Abstract
Acute kidney injury (AKI) induces adaptive responses within proximal tubular (PT) cells that serve to protect them from further ischemic or toxic damage. However, it is not known whether uremia, a potential consequence of AKI, independently alters susceptibility to tubular injury. To address this issue, we subjected CD-1 mice to bilateral ureteral transection (BUTx), which produces uremia (blood urea nitrogen ∼150 mg/dl) in the absence of direct renal damage. PT segments were then isolated from BUTx and control mice and subjected to in vitro hypoxic injury. Additionally, “in vitro uremia” was modeled in isolated tubules or in cultured PT (HK-2) cells by addition of 1) peritoneal dialysate (obtained from mice with bilateral ureteral obstruction), 2) peritoneal fluid (from BUTx mice), or 3) normal human urine (pH 7.4, with and without boiling). Effects on injury severity (lactate dehydrogenase release) were assessed. Finally, because uremia is a prooxidant state, it was hypothesized that BUTx would increase renal lipid peroxidation (malondialdehyde) and induce heme oxygenase-1 (HO-1), a redox-sensitive cytoprotective protein. BUTx conferred striking protection against hypoxic damage. This could be partially modeled in tubules and HK-2 cells by induction of in vitro uremia. Urine's protective action was heat labile (largely destroyed by boiling). BUTx caused a tripling of renal malondialdehyde and HO-1 protein levels. Increased HO-1 transcription was likely involved, as indicated by a tripling of HO-1 mRNA and RNA polymerase II binding along the HO-1 gene (chromatin immunoprecipitation assay). “Gene-activating” histone modifications [H3K4 trimethylation (H3K4m3) and histone 2 variant (H2A.Z)] at HO-1 gene loci were also observed. Uremia, per se, can contribute to the AKI-induced cytoresistance. Low-molecular-weight, heat-labile, cytoprotective factor(s) and uremia-induced renal stress responses (e.g., HO-1 gene activation) are likely involved. Finally, renal HO-1 induction following AKI may reflect direct cell injury effects and adaptations to uremia.
Keywords: acute renal failure, azotemia, bilateral ureteral transection, cytoprotection
it has been long been recognized that acute kidney injury (AKI), e.g., as induced by ischemia-reperfusion, nephrotoxins, heat shock, or urinary tract obstruction, renders proximal tubules (PTs) relatively resistant to subsequent attack (5, 6, 10, 16, 19, 20, 27, 28, 32, 34, 38). For example, if rodents are subjected to 25 min of transient renal ischemia, within 18–24 h they manifest relative resistance to a second, and more severe, bout (e.g., 40 min) of ischemic renal damage (34). This phenomenon has been termed “acquired cytoresistance,” “ischemic preconditioning,” the “heat shock response,” and “acquired resistance to acute renal failure” (6). It has been amply demonstrated that this state is conferred, in part, by a variety of cytoprotective proteins that are upregulated in response to the first bout of renal damage. These include the heat shock protein family (10, 27, 38), including heme oxygenase-1 (HO-1) (1, 3, 16, 28), antiapoptotic proteins (e.g., Bcl2) (18), antioxidant enzymes (e.g., catalase, superoxide dismutase, and ferritin) (3, 24, 29, 35), and proteins that regulate cell cycle progression (e.g., p21) (15, 21, 30, 46). In addition to “stress”-induced proteins, changes in lipid homeostasis, e.g., increases in membrane cholesterol (33, 37), unsaturated fatty acids (2, 45), sphingolipid metabolites (4, 6, 7, 9, 11, 17, 36), and lipid peroxidation (43), may also be involved.
A fundamental, but unanswered, question is whether azotemia, a potential consequence of a first renal insult, alters tubule responsiveness to further damage. For example, might the retention of specific uremic solute(s) exert a direct cytoprotective action? Alternatively, might tubular adaptations to a uremic milieu alter cell injury responses? Azotemia is not a prerequisite for the emergence of the post-renal-injury cytoprotected state (e.g., it can be induced by heat shock, which does not cause renal failure). However, uremia might exert its own protective effect.
The goal of this study was to directly test the above-described hypothesis. Acute renal failure (ARF) was induced in mice by bilateral ureteral transection (BUTx), which produces severe azotemia in the absence of primary renal damage. By isolating PTs from these azotemic mice and from sham-operated controls, relative sensitivity to in vitro hypoxic renal injury, as well as potential tubular adaptations to uremia, could be assessed.
METHODS
Uremia Model
Male CD-1 mice (25–30 g body wt; Charles River Laboratories, Wilmington, MA) were maintained under normal vivarium conditions with free access to food and water. After induction of deep anesthesia with pentobarbital sodium (40–50 mg/kg), the mice were subjected to a midline abdominal incision, and both ureters were exposed. Half of the mice underwent BUTx, which was performed approximately half the distance from the ureteral origin at the renal pelvis. The other half of the mice (controls) underwent sham surgery. The abdominal walls were then sutured in two layers, and the mice were allowed to recover from anesthesia. After ∼18 h, the mice were reanesthetized with pentobarbital sodium, a blood sample was obtained from the inferior vena cava for blood urea nitrogen (BUN) determination, and both kidneys were resected and iced. The animal surgery protocols were approved by the Institutional Animal Care and Use Committee at the Fred Hutchinson Cancer Research Center.
Preparation of Isolated PT Segments
PT segments were prepared from mice that were subjected to BUTx or sham surgery (controls) as previously described (31). Briefly, after bilateral renal resection, the kidneys were iced and both cortices were resected. The tissues were minced with a razor blade, digested with collagenase, passed through a stainless steel sieve, and pelleted by centrifugation (4°C). PTs were recovered by centrifugation through 32% Percoll (Pharmacia, Piscataway, NJ). After they were washed multiple times in iced buffer, the PTs were suspended (∼2–3 mg protein/ml) in experimentation buffer (100 mM NaCl, 2.1 mM KCl, 25 mM NaHCO3, 2.4 mM KH2PO4, 1.2 mM MgSO4, 1.2 mM CaCl2, 5 mM glucose, 1 mM alanine, 4 mM sodium lactate, 10 mM sodium butyrate, and 0.6% 36-kDa dextran, gassed with 95% O2-5% CO2, final pH 7.4). They were rewarmed from 4°C to 37°C in a heated shaking water bath for 15 min and then divided into four to six equal aliquots for experimentation (see below). Each preparation consisted of tubules isolated from a single mouse.
Effect of BUTx on Response to Hypoxic Injury
PTs were prepared from mice that were subjected to BUTx or sham surgery (n = 5 from each group). One set of tubules from each of the BUTx and control groups was studied simultaneously on any given day. The following groups were prepared: 1) control tubules incubated for 45 min under oxygenated conditions (95% O2-5% CO2), 2) BUTx tubules incubated for 45 min under oxygenated conditions, 3) control tubules subjected to 15 min of hypoxia (gassed with 95% N2-5% CO2), and 4) BUTx tubules subjected to 15 min of hypoxia. After the incubations were completed, lethal cell injury was assessed by calculation of percent lactate dehydrogenase (LDH) release (LDH concentration in tubule incubation buffer after centrifugation ÷ LDH concentration in total tubule suspension).
Effect of Uremic Environment on PT Susceptibility to Injury
The ∼0.5 ml of intraperitoneal fluid (IPF) that accumulated in BUTx mice was saved. Its urea concentration closely matched that observed in terminal blood samples (∼150 mg/dl; see results), implying approximate dialytic equilibrium. The following experiment was undertaken to ascertain whether this “uremic” IPF was able to confer a direct cytoprotective effect. Five sets of tubules were prepared from normal mice, and each was divided into aliquots as follows: 1) control oxygenated incubation (with addition of 0.25 ml of saline, 1 ml final incubation volume), 2) oxygenated incubation in the presence of 0.25 ml of IPF (after passage through a 50,000-Da-cutoff filter to remove high-molecular-weight molecules), 3) saline addition followed by 15 min of hypoxic challenge, and 4) filtered IPF addition followed by 15 min of hypoxic challenge. After the incubations were completed, percent LDH release was assessed. [The lack of effect of urea on tubule response to injury was assessed in subsequent experiments (see below).]
Effect of a Uremic Environment on HK-2 Cell Susceptibility to Injury
The HK-2 immortalized human PT cell line (25) was employed to test whether the results observed in PT segments could be recapitulated in a cell culture system. Briefly, the cells were maintained in T75 Costar flasks with keratinocyte serum-free medium (K-SFM) supplemented with glutamine and pituitary extract, as previously described (25). On the day before experimentation, the cells were trypsinized and then seeded into 24-well Costar plates with K-SFM. On the next day, the following experimental groups were prepared: 1) control incubation, 2) incubation with IPF obtained from mice subjected to BUTx (40% IPF-60% K-SFM), 3) ATP depletion injury induced by inhibition of mitochondrial respiration and glycolysis [with 7.5 μM antimycin + 5 μM Ca ionophore A-23187 + 20 mM 2-deoxyglucose (8)], 4) ATP depletion in the presence of IPF, 5) oxidant injury induced by 10 μM ferrous ammonium sulfate complexed to equimolar hydroxyquinoline (FeHQ) to permit Fe to gain intracellular access, and 6) FeHQ-mediated oxidant injury in the presence of IPF (n = 6 replicates, 3 different occasions). After ∼18 h, lethal cell injury was assessed as percent LDH release.
Effect of Peritoneal Dialysis Fluid on HK-2 Cell Injury
The following experiment was undertaken to ascertain whether a dialyzable uremic solute can confer a cytoprotective effect. Acute uremia was induced in normal mice by bilateral ureteral ligation (performed through a midline abdominal incision with ligation of the ureters at the approximate midpoint with silk ligatures). After ∼18 h, when severe uremia was present (BUN ∼150 mg/dl), the mice were reanesthetized with pentobarbital sodium and injected intra-abdominally with 2 ml of 0.9% saline at 37°C. After 30 min, the abdominal cavity was opened, ∼0.5 ml of peritoneal fluid was recovered, and the urea nitrogen concentrations were determined. This fluid was utilized in the HK-2 cell injury protocol as described above (with substitution of 40% dialysate fluid for IPF). The control cells were treated in the same fashion, with substitution of 0.9% saline for dialysate fluid. (The obstruction, rather than the BUTx, model of ARF was used, because peritoneal dialysate in the latter model would be contaminated with spontaneously occurring IPF.)
Effect of Urea Addition on HK-2 Cell and Isolated PT Injury
The following two experiments tested whether urea loading, a result of IPF or peritoneal dialysate addition, accounted for the protection that was observed.
HK-2 cells.
HK-2 cells on 24-well plates were divided into treatment groups: 1) control incubation, 2) the ATP depletion protocol described above, 3) incubation with 10 μM FeHQ, 4) addition of urea (40% of a 300 mg/dl stock urea solution, with final concentration of 120 mg/dl, thereby approximating urea concentrations produced in the above-described HK-2 IPF or dialysate addition experiments), 5) the ATP depletion protocol in the presence of urea, and 6) the FeHQ protocol in the presence of urea. After 18 h, the extent of lethal cell injury (percent LDH release) was assessed (n = 5 determinations of each treatment).
Isolated PTs.
PTs were prepared from five normal mice, and each PT was divided into four aliquots: 1) control incubation, 2) urea addition (final concentration 120 mg/dl), 3) 15 min of hypoxia, and 4) hypoxia in the presence of urea. After 15 min of incubation, percent LDH release was determined.
Initial Characterization of “In Vitro Uremic Cytoprotective Effect”
Addition of human urine aliquots to isolated tubules.
The following experiment assessed whether exposure of isolated tubules to an “experimental uremic milieu” via the addition of human urine samples might transfer the above-observed cytoprotective effects. If so, this system might facilitate initial characterization of this uremic property. Nine sets of tubules were prepared from normal mouse kidneys and divided into four aliquots: 1) control oxygenated incubation for 15 min, 2) incubation with urine samples obtained from three healthy individuals (20% urine-80% incubation buffer), 3) 15 min of hypoxia, and 4) 15 min of hypoxia in the presence of 20% human urine. Lethal cell injury was then assessed by determination of percent LDH release. Because acidosis can confer an independent cytoprotective effect (44), pH of the urine samples was adjusted to 7.4 to match that of the tubule incubation medium. Non-urine-treated samples were exposed to equal-volume 0.9% saline additions to maintain a constant volume.
Size exclusion filtration.
The above-described 20% urine addition experiment was repeated with urine samples that had first been passed through a 3,000-Da-cutoff filter to remove macromolecular species (n = 5).
Heat stability.
Urine samples obtained after 3,000-mol-wt filtration were boiled for 30 min or subjected to three freeze-thaw cycles. The relative ability of these boiled or freeze-thawed samples to mitigate hypoxic injury was then compared with that of the same urine samples not subjected to these treatments (n = 5 each).
Effects of Uremia on Renal Oxidative Stress and HO-1 Expression
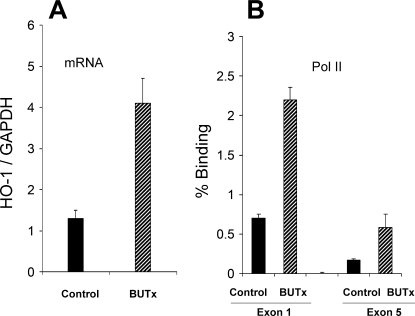
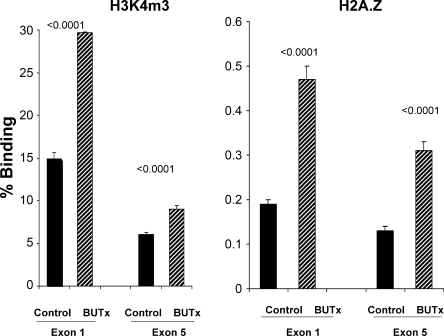
Uremia is recognized as a prooxidant state (22, 23). Furthermore, preexistent oxidative tissue damage, as manifested by malondialdehyde (MDA) elevations, is known to usher in a cytoresistant state (43). The following experiments were undertaken to assess whether 1) BUTx evokes oxidative stress in renal cortex and 2) there is an associated change in expression of HO-1 [a dominant cytoprotective enzyme expressed in renal tissues (16)]. Mice were subjected to BUTx or sham surgery (n = 8 each). After 18 h, bilateral nephrectomies were performed. The renal cortices were dissected at 4°C, and 1) MDA concentrations were assessed as an index of lipid peroxidation (43), 2) protein was extracted for subsequent HO-1 Western blotting (40), 3) total mRNA was extracted for subsequent assessment of HO-1 mRNA levels by competitive PCR [with values expressed as a ratio to simultaneously determined GAPDH mRNA (40)], and 4) chromatin was isolated for subsequent chromatin immunoprecipitation (ChIP) assay. ChIP assays were performed as previously described (13, 14, 41) to assess 1) RNA polymerase II (Pol II) binding to HO-1 gene loci [start and end exons; a correlative index of gene transcription rates (12)] and 2) histone modifications at the start (exon 1) and end (exon 5) of the HO-1 gene [H3K4 trimethylation (H3K4m3) and histone 2 variant (H2A.Z)].
Renal Cortical Cholesterol Content
Renal cortical cholesterol synthesis is a component of the renal stress response, and the resulting increases can help mediate the cytoresistant state (33, 37). This experiment assessed whether uremia can evoke cholesterol accumulation in otherwise normal kidney tissue. Renal cortical tissues were obtained from eight BUTx and eight sham-operated mice and subjected to lipid extraction (42). Cholesterol (free cholesterol and cholesteryl esters) was analyzed by gas chromatography as previously described and expressed as nanomoles per micromole of phospholipid phosphate (42).
Calculations and Statistics
Values are means ± SE. Statistical comparisons were performed by unpaired Student's t-test. Significance was judged by P < 0.05.
RESULTS
BUTx Protects Against Hypoxic Injury
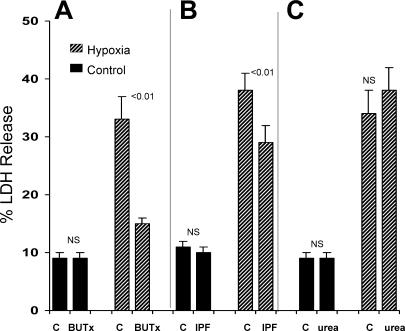
After 45-min control (oxygenated) incubations, percent LDH release was identical in isolated tubules harvested from control and BUTx mice (Fig. 1A). However, susceptibility to hypoxic injury was markedly reduced in tubules from BUTx mice (Fig. 1A): whereas control tubule LDH release rose from a baseline of 9% to 34% with hypoxia (Δ = 25%), the increase in BUTx tubules was only 5%. BUN concentrations from the BUTx mice were markedly elevated compared with values from normal animals (152 ± 4 vs. 22 ± 1 mg/dl).
Fig. 1.
Bilateral ureteral transection (BUTx) or exposure to intraperitoneal fluid (IPF) obtained from BUTx mice protects proximal tubules (PTs) from hypoxic injury. A: BUTx did not alter extent of lactate dehydrogenase (LDH) release under control (C) oxygenated incubation conditions. However, an ∼80% reduction in the severity of hypoxia-mediated cell death was observed in BUTx tubules (i.e., 26% and 5% increases in LDH release over baseline values for control and BUTX tubules, respectively). B: addition of IPF to isolated tubules, harvested from normal mice, did not alter LDH release under control conditions. However, IPF significantly reduced LDH release in response to hypoxia. C: cytoprotective effects in A and B could not be attributed to urea, inasmuch as equal amounts of pure urea conferred no protection. NS, not significant.
IPF From BUTx Mice Protects Against Hypoxic Cell Injury
Urea nitrogen concentration in IPF was essentially identical to that in serum (148 ± 4 mg/dl), implying dialytic equilibration. Addition of IPF to normal tubules did not alter percent LDH release under control oxygenated incubation conditions (Fig. 1B). However, IPF did confer a modicum of protection against hypoxic cell death (Fig. 1B). This protection could not be ascribed to a direct urea effect, inasmuch as addition of pure urea, equal to that in IPF, tended to slightly increase, not decrease, hypoxia-mediated LDH release (Fig. 1C).
HK-2 Cell Experiments
IPF addition.
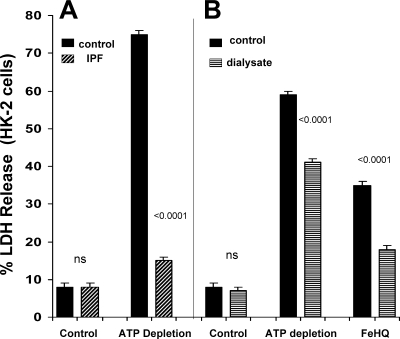
Addition of IPF to HK-2 cells did not exert an independent effect on cell viability, as assessed by percent LDH release (Fig. 2A). However, IPF virtually eliminated ATP depletion-mediated cell death: 8 ± 1% in the control condition, 75 ± 1% with ATP depletion, and 15 ± 1% with ATP depletion in presence of IPF (Fig. 2A).
Fig. 2.
IPF (obtained from mice with BUTx) and peritoneal dialysate protected HK-2 cells against ATP depletion injury and Fe-mediated oxidative cell death. A: addition of IPF did not independently alter HK-2 cell viability, as assessed by LDH release. However, it virtually eliminated ATP depletion injury, as induced by combined mitochondrial electron transport (antimycin A) and glycolytic blockade (2-deoxyglucose). B: peritoneal dialysate fluid, obtained from mice with bilateral ureteral obstruction, conferred significant cytoprotection against ATP depletion injury and Fe-induced oxidative stress [induced by addition of ferrous ammonium sulfate complexed to hydroxyquinoline (FeHQ)].
Peritoneal dialysate addition.
Addition of dialysate did not independently alter HK-2 viability under control conditions (Fig. 2B). However, it did decrease the extent of ATP depletion-mediated and FeHQ-mediated oxidant cell death (Fig. 2B).
Urea addition.
IPF- or dialysate-mediated protection could not be ascribed to a direct effect of urea, because addition of urea did not alter the extent of ATP depletion or Fe-mediated cell death: 73 ± 1% and 72 ± 1% LDH release without and with urea, respectively, for ATP depletion and 24 ± 1 and 29 ± 1% LDH release without and with urea, respectively, for Fe-mediated injury.
Initial Characterization of Uremia-Induced Cytoprotective Effect
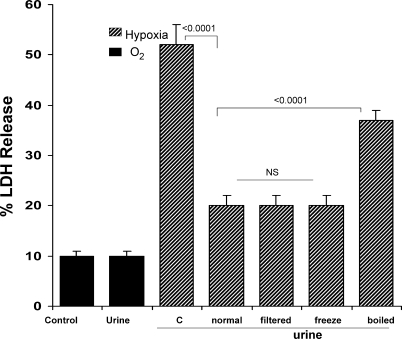
As shown in Fig. 3, the addition of normal human urine samples to isolated PTs did not alter LDH release under control (oxygenated) incubation. However, each of the urine samples markedly diminished hypoxic injury, as denoted by a reduction of LDH release from ∼52% to ∼20% (Fig. 3). The cytoprotective factor(s) were low molecular weight, given that filtering the urine samples through 3,000-kDa-cutoff filters did not diminish urine's cytoprotective effect. Freeze-thawing also did not impact urine's cytoprotective property. However, the latter was largely destroyed by boiling, indicating that the active property was neither an inorganic salt nor urea.
Fig. 3.
Normal human urine confers cytoprotection on isolated tubules exposed to hypoxic injury. Addition of normal human urine samples to normal isolated tubules did not independently alter LDH release. However, urine samples dramatically reduced hypoxia-induced tubular cell death. This cytoprotective effect was due to low-molecular-weight (3,000) substance(s), because filtration through a 3,000-Da-cutoff filter did not diminish urine-mediated protection. Protective property was not freeze-thaw sensitive. However, it was heat sensitive, inasmuch as boiling urine samples markedly reduced urine's cytoprotective effects.
Evaluation of Uremia-Induced Renal Cortical Oxidative Stress
MDA and HO-1 expression in response to BUTx.
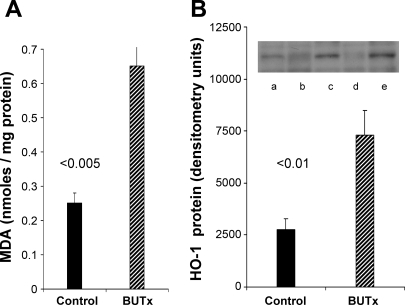
BUTx induced an approximately threefold increase in renal cortical MDA (Fig. 4A). To assess whether this change was associated with a change in HO-1 protein levels, the latter was assessed by Western blotting, and a threefold increase was observed. A quantitatively comparable (∼3-fold) increase in HO-1 mRNA was observed (Fig. 5A).
Fig. 4.
BUTx induces lipid peroxidation and increases heme oxygenase-1 (HO-1) protein levels. A: ∼3-fold elevation in renal cortical malondialdehyde (MDA) concentrations in BUTx mice indicates lipid peroxidation. B: corresponding 3-fold increase in tissue HO-1 protein levels, as assessed by Western blotting (lanes a, c, and e represent BUTx; lanes b and d represent sham).
Fig. 5.
BUTx increases HO-1 mRNA and RNA polymerase II (Pol II) binding to the HO-1 gene. A: BUTx induced an ∼3-fold increase in HO-1 mRNA levels. B: This likely arose from increased HO-1 gene transcription, because 3-fold increases in Pol II binding to HO-1 start and end exons (exons 1 and 5, respectively) were observed.
To further assess whether BUTx leads to activation of the HO-1 gene, we assessed Pol II recruitment to HO-1 gene loci. BUTx induced a threefold increase in Pol II binding at the start (exon 1) and end (exon 5) of the HO-1 gene (Fig. 5B).
Histone modifications at the HO-1 gene.
To ascertain whether BUTx might also alter histone expression at HO-1 gene loci, we assessed a representative histone modification [trimethylation of H3K4 (H3K4m3)] at HO-1 exons 1 and 5. An approximate doubling was observed at both loci (Fig. 6A). To assess whether a normally repressed histone variant (H2A.Z) might be upregulated in BUTx, we determined H2A.Z levels. An approximate doubling at HO-1 exons 1 and 5 was observed.
Fig. 6.
BUTx modifies selected histone expression at HO-1 exons 1 and 5. To assess whether BUTx can alter histone expression at the HO-1 gene, a representative histone modification, trimethylation of H3K4 (H3K4m3), and the levels of a normally repressed histone 2 variant (H2A.Z) were assessed at HO-1 start and end exons. BUTx induced increases in both instances and at both exons.
Renal Cortical Cholesterol Content
Free cholesterol levels were significantly higher in renal cortex obtained from BUTx mice than sham-operated controls: 292 ± 6 vs. 268 ± 7 nmol/μmol phospholipid phosphate (P < 0.005). Renal cortical cholesteryl esters (a reflection of cellular cholesterol excess, i.e., cholesterol flux into the ester storage pools) were approximately twice as great in the BUTx as in the control tissue samples: 9.3 ± 0.4 vs. 4.0 ± 0.3 nmol/μmol phospholipid phosphate (P < 0.0001). Thus, similar to HO-1, a lipid cytoprotectant was also induced in renal cortex by the uremic environment.
DISCUSSION
It has been widely demonstrated that diverse forms of AKI can confer cytoprotection against subsequent renal damage (for review see Ref. 6). However, whether uremia, a potential consequence of AKI, independently influences tubule susceptibility to injury has not, to our knowledge, been defined. As an initial exploration of this issue, mice were subjected to BUTx to produce uremia (BUN ∼150 mg/dl) in the absence of direct, or primary, renal damage. By extraction of tubules from these mice, it was possible to study them in vitro to directly assess cellular susceptibility to hypoxia compared with tubules obtained from sham-operated controls. Uremia-induced nephron solute loading can protect against ARF via a diuretic effect (38). By studying tubules in vitro, it was possible to eliminate the potential confounding variable of by a solute diuresis.
The results of these experiments clearly indicate that uremia, by itself, can confer a direct PT cytoprotective effect. As shown in Fig. 1A, whereas BUTx did not alter tubule viability during control incubations, when tubules were subjected to hypoxia, a marked reduction in lethal cell injury (LDH release) was observed. At least part of the cytoprotection observed in BUTx tubules could be rapidly transferred to normal tubules by exposure to an experimental “uremic milieu.” For example, as shown in Fig. 1B, within minutes of addition of small amounts of IPF (which was in dialytic equilibrium with plasma), hypoxic tubular injury was reduced. However, the extent of this protection was only ∼50% of that observed in tubules harvested from BUTx mice. This suggests that, in addition to tubule exposure to “uremic molecules,” secondary tubular adaptations may also contribute to the cytoprotected state (see below).
Because the extraction of PTs from renal cortex induces some baseline cell injury, the impact of a uremic milieu on tubule injury susceptibility was also assessed in a cell culture system, i.e., where no isolation injury exists. Cultured HK-2 cells were exposed to a uremic environment for 18 h (via IPF addition), during which time ATP depletion injury was imposed by mitochondrial + glycolytic blockade. As shown in Fig. 2A, IPF conferred almost complete protection against ATP depletion injury, reducing LDH release from 73% to 12%. To underscore that the uremic environment can, indeed, protect against injury, ARF was induced in mice by bilateral ureteral obstruction, and 18 h later, peritoneal dialysis was performed. When the resulting dialysate was added to HK-2 cells, protection against hypoxic injury was observed. This protection against ATP depletion injury and oxidant (Fe-mediated) injury indicates a potentially broad-based cytoprotective effect. Finally, it is notable that equimolar urea exposure had no effect on ATP depletion injury, whether imposed on HK-2 cells or isolated PTs. Hence, the protection conferred by a uremic milieu cannot simply be attributed to a urea-induced hyperosmolar state.
The ability of peritoneal dialysate to directly transfer cytoprotection to tubular cells indicates that the responsive molecule(s) are dialyzable; hence, these compounds are likely of low molecular weight and undergo urinary excretion. To test this concept, PTs were exposed to normal human urine samples (pH corrected to 7.4) and then their impact on hypoxic tubule damage was assessed. As shown in Fig. 3, a dramatic reduction in hypoxic injury was observed. To confirm that this protection was, indeed, imparted by low-molecular-weight compound(s), the urine samples were passed through 3,000-Da-cutoff filters and then retested. A complete preservation of cytoprotection was observed. The responsible urine factor(s) was highly heat labile, as indicated by a dramatic loss of protective activity by boiling. This observation clearly indicates that inorganic urinary salts (e.g., Na, K, Cl, phosphates, and sulfates, which are heat stable) are not responsible for urine's protective property. The exact uremic/urinary component(s) that elicits the cytoresistance is obviously of great experimental and potential pharmacological interest. As just one example, it seems possible that the excretion of a naturally occurring cytoprotectant molecule could help tubular cells withstand the low oxygen tensions that normally exist in the renal medulla (i.e., which is said to exist “on the brink of anoxia”). Also, these factor(s) might protect against the rapid osmotic shifts that are natural consequences of urinary concentration and dilution. Unfortunately, despite considerable efforts undertaken by this laboratory, the exact nature of these compound(s) remains to be defined. Hence, it is premature to speculate as to this issue.
PTs that are harvested from BUTx mice and incubated in normal experimentation buffer are no longer exposed to a uremic environment. Nevertheless, these tubules manifested cytoresistance to hypoxic damage (see above). This finding suggests that prior in vivo exposure to uremia evoked secondary tubular adaptations that contributed to the cytoprotected state. Stated differently, cytoresistance might arise from two phenomena: 1) direct exposure to cytoprotective uremic compounds (e.g., as present in urine, IPF, or dialysate) and 2) secondary cellular adaptations to in vivo uremia. It is well known that uremia represents a prooxidant state (22, 23). This suggests that uremia, per se, induces renal oxidative stress, which leads to the induction of stress-activated cytoprotective molecules, e.g., HO-1. To test this hypothesis, MDA concentrations, a marker of lipid peroxidation, were measured in renal cortices obtained from BUTx and control animals. Threefold-higher levels were observed in BUTx than in control tissue samples. This observation directly supports the concept that uremia-induced renal oxidant stress occurred. To explore whether there was a secondary increase in HO-1, HO-1 protein and its mRNA were measured. In both instances, approximately threefold elevations were observed. To test whether these HO-1 mRNA and protein increases reflected increased HO-1 transcription, Pol II recruitment to the start and end exons of the HO-1 gene was assessed (by ChIP assay). Pol II recruitment to a particular gene can both drive and reflect gene transcription rates (12–14, 41). As with HO-1 protein and HO-1 mRNA, approximately threefold increases in Pol II levels at HO-1 start and end exons strongly suggest that increased HO-1 transcription caused, or contributed to, the HO-1 protein increases. Pol II recruitment to, and activity at, specific gene loci can be strongly influenced by epigenetic modifications at these sites. Two potential gene-activating histone modifications are trimethylation of H3K4 (H3K4m3) and increases in the histone-2 variant H2A.Z (13, 14). Hence, these two histone marks were quantified in control and BUTx tissue samples, and approximately twofold increases in both at start and end HO-1 exons were observed. The molecular inducers of these histone marks and whether they mechanistically contribute to HO-1 transcription remain unknown at this time. However, one additional point does seem clear: when AKI leads to increased renal HO-1 expression, direct cell injury effects, as well as the secondary effects of associated uremia, may each play a role.
It is highly unlikely that increased HO-1 expression is the only component of the renal “stress response” that is induced by BUTx-induced uremia. For example, many other cytoprotective pathways might also respond. As just one example, increased renal cholesterol synthesis is part of the acute renal stress response (39), and the cholesterol increases can help mediate a cytoresistant state. To test this hypothesis, free cholesterol and cholesteryl ester levels were measured in renal cortex obtained from BUTx and control renal tissues, and substantial increases in each were observed in the BUTx samples. These data simply serve to underscore that uremia-induced cytoresistance likely reflects the impact of multiple, and potentially interactive, components of a renal stress response, rather than any single cellular adaptive event.
In conclusion, the present study demonstrates, we believe for the first time, that uremia, in the absence of primary kidney injury, exerts a cytoprotective effect on renal PTs. Thus the so-called phenomenon of acquired resistance to acute renal failure, whereby one renal insult confers protection against another, is not necessarily a sole consequence of direct tubular damage. Rather, if AKI induces uremia, the latter may also evoke cytoprotective effects. Uremia-initiated cytoresistance may arise from two general pathways: 1) the accumulation of yet-to-be-defined low-molecular-weight heat-labile cytoprotectants that are normally excreted in urine and 2) a uremia-initiated stress response within the renal tubules. Uremia-induced renal oxidant stress, with corresponding increases in HO-1 gene expression, and renal tubular cholesterol accumulation are illustrative of this latter pathway. Finally, because the present study documented heterogeneous biological adaptations within normal tubules in response to uremia (e.g., epigenetic modifications at, or Pol II recruitment to, the HO-1 gene or cholesterol accumulation) it would appear that the BUTx mouse model has substantial utility for studying interactions between uremia and renal homeostasis.
GRANTS
This project was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-R37-38432 and DK-68520.
Acknowledgments
The author thanks A. C. M. Johnson, S. Lund, K. Nayeon, and M. Naito for expert technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Agarwal A, Balla J, Alam J, Croatt AJ, Nath KA. Induction of heme oxygenase in toxic renal injury: a protective role in cisplatin nephrotoxicity in the rat. Kidney Int 48: 1298–1307, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Alkhunaizi AM, Yaqoob MM, Edelstein CL, Gengaro PE, Burke TJ, Nemenoff RA, Schrier RW. Arachidonic acid protects against hypoxic injury in rat proximal tubules. Kidney Int 49: 620–625, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Balla J, Nath KA, Balla G, Juckett MB, Jacob HS, Vercellotti GM. Endothelial cell heme oxygenase and ferritin induction in rat lung by hemoglobin in vivo. Am J Physiol Lung Cell Mol Physiol 268: L321–L327, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Basnakian AG, Ueda N, Hong X, Galitovsky VE, Yin X, Shah SV. Ceramide synthase is essential for endonuclease-mediated death of renal tubular epithelial cells induced by hypoxia-reoxygenation. Am J Physiol Renal Physiol 288: F308–F314, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bonventre JV Kidney ischemic preconditioning. Curr Opin Nephrol Hypertens 11: 43–48, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Honda N, Hishida A, Ikima K, Yonemura K. Acquired resistance to acute renal failure. Kidney Int 31: 1233–1238, 1987. [DOI] [PubMed] [Google Scholar]
- 7.Iwata M, Herrington J, Zager RA. Sphingosine: a mediator of acute renal tubular injury and subsequent cytoresistance. Proc Natl Acad Sci USA 92: 8970–8974, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwata M, Herrington J, Zager RA. Protein synthesis inhibition induces cytoresistance in cultured human proximal tubular (HK-2) cells. Am J Physiol Renal Fluid Electrolyte Physiol 268: F1154–F1163, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Jin ZQ, Karliner JS, Vessey DA. Ischaemic postconditioning protects isolated mouse hearts against ischaemia/reperfusion injury via sphingosine kinase isoform-1 activation. Cardiovasc Res 79: 134–140, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Jo SK, Ko GJ, Boo CS, Cho WY, Kim HK. Heat preconditioning attenuates renal injury in ischemic ARF in rats: role of heat-shock protein 70 on NF-κB-mediated inflammation and on tubular cell injury. J Am Soc Nephrol 17: 3082–3092, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Lochhead KM, Zager RA. Fluorinated anesthetic exposure “activates” the renal cortical sphingomyelinase cascade. Kidney Int 54: 373–381, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Marx J Transcription enzyme structure solved. Science 292: 411–414, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Naito M, Bomsztyk K, Zager RA. Endotoxin mediates recruitment of RNA polymerase II to target genes in acute renal failure. J Am Soc Nephrol 19: 1321–1330, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naito M, Bomsztyk K, Zager RA. Renal ischemia induced cholesterol loading: transcription factor recruitment and chromatin remodeling along the HMG CoA reductase gene. Am J Pathol. In press. [DOI] [PMC free article] [PubMed]
- 15.Nath KA Provenance of the protective property of p21. Am J Physiol Renal Physiol 289: F512–F513, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest 190: 267–270, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishino Y, Webb I, Marber MS. Sphingosine kinase isoforms and cardiac protection. Cardiovasc Res 76: 3–4, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Ortiz A, Lorz C, Catalán MP, Danoff TM, Yamasaki Y, Egido J, Neilson EG. Expression of apoptosis regulatory proteins in tubular epithelium stressed in culture or following acute renal failure. Kidney Int 57: 969–981, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Park KM, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury and JNK, p38, and MAPK kinase activation by remote ischemic pretreatment. J Biol Chem 276: 11870–11876, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Park KM, Kramers C, Vayssier-Taussat M, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury, MAPK and MAPK kinase activation, and inflammation by remote transient ureteral obstruction. J Biol Chem 277: 2040–2049, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Price PM, Megyesi J, Saf Irstein RL. Cell cycle regulation: repair and regeneration in acute renal failure. Kidney Int 66: 509–514, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Pupim LB, Himmelfarb J, McMonagle E, Shyr Y, Ikizler TA. Influence of initiation of maintenance hemodialysis on biomarkers of inflammation and oxidative stress. Kidney Int 65: 2371–2379, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Ramos LF, Shintani A, Ikizler TA, Himmelfarb J. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol 19: 593–599, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubinstein I, Abassi Z, Milman F, Ovcharenko E, Coleman R, Winaver J, Better OS. Hyperbaric oxygen treatment improves GFR in rats with ischaemia/reperfusion renal injury: a possible role for the antioxidant/oxidant balance in the ischaemic kidney. Nephrol Dial Transplant. In press. [DOI] [PMC free article] [PubMed]
- 25.Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int 45: 48–57, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Sogabe K, Roeser NF, Venkatachalam MA, Weinberg JM. Differential cytoprotection by glycine against oxidant damage to proximal tubule cells. Kidney Int 50: 845–854, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Van Why SK, Siegel NJ. Heat shock proteins in renal injury and recovery. Curr Opin Nephrol Hypertens 7: 407–412, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Vogt BA, Alam J, Croatt AJ, Vercellotti GM, Nath KA. Acquired resistance to acute oxidative stress. Possible role of heme oxygenase and ferritin. Lab Invest 72: 474–183, 1995. [PubMed] [Google Scholar]
- 29.Yamanobe T, Okada F, Luchi Y, Onuma K, Tomita Y, Fujii J. Deterioration of ischemia/reperfusion-induced acute renal failure in SOD1-deficient mice. Free Radic Res 41: 200–207, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Yu F, Megyesi J, Safirstein RL, Price PM. Identification of the functional domain of p21WAF1/CIP1 that protects cells from cisplatin cytotoxicity. Am J Physiol Renal Physiol 289: F514–F520, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Zager RA Heme protein induced cytoresistance: expression at the plasma membrane level. Kidney Int 47: 1336–1345, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Zager RA Obstruction of proximal tubules initiates cytoresistance against hypoxic damage. Kidney Int 47: 628–637, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Zager RA Plasma membrane cholesterol: a critical determinant of cellular energetics and tubular resistance to attack. Kidney Int 58: 193–205, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Zager RA, Baltes LA, Sharma HM, Jurkowitz MS. Responses of the ischemic acute renal failure kidney to additional ischemic events. Kidney Int 26: 689–701, 1984. [DOI] [PubMed] [Google Scholar]
- 35.Zager RA, Burkhart K. Decreased expression of mitochondrial-derived H2O2 and hydroxyl radical in cytoresistant proximal tubules. Kidney Int 52: 942–952, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Zager RA, Burkhart KM, Johnson A. Sphingomyelinase and membrane sphingomyelin content: determinants of proximal tubule cell susceptibility to injury. J Am Soc Nephrol 11: 894–902, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Zager RA, Burkhart KM, Johnson AC, Sacks BM. Increased proximal tubular cholesterol content: implications for cell injury and “acquired cytoresistance.” Kidney Int 56: 1788–1797, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Zager RA, Iwata M, Burkhart KM, Schimpf BA. Post-ischemic acute renal failure protects proximal tubules from O2 deprivation injury, possibly by inducing uremia. Kidney Int 45: 1760–1768, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Zager RA, Johnson AC. Renal cortical cholesterol accumulation is an integral component of the systemic stress response. Kidney Int 60: 2299–2310, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Zager RA, Johnson AC, Hanson SY. Parenteral iron therapy exacerbates experimental sepsis. Kidney Int 65: 2108–2112, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Zager RA, Johnson AC, Naito M, Lund SR, Kim N, Bomsztyk K. Growth and development alter susceptibility to acute renal injury. Kidney Int 74: 674–678, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zager RA, Kalhorn TF. Changes in free and esterified cholesterol: hallmarks of acute renal tubular injury and acquired cytoresistance. Am J Pathol 157: 1007–1016, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zager RA, Schimpf BA, Bredl CR, Gmur DJ. Inorganic iron effects on in vitro hypoxic proximal renal tubular cell injury. J Clin Invest 91: 702–708, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zager RA, Schimpf BA, Gmur DJ. Physiological pH: effects on posthypoxic proximal tubular injury. Circ Res 72: 837–846, 1993. [DOI] [PubMed] [Google Scholar]
- 45.Zager RA, Schimpf BA, Gmur DJ, Burke TJ. Phospholipase A2 activity can protect renal tubules from oxygen deprivation injury. Proc Natl Acad Sci USA 90: 8297–8231, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou H, Kato A, Yasuda H, Miyaji T, Fujigaki Y, Yamamoto T, Yonemura K, Hishida A. The induction of cell cycle regulatory and DNA repair proteins in cisplatin-induced acute renal failure. Toxicol Appl Pharmacol 200: 111–120, 2004. [DOI] [PubMed] [Google Scholar]