Abstract
Background: Soy and some of its constituents, such as isoflavones, have been shown to have cancer-inhibitory activities in experimental studies. Data from epidemiologic studies linking usual soy food intake with colorectal cancer are limited and inconsistent.
Objective: The objective was to investigate whether soy food intake is associated with colorectal cancer risk.
Design: We prospectively examined 68,412 women aged 40–70 y and free of cancer and diabetes at enrollment. Usual soy food intake was assessed at baseline (1997–2000) and reassessed during the first follow-up (2000–2002) through in-person interviews with a validated food-frequency questionnaire. We excluded the first year of observation to minimize lifestyle changes related to preclinical disease.
Results: During a mean follow-up of 6.4 y, 321 incident colorectal cancer cases were identified. After adjustment for potential confounding factors, total soy food intake was inversely associated with colorectal cancer risk. Each 5-g/d increment in intake of soy foods as assessed by dry weight [equivalent to ≈1 oz (28.35 g) tofu/d] was associated with an 8% reduction in risk (95% CI: 3%, 14%). Women in the highest tertile of intake had a multivariate relative risk of 0.67 (95% CI: 0.49, 0.90) compared with those in the lowest tertile (P for trend = 0.008). This inverse association was primarily confined to postmenopausal women. Similar results were also found for intakes of soy protein and isoflavones.
Conclusion: This prospective study suggests that consumption of soy foods may reduce the risk of colorectal cancer in postmenopausal women.
INTRODUCTION
Historically, the incidence of colorectal cancer is much lower in Asian countries, such as China and Japan, than in Western societies (1). Migration and ecologic studies have suggested that environmental factors have been the main contributors to this variation. Diet, in particular, is believed to play an important role in disease risk (2–4). Growing evidence from in vitro and animal studies has implicated soy and certain soy constituents, such as isoflavones, in cancer protection (5–9). It has been suggested that a high intake of soy products in Asian populations may have contributed to the low risk of colorectal cancer in these populations. However, data linking dietary soy intake with colorectal cancer risk in humans has been limited and inconsistent (9, 10). Intakes of soy foods in previous studies, most of which had a case-control study design, were assessed by using instruments that either had few questions on consumption or had questions that were in nonquantitative format (9, 11), which raises concerns about the completeness and accuracy of the soy intake data. Additionally, soy consumption is low in most Western populations (12, 13), which hinders epidemiologic studies of the health effects of soy consumption.
The Shanghai Women's Health Study, a large prospective cohort study, was conducted in a population that has high but varied levels of soy food consumption. Comprehensive dietary data, covering virtually all soy foods consumed in the study population, were collected at baseline and again during follow-up (14, 15). This cohort study provides a unique opportunity to evaluate the hypothesis that intake of soy and certain soy constituents may reduce the risk of colorectal cancer in women.
SUBJECTS AND METHODS
Cohort of the Shanghai Women's Health Study
The design and methods of the Shanghai Women's Health Study have been described in depth in previous reports (14, 15). Briefly, the study recruited 74,942 women aged 40–70 y between 1996 and 2000 from 7 urban communities of Shanghai, with a participation rate of 92.7%. All women completed a detailed baseline survey that collected information on demographic characteristics, lifestyle and dietary habits, medical history, and family history of cancer, among others. Anthropometric measurements, including current weight, height, and circumferences of the waist and hips, were also taken. The study was approved by the relevant institutional review boards for human research in both China and the United States. Written informed consent was obtained from all study participants.
Assessment of diet
Usual dietary intake over the 12 mo before the interview was assessed at baseline for all cohort members and was reassessed 2–3 y after the baseline survey for ≈91% of cohort members with the use of a comprehensive, quantitative food-frequency questionnaire (FFQ). The questionnaire included 11 soy food items covering virtually all soy foods commonly consumed in Shanghai, including soy milk, tofu, fried tofu, dried or pressed tofu, fresh green soy beans, dry soy beans, soy sprouts, and other soy products. During an in-person interview, each participant was first asked how often, on average, she had consumed a specific food or food group (the possible responses were daily, weekly, monthly, yearly, or never) over the 12 mo preceding the dietary survey, which was followed by a question on the amount consumed in liang (Chinese unit, 1 liang = 50 g) per unit of time. For seasonal foods such as fresh soybeans, participants were asked to describe their consumption during the season when the foods were available. All interviews were tape-recorded and selectively checked by quality-control staff to monitor the quality of the interview. Nutrient intakes were calculated by multiplying the amount of each food consumed by the nutrient content per gram of the food as obtained from the China Food Composition Tables 2002 (16), and summed over all relevant foods. Because the water content of the various soy foods varies widely (from 96.4% for soy milk, 82.8% for tofu, and 65.2% for dried/pressed tofu to 10.2% for dry soybeans), we also calculated total intake of the dry weight of soy foods by multiplying the amount of each soy food consumed by (1 – percentage water content of the food item) (16) and summing over all soy foods. Because neither soy protein isolate nor isoflavone supplements are commonly consumed in the study population, neither was included in the assessment of soy intake.
The FFQ used in the study was validated in a random sample of 200 cohort participants (15). The Pearson correlation coefficients between soy food intake derived from the FFQ and the mean of 24 d of 24-h dietary recalls (the recalls were conducted twice per month over a 12-mo period) was 0.49.
Outcome ascertainment
The cohort was followed for the occurrence of cancer and other chronic diseases by a combination of active surveys conducted every 2 y and annual record linkage of the study population to cancer case data collected by the population-based Shanghai Cancer Registry, and death certificates were collected by the Shanghai Municipal Center for Disease Control and Prevention. Nearly all cohort members were successfully followed; the response rates for the first (2000–2002), second (2002–2004), and third (2004–2007) in-person follow-up surveys were 99.8%, 98.7%, and 96.7%, respectively. The matching criteria used for record linkage to identify cancer cases and deceased subjects in the cohort were citizen identification number, name, date of birth, and address. All possible incident cancer cases were verified by conducting home visits. Medical charts from the diagnostic hospital were reviewed to verify the diagnosis, and pathological characteristics of the tumor were recorded. Evidence for histopathologic diagnosis was obtained from most of the cases (n = 307, 95.6%).
Statistical analysis
I n this analysis, we excluded subjects who reported a history of cancer (n = 1490), diabetes (n = 3302), or familial adenomatous polyposis (n = 86) at baseline. We also excluded subjects who had extreme total energy intake (<500 or > 3500 kcal/d; n = 44), were lost to follow-up since enrollment (n = 10), or took postmenopausal hormones (n = 1653). Hormone users were excluded to avoid potential confounding of exogenous hormones on the effect of soy or soy isoflavone intake. After these exclusions (not mutually exclusive), a total of 68,412 women remained for the present analysis.
To better estimate usual dietary intake, we used the average intake on the first FFQ at baseline and the second FFQ conducted 2–3 y after the baseline survey. Given the possible presence of prediagnosed diseases that may have resulted in changes in dietary intake and lifestyle, which would bias the risk estimates associated with soy consumption, we excluded the first year of observation and incident cases diagnosed during this period from the analysis. A total of 68,412 participants included in the study contributed 438,221 person-years in a mean follow-up of 6.4 y between 1998 and 2005. For individuals who provided no second FFQ data or whose cancer was diagnosed between the baseline FFQ and the first year of the second FFQ survey (n = 5978, 8.7%), only the baseline dietary intake was used as the exposure.
Cox proportional hazards modeling was used, with age as the time scale, to compute relative risks (RRs) of developing colorectal cancer and their 95% CIs associated with soy food intake and to adjust for potential confounders. Entry time was defined as age at enrollment and exit time was defined as age at colorectal cancer diagnosis or censoring. Potential confounders adjusted for in multivariate models included birth calendar year, education, household income, body mass index (BMI; calculated as weight in kilograms divided by the square of height in meters), physical activity [measured as metabolic equivalent hours (MET-h) per week per year] (17), colorectal cancer in first-degree relatives, menopausal status, and dietary intakes of total calories, red meat, total fruit and vegetables, nonsoy fiber, nonsoy calcium, and nonsoy folic acid. Menopausal status updated during follow-up was considered as a time-varying covariate in the model. Additional adjustments for cigarette smoking, alcohol consumption, aspirin or other nonsteroidal antiinflammatory drug use, prior history of colorectal polyps and chronic ulcerative colitis, and intakes of fat and nonsoy protein did not appreciably alter the results; these variables were therefore not included in the final model. Nutrient (soy protein and isoflavones) and soy food intakes were grouped into tertiles based on distributions of intake in the entire cohort, with the lowest tertile serving as the reference. Tests for linear trend across levels of soy food consumption were performed by using the median value for each tertile of soy consumption and modeling them as continuous variables. We also used a restricted cubic spline Cox regression analysis (18), a flexible statistical technique, to evaluate the association between colorectal cancer risk and soy food intake on a continuous basis. Knots were placed at the 5th, 50th, and 95th percentiles of the distribution of soy food consumption in the cohort. We excluded subjects whose soy food intake was above the 99th percentile from the spline model to minimize the influence of outliers.
We conducted stratified analyses to evaluate potential effect modification by age, BMI, physical activity, menopausal status, anatomic subsite of tumor (colon compared with rectum), and dietary factors, such as intake of red meat, fruit, vegetables, fiber, calcium, and folic acid. Multiplicative interactions were determined by using the likelihood ratio test between the main effect model and models with a cross-product term. Statistical analyses were carried out by using SAS version 9.1 (SAS Institute, Cary, NC). All statistical tests were based on 2-sided probability.
RESULTS
The mean (± SD) age of the study population was 51.6 ± 9.0 y at enrollment. Age-adjusted characteristics, separately for women at each tertile of total soy food intake, are shown in Table 1. Women with higher intakes of soy foods were slightly older and were more likely to exercise regularly, consume more fruit and vegetables, and have a higher intake of total calories, fat, protein, dietary fiber, calcium, and folic acid, but a lower intake of carbohydrates. Women with higher intakes of soy foods were also likely to achieve higher education, have a higher BMI, but consume less red meat. These differences, although statistically significant, were small and unlikely to have biological relevance. There were no significant differences across tertiles of soy food intake in household income or family history of colorectal cancer. Few women in this cohort ever smoked cigarettes (2.7%), consumed alcoholic beverages (2.3%), or used aspirin or other nonsteroidal antiinflammatory drugs regularly (1.8%).
TABLE 1.
Characteristics of study participants according to total soy food intake: Shanghai Women's Health Study (1997–2005)1
| Tertiles of soy food intake (g/d)23 |
|||||
| All subjects (n = 68,412) | ≤12.8 (n = 22,402) | 12.9–21.0 (n = 22,815) | >21.0 (n = 23,195) | P for trend4 | |
| Baseline characteristics | |||||
| Age (y) | 51.6 ± 9.05 | 51.2 | 51.5 | 52.2 | <0.0001 |
| Education, college and above (%) | 13.2 | 12.9 | 13.4 | 13.2 | 0.002 |
| Household income >30,000 yuan/y (%)6 | 17.5 | 17.6 | 17.9 | 16.9 | 0.15 |
| Cigarette smoking (%) | 2.7 | 3.2 | 2.4 | 2.5 | <0.0001 |
| Alcohol consumption (%) | 2.3 | 2.2 | 2.2 | 2.6 | 0.009 |
| Physical activity >100 MET7-h/wk per year (%) | 50.8 | 47.4 | 50.1 | 54.8 | <0.0001 |
| BMI (kg/m2) | 24.0 ± 3.4 | 23.7 | 23.9 | 24.3 | <0.0001 |
| Family history of colorectal cancer (%) | 2.2 | 2.1 | 2.3 | 2.1 | 0.97 |
| Dietary intake3 | |||||
| Fruit (g/d) | 256 ± 150 | 248 | 256 | 263 | <0.0001 |
| Vegetables (g/d) | 303 ± 151 | 261 | 295 | 351 | <0.0001 |
| Red meat (g/d) | 48.3 ± 30.1 | 48.2 | 48.9 | 47.8 | 0.02 |
| Total calories (kcal/d) | 1657 ± 351 | 1496 | 1638 | 1832 | <0.0001 |
| Fat (g/d) | 29.6 ± 11.5 | 27.2 | 29.3 | 32.1 | <0.0001 |
| Carbohydrates (g/d) | 278.8 ± 58.1 | 289.1 | 280.2 | 267.6 | <0.0001 |
| Protein (g/d) | 66.7 ± 18.5 | 61.8 | 66.0 | 72.2 | <0.0001 |
| Soy protein (g/d) | 9.1 ± 5.4 | 4.6 | 8.2 | 14.3 | <0.0001 |
| Isoflavones (mg/d) | 30.8 ± 18.6 | 15.1 | 27.9 | 48.9 | <0.0001 |
| Fiber (g/d)8 | 10.5 ± 3.5 | 9.2 | 10.3 | 11.9 | <0.0001 |
| Calcium (mg/d)8 | 479 ± 180 | 407 | 464 | 563 | <0.0001 |
| Folic acid (mg/d)8 | 290 ± 90 | 249 | 286 | 334 | <0.0001 |
Except for the mean age, data were standardized to the age distributions at baseline; data on dietary variables, except for total calories, were further standardized to total calorie intake.
The amount of soy food intake was assessed by dry weight.
Data are the average intakes from the baseline and second quantitative food-frequency questionnaires.
Generalized linear models were used for continuous variables and chi-square tests for categorical variables.
Mean ± SD (all such values).
1 US$ = ≈8 yuan (at time of recruitment).
MET, metabolic equivalents.
Soy foods contributed 15%, 26%, and 18% to the total intake of dietary fiber, calcium, and folic acid, respectively.
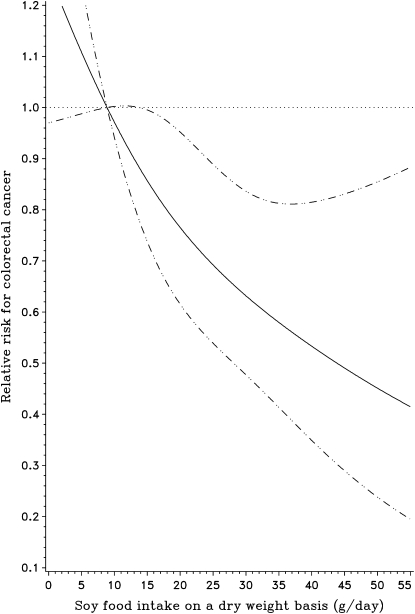
During a mean follow-up of 6.4 y, 321 incident cases of colorectal cancer, including 195 colon and 126 rectal cancers, were identified. After adjustment for age, birth calendar year, and total energy intake, consumption of soy foods was significantly associated with a decreased risk of colorectal cancer (Table 2). Compared with women in the lowest tertile of soy food intake, women in the highest tertile of intake had an RR of 0.71 (95% CI: 0.53, 0.95; P for trend = 0.02). Further adjustment for lifestyle and other dietary factors did not appreciably alter the results. When intake of soy foods was analyzed on a continuous basis in a restricted cubic spline Cox regression model, the risk of colorectal cancer significantly deceased with increasing soy food consumption (Figure 1), with a trend appearing monotonic and approximately linear (P for nonlinear relation = 0.69, P for overall significance of curve = 0.004, and P for linear relation = 0.0009). Each 5-g increment in intake per day of dry weight soy foods [equivalent to ≈1 oz (28.35 g) tofu/d] was associated with a reduction in RR of 8% (95% CI: 3%, 14%) after fully adjusting for potential confounding factors. Similar inverse associations were also found for both dietary soy protein and isoflavone intake; the corresponding RRs for the highest tertile of intake were 0.66 (95% CI: 0.49, 0.89; P for trend = 0.007) and 0.76 (95% CI: 0.56, 1.01; P for trend = 0.06), respectively.
TABLE 2.
Relative risks (RRs) and 95% CIs for colorectal cancer associated with average intakes of soy foods, soy protein, and isoflavones: Shanghai Women's Health Study (1997–2005)1
| Tertiles of intakes2 |
||||
| 1 | 2 | 3 | P for trend3 | |
| Soy foods | ||||
| Person-years | 166,056 | 169,383 | 171,193 | |
| No. of cases | 113 | 108 | 100 | |
| Age- and energy-adjusted RR (95% CI) | 1.00 | 0.88 (0.67, 1.15) | 0.71 (0.53, 0.95) | 0.02 |
| Multivariate RR (95% CI)4 | 1.00 | 0.86 (0.66, 1.13) | 0.67 (0.49, 0.90) | 0.008 |
| Soy protein | ||||
| Person-years | 168,425 | 167,884 | 170,324 | |
| No. of cases | 115 | 105 | 101 | |
| Age- and energy-adjusted RR (95% CI) | 1.00 | 0.86 (0.66, 1.13) | 0.71 (0.53, 0.95) | 0.02 |
| Multivariate RR (95% CI)4 | 1.00 | 0.84 (0.64, 1.10) | 0.66 (0.49, 0.89) | 0.007 |
| Isoflavones | ||||
| Person-years | 167,031 | 167,695 | 171,908 | |
| No. of cases | 110 | 103 | 108 | |
| Age- and energy-adjusted RR (95% CI) | 1.00 | 0.91 (0.69, 1.19) | 0.80 (0.60, 1.07) | 0.13 |
| Multivariate RR (95% CI)4 | 1.00 | 0.89 (0.68, 1.17) | 0.76 (0.56, 1.01) | 0.06 |
The first year of observation was omitted from the analysis.
Tertile cutoffs were assessed by dry weight for soy foods (12.8 and 21.0 g/d), soy protein (6.3 and 10.2 g/d), and isoflavones (20.9 and 34.8 mg/d).
Based on the median value of each intake category and modeled as continuous variables in a Cox proportional hazard model.
Multivariable Cox proportional hazard model was adjusted for age, education, household income, physical activity, BMI, menopausal status, family history of colorectal cancer, total calorie intake, and average intakes of fruit, vegetables, red meat, nonsoy calcium, nonsoy fiber, and nonsoy folic acid and was stratified by birth year.
FIGURE 1.
Smoothed plot of the relative risk of colorectal cancer according to intake of soy foods. The amount of soy food intake was assessed by dry weight (g/d). The relative risks were estimated by restricted cubic-spline Cox regression analysis with knots placed at the 5th, 50th, and 95th percentiles of intake, after adjustment for age, birth year, education, household income, physical activity, BMI, menopausal status, family history of colorectal cancer, total calorie intake, and average intakes of fruit, vegetables, red meat, nonsoy calcium, nonsoy fiber, and nonsoy folic acid. The median value of the first tertile of intake was treated as the reference point. Participants with an intake of soy foods above the 99th percentile were not plotted. Point estimates are indicated by a solid line and 95% CIs by dashed lines. P for nonlinear relation = 0.69, P for overall significance of curve = 0.004, and P for linear relation = 0.0009.
Because the value of soy food intake for ≈9% of the subjects was based on the baseline FFQ only, we further restricted analyses to subjects (n = 62,401) who completed both the first and second FFQs and found RR estimates similar to those observed in the entire population. The multivariate RR for the highest compared with the lowest tertile of soy food intake was 0.63 (95% CI: 0.44, 0.92; P for trend = 0.02; data not shown in Tables). Likewise, results were comparable when only the baseline intake was analyzed for the entire cohort, with a corresponding RR of 0.70 (95% CI: 0.52, 0.94) for the highest tertile of soy food intake (P for trend = 0.02). Furthermore, a consistent and graded inverse association was also observed in a nutrient residual (energy-adjusted) model (19), with an RR of 0.74 (95% CI: 0.57, 0.97) for the top tertile of soy food intake (P for trend = 0.03).
The soy and colorectal cancer association was not modified by traditional risk factors for colorectal cancer, including BMI, physical activity, and consumption of red meat, fruit, and vegetables (data not shown). Furthermore, the association did not vary significantly by anatomic subsite (colon compared with rectum; P for heterogeneity = 0.31), although the magnitude of the association appeared to be somewhat stronger with rectal cancer (Table 3). Analyses stratified by menopausal status at cancer diagnosis, however, showed that the inverse association between soy food intake and colorectal cancer risk was primarily confined to postmenopausal women (P for interaction = 0.055). We also conducted analyses excluding individuals who had ever smoked cigarettes, consumed alcoholic beverages, or regularly used nonsteroidal antiinflammatory drugs and found no material changes in the risk estimates. RRs for the highest compared with the lowest tertiles of soy food intake ranged from 0.65 to 0.66.
TABLE 3.
Multivariate relative risks (RRs) and 95% CIs for colorectal cancer in association with soy food intake, stratified by age, menopausal status, and anatomic site and stage of tumor: Shanghai Women's Health Study (1997–2005)1
| RR (95% CI)2, by tertile of average intake of soy foods (g/d)3 |
||||||
| No. of events | ≤12.8 | 12.9–21.0 | >21.0 | P for trend4 | P for interaction | |
| Age5 | 0.21 | |||||
| <52 y | 79 | 1.00 | 0.94 (0.54, 1.61) | 0.93 (0.51, 1.70) | 0.83 | |
| ≥52 y | 242 | 1.00 | 0.85 (0.63, 1.16) | 0.62 (0.44, 0.87) | 0.006 | |
| Menopausal status | 0.06 | |||||
| Premenopausal | 76 | 1.00 | 0.84 (0.39, 1.79) | 1.28 (0.60, 2.70) | 0.44 | |
| Postmenopausal | 273 | 1.00 | 0.87 (0.65, 1.16) | 0.60 (0.43, 0.83) | 0.002 | |
| Anatomic site | 0.316 | |||||
| Colon | 195 | 1.00 | 0.98 (0.69, 1.40) | 0.76 (0.52, 1.13) | 0.15 | |
| Rectum | 126 | 1.00 | 0.73 (0.48, 1.12) | 0.55 (0.34, 0.90) | 0.02 | |
The first year of observation was omitted from the analysis.
Multivariable Cox proportional hazard model was adjusted for age, education, household income, physical activity, BMI, menopausal status, family history of colorectal cancer, total calorie intake, and average intakes of fruit, vegetables, red meat, nonsoy calcium, nonsoy fiber, and nonsoy folic acid and was stratified by birth year.
Intake assessed by dry weight.
Based on the median value of each intake category and modeled as continuous variables in a Cox proportional hazard model.
Stratified based on the mean value.
The Wald statistic was used to test for homogeneity (20).
DISCUSSION
In this large, population-based, prospective cohort of women with high but varied soy intakes, we found that the risk of colorectal cancer decreased with increasing soy food intake, primarily in postmenopausal women. The risk decreased by >30% among women who were in the top tertile of soy food intake compared with women in the bottom tertile of soy food intake. This association was independent of traditional risk factors and was not explained by differences in consumption of other dietary factors such as red meat, fruit, and vegetables.
Soy foods are rich in fiber, calcium, and folic acid and contributed 15%, 26%, and 18%, respectively, to the total intake of these nutrients in this study population. These compounds are believed to mediate many of the cancer protective effects of soy (9, 21–23), including binding to carcinogenic bile acid and ionized fatty acid (24), increasing stool bulk with subsequent dilution of colonic luminal carcinogens (25), and maintaining intracellular normal DNA synthesis and methylation (26). Previous studies, including some large prospective cohort studies, have shown that diets poor in these nutrients are associated with an increased risk of colorectal cancer, although the evidence is still not conclusive (2, 27).
Cumulative evidence from epidemiologic studies suggests a protective effect of postmenopausal hormone replacement therapy (HRT) on colorectal cancer (28, 29). This protective effect was further supported by the results of the Women's Health Initiative, the first randomized clinical trial on the health effects and safety of HRT use (29). However, the trial also reported adverse effects in HRT users, including an increased risk of stroke, pulmonary thromboembolic events, and breast cancer, which appear to outweigh the benefits of HRT use (30, 31). Recently, plant-derived estrogens, especially soy isoflavones, have attracted considerable attention as a natural alternative to HRT. Soy and its products are the most abundant source of isoflavones in human diets. Isoflavones have a diphenolic structure similar to that of 17β-estradiol and have been shown to bind preferentially to estrogen receptor-β (ER-β) but weakly to estrogen receptor-α (ER-α) (32–34). Levels of ER-β, the predominant ER form in the human colon, are significantly lower in colonic tumors than in normal mucosa (35). Loss of ER-β is associated with elevated cell proliferation, tumor cell dedifferentiation, and advanced stages of colon cancer, which suggests that ER-β–mediated processes in the colonic epithelium may play a role in colorectal tumorigenesis (35–39). It has been shown that the biologic behavior of isoflavones may be modulated by an individual's endogenous concentration of estrogens. In vitro studies have shown that isoflavones can act primarily as estrogen agonists in a low-estrogen environment, whereas they can act as estrogen antagonists in a high estrogen environment (40). Our finding of an inverse association of soy consumption with colorectal cancer risk was predominantly seen among postmenopausal women, which suggests that the effect of soy on colorectal cancer may, at least in part, be due to its estrogen-like effect.
Soy or isoflavones have also shown other anticarcinogenic activities in in vitro and animal studies (41), including decreasing oxidative stress (42–44) and modulating multiple signaling pathways in neoplastic transformation (45–49). These actions can inhibit cell growth and transformation, induce apoptosis, and inhibit angiogenesis (41, 47, 50, 51). The colorectum has been suggested as a good target for potential tumor inhibition by soy because of the high bioavailability of soy constituents that are orally administered. Administration of isoflavones or soy products has been shown to inhibit the growth of human cancer cells and the formation of animal tumors, including tumors of the intestine (21, 22, 52). Some small-scale trials have provided evidence that soy or certain soy components may be effective in lowering biomarkers of cell proliferation (41), although the data have not been entirely consistent (53, 54). On the other hand, most of these trials and animal studies investigated the effects of specific soy components given as supplements at a relatively high dose for a short period of time. Data are scanty on the relation of usual dietary intake of soy foods with colorectal cancer risk in general populations.
Previous epidemiologic studies of usual dietary soy intake and colorectal cancer risk have generated mixed results, but generally pointed toward an inverse association (9). High consumption of soy-containing foods was found to be associated with a reduced risk of colon cancer in a case-control study of multiethnic populations in Hawaii (55), a reduced risk of rectal cancer in case-control studies conducted in China (56) and Japan (57), and a reduced risk of colorectal adenomas, precursors to colorectal cancer, in a case-control study of multiethnic populations in Southern California (12). Recently, a prospective cohort study in Japan reported an inverse association between soy food intake and colon cancer risk (58). Women in the top tertile of soy food intake had about half the risk of colon cancer compared with women in the bottom tertile of intake. On the other hand, there have been several reports of a null association (59–61), including an earlier Japanese cohort study initiated in the mid-1960s on miso consumption and mortality from cancer (11). A small-scale, hospital-based case-control study (involving 51 rectal cancer patients and their controls) even suggested an adverse effect of soy consumption (62). It has been noted that the study tools used in previous studies may have had a limited ability to capture major food sources that contribute significantly to total soy intake or variance in soy consumption (9). Some of the studies did not make any distinction between fresh and preserved (fermented) soy foods (such as miso soup in Japan) in their dietary assessment and/or data analysis. Preserved foods contain high levels of N-nitroso compounds, which are potential carcinogens for many human cancers (63). In this population, soy consumption is primarily limited to fresh soy products. In addition, insufficient statistical power and/or inadequate controlling for potential confounding may also have contributed to the inconsistency.
Several features distinguish this study from previous investigations. This was the first study to prospectively evaluate the relation between soy food consumption and incident colorectal cancer risk with repeated dietary assessment of soy food intake. The FFQ used in this study covered virtually all soy foods consumed in the study population. The study population is well suited to the study of soy and colorectal cancer associations given its high yet diverse intake of soy foods. Other strengths of our study included the high participation (92% at enrollment) and follow-up (>96% for active, in-person follow-up) rates and the use of an in-person interview. Additionally, we carefully adjusted for a wide range of dietary and nondietary factors that are potential confounders of the soy and colorectal cancer association.
As with all nutritional epidemiology studies, measurement error in assessing soy food intake is a possible concern. However, the Shanghai Women's Health Study FFQ was previously shown to have good validity for the measurement of soy food intake as compared with intake assessed by 24 d (2 d/mo) of 24-h dietary recalls (15). The validity of the FFQ in assessing usual soy food intake was also supported by our recent work in 48 men, in which a moderate correlation (γ = 0.48) was found between soy food intake derived from the FFQ and the mean level of isoflavone excretion measured in 4 spot urine samples collected over a 1-y period preceding the FFQ survey (one sample was collected in each quarter of the year) (64). In addition, because the exposure assessment was conducted prospectively and before cancer diagnosis, errors in measurement of soy consumption are more likely to be nondifferential, which would tend to attenuate the true association between soy consumption and colorectal cancer.
In conclusion, this prospective cohort study provides strong evidence in humans that soy food consumption may protect against colorectal cancer, particularly against rectal cancer among postmenopausal women. Given the fact that colorectal cancer is one of the most common cancers worldwide and that soy can be readily incorporated into most diets, our findings have important public health implications in the prevention of this common malignancy.
Acknowledgments
We are grateful to the participants and research staff of the Shanghai Women's Health Study for their contributions to the study.
The authors' responsibilities were as follows—GY, X-OS, and WZ: study design, data collection, statistical analyses, and manuscript writing; HL and HC: data collection and management; XZ: statistical analyses and manuscript writing; and W-HC and Y-TG: data collection and manuscript revision. None of the authors had any financial conflicts of interest to declare.
REFERENCES
- 1.International Agency for Research on Cancer Cancer incidence in five continents. Lyon, France: IARC, 2003 [Google Scholar]
- 2.Potter JD, Slattery ML, Bostick RM, Gapstur SM. Colon cancer: a review of the epidemiology. Epidemiol Rev 1993;15:499–545 [DOI] [PubMed] [Google Scholar]
- 3.Whittemore AS. Colorectal cancer incidence among Chinese in North America and the People's Republic of China: variation with sex, age and anatomical site. Int J Epidemiol 1989;18:563–8 [DOI] [PubMed] [Google Scholar]
- 4.Whittemore AS, Wu-Williams AH, Lee M, et al. Diet, physical activity, and colorectal cancer among Chinese in North America and China. J Natl Cancer Inst 1990;82:915–26 [DOI] [PubMed] [Google Scholar]
- 5.Schabath MB, Hernandez LM, Wu X, Pillow PC, Spitz MR. Dietary phytoestrogens and lung cancer risk. JAMA 2005;294:1493–504 [DOI] [PubMed] [Google Scholar]
- 6.Wu AH, Koh WP, Wang R, Lee HP, Yu MC. Soy intake and breast cancer risk in Singapore Chinese Health Study. Br J Cancer 2008;99:196–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu WH, Zheng W, Xiang YB, et al. Soya food intake and risk of endometrial cancer among Chinese women in Shanghai: population based case-control study. BMJ 2004;328:1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shu XO, Jin F, Dai Q, et al. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev 2001;10:483–8 [PubMed] [Google Scholar]
- 9.Spector D, Anthony M, Alexander D, Arab L. Soy consumption and colorectal cancer. Nutr Cancer 2003;47:1–12 [DOI] [PubMed] [Google Scholar]
- 10.Badger TM, Ronis MJ, Simmen RC, Simmen FA. Soy protein isolate and protection against cancer. J Am Coll Nutr 2005;24:146S–9S [DOI] [PubMed] [Google Scholar]
- 11.Hirayama T. Contribution of a long-term prospective cohort study to the issue of nutrition and cancer with special reference to the role of alcohol drinking. Prog Clin Biol Res 1990;346:179–87 [PubMed] [Google Scholar]
- 12.Witte JS, Longnecker MP, Bird CL, Lee ER, Frankl HD, Haile RW. Relation of vegetable, fruit, and grain consumption to colorectal adenomatous polyps. Am J Epidemiol 1996;144:1015–25 [DOI] [PubMed] [Google Scholar]
- 13.Keinan-Boker L, van Der Schouw YT, Grobbee DE, Peeters PH. Dietary phytoestrogens and breast cancer risk. Am J Clin Nutr 2004;79:282–8 [DOI] [PubMed] [Google Scholar]
- 14.Zheng W, Chow WH, Yang G, et al. The Shanghai Women's Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol 2005;162:1123–31 [DOI] [PubMed] [Google Scholar]
- 15.Shu XO, Yang G, Jin F, et al. Validity and reproducibility of the food frequency questionnaire used in the Shanghai Women's Health Study. Eur J Clin Nutr 2004;58:17–23 [DOI] [PubMed] [Google Scholar]
- 16.Yang YX, Wang GY, Pan XC.eds China food composition tables 2002. Beijing, China: Beijing University Medical Press, 2002 [Google Scholar]
- 17.Matthews CE, Shu XO, Yang G, et al. Reproducibility and validity of the Shanghai Women's Health Study physical activity questionnaire. Am J Epidemiol 2003;158:1114–22 [DOI] [PubMed] [Google Scholar]
- 18.Harrell FJ., Jr Regression modeling strategies: with applications to linear models, logistic regression, survival analysis. New York, NY: Springer-Verlag, 2001 [Google Scholar]
- 19.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–8S [DOI] [PubMed] [Google Scholar]
- 20.Greenland S, Rothman KJ. Introduction to stratified analysis. Rothman KJ, Greenland S.eds Modern epidemiology. Philadelphia, PA: Lippincott-Raven, 1998275–7 [Google Scholar]
- 21.Linz AL, Xiao R, Parker JG, Simpson PM, Badger TM, Simmen FA. Feeding of soy protein isolate to rats during pregnancy and lactation suppresses formation of aberrant crypt foci in their progeny's colons: interaction of diet with fetal alcohol exposure. J Carcinog 2004;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo JY, Li X, Browning JD, Jr, et al. Dietary soy isoflavones and estrone protect ovariectomized ERalphaKO and wild-type mice from carcinogen-induced colon cancer. J Nutr 2004;134:179–82 [DOI] [PubMed] [Google Scholar]
- 23.Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey A, Harper P. Dietary phytoestrogen intake is associated with reduced colorectal cancer risk. J Nutr 2006;136:3046–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer 2003;3:601–14 [DOI] [PubMed] [Google Scholar]
- 25.Courtney ED, Melville DM, Leicester RJ. Review article: chemoprevention of colorectal cancer. Aliment Pharmacol Ther 2004;19:1–24 [DOI] [PubMed] [Google Scholar]
- 26.Mason JB, Levesque T. Folate: effects on carcinogenesis and the potential for cancer chemoprevention. Oncology 1996;10:1727–36 [PubMed] [Google Scholar]
- 27.Potter JD. Colorectal cancer: molecules and populations. J Natl Cancer Inst 1999;91:916–32 [DOI] [PubMed] [Google Scholar]
- 28.Newcomb PA, Storer BE. Postmenopausal hormone use and risk of large-bowel cancer. J Natl Cancer Inst 1995;87:1067–71 [DOI] [PubMed] [Google Scholar]
- 29.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med 2004;350:991–1004 [DOI] [PubMed] [Google Scholar]
- 30.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA 2003;289:3243–53 [DOI] [PubMed] [Google Scholar]
- 31.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321–33 [DOI] [PubMed] [Google Scholar]
- 32.Lechner D, Kallay E, Cross HS. Phytoestrogens and colorectal cancer prevention. Vitam Horm 2005;70:169–98 [DOI] [PubMed] [Google Scholar]
- 33.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest 2006;116:561–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998;139:4252–63 [DOI] [PubMed] [Google Scholar]
- 35.Foley EF, Jazaeri AA, Shupnik MA, Jazaeri O, Rice LW. Selective loss of estrogen receptor beta in malignant human colon. Cancer Res 2000;60:245–8 [PubMed] [Google Scholar]
- 36.Martineti V, Picariello L, Tognarini I, et al. ERbeta is a potent inhibitor of cell proliferation in the HCT8 human colon cancer cell line through regulation of cell cycle components. Endocr Relat Cancer 2005;12:455–69 [DOI] [PubMed] [Google Scholar]
- 37.Jassam N, Bell SM, Speirs V, Quirke P. Loss of expression of oestrogen receptor beta in colon cancer and its association with Dukes' staging. Oncol Rep 2005;14:17–21 [PubMed] [Google Scholar]
- 38.Konstantinopoulos PA, Kominea A, Vandoros G, et al. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour's dedifferentiation. Eur J Cancer 2003;39:1251–8 [DOI] [PubMed] [Google Scholar]
- 39.Campbell-Thompson M, Lynch IJ, Bhardwaj B. Expression of estrogen receptor (ER) subtypes and ERbeta isoforms in colon cancer. Cancer Res 2001;61:632–40 [PubMed] [Google Scholar]
- 40.Hwang CS, Kwak HS, Lim HJ, et al. Isoflavone metabolites and their in vitro dual functions: they can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J Steroid Biochem Mol Biol 2006;101:246–53 [DOI] [PubMed] [Google Scholar]
- 41.Bennink MR. Dietary soy reduces colon carcinogenesis in human and rats: soy and colon cancer. Adv Exp Med Biol 2001;492:11–7 [DOI] [PubMed] [Google Scholar]
- 42.Raschke M, Rowland IR, Magee PJ, Pool-Zobel BL. Genistein protects prostate cells against hydrogen peroxide-induced DNA damage and induces expression of genes involved in the defence against oxidative stress. Carcinogenesis 2006;27:2322–30 [DOI] [PubMed] [Google Scholar]
- 43.Djuric Z, Chen G, Doerge DR, Heilbrun LK, Kucuk O. Effect of soy isoflavone supplementation on markers of oxidative stress in men and women. Cancer Lett 2001;172:1–6 [DOI] [PubMed] [Google Scholar]
- 44.Chan WH, Yu JS. Inhibition of UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermal carcinoma A431 cells by genistein. J Cell Biochem 2000;78:73–84 [DOI] [PubMed] [Google Scholar]
- 45.Li M, Zhang Z, Hill DL, Chen X, Wang H, Zhang R. Genistein, a dietary isoflavone, down-regulates the MDM2 oncogene at both transcriptional and posttranslational levels. Cancer Res 2005;65:8200–8 [DOI] [PubMed] [Google Scholar]
- 46.Cross HS, Kallay E, Lechner D, Gerdenitsch W, Adlercreutz H, Armbrecht HJ. Phytoestrogens and vitamin D metabolism: a new concept for the prevention and therapy of colorectal, prostate, and mammary carcinomas. J Nutr 2004;134:1207S–12S [DOI] [PubMed] [Google Scholar]
- 47.Kim EJ, Shin HK, Park JH. Genistein inhibits insulin-like growth factor-I receptor signaling in HT-29 human colon cancer cells: a possible mechanism of the growth inhibitory effect of genistein. J Med Food 2005;8:431–8 [DOI] [PubMed] [Google Scholar]
- 48.Jun M, Hong J, Jeong WS, Ho CT. Suppression of arachidonic acid metabolism and nitric oxide formation by kudzu isoflavones in murine macrophages. Mol Nutr Food Res 2005;49:1154–9 [DOI] [PubMed] [Google Scholar]
- 49.Frey RS, Singletary KW. Genistein activates p38 mitogen-activated protein kinase, inactivates ERK1/ERK2 and decreases Cdc25C expression in immortalized human mammary epithelial cells. J Nutr 2003;133:226–31 [DOI] [PubMed] [Google Scholar]
- 50.Lo FH, Mak NK, Leung KN. Studies on the anti-tumor activities of the soy isoflavone daidzein on murine neuroblastoma cells. Biomed Pharmacother 2007;61:591–5 [DOI] [PubMed] [Google Scholar]
- 51.Hwang JT, Ha J, Park OJ. Combination of 5-fluorouracil and genistein induces apoptosis synergistically in chemo-resistant cancer cells through the modulation of AMPK and COX-2 signaling pathways. Biochem Biophys Res Commun 2005;332:433–40 [DOI] [PubMed] [Google Scholar]
- 52.Thiagarajan DG, Bennink MR, Bourquin LD, Kavas FA. Prevention of precancerous colonic lesions in rats by soy flakes, soy flour, genistein, and calcium. Am J Clin Nutr 1998;68:1394S–9S [DOI] [PubMed] [Google Scholar]
- 53.Adams KF, Lampe PD, Newton KM, et al. Soy protein containing isoflavones does not decrease colorectal epithelial cell proliferation in a randomized controlled trial. Am J Clin Nutr 2005;82:620–6 [DOI] [PubMed] [Google Scholar]
- 54.Petrakis NL, Barnes S, King EB, et al. Stimulatory influence of soy protein isolate on breast secretion in pre- and postmenopausal women. Cancer Epidemiol Biomarkers Prev 1996;5:785–94 [PubMed] [Google Scholar]
- 55.Le Marchand L, Hankin JH, Wilkens LR, Kolonel LN, Englyst HN, Lyu LC. Dietary fiber and colorectal cancer risk. Epidemiology 1997;8:658–65 [DOI] [PubMed] [Google Scholar]
- 56.Hu JF, Liu YY, Yu YK, Zhao TZ, Liu SD, Wang QQ. Diet and cancer of the colon and rectum: a case-control study in China. Int J Epidemiol 1991;20:362–7 [DOI] [PubMed] [Google Scholar]
- 57.Hoshiyama Y, Sekine T, Sasaba T. A case-control study of colorectal cancer and its relation to diet, cigarettes, and alcohol consumption in Saitama Prefecture, Japan. Tohoku J Exp Med 1993;171:153–65 [DOI] [PubMed] [Google Scholar]
- 58.Oba S, Nagata C, Shimizu N, et al. Soy product consumption and the risk of colon cancer: a prospective study in Takayama, Japan. Nutr Cancer 2007;57:151–7 [DOI] [PubMed] [Google Scholar]
- 59.Seow A, Quah SR, Nyam D, Straughan PT, Chua T, Aw TC. Food groups and the risk of colorectal carcinoma in an Asian population. Cancer 2002;95:2390–6 [DOI] [PubMed] [Google Scholar]
- 60.Inoue M, Tajima K, Hirose K, et al. Subsite-specific risk factors for colorectal cancer: a hospital-based case-control study in Japan. Cancer Causes Control 1995;6:14–22 [DOI] [PubMed] [Google Scholar]
- 61.Haenszel W, Berg JW, Segi M, Kurihara M, Locke FB. Large-bowel cancer in Hawaiian Japanese. J Natl Cancer Inst 1973;51:1765–79 [DOI] [PubMed] [Google Scholar]
- 62.Tajima K, Tominaga S. Dietary habits and gastro-intestinal cancers: a comparative case-control study of stomach and large intestinal cancers in Nagoya. Japan. Jpn J Cancer Res 1985;76:705–16 [PubMed] [Google Scholar]
- 63.Wu AH, Yang D, Pike MC. A meta-analysis of soyfoods and risk of stomach cancer: the problem of potential confounders. Cancer Epidemiol Biomarkers Prev 2000;9:1051–8 [PubMed] [Google Scholar]
- 64.Lee SA, Wen W, Xiang YB, et al. Assessment of dietary isoflavone intake among middle-aged Chinese men. J Nutr 2007;137:1011–6 [DOI] [PMC free article] [PubMed] [Google Scholar]