Abstract
Bone is the most common site of metastases from prostate cancer. The mechanism by which prostate cancer cells metastasize to bone is not fully understood, but interactions between prostate cancer cells and bone cells are thought to initiate the colonization of metastatic cells at that site. Here we show that cadherin-11 (also known as osteoblast-cadherin) was highly expressed in prostate cancer cell line derived from bone metastases and had strong homophilic binding to recombinant cadherin-11 in vitro. Downregulation of cadherin-11 in bone metastasis-derived PC3 cells with cadherin-11-specific shRNA (PC3-shCad-11) significantly decreased the adhesion of those cells to cadherin-11 in vitro. In a mouse model of metastasis, intracardiac injection of PC3 cells led to metastasis of those cells to bone. However, the incidence of PC3 metastasis to bone in this model was reduced greatly when the expression of cadherin-11 by those cells was silenced. The clinical relevance of cadherin-11 in prostate cancer metastases was further studied by examining the expression of cadherin-11 in human prostate cancer specimens. Cadherin-11 was not expressed by normal prostate epithelial cells but was detected in prostate cancer, with its expression increasing from primary to metastatic disease in lymph nodes and especially bone. Cadherin-11 expression was not detected in metastatic lesions that occur in other organs. Collectively, these findings suggest that cadherin-11 is involved in the metastasis of prostate cancer cells to bone.
Keywords: prostate cancer, bone metastasis, cadherin-11, osteoblast
Introduction
Prostate cancer is the most common cancer among men. The mortality from this disease results mostly from the metastasis of tumor cells to secondary sites, particularly bone. Prostate cancer metastasizes to the bone with high frequency, causing significant morbidity and mortality (1). Jacobs et al. (2) reported that 80% of men with prostate cancer had bone metastases at autopsy. A more recent rapid-autopsy study also reported that about 80% of patients who die from prostate cancer have metastases in bone (3), further confirming the prevalence of bone metastasis in prostate cancer. Delineating the biological basis of the proclivity of prostate cancer cells for bone may lead to strategies to prevent or treat prostate cancer metastasis.
Metastasis of cancer cells to distant sites is a multistep process that involves the cancer cells becoming dislodged from primary site, surviving in circulation, attaching to a distant target organ, and growing in the target organ (4, 5). Exactly how circulating cancer cells disseminate to specific organ sites is not clear. Adhesion molecules that mediate the interactions between the metastatic cancer cells and cells present in the target organs are likely to play a central role in cancer cell dissemination. The tropism of prostate cancer cells to bone suggests that prostate cancer cells may preferentially interact with specific cells in the bone microenvironment (6–48), the most likely candidates of which are osteoblasts. Specifically, the interaction of disseminated prostate cancer cells with osteoblasts may be one of the steps that lead to colonization of bone by prostate cancer cells.
To search for molecules involved in metastasis to bone, we performed a gene array analysis to identify genes that are differentially expressed between acinar carcinoma of prostate, which tend to metastasize to bone, and ductal carcinoma of prostate, which tend to metastasize to other organ sites (9). We found that a series of osteoblast related genes including cadherin-11 (also known as osteoblst-cadherin or OB-cadherin) were upregulated in acinar PCa specimens. Because cadherin-11 is a homophilic cell adhesion molecule originally identified in the osteoblast, this observation led us to hypothesize that PCa cells expressing cadherin-11 may have an increased ability to interact with osteoblasts.
Cadherin-11, also known as osteoblast-cadherin, is an adhesion molecule highly expressed in primary osteoblasts, although weak signals have been detected in brain, lung, and testis tissue (10). Cadherin-11 mediates homophilic cell adhesion in a calcium-dependent manner (10). Its expression is associated with osteoblast differentiation and may function in cell sorting, migration, and alignment during the maturation of osteoblasts (11).
Altered expression of E-cadherin, another cadherin that mediates adhesion between differentiated epithelial cells, has been linked with prostate cancer progression. Specifically, E-cadherin was found to be expressed at high levels in normal prostate tissue, at lower levels in low-grade (well-differentiated) tumors, and at the lowest levels in high-grade (poorly differentiated) prostate cancer (12). Indeed, loss of E-cadherin expression has been found by others to correlate with the invasiveness of prostate cancer and the ratio of E-cadherin to matrix metalloproteinase 9 to predict metastasis potential (13). Although one report (14) indicated that loss of E-cadherin expression was accompanied by upregulation of cadherin-11 in prostate cancer cells within metastatic lesions in lymph nodes in men with advanced prostate cancer, the role of cadherin-11 in the metastasis of prostate cancer to bone has not been explored. We hypothesize that cadherin-11 participates in the dissemination of prostate cancer cells to bone by mediating the adhesion of the metastatic prostate cancer cells and osteoblasts. In this study, we show that a bone-derived prostate cancer cell line (PC3) expressed high levels of cadherin-11 and frequently metastasized to bone upon intracardiac injection in mice. Knocking down cadherin-11 in PC3 cells suppressed this process. These results implicate that cadherin-11plays a role in the metastasis of prostate cancer cells to bone.
Results
Cadherin-11 expression in human prostate cancer cell lines
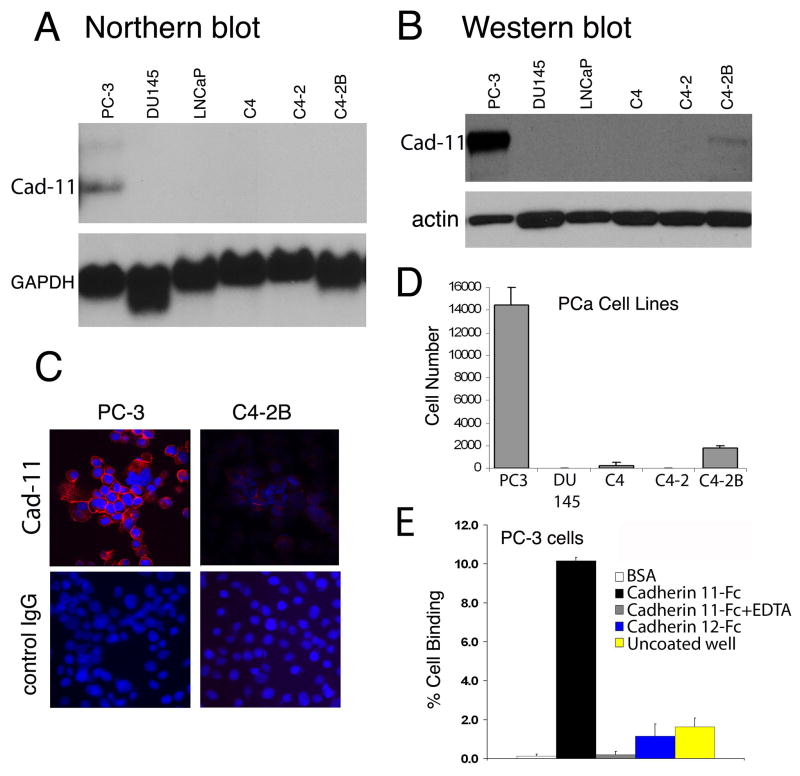
To assess the endogenous expression of cadherin-11 in prostate cancer cell lines from a variety of anatomic metastases, we used northern blot analysis and found cadherin-11 transcripts only in prostate cancer cells derived from human bone metastases (PC3) but not in those derived from lymph node metastases (LNCaP) and its derivatives (C4, C4-2, C4-2B) or brain metastases (DU145) (Fig. 1A).
Figure 1.
Expression of cadherin-11 in human prostate cancer cell lines evaluated by A, northern blotting, B, western blotting, and C, immunocytochemical staining. Cadherin-11 was expressed in cell membranes in areas of cell-cell contact in PC3 and C4–2B cells. D, Binding of prostate cancer cell lines to cadherin-11-Fc–coated wells, indicated by luciferase activity plotted versus cell number. The bone-derived PC3 cells show the strongest binding. E, Analysis of PC3 binding to different substratum. Strong binding of PC3 was observed with cadherin 11-Fc and this binding was inhibited by the addition of 5 mM EDTA. PC3 showed minimal binding to BSA, cadherin 12-Fc, or uncoated well.
We next examined cadherin-11 protein expression by using a monoclonal antibody specific to cadherin-11 (Fig. 1B). Western blotting revealed a single band of apparent molecular weight ~100 kDa from the PC3. We also detected a very low level of cadherin-11 protein in C4-2B cells in western blot. The detection of cadherin-11 by western blot but not by Northern blot is either indicative of the greater sensitivity of the western blot assay versus northern blot or the greater stability of protein versus the transcript.
Immunocytochemical staining used to localize cadherin-11 within the cell revealed that in the PC3, cadherin-11 protein was expressed on the cell membrane, mainly at sites of cell-cell contact (Fig. 1C). Similar to those observed in Western blot, very weak staining of cadherin-11 was detected in C4-2B cells. No nonspecific staining with normal mouse IgG was noted (Fig. 1C).
To examine whether cadherin-11 expressed in PC3 cells can mediate adhesion, we used a cell-to-substrate adhesion assay to assess the ability of prostate cancer cells to bind to recombinant cadherin-11-Fc coated onto 96-cell plates. Prostate cancer cells were transduced with retroviral vector containing the Luc gene so that cells could be quantified in terms of Luc activity. PC3 cells demonstrated high adhesion activity, and C4-2B cells low adhesion activity, to cadherin-11-Fc; DU145, C4, and C4-2 cells showed no detectable adhesion activity (Fig. 1D). We further characterized the specificity of PC3 binding to cadherin. PC3 cells bound to cadherin-11-Fc, but not to BSA or uncoated well (Fig. 1E). The binding was abolished by EDTA, suggesting that the binding is Ca2+-dependent. In addition, PC3 did not bind to cadherin-12-Fc, indicating that the binding is specific. Hence the ability of prostate cancer cells to bind to cadherin-11-Fc correlated with the levels of cadherin-11 expressed in the cells. Collectively, these observations demonstrate that cadherin-11 expressed in PC3 and C4-2B cells functions as a homophilic cell adhesion molecule.
PC3 cells disseminate to bone after intracardiac injection
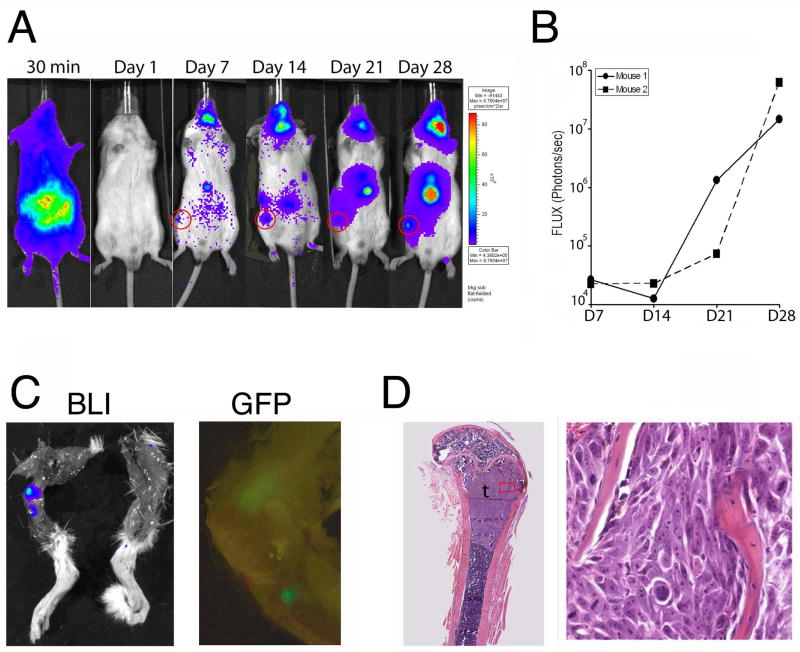
Given our findings that cadherin-11 is expressed by PC3 cells, we next tested whether cadherin-11 could participate in the metastasis of prostate cancer cells to bone in vivo by using an experimental metastasis model involving intracardiac injection of PC3 tumor cells in SCID mice. This approach models the hematogenous dissemination of cancer cells and allows examination of the process of metastatic colonization at various organ sites. After their initial adhesion at the distant organ site, the disseminated tumor cells must be able to proliferate there so that sufficient tumor cells are present for subsequent detection. We transduced PC3 cells with a retroviral vector containing genes for Luc and GFP genes and examined the ability of the resulting PC3-Luc cells to metastasize to the skeleton after being injected into the left ventricles of male SCID mice. The distribution of PC3 cells to the various organs and their growth therein were followed by bioluminescence imaging. Only those mice that showed whole-body distribution of PC3-Luc cells at 30 min after injection were used for further analysis. Although whole-body dissemination of PC3-Luc cells was evident as early as 30 min after the injection, the bioluminescence signal was significantly weakened by 60 min after injection (not shown) and became undetectable at 24 hours (Fig. 2A), suggesting that most of the PC3-Luc cells did not survive in the circulation in vivo and that only a small fraction disseminated to the tissues. Notably, the PC3-Luc cells established colonies in the bone compartment of the hind leg (Figs. 2A and 2C). Indeed, tumors in femurs and tibiae were detected by both bioluminescence and GFP imaging (Fig. 2C), and the intensity of the luminescence indicated exponential growth between weeks 3 and 4 (Fig. 2B). Bioluminescence imaging revealed metastatic colonization in bone in two of the three mice successfully injected with PC3-Luc cells, for a bone metastasis rate of 67%. Histologic analysis showed that the femoral or tibial tumors generally occupied the primary spongiosum (trabecular epiphysis) region of the bone and displaced the bone marrow cells (Fig. 2D). PC3-Luc cells were also detected at several other organ sites, including sublingual glands, mandible, and adrenal glands (Fig. 2A). These observations indicate that intracardiac injection of PC3-Luc cells is a model for studying bone homing of prostate cancer cells.
Figure 2.
Metastasis of PC3 cells in vivo after intracardiac injection. PC3-Luc cells were transduced with genes for luciferase [Luc] and green fluorescent protein [GFP]) and injected into the left ventricles of mice. A, Bioluminescence images of a single mouse at various times after tumor cell inoculation. B, Bioluminescence tracings for two mice show exponential increases in Luc activity in hind leg. C, Ex vivo detection of tumor in mouse femur by bioluminescence (BLI) and GFP imaging. D, Histologic analysis of tumor in bone at low (left) and high (right) magnification.
Knockdown of cadherin-11 in PC3 cells inhibits their dissemination to bone
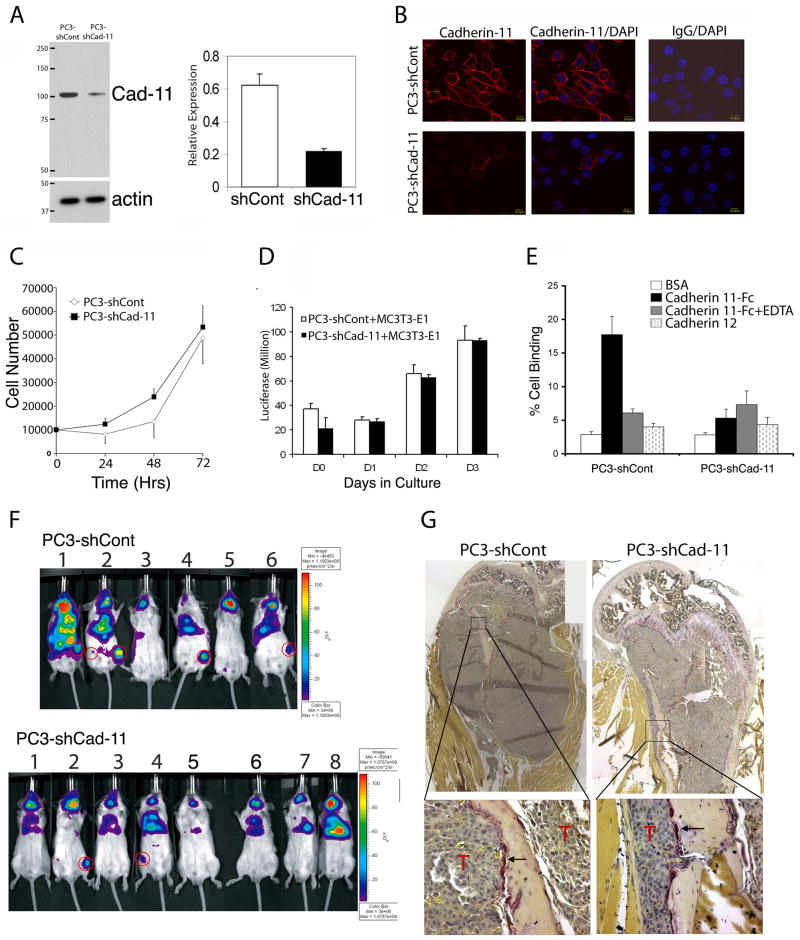
To test whether cadherin-11 is involved in the dissemination of prostate cancer cells to bone, we used retroviral delivery of shRNA to downregulate cadherin-11 in PC3-Luc cells. Cells were then selected with puromycin to enrich for the transduced cells, and pools of puromycin-resistant cells were combined. We found that the PC3-shCad-11 cells showed a reduction in expression of cadherin-11 by about 65% compared with that of the control PC3-shCont cells as analyzed by western blotting (Fig. 3A). Similarly, immunostaining showed considerably less cadherin-11 in the plasma membranes of PC3-shCad-11 cells than in the control PC3-shCont cells (Fig. 3B). Knockdown of cadherin-11 did not affect cell proliferation (Fig. 3C). To examine whether cadherin-11 expression may have an effect on tumor-bone interaction, we co-cultured PC3-shCont or PC3-shCad-11 cells with an osteoblast cell line MC3T3-E1. We found that there is no significant difference in the growth rate when these cells were co-cultured with MC3T3-E1 cells (Fig. 3D). However, knockdown of cadherin-11 was accompanied by lesser adhesion to cadherin-11-Fc by PC3-shCad-11 cells than by PC3-shCont cells (Fig. 3E). The adhesion activity of the PC3-shCont cells was similar to that of the PC3-Luc cells (data not shown). Binding specificity to cadherin-11-Fc was confirmed by the lack of binding to cadherin-12-Fc (Fig. 3E). Furthermore, binding to cadherin-11-Fc was abolished in the presence of EDTA, a finding consistent with calcium being required for homophilic binding.
Figure 3.
Knockdown of cadherin-11 in PC3 cells blocks adhesion of those cells to cadherin-11 and reduces the incidence of metastasis to hind legs in a mouse model. A,, Western blotting shows decreased cadherin-11 expression in PC3 cells treated with shRNA to cadherin-11 (quantification shown at right). B, Immuncytochemical staining of cadherin-11 in PC3-shCont and PC3-shCad-11 cells. Cy3 labelled cadherin-11 at the membrane of PC3-shCont and PC3-shCad-11 cells (left). Overlay of Cy3 labelled-cadherin-11 with nuclear staining by DAPI to localize cells (middle). Negative control mouse IgG staining with DAPI overlay (right). C, Proliferation rates were not different for PC3-shCont and PC3-shCad-11 cells in vitro. D, Coculture of PC3-shCont or PC3-shCad-11 with MC3T3-E1 osteoblast cells. The presence of osteoblast does not affect the proliferation of PC3-shCont and PC3-shCad-11 cells. E, A cell-to-substrate assay indicates that knockdown of cadherin-11 reduced adhesion to cadherin-11-Fc. BSA, bovine serum albumin. F, PC3-Luc cells treated with shRNA to cadherin-11 formed fewer skeletal metastases in hind legs of mice after intracardiac injection than did cells with a scrambled shRNA control vector. G, Tartrate-resistant alkaline phosphatase staining of PC3-shCont and PC3-shCad-11 bones showed that both type of cells induced osteolytic bone lesion. Magnified region showing the presence of tartrate-resistant alkaline phosphatase positive cells (purple staining, arrow) were observed on the bone surface adjacent to tumor cells (T).
To investigate the effect of cadherin-11 on the ability of PC3-Luc cells to metastasize to bone, we injected PC3-shCad-11 and PC3-shCont cells into the left ventricles of SCID mice and followed the distribution of those cells with bioluminescence imaging. Whole-body distribution of the cells, indicating successful injection, was apparent in about 80% of the mice. Images were then obtained at 10, 23, and 33 days after injection to monitor tumor cell colonization, and the mice were euthanized on day 36. As noted in the previous section, the preferred skeletal site for the PC-Luc cells was the hind limb (femur or tibia). Approximately 67% of the mice injected with PC3-shCont cells developed tumors in the femur or tibia, but only 25% of the mice injected with PC3-shCad-11 cells developed tumors at these sites (Fig. 3F). Macroscopic dissection and histologic analysis of the hind limbs from the injected mice (as done with the mice injected with PC3-Luc cells [Figs. 2C and 2D]) confirmed that the Luc signal corresponded with the presence of tumor cells (data not shown). Both the PC3-shCont and PC3-shCad-11 cell lines colonized the submandibular glands, mandibles, and adrenal glands at frequencies similar to the PC3-Luc cells (Table 1). These experiments were repeated twice, and similar results were obtained. In total, nine of 15 mice injected with PC3-shCont cells (60%) showed metastases in bone, whereas only three of 13 mice injected with PC-shCad-11 cells (23%) showed bone metastases (P = 0.049, χ2 test). We also collected all organs that exhibited luciferase signals from injected mice and examined the presence of tumor cells at the histology sections. We found that both cell lines colonized in sublingual gland, mandible, and adrenal gland with similar frequency (Table 1). Tumors were also observed in the chest cavity in most mice irrespective of the levels of cadherin-11, likely due to tumor cells spill during the heart injection. These observations suggest that downregulation of cadherin-11 in PC3-Luc cells reduced the incidence of metastasis to the femur or tibia in this model but not to other sites.
Table 1.
Incidence of metastasis to organ sites
| Cell Lines | Hind Limb | Adrenal gland | Liver | Pancreas | Lymph Node | Sublingual Gland | Mandible | Thoracic Cavity |
|---|---|---|---|---|---|---|---|---|
| PC3-shCont | 9/15 (60%) | 10/12 (83%) | 5/12 (42%) | 6/12 (50%) | 1/9 (11%) | 12/12 (100%) | 12/12 (100%) | 6/9 (66%) |
| PC3-shCad-11 | 3/13 (23%) | 7/11 (64%) | 3/9 (33%) | 2/9 (22%) | 1/6 (17%) | 11/11 (100%) | 11/11 (100%) | 7/8 (87%) |
| P value* | 0.049 | 0.28 | 0.7 | 0.19 | 0.76 | 1.00 | 0.33 | 0.31 |
Chi-square test
Although the clinical prostate cancer bone metastases have the predominant osteoblastic phenotype mixed with osteolytic components, PC3 cell line induces mainly osteolytic lesion, likely due to the secretion of DKK1 (15). To assess whether knockdown of cadherin-11 have an effect on the osteolytic phenotype of PC3 cells, we evaluated mouse bones with PC3-shCont and PC3-shCad-11 cells. Femurs/tibia in the PC3-shCont group and in the PC3-shCad-11 group were assessed by both histological analysis and staining for tartrate-resistant alkaline phosphatase, which is an osteoclast marker. Both the PC3-shCont and PC3-shCad-11 cells induced osteolytic lesions with osteoclasts present in bone area adjacent to tumor (Fig. 3G). These observations suggest that down regulation of cadherin-11 does not affect PC3-induced osteolytic lesions.
Cadherin-11 expression in human prostate cancer specimens
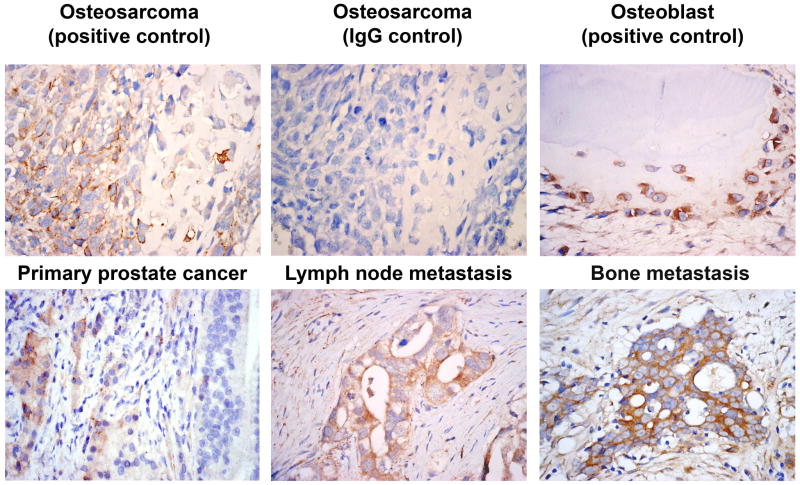
A previous report by Tomita et al. (14) indicated that loss of E-cadherin expression was accompanied by upregulation of cadherin-11 in prostate cancer specimens in primary tumor and lymph node metastasis. To extend the findings to bone metastases, we examined cadherin-11 expression by immunohistochemical staining of specimens from men with localized or metastatic prostate cancer. Specimens of normal human bone and osteosarcoma were used as positive controls. As expected, an anti-cadherin-11 antibody stained osteoblasts and osteosarcoma cells. An IgG control antibody showed no staining (Fig. 4, top row). Of 50 specimens of prostate tissue from men with localized prostate cancer, seven (14%) showed positive staining for cadherin-11 in epithelial cells; of 19 specimens of prostate cancer cells within lymph nodes, five (26%) showed cadherin-11 staining; and of 18 specimens of prostate cancer cells within bone, nine (50%) showed cadherin-11 staining (representative images shown in Fig. 4, bottom row). Cadherin-11 expression was not detected in metastatic lesions that occur in other organs (10 specimens). A two-sided Fisher’s exact test to assess differences in cadherin-11 expression among the four specimen groups (i.e., primary prostate tumor, lymph node metastases, bone metastases, and other metastases) showed a significant (P = 0.005) difference, indicating that at least one specimen group was different from the others. Three post hoc comparisons with two-sided Fisher’s exact tests were then implemented by comparing the primary prostate cancer specimens with the metastases. Only the staining in bone metastases (50%) relative to that in the primary prostate tumor (14%) showed a significant difference (P = 0.004) (Table 2). These observations suggest that cadherin-11 expression is associated with metastasis of prostate cancer to bone.
Figure 4.
Staining of human prostate cancer specimens with antibody to cadherin-11. Top row, Osteosarcoma specimens show positive staining for cadherin-11 and negative staining for an IgG control; cadherin-11 is also expressed in osteoblasts. Bottom row, Cadherin-11 is expressed in a primary prostate cancer specimen (Gleason score 5+5). Adjacent prostate intraepithelial neoplasia did not express cadherin-11. Cadherin-11 is also evident in metastatic prostate cancer cells in lymph nodes and, to a much greater extent, in metastatic prostate cancer cells in bone.
Table 2.
Pathologic Characteristics and Cadherin-11 Staining of Human Prostate Cancer Samples
| Specimen Type | Specimen Source | Total No.Samples | No. of Samples (Gleason Score) | Cadherin-11–Positive | P Value* | |
|---|---|---|---|---|---|---|
| No. of samples | % | |||||
| Primary prostate tumor | Radical prostatectomy | 9 | 4 (6) | 1 | 7/50 (14%) | |
| 1 (7) | 0 | |||||
| 2 (8) | 0 | |||||
| 2 (10) | 0 | |||||
| Transurethral prostatectomy | 41 | 8 (6) | 1 | |||
| 6 (7) | 1 | |||||
| 6 (8) | 1 | |||||
| 15 (9) | 2 | |||||
| 6 (10) | 1 | |||||
| Lymph node metastases | Proximal and distal | 19 | N/A | 5 | 5/19 (26%) | P = 0.289 |
| Bone metastases | Vertebrae and long bone | 18 | N/A | 9 | 9/18 (50%) | P = 0.004 |
| Other metastases | 10 | N/A | 0 | 0/10 (0%) | P = 0.589 | |
Abbreviation: N/A, not applicable.
From two-tailed Fisher’s exact tests.
Discussion
Our findings indicate that cadherin-11 is likely to be one of perhaps several adhesion molecules involved in the metastasis of prostate cancer to bone. Specifically, cadherin-11 was expressed in prostate cancer cell lines derived from bone metastasis (PC3) but not in those from lymph node (LNCaP) or brain (DU145) metastases; the cadherin-11 in PC3 cells was located mostly at cell-cell junctions; and the PC3 cells showed stronger adhesion to cadherin-11 in vitro than did the other prostate cancer cell lines. Moreover, cadherin-11-expressing PC3 cells showed a high rate of colonization in bone after intracardiac injection, but knockdown of cadherin-11 with shRNA in such cells decreased it. Finally, human specimens showed that prostate cancer cells in bone expressed cadherin-11 more often than did cells in lymph nodes, while metastatic cancer cells at other organ sites expressed no cadherin-11. Together, our findings implicate cadherin-11 in the metastasis of prostate cancer to bone.
The exact mechanism on how cadherin-11 participates in this process remains unclear. One possibility is that cadherin-11 increases the potential for prostate cancer to metastasize to bone by increasing the binding of cancer cells to osteoblasts in the skeleton. The enhanced retention of cancer cells in bone could then facilitate molecular interactions between cancer cells and the osteoblasts through paracrine factors, a process thought to be crucial for the survival and growth of prostate cancer cells in bone (16, 17). In addition, adhesion via cadherin-11 may well bring prostate cancer cells and osteoblasts into closer physical contact that would allow juxtacrine interactions in which ligands would remain membrane bound and non-diffusible (e.g. Notch-Delta signaling).
In addition to adhesion, signal transduction through cadherin-11 could also be expected to modulate cellular functions of the cancer cells, the osteoblasts, or both. Like other cadherin family members, cadherin-11 contains a cytoplasmic tail that can potentially bind to catenins (18, 19). Catenins have been demonstrated to function in cellular signaling and also to link cadherins to the cytoskeleton. Several studies have also shown cadherins to modulate receptor tyrosine kinase signaling (18). Most of these studies have focused on E-cadherin and, to a lesser extent, N-cadherin and VE-cadherin. Orlandini and Oliviero (20) reported that in fibroblasts, cell-cell contact mediated by cadherin-11 induced the expression of vascular endothelial growth factor (VEGF)–D. In testing this possibility in prostate cancer cells, we found that overexpression of cadherin-11 in LNCaP cells did not increase VEGF-D expression (data not shown). In breast carcinoma cells, the expression of cadherin-11 by transfection was shown to increase the invasive and migratory properties of these cells in vitro (21). However, overexpression of cadherin-11 in LNCaP cells did not have effects on the invasiveness or migratory properties these cells as measured by matrigel invasion assay and wound healing assay, respectively (data not shown). These observations suggest that cadherin-11 induces different cellular responses in different cell types. In osteoblasts, cell-to-cell adhesion and signaling via N-cadherin or cadherin-11 have been implicated in osteoblast differentiation in vitro and in vivo (19, 22). Because cadherins respond differently depending on their subtype and the cell types that express them, exactly how cadherin-11 transduces signals in interactions between different cell types (i.e., prostate cancer cells and osteoblasts) has yet to be determined.
The mechanism by which cadherin-11 expression is increased in prostate cancer cells is not clear. It has been reported that prostate cancer cells undergo a switch from expressing epithelial E-cadherin to mesenchymal cadherin-11 (14), probably through an epithelial-to-mesenchymal transition (EMT) commonly observed in tumorigenesis (23). Intriguingly, prostate cancer cells have a propensity to express molecules that are usually expressed by osteoblasts, including osteocalcin (24, 25), osteonectin (26), osteopontin (27–29), bone sialoprotein (24, 29), the receptor activator of NF-κB ligand, and osteoprotegerin (30). This phenomenon, termed osteomimicry, was first described by Koeneman et al., who hypothesized that it contributes to the preferential growth of prostate cancer cells in bone (31). The EMT is triggered by the interplay of extracellular signals, including growth factors and extracellular matrix (32). Of the many factors, and thus many signaling pathways, involved in the EMT during prostate cancer progression, Huang et al. (33) recently identified β2-microglobin (β2M) as one factor that maintains the bone phenotypes exhibited by prostate cancer cells. Furthermore, Zayzafoon et al. (34) showed that Notch activation is crucial for prostate cancer cells to be able to acquire “osteoblast-like” properties. Whether β2M, Notch signaling, or other factors are involved in the expression of cadherin-11 in prostate cancer cells remain to be demonstrated.
The interaction between metastatic prostate cancer cells and organ-specific endothelial cells is also considered an important step in organ-specific metastasis, because the circulating prostate cancer cells must first arrest at sinusoids by adhering to the bone marrow endothelial cells and then migrate through the endothelial layer before settling into the bone environment (6, 7). Integrins present on the prostate cancer cell membrane have been shown to facilitate the adhesion of those cells to marrow endothelial cells (5). Another membrane protein, CXCR4, the receptor for stromal cell–derived factor-1 (SDF-1 or CXCL12), is also expressed by prostate cancer cells (35). Because endothelial cells, osteoblasts and some stromal cells express SDF-1, the CXCR4/SDF-1 pathway may be involved in prostate cancer metastasis. These observations suggest that additional interactions between metastatic prostate cancer cells and other host cells likely contribute to the tropism of circulating prostate cancer cells for bone. In our study, immunostaining of human prostate cancer specimens showed that only 50% of the human bone metastasis specimen showed cadherin-11 staining. Thus, cadherin-11 is one of many factors involved in the bone homing of prostate cancer cells.
In terms of clinical applications, cadherin-11 may be a candidate marker for the diagnosis of bone metastatic progression in prostate cancer patients. The inability to predict which patients will develop metastatic disease remains a major challenge for prostate cancer management and has resulted in excessive therapy for some patients and delayed or insufficient therapy for others. Although the presence of circulating prostate cancer cells in the blood is an indication that tumor cells have disseminated from the primary site, the nature of the association between circulating tumor cells and bone metastasis remains controversial (36). It is possible that only a subset of circulating tumor cells possess the necessary properties to target bone. Our findings here indicate that cadherin-11 has a role in the metastasis of prostate cancer to bone. Further development of cadherin-11 in clinical applications involving prostate cancer bone metastasis is warranted.
Materials and Methods
Cells, animals
The human prostate cancer cell lines LNCaP and DU145 were obtained from the American Type Culture Collection (Manassas, VA). The bone-metastatic cell line PC3 was kindly provided by Dr. I. J. Fidler (M. D. Anderson Cancer Center). The LNCaP derivatives C4, C4-2, and C4-2B (37) were kind gifts from Dr. L. Chung (Emory University). All prostate cancer cell lines were grown at 37°C with 5% CO2 in RPMI medium (Invitrogen) containing 10% fetal bovine serum.
SCID mice were purchased from Jackson Laboratory and maintained in M. D. Anderson’s animal facilities. All experimental procedures involving animals were performed in compliance with institutional and governmental requirements and approved by M. D. Anderson’s Animal Care and Use Committee.
Western blotting
Cells were lysed in cell lysis buffer (10 mM Tris–HCl [pH 7.5] containing 0.5% Nonidet P-40, 0.5 mM CaCl2, and protease inhibitors (Roche)). Whole cell extracts were separated by gel electrophoresis on 4%–12% NuPAGE Bis-Tris gradient gel (Novex/Invitrogen) and transferred to a Protran nitrocellulose membrane (Schleicher & Schnell). Mouse anti-Cad11 (5B2H5; Invitrogen) was used for western blotting, and signals were detected with a chemiluminescent detection kit (Pierce Biotechnology). Membranes were re-probed with a goat anti-actin antibody (Santa Cruz Biotechnology).
Immunocytochemical analysis
Cells were grown on Lab-Tek II chamber slides (Nunc) for 48 h, washed with phosphate-buffered saline (PBS), fixed with cold methanol for 10 min, washed with PBS, permeabilized with PBS plus 0.1% Triton X-100 for 10 min, washed with PBS, and blocked with 5% normal donkey serum, 1% bovine serum albumin (BSA), and 0.01% Triton X-100 in PBS for 30 min at room temperature. Mouse anti-cad11 (1:50) in 0.5% normal donkey serum and 0.01% BSA in PBS, with mouse IgG (sc-45051, Santa Cruz Biotechnology) used as a control. Cells were incubated with primary antibody overnight at 4°C, followed by a wash with Tris-buffered saline (pH 7.8) containing 0.1% Triton X-100. The slides were then incubated with Cy3-conjugated donkey anti-mouse secondary antibody (Jackson ImmunoResearch). Slides were mounted with Vectashield mounting medium plus 4′,6-diamidino-2-phenylindole and examined with an Olympus confocal microscope.
Retrovirus production and transduction
The plasmid pBMN-I-GFP (from Gary Nolan, Stanford University) is a bicistronic retroviral vector that allows the expression of a gene of interest in tandem with the reporter gene green fluorescent protein (GFP) through an internal ribosome entry site. The pBMN-Luc-I-GFP plasmid is a pBMN-I-GFP plasmid containing firefly luciferase (Luc) upstream of the internal ribosome entry site sequence. The pBMN-based plasmids were transfected into Pheonix cell by using Fugene 6 (Roche). To knockdown cadherin-11, shRNA against cadherin-11 were purchased from Open Biosystems (clone ID V2HS_150474) and Sigma-Aldrich (TRCN0000054334). The former targets bases 1997–2017 while the latter targets bases 2405–2422 of the human cadherin-11 (NM001797). The mouse stem cell virus containing human cadherin-11 shRNA knockdown construct (Open Biosystems) or control non-silencing plasmid were transfected into LinX cells by using Fugene 6. Similarly, lentivirus for the shRNA from Sigma-Aldrich was co-transfected with the Virapower DNA (Invitrogen) into 293FT cells using Fugene 6(Roche). The virus-containing supernatant was collected 48 h later and concentrated by using a Centriplus concentrator (Millipore). Prostate cancer cells were transduced with the recombinant retrovirus in the presence of 8 μg/μL polybrene at 32°C for 24 h. Cells transduced with the Luc-I-GFP retrovirus were further selected by fluorescence-activated cell sorting on a FACScan (Becton Dickinson). Cells transduced with mouse stem cell virus for knockdown studies were further selected with 1μg/ml puromycin for 14 days and pools of cells were screened by western blotting.
Cell-to-substrate adhesion assay
To assess the ability of prostate cancer cells to bind to recombinant cadherin-11, prostate cancer cells containing the Luc gene were lifted from plates with 1 mmol/L ethylenediamine tetraacetic acid (EDTA) in PBS, washed once with binding buffer (10 mM HEPES [pH 7.4], 137 mM NaCl, 5.4 mM KCl, 0.34 mM Na2HPO4, 5.5 mM glucose, and 2 mM CaCl2), and used in a cell-to-substrate adhesion assay as follows. The Luc-labeled cells were diluted to 5 ×105/mL and plated in 96-well plates that had been coated with Cad11-Fc or Cad12-Fc (R&D Systems). Adhesion was allowed to continue for 30 min at 37°C, after which the wells were washed once with binding buffer and the total Luc per well was measured with a luminometer (Turner BioSystems).
Cell proliferation assay
PC3-shCont and PC3-shCad-11 cells were seeded in six-well plates and counted daily for 3 days with a hematocytometer. For co-culture study, PC3-shCont or PC3-shCad-11 were cocultured with MC3T3-E1 at a 1:1 ratio and added to a 24 well plate in triplicate. At specific time points, cells were lysed with 200μL of Passive Lysis Buffer (Promega) and 5μL was used to measure the Luciferase activity.
Xenograft model of metastasis
Intracardiac injection of prostate cancer cells followed with in vivo bioluminescence imaging was used to examine the ability of the prostate cancer cells to home to bone. Male SCID mice (5–6 weeks old) were anesthetized with isofluorene gas and PC3-Luc, PC3-shCont, or PC3-shCad-11 cells (1 × 106 cells/mouse) were injected into the left ventricle. Bioluminescence imaging was performed 30 min after the intracardiac injection to detect the distribution of prostate cancer cells. Mice that showed whole-body bioluminescence signal were further monitored with weekly bioluminescence imaging. Mice were injected with 150 μL of D-Luciferin solution (150mg/ml) intraperitoneally. Images were acquired and analyzed with an IVIS 200 Imaging System (Xenogen). Ex vivo images of tumor-bearing tissues excised from the mice at necropsy were also obtained.
Histologic analysis of mouse tissues
Tumor-bearing tissues were fixed in cold 4% paraformaldehyde. Histologic analysis was used for further confirmation of the presence of tumor cells in the specific organs at the end of the experiment. Bone specimens were decalcifed in 10% EDTA in PBS for 14 days. The decalcified bones were cut at the midpoint and embedded in paraffin blocks. Serial paraffin sections were stained with hematoxylin and eosin. Tartrate-resistant alkaline phosphatase staining was performed using an Acid Phosphatase Kit (Sigma-Aldrich, 386A-1KT) as described in the manufacture’s instruction.
Immunostaining of human prostate cancer specimens
Formalin-fixed, paraffin-embedded tissue samples representing a spectrum of localized and metastatic prostate cancer, including specimens from radical or transurethral prostatectomy, lymph nodes, and bone with prostate cancer metastases (Table 1), were selected from a prostate cancer tissue bank (supported by a Specialized Program of Research Excellence Award to The University of Texas M. D. Anderson Cancer Center) or from Taipei Medical University and Hospital.
A goat anti-cadherin-11 antibody (R&D Systems) against the extracellular domain of cadherin-11 was used for immunohistochemical analysis as follows. Four-μm-thick sections were dewaxed with xylene, rehydrated in graded concentrations of alcohol, treated with 3% hydrogen peroxide in methanol for 15 min, washed with PBS, blocked with normal horse serum for 30 min, and incubated at 4°C overnight with anti-cadherin-11 (2 μg/ml). Antibody binding was detected by using a labeled streptavidin-biotin kit with 3,3′-diaminobenzidine as the chromogen (DAKO). Hematoxylin was used as the counterstain. The immunostaining was considered positive when more than 10% of the tumor cells were immunoreactive.
Statistical analysis
Two-sided Fisher’s exact tests were used to test for significant differences in cadherin-11 expression among the human specimens from various organ sites (primary prostate tumor, lymph node metastases, bone metastases, and other metastases). P values less than 0.05 were considered statistically significant.
Acknowledgments
We thank Christine Wogan of the Department of Scientific Publications at M. D. Anderson Cancer Center for editing this manuscript. This work was supported by grants from the National Institutes of Health (CA111479, P50 CA90270, and DK53176), the Prostate Cancer Foundation, the U.S. Department of Defense (PC061279), and a Young Investigator Award from the American Society of Clinical Oncology (A.J. Zurita).
References
- 1.Tu S-M, Lin S-H. Clinical Aspects of Bone Metastases in Prostate Cancer. In: Keller ET, Chung LW, editors. The Biology of Bone Metastases. Boston, MA: Kluwer Academic Publishers; 2004. pp. 23–46. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs SC. Spread of prostatic cancer to bone. Urology. 1983;21:337–44. doi: 10.1016/0090-4295(83)90147-4. [DOI] [PubMed] [Google Scholar]
- 3.Shah RB, Mehra R, Chinnaiyan AM, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64:9209–16. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 4.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 5.Tantivejkul K, Kalikin LM, Pienta KJ. Dynamic process of prostate cancer metastasis to bone. J Cell Biochem. 2004;91:706–17. doi: 10.1002/jcb.10664. [DOI] [PubMed] [Google Scholar]
- 6.Sikes RA, Nicholson BE, Koeneman KS, et al. Cellular interactions in the tropism of prostate cancer to bone. Int J Cancer. 2004;110:497–503. doi: 10.1002/ijc.20153. [DOI] [PubMed] [Google Scholar]
- 7.Lehr JE, Pienta KJ. Preferential adhesion of prostate cancer cells to a human bone marrow endothelial cell line. J Natl Cancer Inst. 1998;90:118–23. doi: 10.1093/jnci/90.2.118. [DOI] [PubMed] [Google Scholar]
- 8.Cooper CR, McLean L, Walsh M, et al. Preferential adhesion of prostate cancer cells to bone is mediated by binding to bone marrow endothelial cells as compared to extracellular matrix components in vitro. Clin Cancer Res. 2000;6:4839–47. [PubMed] [Google Scholar]
- 9.Tu S-M, Reyes A, Maa A, et al. Prostate carcinoma with testicular or penile metastases. Cancer. 2002;94:2610–7. doi: 10.1002/cncr.10546. [DOI] [PubMed] [Google Scholar]
- 10.Okazaki M, Takeshita S, Kawai S, et al. Molecular cloning and characterization of OB-cadherin, a new member of cadherin family expressed in osteoblasts. J Biol Chem. 1994;269:12092–8. [PubMed] [Google Scholar]
- 11.Kawaguchi J, Kii I, Sugiyama Y, Takeshita S, Kudo A. The transition of cadherin expression in osteoblast differentiation from mesenchymal cells: consistent expression of cadherin-11 in osteoblast lineage. J Bone Miner Res. 2001;16:260–9. doi: 10.1359/jbmr.2001.16.2.260. [DOI] [PubMed] [Google Scholar]
- 12.Umbas R, Schalken JA, Adlders TW, et al. Expression of the cellular adhesion molecule E-cadherin is reduced or absent in high-grade prostate cancer. Cancer Res. 1992;52:5104–9. [PubMed] [Google Scholar]
- 13.Kuniyasu H, Troncoso P, Johnston D, et al. Relative expression of type IV collagenase, E-cadherin, and vascular endothelial growth factor/vascular permeability factor in prostatectomy specimens distinguishes organ-confined from pathologically advanced prostate cancers. Clin Cancer Res. 2000;6:2295–308. [PubMed] [Google Scholar]
- 14.Tomita K, van Bokhoven A, van Leenders GJ, et al. Cadherin switching in human prostate cancer progression. Cancer Res. 2000;60:3650–4. [PubMed] [Google Scholar]
- 15.Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005;65(17):7554–60. doi: 10.1158/0008-5472.CAN-05-1317. [DOI] [PubMed] [Google Scholar]
- 16.Logothetis C, Lin S-H. Osteoblasts in prostate cancer metastasis to bone. Nature Reviews Cancer. 2005;5:21–8. doi: 10.1038/nrc1528. [DOI] [PubMed] [Google Scholar]
- 17.Chung LW. Prostate carcinoma bone-stroma interaction and its biologic and therapeutic implications. Cancer. 2003;97:772–8. doi: 10.1002/cncr.11140. [DOI] [PubMed] [Google Scholar]
- 18.Wheelock MJ, Johnson KR. Cadherin-mediated cellular signaling. Curr Opin Cell Biol. 2003;15:509–14. doi: 10.1016/s0955-0674(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 19.Mbalaviele G, Shin CS, Civitelli R. Cell-cell adhesion and signaling through cadherins: connecting bone cells in their microenvironment. J Bone Miner Res. 2006;21:1821–7. doi: 10.1359/jbmr.060811. [DOI] [PubMed] [Google Scholar]
- 20.Orlandini M, Oliviero S. In fibroblasts Vegf-D expression is induced by cell-cell contact mediated by cadherin-11. J Biol Chem. 2001;276:6576–81. doi: 10.1074/jbc.M009573200. [DOI] [PubMed] [Google Scholar]
- 21.Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147:631–44. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawaguchi J, Azuma Y, Hoshi K, et al. Targeted disruption of cadherin-11 leads to a reduction in bone density in calvaria and long bone metaphyses. J Bone Miner Res. 2001;16:1265–71. doi: 10.1359/jbmr.2001.16.7.1265. [DOI] [PubMed] [Google Scholar]
- 23.Maeda M, Johnson KR, Wheelock MJ. Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci. 2005;118:873–87. doi: 10.1242/jcs.01634. [DOI] [PubMed] [Google Scholar]
- 24.Huang WC, Xie Z, Konaka H, Sodek J, Zhau HE, Chung LW. Human osteocalcin and bone sialoprotein mediating osteomimicry of prostate cancer cells: role of cAMP-dependent protein kinase A signaling pathway. Cancer Res. 2005;65:2303–13. doi: 10.1158/0008-5472.CAN-04-3448. [DOI] [PubMed] [Google Scholar]
- 25.Ou YC, Chen JT, Yang CR, Ko JL, Hsieh YS, Kao C. Expression of osteocalcin in prostate cancer before and after hormonal therapy. Anticancer Res. 2003;23:3807–11. [PubMed] [Google Scholar]
- 26.Thomas R, True LD, Bassuk JA, Lange PH, Vessella RL. Differential expression of osteonectin/SPARC during human prostate cancer progression. Clin Cancer Res. 2000;6:1140–9. [PubMed] [Google Scholar]
- 27.Tozawa K, Yamada Y, Kawai N, Okamura T, Ueda K, Kohri K. Osteopontin expression in prostate cancer and benign prostatic hyperplasia. Urol Int. 1999;62:155–8. doi: 10.1159/000030381. [DOI] [PubMed] [Google Scholar]
- 28.Khodavirdi AC, Song Z, Yang S, et al. Increased expression of osteopontin contributes to the progression of prostate cancer. Cancer Res. 2006;66:883–8. doi: 10.1158/0008-5472.CAN-05-2816. [DOI] [PubMed] [Google Scholar]
- 29.Carlinfante G, Vassiliou D, Svensson O, Wendel M, Heinegard D, Andersson G. Differential expression of osteopontin and bone sialoprotein in bone metastasis of breast and prostate carcinoma. Clin Exp Metastasis. 2003;20:437–44. doi: 10.1023/a:1025419708343. [DOI] [PubMed] [Google Scholar]
- 30.Roato I, Grano M, Brunetti G, et al. Mechanisms of spontaneous osteoclastogenesis in cancer with bone involvement. FASEB J. 2005;19:228–30. doi: 10.1096/fj.04-1823fje. [DOI] [PubMed] [Google Scholar]
- 31.Koeneman KS, Yeung F, Chung LWK. Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate. 1999;39:246–61. doi: 10.1002/(sici)1097-0045(19990601)39:4<246::aid-pros5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 32.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 33.Huang WC, Wu D, Xie Z, et al. beta2-microglobulin is a signaling and growth-promoting factor for human prostate cancer bone metastasis. Cancer Res. 2006;66:9108–16. doi: 10.1158/0008-5472.CAN-06-1996. [DOI] [PubMed] [Google Scholar]
- 34.Zayzafoon M, Abdulkadir SA, McDonald JM. Notch signaling and ERK activation are important for the osteomimetic properties of prostate cancer bone metastatic cell lines. J Biol Chem. 2004;279:3662–70. doi: 10.1074/jbc.M308158200. [DOI] [PubMed] [Google Scholar]
- 35.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LS. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–7. [PubMed] [Google Scholar]
- 36.Morgan TM, Lange PH, Vessella RL. Detection and characterization of circulating and disseminated prostate cancer cells. Front Biosci. 2007;12:3000–9. doi: 10.2741/2290. [DOI] [PubMed] [Google Scholar]
- 37.Wu TT, Sikes RA, Cui Q, et al. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer. 1998;77:887–94. doi: 10.1002/(sici)1097-0215(19980911)77:6<887::aid-ijc15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]