Abstract
Foxp3 plays a critical role in development of CD4+ regulatory T lymphocytes (Tregs). It was originally proposed as a specific marker for Tregs, but recent studies have shown that Foxp3 can be expressed in proliferating CD8+ and CD4+ T lymphocytes. We further investigated the association between Foxp3 expression and proliferation of peripheral blood CD4+ and CD8+ T lymphocytes and focused on virus-specific memory CD8+ T lymphocytes. We found that resting peripheral blood bulk and cytomegalovirus- or HIV-1-specific CD8+ T lymphocytes do not normally express Foxp3. However, stimulation in vitro triggered these cells to express Foxp3 as well as CD25, and the addition of interleukin-2 possibly enhanced the expression of Foxp3. These data demonstrate that proliferation itself is sufficient to induce the Treg-like phenotype. Given that others have demonstrated Treg functional activity in such “induced Tregs,” these results suggest that virus-specific CD8+ T lymphocytes have the capacity to acquire regulatory functions. Although the implications of Foxp3 expression in virus-specific CD8+ T lymphocytes in the immunologic control of persistent HIV-1 viremia remain to be determined, our results are consistent with Foxp3 expression playing an essential role in regulation of cell proliferation and functional outcomes for HIV-1-specific CD8+ T lymphocytes.
Introduction
The earliest attempts to classify the functions of T lymphocytes in the 1980s originally distinguished CD4+ T lymphocytes as “helper” and CD8+ T lymphocytes as “suppressor,” but it rapidly became apparent that the latter mostly represent lymphocytes with the capacity to serve as HLA class I-restricted effector cells. Eventually, true immunosuppressive regulatory T lymphocytes (Tregs) with clear roles in autoimmunity and malignancy were identified in mice1 and then humans.2 Interestingly, these were found to be a subset of activated CD4+ T lymphocytes expressing CD25, although specific markers were elusive.
Foxp3 has been proposed as an additional specific marker for Tregs within the CD4+CD25+ T-lymphocyte population. Foxp3 is a member of the forkhead/winged family of transcription factors,3 and acting through NFAT (nuclear factor of activated T lymphocytes) has been postulated to control key genes to specifically drive Treg development.4,5 However, recent data have cast doubt on this initial hypothesis. Foxp3 appears to be universally expressed in all proliferating T lymphocytes, including CD4+ and CD8+ subsets,6 and does not necessarily confer a Treg phenotype when expressed in CD4+ T lymphocytes.7,8
Given the generality of Foxp3 expression in proliferating CD4+ and CD8+ T lymphocytes, the factors determining Treg activity and the involvement of Foxp3 thus remain unclear. It is unknown whether CD8+ T lymphocytes can be regulatory. It has been suggested that a CD8+CD28− T-lymphocyte subset has regulatory functions.9 Regarding antigen-specific CD8+ T cells, it has been reported that some HIV-1-specific CD8+ T lymphocytes exert an immunosuppressive effect through secretion of transforming growth factor-β10 or interleukin (IL)-10.11 More recently, three studies have demonstrated that proliferation of human CD8+ T lymphocytes after in vitro stimulation induces negative regulatory functions associated with Foxp3 expression, albeit at lower levels than natural CD4+ Tregs in vivo.8,12,13 These studies raise questions regarding the functional implications of Foxp3 expression in CD8+ T lymphocytes and markers related to Treg activity. Whether antigenic stimulation of CD8+ T lymphocytes causes upregulation of Foxp3 in a manner that exerts negative regulatory function in vivo is unknown. In this study, we examine Foxp3 expression in proliferating bulk and antigen-specific CD8+ T lymphocytes in vivo (compared with classical CD4+ Tregs) and the same cells stimulated in vitro to assess the potential determinants of natural versus induced Tregs.
Materials and Methods
Study participants and isolation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood samples from 17 total study participants (all of whom were enrolled in the Multicenter AIDS Cohort Study) by Ficoll-Hypaque (Sigma, St. Louis, MO) density gradients. Four participants were HIV-1-infected individuals, and the remaining participants were HIV-1-uninfected. The study was approved by the Institutional Review Board at the University of California, Los Angeles.
Peptides
Peptides corresponding to epitopes from cytomegalovirus (CMV) pp65 (A2CMV, NLVPMVATV, 493–503), HIV-1 p17 (SL9; SLYNTVATL, 77–85), HIV-1 p24 (KF11, KAFSPE-VIPMF, 30–40, and IW9, ISPRTLNAW, 15–23) were purchased from the Protein Chemistry Core facility at University of Illinois.
Cell culture
Cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, L-glutamine, and penicillin-streptomycin (Sigma). IL-2 was provided by the NIH AIDS Reagent Repository. OKT3 and anti-human CD28 (clone CD28.2) monoclonal antibodies were obtained from BD Biosciences.
Antibody and tetramer staining of PBMCs
The following fluorochrome-conjugated antibodies were used: Foxp3-phycoerythrin (PE) (eBiosciences, San Diego, CA), and Ki-67-fluorescein isothiocyanate, CD107a-PE, CD25-PECy7, CD4-PerCP, CD3-allophycocyanin (APC), and CD8-APCCy7 (BD Biosciences, San Jose, CA). PBMCs were stained as described previously.14,15 Briefly, cells were washed and resuspended in phosphate-buffered saline with 4%fetal calf serum (FCS) and 0.1% sodium azide and incubated with a cocktail of monoclonal antibodies for 30 min at 4°C. For the MHC class I tetramer staining, 2–5 × 106 fresh PBMCs were prestained with 1/50-1/200 dilution of the appropriate tetramer in RPMI 1640 medium with 10% FCS at 37°C for 25 min. After the final wash, cells were fixed with 1% paraformaldehyde for analysis.
T-lymphocyte proliferation assay
PBMCs were labeled with 0.65 μM carboxyfluorescein diacetate succinimidyl ester (Invitrogen) in RPMI 1640 medium (GIBCO, Grand Island, NY) for 8 min at 37°C. The cells were immediately washed and plated in a 24-well plate. For bulk stimulation, cells were stimulated with 1 μg/ml OKT3. For antigen-specific stimulation, PBMCs were stimulated in the presence of 2 μM peptides in the presence or absence of IL-2 (50 U/ml). Cells were incubated for 6 days and then were harvested and prepared for flow cytometric analysis.
Intracellular Foxp3 staining
Intracellular Foxp3 staining was performed by using the Human Foxp3 Staining Kit (eBiosciences) and carried out according to the manufacturer's protocol.
Flow cytometry
A FACS Canto flow cytometer (Becton Dickinson) was calibrated for laser fluctuation/alignment and photomultipeer tube voltage adjustment by using chicken red blood cells (Biosure Inc., Grass Valley, CA) and Ultra Rainbow Calibration beads (Spherotech Inc., Libertyville, IL) prior to the sample acquisition. A six-color compensation matrix was created by six singly stained PBMC samples. We used a compounded gating scheme previously described14,16 with modifications. For visualization of ex vivo stained cells, CD4+ or CD8+ T lymphocytes were first gated on the CD3+ population on a CD3-APC and SS-Log plot, followed by a lymphocyte gate on an FS and SSLog plot. After potential doublets were excluded based on a FS-A and FS-H plot, the CD4+ and CD8+ population were gated separately on a CD4-PerCP versus CD8-APCCy7 plot for determining CD25, Foxp3, and/or Ki-67 expression. A similar gating strategy was used to visualize Foxp3 and CD25 expression on the tetramer+ population except these cells were gated first on the CD8high population on CD8-APCCy7 versus SS-Log, and the tetramer population was visualized on tetramer-APC versus SSLog plot. A minimum of 500 total tetramer+CD8high events were collected for the fully stained sample, necessitating collection of approximately 1–3 × 106 total events. Proper gating was established by using tetramer and CD8-PECy7 doubly stained cells in the presence of fluorescent-labeled isotype controls. Data analysis and graphic representations were done with FlowJo v.8.5.2 (TreeStar, Ashland, OR). This program was also used to compare the fluorescence intensities of the Foxp3+CD25+ cells stimulated in the presence or absence of IL-2. For this, we used a probability binning algorithm for the multivariate parameter to calculate the T(X) value.17,18
Statistical analysis
Analysis of variance and the Tukey-Kramer honestly statistical difference (HSD) test were employed for determining statistical significance between and among group means. Results are expressed as the mean ± standard error of mean unless otherwise noted. p < 0.05 was considered statistically significant. Statistical analysis and graphical representation were done using JMP v6.0.3 (JMP Sales, Cary, NC).
Results
Foxp3 and CD25 are coexpressed in about 5% of CD4+ and 0.2% of CD8+ peripheral blood T lymphocytes
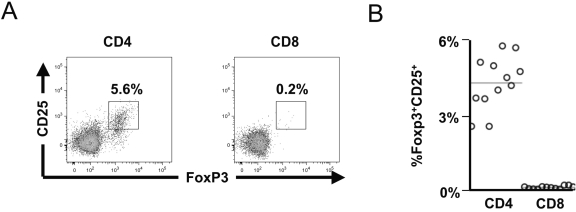
Peripheral blood CD4+ and CD8+ subsets of T lymphocytes were surveyed for Foxp3 and the activation marker CD25, to characterize the usual distribution of Foxp3 in T lymphocytes in vivo. In an evaluation of healthy (HIV-1-uninfected) individuals, it was notable that a significant subpopulation of the CD4+ T-lymphocyte subset coexpressed Foxp3 and CD25 (presumed to reflect natural Tregs), but that this phenotype was minimal or absent in the CD8+ T-lymphocyte subset (Fig. 1A). Across all individuals, 5.3 ± 0.3% of peripheral blood CD4+ T lymphocytes coexpressed Foxp3 and CD25, while this phenotype only represented 0.15 ± 0.01% of the CD8+ T-lymphocyte subset (Fig. 1B). These results were similar to prior studies defining Tregs as CD4+CD25+Foxp3+ T lymphocytes, which noted few if any CD8+ T lymphocytes with the CD25+Foxp3+ phenotype.
FIG. 1.
Only rare CD8+ T lymphocytes express CD25 and Foxp3 within peripheral blood. Peripheral blood mononuclear cells from 12 healthy (HIV-1-uninfected) subjects were analyzed for expression of Foxp3 and CD25 in CD4+ and CD8+ T-lymphocyte subsets by flow cytometry. (A) Representative plots are shown (gated on CD4+ and CD8+ T-lymphocyte populations). (B) Data from all 12 individuals are summarized.
Proliferation of peripheral blood T lymphocytes in healthy individuals in vivo is not sufficient to induce Foxp3 and CD25 expression
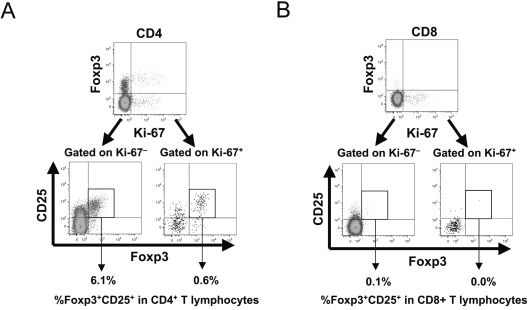
Given recent observations that Foxp3 can be upregulated in activated/proliferating T lymphocytes after in vitro stimulation,7,13 we evaluated the expression of Foxp3 and CD25 in relation to the proliferation status of CD4+ and CD8+ T lymphocytes in the peripheral blood of healthy (HIV-1-uninfected) individuals, using Ki-67 expression as a marker of proliferation.19 The percentages of proliferating cells were similar between CD4+ and CD8+ T lymphocytes (Fig. 2).
FIG. 2.
Foxp3 and CD25 expression does not correlate to proliferation in peripheral blood CD8+ and CD4+ T lymphocytes. Peripheral blood mononuclear cells from 13 healthy (HIV-1-uninfected) subjects were assessed for proliferative status (Ki-67 expression) and CD25/Foxp3 expression in CD4+ and CD8+ T lymphocytes by flow cytometry. Ki-67 expression was observed in 1.82 ± 0.17% versus 1.67 ± 0.15% of CD4+ and CD8+ T lymphocytes respectively (not shown). (A, B) Representative plots are shown for CD4+ (A) and CD8+ (B) T lymphocytes. The upper plots show Foxp3 versus Ki-67 expression, and the lower subplots show Foxp3 and CD25 expression on gated Ki-67+ and Ki-67− subpopulations (percentages indicate frequency within total CD4+ or CD8+ T lymphocytes). (C) Data from all 13 subjects are summarized.
The proliferation status of T lymphocytes appeared unrelated to Foxp3 and CD25 expression for both CD4+ and CD8+ T lymphocytes. Within the CD4+ T-lymphocyte compartment, most Foxp3+CD25+ cells were not proliferating, and most proliferating cells did not express Foxp3 and CD25 (Fig. 2A). Foxp3+CD25+Ki-67− cells accounted for 4.3 ± 0.3%, whereas Foxp3+CD25+Ki-67+ cells accounted for only 0.4 ± 0.04% of the total CD4+ T-lymphocyte population (Fig. 2C). Thus, the vast majority of CD4+ T lymphocytes that appeared to have the natural Treg phenotype were not proliferating. Within the CD8+ T-lymphocyte compartment, the rare Foxp3+CD25+ cells were mostly not proliferating, and most proliferating cells did not express Foxp3 and CD25 (Fig. 2B). Foxp3+CD25+Ki-67+ and Foxp3+CD25+Ki-67− cells accounted for 0.01 ± 0.01% and 0.22 ± 0.01% of the total CD8+ T-lymphocyte population, respectively (Fig. 2C).
These data suggested that proliferation of T lymphocytes in peripheral blood under normal physiologic conditions in vivo does not drive high levels of Foxp3 expression and that the mechanism of proliferation-driven transcriptional regulation for Foxp3 appears to require additional factors. Also, the data demonstrated that the majority of classical CD4+ Tregs are not proliferating in vivo, and a rare subset of CD8+ T lymphocytes may have a similar phenotype.
In vitro stimulation and proliferation of bulk T lymphocytes drives CD25 and Foxp3 expression, which is enhanced by IL-2
It has been reported that driving T lymphocytes to proliferate in vitro can induce expression of Foxp3,7,13 but the determinants of this phenomenon are poorly understood in light of our above observation that most proliferating cells in vivo do not express Foxp3 (Fig. 2). Because IL-2 is believed to upregulate Foxp3 expression through Stat5,20–22 we tested whether IL-2 affects the expression of Foxp3 in peripheral blood T lymphocytes during in vitro stimulation by CD3 signaling, using cells from healthy (HIV-1-uninfected) donors.
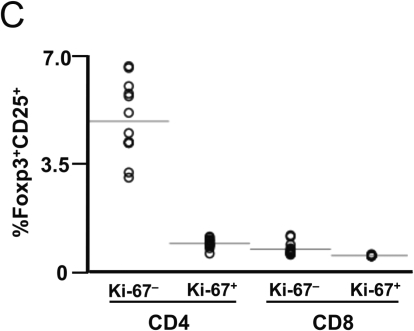
Among CD4+ T lymphocytes (Fig. 3A), a fraction of cells corresponding to natural Tregs expressed Foxp3 in the unstimulated control (Figs. 3A and 3C), and upon stimulation, a majority of these cells did not proliferate even in the presence of IL-2, consistent with our above observation that natural Tregs are not proliferating in vivo, and further suggesting that they may lack proliferative capacity. In contrast, a population of proliferating cells clearly demonstrated coexpression of CD25 and Foxp3 (Figs. 3A and 3C). Across subjects, 28 ± 3% and 32 ± 4% of CD4+ T lymphocytes proliferated and expressed CD25 and Foxp3 in the absence and presence of IL-2, respectively (Fig. 3C). This increase in the presence of IL-2 was not statistically significant; however, there was also a trend for increased intensity of Foxp3 expression with IL-2 (Fig. 3A).
FIG. 3.
Subsets of proliferating bulk CD4+ and CD8+ T lymphocytes express Foxp3 after anti-CD3 stimulation, with enhancement by added interleukin (IL)-2. Peripheral blood mononuclear cells from eight healthy (HIV-1-uninfected) subjects were assessed for proliferation after stimulation with anti-CD3 antibody in the absence and presence of added IL-2. (A, B) Representative plots are shown for each of the four conditions (Uns = unstimulated; Uns + IL-2 = unstimulated with added 50 U/ml IL-2; St = stimulated by anti-CD3 antibody; St + IL-2 = stimulated with added 50 U/ml IL-2). Proliferation was determined by carboxyfluorescein succinyl ester dilution assessment by flow cytometry, with costaining of the CD4+ (A) and CD8+ (B) T lymphocytes for CD25 and Foxp3. (C) The results are summarized for all eight subjects. (D) Foxp3 were generally coexpressed in all analyses; a representative contour plot is shown to demonstrate coexpression.
Among the CD8+ T lymphocytes (Fig. 3B), rare cells expressed Foxp3 in the unstimulated control cultures. A clear subset proliferated after stimulation, and some of these expressed both CD25 and Foxp3. Similar to the CD4+ T lymphocytes, after stimulation, 37 ± 2% and 39 ± 4% of the CD8+ T lymphocytes proliferated in the absence and presence of IL-2 (Fig. 3C). Again, there were trends for increased percentage and intensity of Foxp3 expression the presence of IL-2 (Fig. 3B), suggesting that the mechanism of proliferation-induced Foxp3 expression in CD8+ T lymphocytes parallels that of CD4+ T lymphocytes.
Overall, these results agreed with the findings of Pillai et al.13 demonstrating similar percentages of upregulation of Foxp3 in proliferating CD8+ T lymphocytes in vitro and additionally suggested that IL-2 enhances this phenomenon. However, the observation that only a portion of proliferating T lymphocytes expressed Foxp3 raised the question of whether a distinct subset of cells possessed this capacity. Given the function of memory T lymphocytes to proliferate more rapidly in response to stimulation than naïve T lymphocytes, this suggested that antigen-specific memory T lymphocytes might have larger capacities to express Foxp3 upon stimulation.
Virus-specific memory CD8+ T lymphocytes can express CD25 and Foxp3 when stimulated to proliferate
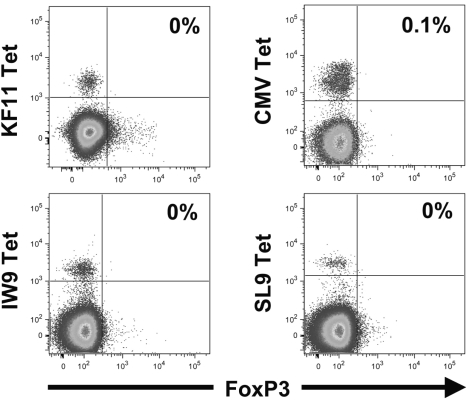
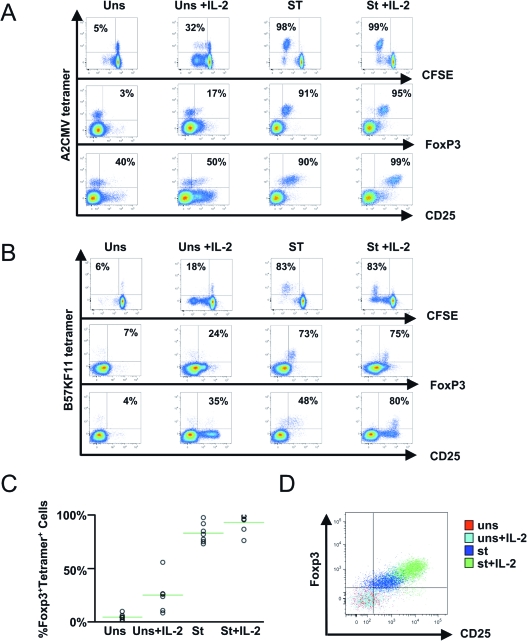
To examine whether antigen-specific memory CD8+ T lymphocytes were the population that expressed Foxp3 upon stimulation, peripheral blood virus-specific CD8+ T lymphocytes from persons with chronic CMV and/or HIV-1 infection were analyzed. In vivo, peripheral blood virus-specific CD8+ T lymphocytes did not express Foxp3 (Fig. 4), indicating that memory cells even in a persistent antigenic stimulation (chronic HIV-1 infection) do not express detectable levels of Foxp3. In contrast, epitope-specific stimulation in vitro efficiently induced Foxp3 expression; nearly all CMV and HIV-1 epitope-specific CD8+ T lymphocytes proliferated and expressed both CD25 and Foxp3 (Figs. 5A and 5B). For the seven distinct epitope-specific CD8+ T-lymphocyte populations examined, 83 ± 3% of the proliferating cells coexpressed these markers in the absence of added IL-2, and the addition of IL-2 increased the percentage to 92 ± 3% (Fig. 5C). Moreover, the intensity of Foxp3 expression in the stimulated virus-specific cells was significantly elevated by added IL-2 (Fig. 5D, T(^) median 51, range 12–119).
FIG. 4.
Peripheral blood memory cytomegalovirus (CMV)-and HIV-1-specific CD8+ T lymphocytes do not express Foxp3 in vivo despite the presence of ongoing viral infection. Peripheral blood mononuclear cells from two HIV-1-infected subjects were stained with peptide/MHC-I tetramers to identify CD8+ T lymphocytes directed against epitopes from CMV or HIV-1 (KF11, IW9, SL9). Foxp3 expression is plotted, and the percentages of Foxp3+ tetramer+ CD8+ T lymphocytes are indicated.
FIG. 5.
Virus-specific memory CD8+ T lymphocytes efficiently express CD25 and Foxp3 when proliferating in response to cognate epitopes in vitro, with enhancement by interleukin (IL)-2. Peripheral blood mononuclear cells from four persons with HIV-1 and cytomegalovirus (CMV) infection and one with CMV infection were stimulated with peptide epitopes in the absence or presence of added IL-2 and assessed for proliferation and expression of CD25 and Foxp3 as described in Figure 3. (A, B) Representative plots are shown for CMV (A) and HIV-1 (B) epitopes (seven tested). The results were similar for CMV and HIV-1 epitopes across all individuals. (C) Data are summarized across all epitopes and subjects. (D) An overlay of dot plots depicting Foxp3 versus CD25 expression demonstrates the intensity of expression across different conditions for the experiment plotted in panel A.
These results indicated that antigen-specific memory CD8+ T lymphocytes (and possibly memory cells in general) have the capacity to express Foxp3 during antigen-driven proliferation under certain stimulatory conditions, particularly in the presence of IL-2. Overall, these data suggested that memory T lymphocytes are the major subpopulation within the bulk PBMC population that can develop the induced Treg-like CD25+Foxp3+ phenotype upon stimulation.
Discussion
After the discovery of CD4+ regulatory T lymphocytes, there has been considerable interest in identifying specific marker(s) both to have a tool for accurate quantitation in pathogenesis studies as well as to better understand the biology of their action. Despite the initial optimism for the activation marker CD25, and subsequently CD25 with Foxp3, being specific markers for Tregs,23,24 it has become clear that features governing development of these cells is complex and cannot be defined solely by examining these two markers. It is clear, however, that Foxp3 contributes an important role, given studies showing that this protein represses important genes for T-lymphocyte activation and proliferation and activates genes involving regulatory function.4,5
Despite being a central player in the development of Treg activity in CD4+ T lymphocytes, the factors that determine the impact of Foxp3 expression remain undefined. It is apparent that perhaps all CD4+ and CD8+ T lymphocytes normally express Foxp3 in the setting of stimulation and/or proliferation,8,12,13,25–27 suggesting that Foxp3 expression itself does not commit T lymphocytes to a Treg phenotype. Interestingly, however, transduced constitutively high expression of Foxp3 in CD4+ T lymphocytes in humans28 and transgenic mice29 can drive these cells to acquire a fully functional Treg phenotype. These data suggest that the timing and/or level of Foxp3 expression may determine its impact on CD4+ T-lymphocyte function.
Data on the expression of Foxp3 in CD8+ T lymphocytes are more limited. In contrast to peripheral blood, significant levels of CD8+ T lymphocytes expressing CD25 and Foxp3 with regulatory functions resembling natural CD4 Tregs have been observed in the human thymus in vivo.30 In vitro, experimental stimulation of bulk CD8+ T lymphocytes has been observed to drive Foxp3 expression,8,12,13,25–27,31 although the relationship of the Foxp3-expressing cells to memory antigen-specific CD8+ T lymphocytes has not been described.
In the present study, we found that only rare peripheral blood antigen-specific CD8+ T lymphocytes expressed CD25 and Foxp3 (the classical natural Treg phenotype), even in the setting of chronic persistent viral infection and antigenic stimulation. In contrast, in vitro antigenic stimulation of CMV- and HIV-1-specific CD8+ T lymphocytes drove a majority of these cells to express Foxp3 and CD25, providing the novel observation that virus-specific memory/effector cells can adopt this Treg-like phenotype under certain conditions. Thus memory T lymphocytes are likely to be the predominant subset of cells within bulk PBMCs that express these markers during ex vivo stimulation and proliferation. Interestingly, the enhancement of expression by IL-2 was most pronounced in the proliferating virus-specific CD8+ T lymphocytes. This could be due to IL-2 enhancing proliferation/activation and indirectly promoting higher Foxp3 expression or, alternatively, directly enhancing foxp3 transcription via activating Stat5.20–22
This apparent discrepancy between our observations that CD8+ T lymphocytes driven to proliferate by anti-CD3 stimulation with IL-2 acquire a Treg-like phenotype, while circulating recently proliferated CD8+ T lymphocytes do not have this phenotype, underscores the fact that the precise requirements to induce Foxp3 expression by CD8+ T lymphocytes in vivo are unclear. Despite the prominent upregulation of CD25 and Foxp3 in response to in vitro stimulation, few proliferating (Ki-67+) T lymphocytes in blood in vivo express these molecules. A potential explanation could be that Foxp3 level falls more rapidly than Ki-67 during transition from a proliferative state in the periphery to a quiescent memory state in the circulation; proliferating CD8+ and CD4+ T lymphocytes appear to transiently express Foxp3 after stimulation in vitro.8,13 Another explanation could be that a quantitative or qualitative difference in stimulation determines Foxp3 expression and that memory cells in a highly inflammatory milieu are driven to express Foxp3. Preliminary evaluations of lymphocytes from the gut mucosal compartment indeed observe significant levels of CD8+ T lymphocytes that coexpress CD25 and FoxP3 (data not shown), consistent with either of these hypotheses.
An interesting novel side observation is that the majority of CD4+ T lymphocytes with the natural Treg CD25+Foxp3+ phenotype are nonproliferative in vivo and in vitro. This suggests that true Tregs differ in their mechanism of Foxp3 expression compared with proliferating memory T lymphocytes. Functional testing will be required to examine whether proliferation versus nonproliferation is a distinguishing factor separating Treg from non-Treg CD4+ T lymphocytes.
Although the functional implications of the observed CD25 and Foxp3 expression on CMV- and HIV-1-specific CD8+ T lymphocytes are unknown, there are data to suggest that these cells could have Treg functions. Others have found that memory antigen-specific CD8+ T lymphocytes that are driven to proliferate in vitro acquire transient Treg activity.12,13 Although this phenomenon has been observed solely in vitro to date, there are potentially significant in vivo ramifications. There are examples of “induced” or “adaptive” human CD8+ Tregs32 that are proposed to play key roles in pathogenic processes involving infection and organ transplantation. For example, Epstein Barr virus-specific CD8+ T lymphocytes are believed to play a key role in dampening antiviral immunity, leading to posttransplant lymphoproliferative diseases in organ transplant patients.33
Additionally, significant regulatory roles of CD8+ Tregs have been implicated in antiviral immune responses to persistent simian immunodeficiency virus (SIV) infection. During acute SIV infection in African green monkeys, a period of massive immune activation and CD8+ T-lymphocyte expansion, a rapid rise in a number of putative CD8+ Tregs was observed.34 Although this observation suggests a protective role of the CD8+ Tregs in progressive SIV infection, a recent report by Karlsson et al.35 showed a significant association between expansion of CD8+ Tregs during acute SIV infection in cynomologus macaques and rapid disease progression. These observations imply similar participation of the CD8+ Tregs in HIV-1 pathogenesis, and our data raise a possibility that a majority of CD8+ Tregs appearing during acute infection are the induced Tregs. The induced Tregs could largely consist of rapidly proliferating HIV-1-specific CD8+ T lymphocytes, and more importantly, their regulatory functions will be intensified significantly by the presence of IL-2. Further studies are needed to evaluate whether this occurs in acute HIV-1 infection in humans and to define the regulation and implications of Foxp3 expression in HIV-1-specific CD8+ T lymphocytes in vivo.
Acknowledgments
We are grateful to the Los Angeles MACS study participants for their blood donations. This work was supported by PHS grant AI043203. IL-2 was provided by the NIH AIDS Reagent Repository. The Multicenter AIDS Cohort Study (MACS) includes the following sites: The Johns Hopkins University Bloomberg School of Public Health: J.B. Margolick (Principal Investigator), H. Armenian, B. Crain, A. Dobs, H. Farzadegan, J. Gallant, J. Hylton, L. Johnson, S. Lai, J. McArthur, N. Sacktor, O. Selnes, J. Shepard, C. Thio; Howard Brown Health Center, Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: J.P. Phair (Principal Investigator), J.S. Chmiel (Co-Principal Investigator), S. Badri, B. Cohen, C. Conover, M. O'Gorman, D. Ostrow, F. Palella, D. Variakojis, S.M. Wolinsky; University of California, UCLA Schools of Public Health and Medicine: R. Detels (Principal Investigator), B.R. Visscher (Co-Principal Investigator), A. Aronow, R. Bolan, E. Breen, A. Butch, T. Coates, R. Effros, J. Fahey, B. Jamieson, O. Martínez-Maza, E.N. Miller, J. Oishi, P. Satz, G. Vaccaro, H. Vinters, D. Wiley, M. Witt, O. Yang, S. Young, Z.F. Zhang; University of Pittsburgh, Graduate School of Public Health: C.R. Rinaldo (Principal Investigator), L. Kingsley (Co-Principal Investigator), J.T. Becker, R.L. Cook, R.W. Evans, J. Mellors, S. Riddler, A. Silvestre; Data Coordinating Center, The Johns Hopkins University Bloomberg School of Public Health: L.P. Jacobson (Principal Investigator), A. Muñoz (Co-Principal Investigator), H. Chu, S.R. Cole, C. Cox, S.J. Gange, J. Schollenberger, E.C. Seaberg, S. Su; NIH: R.E. Huebner (NIAID), G. Dominguez (NCI), C. McDonald (NHLBI). Funding for the MACS is provided by PHS grants UO1-AI-35042, 5-MO1-RR-00722 (GCRC), UO1-AI-35043, UO1-AI-37984, UO1-AI-35039, UO1-AI-35040, UO1-AI-37613, UO1-AI-35041. The MACS website is located at http://www.statepi.jhsph.edu/macs/macs.html.
References
- 1.Sakaguchi S. Sakaguchi N. Asano M. Itoh M. Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 2.Dieckmann D. Plottner H. Berchtold S. Berger T. Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wildin RS. Ramsdell F. Peake J. Faravelli F. Casanova JL. Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Y. Josefowicz SZ. Kas A. Chu TT. Gavin MA. Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 5.Marson A. Kretschmer K. Frampton GM. Jacobsen ES. Polansky JK. MacIsaac KD, et al. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–935. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziegler SF. FOXP3: not just for regulatory T cells anymore. Eur J Immunol. 2007;37:21–23. doi: 10.1002/eji.200636929. [DOI] [PubMed] [Google Scholar]
- 7.Bacchetta R. Passerini L. Gambineri E. Dai M. Allan SE. Perroni L, et al. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006;116:1713–1722. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavin MA. Torgerson TR. Houston E. DeRoos P. Ho WY. Stray-Pedersen A, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortesini R. LeMaoult J. Ciubotariu R. Cortesini NS. CD8+CD28− T suppressor cells and the induction of antigen-specific, antigen-presenting cell-mediated suppression of Th reactivity. Immunol Rev. 2001;182:201–206. doi: 10.1034/j.1600-065x.2001.1820116.x. [DOI] [PubMed] [Google Scholar]
- 10.Garba ML. Pilcher CD. Bingham AL. Eron J. Frelinger JA. HIV antigens can induce TGF-beta(1)-producing immunoregulatory CD8+ T cells. J Immunol. 2002;168:2247–2254. doi: 10.4049/jimmunol.168.5.2247. [DOI] [PubMed] [Google Scholar]
- 11.Elrefaei M. Barugahare B. Ssali F. Mugyenyi P. Cao H. HIV-specific IL-10-positive CD8+ T cells are increased in advanced disease and are associated with decreased HIV-specific cytolysis. J Immunol. 2006;176:1274–1280. doi: 10.4049/jimmunol.176.2.1274. [DOI] [PubMed] [Google Scholar]
- 12.Bisikirska B. Colgan J. Luban J. Bluestone JA. Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. J Clin Invest. 2005;115:2904–2913. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pillai V. Ortega SB. Wang CK. Karandikar NJ. Transient regulatory T-cells: a state attained by all activated human T-cells. Clin Immunol. 2007;123:18–29. doi: 10.1016/j.clim.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoji A. Rinaldo CRJ. Human CD8+ T cells specific for influenza A virus M1 display broad expression of maturation-associated phenotypic markers and chemokine receptors. Immunology. 2005;115:239–245. doi: 10.1111/j.1365-2567.2005.02135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoji A. Connolly NC. Buchanan WG. Rinaldo CRJ. CD27 and CD57 expression reveals atypical differentiation of human immunodeficiency virus type 1-specific memory CD8+ T cells. Clin Vaccine Immunol. 2007;14:74–80. doi: 10.1128/CVI.00250-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann TK. Donnenberg VS. Friebe-Hoffmann U. Meyer EM. Rinaldo CR., Jr DeLeo AB, et al. Competition of peptide-MHC class I tetrameric complexes with anti-CD3 provides evidence for specificity of peptide binding to the TCR complex. Cytometry. 2000;41:321–328. [PubMed] [Google Scholar]
- 17.Roederer M. Moore W. Treister A. Hardy RR. Herzenberg LA. Probability binning comparison: a metric for quantitating multivariate distribution differences. Cytometry. 2001;45:47–55. doi: 10.1002/1097-0320(20010901)45:1<47::aid-cyto1143>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 18.Roederer M. Treister A. Moore W. Herzenberg LA. Probability binning comparison: a metric for quantitating univariate distribution differences. Cytometry. 2001;45:37–46. doi: 10.1002/1097-0320(20010901)45:1<37::aid-cyto1142>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Kovacs JA. Lempicki RA. Sidorov IA. Adelsberger JW. Herpin B. Metcalf JA, et al. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J Exp Med. 2001;194:1731–1741. doi: 10.1084/jem.194.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burchill MA. Yang J. Vogtenhuber C. Blazar BR. Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 21.Murawski MR. Litherland SA. Clare-Salzler MJ. Davoodi-Semiromi A. Upregulation of Foxp3 expression in mouse and human Treg is IL-2/STAT5 dependent: implications for the NOD STAT5B mutation in diabetes pathogenesis. Ann N Y Acad Sci. 2006;1079:198–204. doi: 10.1196/annals.1375.031. [DOI] [PubMed] [Google Scholar]
- 22.Zorn E. Nelson EA. Mohseni M. Porcheray F. Kim H. Litsa D, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hori S. Nomura T. Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 24.Fontenot JD. Gavin MA. Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 25.Manavalan JS. Kim-Schulze S. Scotto L. Naiyer AJ. Vlad G. Colombo PC, et al. Alloantigen specific CD8+CD28-FOXP3+ T suppressor cells induce ILT3 + ILT4 + tolerogenic endothelial cells, inhibiting alloreactivity. Int Immunol. 2004;16:1055–1068. doi: 10.1093/intimm/dxh107. [DOI] [PubMed] [Google Scholar]
- 26.Morgan ME. van Bilsen JH. Bakker AM. Heemskerk B. Schilham MW. Hartgers FC, et al. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Roncador G. Brown PJ. Maestre L. Hue S. Martinez-Tor-recuadrada JL. Ling KL, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–1691. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 28.Oswald-Richter K. Grill SM. Shariat N. Leelawong M. Sundrud MS. Haas DW, et al. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2004;2:E198. doi: 10.1371/journal.pbio.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khattri R. Cox T. Yasayko SA. Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 30.Cosmi L. Liotta F. Lazzeri E. Francalanci M. Angeli R. Mazzinghi B, et al. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood. 2003;102:4107–4114. doi: 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]
- 31.Jarvis LB. Matyszak MK. Duggleby RC. Goodall JC. Hall FC. Gaston JS. Autoreactive human peripheral blood CD8+ T cells with a regulatory phenotype and function. Eur J Immunol. 2005;35:2896–2908. doi: 10.1002/eji.200526162. [DOI] [PubMed] [Google Scholar]
- 32.Bluestone JA. Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 33.Popescu I. Macedo C. Abu-Elmagd K. Shapiro R. Hua Y. Thomson AW, et al. EBV-specific CD8+ T cell reactivation in transplant patients results in expansion of CD8+ type-1 regulatory T cells. Am J Transplant. 2007;7:1215–1223. doi: 10.1111/j.1600-6143.2007.01740.x. [DOI] [PubMed] [Google Scholar]
- 34.Kornfeld C. Ploquin MJ. Pandrea I. Faye A. Onanga R. Apetrei C, et al. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest. 2005;115:1082–1091. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karlsson I. Malleret B. Brochard P. Delache B. Calvo J. Le Grand R, et al. FoxP3+ CD25+ CD8+ T-cell induction during primary simian immunodeficiency virus infection in cynomolgus macaques correlates with low CD4+ T-cell activation and high viral load. J Virol. 2007;81:13444–13455. doi: 10.1128/JVI.01466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]