Abstract
Nanodisks (ND) are nanometer scale complexes of phospholipid and apolipoprotein that have been shown to function as drug delivery vehicles. ND harboring significant quantities of the antifungal agent, amphotericin B, or the bioactive isoprenoid, all trans retinoic acid, have been generated and characterized. As currently formulated, ND possess limited targeting capability. In the present study we constructed a single chain variable antibody (scFv)•apolipoprotein chimera and assessed the ability of this fusion protein to form ND and recognize the antigen to which the scFv is directed. Data obtained revealed that α-vimentin scFv•apolipoprotein A-I is functional in ND formation and antigen recognition, opening the door to the use of such chimeras in targeting drug-enriched ND to specific tissues.
Keywords: single chain variable antibody, apolipoprotein, chimera, nanodisk, targeting, drug delivery, phospholipid
Introduction
Nanodisks are discrete nanoscale-sized ternary complexes of apolipoprotein (apo), phospholipid and incorporated hydrophobic biomolecules. These disk-shaped complexes have been formulated with the antifungal agent, amphotericin B (AMB), as well as the retinoid, all trans retinoic acid (ATRA) [1]. In the case of AMB, ND were able to solubilize significant quantities of the antibiotic with retention of potent biological activity [2]. Likewise, ATRA was efficiently solubilized in ND and was effective at inducing apoptosis in cultured hepatocarcinoma cells [3].
An advantage of the ND platform compared to liposomes or other delivery vehicles is the presence of intrinsically associated protein. ND exist as disk-shaped particles that are far smaller that liposomes (10 – 20 nm versus 75–250 nm). The protein component of ND serves as a scaffold that circumscribes the perimeter of the discoidal bilayer, interacting with the hydrophobic fatty acyl chains of phospholipids at the edge of the disk. Members of the class of plasma apolipoproteins (apo) are well suited to this function since they are generally comprised of a series of amphipathic α-helices. Thus, hydrophobic interactions between the non polar face of apolipoprotein α-helices and disk phospholipids stabilize the particle while complementary interactions of the polar face of these helices with the aqueous milieu confers water solubility.
In an effort to expand the targeting capability of ND, we have explored the concept that a single chain variable antibody (scFv)•apolipoprotein chimera may provide a means to confer targeting to ND. In the present study we have generated a α-vimentin (VIM) scFv•apoA-I chimera. The fusion protein was expressed in bacteria, isolated and shown to retain ND formation capability. Furthermore, the scFv component of the chimera recognized VIM on immunoblots. Thus, we conclude that scFv•apoA-I chimeras represent a potentially feasible means to target drug bearing ND to specific tissues.
Material and Methods
Chimera cDNA Construction
The α-VIM scFv•apoA-I chimeric gene fusion was constructed uisng an α-VIM scFv cDNA (provided by Dr. Jill Winter, Children’s Hospital Oakland Research Institute). A polylinker was introduced at the 3′ terminus in addition to two restriction sites to allow joining of the scFv and apoAI cDNAs. A 3′ terminal His-tag encoding sequence was introduced to facilitate downstream processing of the protein product. Briefly, NdeI and ClaI restriction sites were introduced into the 5′ and 3′ terminal ends respectively of the α-VIM scFv nucleotide sequence by amplification with the following primers: GGTACCAAAAGCTGGCATATGAAACAAAGCACTATTGCAC (forward primer containing NdeI site) and CGGCGGATCATCGATACTGCCCCCTCCACCTGAGCCACCTCCCCCGAATTCCTGGCCAAG AACGGTTAA (reverse primer containing ClaI site). The reverse primer also included nucleotide sequences (underlined) encoding a gly(4)-ser-gly(4)-ser peptide linker to serve as a spacer between the scFv and apoA-I components of the fusion protein. PCR reactions employed Thermo Start DNA polymerase (Thermo Scientific) and consisted of 1X reaction buffer (1.5 mM MgCl2), 0.2mM dNTPs, 300nM forward and reverse primers and 5U of polymerase in a 50 μl reaction. Thermal cycling consisted of a 10 min activation of the thermal start polymerase at 95 °C followed by 35 cycles at: 95 °C for 30 sec, 65 °C for 20 sec, 72 °C for 2.5 min. Amplified scFv gene product was double digested with NdeI and ClaI (New England Biolabs) according to the manufacturers guidelines and ligated into the NdeI/ClaI double digest cloning site located at the 5′ terminus of the apoA-I plasmid sequence [4]. The resulting scFv•apoA-I cDNA fusion clone was released from the plasmid vector by NdeI/HindIII double digestion and ligated into the NdeI/HindIII polylinker region of pET41b(+) expression vector (Novagen) containing an in-frame 3′ terminal His-tag. The final gene construct encoded a chimeric protein containing the alkaline phosphatase A (PhoA) signal peptide, α-VIM scFv, gly(4)-ser-gly(4)-ser peptide linker, apoA-I, His-tag, as follows: NH2-PhoA•α-VIM scFv•linker•apoAI•His-tag-COOH, hereafter referred to as α-VIM scFv•apoA-I.
Expression, purification and characterization of the scFv•apoA-I chimera
scFv•apoA-I chimera and control apoA-I expression and purification were performed according to the Ryan et al. [4] with some modification. Briefly, E. coli BL21(DE3)pLysS cells bearing the pET/V-A plasmid were cultured in 500 mL NCZYM medium (with 50 μg/mL kanamycin, 10 mM MgCl2 and 20 mM MOPS buffer, pH 7.0) at 37 °C. When the culture OD600 reached 0.8, it was quickly cooled to room temperature in an ice water bath. Protein synthesis was induced by the addition of isopropyl thiogalactopyranoside to a final concentration of 1.0 mM and aeration continued at room temperature overnight. The bacteria were pelleted by centrifugation, resuspended in 50 mM NaH2PO4/150 mM NaCl, pH 8.0 (PBS) containing Complete, EDTA-free protease inhibitors (Roche) and 1 μg/ml DNAse I, Type II (Sigma). Periplasmic proteins were released with 3 freeze-thaw cycles by incubation in liquid nitrogen for 10 min followed by 15 min in a 37 °C water bath. The cell lysate was centrifuged at 20000g for 30 min at 4 °C. The supernatant containing the periplasmic fraction was then mixed with an equal volume of PBS and applied to a 5 mL bed volume Hi-Trap affinity column (Amersham Pharmacia Biotech), washed with 2 column volumes of buffer and 40 mM imidazole, and eluted in a buffer containing 500 mM imidazole. Fractions containing protein were pooled and dialyzed against PBS, resulting in precipitation of the protein. The sample was re-dissolved in 0.05% trifluoroacetic acid in water (or 0.1 N HCl) and further purified on a Perkin-Elmer Series 200 high-pressure liquid chromatograph. The sample was applied to an RXC-8 Zorbax 300SB semipreparative column and eluted with a linear AB gradient of 2% B/min, where solvent A was 0.05% trifluoroacetic acid in water and solvent B was 0.05% trifluoroacetic acid in acetonitrile. Fractions were monitored at 230 nm, and those containing α-VIM scFv•apoA-I were pooled, lyophilized, and stored at −20 °C.
Nanodisk formulation
Nanodisks were prepared according to the cholate dialysis method described by Jonas [5].
Analytical methods
Protein concentrations were determined by the bicinchoninic acid assay (Pierce Chemical Co.) with bovine serum albumin as standard. Choline containing phospholipids were quantified by an enzyme-based colorimetric assay (Wako). Nondenaturing PAGE was performed on 4–20% acrylamide slab gels. Samples were electrophoresed at 60 V constant voltage for 16 h. Gels were stained with Imperial Protein Stain (Pierce Chemical Co.), and the relative mobility of ND complexes was compared with those of standards of known size, including albumin, lactate dehydrogenase, catalase, horse ferritin, and thyroglobulin.
For immunoblotting, protein samples were run on a 4 to 20% acrylamide gradient, Tris-glycine SDS slab gel (Invitrogen Life Tech). Separated proteins were transferred to a 0.2 μm PVDF membrane using the blot module Electro-Eluter (Bio-Rad Laboratories) at a constant current of 150 mA for 3 h. Nonspecific binding sites on the membrane were blocked with 0.1% TTBS [0.1% Tween 20, 20 mM Tris, and 150 mM NaCl (pH 7.2)] overnight, at room temperature while the mixture was being rotated. Either α-VIM scFv or α-VIM scFv•apoA-I chimera (1:10000 dilution in 0.1% TTBS) was incubated with the membrane as the 1° Ab for 60 min with rotation. After washing (3X) in TTBS, rabbit anti-6XHisTag Ab (Abcam, diluted 1:10000 in 0.1% TTBS) was incubated with the membrane for 60 min (2° Ab). Subsequently, the membrane was washed in TTBS and horseradish peroxidase labeled mouse anti-IgG (3° Ab) was incubated with the membrane for 60 min followed by washing. Lastly, membranes were incubated with SuperSignal West Femto Maximum Sensitivity Substrate (Pierce Chemical Co.) in which both reagents were diluted by adding 0.5 mL in 9 mL of dH2O. The substrate was incubated for 2–5 min at room temperature and exposed to CL-Xposure Film (Pierce Chemical Co.) for 60 s. Film was developed using a Kodak M35A X-OMAT processor.
Results and Discussion
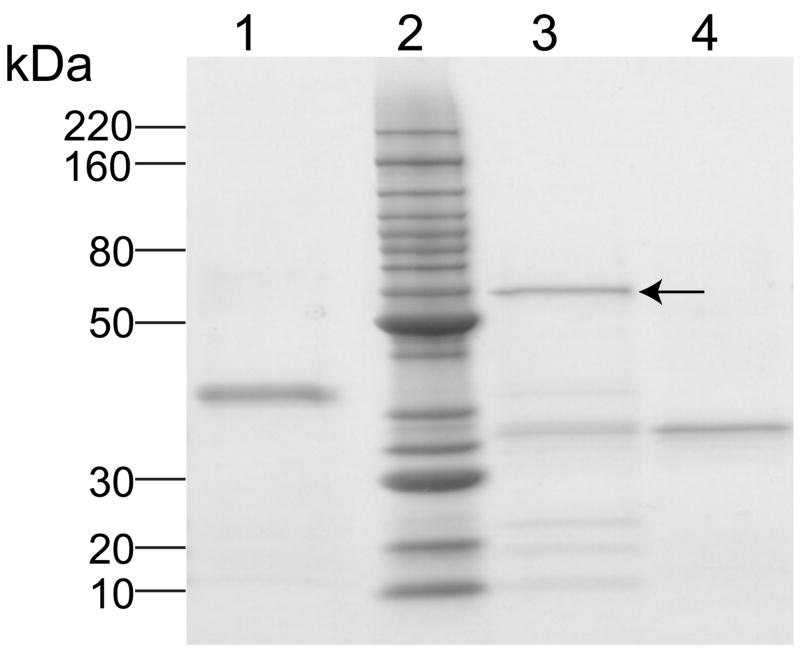
ND provide a versatile platform for solubilization and transport of hydrophobic biomolecules [1]. To extend the utility of this vehicle to cell type specific targeting, a protein engineering approach was employed. The apolipoprotein component of ND is intrinsically associated with the particle, circumscribing the perimeter of the bilayer disk. To confer targeting to the apolipoprotein, protein engineering was employed to fuse it to an scFv, creating a chimera (Figure 1). For the present study an scFv directed against the cellular matrix protein, VIM, was employed. Following construction of the chimera encoding cDNA and expression of the fusion protein in E. coli, the fusion protein was isolated and characterized by SDS-PAGE (Figure 2). Compared to either control apoA-I (lane 4, ~28 kDa), or control scFv (lane 1, ~32 kDa) the α-VIM scFv•apoA-I was larger in size (lane 3 arrow, ~ 60 kDa), in agreement with its predicted molecular weight of 57.7 kDa.
Figure 1. Cartoon depicting nanodisk technology.
Chimeric cDNA construct and protein product composed of α-VIM scFv•apoA-I with spacers, and N and C terminal extensions. Also depicted is the fusion protein associated with a nanodisk particle.
Figure 2. SDS-PAGE analysis of α-VIM scFv•apoA-I fusion protein.
Samples were electrophoresed on a 4–20% acrylamide gradient SDS slab gel under reducing conditions and stained with Imperial Protein Stain. Lane 1, α-VIM scFv; lane 2, Invitrogen Benchmark protein ladder molecular weight standards; lane 3, α-VIM scFv•apoA-I fusion protein (see arrow); lane 4, apoA-I.
Subsequently, studies were designed to assess whether the α-VIM scFv•apoA-I chimera possessed the capacity to form ND. Using apoA-I as a positive control, experiments revealed that fusion of α-VIM scFv to apoA-I did not compromise its ND formation properties. Nondenaturing PAGE analysis (Figure 3) revealed that the ND particles generated with α-VIM scFv•apoA-I fusion protein (lanes 3–6, increasing load volume) displayed a molecular weight (>232 kDa) significantly larger than that of the fusion protein alone (lane 2) and that fusion protein-containing ND particles are similar in size to those generated with wild type apoA-I (lane 8). Thus, it is evident that the present N-terminal extension of apoA-I does not abolish the lipid binding properties of the protein.
Figure 3. Native PAGE of nanodisks.
Samples were electrophoresed on a 4–20% acrylamide gel under native conditions and stained with Imperial Protein Stain. Lane 1, Native protein molecular weight standards; lane 2, α-VIM scFv•apoA-I fusion protein; lanes 3–6, increasing amounts of α-VIM scFv•apoA-I ND; lane 7, apoA-I; lane 8, apoA-I ND.
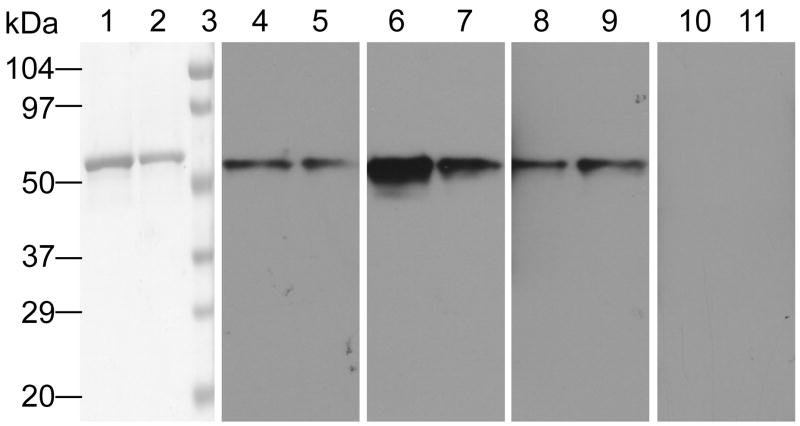
Another question relates to the antigen recognition properties of the α-VIM scFv component of the chimera. To address this, an immunoblot experiment was performed. Figure 4 shows a Western blot of VIM protein probed with α-VIM scFv, α-VIM scFv•apoA-I, α-VIM scFv•apoA-I ND as well as secondary/tertiary antibody controls. The data indicate that fusion of α-VIM scFv to apoA-I (lanes 6 and 7) or integration of the fusion protein into ND (lanes 8 and 9) does not abolish the antigen recognition properties of the scFv (lanes 4 and 5).
Figure 4. Western Blot Analysis.
VIM was electrophoresed (heavy and light load volumes) on 4–20% gradient SDS slab gels under reducing conditions and a) stained with Coomassie Blue (lanes 1 and 2 and lane 3, Molecular weight standards) or b) transferred to PVDF membrane and probed with the following antibodies (a specified 1° Ab), followed by 2° (α-His-tag) and 3° (α-IgG-HRP). Lanes 4 and 5, 1° Ab = α-VIM scFv; lanes 6 and 7, 1° Ab = α-VIM scFv•apoA-I; lanes 8 and 9, 1° Ab = α-VIM scFv•apoA-I ND; lanes 10 and 11, no 1° Ab.
The development of antibody-based therapies has expanded treatment strategies for neoplastic disease [6]. Specifically, the administration of anti-tumor monoclonal antibodies (mAbs) has shown efficacy in a variety of model systems, and benefits have been observed in human clinical trials of various lymphoma tumors. Based on this, strategies have been developed to optimize mAbs for clinical application. Extensive research on this topic has led to the development of scFv reagents [7]. These engineered antibody fragments, derived by protein design or phage display methods, retain the antigen recognition properties of the parent antibody. At the same time, their compact size and structure offer potential advantage in terms of bio-distribution, tumor penetration and immunogenicity. scFv are comprised of a single polypeptide chain containing a heavy chain variable region and a light chain variable region that are connected to one another by a polypeptide linker. Because scFv antibodies are composed of a linear polypeptide chain, they represent suitable molecules for engineering into chimeric fusion proteins. On this basis, we have explored a strategy to employ scFv as a targeting agent for drug payload delivery. ND are ideally suited for this function because they possess a high binding capacity for hydrophobic bioactive agents and an intrinsically associated apolipoprotein [1].
The hypotheses tested in the present study were whether an scFv fused to a model apolipoprotein would a) interfere with the ND formation capability of the apolipoprotein or b) alter the antigen recognition properties of the scFv. This latter question was examined for the α-VIM scFv•apoA-I chimera as a free protein and as a component of ND. The data presented clearly demonstrate that the α-VIM scFv•apoA-I fusion protein possesses the capacity to form ND and that the α-VIM scFv•apoA-I chimera recognizes antigen, both as a free protein and as a component of ND. Thus, the present study constitutes a proof of principle, demonstrating that tethering an scFv to ND via fusion to the apolipoprotein scaffold protein retains the antigen recognition properties of the scFv. As such, these studies permit expansion of ND technology toward targeted delivery in vivo. Given the success of mAb in cancer chemotherapy, a logical system to pursue would be an scFv directed against a cell surface antigen expressed solely or predominantly on malignant cells. By combining this scFv with apoA-I and formation of ND harboring one or more hydrophobic chemotherapeutic agents, it is reasonable to anticipate synergy will be observed. Based on recent success incorporating the anti cancer agent ATRA into ND [3] suggests targeting these particles via an scFv•apolipoprotein chimera would constitute a feasible extension of this technology.
Acknowledgments
We wish to thank Jianhui Zho for helpful discussions related to periplasmic expression, Dr. Jill Winter for providing the α-vimentin scFv encoding template DNA, and to John F. Hess for providing vimentin protein.
Footnotes
This work was supported by a grant from the National Institutes of Health (AI61354).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ryan RO. Nanodisks: hydrophobic drug delivery vehicle. Expert Opin Drug Deliv. 2008;5:343–351. doi: 10.1517/17425247.5.3.343. [DOI] [PubMed] [Google Scholar]
- 2.Oda MN, Hargreaves PL, Beckstead JA, Redmond KA, van Antwerpen R, Ryan RO. Reconstituted high-density lipoprotein enriched with the polyene antibiotic, amphotericin B. J Lipid Res. 2006;47:260–267. doi: 10.1194/jlr.D500033-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Redmond KA, Nguyen TS, Ryan RO. All-trans retinoic acid nanodisks. Int J Pharm. 2007;339:246–250. doi: 10.1016/j.ijpharm.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan RO, Forte TM, Oda MN. Optimized bacterial expression of human apolipoprotein A-I. Protein Expr Purif. 2003;27:98–103. doi: 10.1016/s1046-5928(02)00568-5. [DOI] [PubMed] [Google Scholar]
- 5.Jonas A. Reconstitution of high-density lipoproteins. Methods Enzymol. 1986;128:553–582. doi: 10.1016/0076-6879(86)28092-1. [DOI] [PubMed] [Google Scholar]
- 6.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–5283. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 7.Filpula D. Antibody engineering and modification technologies. Biomol Eng. 2007;24:201–215. doi: 10.1016/j.bioeng.2007.03.004. [DOI] [PubMed] [Google Scholar]