Abstract
IA-2 and IA-2β, major autoantigens in type 1 diabetes, are transmembrane proteins in dense-core vesicles, and their expression influences the secretion of hormones and neurotransmitters. The present experiments were performed to examine whether IA-2 and IA-2β modulate the release of renin from dense-core vesicles of juxtaglomerular granular cells in the kidney. Plasma renin concentration (PRC; ng angiotensin I·ml−1·h−1) was significantly reduced in mice with null mutations in IA-2, IA-2β, or both IA-2 and IA-2β compared with wild-type mice (876 ± 113, 962 ± 130, and 596 ± 82 vs. 1,367 ± 93; P < 0.01, P < 0.02, and P < 0.001). Renin mRNA levels were reduced to 26.4 ± 5.1, 39 ± 5.4, and 35.3 ± 5.5% of wild-type in IA-2−/−, IA-2β−/−, and IA-2/IA-2β−/− mice. Plasma aldosterone levels were not significantly different among genotypes. The regulation of PRC by furosemide and salt intake, and of aldosterone by salt intake, was maintained in all genotypes. IA-2 and IA-2β expression did not colocalize with renin but showed overlapping immunoreactivity with tyrosine hydroxylase. While propranolol reduced PRC in wild-type mice, it had no effect on PRC in IA-2/ IA-2β−/− mice. Renal tyrosine hydroxylase mRNA and immunoreactivity were reduced in IA-2/IA-2β−/− mice as was the urinary excretion of catecholamines. We conclude that IA-2 and IA-2β are required to maintain normal levels of renin expression and renin release, most likely by permitting normal rates of catecholamine release from sympathetic nerve terminals.
Keywords: knockout mice, renin mRNA, propranolol, blood pressure, heart rate, salt intake
ia-2 (ica512) and IA-2β (phogrin) have been identified as major autoantigens in type I diabetes mellitus (11, 12, 16). Structurally, these proteins are members of the tyrosine phosphatase family although they do not appear to have enzymatic activity. They are expressed as integral proteins of dense-core vesicle membranes in neuroendocrine cells of the pancreas, brain, pituitary gland, and adrenal medulla (11, 19). Mice with null mutations of IA-2 and IA-2β have reduced glucose tolerance and decreased glucose-induced insulin secretion, indicating that these proteins play a role in the regulated secretion of insulin (8, 17). A second secretory defect has been documented in female IA-2/IA-2β double knockout mice, which are infertile as a result of an absent LH surge in the proestral phase of the estrous cycle (9, 10). Because of the widespread expression of IA-2 and IA-2β in dense-core vesicles of secretory cells throughout the body, it is a reasonable hypothesis that regulation of exocytosis of dense-core vesicles may be a general role of these proteins.
Renin is an aspartic protease that is primarily synthesized by modified smooth muscle cells in afferent arterioles of the kidney and stored in dense-core vesicles of juxtaglomerular granular (JG) cells (4). It therefore seemed of interest to examine whether IA-2 and/or IA-2β might participate in the secretory process of renin. The present studies were performed in mice lacking either IA-2, IA-2β, or both IA-2 and IA-2β to test the validity of this notion. Since the renin-angiotensin system plays a critical role in the maintenance of arterial blood pressure, we also determined the effect of IA-2 deficiencies on arterial blood pressure levels.
Our data show that mice with null mutations in the genes for IA-2 and IA-2β have significantly reduced levels of plasma renin and renal renin mRNA, suggesting that these proteins exert a tonic stimulatory influence on renin-secreting JG cells. The effect on renin secretion does not appear to be the result of a direct interaction between IA-2 or IA-2β and JG cells since the expression of IA-2 and IA-2β did not colocalize with renin. Rather, a reduction in tonic sympathetic transmitter release is the most likely mechanism responsible for the suppression of the renin-angiotensin system in IA-2/IA-2β-deficient mice.
METHODS
Experiments were performed using male and female mice of the IA-2, IA-2β, and IA-2/IA-2β null mutant mice generated as described previously (9). Wild-type (WT) and mutant mice have a C57BL/6NCI congenic background. Mice were fed a standard laboratory chow, and they were housed under 12:12-h light-dark conditions. Acute responsiveness of renin secretion was tested in conscious mice by intraperitoneal injections of furosemide (40 mg/kg, Lasix, Hoechst) or propranolol (10 mg/kg), with blood collections made before and 60 min after the injection. To study the effect of variations of NaCl intake, mice (2–3 mo old) were placed on a normal-, high-, or low-salt diet (0.4, 8, or 0.03% NaCl, respectively) for 7 days. At the end of the experiments, kidneys were harvested under ketamine/xylazine anesthesia. Animal care and experimentation were approved by the National Institute of Diabetes and Digestive and Kidney Diseases Animal Care and Use Committee and carried out in accordance with National Institutes of Health principles and guidelines for the Care and Use of Laboratory Animals.
Blood collection and renin determination.
Blood was taken from conscious mice by tail vein puncture and collected in a 75-μl hematocrit tube that contained 1 μl of 125 mM EDTA in its tip. Tubes were centrifuged to separate red blood cells and plasma, and plasma was frozen until used for renin determination. Using a fivefold dilution of 2 μl of plasma, renin concentration was measured by radioimmunoassay (Gammacoat, DiaSorin, Stillwater, MN) as the generation of angiotensin I (ANG I) after addition of excess rat substrate, with final plasma dilutions varying between 1:500 and 1:1,000. ANG I generation was determined for a 1-h incubation period at 37°C and expressed as an hourly average. In each assay, background ANG I formation was determined by incubating substrate without plasma for the same time and subtracting any ANG I from the plasma containing samples if necessary. In addition, background ANG I levels were determined in a plasma aliquot kept frozen without the addition of substrate until assay. All plasma samples were stored at −20°C until assay. Blood collections for PRC measurements were usually made between 10 and 12 AM.
Plasma aldosterone was measured by RIA (Coat-A Count Aldosterone Kit TKAL1, DPC, Los Angeles, CA).
Urinary catecholamines.
Age-matched WT and IA-2/IA-2β-deficient mice were housed in metabolic cages, and urine was collected for 24 h into vials containing 20 μl of 6 N HCl. Urinary epinephrine and norepinephrine concentrations were determined using an enzyme immunoassay kit according to the manufacturer's instructions (BiCat EIA kit, ALPCO Diagnostics, Salem, NH).
RNA quantification.
PCR (total volume of 10 μl) included cDNA, 900-nM primers, a 250-nM probe, and 5 μl of TaqMan MasterMix (Applied Biosystems, Foster City, CA). Total RNA was extracted (RNeasy Mini kit, Qiagen), and treated with DNase I (Invitrogen) at room temperature for 15 min. Reverse transcription was performed using SuperScript II (SuperScript II first-strand synthesis system for RT-PCR, Invitrogen). Renin mRNA levels were assessed by real-time PCR using β-actin cDNA (primers and probe from Applied Biosystems) as an internal control. The following primers were used to amplify a 98-bp renin product: sense 5′-CACACTCAGCAGTACGGACTACGT (9952–9975), antisense 5′-CAGTGGGTGGTGGGATGTC (10613–10595), and probe CTACAGT ATCCCAACAGGA (9977–10559). Tyrosine hydroxylase mRNA was determined using commercial primers and a probe from Applied Biosystems (assay ID Mm00447557 m1).
Isolated JG cells.
JG cells were isolated from C57/B6 mice (6–8 wk) as described previously (1). After Percoll gradient centrifugation, the cells were washed with PBS, and total RNA was extracted (minikit). RNA (0.5 μg) was reverse transcribed, and cDNA (25 ng) was amplified with the ABI Prism 7900 Sequence Detection System using SYBR green Master buffer (Applied Biosystems). Cycling profiles were 50°C for 2 min, 95°C for 10 min followed by 40 cycles of 95°C for 0.15 min and 60°C for 1 min. Primer sequences were as follows: IA-2 sense 5′-tacttttgagtaccaggacct-3′, and IA-2 antisense 5′-gtcgctgaactgggaggaca-3′; IA-2-β sense 5′-gccattctcagcaactgctt-3′, and IA-2-β antisense 5′-aaatccttcaggagactctgc-3′; β-actin sense 5′-gctctggctcctagcaccat-3′, and β-actin antisense 5′-gccaccgatccacacagagt-3′.
Immunohistochemistry.
Mice were anesthetized by an intraperitoneal injection of ketamine/xylazine. Kidneys were perfused via the abdominal aorta using 3% paraformaldehyde dissolved in PBS. For cryostat sectioning, tissues were protected from freezing artifacts by 800 mosM sucrose/PBS, shock-frozen in cooled isopropane, and stored at −80°C. Alternatively, tissues were postfixed in 3% PFA/PBS, dehydrated, and standard paraffin embedded. For ultrastructural analysis, kidney specimens were postfixed in 1.5% PFA/PBS containing 1.5% glutaraldehyde and 0.05% picric acid, rinsed, and stored in PBS until embedding in Epon (Serva, Heidelberg, Germany).
Immunohistochemical staining was performed on cryostat or on paraffin sections. Sections were blocked with 5% skim milk/PBS, incubated with the respective primary antibody followed by incubation with suitable horseradish peroxidase (DAKO, Hamburg, Germany)- or cy-3-coupled secondary antibodies (Dianova, Hamburg, Germany). In double-labeling experiments, the different primary antibodies were administered consecutively. Specificity of the double-staining procedures was controlled by parallel incubation of consecutive sections, each incubated only with one single probe. Sections were counterstained and analyzed using a Leica DMRB microscope or multilaser confocal scanning microscope (TCS SP-2, Leica Microsystems, Bernsheim, Germany). The following primary antibodies were used: mouse monoclonal anti-IA-2 (SK1, Saeki), mouse monoclonal anti-IA-2β (KO6, Saeki), rabbit anti-cyclooxygenase-2 (COX-2, Cayman Chemical), rabbit anti-renin (gift of A. Kurtz), rabbit anti-tyrosine hydroxylase (A&D Serotec, Düsseldorf, Germany), and goat anti-vimentin (Sigma-Aldrich, Munich, Germany).
Renin and COX-2 signals were semiquantitatively evaluated with a ×20 and ×40 objective by counting the number of renin-positive JG apparatus (JGA) sites or COX-2-positive macula densa cells within a total of about 150 glomeruli (20). Sympathetic terminals were quantified with a ×40 objective by counting tyrosine hydroxylase-positive arterioles counterstained with vimentin in close proximity to glomeruli. Approximately 150 glomeruli were evaluated.
Blood pressure telemetry.
Telemetric transmitters were magnetically activated >24 h before implantation. Mice were anesthetized with ketamine and xylazine (90 and 10 mg/kg, respectively), and the left carotid artery was isolated. The telemetric catheter was inserted into the left carotid artery and advanced to reach the aortic arch, and the telemeter body (model TA11PA-C20, Data Sciences International, St. Paul, MN) was placed in a subcutaneous pocket on the right flank. One day after surgery, each animal was returned to its home cage with ad libitum food and water for the duration of the study. The telemetric signal was detected using a model RPC-1 receiver, a 20-channel data-exchange matrix, APR-1 ambient pressure monitor, and a Dataquest ART 2.3 acquisition system (Data Sciences International). The system was programmed to acquire data for 10 s every 2 min and to calculate 10-min averages of the mean, systolic and diastolic blood pressure, pulse pressure, heart rate, and activity. The recording room was maintained at 21–22°C with a 12:12-h light-dark cycle. The implanted telemeter was activated on the morning of day 10, and the mice were left undisturbed for at least 5 h while their blood pressure was recorded.
Glomerular filtration rate.
Glomerular filtration rate (GFR) was measured by single-injection FITC-inulin clearance as described by Qi et al. (3, 15) modified to minimize plasma volume. FITC-inulin (5%), dialyzed overnight against 0.9% NaCl (final concentration ∼3%), was injected at 3.7 μl/g body wt into the retroorbital plexus during brief isoflurane anesthesia (recovery within ∼20 s). At 3, 7, 10, 15, 35, 55, and 75 min, mice were placed in a restrainer, the tail vein was punctured with a 30-g atraumatic needle, and ∼2 μl of blood was collected by capillarity into heparinized 5-μl microcaps (Drummond Scientific, Broomall, PA). Five hundred nanoliters of plasma was diluted 1:10 in 500 mmol HEPES (pH 7.4) and measured against a standard curve (1 μl of ∼3%-FITC-inulin diluted 1:50, 1:100, and 1:500 in 500 mmol HEPES). Fluorescence was determined in 1.7 μl in a Nanodrop-ND-3300 spectrometer (Nanodrop Technologies, Wilmington, DE). GFR was calculated using a two-compartment model of two-phase exponential decay (15).
Statistics.
Data are expressed as means ± SE. Statistical comparisons were done by a paired t-test for comparisons of PRC before and after an intervention in the same animals and by an unpaired t-test for comparisons between two groups of animals, for example of different genotypes. ANOVA with repeated measures was used for comparisons of more than two data sets. P values <0.05 were considered to indicate a significant difference. Quantitative immunohistological data are presented as means ± SD. For statistical comparison, the Mann-Whitney rank sum test was employed. P values of <0.05 were considered statistically significant.
RESULTS
Plasma renin.
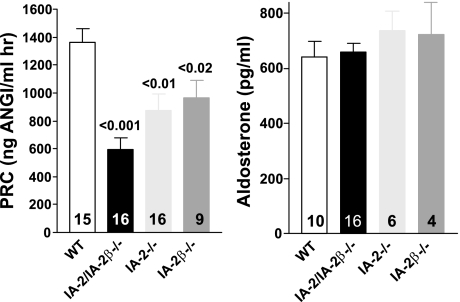
Plasma renin concentrations (PRC) of individual mice deficient in one or both of the IA-2 isoforms are shown in Fig. 1. The mean PRC (ng ANG I·ml−1·h−1) of 596.5 ± 82 in IA-2/IA-2β-deficient mice (n = 16) was significantly lower than the mean of 1,367 ± 93 in WT animals (n = 15; P < 0.001). PRC was also significantly lower than WT in mice deficient in either IA-2 (876 ± 113; n = 16; P < 0.01), or IA-2β (962 ± 130; n = 9; P < 0.02). Despite the reduction of plasma renin, plasma aldosterone concentrations were found to be comparable between WT animals and mice with single or double null mutations of IA-2 and IA-2β (Fig. 1).
Fig. 1.
Left: plasma renin concentration (PRC) in wild-type mice (WT) and in mice with null mutations of IA-2 (IA-2−/−), IA-2β (IA-2β−/−), and both (IA-2/IA-2β; IA-2/IA-2β−/−). Right: plasma aldosterone concentrations (pg/ml) in WT and in mice with null mutations of IA-2, IA-2β, and both IA-2/IA-2β. Values are means ± SE; numbers inside columns indicate number of animals in each group. Significances are given for comparison with WT (ANOVA with Bonferroni's post hoc test).
Renin expression.
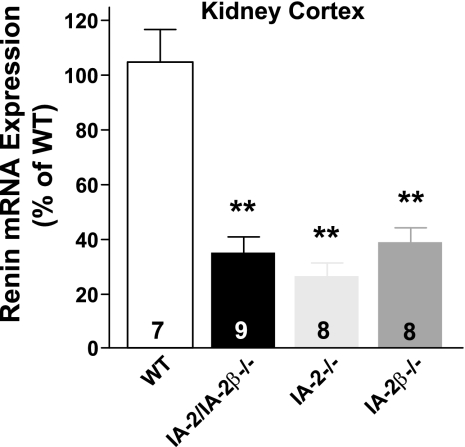
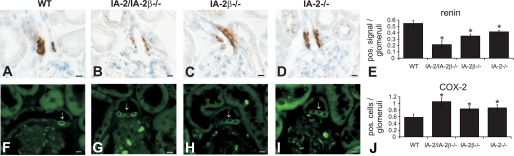
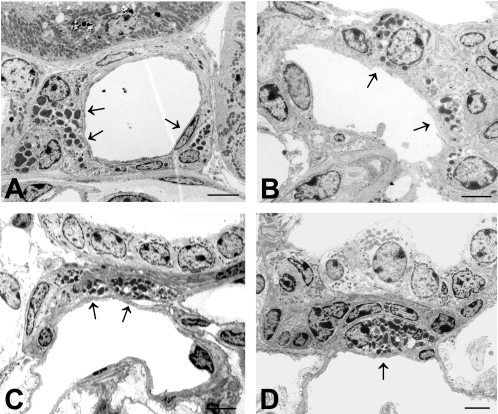
Renal expression of renin was found to be reduced in IA-2 mutant mice at both the mRNA and protein level. As shown in Fig. 2, renin mRNA expression in the renal cortex expressed as the percentage of WT was significantly reduced to 35 ± 5.5% in IA-2/IA-2β double-mutant animals (P < 0.001), to 26.4 ± 5.1% in IA-2 single mutants (P < 0.001), and to 39 ± 5.4% in IA-2β-deficient mice. Renin expression in the inner medulla of WT mice was <1% of that in cortex, and it was even lower in the mutant animals (data not shown). As shown in Fig. 3, immunocytochemical quantification of renin protein expression revealed significant reductions of the fraction of renin-positive JGAs in IA-2/IA-2β−/− (0.21 ± 0.06), IA-2β−/− (0.35 ± 0.03), and IA-2−/− mice (0.41 ± 0.02) compared with WT animals (0.55 ± 0.1; P < 0.05 for all comparisons with mutant animals). Electron microscopy showed that the number of renin-containing cells was reduced in IA-2/IA-2β−/− mice but that the fine structure of the JGA and the glomerular tuft was well maintained in all mutant strains (Fig. 4).
Fig. 2.
Renin mRNA levels in the kidney cortex of WT and mice with null mutations of IA-2, IA-2β, and both IA-2/IA-2β. Values are means ± SE expressed as % of WT levels. Numbers in columns indicate numbers of animals. **P < 0.01 compared with WT (ANOVA with Bonferroni's post hoc test).
Fig. 3.
Immunofluorescent labeling with antibodies against renin and cyclooxygenase-2 (COX-2) in WT and IA-2/IA-2β−/−, IA-2β−/−, and IA-2−/− mice. A–E: renin expression of the afferent arteriole is reduced in IA-2/IA-2β−/− (B), IA-2β−/− (C), and IA-2−/− mice (D). The fraction of renin-positive signals per 150 glomeruli is shown in E. F–K: COX-2 expression in the macula densa of WT (F), IA-2/IA-2β−/− (G), IA-2β−/− (H), and IA-2−/− mice (I). Arrows point to COX-2-positive macula densa cells. Numerical evaluation of COX-2-positive cells/macula densa is shown in J.
Fig. 4.
Electron microscopic images of renin granular cells of WT (A), IA-2/IA-2β−/− (B), IA-2β−/− (C), and IA-2−/− mice (D). Arrows point to renin-containing cells. Calibration bars = 2 μm.
In contrast, the average number of COX-2-positive MD cells (Fig. 3) was significantly increased in IA-2/IA-2β−/− (1.06 ± 0.12), IA-2β−/− (0.84 ± 0.06), and IA-2−/− mice (0.87 ± 0.09) compared with WT animals (0.58 ± 0.12; P < 0.05).
Regulation of plasma renin.
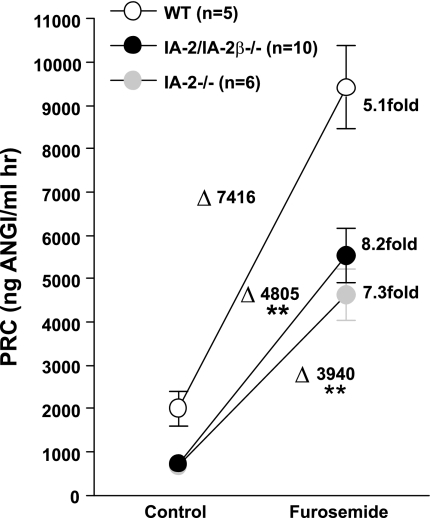
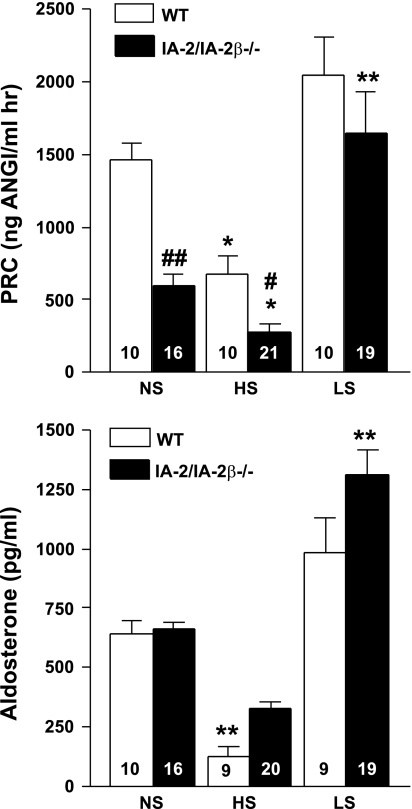
To examine whether the absence of IA-2 affects the acute renin secretory response, we assessed the effect of furosemide on renin release in WT, IA-2/IA-2β−/−, and IA-2−/− mice (Fig. 5). We found that the stimulatory effect of furosemide was maintained in all three genotypes, but that the absolute magnitude of the increase of plasma renin was less in the IA-2-deficient animals. Additional studies were performed to examine whether the regulation of plasma renin and aldosterone by changes in dietary salt intake is affected by the absence of IA-2 and IA-2β. As summarized in Fig. 6, PRC fell significantly in both WT and IA-2/IA-2β−/− mice with a change from a normal- to a high-salt diet while the increase in PRC with low salt intake was significant only in the knockout mice. Changes in salt intake caused directionally similar changes in plasma aldosterone in both strains of mice. Interestingly, the effect of low salt intake was again only significant in the knockout animals. Thus the regulation of renin secretion and aldosterone synthesis by salt intake is maintained in the absence of IA-2 and IA-2β.
Fig. 5.
Effect of an acute administration of furosemide (40 mg/kg) on PRC in WT, IA-2/IA-2β−/−, and IA-2−/− mice (values in all genotypes are significantly different from control at P < 0.001). Changes in PRC caused by furosemide in the 3 genotypes are given by Δ values. **P < 0.01 compared with WT (ANOVA).
Fig. 6.
PRC (top) and plasma aldosterone concentration (bottom) in WT (open bars) and IA-2/IA-2β−/− mice (filled bars) maintained on diets with normal (NS), high (HS), or low (LS) NaCl content. Numbers inside columns indicate numbers of animals in each group.*P < 0.05, **P < 0.01 compared with NS (ANOVA and Bonferroni's post hoc test). #P < 0.05, ##P < 0.01 compared with WT (ANOVA).
Blood pressure and GFR.
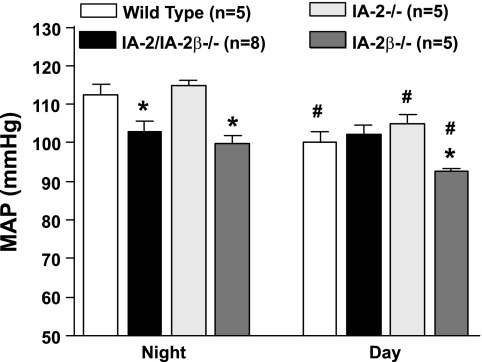
Twenty-four-hour mean arterial blood pressure (MAP) was not significantly different between WT (n = 5), IA-2−/− (n = 5), IA-2β−/− (n = 5), and IA-2/ IA-2β double-mutant mice (n = 8), but it was significantly lower in IA-2β single mutants (96.4 ± 1.2 vs. 106.3 ± 2.6 mmHg). Similarly, heart rate was significantly lower in IA-2β-deficient compared with WT mice (536 ± 2.6 vs. 606 ± 22 beats/min) whereas there were no differences between WT and IA-2−/− or IA-2/ IA-2β−/− mutants. Analysis of MAP for the 12-h nighttime (6 PM to 6 AM) and daytime periods (6 AM to 6 PM) showed lower daytime MAP in all strains except in IA-2/IA-2β−/− mutants (Fig. 7). In a comparison of WT and mutant animals, only IA-2β−/− mice had both lower daytime and nighttime MAP results whereas the MAP in IA-2/ IA-2β−/− animals was lower than WT only during the night. Neither the daytime nor nighttime MAP results in IA-2−/− animals were different from WT.
Fig. 7.
Mean arterial blood pressure (MAP) in WT and in mice with null mutations of IA-2, IA-2β, and both (IA-2/IA-2β) measured by radiotelemetry. Data are given as means for the 12-h nighttime (left) and 12-h daytime periods (right). *P < 0.05 compared with WT. #P < 0.05 compared with nighttime (ANOVA).
The GFR as an index of renal function was found to be unaltered by the absence of IA-2 and IA-2β. It averaged 352 ± 91 μl/min in WT (n = 6) and 345 ± 65 μl/min in IA-2/ IA-2β-deficient mice (n = 6). Since mean body weights were comparable (30.7 ± 3.9 and 28.5 ± 3.6 g in WT and mutant mice, respectively), correction for body weight did not alter the conclusion that GFR was unaffected by the consequences of IA-2/IA-2β deficiency. Hematocrit was significantly higher in IA-2/IA-2β-deficient (52.6 ± 1.4%, n = 6) than WT animals (46.4 ± 2.7%, n = 6; P < 0.001). Ambient urine osmolarity averaged 2,250 ± 372 mosmol/l in WT (n = 13) and 1,799 ± 171 mosmol/l in IA-2/ IA-2β-deficient mice (n = 16; P > 0.05).
Localization of IA-2 and IA-2β in the kidney.
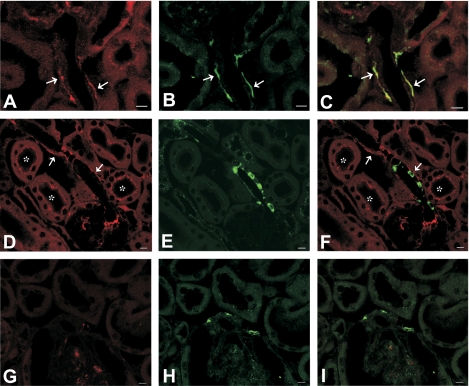
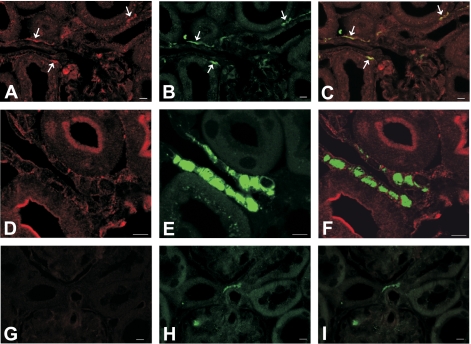
Immunoreactivity of IA-2 was observed along afferent arterioles (Fig. 8A). Arteriolar IA-2 did not colocalize with renin (Fig. 8F) but overlapped with anti-tyrosine hydroxylase (Fig. 8C), suggesting that it was expressed in sympathetic nerve terminals. In addition, IA-2 positivity in a punctate pattern was observed in proximal tubules (Fig. 8D). Expression of IA-2β was similar in that it also localized to afferent arterioles without overlapping with renin positivity in renin granules (Fig. 9, D–F). Instead, double labeling revealed colocalization with tyrosine hydroxylase (Fig. 9, A–C). In addition, IA-2β immunoreactivity was found in the apical plasma membrane of several nephron segments, including in the connecting and collecting tubule (Fig. 9D). Antibody specificity was verified by the absence of consistent staining in sections from IA-2- and IA-2β-deficient mice (Figs. 8G and 9G). The immunohistochemical evidence is consistent with the absence of IA-2 and IA-2β mRNA in isolated JG cells as assessed by real-time RT-PCR. In four separate JG cell preparations, Ct values for IA-2 and IA-2β were either undetermined (n = 2 for each target) or not different from water (n = 2; 35.2 cycles for water, 35.4 cycles for IA-2, and 35.9 cycles for IA-2β).
Fig. 8.
Localization of IA-2, tyrosine hydroxylase, and renin in the kidney (calibration bars = 10 μm). A–C: double labeling of IA-2 (red, A) and tyrosine hydroxylase (green, B) in a WT mouse; the merged image (C) suggests colocalization of both signals in afferent arteriolar walls, presumably sympathetic terminals (arrows). D–F: double labeling with IA-2 (red, D) and renin (green, E) in a WT mouse; merged image (F) indicates lack of colocalization (arrows). IA-2 labeling is also seen in proximal tubules in a punctate pattern, probably associated with endocytotic vesicles (asterisks). G and H: double labeling of IA-2 (G) and tyrosine hydroxylase (H) in an IA-2−/− mouse; no consistent labeling of IA-2 was seen (I: merged image).
Fig. 9.
Localization of IA-2β, tyrosine hydroxylase, and renin in the kidney (calibration bars = 10 μm). A–C: double labeling of IA-2β (red, A) and tyrosine hydroxylase (green, B) in a WT mouse; the merged image (C) suggests colocalization of both signals in afferent arteriolar walls, presumably sympathetic terminals (arrows). D–F: double labeling with IA-2β (red, D) and renin (green, E) in a WT mouse; merged image (F) indicates lack of colocalization. IA-2 labeling is also seen in the apical membranes of tubules. G and H: double labeling of IA-2β (G) and tyrosine hydroxylase (H) in an IA-2β−/− mouse; no labeling of IA-2β was seen (I: merged image).
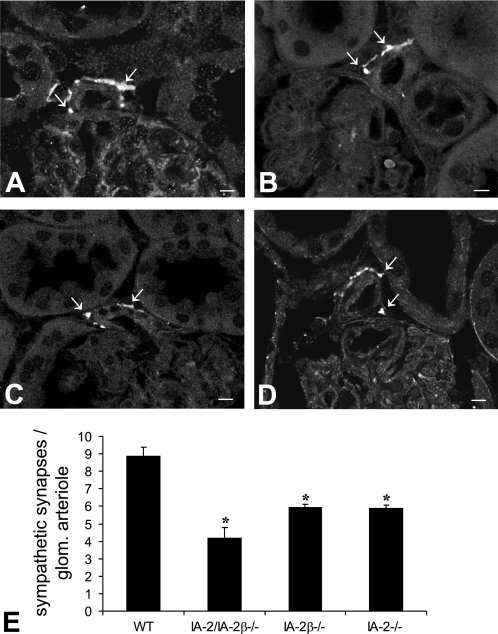
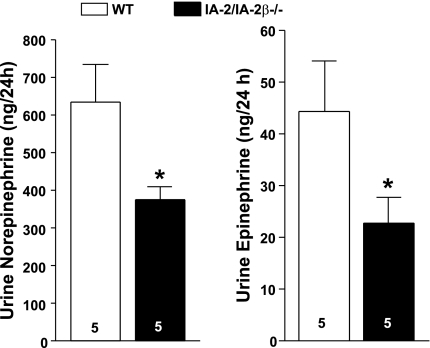
Quantification of tyrosine hydroxylase positivity (Fig. 10) revealed that the density of sympathetic synapses on glomerular arterioles was diminished in IA-2/IA-2β−/− (4.15 ± 0.64), IA-2β−/− (5.9 ± 0.2), and IA-2−/− mice (5.9 ± 0.56) compared with WT animals (8.9 ± 0.5; P < 0.05). Real time PCR assessment of tyrosine hydroxylase mRNA expression showed a significant reduction in kidneys of IA-2/IA-2β−/− mice, confirming that the density of sympathetic synapses is affected by IA-2 and IA-2β (WT: 100.6% ± 6.6, n = 4; IA-2/IA-2β−/−: 26% ± 5.2, n = 4; P < 0.002). Measurements of urinary excretion of catecholamines showed reduced levels of both norepinephrine and epinephrine in IA-2/IA-2β−/− compared with WT mice (Fig. 11).
Fig. 10.
Immunofluorescent labeling with antibodies against tyrosine hydroxylase. Staining shows sympathetic synapses near afferent arterioles of WT (A), IA-2/IA-2β−/− (B), IA-2β−/− (C), and IA-2−/− mice (D). Numerical evaluation of tyrosine hydroxylase expressed as immunopositive signals per 150 arterioles is shown in E.
Fig. 11.
Excretion of norepinephrine (left) and epinephrine (right) in the 24-h urine of WT (open bars) and IA-2/IA-2β−/− mice (filled bars). *P < 0.05 compared with WT.
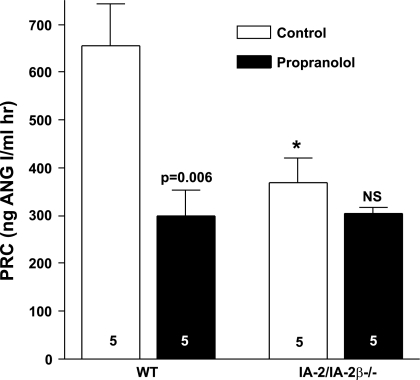
To assess whether the reduced renin secretion in IA-2-deficient mice may be related to diminished tonic β-adrenergic stimulation, we determined the acute effect of the β-adrenergic blocker propranolol on PRC to test whether the renin-lowering effect of β-adrenergic blockade would be diminished in mutant compared with WT mice. As shown in Fig. 12, the effect of propranolol (10 mg/kg) on PRC was significantly lower in IA-2/IA-2β−/− than WT (P = 0.04) mice. Whereas acute β-adrenergic blockade caused a significant reduction of PRC in WT mice (P = 0.039), it caused no change in the IA-2/IA-2β−/− animals (n = 5 in each strain).
Fig. 12.
Effect of an acute administration of the β-adrenergic receptor antagonist propranolol (10 mg/kg ip) on PRC in WT and IA-2/IA-2β−/− mice. Numbers in bars indicate number of experiments. Significance level is shown for comparison with control. *P < 0.05 compared with WT.
DISCUSSION
The purpose of the present study was to investigate the possible role of the dense-core vesicle proteins IA-2 and IA-2β in renin synthesis and secretion. We found significant reductions of plasma renin in mice with null mutations in one or both of these proteins. These results indicate that the maintenance of normal rates of basal renin secretion requires the presence of IA-2 proteins. IA-2/IA-2β deficiency did not abolish the acute upregulation of renin release by furosemide nor its chronic downregulation by a high-NaCl diet. Like in other states of reduced basal renin expression and release, however, the absolute magnitude of the change caused by acute or chronic stimulation was diminished, consistent with a nonspecific effect of basal expression levels on the releasable renin pool (6, 7).
The mechanism of this novel effect of IA-2 proteins on renin release and expression is not clear. Given the documented presence of IA-2 in dense-core vesicles and its effect on secretion of other proteins (2, 5, 8, 17), an obvious possibility is a direct interaction with JG cells, resulting in stimulation of renin secretion. A prerequisite of such an interaction would be the expression of IA-2 and/or IA-2β in JG cells. Consistent with the failure of previous attempts to identify IA-2 mRNA in the kidney, total renal mRNA expression of IA-2 and IA-2β is rather low (11, 18). Immunocytochemical double staining with antibodies against renin and IA-2β or IA-2 did not reveal coexpression but showed the presence of IA-2 and IA-2β along afferent arterioles and in proximal tubules, a result consistent with failure to detect IA-2 or IA-2β mRNA in isolated JG cells.
Thus indirect mechanisms need to be considered for the reduced plasma renin levels in IA-2-deficient mice. Measurements of arterial blood pressure by radiotelemetry did not yield a useful clue since there was no increase in blood pressure during either day or night in any of the IA-2/IA-2β mutant mice that could explain the decrease in PRC. In fact, IA-2β null mutants had lower blood pressures during both day and night, an effect that may have blunted the full extent of the inhibitory action of IA-2β deficiency. The accidental finding of reduced blood pressure in IA-2β mice has not been further explored at this time, but it is an interesting aspect that merits further investigation. In view of these results, it seems highly unlikely that renal baroreceptor activation, either directly or indirectly through inhibition of sympathetic nervous output, was responsible for the marked reduction of renin release. The volume status of the mutant mice is not known, but the unchanged GFR and unchanged urine osmolarity do not support the possibility that extracellular volume expansion could be responsible for the fall in PRC. In addition, the increased hematocrit in IA-2/IA-2β knockout mice and the elevated plasma protein concentration would be more consistent with volume contraction rather than expansion. One could also argue that the levels of plasma aldosterone in IA-2-deficient mice are inappropriately high for the reduced levels of plasma renin. The reasons for the dissociation between the changes in renin and aldosterone have not been studied in the present experiments. COX-2 activity in the macula densa and PGE2 production have been shown previously to be critical for the regulation of renin release, specifically through the macula densa mechanism (14, 21). However, since COX-2 expression appears to be elevated in IA-2-deficient mice (Fig. 4), the decrease in renin was apparently COX-2 independent. ANG II has been shown to exert an inhibitory effect on COX-2 expression so that the increased COX-2 level is probably a reflection of a primary decrease in renin and angiotensin levels and reduced inhibition of COX-2 expression by angiotensin (1).
The widespread presence of IA-2 and IA-2β in neuronal cells suggests that it could play a role in neurotransmitter release (9, 18). In this regard, the presence of IA-2 immunoreactivity has been shown in autonomic nerves of the pancreas and gastric mucosa and in presynaptic varicosities of hypothalamic neurons in primary culture (19). Furthermore, recent work has shown expression of IA-2 and IA-2β in synaptic vesicles of neuronal cells as well as reduced levels of catecholamines in brain tissue, suggesting that IA-2 and IA-2β expression is not restricted to dense-core vesicles (Nishimura T, Kubosaki A, Ito Y, Notkins AL, unpublished observations). It is conceivable therefore that the effect of IA-2 deficiency on renin secretion is a reflection of reduced release of catecholamines from sympathetic nerve terminals. In fact, the absence of a significant reduction of renin release in response to acute β-adrenergic inhibition with propranolol in IA-2/IA-2β−/− mice indicates that the normal tonic stimulation of renin by sympathetic input is diminished in the IA-2-deficient mice. The requirement of tonic β-adrenergic input for the maintenance of normal levels of renin mRNA and plasma renin has recently been documented in β1/β2-adrenergic receptor knockout mice in which PRC and renin mRNA were greatly reduced (6). Furthermore, like β-receptor deficiency, the absence of IA-2 proteins affects basal renin expression and release levels, but not the ability of the renin system to respond to regulatory challenges (6). Insufficient production of catecholamines in IA-2/IA-2β−/− mice is suggested by the reduced levels of renal tyrosine hydroxylase, by the immunocytochemical evidence for reduced sympathetic terminals in glomerular arterioles, and by the reduced levels of urinary catecholamine excretion. The ability of IA-2 to induce structural adaptations has been documented previously in insulin-secreting MIN-6 cells where increases and decreases in IA-2 expression caused parallel changes in the number of insulin-containing vesicles (5). Furthermore, the regeneration of β-cells following partial pancreatectomy was found to be impaired in IA-2-deficient mice, suggesting that IA-2 may play a role in β-cell replication (13).
In conclusion, the present results indicate that renin secretion and renin mRNA levels are markedly reduced in mice that lack functional IA-2 and/or IA-2β genes. This effect appears to be indirect, probably reflecting reduced tonic stimulation of renin secretion by impaired sympathetic transmitter release. In addition to their role in dense-core secretory vesicles, IA-2 and IA-2β may affect the release of neurotransmitters from presynaptic vesicles of autonomic nerve terminals.
GRANTS
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and National Institute of Dental and Craniofacial Research (NIDCR), and by the Deutsche Forschungsgemeinschaft through Forschergruppe 667.
Acknowledgments
We thank Kerstin Riskowsky for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Cheng HF, Wang JL, Zhang MZ, Miyazaki Y, Ichikawa I, McKanna JA, Harris RC. Angiotensin II attenuates renal cortical cyclooxygenase-2 expression. J Clin Invest 103: 953–961, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doi A, Shono T, Nishi M, Furuta H, Sasaki H, Nanjo K. IA-2beta, but not IA-2, is induced by ghrelin and inhibits glucose-stimulated insulin secretion. Proc Natl Acad Sci USA 103: 885–890, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faulhaber-Walter R, Chen L, Oppermann M, Kim SM, Huang Y, Hiramatsu N, Mizel D, Kajiyama H, Zerfas P, Briggs JP, Kopp JB, Schnermann J. Lack of A1 adenosine receptors augments diabetic hyperfiltration and glomerular injury. J Am Soc Nephrol 19: 722–730, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev 70: 1067–1116, 1990. [DOI] [PubMed] [Google Scholar]
- 5.Harashima S, Clark A, Christie MR, Notkins AL. The dense core transmembrane vesicle protein IA-2 is a regulator of vesicle number and insulin secretion. Proc Natl Acad Sci USA 102: 8704–8709, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SM, Chen L, Faulhaber-Walter R, Oppermann M, Huang Y, Mizel D, Briggs JP, Schnermann J. Regulation of renin secretion and expression in mice deficient in beta1- and beta2-adrenergic receptors. Hypertension 50: 103–109, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Kim SM, Chen L, Mizel D, Huang YG, Briggs JP, Schnermann J. Low plasma renin and reduced renin secretory responses to acute stimuli in conscious COX-2-deficient mice. Am J Physiol Renal Physiol 292: F415–F422, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Kubosaki A, Gross S, Miura J, Saeki K, Zhu M, Nakamura S, Hendriks W, Notkins AL. Targeted disruption of the IA-2beta gene causes glucose intolerance and impairs insulin secretion but does not prevent the development of diabetes in NOD mice. Diabetes 53: 1684–1691, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Kubosaki A, Nakamura S, Clark A, Morris JF, Notkins AL. Disruption of the transmembrane dense core vesicle proteins IA-2 and IA-2beta causes female infertility. Endocrinology 147: 811–815, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Kubosaki A, Nakamura S, Notkins AL. Dense core vesicle proteins IA-2 and IA-2beta: metabolic alterations in double knockout mice. Diabetes 54, Suppl 2: S46–S51, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Lan MS, Lu J, Goto Y, Notkins AL. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol 13: 505–514, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Lu J, Li Q, Xie H, Chen ZJ, Borovitskaya AE, Maclaren NK, Notkins AL, Lan MS. Identification of a second transmembrane protein tyrosine phosphatase, IA-2β as an autoantigen in insulin-dependent diabetes mellitus: precursor of the 37-kDa tryptic fragment. Proc Natl Acad Sci USA 93: 2307–2311, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mziaut H, Kersting S, Knoch KP, Fan WH, Trajkovski M, Erdmann K, Bergert H, Ehehalt F, Saeger HD, Solimena M. ICA512 signaling enhances pancreatic beta-cell proliferation by regulating cyclins D through STATs. Proc Natl Acad Sci USA 105: 674–679, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peti-Peterdi J, Komlosi P, Fuson AL, Guan Y, Schneider A, Qi Z, Redha R, Rosivall L, Breyer MD, Bell PD. Luminal NaCl delivery regulates basolateral PGE2 release from macula densa cells. J Clin Invest 112: 76–82, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286: F590–F596, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Rabin DU, Pleasic SM, Shapiro JA, Yoo-Warren H, Oles J, Hicks JM, Goldstein DE, Rae PM. Islet cell antigen 512 is a diabetes-specific islet autoantigen related to protein tyrosine phosphatases. J Immunol 152: 3183–3188, 1994. [PubMed] [Google Scholar]
- 17.Saeki K, Zhu M, Kubosaki A, Xie J, Lan MS, Notkins AL. Targeted disruption of the protein tyrosine phosphatase-like molecule IA-2 results in alterations in glucose tolerance tests and insulin secretion. Diabetes 51: 1842–1850, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu S, Saito N, Kubosaki A, SungWook S, Takeyama N, Sakamoto T, Matsumoto Y, Saeki K, Matsumoto Y, Onodera T. Developmental expression and localization of IA-2 mRNA in mouse neuroendocrine tissues. Biochem Biophys Res Commun 288: 165–171, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Solimena M, Dirkx R Jr, Hermel JM, Pleasic-Williams S, Shapiro JA, Caron L, and Rabin DU. ICA 512, an autoantigen of type I diabetes, is an intrinsic membrane protein of neurosecretory granules. EMBO J 15: 2102–2114, 1996. [PMC free article] [PubMed] [Google Scholar]
- 20.Theilig F, Campean V, Paliege A, Breyer M, Briggs JP, Schnermann J, Bachmann S. Epithelial COX-2 expression is not regulated by nitric oxide in rodent renal cortex. Hypertension 39: 848–853, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Traynor TR, Smart A, Briggs JP, Schnermann J. Inhibition of macula densa-stimulated renin secretion by pharmacological blockade of cyclooxygenase-2. Am J Physiol Renal Physiol 277: F706–F710, 1999. [DOI] [PubMed] [Google Scholar]