. Eur J Cancer 32: 30–38, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Droge W Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson A, Siegbahn A, Westermark B, Heldin CH, Claesson-Welsh L. PDGF alpha- and beta-receptors activate unique and common signal transduction pathways. EMBO J 11: 543–550, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finkel T Oxygen radicals and signaling. Curr Opin Cell Biol 10: 248–253, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Rubio M, Voit S, Rodriguez-Puyol D, Weber M, Marx M. Oxidative stress induces tyrosine phosphorylation of PDGF alpha-and beta-receptors and pp60c-src in mesangial cells. Kidney Int 50: 164–173, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Gorin Y, Kim NH, Feliers D, Bhandari B, Choudhury GG, Abboud HE. Angiotensin II activates Akt/protein kinase B by an arachidonic acid/redox-dependent pathway and independent of phosphoinositide 3-kinase. FASEB J 15: 1909–1920, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol 285: F219–F229, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Grandaliano G, Choudhury GG, Biswas P, Abboud HE. Mitogenic signaling of thrombin in mesangial cells: role of tyrosine phosphorylation. Am J Physiol Renal Fluid Electrolyte Physiol 267: F528–F536, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Grandaliano G, Valente AJ, Rozek MM, Abboud HE. Gamma interferon stimulates monocyte chemotactic protein (MCP-1) in human mesangial cells. J Lab Clin Med 123: 282–289, 1994. [PubMed] [Google Scholar]
- 25.Hallbrucker C, Ritter M, Lang F, Gerok W, Haussinger D. Hydroperoxide metabolism in rat liver. K+ channel activation, cell volume changes and eicosanoid formation. Eur J Biochem 211: 449–458, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Heldin CH, Ernlund A, Rorsman C, Ronnstrand L. Dimerization of B-type platelet-derived growth factor receptors occurs after ligand binding and is closely associated with receptor kinase activation. J Biol Chem 264: 8905–8912, 1989. [PubMed] [Google Scholar]
- 27.Heldin CH, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta 1378: 79–113, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Hodgson L, Dong C. [Ca2+]i as a potential downregulator of α2β1-integrin-mediated A2058 tumor cell migration to type IV collagen. Am J Physiol Cell Physiol 281: C106–C113, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horgan AM, Copenhaver PF. G protein-mediated inhibition of neuronal migration requires calcium influx. J Neurosci 18: 4189–4200, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston RB Measurement of O2− secreted by monocytes and macrophages. Methods Enzymol 105: 365–369, 1984. [DOI] [PubMed] [Google Scholar]
- 31.Junn E, Lee KN, Ju HR, Han SH, Im JY, Kang HS, Lee TH, Bae YS, Ha KS, Lee ZW, Rhee SG, Choi I. Requirement of hydrogen peroxide generation in TGF-beta 1 signal transduction in human lung fibroblast cells: involvement of hydrogen peroxide and Ca2+ in TGF-beta 1-induced IL-6 expression. J Immunol 165: 2190–2197, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Kawakami M, Okabe E. Superoxide anion radical-triggered Ca2+ release from cardiac sarcoplasmic reticulum through ryanodine receptor Ca2+ channel. Mol Pharmacol 53: 497–503, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Klinghoffer RA, Mueting-Nelsen PF, Faerman A, Shani M, Soriano P. The two PDGF receptors maintain conserved signaling in vivo despite divergent embryological functions. Mol Cell 7: 343–354, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Koyama N, Kinsella MG, Wight TN, Hedin U, Clowes AW. Heparan sulfate proteoglycans mediate a potent inhibitory signal for migration of vascular smooth muscle cells. Circ Res 83: 305–313, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Kumasaka S, Shoji H, Okabe E. Novel mechanisms involved in superoxide anion radical-triggered Ca2+ release from cardiac sarcoplasmic reticulum linked to cyclic ADP-ribose stimulation. Antioxid Redox Signal 1: 55–69, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, Rhee SG. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci USA 101: 16419–16424, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leavesley DI, Schwartz MA, Rosenfeld M, Cheresh DA. Integrin beta 1- and beta 3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol 121: 163–170, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev 8: 1875–1887, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Ponten A, Aase K, Karlsson L, Abramsson A, Uutela M, Backstrom G, Hellstrom M, Bostrom H, Li H, Soriano P, Betsholtz C, Heldin CH, Alitalo K, Ostman A, Eriksson U. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol 2: 302–309, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Lindahl P, Hellstrom M, Kalen M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C. Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development 125: 3313–3322, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Margolis B, Skolnik EY. Activation of Ras by receptor tyrosine kinases. J Am Soc Nephrol 5: 1288–1299, 1994. [DOI] [PubMed] [Google Scholar]
- 42.Marumo T, Schini-Kerth VB, Fisslthaler B, Busse R. Platelet-derived growth factor-stimulated superoxide anion production modulates activation of transcription factor NF-kappaB and expression of monocyte chemoattractant protein 1 in human aortic smooth muscle cells. Circulation 96: 2361–2367, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Matsui T, Heidaran M, Miki T, Popescu N, La Rochelle W, Kraus M, Pierce J, Aaronson S. Isolation of a novel receptor cDNA establishes the existence of two PDGF receptor genes. Science 243: 800–804, 1989. [DOI] [PubMed] [Google Scholar]
- 44.Nakashio A, Fujita N, Tsuruo T. Topotecan inhibits VEGF- and bFGF-induced vascular endothelial cell migration via downregulation of the PI3K-Akt signaling pathway. Int J Cancer 98: 36–41, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Nister M, Hammacher A, Mellstrom K, Siegbahn A, Ronnstrand L, Westermark B, Heldin CH. A glioma-derived PDGF A chain homodimer has different functional activities from a PDGF AB heterodimer purified from human platelets. Cell 52: 791–799, 1988. [DOI] [PubMed] [Google Scholar]
- 46.Okabe E, Kato Y, Sasaki H, Saito G, Hess ML, Ito H. Calmodulin participation in oxygen radical-induced cardiac sarcoplasmic reticulum calcium uptake reduction. Arch Biochem Biophys 255: 464–468, 1987. [DOI] [PubMed] [Google Scholar]
- 47.Okabe E, Kuse K, Sekishita T, Suyama N, Tanaka K, Ito H. The effect of ryanodine on oxygen free radical-induced dysfunction of cardiac sarcoplasmic reticulum. J Pharmacol Exp Ther 256: 868–875, 1991. [PubMed] [Google Scholar]
- 48.Okabe E, Sugihara M, Tanaka K, Sasaki H, Ito H. Calmodulin and free oxygen radicals interaction with steady-state calcium accumulation and passive calcium permeability of cardiac sarcoplasmic reticulum. J Pharmacol Exp Ther 250: 286–292, 1989. [PubMed] [Google Scholar]
- 49.Ricono JM, Arar M, Choudhury GG, Abboud HE. Effect of platelet-derived growth factor isoforms in rat metanephric mesenchymal cells. Am J Physiol Renal Physiol 282: F211–F219, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Ricono JM, Xu YC, Arar M, Jin DC, Barnes JL, Abboud HE. Morphological insights into the origin of glomerular endothelial and mesangial cells and their precursors. J Histochem Cytochem 51: 141–150, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Seifert RA, Alpers CE, Bowen-Pope DF. Expression of platelet-derived growth factor and its receptors in the developing and adult mouse kidney. Kidney Int 54: 731–746, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Seifert RA, van Koppen A, Bowen-Pope DF. PDGF-AB requires PDGF receptor alpha-subunits for high-affinity, but not for low-affinity, binding and signal transduction. J Biol Chem 268: 4473–4480, 1993. [PubMed] [Google Scholar]
- 53.Simpson PB, Russell JT. Role of mitochondrial Ca2+ regulation in neuronal and glial cell signalling. Brain Res Brain Res Rev 26: 72–81, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Soriano P Abnormal kidney development and hematological disorders in PDGF b-receptor mutant mice. Genes Dev 8: 1888–1896, 1994. [DOI] [PubMed] [Google Scholar]
- 55.Soriano P The PDGF a receptor is required for neural crest cell development and for normal patterning of the somites. Development 124: 2691–2700, 1997. [DOI] [PubMed] [Google Scholar]
- 56.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270: 296–299, 1995. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med 22: 269–285, 1997. [DOI] [PubMed] [Google Scholar]
- 58.Valentin F, Bueb J, Capdeville-Atkinson C, Tschirhart E. Rac-1-mediated O2− secretion requires Ca2+ influx in neutrophil-like HL-60 cells. Cell Calcium 29: 409–415, 2001. [DOI] [PubMed] [Google Scholar]
- 59.Valius M, Kazlauskas A. Phospholipase C-γ1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor's mitogenic signal. Cell 73: 321–334, 1993. [DOI] [PubMed] [Google Scholar]
- 60.Wagner B, Ricono JM, Gorin Y, Block K, Arar M, Riley D, Choudhury GG, Abboud HE. Mitogenic signaling via platelet-derived growth factor beta in metanephric mesenchymal cells. J Am Soc Nephrol 18: 2903–2911, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Wang XT, McCullough KD, Wang XJ, Carpenter G, Holbrook NJ. Oxidative stress-induced phospholipase C-gamma 1 activation enhances cell survival. J Biol Chem 276: 28364–28371, 2001. [DOI] [PubMed] [Google Scholar]
- 62.Wennstrom S, Siegbahn A, Yokote K, Arvidsson AK, Heldin CH, Mori S, Claesson-Welsh L. Membrane ruffling and chemotaxis transduced by the PDGF beta-receptor require the binding site for phosphatidylinositol 3′ kinase. Oncogene 9: 651–660, 1994. [PubMed] [Google Scholar]
- 63.Yarden Y, Escobedo JA, Kuang WJ, Yang-Feng TL, Daniel TO, Tremble PM, Chen EY, Ando ME, Harkins RN, Francke U, Fried VA, Ullrich A, Williams LT. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature 323: 226–232, 1986. [DOI] [PubMed] [Google Scholar]
- 64.Yokote K, Mori S, Siegbahn A, Ronnstrand L, Wernstedt C, Heldin CH, Claesson-Welsh L. Structural determinants in the platelet-derived growth factor alpha-receptor implicated in modulation of chemotaxis. J Biol Chem 271: 5101–5111, 1996. [DOI] [PubMed] [Google Scholar]