Abstract
Coronary artery disease (CAD) is the leading cause of mortality in diabetic patients. Because of the diffuse nature of their disease, diabetic patients may be at risk for incomplete revascularization, highlighting a potential role for proangiogenic therapy in this group. This study investigates molecular mechanisms of angiogenesis in diabetic patients. Myocardial tissue was harvested from patients undergoing coronary artery bypass grafting [nondiabetic (ND) 11, type 2 diabetic (DM) 10]. Expression of angiostatin, endostatin, their precursors (plasminogen and collagen XVIII, respectively), enzymes leading to their production [matrix metalloprotease (MMP)-2 and -9, cathepsin L], and an inhibitor of MMPs (tissue inhibitor of metalloproteinase) was assessed with Western blotting. MMP activity was assessed. Coronary collateralization was graded by Rentrop scoring of angiograms. Plasminogen and collagen XVIII expression were similar between groups. Angiostatin expression trended to increase 1.24-fold (P = 0.07), and endostatin expression increased 2.02-fold in DM patients relative to ND (P = 0.02). MMP-9 expression was no different between groups, whereas MMP-2 expression decreased 1.8-fold in diabetics (P = 0.003). MMP-2 and -9 activity decreased 1.33-fold (P = 0.03) and 1.57-fold (P = 0.04), respectively, in diabetic patients. Cathepsin L expression was 1.38-fold higher in diabetic patients (P = 0.02). Coronary collateralization scores were ND 2.1 ± 0.37 vs. DM 1.0 ± 0.4 (P = 0.05). Myocardial endostatin expression correlated strongly with the percentage of hemoglobin A1c (r = 0.742, P = 0.0001). Myocardial expression of angiostatin and endostatin demonstrated significant negative linear correlations with coronary collateralization (angiostatin r = −0.531, P = 0.035, endostatin r = −0.794, P = 0.0002). Diabetic patients with CAD exhibit increased levels of the antiangiogenic proteins angiostatin and endostatin and differential regulation of the enzymes governing their production relative to ND patients. Myocardial levels of these proteins show significant correlation to coronary collateralization. These findings offer potential new therapeutic targets for enhancing proangiogenic therapy and insight into the angiogenic impairments seen in diabetes.
Keywords: matrix metalloprotease, tissue inhibitor of metalloprotease
over 20 million individuals suffer from diabetes mellitus (DM) in the United States (29a). These individuals carry an eightfold increase in risk for suffering from an adverse cardiovascular event (15), and cardiovascular events account for 65% of all deaths in this population making it the leading cause of mortality in this group (29a). Despite lifestyle modification and aggressive strategies aimed at improving glucose control, many of these patients suffer from advanced coronary artery disease (CAD) necessitating myocardial revascularization. Although percutaneous intervention may be an option for some of these patients, studies have demonstrated improved outcomes with coronary artery bypass grafting (CABG) (7, 30). Despite continued refinements in surgical technique, adequate distal coronary artery bypass targets may be limited in diabetic patients secondary to the diffuse distribution of their disease and the presence of vascular calcifications. The question is then raised, are these patients the ideal candidates for surgically delivered proangiogenic therapy? While initial preclinical trials in healthy large animal models demonstrated significant benefit in inducing coronary collateral formation utilizing vascular endothelial growth factor (18) and fibroblast growth factor-2 (39), the majority of the subsequent randomized, double-blind, placebo-controlled clinical trials failed to demonstrate significant objective benefit (reviewed in Ref. 4).
These findings, combined with evidence that diabetic patients exhibit impaired endogenous coronary collateral formation in response to chronic ischemia (1), highlighted the need for an improved understanding of the effects of diabetes on molecular angiogenic signaling pathways influencing coronary collateral formation. To address this issue, our group and others have investigated angiogenic signaling in the setting of diabetes in preclinical animal models of hyperglycemia (46) and diabetes (5). A key finding of these studies indicated hyperglycemia is associated with increased antiangiogenic signaling, specifically implicating two well-studied proteins in the oncology literature, angiostatin and endostatin.
J. Folkman originally published his hypothesis that the regulation of angiogenesis in vivo is comprised of a balance between angiogenic and angiostatic factors (14). Twenty years subsequent to that publication, his laboratory reported on the discovery of an endogenous inhibitor of angiogenesis (angiostatin) followed thereafter by their discovery of endostatin in 1997 (32, 33). These antiangiogenic proteins are derived from plasminogen and collagen XVIII, respectively, through a series of cleavage steps, ultimately by matrix metalloproteinases (MMPs). This study investigates the relationship between diabetes, angiostatin and endostatin, and coronary collateral formation.
MATERIALS AND METHODS
Patient tissue harvest.
Myocardial tissue samples were obtained from the right atrial appendage of 21 patients [11 nondiabetic (ND), 10 DM] undergoing initial elective CABG at the start of the surgical procedure. Tissue was immediately placed in liquid nitrogen and stored at −80°C for use in molecular studies. Patient samples were not harvested if any history of malignancy was reported. The study was approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center (Boston, MA). Written, informed consent was obtained from each study patient.
Assessment of coronary collateralization.
Standard coronary angiography using four or more views of the left coronary system and two views of the right coronary system was performed before surgery. Degree of coronary collateralization was assessed by a blinded board-certified cardiologist with a subspecialty in interventional cardiology. Collateral flow before revascularization was graded using the classification developed by Rentrop et al. (38): grade 0 = no visible filling of any collateral channels; grade 1 = filling of side branches of the infarct artery with no dye reaching the epicardial segment; grade 2 = partial filling of the epicardial vessel; and grade 3 = complete filling of the epicardial vessel by collateral vessels.
Western blotting.
Whole cell lysates were isolated from the homogenized myocardial samples with a RIPA buffer (Boston Bioproducts, Worcester, MA) and centrifuged at 12,000 g for 10 min at 4°C to separate soluble from insoluble fractions. Protein concentration was measured spectrophotometrically at a 595-nm wavelength with a DC protein assay kit (Bio-Rad, Hercules, CA). Total protein (40–80 μg) was fractionated by 4–20% gradient and SDS-PAGE (Invitrogen, San Diego, CA) and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). Each membrane was incubated with specific antibodies as follows: anti-plasminogen antibody (dilution 1:500) (Calbiochem, San Diego, CA), anti-angiostatin antibody (1:2,500) (BD Biosciences, San Jose, CA), anti-collagen XVIII antibody (1:300) (Santa Cruz Biotech, Santa Cruz, CA), anti-endostatin antibody (1:1,000) (Upstate, Chicago, IL), anti-MMP-2 antibody (1:500) (Calbiochem), anti-MMP-9 (1:500) (Millipore), anti-tissue inhibitor of metalloproteinase-2 (TIMP-2) antibody (1:300) (Calbiochem), and anti-cathepsin L antibody (1:300) (Calbiochem). Membranes were subsequently incubated for 1 h in diluted appropriate secondary antibody (Jackson Immunolab, West Grove, PA). Immune complexes were visualized with the enhanced chemiluminescence detection system (Amersham, Piscataway, NJ). Bands were quantified by densitometry of radioautograph films. Ponceau S staining was performed to confirm equivalent protein loading.
Serum measurement of hemoglobin A1c.
Hemoglobin A1c (HbA1c) measurements were performed at the Beth Israel Deaconess Medical Center Clinical Laboratory. Laboratory ranges for normal in ND patients are 4.8–5.9% HbA1c. On average, each 1% increase in glycated hemoglobin represents roughly a 30 mg/dl increase in mean blood glucose. Thus a glycated hemoglobin of 10% corresponds to a mean blood glucose of 250 mg/dl: a normal 5% glycated hemoglobin corresponds to a normal 100 mg/dl mean blood glucose; an increment of 5% (to get to 10%) corresponds to 5 × 30 = 150 mg/dl glucose; 100 + 150 = 250.
Gelatin zymography for MMP activity.
To determine the degree of MMP-2 and MMP-9 activity in myocardial specimens, gelatin zymography was performed. Protein (40 μg) in 20 μl of zymogram buffer (Bio-Rad) was loaded from tissue homogenates on to a 10% SDS-PAGE gel copolymerized with gelatin (1 mg/ml). Proteins were electrophoretically separated at 120 volts constant current and then washed for 1 h in 2.5% Triton X-100 (Renaturation Buffer; Bio-Rad) at room temperature and incubated overnight in 50 mmol/l Tris·HCl (pH 7.5), 200 mmol/l NaCl, 5 mmol/l CaCl2, and 0.02% Brij-35 (Development Buffer; Bio-Rad). Gels were stained with 0.5% Coomassie blue in 30% methanol and 10% glacial acetic acid and destained in the same solution lacking Coomassie blue. The gelatinolytic activity was identified as transparent bands against the background of Coomassie blue-stained gelatin. Band size (based on migration) and loading of control purified MMP-2 and MMP-9 were used to identify bands of interest.
Regression analysis.
Linear regression analyses were performed utilizing Pearson's correlation coefficient to determine if an association existed between tissue levels of angiostatin and endostatin and percent HbA1c and coronary collateralization (as quantified by Rentrop scoring).
Data analysis.
Data are reported as means ± SE. Immunoblots are expressed as a ratio of protein to loading band density and were analyzed after digitization and quantification of X-ray films with ImageJ 1.33 (National Institutes of Health). Blots were analyzed using ANOVA. Bonferroni corrections were applied to multiple tests, and probability values <0.05 were considered statistically significant.
RESULTS
Patient characteristics.
Patient groups (ND and DM) were similar in terms of age, gender, and comorbidities. Based on %HbA1c, the calculated mean blood sugar of patients in the ND group was 118 and 184 mg/dl in the DM group. One patient in the DM group was a type 2 diabetic utilizing insulin therapy. The average level of inflow occlusion was similar between the ND group (82.5 ± 3.1%) and the DM group (80.7 ± 5.1%), P = 0.53 (Table 1).
Table 1.
Baseline patient characteristics
| Nondiabetic | Diabetic | P Value | |
|---|---|---|---|
| Age, yr | 73.3±3.1 | 67.0±2.6 | 0.14 |
| Males, % | 64 | 70 | 0.77 |
| HbA1c, % | 5.6±0.11 | 7.8±0.9 | 0.02* |
| Hypertension, % | 64 | 60 | 0.87 |
| Hyperlipidemia, % | 45 | 40 | 0.81 |
| Chronic renal insufficiency, % | 0 | 0 | NS |
| History of smoking, % | 27 | 40 | 0.31 |
| History of stroke, % | 0 | 0 | NS |
| b-Blocker, % | 73 | 70 | 0.90 |
| Calcium channel blocker, % | 18 | 10 | 0.61 |
| ACE inhibitor, % | 27 | 30 | 0.90 |
| Statin, % | 36 | 50 | 0.85 |
| Aspirin, % | 64 | 60 | 0.87 |
ACE, angiotensin-converting enzyme; NS, not significant.
P < 0.05.
Myocardial expression of plasminogen and collagen XVIII.
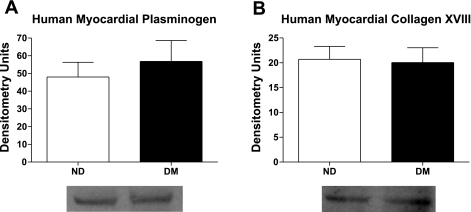
Myocardial levels of plasminogen [ND 48.0 ± 8.3 densitometry units (DU) vs. DM 56.8 ± 11.8 DU, P = 0.58] and collagen XVIII (ND 20.7 ± 2.6 DU vs. DM 20.1 ± 2.9 DU, P = 0.87) were similar between groups (Fig. 1).
Fig. 1.
A: myocardial expression of plasminogen, the precursor of angiostatin, was similar between diabetic and nondiabetic patients. Similarly, myocardial expression of collagen XVIII (B), the precursor of endostatin, remained similar between groups.
Myocardial expression of angiostatin and endostatin.
Myocardial expression of angiostatin (Fig. 2A) trended to a 1.24-fold increase in DM patients relative to ND patients (ND 76.6 ± 7.6 DU vs. DM 95.2 ± 5.9 DU, P = 0.07). Endostatin expression in the myocardium (Fig. 2B) was 2.02-fold higher in DM compared with ND subjects (ND 24.7 ± 6.7 DU vs. 50.0 ± 5.9 DU, P = 0.02).
Fig. 2.
Myocardial expression of angiostatin (A) and endostatin (B) was increased in diabetic patients. +P = 0.07 and *P < 0.05.
Myocardial expression of MMP-2 and -9.
A 1.8-fold reduction in expression of the active form of MMP-2 was observed in DM relative to ND patients (ND 86.2 ± 5.5 DU vs. DM 47.8 ± 10.4 DU, P = 0.003), whereas expression of the active form of MMP-9 remained similar between groups (ND 27.50 ± 5.2 DU vs. DM 24.2 ± 9.1, P = 0.76) (Fig. 3, A and B). Latent forms of MMP-2 and MMP-9 were assessed and demonstrated no significant differences (data not shown).
Fig. 3.
Expression of the active form of matrix metalloproteinase (MMP)-2 was significantly lower in diabetic patients (A), whereas expression of the active form of MMP-9 remained similar between patient groups (B). *P < 0.05.
Myocardial MMP activity.
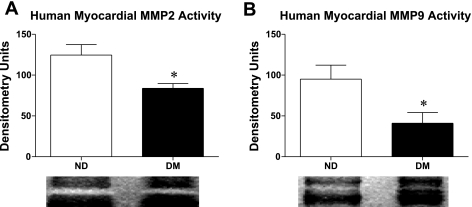
A 1.33-fold decrease in MMP-2 activity was seen in the myocardium of DM patients relative to ND patients (ND 124.5 ± 12.9 DU vs. DM 83.8 ± 5.9 DU, P = 0.03). MMP-9 activity decreased by 1.57-fold in DM relative to ND patients (ND 95.0 ± 17.1 DU vs. 41.00 ± 13.3 DU, P = 0.04) (Fig. 4, A and B).
Fig. 4.
MMP-2 (A) and MMP-9 (B) activity was significantly lower in the myocardium of diabetic patients. *P < 0.05.
Myocardial expression of cathepsin L.
A 1.38-fold increase in cathepsin L expression was seen in the myocardium of DM patients relative to ND patients (ND 588.9 ± 61.6 DU vs. DM 815.1 ± 48.2 DU, P = 0.02) (Fig. 5).
Fig. 5.
Myocardial expression of cathepsin L was increased in diabetic patients. *P < 0.05.
Myocardial expression of TIMP.
TIMP-2 expression remained similar between DM and ND patients (ND 23.3 ± 1.9 DU vs. 24.3 ± 1.7 DU, P = 0.69) (Fig. 6).
Fig. 6.
Myocardial expression of tissue inhibitor of metalloproteinase (TIMP)-2, an inhibitor of MMP-2 and MMP-9 activity, remained similar between diabetic and nondiabetic patients.
Coronary collateralization.
Coronary collateralization was impaired in DM patients compared with ND patients, with a mean Rentrop score in the ND group 2.1 ± 0.37 vs. 1.0 ± 0.4 in the DM group (P = 0.05). Two patients were excluded from scoring analysis (1 ND, 1 DM) because coronary stenosis <80%, which is generally regarded as necessary for collateral development (Fig. 7).
Fig. 7.
Coronary collateralization, as quantified by Rentrop scoring, in nondiabetic (ND) vs. diabetic (DM) patients. +P = 0.05.
Regression analysis.
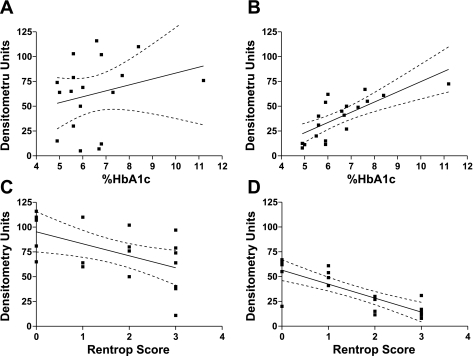
Myocardial expression of angiostatin and endostatin demonstrated strong negative linear correlations with coronary collateralization in patients, as assessed by Rentrop scoring (myocardial angiostatin vs. Rentrop score r = −0.531, P = 0.035, myocardial endostatin vs. Rentrop score r = −0.794, P = 0.0002).
Myocardial expression of endostatin demonstrated a positive linear correlation with %HbA1c (Table 2 and Fig. 8).
Table 2.
Regression analysis
| r2 | r | P Value | |
|---|---|---|---|
| Myocardial endostatin | |||
| HbA1c, % | 0.551 | +0.742 | <0.001* |
| Rentrop score | 0.63 | −0.794 | 0.0002* |
| Myocardial angiostatin | |||
| HbA1c, % | 0.060 | +0.245 | 0.360 |
| Rentrop score | 0.2821 | −0.531 | 0.035* |
Fig. 8.
Linear regression analysis displaying angiostatin vs. %hemoglobin A1c (HbA1c), r = 0.245 (A), endostatin vs. % HbA1c, r = 0.742 (P < 0.05) (B), angiostatin vs. Rentrop score, r = −0.531 (P < 0.05) (C), and endostatin vs. Rentrop score, r = −0.794 (P < 0.05) (D).
DISCUSSION
The primary novel findings of this study indicate myocardial levels of the antiangiogenic proteins angiostatin and endostatin are increased in diabetic patients. The myocardial levels of angiostatin and endostatin have a strong negative correlation to coronary collateralization in patients and a positive correlation to blood glucose levels in the case of endostatin. The myocardial levels of the two proteins is not regulated at the level of production of their precursors, plasminogen and collagen XVIII, but may be at the level of enzymes responsible for their production through cleavage. Of these enzymes, MMP-2 and MMP-9 exhibit diminished activity in the myocardium of diabetic patients, whereas the expression of cathepsin L is increased in the diabetic myocardium. There does not appear to be a difference in an inhibitor of their activity, TIMP-2.
The development of coronary collateral vessels represents an endogenous adaptive response to chronic myocardial ischemia. The presence of adequate coronary collateralization can limit infarct size after coronary occlusion and may provide a survival benefit (16, 17, 19, 47). Given the importance of coronary collateral formation, the findings that diabetic patients manifest impaired coronary collateralization (1) highlight the importance of understanding the molecular basis for this impaired response, especially if future clinical trials of proangiogenic growth factors are to be successful.
Angiostatin.
Angiostatin (33) has been the focus of intensive study for the treatment of cancer where it has been shown to be an effective antiangiogenic agent (13, 22). A cleavage product of plasminogen, angiostatin, is produced after a series of cleavage steps involving plasmin, disulfide reductase, and terminally, MMP-2 and -9 (45). The molecular mechanism by which it exerts its antiangiogenic effects have yet to be fully elucidated but presently center around endothelial cells. Angiostatin has been shown to induce endothelial cell death through apoptotic pathways (9, 28, 29, 43) and impair endothelial cell proliferation (40), migration, and tube formation by binding to angiomotin (44). Despite these findings, numerous angiostatin-binding partners exist for which functionality has yet to be determined (45).
Mechanistically, it is unclear how diabetes or hyperglycemia induces increases in angiostatin. There is evidence the plasmin reductase involved in plasmin processing is phosphoglycerate kinase (PGK), a glycolytic enzyme (25). Interestingly, PGK activity is increased diabetes (42), providing a possible explanation for increased angiostatin in this setting. This increased PGK activity is upstream of the MMP, which may explain why increases in angiostatin are seen despite decreases in MMP-2 and MMP-9 activity. Future studies investigating PGK activity in the myocardium of diabetic patients may be of value.
Two prior reports by other groups have demonstrated angiostatin expression is increased in the setting of hyperglycemia (46) and diabetes (10). The first of these reports utilized a canine model of hyperglycemia (via continuous glucose infusions) for 21 days and examined myocardial interstitial fluid (MIF) and found increases in angiostatin levels in MIF at day 21. Chung et al. (10) examined internal mammary artery samples of diabetic and nondiabetic patients and found a 1.62-fold increase in angiostatin expression in diabetic mammary arteries. These results are consistent with those of our study which demonstrate increases in myocardial tissue levels of angiostatin.
Endostatin.
Endostatin, the 20-kDa cleavage product of collagen XVIII, has been shown to be a potent inhibitor of angiogenesis (32). Its mechanism of action remains the subject of investigation. Our current understanding of endostatin's mechanism of action indicates it can induce endothelial cell apoptosis through multiple signaling pathways (11, 21) and diminish cell migration, adhesion, and proliferation (23, 37, 41). These findings are likely only a few of those relevant to endostatin function, since microarray studies of endothelial cells have show endostatin can regulate 12% of the genome, including a significant number of antiangiogenic genes (which are upregulated) and proangiogenic genes (which are downregulated) (2, 3).
Investigation into MMP activity and TIMP expression, as discussed below, did not explain why endostatin expression is increased in diabetic subjects. Although previous reports indicate that MMPs may be a responsible enzyme for this as discussed below, our results demonstrate cathepsin L, which can generate endostatin from collagen XVIII (12), may also play a role in the differential expression of endostatin observed. Other investigators have demonstrated that cathepsin activity is altered in the setting of diabetes (20, 24). Reddy et al. (36) have shown collagen breakdown is differentially regulated between diabetic and nondiabetic subjects. This variability in collagen breakdown and the role of extracellular matrix glycation in diabetes warrant future study as potential mechanisms for changes in endostatin production.
MMPs and TIMP-2.
MMP-2 and MMP-9 are both able to cleave plasminogen and collagen XVIII to generate angiostatin and endostatin, respectively. Interestingly, these proteases act as proangiogenic mediators initially, allowing for degradation of the extracellular matrix to facilitate collateralization but can ultimately lead to inhibition of angiogenesis through generation of angiostatin and endostatin, revealing the fine dynamic balance of the angiogenic process. Controversy exists as to whether diabetes induces an upregulation or downregulation of MMP activity. Large animal studies in canines made hyperglycemic via exogeous glucose infusion have demonstrated increases in MMP-9 activity in the MIF of these dogs (46). Although not in the setting of diabetes, previous authors have examined pericardial fluid levels of angiostatin and MMP activity. Although pericardial angiostatin levels were increased in patients with CAD and limited coronary collateralization, MMP-2 and -9 activity remained unchanged (27). The two prior studies investigating MMP activity in diabetic human samples (mammary artery) reached opposite conclusions; MMP-2 and MMP-9 activity is increased (10) and decreased (35) in diabetic mammary arteries. Our results are consistent with the latter, although comparison is limited because of the samples examined (myocardium vs. mammary artery). It is unknown whether these differences are the result of different patient variables, or methodology. Notably, endostatin, which our study demonstrates is increased in diabetes, can act as an inhibitor of MMP-2 and MMP-9 (26, 31), which may account for the decreases in MMP activity seen in our investigation.
To investigate molecular signaling governing MMP activity, we examined expression of TIMP-2, a protein capable of inhibiting MMP-2 and -9 and which has been shown to be downregulated in the setting of diabetes (10). Our study did not demonstrate any significant differences in TIMP-2 expression in the myocardium of nondiabetic vs. diabetic patients. This may reflect differences in the tissue studied from the previous reference. At present, no exhaustive approach investigating which TIMPs inhibit which MMPs most effectively has been undertaken (8), leaving the possibility alternative TIMPs may play a role in inhibiting MMP-2 and -9 in the myocardium and be differentially expressed in diabetes. Alternately, inhibition of MMP activity may occur primarily through negative feedback inhibition, as discussed above, by endostatin (26, 31).
Angiostatin, endostatin, and coronary collateralization.
Angiostatin and endostatin have been the subject of several studies investigating coronary collateralization. Matsunaga et al. (27) analyzed pericardial fluid obtained from patients undergoing CABG and found increased pericardial fluid levels of angiostatin were associated with lower Rentrop scores. In a similar study investigating pericardial fluid levels of endostatin, Panchal et al. (34) found increased pericardial fluid levels of endostatin correlated to reduced collateralization. In patients undergoing angiography, Mitsuma and colleagues (27a) found increased differences in left ventricular blood vs. coronary sinus blood concentrations of endostatin were associated with poor collateralization. Our study, which focused on diabetes and myocardium, demonstrated myocardial levels of endostatin, but not angiostatin, correlate positively with %HbA1c levels. The myocardial levels of angiostatin and endostatin in patients both demonstrated significant negative linear correlations with coronary collateralization, but notably the relationship for endostatin was significantly stronger. Taken in sum, the results suggest that myocardial levels of endostatin may have greater relevance to coronary collateralization over angiostatin levels in diabetic patients.
Limitations.
The patient studies used nondiabetic patients with CAD as a control, whereas, ideally, a group of healthy age-matched controls would have been studied. Additionally, the associations observed between angiostatin, endostatin, and collateral formation cannot be categorized as causative.
In conclusion, diabetes mellitus is associated with an increase in myocardial levels of the potent antiangiogenic proteins angiostatin and endostatin. These increases, more so for endostatin, correlate strongly with coronary collateralization in patients. Targeting these two proteins may have significant therapeutic utility in enhancing both endogenous and exogenous growth factor-induced coronary collateral development.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant HL-46716. N. R. Sodha is supported, in part, by NHLBI Grant T-32HL076130-02 and the Irving Bard Memorial Fellowship.
Acknowledgments
We thank Dr. Vikas Sukhatme (Beth Israel Deaconess Medical Center, Boston, MA) for valuable insight and advice.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinc H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation 99: 2239–2242, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Abdollahi A, Hahnfeldt P, Maercker C, Grone HJ, Debus J, Ansorge W, Folkman J, Hlatky L, Huber PE. Endostatin's antiangiogenic signaling network. Mol Cell 13: 649–663, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Abdollahi A, Hlatky L, Huber PE. Endostatin: the logic of antiangiogenic therapy. Drug Resist Updat 8: 59–74, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Boodhwani M, Sodha NR, Laham RJ, Sellke FW. The future of therapeutic myocardial angiogenesis. Shock 26: 332–341, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Boodhwani M, Sodha NR, Mieno S, Feng J, Clements RT, Ruel M, Sellke FW. Insulin treatment enhances the myocardial angiogenic response in diabetes. JTCVS In press. [DOI] [PMC free article] [PubMed]
- 7.Brener SJ, Lytle BW, Casserly IP, Schneider JP, Topol EJ, Lauer MS. Propensity analysis of long-term survival after surgical or percutaneous revascularization in patients with multivessel coronary artery disease and high-risk features. Circulation 109: 2290–2295, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta 1477: 267–283, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Chen YH, Wu HL, Li C, Huang YH, Chiang CW, Wu MP, Wu LW. Anti-angiogenesis mediated by angiostatin K1–3, K1–4 and K1–4.5 Involvement of p53, FasL, AKT and mRNA deregulation. Thromb Haemost 95: 668–677, 2006. [PubMed] [Google Scholar]
- 10.Chung AW, Hsiang YN, Matzke LA, McManus BM, van Breemen C, Okon EB. Reduced expression of vascular endothelial growth factor paralleled with the increased angiostatin expression resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in human type 2 diabetic arterial vasculature. Circ Res 99: 140–148, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Dhanabal M, Ramchandran R, Waterman MJ, Lu H, Knebelmann B, Segal M, Sukhatme VP. Endostatin induces endothelial cell apoptosis. J Biol Chem 274: 11721–11726, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Felbor U, Dreier L, Bryant RA, Ploegh HL, Olsen BR, Mothes W. Secreted cathepsin L generates endostatin from collagen XVIII. Embo J 19: 1187–1194, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folkman J Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29: 15–18, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Folkman J Tumor angiogenesis: therapeutic implications. N Engl J Med 285: 1182–1186, 1971. [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM, Garber A, Goldberg R, Havas S, Holman R, Lamendola C, Howard WJ, Savage P, Sowers J, Vega GL. Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group IV: lifestyle and medical management of risk factors. Circulation 105: e153–e158, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Habib GB, Heibig J, Forman SA, Brown BG, Roberts R, Terrin ML, Bolli R. Influence of coronary collateral vessels on myocardial infarct size in humans. Results of phase I thrombolysis in myocardial infarction (TIMI) trial The TIMI Investigators. Circulation 83: 739–746, 1991. [DOI] [PubMed] [Google Scholar]
- 17.Hansen JF Coronary collateral circulation: clinical significance and influence on survival in patients with coronary artery occlusion. Am Heart J 117: 290–295, 1989. [DOI] [PubMed] [Google Scholar]
- 18.Harada K, Friedman M, Lopez JJ, Wang SY, Li J, Prasad PV, Pearlman JD, Edelman ER, Sellke FW, Simons M. Vascular endothelial growth factor administration in chronic myocardial ischemia. Am J Physiol Heart Circ Physiol 270: H1791–H1802, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Helfant RH, Vokonas PS, Gorlin R. Functional importance of the human coronary collateral circulation. N Engl J Med 284: 1277–1281, 1971. [DOI] [PubMed] [Google Scholar]
- 20.Huang X, Vaag A, Carlsson E, Hansson M, Ahren B, Groop L. Impaired cathepsin L gene expression in skeletal muscle is associated with type 2 diabetes. Diabetes 52: 2411–2418, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Kang HY, Shim D, Kang SS, Chang SI, Kim HY. Protein kinase B inhibits endostatin-induced apoptosis in HUVECs. J Biochem Mol Biol 39: 97–104, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer 2: 727–739, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Kim YM, Hwang S, Pyun BJ, Kim TY, Lee ST, Gho YS, Kwon YG. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. J Biol Chem 277: 27872–27879, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Kwan CY, Wang RR, Beazley JS, Lee RM. Alterations of elastin and elastase-like activities in aortae of diabetic rats. Biochim Biophys Acta 967: 322–325, 1988. [DOI] [PubMed] [Google Scholar]
- 25.Lay AJ, Jiang XM, Kisker O, Flynn E, Underwood A, Condron R, Hogg PJ. Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase. Nature 408: 869–873, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Lee SJ, Jang JW, Kim YM, Lee HI, Jeon JY, Kwon YG, Lee ST. Endostatin binds to the catalytic domain of matrix metalloproteinase-2. FEBS Lett 519: 147–152, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Matsunaga T, Chilian WM, March K. Angiostatin is negatively associated with coronary collateral growth in patients with coronary artery disease. Am J Physiol Heart Circ Physiol 288: H2042–H2046, 2005. [DOI] [PubMed] [Google Scholar]
- 27a.Mitsuma W, Kodama M, Hanawa H, Ito M, Ramadan MM, Hirono S, Obata H, Okada S, Sanada F, Yanagawa T, Kashimura T, Fuse K, Tanabe N, Aizawa Y. Serum endostatin in the coronary circulation of patients with coronary heart disease and its relation to coronary collateral formation. Am J Cardiol 99: 494–498, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Moser TL, Kenan DJ, Ashley TA, Roy JA, Goodman MD, Misra UK, Cheek DJ, Pizzo SV. Endothelial cell surface F1–F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci USA 98: 6656–6661, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moser TL, Stack MS, Asplin I, Enghild JJ, Hojrup P, Everitt L, Hubchak S, Schnaper HW, Pizzo SV. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci USA 96: 2811–2816, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.National Diabetes Statistics. National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda, MD: Natl Inst Health.
- 30.Niles NW, McGrath PD, Malenka D, Quinton H, Wennberg D, Shubrooks SJ, Tryzelaar JF, Clough R, Hearne MJ, Hernandez F Jr, Watkins MW, O'Connor GT. Survival of patients with diabetes and multivessel coronary artery disease after surgical or percutaneous coronary revascularization: results of a large regional prospective study Northern New England Cardiovascular Disease Study Group. J Am Coll Cardiol 37: 1008–1015, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Nyberg P, Heikkila P, Sorsa T, Luostarinen J, Heljasvaara R, Stenman UH, Pihlajaniemi T, Salo T. Endostatin inhibits human tongue carcinoma cell invasion and intravasation and blocks the activation of matrix metalloprotease-2, -9, and -13. J Biol Chem 278: 22404–22411, 2003. [DOI] [PubMed] [Google Scholar]
- 32.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88: 277–285, 1997. [DOI] [PubMed] [Google Scholar]
- 33.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79: 315–328, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Panchal VR, Rehman J, Nguyen AT, Brown JW, Turrentine MW, Mahomed Y, March KL. Reduced pericardial levels of endostatin correlate with collateral development in patients with ischemic heart disease. J Am Coll Cardiol 43: 1383–1387, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Portik-Dobos V, Anstadt MP, Hutchinson J, Bannan M, Ergul A. Evidence for a matrix metalloproteinase induction/activation system in arterial vasculature and decreased synthesis and activity in diabetes. Diabetes 51: 3063–3068, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Reddy GK Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit achilles tendon. Exp Diabesity Res 5: 143–153, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo C, Pihlajaniemi T, Alitalo K, Vuori K. Interaction of endostatin with integrins implicated in angiogenesis. Proc Natl Acad Sci USA 98: 1024–1029, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol 5: 587–592, 1985. [DOI] [PubMed] [Google Scholar]
- 39.Sellke FW, Li J, Stamler A, Lopez JJ, Thomas KA, Simons M. Angiogenesis induced by acidic fibroblast growth factor as an alternative method of revascularization for chronic myocardial ischemia. Surgery 120: 182–188, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Sharma MR, Tuszynski GP, Sharma MC. Angiostatin-induced inhibition of endothelial cell proliferation/apoptosis is associated with the down-regulation of cell cycle regulatory protein cdk5. J Cell Biochem 91: 398–409, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Shichiri M, Hirata Y. Antiangiogenesis signals by endostatin. FASEB J 15: 1044–1053, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Suhail M, Rizvi SI. Red cell phosphoglycerate kinase in insulin-dependent diabetes mellitus. Indian J Med Res 92: 21–23, 1990. [PubMed] [Google Scholar]
- 43.Tarui T, Miles LA, Takada Y. Specific interaction of angiostatin with integrin alpha(v)beta(3) in endothelial cells. J Biol Chem 276: 39562–39568, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Troyanovsky B, Levchenko T, Mansson G, Matvijenko O, Holmgren L. Angiomotin: an angiostatin binding protein that regulates endothelial cell migration and tube formation. J Cell Biol 152: 1247–1254, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wahl ML, Kenan DJ, Gonzalez-Gronow M, Pizzo SV. Angiostatin's molecular mechanism: aspects of specificity and regulation elucidated. J Cell Biochem 96: 242–261, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Weihrauch D, Lohr NL, Mraovic B, Ludwig LM, Chilian WM, Pagel PS, Warltier DC, Kersten JR. Chronic hyperglycemia attenuates coronary collateral development and impairs proliferative properties of myocardial interstitial fluid by production of angiostatin. Circulation 109: 2343–2348, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Williams DO, Amsterdam EA, Miller RR, Mason DT. Functional significance of coronary collateral vessels in patients with acute myocardial infarction: relation to pump performance, cardiogenic shock and survival. Am J Cardiol 37: 345–351, 1976. [DOI] [PubMed] [Google Scholar]