Abstract
The present experiments were designed to detail factors regulating phosphate transport in cultured mouse proximal tubule cells by determining the response to parathyroid hormone (PTH), dopamine, and second messenger agonists and inhibitors. Both PTH and dopamine inhibited phosphate transport by over 30%. The inhibitory effect of PTH was completely abolished in the presence of chelerythrine, a PKC inhibitor, but not by Rp-cAMP, a PKA inhibitor. By contrast, both chelerythrine and Rp-cAMP blocked the inhibitory effect of dopamine. Chelerythrine inhibited PTH-mediated cAMP accumulation but also blocked the inhibitory effect of 8-bromo-cAMP on phosphate transport. On the other hand, Rp-cAMP had no effect on the ability of DOG, a PKC activator, to inhibit phosphate transport. PD98059, an inhibitor of MAPK, had no effect on PTH- or dopamine-mediated inhibition of sodium-phosphate cotransport. Finally, compared with 8-bromo-cAMP, 8-pCPT-2′-O-Me-cAMP, an activator of EPAC, had no effect on phosphate transport. These results outline significant differences in the signaling pathways utilized by PTH and dopamine to inhibit renal phosphate transport. Our results also suggest that activation of MAPK is not critically involved in PTH- or dopamine-mediated inhibition of phosphate transport in mouse renal proximal tubule cells in culture.
Keywords: parathyroid hormone, PKA, PKC
in recent experiments from this laboratory, we used primary cultures of mouse proximal tubule cells to study phosphate transport with emphasis on elucidating the role of adaptor proteins such as NHERF-1 (7–9, 15, 30, 33). From such studies, we provided evidence that parathyroid hormone (PTH) inhibits phosphate transport utilizing downstream signaling pathways involving activation of protein kinase C (PKC) and protein kinase A (PKA) (7, 8). We have not, however, detailed the role of these pathways in this tissue. This is important since there may be differences in second messenger signaling pathways between model cell systems (3, 22). Moreover, hormones such as PTH have been demonstrated to interact with PTH1 receptors on both the apical and basolateral sides of renal proximal tubule cells and although the receptors are the same, signaling following the binding of PTH differs (5, 19, 26, 31). Accordingly, in the present experiments, we employ inhibitors and activators of specific protein kinases to determine the role of PKC, PKA, and MAPK on both PTH- and dopamine-mediated inhibition of phosphate transport in primary cultures of mouse proximal tubule cells. In addition, we contrasted the effect of cAMP with a specific activator of exchange protein activated by cAMP (EPAC) (10, 16, 27).
METHODS
Animals and preparation of renal proximal tubule cells.
Male C57BL/6 mice 12 to 16 wk were used in the current experiments. To prepare primary renal proximal tubule cell cultures, mice were euthanized by intraperitoneal injection of 100 mg/kg pentobarbital sodium followed by decapitation. The kidneys were removed, and the cortices were dissected, minced, and digested using 1% collagenase type II (Worthington) and 0.025% soy bean trypsin inhibitor, and sedimented on 45% Percoll (7, 9, 33). The proximal tubule cells were grown in an incubator at 37°C in 5% CO2 in DMEM-F12 media containing 50 U/ml of penicillin, 50 μg/ml streptomycin, 10 ng/ml epidermal growth factor, 0.5 μM hydrocortisone, 0.87 μM bovine insulin, 50 μM prostaglandin E1, 50 nM sodium selenite, 5 μg/ml human transferrin, and 5 pM 3,3′,5-triiodo-l-thyronine on Matrigel-coated plastic cell culture dishes. The cultures were left undisturbed for 36 h after which the media were replaced every 2 days until the cells achieved confluence at 5 to 7 days after plating.
Transport assay.
Where studied, cells were treated initially with chelerythrine (10 nM), Rp-cAMP (100 μM), or PD98059 (100 μM) for 20 min before the addition of PTH (10−7 M), dopamine (10 μM), DOG (10 μM), or 8-bromo-cAMP (100 μM) for 60 min. In other studies, cells were incubated in 8-bromo-cAMP (100 μM) or 8-pCPT-2′-O-Me-cAMP (50 μM) for 25 min before study. Phosphate transport was measured by determination of the sodium-dependent uptake of 32P-labeled phosphate (9, 11). The cells were washed three times and preincubated for 5 min in nonradioactive transport medium containing 137 mM NaCl, 5.0 mM KCl, 1.0 mM CaCl2, 1.8 mM MgSO4, and 0.1 mM KH2PO4. Phosphate uptake was initiated by the addition of transport medium containing 32P-radiolabeled orthophosphate. Uptake was continued for 10 min at room temperature, after which the cells were washed with ice-cold medium in which tetramethylammonium chloride was substituted for sodium chloride, 32P-phosphate was omitted, and 0.5 mM sodium arsenate was added. The cells were solubilized in 1% Triton X-100 for 90 min at 4°C and an aliquot was analyzed by liquid scintillation spectroscopy. Each assay was performed in triplicate and averaged to provide a single data point.
Other assays.
Membrane preparations from cultured cells were prepared as previously described (8). The resultant pellet was resuspended in 0.1% SDS, electrophoresed in Laemli buffer, and Western immunoblotting was performed using an antibody specific for Npt2a (8). The intensity of the Npt2a bands was quantitated using laser densitometry. The production of intracellular cAMP in cultured cells in response to 10−7 M PTH was measured by nonacetylation EIA (cAMP Biotrak Assay Kit, Amersham) in the presence of 0.4 mM 3-isobutyl-1-methylxanthine (7). PKA activity was determined using cAMP-Dependent Protein Kinase (PKA) Radioactive Assay Kit (SignaTECT Promega). PKC activity was assayed using Promega's SignaTECT PKC Assay System containing a specific PKC substrate and capture membrane. Protein concentrations were determined using the method of Lowry et al. (24). Statistical comparisons were performed using ANOVA.
RESULTS
In initial experiments, we determined the minimum concentration of chelerythrine, a PKC inhibitor, that blocked the inhibitory effect of 1,2-s,n-dioctanoylglycerol (DOG; 10 μM), an activator of PKC, on phosphate uptake. Chelerythrine was studied over a concentration range of 10 pM to 10 nM. Incubation of proximal tubule cells with 10 nM chelerythrine for 20 min did not affect basal phosphate uptake (% change vs. control = 3 ± 3%, n = 4, P = NS). DOG inhibited phosphate uptake by 27 ± 4% in the absence and 1 ± 3% in the presence of chelerythrine (10 nM). We also studied the effect of Rp-cAMP, an inhibitor of PKA, over a concentration range of 50 to 1,000 μM. Treatment of cells with Rp-cAMP (100 μM) did not affect basal phosphate uptake (% change vs. control = 1 ± 3%, n = 3, P = NS). 8-bromo-cAMP (100 μM) inhibited phosphate uptake by 33 ± 2% in the absence and 1 ± 1% in the presence of Rp-cAMP (100 μM). Accordingly, in the remaining experiments, the PKC inhibitor chelerythrine was used in a concentration of 10 nM and the PKA inhibitor Rp-cAMP was used in a concentration of 100 μM.
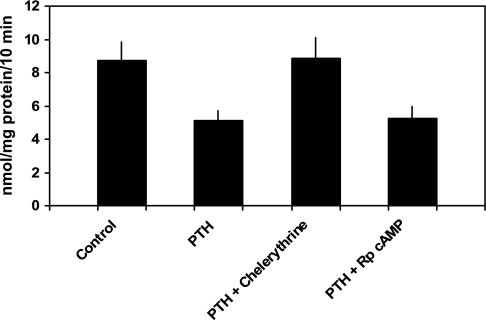
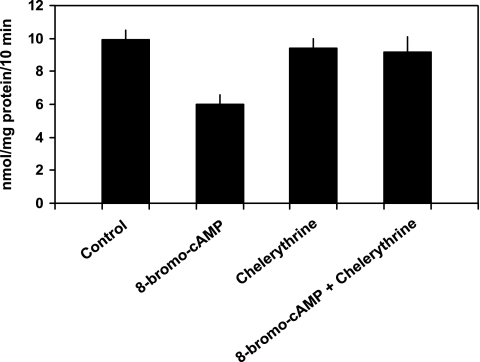
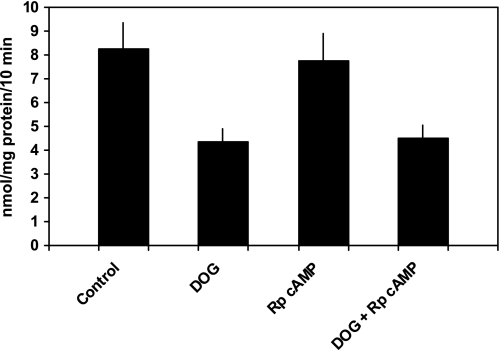
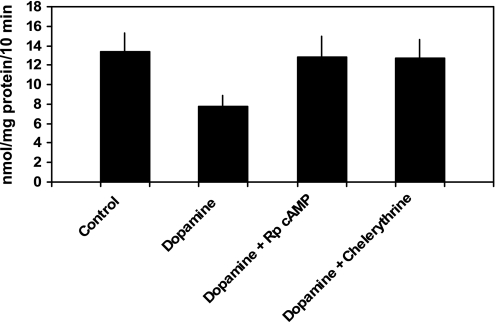
PTH 1–34 (10−7 M) inhibited phosphate transport by 40.1 ± 2.0% from 8.7 ± 1.1 to 5.1 ± 0.6 nmol·mg protein−1·10 min−1 (n = 6, P < 0.01; Fig. 1). Phosphate uptake averaged 8.9 ± 1.2 nmol·mg protein−1·10 min−1 in cells treated with chelerythrine and PTH (P = NS vs. control) and 5.3 ± 0.7 nmol·mg protein−1·10 min−1 in cells treated with Rp-cAMP and PTH (P = NS vs. PTH-treated cells). Thus, chelerythrine completely blocked PTH-associated inhibition of phosphate transport while Rp-cAMP had no effect. In cultured mouse renal proximal tubule cells, PTH activates PKC and stimulates the production of cAMP (7, 8). Independent of PTH, treatment of these cells with 8-bromo-cAMP inhibits phosphate transport. Accordingly, interpretation of the above experiments requires an explanation for why chelerythrine, a putative PKC inhibitor, would also block the predicted inhibitory effect of PTH-generated cAMP accumulation. We first determined whether chelerythrine affected PTH-mediated cAMP generation. cAMP accumulation averaged 55 ± 19 fmol well/OD280 in untreated cells, 4,970 ± 1,019 in PTH-treated cells (P < 0.01 vs. control untreated cells), 72 ± 9 in chelerythrine-treated cells (P = NS vs. untreated cells), and 91 ± 13 in cells treated with chelerythrine and PTH (P = NS vs. untreated cells; n = 4). We also determined the effect of chelerythrine on total cellular cAMP-stimulated PKA activity in these cultured proximal tubule cells. PKA activity averaged 242 ± 76 pmol/μg protein in control cells and 233 ± 94 in cells treated with chelerythrine (n = 4, P = NS vs. control cells). We next examined the effects of inhibition of PKC and PKA on phosphate transport when the second messenger pathways were individually activated. Phosphate transport averaged 9.1 ± 0.6 nmol·mg protein−1·10 min−1 in untreated cells and 6.0 ± 0.6 in cells treated with 8-bromo-cAMP (n = 5, P < 0.01). Phosphate transport was 9.4 ± 0.6 nmol·mg protein−1·10 min−1 in cells treated with chelerythrine (P = NS vs. untreated cells) and 9.2 ± 0.9 in cells treated with chelerythrine and 8-bromo-cAMP (P = NS vs. untreated cells; Fig. 2). By contrast, Rp-cAMP did not block DOG-associated inhibition of phosphate transport. Phosphate transport averaged 8.1 ± 1.1 nmol·mg protein−1·10 min−1 in untreated cells and 4.4 ± 0.6 in cells treated with DOG (n = 6, P < 0.01). Phosphate transport was 7.8 ± 1.1 nmol·mg protein−1·10 min−1 in cells treated with Rp-cAMP (P = NS vs. untreated cells) and 4.5 ± 0.6 in cells treated with Rp-cAMP and DOG (P = NS vs. DOG-treated cells; Fig. 3). These experiments demonstrate that while chelerythrine, in the dose studied, inhibits cAMP production, it had no effect on total cellular PKA activity. Chelerythrine completely blocked the inhibitory effect of 8-bromo-cAMP on phosphate transport, whereas Rp-cAMP did not block the inhibitory effect of DOG. These results indicate that the inhibitory effect of cAMP on phosphate transport proceeds through a pathway that absolutely requires active PKC. In the above model, PTH activation of PKA appears secondary or even redundant to the direct activation of PKC to mediate inhibition of phosphate transport. To determine whether PKA activation was required for the regulation of phosphate transport by other hormones that also elevate intracellular cAMP, we examined the effect of dopamine (Fig. 4). In separate experiments, phosphate transport was 13.4 ± 1.9 nmol·mg protein−1·10 min−1 in control cells and 7.8 ± 1.1 (n = 6, P < 0.01) in cells treated with dopamine (10 μM). The abundance of Npt2a averaged 2.6 ± 0.5 (arbitrary units) in control proximal tubule cells and 1.4 ± 0.3 in cells treated with dopamine (n = 3, P < 0.05). By contrast to the effect of PTH, treatment of cells with both Rp-cAMP [12.8 ± 2.0 nmol·mg protein−1·10 min−1 (P = NS vs. untreated cells)] and chelerythrine [12.7 ± 1.9 nmol·mg protein−1·10 min−1 (P = NS vs. untreated cells)] blocked the inhibitory effect of dopamine. These findings suggest a linear signal transduction pathway whereby dopamine activates adenylyl cyclase followed by PKA activation of PKC. To extend these observations, we measured cAMP accumulation and PKC activation in response to PTH, dopamine, and second messenger agonists. As shown in Table 1, treatment of wild-type proximal tubule cells with PTH resulted in cAMP generation. Dopamine also significantly increased cAMP generation albeit not to the same degree as PTH. Treatment with DOG to activate PKC, on the other hand, did not stimulate cAMP accumulation. Table 2 summarizes the effect of PTH, 8-bromo-cAMP (to activate PKA), and dopamine on PKC activation. PTH, dopamine, and 8-bromo-cAMP all significantly activated PKC in wild-type proximal tubule cells.
Fig. 1.
Inhibitory effect of parathyroid hormone (PTH) on phosphate transport is blocked by chelerythrine, a PKC inhibitor, but not by Rp-cAMP, a PKA inhibitor. Sodium-dependent phosphate uptake was measured in cultured renal proximal tubule cells (n = 6) under control conditions, in the presence of PTH (1–34) (10−7 M), PTH plus chelerythrine (10 nM), and PTH plus Rp-cAMP (100 μM). Results are the mean of means of triplicate determinations ± SE.
Fig. 2.
Chelerythrine, a PKC inhibitor, blocks the inhibitory effect of 8-bromo-cAMP, a PKA activator, on phosphate transport. Sodium-dependent phosphate uptake was measured in cultured renal proximal tubule cells (n = 5) under control conditions, in the presence of 8-bromo-cAMP (100 μM), chelerythrine (10 nM), and 8-bromo-cAMP plus chelerythrine. Results are the mean of means of triplicate determinations ± SE.
Fig. 3.
Rp-cAMP, a PKA inhibitor, does not block the inhibitory effect of 1,2-s,n-dioctanoylglycerol (DOG), a PKC activator, on phosphate transport. Sodium-dependent phosphate uptake was measured in cultured renal proximal tubule cells (n = 6) under control conditions, in the presence of DOG (10 μM), Rp-cAMP (100 μM), and DOG plus Rp-cAMP. Results are the mean of means of triplicate determinations ± SE.
Fig. 4.
Chelerythrine, a PKC inhibitor, and Rp-cAMP, a PKA inhibitor, block the inhibitory effect of dopamine on phosphate transport. Sodium-dependent phosphate uptake was measured in cultured renal proximal tubule cells (n = 6) under control conditions, in the presence of dopamine (10 μM), dopamine plus Rp-cAMP (100 μM), and dopamine plus chelerythrine (10 nM). Results are the mean of means of triplicate determinations ± SE.
Table 1.
Effect of PTH, DOG, and dopamine on cAMP accumulation in primary cultures of mouse proximal tubule cells
Values are the mean of means ± SE. The effect of parathyroid hormone (PTH), 1,2-s,n-dioctanoylglycerol (DOG), and dopamine on cAMP accumulation in mouse renal proximal tubule cells was expressed as cAMP/μg protein per well compared with untreated control cells.
P < 0.05; n = 7.
Table 2.
Effect of PTH, 8-bromo-cAMP, and dopamine on PKC activation in primary cultures of mouse proximal tubule cells
Values are the mean of means ± SE. The effect of PTH, 8-bromo-cAMP, and dopamine on PKC activation in mouse renal proximal tubule cells was noted. The results are expressed as PKC activity/μg protein per well compared with untreated control cells.
P < 0.05; n = 4.
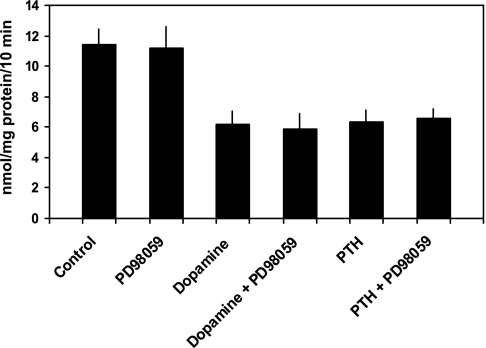
Prior studies showed that MAPK was also activated in response to PTH and to a lesser extent, dopamine (3, 22, 28). Moreover, some studies suggested a pivotal role for MAPK in the inhibition of Npt2a (3, 22). Thus, we also examined the potential role of the MAP kinase pathway in Npt2a inhibition by PTH and dopamine in proximal tubule cells in culture using PD98059, a potent MAPK kinase or MEK inhibitor. In initial experiments, we examined the effect of PD98059 in doses ranging from 10 to 100 μM and found that 100 μM had no effect on basal phosphate uptake. This concentration was then used in subsequent experiments. This concentration was also used by Lederer et al. in studies on OK cells but higher than the concentration used by Bacic et al. (3, 22). Phosphate transport averaged 11.4 ± 1.0 nmol·mg protein−1·10 min−1 in control cells and 11.2 ± 1.4 in cells treated with PD98059 (100 μM; n = 6, P = NS vs. control cells). In cells treated with PTH, phosphate transport was 6.2 ± 0.9 nmol·mg protein−1·10 min−1 (P < 0.01 vs. control cells) in the absence and 5.9 ± 0.9 in the presence of PD98059 (P < 0.01 vs. control cells). In cells treated with dopamine, phosphate transport was 6.4 ± 0.8 nmol·mg protein−1·10 min−1 in the absence (P < 0.01 vs. control cells) and 6.5 ± 0.7 in the presence of PD98059 (P < 0.01 vs. control cells; Fig. 5). Thus, in cultured proximal tubules of mice, inhibition of the MAPK pathway had no effect on the inhibitory effect of either PTH or dopamine on phosphate transport.
Fig. 5.
PD98059, a MAPK inhibitor, does not block the inhibitory effect of PTH or dopamine on phosphate transport. Sodium-dependent phosphate uptake was measured in cultured renal proximal tubule cells (n = 6) under control conditions, in the presence of PD98059 (100 μM), dopamine (10 μM), and dopamine plus PD98059, PTH (10−7 M), and PTH plus PD98059. Results are the mean of means of triplicate determinations ± SE.
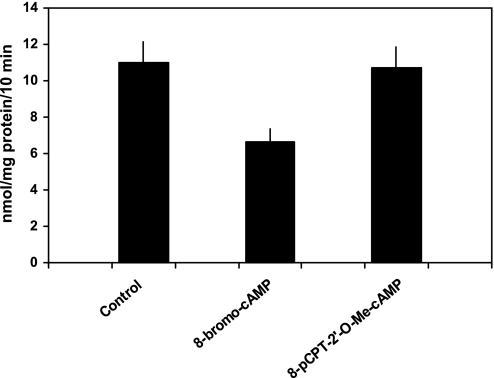
Recent studies showed that EPAC activation by cAMP modulated the function of some renal transporters such as NHE3 (16, 27). Thus, in our final set of experiments, we explored the possibility that the inhibitory effect of cAMP on phosphate transport was the result of activation of EPAC (Fig. 6). Phosphate transport was 11.0 ± 1.2 nmol·mg protein−1·10 min−1 in control cells, 6.7 ± 0.7 in cells treated with 8-bromo-cAMP (100 μM; P < 0.01 vs. control cells), and 10.7 ± 1.1 in cells treated with 8-pCPT-2′-O-Me-cAMP (50 μM), a direct activator of EPAC proteins (P = NS vs. control cells). These results indicate that the effect of cAMP on phosphate transport was not directly linked to EPAC activation but more likely mediated via the activation of PKA.
Fig. 6.
8-bromo-cAMP, an activator of PKA, but not 8-pCPT-2′-O-Me-cAMP, an activator of EPAC, inhibits phosphate transport. Sodium-dependent phosphate uptake was measured in cultured renal proximal tubule cells (n = 6) under control conditions, in the presence of 8-bromo-cAMP (100 μM) and 8-pCPT-2′-O-Me-cAMP (50 μM). Results are the mean of means of triplicate determinations ± SE.
DISCUSSION
The precise downstream signaling pathways utilized by PTH and other hormones that control renal phosphate transport are still poorly understood. Most of the prior studies detailing these pathways have employed OK cells. By contrast, we used primary cultures of mouse proximal tubule cells as a convenient model to study the inhibitory effect of PTH on phosphate transport (7–9, 15, 30, 33). OK cells provided considerable insights into the function of renal proximal tubules and are widely considered the ideal model of proximal tubule cells in culture. It is worth noting, however, that virtually nothing is known about whole kidney function of the animal from which these cells are derived. Other differences between OK cells and other proximal tubules are also documented. For example, OK cells express NHERF-1 but lack NHERF-2, whereas mouse and human proximal tubules express both isoforms (32, 34). This may have important bearings on the actions of PTH and its effect on proximal tubule transporters. NHE3 undergoes endosomal recycling in OK cells, a phenomenon not observed in the intact kidney of the rat (1, 35). Thus, there may be too much reliance on OK cells as the “gold standard” of proximal tubule function. This is particularly germane as it relates to the present studies using primary cultures of proximal tubules from the mouse. The preparation of renal cells used by our laboratory appears to be predominantly proximal tubule cells (7). Less than 5% of cells adapt a spindle shape and we assumed these cells represent fibroblast-like cells. Since these cells were unlikely to express NHE3 or Npt2a activity, we believed their presence had minimal impact on our interpretations. Our cell preparation responds vigorously to PTH with cAMP accumulation and PKC activation (7). By comparison, cAMP accumulation following treatment with vasopressin is orders of magnitude lower than PTH. All but the spindle-like fibroblast cells in this preparation stain positively using antibodies to NHE3 or Npt2a indicative of the proximal tubule origin of these cells (7, 9). Finally, using our published protocol, we find that these cells polarize. Confocal imaging of these primary mouse proximal tubule cells indicates that NHERF-1 and Npt2a are expressed exclusively in the apical membrane while Na-K-ATPase is expressed in the basolateral membrane (33). This cell preparation also demonstrates transport activities found predominantly or exclusively in renal proximal tubules including sodium-hydrogen exchange activity that, using pharmacologic inhibitors, likely represents NHE3 activity, sodium-dependent phosphate transport that correlates with Npt2a abundance, and sodium-dependent glucose transport (7, 33). It is our view, therefore, that the differences in results between preparations cannot be assumed to represent defects in model cell systems other than OK cells.
In our prior experiments, we had not detailed the downstream pathways activated by PTH or other hormones or determined the role of activation of MAPK or EPAC on phosphate transport in primary cultures of proximal tubule cells. Accordingly, the present experiments were designed to examine the effect of PTH and dopamine on the renal transport of phosphate in this preparation and to determine the potential roles of activation of PKC, PKA, MAPK, and EPAC using inhibitors and activators of these biochemical pathways. We initially determined the dose of the PKC inhibitor, chelerythrine, and the PKA inhibitor, Rp-cAMP, that blocked the actions of PKC and PKA agonists while having little or no effect on phosphate transport by themselves. We found that 10 nM chelerythrine and 100 μM Rp-cAMP totally blocked inhibition of phosphate transport by DOG and 8-bromo-cAMP, respectively. PTH-mediated inhibition of phosphate transport was totally blocked by chelerythrine but not by Rp-cAMP, indicating a major role for PKC in the inhibition of phosphate transport following activation of the PTH1 receptor. These results are similar to those reported by Mahon et al. (25) in OK cells using other PKC inhibitors. Remarkably, chelerythrine significantly inhibited PTH-mediated cAMP generation but had no effect on the cellular content of cAMP-stimulated PKA activity. To explore this further, we also studied the effect of these kinase inhibitors on phosphate inhibition by the two second messenger pathways known to be activated by PTH. Surprisingly, chelerythrine totally blocked the inhibitory effect of 8-bromo-cAMP on phosphate transport. Thus, independent of its inhibitory effect on cAMP generation, chelerythrine also inhibited the actions of PKA supporting the conclusion that both PKA and PKC were part of a second pathway activated by PTH with PKC functioning downstream of PKA to inhibit Npt2a. This may occur through the ability of PKA to activate PKC as reported in some nonrenal cells (6, 18, 21, 37) and/or by inhibiting a phosphatase that opposes the actions of PKC, as suggested in our earlier studies (33).
To extend these observations, we studied the effects of dopamine, a hormone known to stimulate cAMP generation and activate PKC in renal proximal tubule cells (11, 17, 28). Dopamine inhibits a number of transporters in renal proximal tubule cells including Na-K-ATPase, Npt2a, and NHE3 (2, 4, 13, 14, 20, 23, 29). It has been suggested that the interplay between these transporters affects their activity. For example, inhibition of NHE3 might lead to downregulation of Na-K-ATPase while inhibition of Na-K-ATPase may inhibit phosphate and Na/H exchange transport. By contrast, studies using isolated brush-border membrane vesicles where an artificial gradient can be imposed and measurements performed examining other sodium-coupled transport systems have indicated that dopamine affects the activity of these three transporters directly (11, 17). It is generally agreed that dopamine activation of the D1-like receptors is the major pathway regulating these transporters (36). There is some disagreement about the D2 receptors. Perrichot et al. (29) reported that activation of the D2 receptors potentiated the inhibitory effect of D1 receptor activation on phosphate transport in OK cells. Lederer et al. (23), on the other hand, reported that D2 activation attenuated the inhibitory effect of D1 activation on phosphate transport in OK cells. Finally, Bacic and co-workers (2) found that D2 activation had no effect on the internalization of Npt2a in mouse kidney slices. The downstream signaling pathways following exposure of proximal tubule cells to dopamine resulting in altered phosphate transport also remain unclear. Dopamine-stimulated cAMP accumulation and cAMP-mediated activation of PKA result in inhibition of phosphate transport. Nonetheless, Lederer et al. using OK cells concluded that the dopamine-mediated cAMP activation was not required for dopamine-mediated inhibition of phosphate transport; results confirmed by Baines and Drangova who demonstrated that PKA inhibitors did not block the effect of dopamine on phosphate transport in OK cells (4, 23). By contrast, Bacic et al. (2) reported that activation of the D1 receptor results in internalization of Npt2a in mouse kidney and in OK cells and that this effect is blocked by inhibitors of PKA. Thus, the role of PKA activation in dopamine-mediated inhibition of phosphate transport is controversial. Dopamine is also known to stimulate PKC activation (17). However, Baines and Drangova (4) reported that inhibition of PKC did not block the inhibitory effect of dopamine in OK cells and Bacic and co-workers (2) reported that dopamine treatment resulted in the endocytosis of Npt2a by a PKA pathway that was not blocked by inhibition of PKC. In addition, studies by Perrichot and co-workers in OK cells suggested that dopamine inhibited phosphate transport via PKA activation but, as assessed by cell calcium measurements as an indirect measure of PKC activity, not by PKC (29).
Our findings need to be compared and contrasted to the above studies. In cultured mouse proximal tubules, we confirm that dopamine inhibits sodium-dependent phosphate transport. The inhibitory effect of dopamine on phosphate transport is blocked by both Rp-cAMP and chelerythrine. This finding is clearly different from the inhibitory effect of PTH that is blocked by chelerythrine but not Rp-cAMP. Accordingly, our data are most consistent with the proposal that dopamine activates PKA via production of cAMP and that PKA sequentially activates PKC. This suggestion is supported by our findings that treatment of cells with 8-bromo-cAMP stimulated PKC activity to the same extent as PTH and dopamine. Activation of PKC, however, did not stimulate cAMP production. It is admittedly difficult to reconcile all the conflicting data regarding the mechanism by which dopamine inhibits phosphate transport but our proposal of sequential activation of PKA followed by activation of PKC is rationalized, at least in part, by consideration of the effect of dopamine on Na-K-ATPase activity. Khundmiri and Lederer and Gomes and Soares-da-Silva (13, 20) reported that dopamine-mediated inhibition of Na-K-ATPase activity was blocked by inhibitors of PKC and PKA. In the Gomes studies (13), it was reported that inhibition of PKC blocked the inhibitory effect of cAMP and suggested that PKA activation led to activation of PKC. Han et al. (14) also reported that both PKA and PKC inhibition blocked dopamine-stimulated uptake of calcium in rabbit proximal convoluted tubule cells in culture. Thus, the proposed biochemical sequence of reactions resulting in dopamine-mediated inhibition of Na-K-ATPase activity and stimulation of calcium uptake in proximal tubule cells resembles the sequence proposed herein for dopamine-mediated inhibition of phosphate transport in mouse proximal tubule cells.
In the present experiments, we also examined the potential role of MAPK activation on PTH-mediated inhibition of phosphate transport in renal proximal tubule cells. The basis for these studies derives from the original observations of Lederer et al. (22) who found that PTH stimulated MAPK activity in OK cells and that the inhibitory effect of PTH could be blocked by an upstream inhibitor of MAPK. In mouse kidney slices, Bacic and co-workers (3) reported smaller PTH-stimulated MAPK activity than in OK cells but also observed that an upstream inhibitor of MAPK partially inhibited PTH-mediated decreases in the retrieval of Npt2a from the apical membranes of proximal tubule cells. In cultured mice proximal tubule cells, on the other hand, we reported that PTH failed to stimulate MAPK activity (8). Consistent with this observation, the MAPK kinase inhibitor PD98059 did not affect either basal transport or impair the inhibitory effect of either PTH or dopamine on phosphate transport. Thus, PTH activation of MAPK, which may be operative in some cells, at best plays a minor role in the inhibition of phosphate transport in response to PTH in primary cultures of mouse proximal tubule cells. We also found that inhibition of MAPK did not block the inhibitory effect of dopamine on phosphate transport. These results confirm the observation of Bacic and co-workers (2) who reported that an inhibitor of MAPK did not affect dopamine-mediated internalization of Npt2a in mouse kidney.
Recent studies have uncovered a novel role for cAMP in the direct binding and activation of an exchanger protein family known as EPAC (10). Activation of EPAC inhibits NHE3 activity in some tissues but had no discernable effect on the internalization of Npt2a in mouse kidney slices (16, 27). Consistent with these findings, 8-pCPT-2′-O-Me-cAMP, a cAMP analog that selectively activates EPAC, had no effect on phosphate transport in cultured mouse proximal tubule cells.
As noted earlier, the present experiments were designed to detail factors regulating phosphate transport in cultured mouse proximal tubule cells. These experiments highlight the unique manner by which PTH and dopamine utilize cellular signaling pathways involving PKC and PKA to inhibit phosphate transport (Fig. 7). While activation of PKC is required for the inhibitory effect of both hormones on phosphate transport, dopamine but not PTH had an absolute requirement for activation of PKA. These experiments also suggest that activation of MAPK is not critical for the inhibitory effect of either PTH or dopamine on phosphate transport in cultured mouse proximal tubule cells and that cAMP serves to activate an inhibitory pathway mediated by PKA rather than EPAC. Recent studies from our laboratory as well as from Deliot et al. (12, 33) indicated that NHERF-1 is a target of the activation of the PTH receptor resulting in the phosphorylation of NHERF-1, dissociation of Npt2a/NHERF-1 complexes, and inhibition of phosphate transport. The downstream targets of occupancy of the dopamine receptor, however, have not been studied and comparison of Npt2a regulatory proteins whose phosphorylation is enhanced by dopamine may shed new light on the difference in the signaling cascades activated by PTH and dopamine and the relative roles of PKA and PKC in the control of renal phosphate transport.
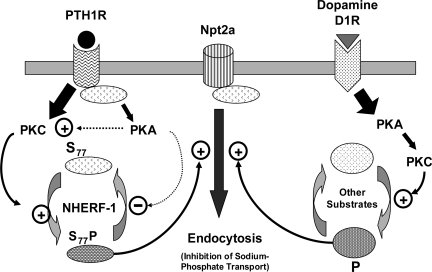
Fig. 7.
In renal proximal tubule cells, PTH1R and Npt2a are both tethered at the plasma membrane through their association with NHERF-1. Following the binding of PTH, signaling by the NHERF-1-bound PTH1R is directed toward the activation of PKC. This promotes the phosphorylation of NHERF-1 at serine77 which lies within the PDZ-1 domain and results in its reduced affinity for Npt2a. The dissociation of NHERF-1/Npt2a complexes enhances the internalization of Npt2a and inhibits sodium-phosphate cotransport. PTH also promotes the generation of cAMP by the PTH1R receptor. cAMP/PKA may activate PKC and/or function by an unknown mechanism to suppress the activity of a protein phosphatase to enhance or prolong the phosphorylation of NHERF-1. Thus, the coordinated actions of PKC and PKA may transduce PTH signals that control the extent and duration of Npt2a inhibition. By contrast, the activation of the dopamine D1 receptor preferentially enhances PKA activity. PKA activation results in activation of PKC with subsequent inhibition of sodium-phosphate transport.
GRANTS
These studies were supported by grants from the National Institutes of Health (NIH) DK-55881 (E. J. Weinman and S. Shenolikar), Research Service, Department of Veterans Affairs (E. J. Weinman), the University of Maryland (R. Cunningham), and the Kidney Foundation of Maryland (R. Cunningham). R. Cunningham is a recipient of a Minority Career Development Award from the NIH and a Harold Amos Faculty Development award from the Robert Wood Johnson Foundation.
Acknowledgments
We thank G. Peng for excellent technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akhter S, Cavet ME, Tse CM, Donowitz M. C-terminal domains of Na+/H+ exchanger isoform 3 are involved in the basal and serum-stimulated membrane trafficking of the exchanger. Biochemistry 39: 1990–2000, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Bacic D, Capuano P, Baum M, Zhang J, Stange G, Biber J, Kaissling B, Moe OW, Wagner CA, Murer H. Activation of dopamine D1-like receptors induces acute internalization of the renal Na+/phosphate cotransporter NaPi-IIa in mouse kidney and OK cells. Am J Physiol Renal Physiol 288: F740–F747, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacic D, Schulz N, Biber J, Kaissling B, Murer H, Wagner CA. Involvement of the MAPK-kinase pathway in the PTH-mediated regulation of the proximal tubule type IIa Na+/Pi cotransporter in mouse kidney. Pflügers Arch 446: 52–60, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Baines AD, Drangova R. Does dopamine use several signal pathways to inhibit Na-Pi transport in OK cells? J Am Soc Nephrol 9: 1604–1612, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Capuano P, Bacic D, Roos M, Gisler SM, Stange G, Biber J, Kaissling B, Weinman EJ, Shenolikar S, Wagner CA, Murer H. Defective coupling of apical PTH receptors to phospholipase C prevents internalization of the Na+-phosphate cotransporter NaPi-IIa in Nherf1-deficient mice. Am J Physiol Cell Physiol 292: C927–C934, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Chio CC, Chang YH, Hsu YW, Chi KH, Lin WW. PKA-dependent activation of PKC, p38 MAPK and IKK in macrophage: implication in the induction of inducible nitric oxide synthase and interleukin-6 by dibutyryl cAMP. Cell Signal 16: 565–575, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham R, Steplock D, Wang F, Huang H, E X, Shenolikar S, Weinman EJ. Defective PTH regulation of NHE3 activity and phosphate adaptation in cultured NHERF−/− renal proximal tubule cells. J Biol Chem 279: 37815–37821, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham R, E X, Steplock D, Shenolikar S, Weinman EJ. Defective PTH regulation of sodium-dependent phosphate transport in NHERF-1−/− renal proximal tubule cells and wild-type cells adapted to low phosphate media. Am J Physiol Renal Physiol 289: F933–F938, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham R, Steplock D, E X, Biswas RS, Wang F, Shenolikar S, Weinman EJ. Adenoviral expression of NHERF-1 in NHERF-1 null mouse renal proximal tubule cells restores Npt2a regulation by low phosphate media and parathyroid hormone. Am J Physiol Renal Physiol 291: F896–F901, 2006. [DOI] [PubMed] [Google Scholar]
- 10.De Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SB, Wittinghoffer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396: 474–477, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Debska-Slizien A, Ho P, Drangova R, Baines AD. Endogenous renal dopamine production regulates phosphate excretion. Am J Physiol Renal Fluid Electrolyte Physiol 266: F858–F867, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Déliot N, Hernando N, Horst-Liu Z, Gisler SM, Capuano P, Wagner CA, Bacic D, O'Brien S, Biber J, Murer H. Parathyroid hormone treatment induces dissociation of type IIa Na+-Pi cotransporter-Na+/H+ exchanger regulatory factor-1 complexes. Am J Physiol Cell Physiol 289: C159–C167, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Gomes P, Soares-da-Silva P. Role of cAMP-PKA-PLC signaling cascade on dopamine-induced PKC-mediated inhibition of renal Na+-K+-ATPase activity. Am J Physiol Renal Physiol 282: F1084–F1096, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Han JY, Heo JS, Lee YJ, Lee JH, Taub M, Han HJ. Dopamine stimulates 45Ca2+ uptake through cAMP, PLC/PKC, and MAPKs in renal proximal tubule cells. J Cell Physiol 211: 486–494, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Hernando N, Déliot N, Gisler SM, Weinman EJ, Lederer E, Biber J, Murer H. PDZ-domain interactions and apical expression of type IIa Na/P(i) cotransporters. Proc Natl Acad Sci USA 99: 11957–11962, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honegger KJ, Capuano P, Winter C, Bacic D, Stange G, Wagner CA, Biber J, Murer H, Hernando N. Regulation of sodium-proton exchanger isoform 3 (NHE3) by PKA and exchange protein directly activated by cAMP (EPAC). Proc Natl Acad Sci USA 103: 803–808, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isaac J, Glahn RP, Appel MM, Onsgard M, Dousa TP, Knox FG. Mechanism of dopamine inhibition of renal phosphate transport. J Am Soc Nephrol 2: 1601–1607, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Jacobowitz O, Iyengar R. Phorbol ester-induced stimulation and phosphorylation of adenylyl cyclase 2. Proc Natl Acad Sci USA 91: 10630–10634, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufmann M, Muff R, Stieger B, Biber J, Murer H, Fischer JA. Apical and basolateral parathyroid hormone receptors in rat renal cortical membranes. Endocrinology 134: 1173–1178, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Khundmiri SJ, Lederer E. PTH and DA regulate Na-K-ATPase through divergent pathways. Am J Physiol Renal Physiol 282: F512–F522, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Lai HL, Yang TH, Messing RO, Ching YH, Lin S, Chem Y. Protein kinase C inhibits adenylyl cyclase type VI activity during desensitization of the A2a-adenosine receptor-mediated cAMP response. J Biol Chem 272: 4970–4977, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Lederer ED, Sohi SS, McLeish KR. Parathyroid hormone stimulates extracellular signal-regulated kinase (ERK) activity through two independent signal transduction pathways: role of ERK in sodium-phosphate cotransport. J Am Soc Nephrol 11: 222–231, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Lederer ED, Sohi SS, McLeish KR. Dopamine regulates phosphate uptake by opossum kidney cells through multiple counter-regulatory receptors. J Am Soc Nephrol 9: 975–985, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951. [PubMed] [Google Scholar]
- 25.Mahon MJ, Cole JA, Lederer ED, Segre GV. Na+/H+ exchanger-regulatory factor 1 mediates inhibition of phosphate transport by parathyroid hormone and second messengers by acting at multiple sites in opossum kidney cells. Mol Endocrinol 17: 2355–2364, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Mahon MJ, Donowitz M, Yun CC, Segre GV. Na+/H+ exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signaling. Nature 417: 858–861, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Murtazina R, Kovbasnjuk O, Zachos NC, Li X, Chen Y, Hubbard A, Hogema BM, Steplock D, Seoder V, Hoque KM, Tse CM, De Jonge HR, Weinman EJ, Donowitz M. Tissue-specific regulation of sodium/proton exchanger isoform 3 activity in Na+/H+ exchanger regulatory factor 1 (NHERF1) null mice. cAMP inhibition is differentially dependent on NHERF1 and exchange protein directly activated by cAMP in ileum versus proximal tubule. J Biol Chem 282: 25141–25151, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Pedrosa R, Gomes P, Soares-da-Silva P. Distinct signalling cascades downstream to Gsα coupled dopamine D1-like NHE3 inhibition in rat and opossum renal epithelial cells. Cell Physiol Biochem 4: 91–100, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Perrichot R, Garcia-Ocaña A, Couette S, Comoy E, Amiel C, Friedlander G. Locally formed dopamine modulates renal Na-Pi cotransport through DA1 and DA2 receptors. Biochem J 312: 433–437, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shenolikar S, Voltz JW, Minkoff CM, Wade JB, Weinman EJ. Targeted disruption of the mouse NHERF-1 gene promotes internalization of proximal tubule sodium-phosphate cotransporter type IIa and renal phosphate wasting. Proc Natl Acad Sci USA 99: 11470–11475, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traebert M, Volkl H, Biber J, Murer H, Kaissling B. Luminal and contraluminal action of 1–34 and 3–34 PTH peptides on renal type IIa Na-Pi cotransporter. Am J Physiol Renal Physiol 278: F792–F798, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Wade JB, Liu J, Coleman RA, Cunningham R, Steplock D, Lee-Kwon W, Pallone TL, Shenolikar S, Weinman EJ. Localization and interaction of NHERF isoforms in the renal proximal tubule of the mouse. Am J Physiol Cell Physiol 285: C1494–C1504, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Weinman EJ, Biswas RS, Peng G, Shen L, Turner CL, E X, Steplock D, Shenolikar S, Cunningham R. Parathyroid hormone inhibits renal phosphate transport by phosphorylation of serine77 of sodium-hydrogen exchanger regulatory factor-1. J Clin Invest 117: 3412–3420, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Weinman EJ, Lakkis J, Akom M, Wali RK, Drachenberg CB, Coleman RA, Wade JB. Expression of NHERF-1, NHERF-2, PDGFR-alpha, and PDGFR-beta in normal human kidneys and in renal transplant rejection. Pathobiology 70: 314–323, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Yang LE, Maunsbach AB, Leong PK, McDonough AA. Differential traffic of proximal tubule Na+ transporters during hypertension or PTH: NHE3 to base of microvilli vs. NaPi2 to endosomes. Am J Physiol Renal Physiol 287: F896–F906, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Yu P, Asico LD, Luo Y, Andrews P, Eisner GM, Hopfer U, Felder RA, Jose PA. D1 dopamine receptor hyperphosphorylation in renal proximal tubules in hypertension. Kidney Int 70: 1072–1079, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Yurko-Mauro KA, Reenstra WW. Prostaglandin F2α stimulates CFTR activity by PKA- and PKC-dependent phosphorylation. Am J Physiol Cell Physiol 275: C653–C660, 1998. [DOI] [PubMed] [Google Scholar]