Abstract
Apoptotic myocyte cell death, diastolic dysfunction, and progressive deterioration in left ventricular pump function characterize the clinical course of diabetic cardiomyopathy. A key question concerns the mechanism(s) by which hyperglycemia (HG) transmits danger signals in cardiac muscle cells. The growth factor adapter protein p66ShcA is a genetic determinant of longevity, which controls mitochondrial metabolism and cellular responses to oxidative stress. Here we demonstrate that interventions which attenuate or prevent HG-induced phosphorylation at critical position 36 Ser residue (phospho-Ser36) inhibit the redox function of p66ShcA and promote the survival phenotype. Adult rat ventricular myocytes obtained by enzymatic dissociation were transduced with mutant-36 p66ShcA (mu-36) dominant-negative expression vector and plated in serum-free media containing 5 or 25 mM glucose. At HG, adult rat ventricular myocytes exhibit a marked increase in reactive oxygen species production, upregulation of phospho-Ser36, collapse of mitochondrial transmembrane potential, and increased formation of p66ShcA/cytochrome-c complexes. These indexes of oxidative stress were accompanied by a 40% increase in apoptosis and the upregulation of cleaved caspase-3 and the apoptosis-related proteins p53 and Bax. To test whether p66ShcA functions as a redox-sensitive molecular switch in vivo, we examined the hearts of male Akita diabetic nonobese (C57BL/6J) mice. Western blot analysis detected the upregulation of phospho-Ser36, the translocation of p66ShcA to mitochondria, and the formation of p66ShcA/cytochrome-c complexes. Conversely, the correction of HG by recombinant adeno-associated viral delivery of leptin reversed these alterations. We conclude that p66ShcA is a molecular switch whose redox function is turned on by phospho-Ser36 and turned off by interventions that prevent this modification.
Keywords: diabetes mellitus, reactive oxygen species
apoptotic myocyte cell death, diastolic dysfunction, and progressive deterioration in left ventricular (LV) pump function characterize the clinical course of diabetic cardiomyopathy (3, 13, 20, 30). Hyperglycemia (HG) and diabetes mellitus (DM) are associated with an exponential increase in reactive oxygen species (ROS) production at the cellular level (4, 15), which may play a causal role in the development of diabetic complications (2, 4). Mitochondria are the primary source of reactive oxygen intermediates and critical determinants of cell death and cell survival (7). HG increases the generation of superoxide anion (O2−) by interfering with the flow of electrons along the mitochondrial electron transport chain (4). In recent communications, our laboratory has provided compelling evidence that signaling molecules of the IGF-1/insulin pathway can attenuate or prevent HG-induced ROS production, oxidative DNA damage, and apoptosis (19, 35). Since adult cardiac muscle cells (CMCs) possess a finite capacity to proliferate, myocyte cell death is a critical determinant of ventricular remodeling and the progression to heart failure. It seems reasonable to infer that strategies, which interrupt or suppress the initiation of the apoptosis program, may offer an innovative approach to preserve the CMC number and LV pump function.
The p66ShcA protein is one of three isoforms encoded at the mammalian ShcA locus. The three overlapping Shc proteins, p66ShcA, p52ShcA, and p46ShcA, all share a COOH-terminal SH2 domain, central collagen homology region (CH), and NH2-terminal phosphotyrosine-binding domain. p46ShcA and p52ShcA are the products of alternative translation initiation sites within the same transcript, whereas p66ShcA is distinguished by a unique NH2-terminal region (CH2), generated by alternative splicing (11, 26). The ShcA family of proteins is the cytoplasmic substrates for the activated IGF-1/insulin receptors. p46 and p52 participate in mitogenesis via the recruitment of the Ras signaling pathway (26), whereas p66ShcA by virtue of its unique NH2-terminal region is a genetic determinant of longevity (9, 28) that controls mitochondrial metabolism (9). In the proposed scheme, phosphorylation at a critical Ser36 (phospho-Ser36) residue position activates p66ShcA redox function by facilitating its translocation to mitochondria where p66ShcA generates ROS via the oxidation of cytochrome c. The latter redox reactions result in the opening of the mitochondrial transition pore, a collapse of mitochondrial transmembrane potential (Δψm), and a cytochrome-c release. We have proposed a model in which p66ShcA redox function is shut down by interrupting HG-induced phosphorylation at the critical Ser36 residue, preventing the translocation to mitochondria where p66ShcA functions as a ROS producer, resulting in organelle dysfunction and cell death.
In the current study, adult rat ventricular myocytes (ARVMs) were genetically engineered to express mutant-36 p66ShcA (mu-36) dominant-negative expression vector to test the hypothesis that mu-36 inhibits p66ShcA redox function by attenuating phospho-Ser36 and promotes the survival phenotype. To evaluate p66ShcA signaling in diabetic myocardium, studies were performed with male Akita mice. Furthermore, Akita mice were treated with recombinant adeno-associated virus vector containing leptin cDNA (33, 36) to test whether the correction of HG protects CMCs from p66ShcA-dependent signals that target mitochondria, oxidizing cytochrome c to generate ROS and promote the activation of the terminal apoptosis program. Our results indicate that p66ShcA is a molecular switch whose redox function is turned on by HG-induced phospho-Ser36 and turned off by interventions that prevent this modification.
MATERIALS AND METHODS
All experiments were performed under a protocol approved by the Institutional Animal Care and Use Committee of the University of Medicine and Dentistry of New Jersey.
Reagents.
Sodium orthovanadate, Triton X-100, EDTA, EGTA, and HEPES buffers were purchased from Sigma-Aldrich. Aprotinin, leupeptin, phenylmethylsulfonyl fluoride (PMSF), and protease inhibitor cocktails (types I and III) were purchased from Calbiochem-Novabiochem.
Isolation of ARVMs and cell culture.
ARVMs were isolated from the hearts of adult male Sprague-Dawley rats (175–225 g) as described (23–25). Briefly, hearts were removed from the chest cavity, suspended, and rinsed with Eagle's minimum essential medium (MEM; Sigma Chemical). Cardiac myocytes were isolated by enzymatic dissociation with collagenase type II (Worthington). Briefly, myocytes were washed with MEM buffer (×2) and resuspended in incubation buffer (MEM plus 0.1 mM BSA) in serum-free medium (SFM) with an incremental addition of CaCl2 at concentrations of 0.25, 0.5, and 1 mM, respectively. Myocytes were washed and resuspended in SFM. Freshly isolated myocytes were plated in laminin-coated petri dishes at a density of 2 × 104 cells/cm2 and incubated in SFM at 37°C in an atmosphere containing 5% CO2-95% room air. Routinely, 80–90% of cells obtained were viable myocytes, as determined by their characteristic morphological shape, striations, and trypan blue staining.
Animals.
Six-week-old wild-type (WT) male C57BL/6J and diabetic nonobese Akita mice were purchased from Jackson Laboratory (Bar Harbor, ME) and were housed one mouse per cage in a temperature (21°C) and light-controlled (10-h light:14-h dark), specific pathogen-free environment with chow diet and water available ad libitum throughout the experiment. The institutional Animal Care and Use Committee of the University of Florida approved the experimental protocols.
Experimental design.
The nonimmunogenic, nonpathogenic, and replicative-deficient recombinant virus vector encoding either leptin (rAAV-Lep) or green fluorescent protein (rAAV-GFP, control) was packaged, purified, concentrated, and titred in the Vector Core laboratory at the University of Florida as described earlier and used in the previous studies (1, 33). GFP in this study was used as an internal control. These vectors were used to investigate the effects of leptin on diabetic hearts. Two weeks after arrival, mice were anesthetized with pentobarbital sodium (60 mg/kg ip), blood samples were collected, and glucose levels were determined. Thereafter, each genotype of mice was divided into subgroups and underwent the following experimental procedures.
Mice were anesthetized and one subgroup of each genotype (n = 6) was injected intracerebroventricularly (1.5 μl) with rAAV-GFP (1.9 × 109 infectious particles) or rAAV-Lep (8.6 × 107 infectious particles) as described (1, 33). Mice were monitored at weekly intervals after intracerebroventricular injection. At 8–10-wk postintracerebroventricular injections, the mice were anesthetized with pentobarbital sodium and euthanized by decapitation and the hearts were dissected out and stored frozen at −80°C for various analyses.
Construction of adenovirus: p66ShcA.
Recombinant adenoviruses were constructed, propagated, and tittered as previously described by Graham and Prevec (10). The viruses were purified on a cesium chloride gradient followed by dialysis against 20 mM Tris-buffered saline with 2% glycerol. As a negative control, the GFP virus was employed. ARVMs were cultured in SFM containing 5 mM glucose (normal glucose) and 25 mM glucose (high glucose) and incubated for 4 h at 37°C. Cells were infected with adenovirus vector (5) expressing dominant-negative mu-36 construct (multiplicity of infection, 10–20). After a 16-h incubation, the cells were used for further studies.
Analysis of DNA fragmentation by ELISA.
Histone-associated DNA fragments were quantified by Cell Death Detection ELISA (Roche Diagnostic, Branchburg, NJ) as previously described (19, 35).
Detection of HG-induced oxidative stress.
Glucose-mediated oxidative stress in cardiac myocytes was studied by the trafficking of 2,3,4,5,6-pentafluorodihydrotetramethylrosamine (Redox Sensor Red CC-1; Molecular Probes) using fluorescence microscopy as previously described (6). Redox Sensor Red CC-1 is oxidized in the presence of O2− and H2O2. Cells were washed with PBS and visualized using a Nikon fluorescence microscope (Nikon Eclipse E800) equipped with a triple filter cube and a charge-coupled device camera (Nikon DXM1200). The staining was performed in quadruplicate for each group, and 30 random fields (average 500 cells) were studied in each replicate. Images were captured using Nikon ACT-1 (Version 1.12) software and combined for publishing format using Adobe Photoshop 7.0 software.
Assessment of Δψm.
Δψm was monitored with the dye, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolylcarbocyanine iodide (JC-1; Molecular Probes) using fluorescence microscopy (19). Cardiac myocytes were seeded and infected with adenovirus vector expressing dominant-negative mu-36 construct as described (19). At the end of the incubation, cells were loaded with JC-1 cationic dye (0.5 μg/ml) added to respective media and incubated at 37°C for 15 min. Cells were washed three times with PBS (1×) and visualized using a Nikon fluorescence microscope. JC-1 dye exhibits a potential dependent accumulation in the mitochondria (J-aggregates; accumulate at high membrane potential), indicated by a fluorescence shift from green to red fluorescence. Green fluorescence reflects the monomeric form of JC-1, appearing in the cytosol after mitochondrial membrane depolarization. The staining was performed in quadruplets for each group as described earlier (19).
Immunoblotting.
ARVMs were harvested from the petri dishes and washed with PBS. The pellets were lysed on ice with 300 μl of lysis buffer [containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 10% EGTA, 10% glycerol, 1% Triton X-100, 1 mM PMSF, 0.2 mM sodium orthovanadate, and 10 mg/ml aprotinin] and protease inhibitor cocktails (types I and III). For the in vivo (Akita mice) study, cardiac tissue samples (20–25 mg) were lysed on ice with 400 μl of lysis buffer. Proteins (50 μg) were separated on 4–15% gradient SDS polyacrylamide gel (Bio-Rad) and transferred to nitrocellulose membranes for Western blot analysis. Blots were probed with primary rabbit polyclonal antibodies for p66shcA and phospho-anti-ShcA (Ser36) mouse monoclonal antibody (1:500 dilution; Calbiochem) to determine the phosphorylation status of Ser36. ARVM cell lysates or tissue homogenates were also analyzed for the expression of phospho-p53 (1:500 dilution; Cell Signaling), superoxide dismutase (SOD) (sheep polyclonal antibody, 1:700 dilution; Calbiochem), catalase (1:500 dilution; Calbiochem), Bax, and cleaved caspase-3 (1:500 dilution; Santa Cruz Biotechnology). Anti-actin antibody (1:500; dilution; Santa Cruz Biotechnology) and mouse monoclonal anti-GFP (1:20,000 dilution) were used as internal controls. Secondary antibody was used at a dilution of 1:5,000. The blots were developed by using Super Signal West Pico Chemiluminiscence Kit (Pierce), and the bands were scanned by using Bio-Rad-1 computerized image analysis (6, 35).
Preparation and immunoblotting of subcellular fractions.
Mouse hearts were fractionated into cytosol and mitochondria by differential centrifugation as described (22). Briefly, hearts were homogenized with a tissue grinder (Tekmar) in a buffer that contained (in mM) 250 sucrose, 10 Tris·HCl (pH 7.4), 2 EDTA, 1 Na3VO4, 10 NaF, and protease inhibitor cocktail I and were centrifuged at 700 g for 10 min at 4°C. The pellet was discarded, and the supernatant was further centrifuged at 16,000 g for 25 min at 4°C. The supernatant (cytosolic fraction) and pellet (mitochondrial fraction) were separated. Protein samples (50 μg) were separated and transferred on nitrocellulose membrane as described in Immunoblotting. Anti-cytochrome-c oxidase subunit 4 (COX4) monoclonal antibody (1:1,000 dilution; Molecular Probes) was used to probe the protein COX4 as a marker in mitochondria, and monoclonal Akt1 antibody (1:500 dilution; Cell Signaling) was used as a marker of cytosol in Western blot analysis. Anti-ShcA (1:500; Cell Signaling) was used to determine the accumulation of p66ShcA in mitochondria. Cytochrome-c expression was determined in cytosolic fraction using Mouse monoclonal Anti-cytochrome-c antibody (1:1,000; BD Biosciences Pharmingen), and the protein was detected by horseradish peroxidase-linked secondary antibody (1:5,000 dilution). The blots were developed using Super Signal West Pico Chemiluminiscence Kit (Pierce), and the bands were scanned using Bio-Rad-1 computerized image system (6, 35).
Immunoprecipitation and immunoblotting of p66ShcA and cytochrome c.
The Mitochondrial fraction of hearts were obtained from WT, rAAV-GFP (GFP group), and rAAV-Lep (LEP group) mice as described in Preparation and immunoblotting of subcellular fractions. Six hundred micrograms of soluble mitochondrial extracts were incubated with 6 μg of rabbit polyclonal anti-p66ShcA antibody (Cell Signaling) and 500 μl of radioimmunoprecipitation assay buffer [containing 0.15 M NaCl; 20 mM Tris (pH 7.4), 1 mM EGTA (pH 7.4), 1 mM EDTA, 1% Triton X-100, and 0.5% P-40] containing the protease inhibitors 0.2 mM PMSF, 2 μg/μl aprotinin, and 0.2 mM Na3VO4 overnight at 4°C. Subsequently, 100 μl of protein A-agarose (Pierce) were added to each sample. After several washings (×3) with a buffer containing (in mM) 20 Tris·HCl (pH 7.4), 300 NaCl, 2 EDTA, and 2 EGTA, the samples were spun at 2,500 rpm for 2 min. A loading buffer was added to each pellet, and the immunoprecipitated proteins were separated by 10% SDS-PAGE. The proteins were transferred to nitrocellulose membranes and exposed to mouse monoclonal anti-mouse cytochrome-c antibodies (BD Biosciences) or rabbit polyclonal anti-mouse p66Shc antibody (Cell Signaling Technology) at a concentration of 1:500 in TBS plus Tween 20. Samples were subjected to Western blot analysis as described in Immunoblotting.
Statistical analysis.
Data are expressed as means ± SD. For multiple comparisons among different groups of data, the significant differences were determined by the Bonferroni method (34). Significance was defined at P ≤ 0.05.
RESULTS
Generation of mu-36 ARVMs.
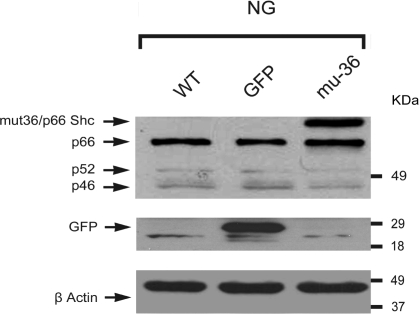
ARVMs were enzymatically dissociated from adult rat hearts (24, 25) and transduced with adenoviral vector expressing the mu-36 p66ShcA. Western blot analysis of Shc isoforms (Fig. 1) shows the position of mu-36 (top band) and p66ShcA protein immediately below. The p52ShcA protein is also shown along with the low expression level of p46ShcA protein.
Fig. 1.
Representative Western blot analysis of Shc isoforms. Adult rat ventricular myocytes (ARVMs) were plated in serum-free medium (SFM) containing 5 mM glucose (normolglycemic; NG) or 25 mM glucose (hyperglycemic; HG). ARVMs [wild-type (WT)], negative control showing green fluorescent protein empty vector (GFP), and ARVMs transduced with (mu-36) mutant-36 p66ShcA (top band) in NG are shown. Also shown are p52ShcA and p46ShcA.
mu-36 inhibits the HG-induced apoptosis signal.
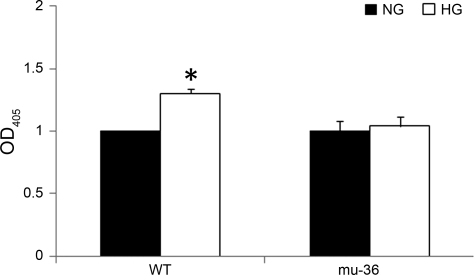
HG induces an exponential increase in the intracellular production of ROS, an event linked to the development of diabetic complications (2, 6). ARVMs possess the genetic program for apoptosis (24), and the DNA double helix is a target for ROS-dependent signals that inflict more than 100 different types of DNA lesions, ranging from base modifications to single-strand breaks and potentially lethal double-strand breaks that trigger the activation of the apoptosis program (12). To test whether the transfection of ARVMs with mu-36 is sufficient to rescue ARVMs from HG-induced DNA damage, ARVMs and mu-36-ARVMs were cultured in SFM containing 5 or 25 mM glucose for 16 h. Apoptosis was evaluated by ELISA cell death assay, which detects histone-associated DNA fragments in the cytosol. As shown in Fig. 2, ARVMs and mu-36 ARVMs at 5 mM glucose exhibit a baseline level of apoptosis due to serum starvation. This parameter increased by 40% in ARVMs at 25 mM glucose, whereas mu-36-ARVMs show no change in apoptotic death from baseline. Taken together, mu-36 prevents the activation of the apoptosis program in ARVMs maintained at HG.
Fig. 2.
Mu-36 p66ShcA attenuates HG-induced apoptosis. Histone-associated DNA fragments were quantified by using ELISA cell death detection kit and presented as optical density at 405 nm (OD405) in 4 different groups. Data represent 7 independent experiments: means ± SD and *P ≤ 0.05.
p66ShcA-dependent regulation of HG-induced oxidant stress.
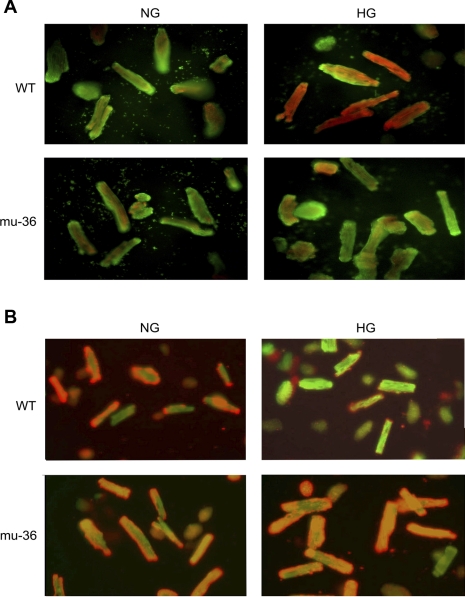
HG alters the redox status of cells through the overproduction of ROS by mitochondria and NADPH oxidase (18, 19). To test whether mu-36 ARVMs exhibit an increased resistance to HG-induced intracellular ROS production, ARVMs and mu-36 ARVMs were loaded with the redox-sensitive probes Red Sensor Red CC-1 and the mitochondria-specific dye Mito-Tracker green FM. As shown in Fig. 3A, at HG, ARVMs exhibit bright yellow-orange fluorescence in the mitochondria (19) due to the colocalization of oxidized red CC-1 and Mito-Tracker green, indicative of augmented ROS production in the mitochondria. Conversely, mu-36 ARVMs at HG show a barely detectable fluorescent signal, indicative that mu-36 induces a strong oxidant resistant phenotype.
Fig. 3.
A: p66ShcA-dependent regulation of HG-induced oxidant stress. ARVMs and mu-36 ARVMs were maintained in SFM containing 5 mM (NG) or 25 mM (HG) glucose for 16 h. Cells were loaded Redox Sensor Red CC-1 and the mitochondrial-specific dye MitoTracker green FM. At HG, ARVMs show reddish-orange fluorescent signal due to colocalization of oxidized red CC-1 and MitoTracker green in mitochondria. B: Mu-36 p66ShcA inhibits HG-induced collapse of mitochondrial transmembrane potential. ARVMs and mu-36 ARVMs were maintained in SFM containing 5 mM (NG) or 25 mM (HG) glucose for 16 h. Cells were loaded with the fluorescent probe JC-1 that exhibits potential dependent accumulation in mitochondria. Under control conditions (5 mM glucose), ARVMs and mu-36 ARVMs showed punctate red stain due to JC-1 accumulation in mitochondria (J-aggregates). At HG, mitochondria of ARVMs depolarize, indicated by release of JC-1 into cytoplasm and shift from red to green fluorescence.
mu-36 inhibits HG-induced collapse of Δψm.
Mitochondria are critical determinants of cell death and cell survival (19). To determine whether mu-36 prevents the collapse of ΔΨm, this parameter was examined in ARVMs and mu-36 ARVMs under control and experimental conditions. Cells were loaded with the fluorescent probe JC-1, which exhibits potential dependent accumulation in mitochondria. Under control conditions (Fig. 3B), ARVMs show punctate red staining due to the accumulation of JC-1 in mitochondria. At HG, the mitochondria of ARVMs (Fig. 3B, top) depolarize, indicated by the reduction in J-aggregates (red/orange fluorescence) and increased JC-1 monomers (green fluorescence). Conversely, mu-36 ARVMs exhibit punctate red/orange staining at 5 and 25 mM glucose. Taken together, mu-36 inhibits HG-induced collapse of ΔΨm, a key event in organelle dysfunction and apoptosis.
mu-36 and leptin downregulate catalase and Cu/Zn SOD.
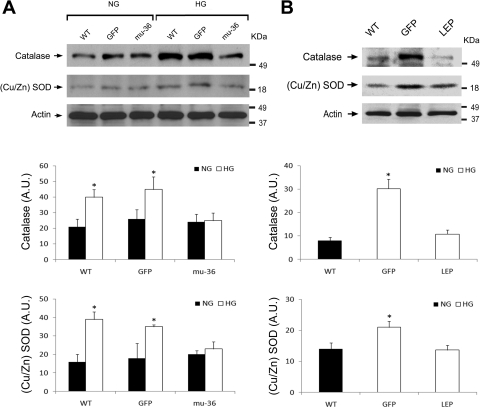
The antioxidant enzymes catalase and Cu/Zn SOD contribute to the defense of cellular redox status. Catalase catalyzes the dismutation of H2O2→O2 + H2O; SOD catalyzes the dismutation of O2−→O2 + H2O2. Western blot analysis (Fig. 4A) of lysates from ARVMs maintained at HG shows an upregulation of catalase and SOD expression, which serve as surrogate markers of oxidative stress. An identical analysis performed with lysates from mu-36 ARVMs did not detect an alteration in the expression levels of catalase and SOD, indicative of increased resistance to the redox stimulus of HG.
Fig. 4.
Mu-36 and leptin inhibit expression of catalase and Cu/Zn SOD. A, top: representative Western blot analysis of Cu/Zn SOD and catalase expression in lysates prepared from ARVMs. Protein extracts from WT ARVMs and ARVMs transduced with GFP and mu-36 were separated by PAGE and nitrocellulose blots probed with specific antibodies for Cu/Zn SOD and catalase. A, middle and bottom: densitometric analyses for catalase and Cu/Zn SOD expression [in arbitrary units (AU)]. Data represent 4 to 5 independent experiments for WT and mu-36 ARVMs and 2 to 3 independent experiments for GFP negative controls: means ± SD and *P ≤ 0.05. B, top: representative Western blot analysis of Cu/Zn SOD and catalase expression in lysates prepared from hearts of WT and Akita mice expressing recombinant adeno-associated viral delivery of GFP (rAAV-GFP) or leptin (rAAV-Lep). Protein extracts from WT, GFP leptin (LEP) groups were separated by PAGE and nitrocellulose blots probed with specific antibodies for Cu/Zn SOD and catalase. B, middle and bottom: densitometric analyses for catalase and Cu/Zn SOD expression. Data represent 3 to 4 independent experiments: means ± SD and *P ≤ 0.05.
We next asked whether the antioxidant enzymes catalase and Cu/Zn SOD are upregulated in the diabetic myocardium and whether a correction of HG turns off the signal for catalase and Cu/Zn-SOD expression. A dominant mutation in the Ins2 gene results in maturity onset diabetes in Akita mice (14). To avoid the confounding effect of insulin on survival pathways to be studied, Akita mice were treated with leptin, which has a potent blood glucose-lowering effect (36). Accordingly, Akita mice received rAAV-containing GFP (GFP group) or leptin cDNA (LEP group). Aged-matched WT mice served as a control. Akita mice were euthanized at 8–10 wk postinjection, hearts were excised, and lysates were prepared for Western blot analysis. Blood glucose levels of the control and experimental groups immediately before euthanasia were as follows: WT (150 ± 20 mg/dl), LEP (120 ± 37 mg/dl), and GFP (568 ± 40 mg/dl; P < 0.05 compared with WT or LEP groups). As shown in Fig. 4B, the expression of catalase increased 3-fold and SOD 1.5-fold in hearts of GFP mice, whereas LEP mice exhibit expression levels of catalase and Cu/Zn SOD comparable with WT control mice. Taken together, we have shown that the transfection of ARVMs with mu-36 or the treatment of Akita mice with leptin transgene turns off the signal for HG induction of catalase and Cu/Zn SOD.
mu-36 and leptin inhibit p66ShcA redox function.
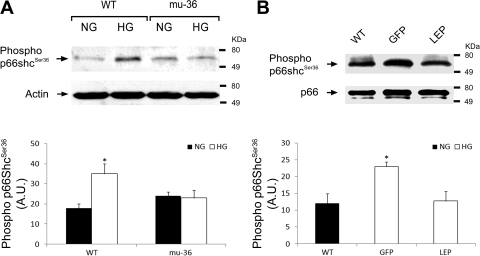
We next asked whether mu-36 quenches HG-induced oxidative stress by inhibiting the redox function of the p66ShcA protein. Since the redox function of the p66ShcA is activated by the ROS-dependent phosphorylation of the Ser36 residue residing at the amino terminus, we hypothesized that mu-36 will inhibit the p66ShcA redox function by interrupting the transmission of signals that target Ser36. To test this hypothesis, Western blot analysis was performed with phospho-anti-ShcA/p66 (phospho-Ser36) mouse monoclonal antibody. This antibody recognizes the 66-kDa isoform of ShcA phosphorylated at Ser36 and does not cross-react with nonphosphorylated p66ShcA, mu-36, or unrelated phosphorylation sites (27). As shown in Fig. 5A, mu-36 ARVMs maintained at HG exhibit no detectable alteration in the phosphorylation status of Ser36, whereas ARVMs show an upregulation in phosphorylation at Ser36 p66ShcA protein, indicative that mu-36 suppresses the transmission of ROS-dependent signals that target Ser36.
Fig. 5.
Mu-36 and leptin inhibit phospho-Ser36 in p66ShcA. A, top: representative Western blot analysis showing phosphorylation status of Ser36 in lysates prepared from ARVMs. Protein extracts from WT ARVMs and ARVMs transduced with GFP and mu-36 were separated by PAGE and nitrocellulose blots probed with mouse monoclonal anti-phospho-serine antibody that recognizes the 66 kDa form of Shc phosphorylated at Ser36. A, bottom: densitometric analysis for phospho-Ser36 expression. Data represent 4 independent experiments: means ± SD and *P ≤ 0.05. B, top: representative Western blot analysis showing phosphorylation status of Ser36 in lysates prepared from hearts of WT and Akita mice expressing rAAV-GFP (GFP) or rAAV-Lep (LEP). Protein extracts from WT, GFP, and LEP groups were separated by PAGE and nitrocellulose blots probed with mouse monoclonal anti-phospho-serine antibody as in A. B, bottom: densitometric analysis for phospho-Ser36. Data represent 3 to 4 independent experiments: means ± SD and *P ≤ 0.05.
We examined the phosphorylation status of Ser36 in hearts from Akita mice treated with rAAV-Lep (LEP group) or rAAV-GFP (GFP group) and WT controls. As shown in Fig. 5B, Western blot analysis of cardiac lysates from control and experimental mice shows increased levels of phospho-Ser36 in GFP mice, whereas phospho-Ser36 levels in LEP mice were comparable with WT controls. Taken together, mu-36 and leptin inhibit the transmission of signals that target the critical Ser36 residue of the p66ShcA protein.
mu-36 and leptin inhibit p66ShcA translocation to mitochondria.
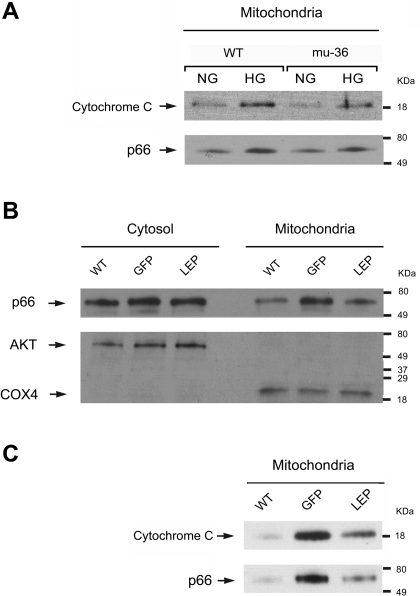
Phosphorylation at Ser36 induces the translocation of the p66ShcA protein to the mitochondria, where p66ShcA interacts with cytochrome c to produce H2O2 (9). We hypothesize mu-36 and leptin will attenuate or prevent p66ShcA/cytochrome-c complexes that result in the generation of H2O2, leading to organelle dysfunction and apoptosis. To test this hypothesis, mitochondria-enriched fractions (19) were prepared from ARVMs maintained under euglycemic and hyperglycemic conditions. Mitochondrial subfractions were immunoprecipitated with anti-p66ShcA and probed with p66ShcA and cytochrome-c antibodies. As shown in Fig. 6A, mitochondrial subfractions of ARVMs maintained at HG show increased levels of p66ShcA/cytochrome-c complexes, whereas mu-36 ARVMs at HG exhibit no detectable alteration from control mice.
Fig. 6.
Mu-36 and leptin inhibit p66ShcA translocation to mitochondria. A: representative Western blot analysis showing expression levels of p66ShcA/cytochrome-c complexes in mitochondria-enriched fractions prepared from WT ARVMs and mu-36 ARVMs. Mitochondrial subfractions were immunoprecipitated with anti-p66ShcA and probed with p66ShcA and cytochrome-c antibodies. Results shown are representative of 3 experiments. B: mitochondria-enriched subfractions prepared from hearts of WT and Akita mice expressing rAAV-GFP (GFP) or rAAV-Lep (LEP). Monoclonal cytochrome-c oxidase subunit 4 (COX4) was used as an internal marker for mitochondria. AKT was used as an internal marker for cytosolic subfraction. C: representative Western blot analysis showing expression levels of p66ShcA/cytochrome-c complexes in mitochondria-enriched fractions prepared from hearts of WT and Akita mice. Lysates were probed with p66ShcA and cytochrome-c antibodies. Results shown are representative of 3 experiments.
Mitochondria enriched fractions were also prepared from the hearts of WT control and Akita mice, expressing rAAV-Lep (LEP group) or rAAV-GFP (GFP group) (Fig. 6B). Akt was used as an internal marker for cytosol and COX4 for mitochondria. As shown in Fig. 6C, LEP mice show a marked reduction in the levels of p66ShcA/cytochrome-c complexes when compared with GFP mice. Taken together, mu-36 and leptin attenuate HG-induced p66ShcA/cytochrome-c complexes that trigger ROS production and organelle dysfunction.
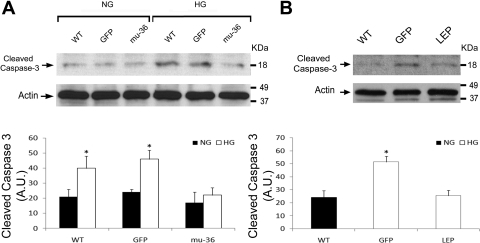
mu-36 and leptin attenuate HG-induced expression of caspase-3 and apoptosis-related proteins.
We next asked whether the inhibition of p66ShcA redox signals prevents the HG-induced activation of the terminal apoptosis program. Cleaved caspase-3 expression was examined by Western blot analysis in lysates from ARVMs and mu-36 ARVMs, maintained at euglycemic and hyperglycemic conditions. As shown in Fig. 7A, at HG, ARVMs show a twofold increase in cleaved caspase-3 expression, whereas mu-36 ARVMs at HG did not affect the expression of this proteolytic protein. This analysis was repeated with lysates prepared from hearts of WT control and Akita diabetic mice expressing rAAV-GFP and rAAV-Lep in the GFP and LEP groups (Fig. 7B). A 2.5-fold increase in cleaved caspase-3 expression was detected in the GFP group, but the LEP group showed levels comparable with the WT control mice.
Fig. 7.
Mu-36 and leptin attenuate HG-induced cleaved caspase-3 expression. A: representative Western blot analysis showing cleaved caspase-3 expression in lysates prepared from ARVMs. Protein extracts from WT ARVMs and ARVMs transduced with GFP and mu-36 were separated by PAGE and nitrocellulose blots probed with antibodies to cleaved caspase-3. Data represent 5 to 6 independent experiments for WT and mu-36 at 5 and 25 mM glucose and 2 to 3 independent experiments for GFP negative controls: means ± SD and *P ≤ 0.05. B: representative Western blot analysis showing cleaved caspase-3 expression in lysates prepared from hearts of WT and Akita mice expressing rAAV-GFP (GFP) or rAAV-Lep (LEP). Protein extracts from WT, GFP, and LEP were separated by PAGE and nitrocellulose blots probed with antibodies to cleaved caspase-3 as in A. Densitometric analysis for phospho-Ser36 is shown (bottom). Data represent 3 to 4 independent experiments: means ± SD and *P ≤ 0.05.
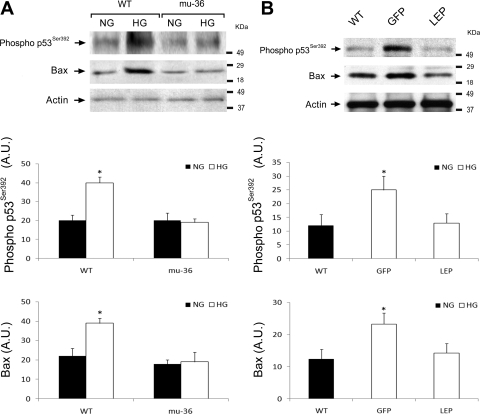
The redox sensitive proapoptosis transcription factor p53 is activated by the stress of HG (8, 19). Ser392 is located at the COOH terminus of p53, and the phosphorylation at this site is correlated with transcriptional activation. Bax is a p53-dependent gene, whose level of expression is increased during myocyte apoptosis (21). As shown in Fig. 8A, Western blot analysis of lysates from ARVMs maintained at HG show a twofold increase in the expression levels of phospho-Ser392 and Bax, whereas mu-36 ARVMs show no detectable alteration in phospho-Ser392 or Bax. This analysis was repeated with lysates prepared from hearts of WT control and Akita mice, expressing rAAV-GFP and rAAV-Lep in the GFP and LEP groups (Fig. 8B). As anticipated, the expression levels of phospho-Ser392 and Bax increased by twofold in the hearts of rAAV-GFP mice, whereas rAAV-Lep mice showed no difference from WT control mice. Taken together, mu-36 and leptin prevent the transmission of HG-induced stress signals that activate the terminal apoptosis program.
Fig. 8.
Mu-36 and leptin attenuate HG-induced p53 activity and expression of apoptosis-related factors. A: representative Western blot analysis showing p53 phospho-Ser392 and Bax expression in lysates prepared from ARVMs. Protein extracts from WT ARVMs and ARVMs transduced with GFP and mu-36 were separated by PAGE and nitrocellulose blots probed with antibodies to cleaved caspase-3. A, middle and bottom: densitometric analysis for phospho-Ser36 and Bax expression. Data represent 5 independent experiments for WT and mu-36 at 5 and 25 mM glucose: means ± SD and *P ≤ 0.05. B: representative Western blot analysis showing p53 phospho-Ser392 and Bax expression in lysates prepared from hearts of WT and Akita mice expressing rAAV-GFP (GFP) or rAAV-Lep (LEP). Protein extracts from WT, GFP, and LEP groups were separated by PAGE and nitrocellulose blots probed with antibodies to p53 phospho-Ser392 and Bax as in A. B, middle and bottom: densitometric analyses for p53 phospho-Ser392 and Bax expression. Data represent 4 to 5 independent experiments: means ± SD and *P ≤ 0.05.
DISCUSSION
The present study demonstrates that p66ShcA is necessary for HG-induced oxidative stress and that interventions which interrupt or prevent phosphorylation of the p66ShcA protein at Ser36 turn off p66ShcA redox activity. We have shown ARVMs transduced with mu-36 construct exhibit an oxidant-resistant phenotype, as judged by the inhibition of HG-induced ROS production, the stabilization of mitochondrial energetics, and the expression of the survival program. To evaluate p66ShcA signaling in vivo, studies were performed with hearts of Akita diabetic mice. The results show an order of progression for phospho-Ser36 and downstream p66ShcA signaling events, identical to that detected in our in vitro studies with ARVMs at HG. To explore whether the correction of HG mimics the p66ShcA expression profile detected in mu-36 ARVMs, Akita mice were treated with the potent blood glucose-lowering hormone leptin. An analysis of hearts from LEP mice showed an order of progression of p66ShcA signaling, identical to that detected in mu-36 ARVMs, indicative that HG stress signals target phospho-Ser36 in the diabetic myocardium to turn on p66ShcA redox activity. Our results indicate that p66ShcA is a molecular switch whose redox function is turned on by HG-induced phosphorylation at Ser36 and turned off by interventions that prevent this modification.
The major objective of the present study was to test whether adult CMCs exhibit increased resistance to HG-induced oxidative stress following interventions that interrupt or prevent phosphorylation at Ser36 of the p66ShcA protein. The p66ShcA protein has emerged as a genetic determinant of longevity in mammals (29) that controls mitochondrial metabolism and cellular responses to oxidative stress, aging, and apoptosis. At the organismal level, the p66ShcA−/− mouse is the unique genetic model of increased resistance to oxidative stress, aging, and apoptosis (9, 29). We hypothesized adenoviral transduction of ARVMs with mu-36 protein would confer a dominant-interfering phenotype, attenuating or preventing the transmission of HG danger signals. Our results show that mu-36 ARVMs exhibit an oxidant-resistant phenotype, as judged by the inhibition of HG-induced ROS production, the attenuation of phospho-Ser36 levels, and the maintenance of ΔΨm. Conversely, ARVMs showed a marked increase in ROS production at HG, an upregulation in phospho-Ser36 levels, and a collapse of ΔΨm. Importantly, the HG-induced component of apoptosis was not detected in mu-36 ARVMs, whereas ARVMs exhibited a 40% increase in this parameter. Taken together, we have identified a pivotal role for p66ShcA redox function as a precursor to HG-induced free radical injury in ARVMs.
A dominant mutation in the Ins2 gene due to an amino acid change results in HG and DM in the Akita mouse. Akita mice have several advantages over inbred mouse strains that require streptozotocin treatment (16, 17), including a better-defined etiology (endoplasmic reticulum stress and proteotoxicity in pancreatic β-cells), along with a more pronounced and durable HG (16, 17). For these reasons, Akita mice were used to evaluate the in vivo consequences of p66ShcA signaling in diabetic myocardium. Our results showed that Akita diabetic mice developed significant HG along with the upregulation in phospho-Ser36 levels that were accompanied by a translocation of p66ShcA protein to mitochondria and an increased formation of p66ShcA/cytochrome-c complexes. Mitochondria are critical determinants of cell death and cell survival. Similar to our in vitro results with ARVMs at HG, the expression of cleaved caspase-3 and the apoptosis-related proteins p53 and Bax were found to be upregulated in hearts of Akita mice. Conversely, rAAV-Lep (LEP) Akita mice were euglycemic, and their hearts showed no detectable alteration in phospho-Ser-36, in p66ShcA/cytochrome-c complexes, or in the expression of terminal components of the apoptosis program. Taken together, we have shown that, in hearts of Akita mice, a single injection of rAAV-Lep (LEP) reverses the effect of HG on the phosphorylation status and redox function of the p66ShcA protein.
The present study has certain limitations, including the necessity to maintain cells under serum-free conditions to eliminate the confounding effects of contained growth factors on signaling pathways linked to cell survival and oxidant stress. Second, we must acknowledge the shortcomings of an in vitro system in simulating a complex metabolic disorder, such as DM. Finally, the precise mechanism(s) by which leptin corrects HG remains to be determined.
Taken into account the above limitations, the present study clearly demonstrates that p66ShcA functions as a potentially harmful regulatory gene, which is required for the generation of HG-induced oxidative stress and apoptosis. Recently, a unifying hypothesis has been proposed for the development of diabetic complications, based on the overproduction of ROS. In support of this hypothesis, Rota et al. (31) report that following the induction of experimental DM, p66ShcA−/− mice express a cardioprotection phenotype characterized by decreased markers of cell senescence and the preservation of CMC number and LV function, whereas WT diabetic mice exhibit cardiac stem cell aging, myocyte apoptosis, and develop heart failure. Whether gene-based strategies that incorporate small-interfering RNA to silence disease-causing genes (32), such as p66ShcA, can be applied in vivo to selectively target cardiac muscle cells remains to be determined.
GRANTS
This work was supported by National Institutes of Health Grants HL-072852 (to A. Malhotra) and RO1-DK-073793 (to L. G. Meggs) and a grant from the Wildwood Foundation (to L. G. Meggs).
Acknowledgments
Part of this work was presented at the Scientific Sessions, American Heart Association meeting in November 2007 held in Orlando, FL (Circulation, Supplement, 116, 2007).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bagnasco M, Dube MG, Katz A, Kalra PS, Kalra SP. Leptin expression in hypothalamic PVN reverses dietary obesity and hyperinsulinemia but stimulates ghrelin, Obesity Res 11, 1463. –1470, 2003. [DOI] [PubMed]
- 2.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48: 1–9, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Bell DS Diabetic cardiomyopathy: a unique entity or a complication of coronary artery disease? Diabetes Care 18: 708–714, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Brownlee M The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54: 1615–1625, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Chen IY, Lypowy J, Pain J, Sayed D, Grinberg S, Alcendor RR, Sadoshima J, Abdellatif M. Histone H2A.z is essential for cardiac myocyte hypertrophy but opposed by silent information regulator 2alpha. J Biol Chem 281: 19369–19377, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Chintapalli J, Yang S, Opawumi D, Goyal SR, Shamsuddin N, Malhotra A, Reiss K, Meggs LG. Inhibition of wild-type p66ShcA in mesangial cells prevents glycooxidant-dependent FOXO3a regulation and promotes the survival phenotype. Am J Physiol Renal Physiol 292: F523–F530, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell 116: 205–219, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Fiordaliso F, Leri A, Cesselli D, Limana F, Safai B, Nadal-Ginard B, Anversa P, Kajstura J. Hyperglycemia activates p53 and p53 regulated genes leading to myocyte cell death. Diabetes 50: 2363–2375, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122: 221–233, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Graham FL, Prevec L. In: Methods in Molecular Biology, ed. by Murray EJ. Clifton, NJ: Humana, 1991, pp. 109–128. [DOI] [PubMed]
- 11.Graiani G, Lagrasta C, Migliaccio E, Spillmann F, Meloni M, Madeddu P, Quaini F, Padura IM, Lanfrancone L, Pelicci P, Emanueli C. Genetic deletion of the p66Shc adaptor protein protects from angiotensin II-induced myocardial damage. Hypertension 46: 433–440, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science 299: 1355–1359, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Heart Outcomes Prevention Evaluation (HOPE). Study investigators effect of ramapril on cardiovascular and microvascular complications in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 355: 253–259, 2000. [PubMed] [Google Scholar]
- 14.Izumi T, Yokota-Hashimoto H, Zhao S, Wang J, Halban PA, Takeuchi T. Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes 52: 409–416, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Kajstura J, Fiordaliso F, Andreoli AM, Li B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, Anversa P. IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes 50: 1411–1424, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Kakoki M, Kizer CM, Yi X, Takahashi N, Kim HS, Bagnell CR, Edgell CJS, Maeda N, Jenette JC, Smithies O. Senescent associated phenotypes in Akita diabetic mice are enhanced by absence of bradykinin B2 receptors. J Clin Invest 166: 1302–1309, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kakoki M, Takahashi N, Jenette JC, Smithies O. Diabetic nephropathy is markedly enhanced in mice lacking the B2 receptor. Proc Natl Acad Sci USA 101: 13302–13305, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang BP, Frencher S, Reddy V, Kessler A, Malhotra A, Meggs LG. High glucose promotes mesangial cell apoptosis by oxidant-dependent mechanism. Am J Physiol Renal Physiol 284: F455–F466, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Kang BP, Urbonas A, Baddoo A, Baskin S, Malhotra A, Meggs LG. IGF-1 inhibits the mitochondrial apoptosis program in mesangial cells exposed to high glucose. Am J Physiol Renal Physiol 285: F1013–F1024, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham Heart Study. JAMA 241: 2035–2038, 1979. [DOI] [PubMed] [Google Scholar]
- 21.Leri A, Liu Y, Wang X, Kajstura J, Malhotra A, Meggs LG, Anversa P. Overexpression of insulin-like growth factor-1 attenuates the myocyte renin angiotensin system in transgenic mice. Circ Res 84: 754–762, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Malhotra A, Begley R, Kang BP, Rana I, Liu J, Yang G, Mochly-Rosen D, Meggs LG. PKCɛ-dependent survival signals in diabetic hearts. Am J Physiol Heart Circ Physiol 289: H1343–H1350, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Malhotra A, Kang BP, Cheung S, Opawumi D, Meggs LG. Angiotensin II promotes glucose-induced activation of cardiac protein kinase C isozymes and phosphorylation of troponin I. Diabetes 50: 1918–1926, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Malhotra A, Kang BP, Hashmi S, Meggs LG. PKCɛ inhibits the hyperglycemia apoptosis signal in adult rat ventricular myocytes. Mol Cell Biochem 268: 169–173, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Malhotra A, Reich D, Nakouzi A, Sanghi V, Geenen DL, Buttrick PM. Experimental diabetes is associated with functional activation of protein kinase Cɛ and phosphorylation of troponin I in the heart, which are prevented by angiotensin II receptor blockade. Circ Res 81: 1027–1033, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lamfrancone L, Pelicci PG. The p66Shc adaptor protein controls oxidative stress and life span in mammals. Nature 402: 309–313, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Napoli C, Martin-Padura I, de Nigris F, Giorgio M, Mansueto G, Somma P, Condorelli M, Sica G, De Rosa G, Pelicci P. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci USA 100: 2112–2116, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, Martin-Padura I, Pelliccia G, Trinei M, Bono M, Puri C, Tacchetti C, Ferrini M, Mannucci R, Nicoletti I, Lanfrancone L, Giorgio M, Pelicci PG. The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J Biol Chem 279: 25689–25695, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F, Wieckowski MR, Del Sal G, Pelicci PG, Rizzuto R. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science 315: 659–663, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigues B, McNeill JH. The diabetic heart: metabolic causes for the development of a cardiomyopathy. Cardiovasc Res 26: 913–922, 1992. [DOI] [PubMed] [Google Scholar]
- 31.Rota M, LeCapitaine N, Hosoda T, Boni A, De Angelis A, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Lüscher TF, Pelicci PG, Anversa P, Leri A, Kajstura J. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res 99: 42–52, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Santiago Y, Chan E, Liu PQ, Orlando S, Zhang L, Urnov FD, Holmes MC, Guschin D, Waite A, Miller JC, Rebar EJ, Gregory PD, Klug A, Collingwood TN. Targeted gene knockout in mammalian cells by using engineered zinc-finger nucleases. Proc Natl Acad Sci USA 105: 5809–5814, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueno N, Inui A, Kalra PS, Kalra SP. Leptin transgene expression in the hypothalamus enforces euglycemia in diabetic, insulin-deficient nonobese Akita mice and leptin-deficient obese ob/ob mice. Peptides 27: 2332–2342, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res 47: 1–9, 1980. [DOI] [PubMed] [Google Scholar]
- 35.Yang S, Chintapalli J, Sodagum L, Baskin S, Malhotra A, Reiss K, Meggs LG. Activated IGF-1R inhibits hyperglycemia-induced DNA damage and promotes DNA repair by homologous recombination. Am J Physiol Renal Physiol 289: F1144–F1152, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci USA 105: 14070–14075, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]