Abstract
A dual childbirth injury model, including vaginal distension (VD) and pudendal nerve crush (PNC), may best represent the injuries seen clinically. The objective of this study was to investigate urethral function, anatomy, and neurotrophin expression after several simulated childbirth injuries. Groups of 140 rats underwent PNC, VD, PNC+VD, or neither (C). Four days after injury, all injury groups had significantly decreased leak-point pressure (LPP) compared with C rats. Ten days after injury, LPP in PNC and PNC+VD rats remained significantly lower than C rats. Three weeks after injury, LPP in all injury groups had recovered to C values. Histological evidence of injury was still evident in the external urethral sphincter (EUS) after VD and PNC+VD 10 days after injury. Three weeks after injury, the EUS of PNC+VD rats remained disrupted. One day after VD, brain-derived neurotrophic factor (BDNF) expression in the EUS was reduced, while neurotrophin-4 (NT-4) and nerve growth factor (NGF) expression was unchanged. BDNF, NT-4, and NGF expression was dramatically upregulated in the EUS after PNC. After PNC+VD, NGF expression was upregulated, and BDNF and NT-4 expression was upregulated somewhat but not to the same extent as after PNC. Ten days after injury, PNC+VD had the least number of normal nerve fascicles near the EUS, followed by PNC and VD. Twenty-one days after injury, all injury groups had fewer normal nerve fascicles, but without significant differences compared with C rats. PNC+VD therefore provides a more severe injury than PNC or VD alone.
Keywords: stress urinary incontinence, vaginal childbirth, pudendal nerve crush, anatomic recovery, rat
during vaginal delivery, the muscles, nerves, ligaments, and other organs of the pelvic floor can be compressed and injured, especially with a prolonged second stage of labor (15, 31, 33). Specifically, the external urethral sphincter (EUS), the striated muscle of the urethra, can be injured during delivery (15). In addition, the pudendal nerve, which courses through Alcock's canal and innervates the EUS, can be trapped and injured during vaginal childbirth. Thus the injuries incurred during vaginal childbirth represent a unique neuromuscular injury: both the target organ (the EUS) and its innervation can be injured simultaneously. These two injuries, along with injuries to other tissues of the pelvic floor, are strongly correlated with later development of stress urinary incontinence (SUI), the involuntary leakage of urine during moments of increased abdominal pressure, such as when laughing, coughing, sneezing, or lifting (1, 20, 35, 36).
Vaginal distension (VD) and pudendal nerve crush (PNC) in rats are two relevant and well-established animal models of SUI of simulated childbirth (10, 17, 22, 26). Numerous studies have demonstrated that these injuries in rats can represent the clinical injuries of vaginal childbirth in women since both lead to decreased urethral closure ability, decreased leak point pressure (LPP), and symptoms of SUI (6, 10, 22, 26, 31). Our recent study demonstrated that functional recovery occurs more slowly after PNC+VD than after either PNC or VD alone (18).
Neurotrophins, or neurotrophic factors, are polypeptides that control the generation, survival, differentiation, and regeneration of neurons in the peripheral and central nervous systems (3, 34). Neurotrophins promote survival of motor neurons in culture and in vivo after nerve transection (16, 21). Brain-derived neurotrophic factor (BDNF) and neurotrophin-4 (NT-4) are upregulated in the target muscle after nerve injury (25). In contrast, the same neurotrophic factors are downregulated after muscle injury to enable neuromuscular junction repair (30). Therefore, the neurotrophic response to an injury such as childbirth, in which both the nerve and its target muscle are injured, is unknown.
The objective of this study was to investigate changes in urethral function, urethral and nerve anatomy, and the expression of neurotrophins after a new dual simulated maternal childbirth injury and compare these with the same outcomes after single childbirth injury models.
MATERIALS AND METHODS
This study was approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic (Cleveland, OH). To decrease possible variations in anatomy and function due to prior deliveries (19), 140 virgin female Sprague-Dawley rats (180- to 200-g weight) were randomized into 4 groups: uninjured control animals (C), PNC, VD, and PNC+VD. Each group was further divided into four subgroups: one subgroup was used to investigate neurotrophin expression 1 day after injury; the other three subgroups were used to investigate urethral function by LPP testing 4, 10, and 21 days after injury. Tissues were harvested after LPP testing for histological assessment of the urethra and pudendal nerve.
PNC.
Bilateral PNC was performed as previously done (19). Rats were anesthetized with a mixture of intraperitoneal (ip) ketamine (100 mg/kg) and xylazine (10 mg/kg). A midline posterior longitudinal skin incision was made, and the ischiorectal fossa was opened bilaterally with retractors. The pudendal nerve was identified, and all branches were crushed twice bilaterally with a Castroviejo needle holder for 30 s. The animals were given buprenorphrine (0.3 mg/kg) subcutaneously for pain control.
VD.
Rats were anesthetized as above and underwent VD as previously described (26). To avoid rupturing the vagina, it was first accommodated to larger capacities by subsequently inserting and removing increasing sizes (24–32 Fr) of Otis Bougie a Boule urethral dilators (Allegiance Health Care, McGaw Park, IL) lubricated with Surgilube (E. Fougera, Melville, NY). A modified 10-Fr Foley catheter (Lifetech, Stafford, TX) was then inserted into the vagina, and the balloon was slowly distended with water to 3 ml for 4 h. The vagina was stitched closed to hold the balloon in place. Buprenorphrine was given as above for pain control.
PNC and VD.
One group of animals underwent PNC followed by VD as described previously (18). The methods of this procedure were the same as above. Age-matched rats served as uninjured controls (C).
Catheter implantation.
Two days before LPP testing, all rats underwent suprapubic bladder catheter implantation as previously described (26). The rats were anesthetized as described above, and a circular purse-string suture was placed on the bladder wall. A small incision was made in the bladder wall, and the catheter (PE-50 tubing with a flared tip) was implanted in the bladder dome. The catheter was then tunneled subcutaneously to the back of neck, where it exited the skin. The catheter was capped, and buprenorphrine was administrated for pain control as above.
LPP.
The animals were anesthetized with urethane (1.2 g/kg body wt ip) and placed supine at the zero-pressure level for LPP testing as previously described (26). Urethane was used because voiding reflexes are maintained best under this anesthetic (5). The bladder catheter was connected via a stopcock to a syringe flow pump (model 200, KD Scientific, New Hope, PA) and pressure transducer (model P300, Grass Instruments, West Warwick, RI). Pressure signals were amplified (model P122, Grass Instruments), recorded on a chart recorder, and digitized for computer data collection (10 samples/s). The bladder was palpated to empty and then filled with saline at 5 ml/h. When 0.3 ml was attained (approximately one-half the capacity for a 200-g rat), gentle pressure was applied to the rat's abdomen to simulate a Credé maneuver, while bladder pressure was recorded and digitized. Pressure was slowly increased until the rat leaked saline through the urethra. At the first indication of leakage at the urethral meatus, the externally applied abdominal pressure was rapidly removed. Peak pressure at leakage in the absence of a detrusor contraction was calculated. LPP was calculated by subtracting bladder baseline pressure from peak bladder pressure. LPP provides a consistent measure of urethral resistance and is not sensitive to volume in the bladder (5, 9). In general, this external application of gentle pressure does not generate a voiding reflex (6, 10, 26). If a voiding reflex occurred, the bladder was emptied and refilled and that test was not included in the results. The study was repeated three times in each rat, and a mean LPP value was calculated for each animal.
Histology and immunohistochemistry.
Intracardiac perfusion fixation was done with 10% formalin. This procedure was performed in 20 rats 1 day after injury to investigate expression of neurotrophins in the EUS by histology and immunohistochemistry (IHC). A 5-mm segment of the urethra, attached to the anterior portion of the vagina (∼5 mm from the urethral orifice), was dissected en bloc. Each piece was then immersed and fixed in 10% formalin, embedded in paraffin, and sectioned transversely (5 μm) for histological and IHC analyses. One section from each specimen was stained with Masson's trichrome for localization of the EUS. Neurotrophins studied using IHC included BDNF (1:150, N-20, Santa Cruz Biotechnology, Santa Cruz, CA), NT-4 (1:50, N-20, Santa Cruz Biotechnology), and nerve growth factor (NGF; 1:50, M-20, Santa Cruz Biotechnology). Tyrosine-specific protein kinase B (1:50, H-181, Santa Cruz Biotechnology), the receptor for both BDNF and NT-4, was tested as well. The sections were incubated with primary antibodies for 32 min on a Ventana IHC slide staining system (Tucson, AZ). Slides were visualized with a diaminobenzidine tetrahydrochloride (DAB) detection kit (Ventana). Sections were subsequently rinsed well with H2O2 for 30 min at room temperature and counterstained with hematoxylin. After extensive rinsing, sections were dehydrated through graded alcohol and xylene. The sections were then mounted in a glycerol-based medium containing p-phenylenediamine. A negative control, created by omission of the primary antibodies, and a positive control using rat brain tissue were both included in the immunostaining procedures. Results were qualitatively compared in a blinded fashion.
A subgroup of rats in each injury group (n = 60) underwent intracardiac perfusion fixation 4, 10, and 21 days after injury as described above. The urethra and vagina were harvested en bloc, prepared for histology, and stained with Masson's trichrome as above. They were analyzed qualitatively in a blinded fashion.
Neuromorphometry.
Four to five animals from each group, 10 and 21 days after injury, were euthanized by perfusion fixation with phosphate-buffered saline (100 ml) mixed with heparin (0.2 ml), followed by cold fixative (400 ml of 2.5% glutaraldehyde and 0.5% paraformaldehyde in 0.1 M cacodylate buffer) for morphometric assessment of nerve recovery from injury, as we have done previously (19). The urethra and anterior vagina (en bloc 5 mm from the urethral orifice) and pudendal nerve (3 mm distal to the crush site) were dissected, postfixed, osmicated in 2% osmium tetroxide, embedded in epon-araldite, sectioned transversely (1 μm), and stained with toluidine blue.
Pudendal nerve morphometry was qualitatively studied in a blinded fashion. Nerve fascicles within 25 μm of the EUS were classified as either normal or abnormal (degenerated). Abnormal nerve fascicles were defined as having one or more of the following characteristics: waviness of axons in the fibers, evidence of myelin breakdown debris, and increased endoneurial cellularity. The total number of nerve fascicles, total number of normal nerve fascicles, and total number of abnormal nerve fascicles were counted and compared.
Statistical analysis.
One-way ANOVA was used to compare response means of LPP or nerve fascicles near the EUS across the C, PNC, VD, and PNC+VD groups. Pairwise mean comparisons were made using the t-test. Bonferroni (for LPP) and Tukey-Kramer (for nerve fascicles) corrections were applied to these post hoc tests, with P < 0.05 indicating a statistically significant difference.
RESULTS
LPP testing.
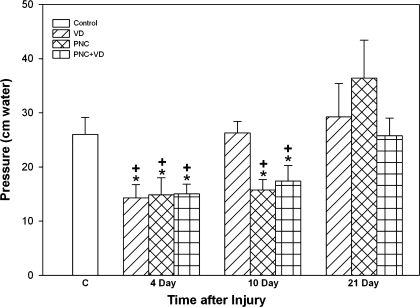
There were no significant differences in baseline pressures among groups. In addition, there were no significant changes in LPP in the C group over time. Therefore, this group was combined into a single C group (Fig. 1). Four days after injury, all three injury groups had significantly reduced LPP compared with the C group (Fig. 1). There were no significant differences in LPP among the injury groups at that time point. Ten days after injury, VD rats had recovered to C values, but LPP of PNC and PNC+VD rats remained significantly decreased. Three weeks after injury, all injury groups had recovered to C values.
Fig. 1.
Leak point pressure (LPP) 4, 10, and 21 days after vaginal distension (VD), pudendal nerve crush (PNC), and pudendal nerve crush and vaginal distension (PNC+VD). C, control group. Values are means ± SE of each experimental group at each time point. +Significantly different compared with control group. *Significantly different compared with similar injury group tested 21 days after injury (P < 0.05).
Histology.
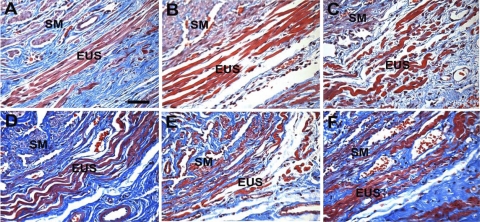
Histological evidence of injury was evident in the EUS of the VD and PNC+VD groups 1 day after injury (Fig. 2). Histology in rats 1 day after PNC was similar to that of the C group and are not shown. Four and 10 days after injury, all injury groups demonstrated striated muscle fiber disruption and atrophy in the EUS (Fig. 2). Twenty-one days after injury, the EUS had regenerated in the VD and PNC groups but remained disrupted in the PNC+VD group (Fig. 2). All injury groups demonstrated evidence of connective tissue infiltration in the EUS.
Fig. 2.
Urethral histology 1, 4, 10, and 21 days after injury. A: control urethra shows tightly packed external urethral sphincter (EUS) muscle fibers. B: 1 day after vaginal distension (VD) shows edema in the EUS. C: 1 day after pudendal nerve crush and VD (PNC+VD), disruption of EUS muscle fibers is evident. D: 10 days after PNC, EUS muscle fibers were wavy and disrupted, indicating denervation injury. Dark blue indicates fibrosis near the EUS. E: 10 days after VD, the EUS showed edema, fiber disruption, and mild waviness. F: 21 days after PNC+VD, the EUS fibers remained disrupted and wavy with fibrosis, indicative of incomplete recovery. SM, smooth muscle. Stain: Masson's trichrome. Bar = 100 μm.
Neurotrophin expression.
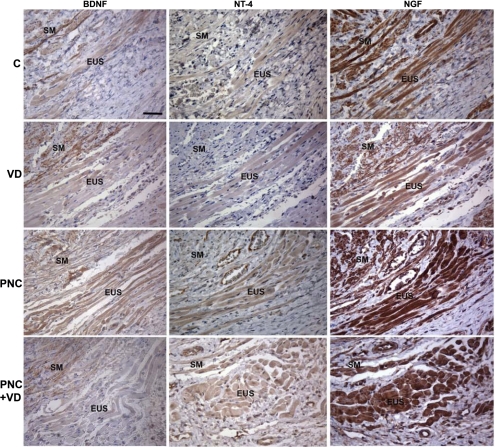
Immunohistochemistry showed low expression of BDNF, NT-4, and tyrosine-specific protein kinase B and relatively higher expression of NGF in the EUS of C rats (Fig. 3). One day after VD, expression of BDNF in the EUS was reduced compared with C rats, while NT-4 expression was low but not different from C animals. NGF expression remained unchanged compared with C rats after VD. BDNF, NT-4, and NGF expression was dramatically upregulated in the EUS of PNC rats (Fig. 3). After PNC+VD, BDNF and NT-4 were upregulated somewhat in the EUS compared with C animals but not to the same extent as after PNC. NGF was upregulated after PNC+VD compared with C animals (Fig. 3). Tyrosine-specific protein kinase B expression was slightly positive in the EUS of all four groups, and there was no discernable difference among groups (data not shown).
Fig. 3.
Neurotrophin expression 1 day after injury. BDNF, brain-derived neurotrophic factor; NGF, nerve growth factor; NT-4, neurotrophin-4; C, control. Section = 5 μm. The antibodies are visualized with diaminobenzidine tetrahydrochloride (DAB) and show as a brown color, indicating expression of neurotrophin. Sections were counterstained with hematoxylin (blue). Bar = 100 μm.
Neuromorphometry.
As noted previously, the sensory branch of the pudendal nerve is the largest branch (19) (Fig. 4A). The motor branch bifurcates into the external anal sphincter (EAS) and EUS branches, the latter of which also forms multiple branches. Ten days after injury, these branches showed signs of Wallerian degeneration, identified by clumps of osmophilic ovoids (Fig. 4B). Damage to the vascular component was also observed.
Fig. 4.
Pudendal nerve in the ischiorectal fossa 3 mm distal from the crush site 10 days after injury (A and B) and the motor branch of the pudendal nerve 3 mm distal to the nerve crush site 21 days after injury [control (C), VD (D), PNC (E), and PNC+VD (F)]. A, artery; S, sensory branch; V, vein; EAS, external anal sphincter branch; EUS, EUS branch; WD, Wallerian degeneration identified by clumps of osmophilic ovoids. Stain: lightly osmicated (A and B), toluidine blue (C–F). Bar = 1.5 mm (A), 0.5 mm (B), 10 μm (C–F).
The motor branch of the pudendal nerve in the C group had a normal appearance with respect to size and number of myelinated axons (Fig. 4C). Twenty-one days after injury, there were many nonmyelinated axons in the endoneurium of all injury groups. The pudendal nerve appeared less normal after VD compared with the C group, as indicated by fewer myelinated axons (Fig. 4D). Nerves from rats that received PNC had an increase in endoneurial nuclei (mostly Schwann cells) and myelin figures, further evidence of Wallerian degeneration. Regenerated axons in PNC animals were smaller and had thinner myelin sheaths than axons in normal animals (Fig. 4E). Animals that underwent PNC+VD showed a worsened appearance, with more extensive Wallerian degeneration and only moderate regeneration compared with PNC or VD alone. Myelinated axons in PNC+VD animals were fewer and smaller, while abnormal myelin segments and endoneurial nuclei were more evident (Fig. 4F).
Nerve fascicles near the EUS.
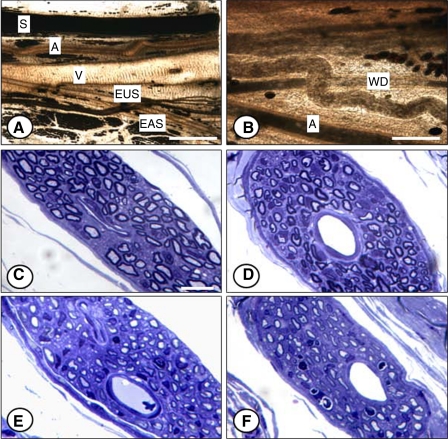
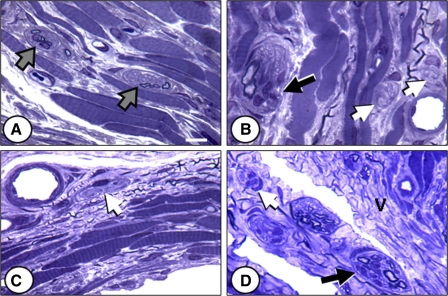
Although normal-appearing nerve fascicles in the region of the EUS 10 days after injury can be seen in all groups, they are an exclusive feature of the C group (Fig. 5A). In the C group, a few myelinated axons and many nonmyelinated axons, with their associated Schwann cells, are bound by a perineurial layer. Animals from all injury groups were found to have more degenerated nerve fascicles than the C group, typically having swollen, pale profiles with a wavy appearance or an increase in the number of endoneurial nuclei (Schwann cells, fibroblasts, and macrophages) (Fig. 5, B–D). Muscle fibers of the injury groups were also damaged; the fibers appeared smaller and more contorted than those of C animals (Fig. 5B). In the PNC+VD group, cells with cytoplasmic vacuoles were observed, possibly macrophages or fibroblasts (Fig. 5D).
Fig. 5.
Nerve fascicles near the EUS 21 days after injury. A: control. B: VD. C: PNC. D: PNC+VD. Gray arrows, normal-appearing fascicles; white arrows, nonmyelinated axons; black arrows, degenerated fascicles, defined as having waviness of axons, evidence of myelin breakdown debris, and/or increased endoneurial cellularity. V, cytoplasmic vacuoles. Stain: toluidine blue. Bar = 10 μm.
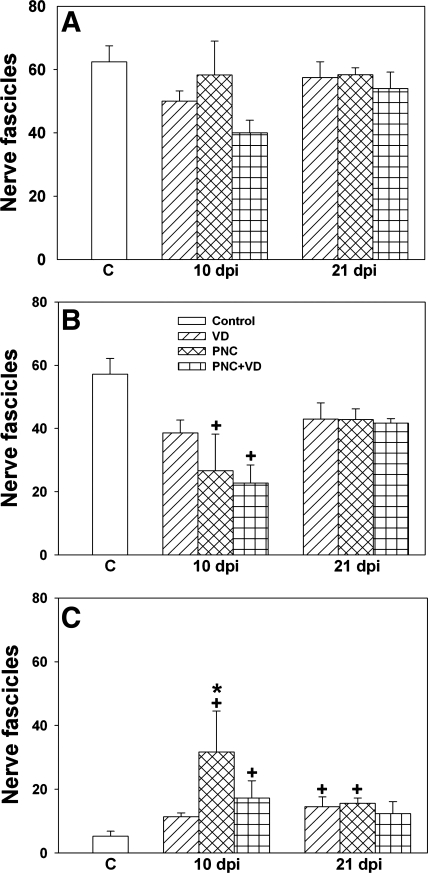
The total number of nerve fascicles in the urethra, near the EUS, showed no significant difference among groups 10 or 21 days after injury (Fig. 6A). However, the number of normal nerve fascicles in the same region 10 days after PNC or PNC+VD was significantly decreased compared with the C group (Fig. 6B). All injury groups 21 days after injury demonstrated fewer normal nerve fascicles, but none of these differences were significantly different from the C group. Moreover, the number of abnormal nerve fascicles in the same region 10 and 21 days after PNC was significantly increased compared with the C group (Fig. 6C).
Fig. 6.
Quantification of nerve fascicles near the EUS after injury, including total number of nerve fascicles (A), number of normal nerve fascicles (B), and number of abnormal nerve fascicles (C). C, control; dpi, days postinjury. Values are means ± SE of each experimental group at each time point. +Significantly different compared with control group (P < 0.05). *Significantly different compared with VD group (P < 0.05).
DISCUSSION
SUI is the most common form of urinary incontinence, accounting for 50% of cases (8). SUI is manifested by “the complaint of involuntary leakage on effort or exertion, or on sneezing or coughing” (1). One clinical study demonstrated that the prevalence of SUI increased from 4.7% in nulliparous women to 12.2% in women who had undergone vaginal delivery (29). Another study showed an increase in the prevalence of moderate to severe SUI after vaginal delivery from 2 to 12% 10 yr after delivery (2). Animal models have demonstrated denervation, hypoxia, and atrophy of the EUS after simulated childbirth injuries (10, 11, 19, 22). A bilateral crush injury to the pudendal nerve decreases urethral closure ability and LPP, resulting in symptoms of SUI, which correlates with clinical data (12, 28, 29, 31, 37). We have further demonstrated in a rat VD model that a longer duration of distension induces a greater severity of SUI and anatomic disruption (26). Both clinical and basic science results suggest a complex etiology in the development of SUI in which repetitive trauma, insufficient regeneration, and biochemical changes with age contribute to urethral hypermobility and/or intrinsic sphincter deficiency.
SUI is considered a deficiency of the urethra to maintain closure caused by weakness in urethral or pelvic floor support. The balance between urethral and bladder pressures is influenced by both intrinsic factors (urethral musculature, blood flow, and innervation) and extrinsic factors (degree of urethral support and the weight and physical activity of the patient) (7). The EUS serves as the striated muscular portion of the urethral closure mechanism (13, 32). LPP reflects urethral function and has been shown to be reproducible when performed in a standardized fashion in both people and animals (4, 24, 26). This study demonstrates that either VD or PNC or both will create temporary urethral dysfunction in rats and produces symptoms similar to SUI.
In this study, LPP was significantly decreased in all three injury groups 4 days after injury, indicative of short-term decreased urethral resistance and urethral dysfunction. Ten days after injury, LPP was significantly decreased only in the PNC and PNC+VD groups, indicating that these injuries need longer time to recover than VD. Twenty-one days after injury, LPP of the VD and PNC groups was not significantly different from the C group, indicating a return to normal function. We have previously demonstrated that LPP returns to C levels 6 wk after PNC+VD (18). This difference from our current study is possibly caused by the different surgical exposures in the two studies. In the previous study, to record EUS electromyography and pudendal nerve motor branch potential, a longer exposure time and larger exposure area surrounding the pudendal nerve and the EUS were required. The chance of injury to the PN and EUS is greater compared with the current experiment. Future experiments will be designed to elucidate the mechanism of these differences.
Vaginal childbirth is a well-known risk factor for the development of SUI (1, 8, 14). VD has been extensively used as an experimental model to serve as a simulation of the tissue damage incurred as a result of vaginal childbirth (6, 10, 17, 22, 26). Histological results in our study demonstrated muscle injuries with increased fiber disruption, atrophy and waviness in the urethra after either PNC or VD. These pathological appearances had notably recovered by 21 days in both the VD and PNC groups. In contrast, the PNC+VD group still had pathological anatomy 21 days after injury, indicating that this dual injury may cause more severe injury to the EUS than either injury alone.
Neurotrophins, including BDNF, NT-4, and NGF, serve to promote nerve regeneration and stimulate axonal growth, as well as reduce neuronal injury by exposure to excitotoxins, glucose deprivation, or ischemia (23). However, during nerve growth, axonal guidance is necessary to reach the appropriate target organs. BDNF is upregulated during the myelination of peripheral nerves, and NT-4 acts as a potent survival factor for motor neurons (16). Our results are in agreement with reports from other similar peripheral nerve injury models (21, 23, 38) and suggest that BDNF and NT-4 are important for neuroregeneration. After axotomy however, NT-4 expression in skeletal muscles is decreased (25), likely because that injury did not allow retrograde axonal transport to occur and, as a result, fewer axons survived.
BDNF and NT-4 are not upregulated in the EUS after VD, suggesting that the lack of upregulation is due to target organ (EUS) injury, which may hamper neuroregeneration after simulated childbirth injuries. After PNC+VD, BDNF and NT-4 expression is relatively upregulated compared with controls, but less upregulated compared with PNC. This suggests that pudendal nerve regeneration may be impaired after dual injury, due to the simultaneous target muscle injury.
Since the number of axons and the number of nerve fascicles in the EUS are strongly correlated with each other and because the nerve fascicles can be observed more reliably under the light microscope, we only counted the number of nerve fascicles (10, 27). We have confirmed the histological appearance of degenerated nerve fascicles 10 and 21 days after PNC, VD, and PNC+VD. Although the total number of nerve fascicles near the EUS 10 days after injury did not show a significant difference among groups, the PNC+VD group had the least nerve fascicles and the C group had the most. Both PNC+VD and PNC groups had significantly fewer normal fascicles than the C group, with PNC+VD having the fewest. This is consistent with the theory of a high rate of nonsurvival among distal nerve fascicles after PNC+VD.
The PNC group had the most abnormal nerve fascicles and significantly more than controls 10 and 21 days after injury. This may indicate that the distal nerve fascicles were not surviving 10 days after PNC+VD. In contrast, they survived but were abnormal after PNC. The occurrence of increasing severity is suggested, although the changes are more subtle than might be expected. It is of interest that the VD group has degenerated nerve fascicles near the EUS, as observed previously (26). Our results suggest that the number of normal nerve fascicles remaining near the EUS is a better structural correlate in parallel with the LPP outcomes than the number of abnormal nerve fascicles. The mechanisms of injury are elusive, but probably involve both ischemic and mechanical compression/stretch damage.
Conclusion
We have observed that simultaneous PNC+VD provides a more severe injury than PNC or VD alone, as indicated by histological assessment. However, urethral resistance recovers at a similar rate as PNC alone, and functional outcome measures following injury of varying severity parallel the structural outcomes. BDNF and NT-4 are upregulated in the EUS after PNC but not as strongly after PNC+VD, suggesting that the pudendal nerve regeneration may be impaired after a dual injury due to the simultaneous target muscle injury.
GRANTS
This material is based on work supported by the office of Research and Development, Rehabilitation Research and Development Service of the Department of Veterans Affairs and National Institute of Child Health and Human Development Grant RO1-HD-038679-08.
Acknowledgments
The authors thank Linda Vargo for immunohistochemical technical support and Dana Williams for histological sectioning.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van KP, Victor A, Wein A, Standardisation Sub-Committee of the International Continence Society. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61: 37–49, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Altman D, Ekstrom A, Gustafsson C, Lopez A, Falconer C, Zetterstrom J. Risk of urinary incontinence after childbirth: a 10-year prospective cohort study. Obstet Gynecol 108: 873–878, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Barde YA Trophic factors and neuronal survival. Neuron 2: 1525–1534, 1989. [DOI] [PubMed] [Google Scholar]
- 4.Bump RC, Elser DM, Theofrastous JP, McClish DK. Valsalva leak point pressures in women with genuine stress incontinence: reproducibility, effect of catheter caliber, and correlations with other measures of urethral resistance. Continence Program for Women Research Group. Am J Obstet Gynecol 173: 551–557, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Cannon TW, Damaser MS. Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sci 69: 1193–1202, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Cannon TW, Wojcik EM, Ferguson CL, Saraga S, Thomas C, Damaser MS. Effects of vaginal distension on urethral anatomy and function. BJU Int 90: 403–407, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Chancellor MB, Perkin H, Yoshimura N. Recent advances in the neurophysiology of stress urinary incontinence. Scan J Urol Nephrol 39: 21–24, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Contreras OO Stress urinary incontinence in the gynecological practice. Int J Gynaecol Obsett 86, Suppl 16, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Conway DA, Kamo I, Yoshimura N, Chancellor MB, Cannon TW. Comparison of leak point pressure methods in an animal model of stress urinary incontinence. Int Urogynecol J 16: 359–363, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Damaser MS, Broxton-King C, Ferguson C, Kim FJ, Kerns JM. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. J Urol 170: 1027–1031, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Damaser MS, Whitbeck C, Chichester P, Levin RM. Effect of vaginal distension on blood flow and hypoxia of urogenital organs of the female rat. J Appl Physiol 98: 1884–1890, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Dolan LM, Hosker GL, Mallett VT, Allen RE, Smith AR. Stress incontinence and pelvic floor neurophysiology 15 years after the first delivery. BJOG 110: 1107–1114, 2003. [PubMed] [Google Scholar]
- 13.Frauscher F, Helweg G, Strasser H, Enna B, Klauser A, Knapp R, Colleselli K, Bartsch G, Zur ND. Intraurethral ultrasound: diagnostic evaluation of the striated urethral sphincter in incontinent females. Eur Radiol 8: 50–53, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Fritel X, Ringa V, Varnoux N, Fauconnier A, Piault S, Breart G. Mode of delivery and severe stress incontinence. a cross-sectional study among 2,625 perimenopausal women. BJOG 112: 1646–1651, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hale DS, Benson JT, Brubaker L, Heidkamp MC, Russell B. Histologic analysis of needle biopsy of urethral sphincter from women with normal and stress incontinence with comparison of electromyographic findings. Am J Obstet Gynecol 180: 342–8, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Henderson CE, Bloch-Gallego E, Camu W, Gouin A, Lemeulle C, Mettling C. Motoneuron survival factors: biological roles and therapeutic potential. Neuromusc Disord 3: 455–458, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Hijaz A, Daneshgari F, Sievert KD, Damaser MS. Animal models of female stress urinary incontinence. J Urol 179: 2103–2110, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang HH, Pan HQ, Gustilo-Ashby AM, Gillb BC, Glaab JB, Zaszczurynski PJ, Damaser MS. Dual simulated childbirth injuries result in slowed recovery of pudendal nerve and urethral function. Neurourol Urodyn. In press. [DOI] [PMC free article] [PubMed]
- 19.Kerns JM, Damaser MS, Kane JM, Sakamoto K, Benson JT, Shott S, Brubaker L. Effects of pudendal nerve injury in the female rat. Neurourol Urodyn 19: 53–69, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Kim HL, Gerber GS, Patel RV, Hollowell CM, Bales GT. Practice patterns in the treatment of female urinary incontinence: a postal and internet survey. Urology 57: 45–48, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Koliatsos VE, Clatterbuck RE, Winslow JW, Cayouette MH, Price DL. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron 10: 359–367, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Lin AS, Carrier S, Morgan DM, Lue TF. Effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology 52: 143–151, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Lykissas MG, Batistatou AK, Charalabopoulos KA, Beris AE. The role of neurotrophins in axonal growth, guidance, and regeneration. Curr Neurovasc Res 4: 143–151, 2007. [DOI] [PubMed] [Google Scholar]
- 24.McGuire EJ, Fitzpatrick CC, Wan J, Bloom D, Sanvordenker J, Ritchey M, Gormley EA. Clinical assessment of urethral sphincter function. J Urol 150: 452–4, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Omura T, Sano M, Omura K, Hasegawa T, Doi M, Sawada T, Nagano A. Different expressions of BDNF, NT3, and NT4 in muscle and nerve after various types of peripheral nerve injuries. J Peripher Nerv Syst 10: 293–300, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Pan HQ, Kerns JM, Lin DL, Liu S, Esparza N, Damaser MS. Increased duration of simulated childbirth injuries results in increased time to recovery. Am J Physiol Regul Integr Comp Physiol 292: R1738–R1744, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandit M, DeLancey JO, Ashton-Miller JA, Iyengar J, Blaivas M, Perucchini D. Quantification of intramuscular nerves within the female striated urogenital sphincter muscle. Obstet Gynecol 95: 797–800, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Retzky SS, Rogers RM Jr. Urinary incontinence in women. Clin Symp 47: 2–32, 1995. [PubMed] [Google Scholar]
- 29.Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S, Norwegian ES. Urinary incontinence after vaginal delivery or cesarean section. N Engl J Med 348: 900–907, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Sakuma K, Watanabe K, Sano M, Uramoto I, Nakano H, Li YJ, Kaneda S, Sorimachi Y, Yoshimoto K, Yasuhara M, Totsuka T. A possible role for BDNF, NT-4 and TrkB in the spinal cord and muscle of rat subjected to mechanical overload, bupivacaine injection and axotomy. Brain Res 907: 1–19, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Snooks SJ, Setchell M, Swash M, Henry MM. Injury to innervation of pelvic floor sphincter musculature in childbirth. Lancet 2: 546–550, 1984. [DOI] [PubMed] [Google Scholar]
- 32.Strasser H, Tiefenthaler M, Steinlechner M, Bartsch G, Konwalinka G. Urinary incontinence in the elderly and age-dependent apoptosis of rhabdosphincter cells. Lancet 354: 918–919, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Sultan AH, Monga AK, Stanton SL. The pelvic floor sequelae of childbirth. Br J Hosp Med 55: 575–579, 1996. [PubMed] [Google Scholar]
- 34.Thoenen H Neurotrophins and activity-dependent plasticity. Prog Brain Res 128: 183–191, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Thom DH, van den Eeden SK, Brown JS. Evaluation of parturition and other reproductive variables as risk factors for urinary incontinence in later life. Obstet Gynecol 90: 983–989, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Turan C, Zorlu CG, Ekin M, Hancerliogullari N, Saracoglu F. Urinary incontinence in women of reproductive age. Gynecol Obstet Invest 41: 132–134, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Viktrup L, Rortveit G, Lose G. Risk of stress urinary incontinence twelve years after the first pregnancy and delivery. Obstet Gynecol 108: 248–254, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Zhang JY, Luo XG, Xian CJ, Liu ZH, Zhou XF. Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur J Neurosci 12: 4171–4180, 2000. [PubMed] [Google Scholar]