Abstract
Low levels (i.e., ≤20 mmHg) of lower body negative pressure (LBNP) have been utilized to unload “selectively” cardiopulmonary baroreceptors in humans, since steady-state mean arterial pressure and heart rate (HR) have been found unchanged at such levels. However, transient reductions in blood pressure (BP), followed by reflex compensation, may occur without detection, which could unload arterial baroreceptors. The purposes of this study were to test the hypothesis that the arterial baroreflex is engaged even during low levels of LBNP and to determine the time course of changes in hemodynamics. Fourteen healthy individuals (age range 20–54 yr) were studied. BP (Portapres and Suntech), HR (ECG), pulmonary capillary wedge pressure (PCWP) or pulmonary artery diastolic pressure (PDP) and right atrial pressure (RAP) (Swan-Ganz catheter) and hemodynamics (Modelflow) were recorded continuously at baseline and −15- and −30-mmHg LBNP for 6 min each. Application of −15-mmHg LBNP resulted in rapid and sustained falls in RAP and PCWP or PDP, progressive decreases in cardiac output and stroke volume, followed subsequently by transient reductions in both systolic and diastolic BP, which were then restored through the arterial baroreflex feedback mechanism after ∼15 heartbeats. Additional studies were performed in five subjects using even lower levels of LBNP, and this transient reduction in BP was observed in three at −5- and in all at −10-mmHg LBNP. The delay for left ventricular stroke volume to fall at −15-mmHg LBNP was about 10 cardiac cycles. An increase in systemic vascular resistance was detectable after 20 heartbeats during −15-mmHg LBNP. Steady-state BP and HR remained unchanged during mild LBNP. However, BP decreased, while HR increased, at −30-mmHg LBNP. These results suggest that arterial baroreceptors are consistently unloaded during low levels (i.e., −10 and −15 mmHg) of LBNP in humans. Thus “selective” unloading of cardiopulmonary baroreceptors cannot be presumed to occur during these levels of mild LBNP.
Keywords: baroreflexes, arterial pressure, hemodynamics
for decades, low levels (i.e., ≤20 mmHg) of lower body negative pressure (LBNP) have been firmly believed to be a useful tool for separating the contribution of cardiopulmonary and arterial baroreflexes to circulatory control in humans (1, 34, 56). They have been utilized “selectively” to unload cardiopulmonary baroreceptors in many studies based on the fact that steady-state mean arterial pressure and heart rate have been found unchanged (5, 26, 28, 40, 46, 54), forearm blood flow and conductance decreased (38, 56), while muscle sympathetic nerve activity increased (16, 17, 27, 38, 43) at such levels. One early study by Zoller et al. (56) investigated not only steady-state but also dynamic responses before and during initiation of mild LBNP and did not reveal any transient changes in arterial pressure, which might have triggered reflexes originating in arterial baroreceptors.
Despite this dogma, direct recordings of aortic nerve activity in anesthetized dogs showed that aortic baroreceptor discharge decreased even when mean arterial pressure remained unchanged during hemorrhage (20). It was also demonstrated previously that mean arterial pressure decreased significantly in sinoaortic-denervated conscious primates, but was not altered in intact animals when a very small amount (i.e., <2%) of blood was removed (12), supporting the notion that a nonhypotensive reduction in venous return unloaded arterial baroreceptors sufficiently to activate the arterial baroreflex.
Some human studies have also challenged the traditional thinking that low levels of LBNP “selectively” unload cardiopulmonary baroreceptors. For example, with magnetic resonance imaging, Taylor et al. (45) found that nonhypotensive hypovolemia induced by mild LBNP reduced ascending aortic dimensions in humans, suggesting an unloading of arterial baroreceptors. Using power spectral analysis, Floras et al. (15) showed that LBNP at −5 mmHg elicited a reflex reduction in the parasympathetic modulation of heart rate in middle-aged men. A previous case report by Aksamit et al. (2) demonstrated, in a patient with impaired arterial baroreflex following neck and mediastinal radiation, that −10-mmHg LBNP caused a sustained fall in arterial pressure, which is not usually seen in healthy humans who have intact arterial baroreflex function, indicating that arterial baroreceptors are involved in blood pressure maintenance during mild LBNP.
Most compellingly, using noninvasive finger photoplethysmography, Hisdal et al. (22, 23) reported recently that mean arterial pressure was transiently but strongly affected by either rapid or slow onset and release of −20-mmHg LBNP in healthy young individuals, providing direct evidence for the engagement of the arterial baroreflex. Whether such transient reductions in blood pressure can also be seen at lower levels (i.e., −5, −10, and −15 mmHg) of LBNP in humans needs to be determined. Moreover, the time course of changes in venous return, cardiac filling, and central hemodynamics during mild LBNP, especially in the initial period of LBNP, needs to be investigated.
With invasive pulmonary artery catheterization, we measured beat-by-beat right atrial pressure and pulmonary capillary wedge pressure or pulmonary artery diastolic pressure (the latter two were used as an index of left atrial and thereby left ventricular end-diastolic pressure) at the onset, as well as during mild LBNP in healthy individuals. We also recorded noninvasively beat-by-beat arterial pressure and heart rate during the entire procedure, while beat-by-beat hemodynamic variables were derived from the Modelflow method (29, 35, 39), calibrated by a modified acetylene rebreathing technique (47). The purposes of this study were 1) to test the hypothesis that the arterial baroreflex is engaged during mild LBNP in humans; and 2) to determine the time course of changes in cardiac filling and central hemodynamics during low levels of LBNP.
METHODS
Subjects
Nineteen healthy volunteers (16 men, 3 women) were studied. They were 35 ± 11 yr old (mean ± SD), 178 ± 10 cm in height, and 77 ± 13 kg body wt. No subject smoked, used recreational drugs, or had significant medical problems. None was an endurance-trained athlete, and subjects were excluded if they exercised for >30 min/day and >3 times/wk (either dynamic or static exercise). No woman was pregnant or in the early follicular phase (menstruation) of her menstrual cycle during the experiments. The subjects were screened with a careful history, physical examination, and 12-lead electrocardiogram. All subjects were informed of the purpose and procedures used in the study and gave their written, informed consent to a protocol approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center at Dallas and Presbyterian Hospital of Dallas.
Measurements
Heart rate and blood pressure.
Heart rate was determined from lead II of the electrocardiogram, and beat-by-beat arterial pressure was measured noninvasively using finger photoplethysmography (Portapres, TNO-BMI, Amsterdam, The Netherlands). Cuff blood pressure was measured by electrosphygmomanometry (model 4240, SunTech Medical Instruments, Raleigh, NC) with a microphone placed over the brachial artery to detect Korotkoff sounds, and arm cuff mean arterial pressure and diastolic blood pressure were used as references for the finger arterial pressure measurements. Respiratory excursions were monitored continuously via a piezoelectric transducer (Pneumotrace II, UFI, Morro Bay, CA).
Cardiac filling pressure.
A 6F balloon-tipped, flow-directed pulmonary arterial catheter (Edwards Swan-Ganz, Edwards Lifesciences, Irvine, CA) was placed under fluoroscopic guidance through a right antecubital vein into the pulmonary artery. Beat-by-beat right atrial pressure was measured at the proximal port of the catheter. Left ventricular end-diastolic pressure was estimated from pulmonary capillary wedge pressure or pulmonary artery diastolic pressure with the balloon of the Swan-Ganz catheter inflated or deflated. All intracardiac pressures were referenced to atmospheric pressure, with the pressure transducer (Transpac IV, CIVCO Medical Instruments, Kalona, IA) zero reading set at 5 cm below the sternal angle in the supine position. Pressure waveforms were amplified (Agilent M1165, Agilent Technologies, Andover, MA) and displayed on a strip-chart recorder (Astromed MT 95000, Astro-Med, West Warwick, RI) with ≤0.5 mmHg resolution.
Hemodynamic variables.
Beat-by-beat cardiac output and stroke volume were derived from the Modelflow method (29, 35, 39), calibrated by the acetylene rebreathing technique (47), so that the baseline Modelflow cardiac output was made equal to the baseline acetylene cardiac output for each subject. Beat-by-beat systemic vascular resistance was calculated as [(mean arterial pressure − right atrial pressure)/cardiac output] × 80 (expressed as dyn·s·cm−5), while beat-by-beat mean arterial pressure was derived from the Beatfast Modelflow program (BeatScope 1.1a, Finapres Medical System BV, Amsterdam, the Netherlands).
Steady-state cardiac output was measured intermittently with the modified acetylene rebreathing technique (model MGA1100, Marquette, Milwaukee, WI) (47). This method has been validated in our laboratory against standard invasive techniques, including thermodilution and direct Fick during orthostatic stress with a typical error (expressed as coefficient of variation) of 4–5% (18). Steady-state stroke volume was calculated from cardiac output and the heart rate measured during the rebreathing. Steady-state systemic vascular resistance was calculated as [(mean arterial pressure − right atrial pressure)/cardiac output] × 80. Mean arterial pressure was calculated using arm cuff blood pressure, where right atrial pressure was averaged during steady state.
Protocol
All experiments were performed in the morning >2 h after a light breakfast and >12 h after the last caffeinated or alcoholic beverage, in a quiet, environmentally controlled laboratory with an ambient temperature of ∼25°C. The subject was placed in a Plexiglas LBNP tank sealed at the level of the iliac crests in the supine position. Suction was provided by a vacuum pump controlled with a variable autotransformer and calibrated against a mercury manometer. After at least 30 min of quiet rest, baseline data were collected for 6 min. Then −15-mmHg LBNP was started over ∼5 s at the end of expiration with breath held for ∼10–15 cardiac cycles in 14 subjects. LBNP −15-mmHg stage lasted for 6 min, followed by −30-mmHg LBNP for 6 min, and then finally by a 3-min recovery period. Heart rate, blood pressure, respiratory waveforms, right atrial pressure, and pulmonary artery diastolic pressure were recorded continuously. Steady-state cardiac output was measured at baseline before data collection, and at the 6th min of −15- and −30-mmHg LBNP. In 9 of the 14 subjects, the balloon of the Swan-Ganz catheter was inflated at baseline and during initiation of −15-mmHg LBNP for ∼20 s each to make sure that pulmonary artery diastolic pressure tracked pulmonary capillary wedge pressure and, therefore, left ventricular end-diastolic pressure accurately.
In a separate study, after ≥30 min of supine rest, LBNP of −5 and −10 mmHg was applied for 3 min each in an additional five healthy young subjects with invasive pulmonary artery catheterization. The balloon of the catheter was inflated in all subjects before and during initiation of LBNP, and, therefore, right atrial pressure and pulmonary capillary wedge pressure were measured continuously. In addition, beat-by-beat finger blood pressure and heart rate were recorded during the entire experimental procedures.
Data Analysis and Statistics
Data were sampled at 200 Hz with a commercial data-acquisition system (Biopac System, Santa Barbara, CA). Baseline data for −5- and −10-mmHg LBNP from five additional subjects were analyzed on a beat-by-beat basis and averaged for every five beats over 40 cardiac cycles before the onset of LBNP. Baseline data for −15- and −30-mmHg LBNP were averaged for 6 min. During −5-, −10-, and −15-mmHg LBNP, data were analyzed on the beat-by-beat basis for the initial 40 heartbeats; however, pulmonary capillary wedge pressure was analyzed for the initial 12 heartbeats, since the balloon of the Swan-Ganz catheter was inflated for only 20 s. Beat-by-beat pulmonary capillary wedge pressure and pulmonary artery diastolic pressure data during −15-mmHg LBNP were combined. Steady-state data during −15- and −30-mmHg LBNP were averaged from the 2nd to the 5th min.
Data are expressed as means ± SD (if normality test passed) or median (25th, 75th percentile) (if normality test failed). Variables at baseline and the initial 40 heartbeats during −15-mmHg LBNP were analyzed using one-way repeated-measures analysis of variance (RM ANOVA), if normality test passed, and using Friedman RM ANOVA on ranks (nonparametric test), if normality test failed. The Holm-Sidak or Dunn's method was used post hoc for multiple comparisons vs. baseline. The correlation between the mean values of combined pulmonary capillary wedge pressure and pulmonary artery diastolic pressure, and pulmonary capillary wedge pressure alone, at baseline and during initiation of −15-mmHg LBNP was analyzed using the least squares linear regression model. Steady-state hemodynamic variables at baseline and during −15- and −30-mmHg LBNP were also analyzed using one-way RM ANOVA or Friedman RM ANOVA on ranks. Both individual and mean data obtained during −5- and −10-mmHg LBNP in five subjects were presented, and no statistics were carried out for these additional data. All statistical analyses were performed with a personal computer-based analysis program (SigmaStat, SPSS). A P value of <0.05 was considered statistically significant.
RESULTS
Beat-by-Beat Hemodynamic Responses
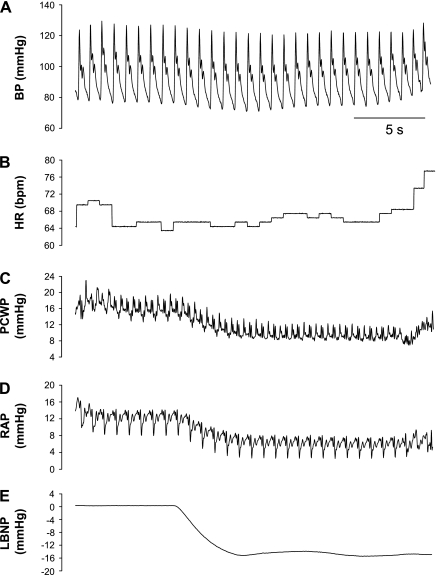
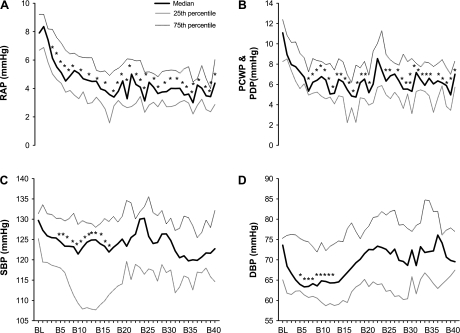
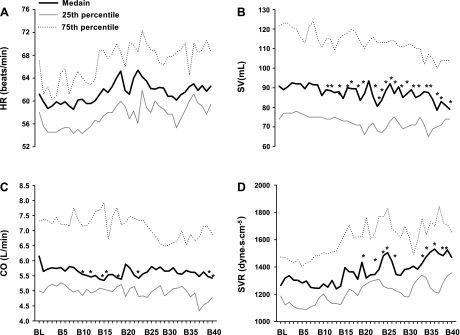
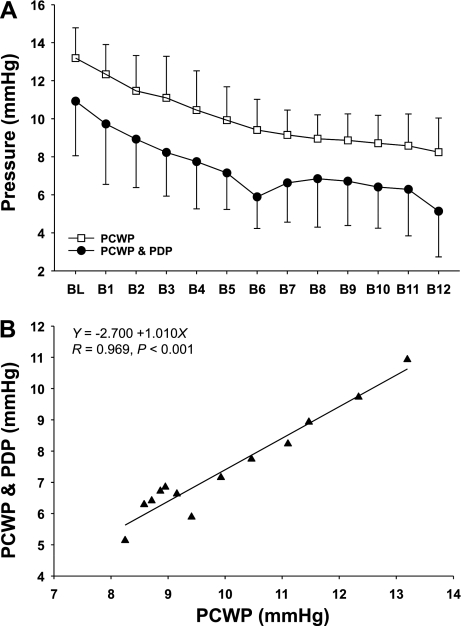
Figure 1 depicts original tracings of beat-by-beat arterial pressure, heart rate, and cardiac filling pressure before and at the onset of −15-mmHg LBNP from one subject. Application of −15-mmHg LBNP resulted in a rapid and sustained fall in right atrial pressure (Fig. 2A), followed subsequently by a fall in pulmonary capillary wedge pressure or pulmonary artery diastolic pressure (Fig. 2B). Systolic and diastolic blood pressures decreased transiently and then were restored through the arterial baroreflex feedback mechanism after ∼15 heartbeats (Fig. 2C and 2D). Heart rate remained stable during −15-mmHg LBNP (Fig. 3A). There was a delay (i.e., 10 cardiac cycles) for stroke volume to fall during application of −15-mmHg LBNP (Fig. 3B). Cardiac output decreased progressively during −15-mmHg LBNP (Fig. 3C). Systemic vascular resistance did not change significantly during initiation of −15-mmHg LBNP, but started to increase after 20 heartbeats (Fig. 3D). The combined pulmonary artery diastolic pressure and pulmonary capillary wedge pressure data tracked pulmonary capillary wedge pressure alone accurately at baseline and during initiation of −15-mmHg LBNP (Fig. 4A). They were highly correlated (Fig. 4B), and the typical error for these two data sets was 5.9%.
Fig. 1.
Original tracings of blood pressure (BP; A), heart rate (HR; B), pulmonary capillary wedge pressure (PCWP; C), right atrial pressure (RAP; D), and lower body negative pressure (LBNP; E) from one representative subject.
Fig. 2.
Beat-by-beat RAP (A), PCWP and pulmonary artery diastolic pressure (PDP; B), systolic blood pressure (SBP; C), and diastolic blood pressure (DBP, D) before and at the onset of −15-mmHg LBNP. BL, baseline; B5–B40: the 5th through 40th heartbeat after initiation of LBNP. Values are expressed as median and 25th as well as 75th percentile. *P < 0.05 compared with baseline.
Fig. 3.
Beat-by-beat HR (A), stroke volume (SV; B), cardiac output (CO; C), and systemic vascular resistance (SVR; D) before and at the onset of −15-mmHg LBNP. Data were derived from the Modelflow method. B5–B40, the 5th through 40th heartbeat after application of LBNP. Values are median and 25th as well as 75th percentile. *P < 0.05 compared with baseline.
Fig. 4.
A: beat-by-beat PCWP (measured with the balloon of the Swan-Ganz catheter inflated) vs. combined PCWP and PDP (measured with the balloon deflated) at baseline and during initiation of −15-mmHg LBNP. B1–B12, the 1st to the 12th heartbeat after application of LBNP. Values are means ± SD. B: the mean values of combined PCWP and PDP, and PCWP, were highly correlated, indicating that PDP could accurately track PCWP and, therefore, left ventricular end-diastolic pressure as well as volume.
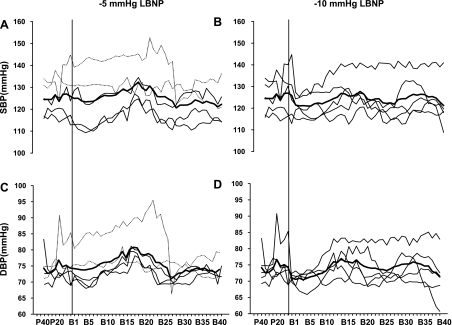
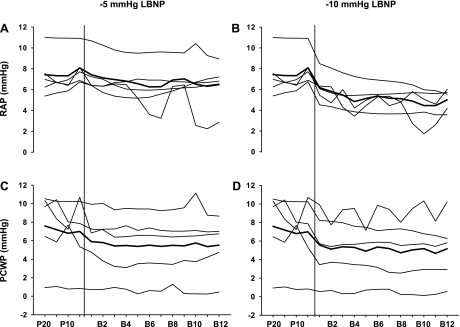
In the additional study, the transient reduction in arterial pressure was observed in three subjects during −5-mmHg LBNP, and in all five subjects during −10-mmHg LBNP (Fig. 5). During −5-mmHg LBNP, systemic vascular resistance increased more rapidly in one of the two subjects who did not have the transient reduction in arterial pressure than those who did; however, the other subject had a similar systemic vascular resistance response to those who had the transient reduction in arterial pressure. Both right atrial pressure and pulmonary capillary wedge pressure decreased at −5- and −10-mmHg LBNP (Fig. 6). Heart rate remained stable during −5- and −10-mmHg LBNP in these subjects.
Fig. 5.
Beat-by-beat individual (thin line) and mean (thick line) SBP and DBP 40 heartbeats before and during initiation of −5- (A and C) and −10-mmHg LBNP (B and D) in an additional 5 healthy young subjects with pulmonary artery catheterization. During −5-mmHg LBNP, two subjects (dotted line) did not have, while three subjects had, transient reductions in arterial pressure. During −10-mmHg LBNP, all subjects showed transient reductions in arterial pressure. P40 and P20, 40 and 20 heartbeats before LBNP, respectively. B1–B40, the 1st through 40th heartbeat after application of LBNP. The vertical line indicates the onset of LBNP.
Fig. 6.
Individual (thin line) and mean (thick line) RAP and PCWP at −5- (A and C) and −10-mmHg LBNP (B and D) in 5 subjects. P20 and P10, 20 and 10 heartbeats before LBNP; B2–B12, the 2nd through 12th heartbeat after application of LBNP. The vertical line indicates the onset of LBNP.
Steady-state Hemodynamic Responses
Table 1 shows steady-state hemodynamic responses. Steady-state cuff systolic blood pressure decreased during −30-mmHg LBNP (P < 0.001), while diastolic pressure did not change significantly during −15- or −30-mmHg LBNP (ANOVA, P = 0.783). Steady-state heart rate remained unchanged during −15-mmHg LBNP (P = 0.226), but increased during −30-mmHg LBNP (P < 0.001). Both right atrial pressure and pulmonary artery diastolic pressure decreased progressively during −15- and −30-mmHg LBNP (both ANOVA, P < 0.001). Steady-state cardiac output and stroke volume decreased during −15-mmHg LBNP and further decreased during −30-mmHg LBNP (both ANOVA, P < 0.001). Systemic vascular resistance increased gradually during −15- and −30-mmHg LBNP in all subjects (ANOVA, P < 0.001).
Table 1.
Steady-state hemodynamic responses during −15- and −30-mmHg LBNP
| Variables | Baseline | −15-mmHg LBNP | −30-mmHg LBNP |
|---|---|---|---|
| Cuff systolic blood pressure, mmHg | 122±8 | 118±10 | 113±10* |
| Cuff diastolic blood pressure, mmHg | 72±7 | 72±8 | 71±7 |
| Heart rate, beats/min | 63±8 | 65±9 | 72±12* |
| Right atrial pressure, mmHg | 8.1±2.1 | 4.1±1.3* | 2.3±0.9* |
| Pulmonary artery diastolic pressure, mmHg | 10.3±2.7 | 6.4±1.7* | 4.8±1.8* |
| Cardiac output, l/min | 6.02±1.05 | 5.23±1.16* | 4.51±0.90* |
| Stroke volume, ml | 90±17 | 74±18* | 58±15* |
| Systemic vascular resistance, dyn·s·cm−5 | 1,101±239 | 1,343±391* | 1,525±361* |
Values are means ± SD. LBNP, lower body negative pressure.
P < 0.05 compared with baseline.
DISCUSSION
The major findings of this study are that 1) application of −15-mmHg LBNP resulted in a rapid and sustained fall in right atrial pressure, a subsequent fall in left ventricular end-diastolic pressure, a progressive decrease in cardiac output, followed by transient reductions in systolic and diastolic blood pressure; 2) both systolic and diastolic pressures were restored, presumably through the arterial baroreflex feedback mechanism, after ∼15 heartbeats; and 3) the transient reduction in arterial pressure was also observed in most subjects during initiation of −5-mmHg LBNP and in all during initiation of −10-mmHg LBNP. These results support our hypothesis and suggest that arterial baroreceptors are unloaded and the arterial baroreflex is consistently engaged during −10- and −15-mmHg of LBNP.
Time Course of Changes in Hemodynamics
We showed the time course of changes in cardiac filling and central hemodynamics during application of mild LBNP in healthy humans. The rapid and sustained fall in right atrial pressure at the onset of LBNP was caused by a quick reduction in venous return due to blood pooling in lower body capacitance vessels (54). Thus right ventricular output to the pulmonary vascular bed must have decreased instantly upon application of LBNP. We did not measure right ventricular output in the present study. However, Wolthuis et al. (55) found a reduction in plasma-bound isotope activity, as detected over the right anterior chest during LBNP, indicating a decrease in pulmonary blood flow and thereby pulmonary blood volume. Followed subsequently, left ventricular end-diastolic pressure decreased, suggesting a reduction in left ventricular end-diastolic volume. Although heart rate remained stable at the onset and during −15-mmHg LBNP, cardiac output decreased progressively.
The transient fall in systolic and diastolic blood pressure during initiation of −15-mmHg LBNP appeared to be attributable to the fall in cardiac output. In the present study, LBNP was started over ∼5 s. Blood pressure was found decreased transiently at either rapid (0.3 s) or slow (15 s) initiation of −20-mmHg LBNP in previous investigations (22, 23). Our data are in agreement with those of Hisdal et al. (22, 23), and together demonstrate convincingly that low levels of LBNP generally do indeed produce a detectable transient reduction in blood pressure. The restoration of arterial pressure later on during low levels of LBNP was due to an increase in systemic vascular resistance.
However, Zoller et al. (56) did not find any transient changes in arterial pressure at the onset of mild (i.e., −10 mmHg) LBNP, which appears to conflict with our findings. One potential explanation for these discrepancies may be attributable to different methodologies utilized to measure blood pressure. Zoller et al. inserted a polyethylene cannula into the brachial artery and advanced it 10–20 cm proximal to the antecubital fossa; thus blood pressure measured was closer to aortic (i.e., central) pressure. In our study, blood pressure was measured noninvasively using finger (i.e., peripheral) photoplethysmography. As suggested by Pawelczyk and Raven (37), it is conceivable that the peripheral amplification of a small change in central/aortic pressure undetected by Zoller et al. (56) may account for the differences between our findings and those of Zoller et al. It is of note that Zoller et al. did not apply −15-mmHg LBNP to their subjects; although −20-mmHg LBNP was applied, they did not report any dynamic changes in arterial pressure during initiation of −20-mmHg LBNP.
Consistent with the results of Hisdal et al. (23), we also observed a delayed (i.e., after 10 heartbeats) reduction in stroke volume during −15-mmHg LBNP in our subjects by using a different (Modelflow vs. ultrasound) method. Similar observations were made by Wieling et al. (41, 53) in healthy individuals on moving from the supine position to upright tilt. A reservoir of blood between the right and left ventricles (24, 50) was likely to account for the delayed fall in left ventricular stroke volume. Some of this blood is undoubtedly stored in the pulmonary circulation (i.e., the upstream reservoir), since Dubois and Marshall (14) showed that, usually during normal or deep respiration, the pulmonary capillary blood flow did not vary. With positron emission tomography, Cai et al. (7) showed that the lung (pulmonary) blood volume decreased by ∼22% (i.e., ∼80 ml) during −20-mmHg LBNP in healthy young men, supporting the concept of pulmonary circulation being a reservoir for filling of left ventricle.
Evidence of Arterial Baroreflex Engagements
We found in this study that application of −10- or −15-mmHg LBNP resulted in universal transient decreases in both systolic and diastolic blood pressures, which were then restored after ∼15 heartbeats. Undoubtedly, the restoration of blood pressure was, at least in part, through the arterial baroreflex feedback mechanism. However, as has been noted for decades, steady-state arterial pressure and heart rate were not altered significantly during mild LBNP, appearing to argue against the notion that the arterial baroreceptors are engaged during this period. Heart rate remained unchanged, despite the fact that the arterial baroreceptors were clearly unloaded at the onset of and during −15-mmHg LBNP. It is possible that vagal withdrawal and sympathetic activation to the heart during the transient reductions in arterial pressure could have been offset or obscured by the simultaneous unloading of atrial, ventricular, or aortic receptors that activate cardiac-specific sympathoexcitatory reflexes. For example, Flores et al. (15) proposed that, if a tonically active Bainbridge reflex was functionally important in humans, nonhypertensive LBNP should exert the opposite effect, i.e., decrease cardiac sympathetic and increase efferent vagal firing. The increase in systemic vascular resistance but not heart rate accounted for the restoration of blood pressure in our subjects. This observation supports the notion that peripheral vascular rather than cardiac mechanisms are essential to maintaining arterial pressure during orthostatic stress in humans (48). Certainly, we cannot exclude the possibility that the cardiopulmonary baroreflex was also involved in the blood pressure restoration.
It has been proposed that baroreceptors sense the mechanical deformation of the arterial wall rather than the level of blood pressure exclusively (3, 9). We did not measure the deformation of aortic and carotid arterial wall in this study. However, using echo-tracking methods, it was observed in humans that stroke changes in common carotid arterial diameter were significantly modified during mild LBNP, suggesting that alterations in carotid geometry might modify the stretch applied at the site of the carotid receptors (30, 31, 36). A deformation of the ascending thoracic aorta was also found during mild LBNP in healthy men with magnetic resonance imaging techniques (45).
In addition, animal studies have shown that aortic baroreceptors may respond not only to changes in aortic pressure and/or deformation, but also to changes in aortic flow (10, 19). The location of arterial baroreceptors in the aortic arch and carotid sinus seems to be ideal for sensing changes in cardiac output or cerebral blood flow. We found in this study that both cardiac output and stroke volume decreased progressively upon application of mild LBNP and reached statistically significant levels during the steady-state period. It is well known that stroke volume is the key variable in the heart rate-stroke volume-total peripheral resistance (“triple-product”) relation that is directly affected by hydrostatic gradients (33), and it is a major determinant of flow in baroreceptive arteries, which modulates baroreceptor activity (19). Stroke volume changes translate into pulse amplitude and pressure changes, and these clearly modulate arterial baroreceptor activity (3, 8). Taken together, the transient fall in arterial pressure, along with the decreases in cardiac output and stroke volume in our subjects, provide strong evidence for unloading of arterial baroreceptors at the onset and during −10- and −15-mmHg LBNP. However, our study does not preclude unloading of cardiopulmonary baroreceptors during low levels of LBNP, although it should be noted that very low levels (i.e., −3 or −6 mmHg) of LBNP do not even consistently result in increased sympathetic nerve activity (13). Moreover, we cannot determine the proportional influence of cardiopulmonary vs. arterial baroreceptors on the restoration of blood pressure during low levels of LBNP. We can say though that arterial baroreceptors are definitely engaged and unloaded at −10- and −15-mmHg LBNP.
Study Limitations
There are at least two limitations in this study. First, arterial pressure was measured noninvasively and indirectly with the Portapres. This method might have reflected waves that do not reflect true central arterial pressure that the arterial baroreceptors see. Further work with left heart catheterization is necessary to confirm our findings. Second, in the present study, beat-by-beat hemodynamic variables were derived from the Modelflow method. Although this method has been validated in different populations under different conditions (4, 6, 11, 32, 39, 52), the absolute values of cardiac output obtained have never been shown to be the same as those from “gold-standard” invasive methods. Nevertheless, it has been shown that the Modelflow method tracks fast changes in hemodynamics during various experimental protocols, including postural stress and exercise (21, 25, 42, 44, 49, 51). In this study, tracking of changes rather than getting the absolute values are more important. Furthermore, we used the modified acetylene rebreathing technique as a reference and calibrated the Modelflow values to this external standard. Thus the proportion and time course of changes in hemodynamics reported in this study are likely to be valid.
In summary, we found, in the present study, that application of −15-mmHg LBNP resulted in a rapid and sustained fall in right atrial pressure, a fall in pulmonary capillary wedge pressure or pulmonary artery diastolic pressure (indicating a decrease in left atrial pressure, and then left-ventricular end-diastolic pressure), progressive decreases in cardiac output and stroke volume, followed by transient reductions in both systolic and diastolic pressures, which were restored through the arterial baroreflex feedback mechanism after ∼15 heartbeats. This transient reduction in arterial pressure was even observed in most subjects at −5- and in all at −10-mmHg LBNP. These results suggest that arterial baroreceptors are unloaded during low levels (i.e., −10 and −15 mmHg) of LBNP. Thus “selective” unloading of cardiopulmonary baroreceptors cannot be presumed to occur during low levels of LBNP in healthy humans.
GRANTS
This study was supported by National Institute on Aging Grant AG17479 and National Aeronautics and Space Administration Grant CA00701.
Acknowledgments
The time and effort put forth by the subjects is greatly appreciated. The authors thank Paul S. Bhella, Eric L. Pacini, Tiffany B. VanGundy, Daniel L. Creson, Diane Bedenkop, Peggy Fowler, Murugappan Ramanathan, and Cyrus Oufic for valuable assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abboud FM, Mark AL. Cardiac baroreceptors in circulatory control in humans. In: Cardiac Receptors, edited by Hainsworth R, Kidd C, and Linden RJ. Oxford, UK: Cambridge University Press, 1979, p. 437–462.
- 2.Aksamit TR, Floras JS, Victor RG, Aylward PE. Paroxysmal hypertension due to sinoaortic baroreceptor denervation in humans. Hypertension 9: 309–314, 1987. [DOI] [PubMed] [Google Scholar]
- 3.Angell James JE The effects of changes of extramural, “intrathoracic”, pressure on aortic arch baroreceptors. J Physiol 214: 89–103, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azabji Kenfack M, Lador F, Licker M, Moia C, Tam E, Capelli C, Morel D, Ferretti G. Cardiac output by Modelflow method from intra-arterial and fingertip pulse pressure profiles. Clin Sci (Lond) 106: 365–369, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Berdeaux A, Duranteau J, Pussard E, Edouard A, Giudicelli JF. Baroreflex control of regional vascular resistances during simulated orthostatism. Kidney Int Suppl 37: S29–S33, 1992. [PubMed] [Google Scholar]
- 6.Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol 90: 437–446, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Cai Y, Holm S, Jenstrup M, Stromstad M, Eigtved A, Warberg J, Hojgaard L, Friberg L, Secher NH. Electrical admittance for filling of the heart during lower body negative pressure in humans. J Appl Physiol 89: 1569–1576, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Chapleau MW, Abboud FM. Determinants of sensitization of carotid baroreceptors by pulsatile pressure in dogs. Circ Res 65: 566–577, 1989. [DOI] [PubMed] [Google Scholar]
- 9.Chapleau MW, Abboud FM. Mechanisms of adaptation and resetting of the baroreceptor. In: Cardiovascular Reflex Control in Health and Disease, edited by Hainsworth R and Mark AL. Philadelphia, PA: Saunders, 1993, p. 165–194.
- 10.Charlton JD, Baertschi AJ. Responses of aortic baroreceptors to changes of aortic blood flow and pressure in rat. Am J Physiol Heart Circ Physiol 242: H520–H525, 1982. [DOI] [PubMed] [Google Scholar]
- 11.Claydon VE, Schroeder C, Norcliffe LJ, Jordan J, Hainsworth R. Water drinking improves orthostatic tolerance in patients with posturally related syncope. Clin Sci (Lond) 110: 343–352, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Cornish KG, Gilmore JP, McCulloch T. Central blood volume and blood pressure in conscious primates. Am J Physiol Heart Circ Physiol 254: H693–H701, 1988. [DOI] [PubMed] [Google Scholar]
- 13.Cui J, Wilson TE, Crandall CG. Muscle sympathetic nerve activity during lower body negative pressure is accentuated in heat-stressed humans. J Appl Physiol 96: 2103–2108, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Dubois AB, Marshall R. Measurements of pulmonary capillary blood flow and gas exchange throughout the respiratory cycle in man. J Clin Invest 36: 1566–1571, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Floras JS, Butler GC, Ando SI, Brooks SC, Pollard MJ, Picton P. Differential sympathetic nerve and heart rate spectral effects of nonhypotensive lower body negative pressure. Am J Physiol Regul Integr Comp Physiol 281: R468–R475, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Fu Q, Iwase S, Niimi Y, Kamiya A, Michikami D, Mano T, Suzumura A. Age-related changes in vasomotor reflex control of calf venous capacitance response to lower body negative pressure in humans. Jpn J Physiol 52: 69–76, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Fu Q, Iwase S, Niimi Y, Kamiya A, Michikami D, Mano T, Suzumura A. Age-related influences of leg vein filling and emptying on blood volume redistribution and sympathetic reflex during lower body negative pressure in humans. Jpn J Physiol 52: 77–84, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol 289: R109–R116, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Hajduczok G, Chapleau MW, Abboud FM. Rheoreceptors in the carotid sinus of dog. Proc Natl Acad Sci USA 85: 7399–7403, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hakumaki MO, Wang BC, Sundet WD, Goetz KL. Aortic baroreceptor discharge during nonhypotensive hemorrhage in anesthetized dogs. Am J Physiol Heart Circ Physiol 249: H393–H403, 1985. [DOI] [PubMed] [Google Scholar]
- 21.Harms MP, Wesseling KH, Pott F, Jenstrup M, Van Goudoever J, Secher NH, Van Lieshout JJ. Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci (Lond) 97: 291–301, 1999. [PubMed] [Google Scholar]
- 22.Hisdal J, Toska K, Flatebo T, Walloe L. Onset of mild lower body negative pressure induces transient change in mean arterial pressure in humans. Eur J Appl Physiol 87: 251–256, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Hisdal J, Toska K, Walloe L. Beat-to-beat cardiovascular responses to rapid, low-level LBNP in humans. Am J Physiol Regul Integr Comp Physiol 281: R213–R221, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman JI, Guz A, Charlier AA, Wilcken DE. Stroke volume in conscious dogs; effect of respiration, posture, and vascular occlusion. J Appl Physiol 20: 865–877, 1965. [DOI] [PubMed] [Google Scholar]
- 25.Ide K, Pott F, Van Lieshout JJ, Secher NH. Middle cerebral artery blood velocity depends on cardiac output during exercise with a large muscle mass. Acta Physiol Scand 162: 13–20, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Imam K, Maddens M, Mohanty PK, Felicetta JV, Sowers JR. Atrial natriuretic peptide attenuates the reflex sympathetic responses to lower body negative pressure. Am J Med Sci 298: 1–7, 1989. [DOI] [PubMed] [Google Scholar]
- 27.Jacobsen TN, Morgan BJ, Scherrer U, Vissing SF, Lange RA, Johnson N, Ring WS, Rahko PS, Hanson P, Victor RG. Relative contributions of cardiopulmonary and sinoaortic baroreflexes in causing sympathetic activation in the human skeletal muscle circulation during orthostatic stress. Circ Res 73: 367–378, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Johnson JM, Rowell LB, Niederberger M, Eisman MM. Human splanchnic and forearm vasoconstrictor responses to reductions of right atrial and aortic pressures. Circ Res 34: 515–524, 1974. [DOI] [PubMed] [Google Scholar]
- 29.Krediet CT, van Lieshout JJ, Bogert LW, Immink RV, Kim YS, Wieling W. Leg crossing improves orthostatic tolerance in healthy subjects: a placebo-controlled crossover study. Am J Physiol Heart Circ Physiol 291: H1768–H1772, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Lacolley PJ, Pannier BM, Cuche JL, Hermida JS, Laurent S, Maisonblanche P, Duchier JL, Levy BI, Safar ME. Microgravity and orthostatic intolerance: carotid hemodynamics and peripheral responses. Am J Physiol Heart Circ Physiol 264: H588–H594, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Lacolley PJ, Pannier BM, Slama MA, Cuche JL, Hoeks AP, Laurent S, London GM, Safar ME. Carotid arterial haemodynamics after mild degrees of lower-body negative pressure in man. Clin Sci (Lond) 83: 535–540, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Langewouters GJ, Settels JJ, Roelandt R, Wesseling KH. Why use Finapres or Portapres rather than intra-arterial or intermittent non-invasive techniques of blood pressure measurement? J Med Eng Technol 22: 37–43, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Levine BD, Buckey JC, Fritsch JM, Yancy CW Jr, Watenpaugh DE, Snell PG, Lane LD, Eckberg DL, Blomqvist CG. Physical fitness and cardiovascular regulation: mechanisms of orthostatic intolerance. J Appl Physiol 70: 112–122, 1991. [DOI] [PubMed] [Google Scholar]
- 34.Mark AL, Mancia G. Cardiopulmonary baroreflexes in humans. In: Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Flow. Bethesda, MD: Am. Physiol. Soc., 1983, sect. 2, vol. III, pt. 2, chapt. 21, p. 795–813.
- 35.Matsukawa K, Kobayashi T, Nakamoto T, Murata J, Komine H, Noso M. Noninvasive evaluation of cardiac output during postural change and exercise in humans: comparison between the modelflow and pulse dye-densitometry. Jpn J Physiol 54: 153–160, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Pannier B, Slama MA, London GM, Safar ME, Cuche JL. Carotid arterial hemodynamics in response to LBNP in normal subjects: methodological aspects. J Appl Physiol 79: 1546–1555, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Pawelczyk JA, Raven PB. Reductions in central venous pressure improve carotid baroreflex responses in conscious men. Am J Physiol Heart Circ Physiol 257: H1389–H1395, 1989. [DOI] [PubMed] [Google Scholar]
- 38.Pawelczyk JA, Zuckerman JH, Blomqvist CG, Levine BD. Regulation of muscle sympathetic nerve activity after bed rest deconditioning. Am J Physiol Heart Circ Physiol 280: H2230–H2239, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Reisner A, Xu D, Ryan K, Convertino V, Mukkamala R. Comparison of cardiac output monitoring methods for detecting central hypovolemia due to lower body negative pressure. Conf Proc IEEE Eng Med Biol Soc 1: 955–958, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Robinson T, Potter J. Cardiopulmonary and arterial baroreflex-mediated control of forearm vasomotor tone is impaired after acute stroke. Stroke 28: 2357–2362, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Sprangers RL, Wesseling KH, Imholz AL, Imholz BP, Wieling W. Initial blood pressure fall on stand up and exercise explained by changes in total peripheral resistance. J Appl Physiol 70: 523–530, 1991. [DOI] [PubMed] [Google Scholar]
- 42.Sugawara J, Tanabe T, Miyachi M, Yamamoto K, Takahashi K, Iemitsu M, Otsuki T, Homma S, Maeda S, Ajisaka R, Matsuda M. Non-invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol Scand 179: 361–366, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Sundlof G, Wallin BG. Effect of lower body negative pressure on human muscle nerve sympathetic activity. J Physiol 278: 525–532, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tam E, Azabji Kenfack M, Cautero M, Lador F, Antonutto G, di Prampero PE, Ferretti G, Capelli C. Correction of cardiac output obtained by Modelflow from finger pulse pressure profiles with a respiratory method in humans. Clin Sci (Lond) 106: 371–376, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Taylor JA, Halliwill JR, Brown TE, Hayano J, Eckberg DL. “Non-hypotensive” hypovolaemia reduces ascending aortic dimensions in humans. J Physiol 483: 289–298, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson CA, Ludwig DA, Convertino VA. Carotid baroreceptor influence on forearm vascular resistance during low level lower body negative pressure. Aviat Space Environ Med 62: 930–933, 1991. [PubMed] [Google Scholar]
- 47.Triebwasser JH, Johnson RL, Burpo RP, Campbell JC, Reardon WC, Blomqvist CG. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med 48: 203–209, 1977. [PubMed] [Google Scholar]
- 48.Tyden G Aspects of cardiovascular reflex control in man. An experimental study. Acta Physiol Scand Suppl 448: 1–62, 1977. [PubMed] [Google Scholar]
- 49.van Dijk N, de Bruin IG, Gisolf J, de Bruin-Bon HA, Linzer M, van Lieshout JJ, Wieling W. Hemodynamic effects of leg crossing and skeletal muscle tensing during free standing in patients with vasovagal syncope. J Appl Physiol 98: 584–590, 2005. [DOI] [PubMed] [Google Scholar]
- 50.van Heusden K, Gisolf J, Stok WJ, Dijkstra S, Karemaker JM. Mathematical modeling of gravitational effects on the circulation: importance of the time course of venous pooling and blood volume changes in the lungs. Am J Physiol Heart Circ Physiol 291: H2152–H2165, 2006. [DOI] [PubMed] [Google Scholar]
- 51.van Lieshout JJ, Toska K, van Lieshout EJ, Eriksen M, Walloe L, Wesseling KH. Beat-to-beat noninvasive stroke volume from arterial pressure and Doppler ultrasound. Eur J Appl Physiol 90: 131–137, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Voogel AJ, van Montfrans GA. Reproducibility of twenty-four-hour finger arterial blood pressure, variability and systemic hemodynamics. J Hypertens 15: 1761–1765, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Wieling W, Van Lieshout JJ, Ten Harkel AD. Dynamics of circulatory adjustments to head-up tilt and tilt-back in healthy and sympathetically denervated subjects. Clin Sci (Lond) 94: 347–352, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Wolthuis RA, Bergman SA, Nicogossian AE. Physiological effects of locally applied reduced pressure in man. Physiol Rev 54: 566–595, 1974. [DOI] [PubMed] [Google Scholar]
- 55.Wolthuis RA, Carpentier WA, Leblanc A, Johnson PC, Bergman SA, Hoffler GW. Regional plasma volume re-distribution during lower body negative pressure stress. In: Preprints of Scientific Programs. Las Vegas, NV: Aerospace Medical, 1973, p. 209–210.
- 56.Zoller RP, Mark AL, Abboud FM, Schmid PG, Heistad DD. The role of low pressure baroreceptors in reflex vasoconstrictor responses in man. J Clin Invest 51: 2967–2972, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]