Abstract
The activity of ROMK channels in rat kidney tubule cells was assessed as tertiapin-Q (TPNQ)-sensitive current under whole cell clamp conditions. With an external K+ concentration of 5 mM and an internal K+ concentration of 140 mM and the membrane potential clamped to 0 mV, TPNQ blocked outward currents in principal cells of the cortical collecting duct (CCD) outer medullary collecting duct and connecting tubule (CNT). The apparent Ki was 5.0 nM, consistent with its interaction with ROMK. The TPNQ-sensitive current reversed at voltages close to the equilibrium potential for K+. The currents were reduced when the pipette solution contained ATP. In the CCD, the average TPNQ-sensitive outward current (ISK) was 476 ± 48 pA/cell in control animals on a 1% KCl diet. ISK increased to 1,255 ± 140 pA when animals were maintained on a high-K (10% KCl) diet for 7 days and decreased to 314 ± 46 pA after 7 days on a low-K (0.1% KCl) diet. In the CNT, ISK was 360 ± 30 pA on control, 1,160 ± 110 on high-K, and 166 ± 16 pA on low-K diets. The results indicate that ROMK channel activity is highly regulated by dietary K in both the CCD and the CNT.
Keywords: renal K channels, tertiapin-Q, K adaptation, K depletion
romk (kir1.1) channels form a major pathway for renal K secretion in the distal nephron (15). Consistent with such a role, several studies (19, 21, 27, 28) have shown that the abundance of active channels in the apical membrane of the cortical collecting duct (CCD) of the rat increased when the animals were fed a high-K diet. The responses to a low-K diet were more variable, with some results indicating a decrease in channel abundance, while others showed little or no effect (1, 21, 29). Although the CCD and connecting tubule (CNT) share common transport mechanisms for Na+ and K+, the CNT is probably the most important site of K+ secretion in vivo (16, 20). However, because this segment is more difficult to study in vitro, relatively little information on the regulation of its ion transport systems is available. In a previous study (6), we documented the presence of ROMK channels in the apical membrane of the CNT cells, but we did not see a consistent change in activity with a high-K diet. This may have been related to the difficulty of estimating channel densities in small membrane patches, especially when the channels are not randomly distributed. In the present study, we attempted to circumvent these problems by measuring channel activity under whole cell clamp conditions. Here currents across the entire cell membrane are examined. This affords more quantitative estimates of the absolute activity of the channels as well as a more reliable assessment of changes in response to different physiological conditions.
METHODS
Animals.
All animal-handling procedures were approved by the Institutional Animal Care and Use Committee of Weill Medical College. Sprague-Dawley rats of either gender (150–170 g), raised free of viral infections (Charles River Laboratories, Kingston, NY), were fed rodent chow (Harlan-Teklad, Madison, WI) containing either 1% KCl (control-K diet), 10% KCl (high-K diet) or 0.1% KCl (low-K diet) for 7–9 days before electrophysiological measurements were made.
Electrophysiology.
Measurement of whole cell currents in principal cells of the CCD, the outer medullary collecting duct (OMCD), and the CNT followed procedures described previously (5, 7). Split-open tubules were superfused with solutions prewarmed to 37°C containing (in mM): 135 Na methanesulfonate, 5 KCl, 2 Ca methanesulfonate, 1 MgCl2, 2 glucose, and 10 HEPES adjusted to pH 7.4 with NaOH. The patch-clamp pipettes were filled with solutions containing (in mM): 7 KCl, 123 aspartic acid, 5 EGTA, and 10 HEPES, adjusted to pH 7.4 with KOH. The total concentration of K+ was ∼145 mM. Tertiapin-Q (TPNQ; Sigma-Aldrich, St. Louis, MO) was dissolved in H2O at a concentration of 100 μM and diluted into the bath solution to final concentrations of 1–1,000 nM. Ba acetate was added to the bath solution to a final concentration of 5 mM. In a few experiments, the tubules remained unopened and were treated with 100–500 μg/ml collagenase (Worthington Biochemical, Lakewood, NJ) until the basement membrane was sufficiently digested to form seals on the basolateral membrane (10–20 min). Pipettes were pulled from hematocrit tubing, coated with Sylgard, and fire polished with a microforge. Pipette resistances ranged from 2 to 5 MΩ. Currents were measured with a List EPC-7 amplifier (Heka Elektronik, Lambrecht, Germany). Voltages were controlled and currents were recorded using P-Clamp software and a Digidata 1322A interface (Molecular Devices, Sunnyvale, CA ).
Xenopus oocytes.
cDNAs encoding rat Kir1.1b (ROMK2), human Kir4.1, rat Kir4.1 and rat Kir5.1 were cloned into the pSPORT vector. The plasmids were linearized with NotI restriction enzyme, and cDNA was transcribed with T7 or SP6 RNA polymerase using mMESSAGE mMACHINE kit (Ambion). cRNA pellets were dissolved in nuclease-free water and stored in −70°C before use. Oocytes harvested from Xenopus laevis were incubated in OR2 solution with 2 mg/ml collagenase type II (Worthington Biochemical) and 2 mg/ml hyaluronidase type II (Sigma Chemical) with gentle shaking for 60 min and another 30 min (if necessary) in a fresh enzyme solution at room temperature. Defolliculated oocytes were injected with 10 ng RNA. After the injection, they were stored at 19°C in Leibovitz's L15 medium (Invitrogen) for 1–2 days before measurements were made. Currents in oocytes expressing the channels were measured using a two-electrode voltage clamp (Warner Instruments) driven by Pulse software (HEKA).
Modeling.
We extended a previously developed mathematical model of Na and K transport by the CCD (8) and OMCD (4) to the CNT. We used values of 5,800 nS/mm for basolateral K+ conductance, based on measured values for principal cells of the CCD (9). The calculated fluxes were not very sensitive to modest changes in this parameter. We also chose values of 47.6 nA/mm for maximal basolateral Na/K pump activity and 1,000 nS/mm for the paracellular conductance. Values of apical Na permeability (PNa) and K conductance were varied as described in results. The highest values of PNa used were taken from measurements of whole cell Na currents in CNTs from aldosterone-treated animals (7). PNa calculated from these currents using the Goldman equation is 1 pA/mM·cell. This corresponds to 3.6 × 10−8 cm2/s, assuming 340 principal cells/mm tubule (8). For simulations of transport in tubules, we assumed that fluid entering the CNT contained 30 mM Na and 5 mM K. The number of principal cells was set at 340/mm tubule based on measurements of nuclei in isolated tubules (7). The model was solved sequentially for 10 segments of a 1-mm long tubule. Na and K concentrations were allowed to change, but the fluid volume was held constant. Thus these simulations pertain to the state of water diuresis and do not take into account changes in the concentration of ions in the lumen by water reabsorption.
RESULTS
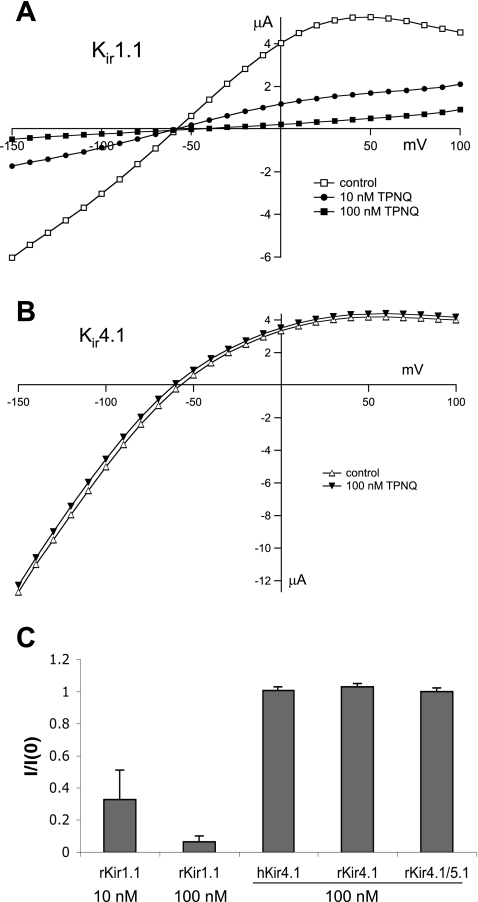
TPNQ, a modified version of the honey-bee venom component tertiapin, is a high-affinity blocker of Kir1.1 (ROMK) channels (10, 11). However, tests of its specificity have been limited. Since under whole cell conditions in the renal tubule cells K currents through both apical and basolateral K channels will be recorded, we examined in Xenopus oocytes whether the toxin could discriminate between the apical Kir1.1 channels and the closely related Kir4.1 and 4.1/5.1 channels that are believed to comprise a major portion of the basolateral K conductance (9, 13). The results are shown in Fig. 1. In oocytes expressing rat ROMK2, TPNQ at a concentration of 10 nM reduced both inward and outward currents substantially (Fig. 1A). At 100 nM, the block was almost complete. A similar dose had no detectable effect on oocytes expressing human Kir4.1 (Fig. 1B) or rat Kir4.1 (not shown). We also coexpressed rat Kir4.1 and Kir5.1 at a cRNA ratio of 1:10. The expression of the 4.1/5.1 heteromultimers was confirmed by lowering the extracellular and intracellular pH using acetate buffer. With an extracellular pH of 6.5, Ba-sensitive currents in Kir4.1-expressing oocytes were reduced to 62 ± 14% of control, while those in the Kir 4.1/5.1-expressing oocytes were reduced to 9.6 ± 1.1% of control. These results are consistent with those reported previously for these channels (24), showing that coexpression of Kir5.1 increases the sensitivity of Kir4.1 to reduction in pH. The Kir4.1/5.1 currents were also completely insensitive to 100 nM TPNQ. Figure 1C summarizes these measurements.
Fig. 1.
Effects of tertiapin-Q (TPNQ) on Kir1.1, Kir4.1, and Kir4.1/5.1 channels in Xenopus oocytes. Currents in oocytes expressing the channels were assayed using the two-electrode voltage clamp. A: current-voltage (I-V) relationships for rat Kir1.1 channels in the absence and presence of 10 and 100 nM TPNQ. External K+ concentration was 10 mM. B: I-V relationships for human Kir4.1 channels in the absence and presence of 100 nM TPNQ. External K+ concentration was 10 mM. C: fraction of control current measured at −100 mV remaining in presence of 10 or 100 nM TPNQ in rat Kir1.1, Kir4.1, and Kir4.1/5.1 and human Kir4.1. Data are means ± SE for 3 or more measurements. I/I0, ratio of current (I) to that measured in the absence of blocker (I0).
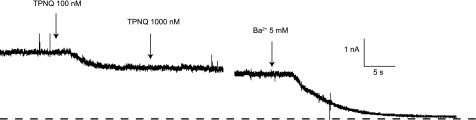
We next used the toxin to estimate ROMK currents in principal cells of the rat CCD. Previous studies (9) have shown that in the presence of high extracellular K, TPNQ reduced whole cell currents by only 10–20%. To increase the relative signal from the apical K channels, we reduced extracellular K to 5 mM and measured outward currents at 0 mV. These conditions reduced basolateral K+ conductance (9). Since Cl− concentrations were the same in the bath and in the pipette fluid, anion currents through basolateral Cl− channels were also eliminated at this membrane voltage. Figure 2 shows whole cell currents in a principal cell of a rat fed a high-K diet. Application of 100 nM TPNQ reduced the current substantially, while increasing the dose to 1 μM had no additional effect. Application of the generic K-channel blocker Ba2+ (5 mM) decreased the current almost to zero. Our interpretation is that the TPNQ blocked only the apical ROMK channels, while Ba2+ blocked both apical and basolateral channels.
Fig. 2.
Effects of TPNQ and Ba2+ on outward currents in a principal cell of a cortical collecting duct (CCD). The tubule was from a rat fed a high-K diet for 1 wk. At the first arrow, TPNQ (100 nM) was added to the superfusate. At the second arrow, the concentration was increased to 1 μM. At the third arrow, the solution was changed to one containing 5 mM Ba2+. Broken line indicates I = 0.
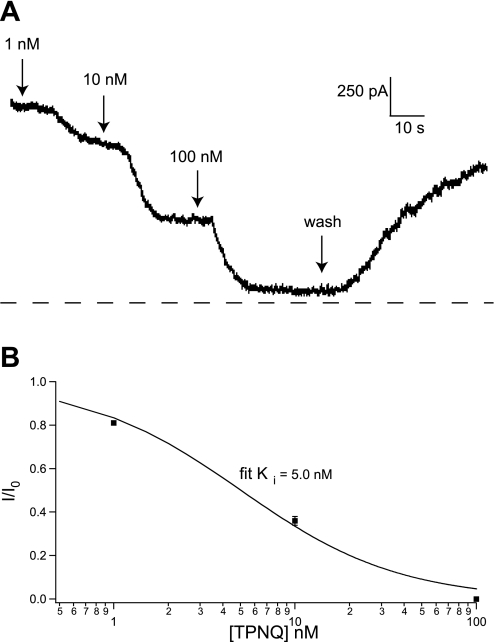
To verify that the effects of the toxin were on ROMK channels, we examined the dose-response relationship. Figure 3A shows a recording from another cell from a CCD of a high-K adapted rat. Application of 1 nM TPNQ produced a small but measurable effect, 10 nM produced a larger one, and 100 nM blocked most of the outward current. The block was largely reversible upon removal of the TPNQ. The dose-response curve is shown in Fig. 3B. The ratio of current (I) to that measured in the absence of blocker (I0) is plotted vs. blocker concentration. One-hundred nanomolar TPNQ was assumed to have a maximal effect as indicated in Fig, 1. The data were fitted with the relationship: I/I0 = 1/(1 + [TPNQ]/Ki) (Eq. 1).
Fig. 3.
Dose-response relationship for TPNQ inhibition of outward currents. A: recording from a principal cell of a CCD from an animal fed a high-K diet for 1 wk. The concentration of TPNQ in the superfusate was increased from 0 to 1, 10, and 100 nM, after which the blocker was washed away. B: fractional inhibition was calculated as [I(TPNQ) − I(100)]/[I(0) − I(100)], where I(0) and I(100) were currents measured in the absence of blocker and in the presence of a maximal concentration (100 nM) of TPNQ. Solid line is the best fit to Eq. 1 with Ki = 5.0 nM.
The value of the inhibition constant Ki obtained from a best fit of the equation to the results was 5.0 nM. This is in reasonable agreement with published values for the inhibition of ROMK channels expressed in Xenopus oocytes (9, 10). This suggests that the TPNQ-blocked currents reflect the activity of ROMK (SK) channels. We designated the TPNQ-sensitive current as ISK.
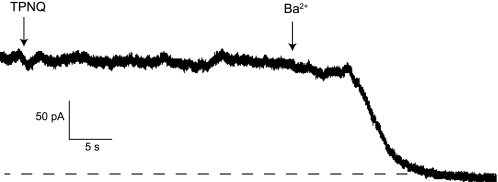
In opened tubules, both apical and basolateral membranes contribute to whole cell currents. To test whether the TPNQ-sensitive currents are associated with the apical membrane, we used unopened tubules in which access to the lumen was limited. Whole-cell clamps were made after sealing to the basolateral membrane of collagenase-treated tubules. Under these conditions, no TPNQ-sensitive current could be detected, although Ba2+−-sensitive currents were preserved (Fig. 4). This observation, which was typical of 10 different cells, suggests that the TPNQ-blocked currents were primarily across the apical membrane, consistent with the presumed location of ROMK channels.
Fig. 4.
Whole cell currents from an intact CCD. A tubule was isolated from a rat fed a normal diet and was not opened. After treatment with collagenase (100 μg/ml, 20 min), a seal was formed on the basolateral membrane and whole cell currents measured at V = 0 mV. There was no detectable response to TPNQ (100 nM), but there was a clear inhibition of outward current by Ba2+ (5 mM).
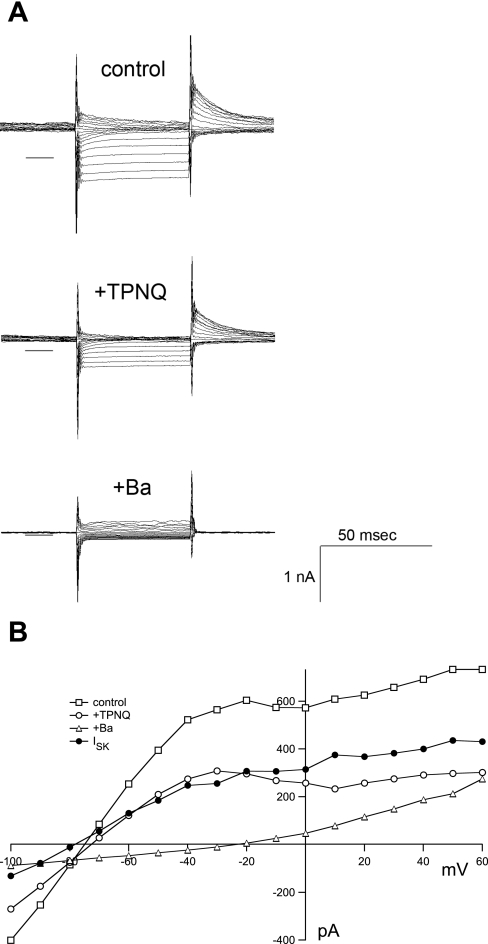
The current-voltage (I-V) relationship for ISK is shown in Fig. 5. Current traces in the absence of blocker and in the presence of either TPNQ or Ba2+ are illustrated. The basic I-V curve is S shaped, with conductance decreasing at strong negative potentials due to the low concentration of the permeant ion, and at positive voltages, indicating inward or anomalous rectification. The effects of Ba2+, but not of TPNQ, are clearly voltage dependent, with large positive voltages giving relief of block. However, both the Ba2+-sensitive and TPNQ-sensitive currents reverse at around −80 mV. This is close to the calculated K+ equilibrium potential of −90 mV, indicating that both agents block a K+-selective conductance, as expected.
Fig. 5.
I-V relationships for TPNQ-sensitive currents. Traces were obtained from a principal cell of a CCD from an animal on a control diet. A: currents obtained at voltages varying from −100 to +60 mV in 10-mV increments. Between test pulses the voltage was returned to the holding potential of 0 mV. The families of traces were obtained in the absence of blocker, in the presence of 100 nM TPNQ, and in the presence of 5 mM Ba2+. Solid lines represent I = 0. B: currents at the end of each trace are plotted as a function of voltage. •, TPNQ-sensitive currents (ISK) obtained by subtracting currents measured with and without the blocker.
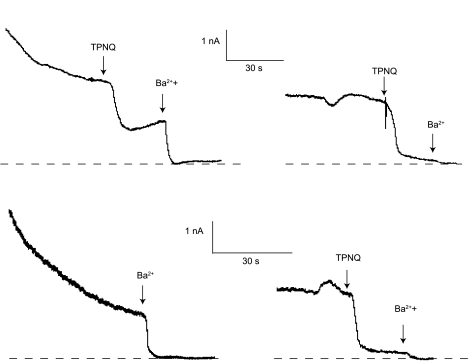
In some cells, the outward current showed a gradual decline with time. This rundown appeared to primarily involve the non-TPNQ-sensitive (i.e., basolateral) K conductance, as illustrated in Fig. 6. These are recordings from two similar cells from the same CCD of a K-loaded rat. In the top traces, current declined over the first minute or so after forming the whole cell clamp. TPNQ sharply decreased the current, and Ba2+ abolished the remainder. After washout, the current recovered to a level lower than the original. A second application of TPNQ caused a similar decrease, while Ba2+ had a smaller additional effect. In the bottom traces, TPNQ was present at the time the whole cell clamp was formed. There was a similar initial outward current that declined with time. Ba2+ again abolished the current, which recovered after washout of the blockers. On readdition of blockers, TPNQ had a robust effect and Ba2+ had a smaller one, similar to the first cell. Our interpretation is that the Ba-sensitive, TPNQ-insensitive K+ channels, presumably mainly in the basolateral membrane, declined with time in both cells, while the TPNQ-sensitive channels, presumably apical ROMK, were relatively stable.
Fig. 6.
Rundown of outward currents. Recordings are from 2 cells from the same CCD of a rat on a high-K diet for 7 days. Outward currents were large immediately after formation of the whole cell clamp and declined over a period of several minutes. Top traces: TPNQ (100 nM) and Ba2+ were added sequentially, washed off, and then added again. The TPNQ-sensitive current declined much less than did the TPNQ-insensitve current. Bottom traces: TPNQ was present during formation of the seal. Initial currents were still high and also declined with time. After washout, TPNQ and Ba2+ were added sequentially, showing the presence of TPNQ-sensitive channels. Broken lines indicate I = 0.
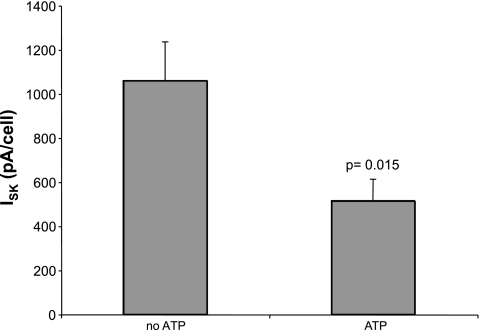
ROMK currents in the CCD are modulated by intracellular ATP (14, 26). We measured ISK in principal cells with either 0 or 3 mM MgATP in the pipette. In most cases, we recorded from two or more cells in the same tubule alternating between the two pipette solutions. As shown in Fig. 7, the currents were ∼2.5-fold smaller in the presence of ATP than in the absence, consistent with single-channel recordings.
Fig. 7.
Effects of ATP on TPNQ-sensitive currents. Data were obtained from CCDs of animals on a high-K diet for 1 wk. Bars show currents with and without 3 mM MgATP in the pipette solution. Data are means ± SE for 12 paired cells under each condition.
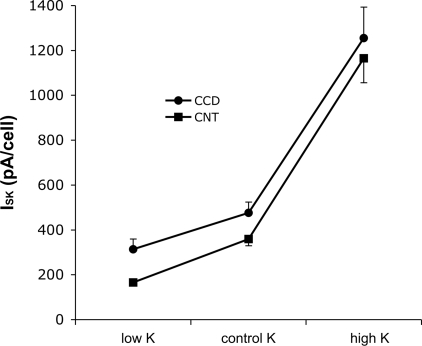
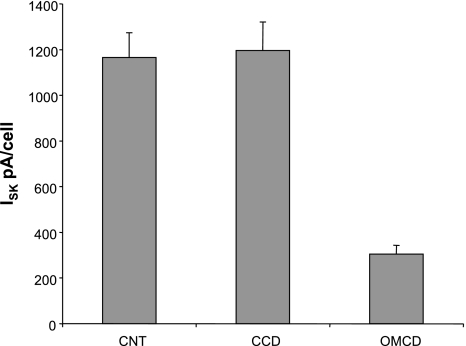
A major goal of this study was to examine the effects of dietary K on ROMK-mediated currents. We first examined the responses to the CCD, since several reports (19, 21, 27, 28) have confirmed the increase in conducting channel density in principal cells during adaptation to a high-K diet. As shown in Fig. 8, after a week on a high-K diet, the mean ISK increased 2.7-fold from 476 to 1,255 pA/cell. Reducing dietary K from 1 to 0.1% KCl had a smaller but statistically significant effect, lowering the mean current by 35% to 314 pA/cell.
Fig. 8.
Effect of diet on TPNQ-sensitive currents in the CCD and the CNT. Animals were maintained on control-K, high-K, or low-K diets for 1 wk before the measurements. Data are means ± SE for the following numbers of cells CCD: low-K 24; control-K 49, and high-K 26; CNT: low-K 25 cells, control-K 21 cells, and high-K 21 cells. Three animals were used for each condition except for the control-K CCD, which includes data from 6 animals.
We next studied the CNT. This segment is more difficult to dissect but is probably more relevant to the process of renal K secretion than the CCD (see Discussion). In fact, ISK was similar in the two segments. Raising dietary K increased channel activity by threefold, while lowering K intake decreased the activity by ∼50%. Overall the range of currents was somewhat larger in the CNT, increasing sevenfold from dietary K restriction to K loading as opposed to a fourfold span for the CCD.
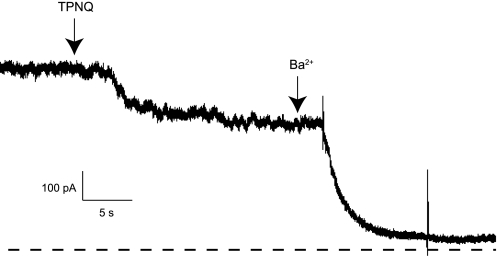
We also examined SK currents in the OMCD. This part of the nephron normally does not secrete K but may reabsorb the ion and contribute to recycling of K within the medulla (22). In OMCDs from animals on a high-K diet, ISK was easily detectable, although considerably smaller than that from the CCD under the same conditions (Fig. 9). The axial distribution of ISK is shown in Fig. 10. We did not study the OMCD in animals with a normal or reduced K intake.
Fig. 9.
TPNQ-sensitive currents in OMCD. A tubule was isolated from a rat fed a high-K diet for 7 days. A whole-cell clamp was formed on a principal cell and the voltage maintained at 0 mV. At the first arrow, TPNQ (100 nM) was added to the superfusate. At the second arrow, the solution was changed to one containing 5 mM Ba2+. Broken line indicates I = 0.
Fig. 10.
Axial distribution of TPNQ-sensitive currents. Measurements were made with tubules from rats fed a high-K diet. Data are means ± SE for 21–45 determinations.
Calculations.
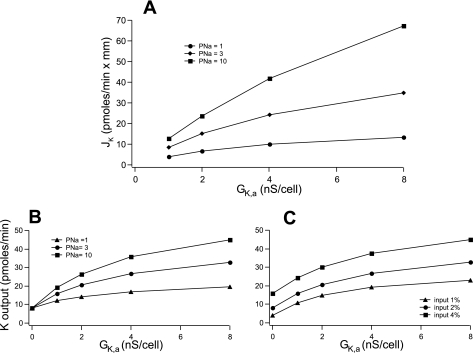
To assess the impact of the changes in K conductance on K secretion, we employed a model of the CCD/CNT described previously (8). We estimated the apical K conductance (GK,a) as the ISK at V = 0 divided by the K reversal potential (−90 mV). We used current values in the CNT shown in Fig. 8, divided by two to account for the effects of intracellular ATP (Fig. 7), which is presumably in the millimolar range in vivo. We first considered a tubule in which the luminal as well as the basolateral ion compositions are fixed (i.e., infinite flow rates). These values are 5 mM K and 30 mM Na in the lumen as an approximation of fluid entering the CNT, and 140 mM Na and 5 mM K on the basolateral side, i.e., values in plasma. GK,a was varied over an eightfold range, corresponding to values found under the extremes of dietary K intake as described above. Apical Na permeability was varied from a high value (10 pA/mM·cell) as found under conditions of high circulating aldosterone (7) to a low value one-tenth that of the high. The results are shown in Fig. 11A. The increased GK,a enhanced K secretion rates, and both the absolute as well as the fractional changes were larger when PNa was high. The fractional increase in flux for an eightfold augmentation of GK,a was fivefold at high PNa and threefold at low PNa. The changes in flux were proportionately less than those of conductance in large part because increased GK,a hyperpolarized the apical membrane, decreasing the driving force for K efflux. However, the simulations clearly suggest that under the likely conditions of the CNT in vitro that apical K conductance can be a limiting factor for K secretion.
Fig. 11.
Model calculations. A: effects of apical GK,a on initial net K+ flux (JK). For this simulation we assume a constant luminal Na+ concentration = 30 mM and K+ concentration = 5 mM. The net rate of K flux across a tubule was calculated as apical conductance (GK,a) was varied over an 8-fold range. Three different values of apical Na permeability (PNa) were used. Other parameters were constant as described in the text. B: effects of apical GK,a and PNa on final K+ output in a tubular epithelium. The efflux of K+ from a 1-mm tubule was calculated for an input rate of 2 nmol/sec. GK,a was varied over an 8-fold range with three different values of PNa Other parameters were constant as described in the text. C: effects of apical GK,a and fluid delivery on final K+ output. GK,a was varied over an 8-fold range with three different values of fluid input from 1–4% of the glomerular filtration rate. PNa was constant at 1. Other parameters were constant as described in the Materials and Methods.
We next extended the model to a tubule with realistic fluid inflow, such that the ion transport processes alter the composition of the luminal fluid. Here we simulated the net addition of K to the tubular fluid, i.e., K secretion. As expected, increasing GK,a increased the amount of K exiting the tubule in a PNa-dependent manner (Fig. 11B). Again the absolute increases were largest at high PNa. The increases in net secretion were blunted by the accumulation of K in the tubular fluid, further decreasing the driving force for K efflux across the apical membrane.
Finally, we examined the effects of increasing delivery of fluid to the CNT from 1 to 4% of the glomerular filtration rate at constant Na and K concentrations (Fig. 11C). Increasing GK,a enhanced the amount of K leaving the CNT at each delivery rate. At the higher rates, the absolute increases in K added to the urine with increasing GK,a were larger, although the fractional increases were smaller. These synergistic effects of flow and K conductance do not take into account any flow dependence of membrane conductances.
The simulations predict that under conditions of high PNa and/or high flow rates the measured values of GK,a are sufficient to account for a significant rate of K secretion by the CNT. The highest rates correspond to K outflows of >3 μmol/min for the two kidneys, accounting for 50% or more of excretion levels with high K intake (see discussion).
DISCUSSION
The main findings reported in this paper are as follows: 1) the toxin TPNQ can be used to assess the density of SK/ROMK channels in the renal tubule, 2) the number of channels per cell can account for significant K secretion by the CNT, and 3) the channel densities vary over a range of around sevenfold depending on dietary K intake. We discuss these findings, and their implication for renal K excretion, below.
Use of TPNQ to assess channel activity.
TPNQ is a derivative of the bee venom toxin tertiapin that has been modified with a methionine to glutamine substitution to reduce effects of oxidation (10). Both tertiapin and TPNQ were shown to be high affinity blockers of ROMK (Kir1.1) channels expressed in oocytes (10, 11). This is, to our knowledge, the first use of the toxin to assess the activity of K channels in cells in which they are naturally expressed. A key aspect of this application is the specificity of the toxin for a given channel type. It was previously shown that TPNQ does not block currents through Kir2.1 channels but Kir3.1/3.4 channels were affected (10, 11). These are not known to be expressed in renal epithelial cells. However, the basolateral membranes of the CCD and CNT have substantial K conductances that are thought to be conferred at least in part by Kir4.1 channels and/or Kir4.1/5.1 heteromultimers (9, 13). These are closely related to the Kir1.1 channels that we want to assay. We therefore tested TPNQ for blockade of these inward rectifiers and found that it was able to discriminate very well between the apical Kir1.1 and the basolateral Kir4.1 and 4.1/5.1 channels. We have not tested for effects on other candidate basolateral channels such as Kir2.3 (31) or Kir7.1 (18). However, the former is more closely related to Kir2.1, which is unaffected by the toxin, and the latter is more distantly related to the other inward rectifiers. These results, together with the findings that the apparent Ki for block of K+ currents in the CCD (5 nM) is consistent with previous determinations on heterologously expressed ROMK channels (9, 10) and of inhibition by ATP, strongly suggest that these TPNQ-sensitive currents are carried by ROMK itself.
Channel densities in the CCD and CNT.
Previous measurements of the density of conducting K channels in renal tubules were obtained from cell-attached patches. This is the most direct and specific assay, but it is difficult to translate such measurements of channels/membrane patch into absolute densities. One problem is that the area of the patch is usually unknown. A second is that many different patches must be sampled to come up with an accurate mean. This difficulty may be exacerbated if the channels are distributed nonrandomly in the membrane. In previous studies (6), the nonuniform pattern of SK channel expression was particularly pronounced in the CNT.
The whole-cell clamp approach is relatively simple to implement and gives information on the entire membrane of the cell. The numbers obtained are in reasonably good agreement with those obtained from single-channel measurements. For the CCD of rats fed a high-K diet, the mean TPNQ-sensitive current was 515 pA/cell in the presence of ATP (Fig. 7). Assuming a single-channel current of 1.2 pA and on open probablility of 0.9 under comparable conditions (8), this predicts 477 channels/cell. For an apical surface are of 200 μ2, this corresponds to 2.4 channels/μ2. Cell-attached patch measurements under similar conditions gave an estimate of ∼2 channels/μ2 (21).
The axial distribution along the distal nephron of SK conductance under conditions of adaptation to high K was CNT ∼ CCD > OMCD. The high density of channels in the CNT presumbably reflects the role of this segment in net K secretion (15). Although the CCD also expresses a high channel density, K secretion by this segment will be smaller for two reasons. First, Na channel density is lower in the CCD, so that the apical membrane is less depolarized and the driving force for K+ secretion is reduced (7). Second, as shown by model calculations, the driving forces will be further reduced, or even reversed, by accumulation of K+ in the lumen of the tubule, particularly in the antidiuretic state when luminal K+ is concentrated by water reabsorption (30).
We show here for the first time the presence of conducting SK channels in the apical membrane of the OMCD. However, the ROMK channel protein was previously detected in this segment, in some cases localized to the luminal membrane (3, 12, 17, 25, 33). Here K is thought to be reabsorbed and to participate in the medullary recycling of K from the collecting duct to the ascending portion of Henle's loop (2, 22). At least a portion of this absorption/recycling probably occurs through apical SK channels.
Effects of diet.
There is general agreement that a very high dietary K intake increases the number of conducting SK channels in the CCD (19, 21, 27, 28). This finding was confirmed by the whole cell measurements of the TPNQ-sensitive current (Fig. 8). The effects of a low-K diet were more variable. Some studies (1) reported a decrease in channel density, while others (21, 29) failed to document a significant effect. In this study, we found a consistent but modest decrease of ∼35% in SK currents in the CCD of K-depleted rats compared with paired controls. This confirms the reduction of channel density with K depletion and perhaps explains why this reduction is difficult to see with single-channel measurements.
Less information was available for the CNT, the presumed major sight of channel-mediated K secretion. Our group found similar densities with high and normal K intake, although we stressed that the clustering of channels with very large numbers in a few patches made this comparison difficult (6). With whole cell currents, we found that the SK channel densities in the CNT increased with a high K intake similar to those of the CCD. Furthermore, we measured for the first time channel densities in the CNT under conditions of K depletion. Here the decrease in density was 50%, somewhat larger than in the CCD.
Implications for K secretion.
Between two extremes of dietary K intake we found a variation in SK channel density of approximately sevenfold. According to model calculations, this can have a significant influence on K secretion by the CNT. Quantitatively, the impact depends on other parameters of the tubular transport apparatus. The greatest impact is when the apical Na permeability is large. This is because high Na influx prevents the increasing K conductance from hyperpolarizing the apical membrane and reducing the driving force for K efflux. The effect of K conductance is also modified by the delivery of fluid to the distal nephron. With an increased delivery, the absolute amount of K added to the fluid increases and is most strongly enhanced by a higher GK. Here larger flows reduce the buildup of K concentrations in the luminal fluid and help maintain the driving force for secretion. This phenomenon is independent of specific regulation of ion conductances by flow (23, 32), which will augment the effect.
At the highest apical SK conductances, the fluid exiting the CNT will contain, at the moderate values of fluid delivery, up to 3.2 μmol/min for two kidneys. This is comparable with the filtered load of K, assuming a glomerular filtration rate of 0.8 ml/min and a plasma K+ concentration of 4 mM. It is ∼50% of the maximum amiloride-sensitive rate of K excretion (Frindt and Palmer, unpublished observations). Thus these channels will contribute in an important way to K handling by the kidney.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-27847.
Acknowledgments
We thank Stephen J. Tucker for the gift of the rat Kir4.1 and 5.1 clones and Diomedes Logothetis for the gift of the human Kir4.1 clone.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Babilonia E, Li D, Wang Z, Sun P, Lin DH, Jin Y, Wang WH. Mitogen-activated protein kinases inhibit the ROMK (Kir 1.1)-like small conductance K channels in the cortical collecting duct. J Am Soc Nephrol 17: 2687–2696, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battilana CA, Dobyan DC, Lacy FB, Bhattacharya J, Johnston PA, Jamison RL. Effect of chronic potassium loading on potassium secretion by the pars recta or descending limb of the juxtamedullary nephron in the rat. J Clin Invest 62: 1093–1103, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ecelbarger CA, Kim GH, Knepper MA, FM, Kachar B. Regulation of potassium channel Kir1.1 (ROMK) abundance in the thick ascending limb of Henle's loop. J Am Soc Nephrol 12: 10–18, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Frindt G, Ergonul Z, Palmer LG. Na channel expression and activity in the medullary collecting duct of rat kidney. Am J Physiol Renal Physiol 292: F1190–F1196, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Frindt G, Masilamani S, Knepper MA, Palmer LG. Activation of epithelial Na channels during short-term Na deprivation. Am J Physiol Renal Physiol 280: F112–F118, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Frindt G, Palmer LG. Apical potassium channels in the rat connecting tubule. Am J Physiol Renal Physiol 287: F1030–F1037, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Frindt G, Palmer LG. Na channels in the rat connecting tubule. Am J Physiol Renal Physiol 286: F669–F674, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Gray DA, Frindt G, Palmer LG. Quantification of K+ secretion through apical low-conductance K channels in the CCD. Am J Physiol Renal Physiol 289: F117–F126, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Gray DA, Frindt G, Zhang YY, Palmer LG. Basolateral K+ conductance in principal cells of rat CCD. Am J Physiol Renal Physiol 288: F493–F504, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Jin W, Lu Z. Synthesis of a stable form of tertiapin: A high-affinity inhibitor of inward-rectifier K+ channels. Biochemistry 38: 14286–14293, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Jin W, Lu Z. A novel high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry 37: 13291–13299, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Kohda Y, Ding W, Phan E, Housini I, JW, Star RA, Huang CL. Localization of the ROMK potassium channel to the apical membrane of distal nephron in rat kidney. Kidney Int 54: 1214–1223, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Lourdel S, Paulais M, Cluzeaud F, Bens M, Tanemoto M, Kurachi Y, Vandewalle A, Teulon J. An inward rectifier K(+) channel at the basolateral membrane of the mouse distal convoluted tubule: similarities with Kir4-Kir5.1 heteromeric channels. J Physiol 538: 391–404, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu M, Leng Q, Egan ME, Caplan MJ, Boulpaep EL, Giebisch G, Hebert SC. CFTR is required for PKA-regulated ATP sensitivity of Kir1.1 potassium channels in mouse kidney. J Clin Invest 116: 797–807, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malnic G, Muto S, Giebisch G. Regulation of potassium excretion. In: The Kidney Physiology and Pathophysiology, edited by Alpern RJ and Hebert SC. Burlington, MA: Academic, 2008.
- 16.Meneton P, Loffing J, Warnock DG. Sodium and potassium handling by the aldosterone-sensitive distal nephron: the pivotal role of the distal and connecting tubule. Am J Physiol Renal Physiol 287: F593–F601, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Mennitt PA, Wade JB, Ecelbarger CA, Palmer LG, Frindt G. Localization of ROMK channels in the rat kidney. J Am Soc Nephrol 8: 1823–1830, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Ookata K, Tojo A, Suzuki Y, Nakamura N, Kimura K, Wilcox CS, Hirose S. Localization of inward rectifier potassium channel Kir7.1 in the basolateral membrane of distal nephron and collecting duct. J Am Soc Nephrol 11: 1987–1994, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol 104: 693–710, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer LG, Frindt G. Na+ and K+ transport by the renal connecting tubule. Curr Opin Nephrol Hypertens 16: 477–483, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Palmer LG, Frindt G. Regulation of apical K channels in rat cortical collecting tubule during changes in dietary K intake. Am J Physiol Renal Physiol 277: F805–F812, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Stokes JB Consequences of potassium recycling in the renal medulla. Effects on ion transport by the medullary thick ascending limb of Henle's loop. J Clin Invest 70: 219–229, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taniguchi J, Imai M. Flow-dependent activation of maxi K+ channels in apical membrane of rabbit connecting tubule. J Membr Biol 164: 35–45, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Tucker SJ, Imbrici P, Salvatore L, D'Adamo MC, Pessia M. pH dependence of the inwardly rectifying potassium channel, Kir5.1, and localization in renal tubular epithelia. J Biol Chem 275: 16404–16407, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Waldegger S, Jentsch TJ. Functional and structural analysis of ClC-K chloride channels involved in renal disease. J Biol Chem 275: 24527–24533, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Giebisch G. Dual effect of ATP on the apical small conductance K channel of rat cortical collecting duct. J Gen Physiol 98: 35–61, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, Schwab A, Giebisch G. Regulation of small conductance K+ channel in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 259: F494–F502, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Wang WH, Lerea KM, Chan M, Giebisch G. Protein tyrosine kinase regulates the number of renal secretory K channels. Am J Physiol Renal Physiol 278: F165–F171, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Wei Y, Bloom P, Lin D, Gu R, Wang WH. Effect of dietary K intake on apical small-conductance K channel in CCD: role of protein tyrosine kinase. Am J Physiol Renal Physiol 281: F206–F212, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein AM A mathematical model of rat distal convoluted tubule. II. Potassium secretion along the connecting segment. Am J Physiol Renal Physiol 289: F721–F741, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Welling PA Primary structure and functional expression of a cortical collecting duct Kir channel. Am J Physiol Renal Physiol 273: F825–F836, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Xu JZ, Hall AE, Peterson LN, Bienkowski MJ, Eesalu TB, Hebert SC. Localization of the ROMK protein on apical membranes of rat kidney nephron segments. Am J Physiol Renal Physiol 273: F739–F748, 1997. [DOI] [PubMed] [Google Scholar]